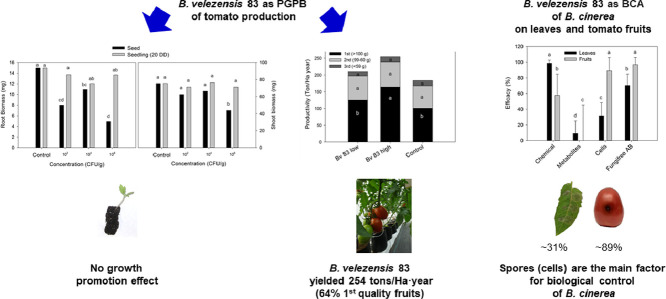
Highlights
-
•
B. velezensis 83 was shown as PGPB for greenhouse grown tomato plants.
-
•
B. velezensis 83 increased by 38% the productivity of tomato plants.
-
•
B. velezensis 83 increased by 19% the production of first quality fruits.
-
•
B. velezensis 83 showed high efficacy of control of B. cinerea on postharvest fruit.
-
•
Spores were the main antagonistic factor of Fungifree AB™ against B. cinerea.
Keywords: Bacillus velezensis 83, Plant growth promotion, Biological control, Greenhouse grown tomato
Abstract
Bacillus spp. are well known plant growth promoting bacteria (PGPB) and biological control agents (BCA) due to their capacity to synthesize a wide variety of phytostimulant and antimicrobial compounds. B. velezensis 83 is a strain marketed in Mexico as a foliar biofungicide (Fungifree AB™) which has been used for biological control of five different genera of phytopathogenic fungi (Colletotrichum, Erysiphe, Botrytis, Sphaerotheca, Leveillula) in crops of agricultural importance such as mango, avocado, papaya, citrus, tomato, strawberry, blueberry, blackberry and cucurbits, among others. In this work, the potential of plant growth promotion of B. velezensis 83 was evaluated on different phenological stages of tomato plants as well as the biocontrol efficacy of B. velezensis 83 formulations (cells and/or metabolites) against B. cinerea infection on leaves and postharvest fruits. Greenhouse grown tomato plants inoculated with a high concentration (1 × 108 CFU/plant) of B. velezensis 83 yielded 254 tons/Ha•year of which the 64% was first quality tomato (≥100 g/fruit), while the control plants produced less than 184 tons/Ha•year with only 55% of first quality tomato. Additionally, in vitro assays carried out with leaves and fruits, shown that the B. velezensis 83 cells formulation had an efficacy of control of B. cinerea infection of ∼31% on leaves and ∼89% on fruits, while the metabolites formulation had an efficacy of control of less than 10%. Therefore, it was concluded that spores (not the metabolites) are the main antagonism factor of Fungifree AB™. The high effectivity of B. cinerea control on fruits by B. velezensis 83, opens the possibility for a postharvest use of this biofungicide.
Graphical abstract
Introduction
Tomato (Solanum lycopersicum L.) is one of the vegetables with the highest production value worldwide. It is consumed in a wide variety of forms and has a beneficial impact on human health mainly due to its high content of lycopene, folic acid, ascorbic acid, flavonoids, α-tocopherol, potassium, and phenolic compounds (Erba et al., 2013). In 2019, world tomato production was 180 million tons and China was the main producing country with 62 million tons, contributing 35% of total world production. In that year, México produced 4 million tons representing 2% of world production and was the country with the highest export level of tomatoes in the world (1.8 million tons), being USA, Germany, France, Russia and the United Kingdom, the main consumers (FAO, 2019, http://www.fao.org/faostat/en/#data/QC). In México, tomato is the most important vegetable produced in protected agriculture (shade net and greenhouse). Greenhouse systems are the preferred production technology for tomato production because, with this cultivation system, two to three production cycles per year can be obtained; an efficient use of water and nutrients are feasible, along with a reduction of the incidence of pests or diseases thanks to the control of environmental variables (Padmanabhan et al., 2016; Hemming et al., 2020) The area of greenhouses around of world is estimated to be 500,000 Ha (RaboResearch, 2018, https://research.rabobank.com/). The United State Department of Agriculture (USDA, 2021, https://www.fas.usda.gov/data/mexico-tomato-annual-4) described in their tomato annual report that in México the total area cultivated with tomato was of 44,814 Ha (agricultural year 2020: October 2019-March 2021). In that year, the open field cultivation represented 66% of the total area planted with tomato, while greenhouse cultivation represented the 16%. However, the harvest of tomato from open field cultivation contributed with 33% of the total tomato produced that year, in contrast the harvest of greenhouse cultivation the contribution was of 40%. Other cultivation technologies such as shade mesh and tunnel contributed with the 27% of the Mexican tomato production.
Currently, farmers are interested in incorporating agroecological practices into the production systems to migrate towards organic agriculture with good yields, promote the efficient use of water and nutrients and to obtain products of high nutritional quality within the normative standards of good agricultural practices and safety. Currently, there are formulations with bacteria that act as biopesticides, biostimulants or biofertilizers, which promote plant health and plant growth, respectively (Chojnacka, 2015, Khatoon et al., 2020; Basu et al., 2021). Among these, several are based on Bacillus species which are well known as PGPB and BCA against various phytopathogens. In the Bacillus amyloliquefaciens operational group (B. amyloliquefaciens, Bacillus siamensis and Bacillus velezensis), B. velezensis species have been recognized as a plant-associated bacteria and they can directly or indirectly establish beneficial relationships with plants (Olanrewaju et al., 2017; Fan et al., 2017). Through direct mechanisms, Bacillus strains promote plant growth because they improve the uptake of nutrients such as nitrogen and phosphate, and/or by the production of phytohormones such as auxins(i.e. indole acetic acid, IAA), enzymes such as ACC deaminase or volatile organic compounds (VOC) such as 2,3-butanediol and acetoin (Asari et al., 2016; Borriss, 2016; Asari et al., 2017; Rabbee et al., 2019; Rabbee and Baek, 2020). On the other hand, by means of indirect mechanisms, Bacillus strains exert biological control through an antibiosis mechanism, due to the production of antimicrobial compounds such as lipopeptides and polyketides (Rabbee et al., 2019; Rabbee and Baek, 2020). Several examples of biological control with Bacillus strains on several species of phytopathogenic bacteria and fungi such as Pseudomonas syringae, Agrobacterium tumefaciens, Xanthomonas campestris, Xanthomonas axonopodis, Erwinia amylovora, Botrytis cinerea, Fusarium oxysporum, Colletotrichum gloeosporioides, Rhizoctonia solani and Penicillium expansum, have been reported (Fira, 2018). The biological control with B. velezensis also involves the competition for space and nutrients (characterized by the biofilm formation) and, in some cases, induced systemic resistance (ISR) in the plant (Fan et al., 2018; Chen et al., 2020). In this way, due to the interaction of the three different biological control mechanisms of B. velezensis, the incidence and severity of diseases in plants can be reduced (Fan et al., 2018; Rabbee et al., 2019). Moreover, Bacillus spp. form spores with high resistance to dehydration and heat, which makes them excellent candidates for formulating bioproducts (Kumar et al., 2011). In México, researchers of Instituto de Biotecnología-UNAM (Universidad Nacional Autónoma de México) have developed a biofungicide based on Bacillus velezensis 83, a formulation that it is marketed as Fungifree AB™, which has been used for the biological control of five different genera of phytopathogenic fungi (Colletotrichum, Erysiphe, Botrytis, Sphaerotheca, Leveillula) in crops of agricultural importance such as mango, avocado, papaya, citrus, tomato, strawberry, blueberry, blackberry, cucurbits (Balderas-Ruíz et al., 2020). However, there are no studies on B. velezensis 83 that provide evidence on its potential as a biostimulant. Therefore, the first objective of this work was to evaluate the biostimulant effect of B. velezensis 83 (Fungifree AB™) applied to the growing media (substrate) on different phenological stages of tomato, over the growth and fruit productivity of tomato plants. Fungifree AB™ has two antagonism factors: 1) B. velezensis 83 spores and 2) the metabolites produced during their submerged liquid culture production. Therefore, the second objective of this work was to evaluate the antagonism factor present in Fungifree AB™ having the major contribution in B. cinerea infection control by means of in vitro tests with tomato leaves and fruits.
Materials and methods
Biologicals
All the assays were carried out with B. velezensis 83 (deposited in the Belgian Coordinated Collection of Micro-organisms (BCCM), accession number LMG S-30921). A powder commercial formulation of B. velezensis 83 (Fungifree AB™ obtained from Agro&Biotecnia S. de R.L. de C.V.) was used. For biological control assays, the phytopathogenic fungus Botrytis cinerea 05 was kindly provided by Dr. Mario A. Serrano Ortega (Centro de Ciencias Genómicas-UNAM). For the tomato (Solanum lycopersicum L.) assay, tomato seeds var. Frodo (Hybrid Tomato, ITSCO, CdMx, México) were used. All treatments described in pots come from 21 days seedlings previously germinated in the presence of 104 CFU of Azospirillum brasilense per seed. A. brasilense was obtained from Instituto de Investigaciones Biomédicas-UNAM (Trujillo-Roldán et al., 2013). It has been previously described that the addition of A. brasilense in seeds provides a better seedling to be transferred to the pots (Mangmang et al., 2015; Reddy et al., 2018).
Effect of the inoculation of different concentrations of B. velezensis 83 on different phenological stages of tomato
To evaluate the effect of the inoculation of B. velezensis 83, the concentrations evaluated were 106, 104 or 102 CFU of B. velezensis 83/g substrate in two treatments: seed germination and seedlings with 20 days of development (20 DD). Each treatment included 25 individuals (seeds or seedlings); the control was the support growing media (Peat Moss) without B. velezensis 83 inoculation, here 24 seeds were used. The support growing media was a commercial Peat Moss based medium (Sunshine Mix 3, Sun Gro Horticulture, Agawam, MA) which was previously sterilized (121 °C/30 min) before use. For this assay, tomato seeds var. Frodo were sown in germination trays and were kept in a culture room with controlled conditions at 25°C and photoperiod of 18 h light/6 h dark. The percentage of germinated seeds in each treatment was evaluated 10 days after sowing, at that time >95% of the control seeds were germinated as declared by the supplier. From the appearance of the first true leaf of the seedlings (after 7 days), these were watered with a Hoagland solution (1/4) according to the requirements of the seedling (every 48 h) (Hoagland and Arnon 1938). The germination process lasted 28 days until the seedlings reached the appropriate size for transfer to pots. The effect of each treatment was evaluated in terms of biomass dry weight (80 °C for 48 h) for root and shoot.
In order to detect the population of B. velezensis 83 in 28 days tomato seedling roots, the population of B. velezensis 83 was quantified by qPCR analysis, for this, two Cq curves were performed. The first curve was a standard curve (Cq vs log [DNA]) to evaluate the amplification efficiency (E) of primers designed to B. velezensis 83 DNA identification. To obtain the DNA from B. velezensis 83 cells, a culture of the strain was incubated in 250 mL flasks with 50 mL of YPG medium, for 12-15 hours at 29 °C and 200 rpm (Innova 4330 refrigerated incubator shaker, New Brunswick Scientific, Edison, NJ, USA). After this time, 1.0 mL of the culture was taken and centrifuged (centrifuge 5810 R, Eppendorf Hamburg, Germany) at 10,000 rpm (rotor F45-30-11 Eppendorf AG, Hamburg, Germany) for 3 min, resulting in 100 μL of the supernatant and the cell pellet in the centrifugation vial. Subsequently, 100 µL of lysozyme (10 mg lysozyme Sigma-Aldrich in 1 mL of TE buffer pH 8.0 Sigma-Aldrich) were added to the samples. Subsequently, this mixture was incubated at 37 °C for 45 min (thermomixer model R, Eppendorf AG, Hamburg, Germany). Once the sample was at room temperature, the DNA of B. velezensis 83 was extracted with the chloroform-phenol method (Moore and Dowhan, 2002). For DNA amplification, the yomR-Bs83F / yomR-Bs83R molecular markers were designed with the Primer Express version 2.0 software, starting from a 221 bp sequence of the yomR gene region of strain 83 this sequence was selected because the less identity with the yomR gen from other B. amyloliquefaciens subsp. plantarum using Blastn analysis. The yomR-Bs83 oligos (yomR-Bs83F: ATGAAACAGCTGCCGGAGC / yomR-Bs83R: CTGCCCTGCATTCCATTTGT) were used as a mixture contained in a concentration of 5 µM each one. All qPCR reactions were carried out by triplicate and the control was a mixture reaction without DNA (NTC) in a thermal cycler equipment (thermal cycler model C1000, Bio-Rad Laboratories Inc., California, USA). An amplification standard curve (Cq vs log [DNA]) was obtained for these primers with a dynamic working range from 60.0 to 0.0006 ng/µL of DNA (y=-3.3793x+20.255; r2=0.9993 and E=97.7%). The detection limit is 0.0006 ng/µL in the Cq 31. The Cq of the NTC was 37.
The second curve was a standard curve (Cq vs log [CFU]) to quantify the B. velezensis 83 CFU/root, for this, firstly a culture of strain 83 was done and serial dilutions were made in order to obtain cell suspensions with different concentrations in the range of 109-101 CFU/mL. With the plate counting method, the value of CFU/mL of these suspensions was determined. Likewise, DNA extraction from 1.0 mL of the same cell suspensions was carried out with the chloroform-phenol method. It is worth mentioning that with suspensions of theoretical concentrations below the order of 101 CFU/mL, the CFU of the bacteria could not be detected with the plate count method. The dynamic working range was from 4.8 × 107 CFU/mL (4.8 × 105 CFU/reaction) to 3.5 × 102 CFU/mL (3.5 CFU/reaction), y =-3.3266x+42.35 with r2=0.992 and E=99.8%. Therefore, the minimum detection limit with oligos yomR-Bs83 F/R was 3.5 × 102 CFU/mL in Cq 33, which would correspond to a concentration of 0.00012 ng of B. velezensis 83 DNA. The samples were analyzed in triplicate and the control was a mixture without DNA (NTC). Cq of the NTC was 37. To detect the population of B. velezensis 83 in the seedling roots, all the root of each seedling grown during 28 days in a germination tray was used. The root wet weight was recorded and later the root was subjected to a sonication (3 min, Branson Ultrasonics M3800 Thermo Fisher Scientific, USA) washing process in an Corning 10 mL tube containing 5 mL of deionized water, from this cellular suspension 1 mL was used for B. velezensis 83 cell disruption by performing 3 freezing-heating cycles by placing the tube in liquid nitrogen (1 min)-hot water (3 min, 55 °C, thermomixer model R, Eppendorf AG, Hamburg, Germany). A reaction volume of 10 µL was used using SYBR Green I reagent (SYBR 5 µL, water 3 µL, primers mix 1 µL, DNA sample 1 µL). The samples were analyzed in triplicate and the control was a mixture without DNA (NTC). Cq of the NTC was 37. Finally, the population of B. velezensis 83 was calculated using the y =-3.3266x+42.35 equation obtained from the standard curve of Cq vs log [CFU], the result was multiplied by 5 (the water volume used for the root washing process) and reported as CFU/root.
Effect of the application of B. velezensis 83 in tomato plants grown in greenhouse
This assay was carried out in a greenhouse (area of 360 m2) located in the Centro de Desarrollo Tecnológico from FIRA (Fideicomisos Instituidos en Relación con la Agricultura ), in Morelos, México. The greenhouse was designed and built for the implementation of a 12-point monitoring system coupled to a PID (proportional integral derivative) feedback control system for temperature, a solar curtain control system, a control system for wet wall operation, a sprinkler system, and a fertigation system, coupled to three exhaust fans and two active ventilation walls which allowed maintaining the tomato crop in controlled environmental conditions. The information generated in the automated monitoring and control process was stored and processed in a central concentrator (PC computer). Each of the 12 monitoring points measured temperature, relative humidity (RH), solar radiation, pH and conductivity in the substrate. The control was programmed to avoid abrupt departures on extremely hot or cold days by using reference ranges of temperature (14 °C to 34 °C), RH (23% to 83%) and a maximum light near of 3000 footcandles (∼33,000 lux). The fertigation input pH was controlled between 6.3 to 6.4, with a percolation pH between 7.5 to 8.1, as well as an electrical conductivity between 0.5 dS/m up to 3.0 dS/m, depending on the cultural stage of the tomato (Table 1).
Table 1.
Nutritional requirements of the tomato crop (Solanum lycopersicum L.) in parts per million (ppm) by phenological stage proposed by FIRA staff.
| Steiner Nutritive solution used by phenological stage (ppm) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenological state | σ (dS/m) | N | P | K | Ca | Mg | S | Fe | Mn | Zn | Cu | B | Mo |
| Transplant | 0.5 | 42 | 8 | 68 | 45 | 12 | 28 | 3 | 0.5 | 0.05 | 0.5 | 0.025 | 0.002 |
| Vegetative-Flowering | 1.0 | 84 | 16 | 137 | 90 | 24 | 56 | ||||||
| Flowering – start of fruiting | 1.5 | 126 | 23 | 205 | 135 | 36 | 84 | ||||||
| 2.0 | 168 | 31 | 273 | 180 | 48 | 112 | |||||||
| fruiting – 1st harvest | 2.5 | 210 | 39 | 341 | 225 | 60 | 140 | ||||||
| Harvest | 3.0 | 252 | 47 | 410 | 270 | 72 | 168 | ||||||
σ (dS/m): electrical conductivity (decisiemens per meter)
Tomato seedlings var. Frodo with 21 days of development were transplanted to 15 L pots in the greenhouse with a mixture of substrate based on coconut fiber: tezontle (30:70). For each pot, two seedlings were placed. The density of the crop was 2.8 plants/m2 with 60 plants/treatment and 61 plants for the control. The crop had a cycle (winter cycle) of 150 days. In the assay, two biostimulation treatments were applied by drench (100 mL) to the substrate close to the root system. The effect of B. velezensis 83 treatments with high or low CFU/plant were evaluated (Table 2). The biological treatments also included the foliar aspersion to the shoot system. These treatments were applied from the beginning (02nd October, 2017, being the day of seedling transplanting) to the end (5th March, 2018) of the tomato cultivation cycle in the greenhouse in order to cover all the phenological stages the tomato plant, therefore the plants had six applications of B. velezensis 83 substrate treatment (applied at day 25, 50, 75, 100, 125 and 150 after seedling transplanting) and ten of B. velezensis 83 foliar aspersions (applied at day 14, 28, 42, 56, 70, 84, 98, 112, 126 and 140 after seedling transplanting). The sprayed volume of each suspension treatment was increasing during the experiment depending on the growth of the plants in the greenhouse in order to moisten all the foliage of each plant included in the experiment. A minimum of 2 L/treatment (33 mL/plant) were applied to the plants at the beginning of the experiment and 4 L/treatment (66 mL/plant) at the end, thereby with a theoretical calculation it was estimated that a minimum of 6.7 × 107 CFU/plant and maximum 1.3 × 108 CFU/plant were applied. The control were plants without bacterial inoculation (without biological treatment to substrate nor shoot) but with chemical fungicides (Previcur energy™, Bayer Crop Science, Germany and Velsul 725™, Velsimex, México) as preventive treatment. The chemicals were applied only once in the tomato cultivation cycle (seven days after transplanting of seedlings).
Table 2.
Treatments used in greenhouse grown tomato plants.
| Treatment | Active ingredient | Concentration | Application interval |
|---|---|---|---|
| Fungifree AB™ substrate low +shoot |
B. velezensis 83 | 1 × 106 CFU/plant + 6.7 × 107 < 1.3 × 108 CFU/plant |
substrate (to the root system) every 25 days + foliar aspersion (to the shoot system) every 14 days |
| Fungifree AB™ substrate high +shoot |
B. velezensis 83 | 1 × 108 CFU/plant + 6.7 × 107 < 1.3 × 108 CFU/plant |
substrate (to the root system) every 25 days + (to the shoot system) foliar aspersion every 14 days |
| Control | Chemical Fungicides |
Previcur energy™ (0.5 mL/L) Velsul 725™ (3 mL/L) |
foliar aspersion (to the shoot system) |
The vegetative variables of tomato growth as heigh (cm) and stem diameter (cm) were measured from 40 to 90 days after transplanting, this interval of time was considered to cover the vegetative plant growth phase. The diameter was measured in the first centimeter of the base of the steam. The productive variables were the number of fruits per plant and weight of fruit per plant. The tomato harvest lasted 65 days (from 30th December until 5th March), the fruits were harvested every three or four days. For year-productivity calculation two identical complete cycles were supposed.
In vitro biological control assays using B. velezensis 83 antagonism factors vs B. cinerea in tomato leaves and postharvest fruit
Fungifree AB™ is a powder formulation that contains two antagonism factors: spores of B. velezensis 83 and metabolites synthesized during the production process of the spores. Therefore, in vitro tests for biological control of B. cinerea 05 were performed using tomato leaves and fruits using treatments that included: 1) B. velezensis 83 spores (spores + inert powder support), 2) B. velezensis 83 metabolites (supernatant + inert powder support) and 3) Fungifree AB™ formulation (spores + supernatant + inert powder support). Fungifree AB™ inert powder support was used as control. The treatments suspension with cells or with metabolites of B. velezensis 83 were prepared according to the recommendation of use for foliar application of Fungifree AB™ (2 g/L). The metabolite suspension was filtered (0.20 µm membrane) in order to evaluate the metabolites inhibition effect without residual cells interference. Chlorothalonil (2,4,5,6-tetrachloroisophthalonitrile) (3 g/L) treatment was included as a positive control. A B. cinerea 05 spores suspension (1 × 106 spores/mL) was used as infective inoculum, for leaves the suspension was prepared with using PDB media (at 25%) to favor the fungal growth and for fruit it was prepared using sterile water.
For biological control in vitro assays carried out with leaves, healthy tomato seedling leaves with 30 days of growth were used. To carried out the experiment, two leaves were placed in a petri dish with agar (13 g/L) to make a system that maintain RH and three petri dishes were used for each treatment. Each leaf was divided in half (considering the central midrib of the leaf as a natural division) and three 10 μL drops of the corresponding treatment or control were applied on each side of the leaf. Once the applied drops had dried, the leaves were placed inside a Petri dish with agar and were sealed with Parafilm™ and placed in an incubator (Benchtop Environ-Cab 680, Lab-Line Instruments, Inc., USA) at 29 °C for 48 h. After this incubation time, three 10 μL drops of a spore suspension of B. cinerea 05 were applied on each side of the leaf, trying to inoculate in the same site of application of the treatments. Once the drops were dry (approx. 10 min), the Petri dishes were again sealed with Parafilm™ and wrapped with brown paper to maintain the RH in the Petri dish and avoid light filtration (to favors the development of infection of the phytopathogen). Finally, the Petri dishes were again stored in the incubator (Incubator Heratherm iGS400, Thermo Fisher Scientific Inc., Waltham, USA) at 25 °C at 90 RH% for 72 h, these conditions were used to favor the development of infection of the fungus. The area of lesion caused by B. cinerea 05 on the leaf was measured by using an image analysis technique using the Image ProPlus™ system (Media Cybernetics, Rockville, USA). The method used is based on the identification of color contrasts between the infected and non-infected areas of the leaf. This allowed us to have high precision when determining the severity of the infection caused by B. cinerea 05 on the leaf.
For biological control in vitro assays carried out with fruits, healthy tomatoes with four days postharvest were used. To carried out the experiment, 10 fruits were used for each treatment. All the fruits were superficially disinfected with commercial chlorine (5 mL/L for 15 min), once disinfected, each fruit was wounded in two equidistant points using a sterile toothpick (a wound of 3 mm deep). One point was inoculated with 10 μL of the treatments (cells, metabolites or Fungifree AB™), and the other point was inoculated with 10 μL of the control (inert powder support in distilled water, 2 g/L). Then the fruits were carefully placed in beakers (5 L), next the beaker was wrapped with paper to avoid light exposition and finally stored in an incubator (Benchtop Environ-Cab 680, Lab-Line Instruments, Inc., IL, USA) at 29 °C for 12 h. After this time, the wounds were inoculated with 10 μL of spore suspension of B. cinerea 05. The tomatoes were again stored in the beakers, this time were wrapped with paper to avoid light exposition and finally were placed in an incubator (Incubator Heratherm iGS400, Thermo Fisher Scientific Inc., Waltham, USA) at 25 °C for 3 days at 90% RH. The fungal infection on the fruits was evaluated by measuring the diameter (average of horizontal and vertical diameter) of injury on fruit and then infection area was calculated assuming a circumference.
The efficacy of inhibition of B. velezensis 83 against B. cinerea 05 in leaves and fruit was calculated according to:
Where:
IT= area in the control
it= area in the treatment
Profitability
The yields and the unit cost of production (CUP) of the greenhouse grown tomato were calculated for each treatment. The cost of production involved the variable and the fixed costs. The variable costs were constituted by the cost of inputs (biological and chemical products) and the direct labor cost. The fixed costs were: greenhouse rent with all the services, equipment and tools. In order to calculate the income, a sale price of 0.3 USD/kg was considered (SIIN, 2018, http://www.economia-sniim.gob.mx/).
Statistical analysis
All data were analyzed by one-way analysis of variance (ANOVA) and Tukey comparison procedure assuming equal variances using Minitab™ 17 Statistical Software (Minitab, LLC, Pennsylvania, USA). In the graphics the mean (bars) and the standard deviation (error bars) are reported. Significance was set at p ≤ 0.05.
Results and discussion
Effect of B. velezensis 83 inoculation on tomato seed germination
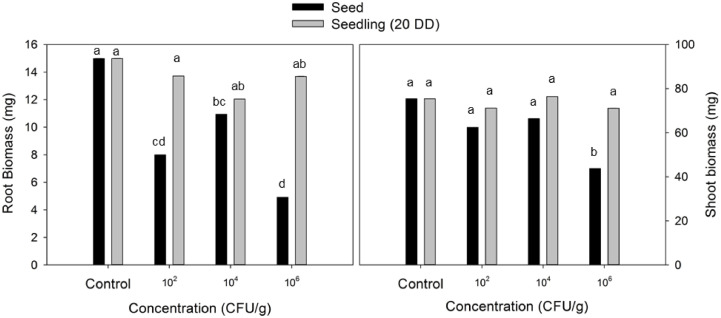
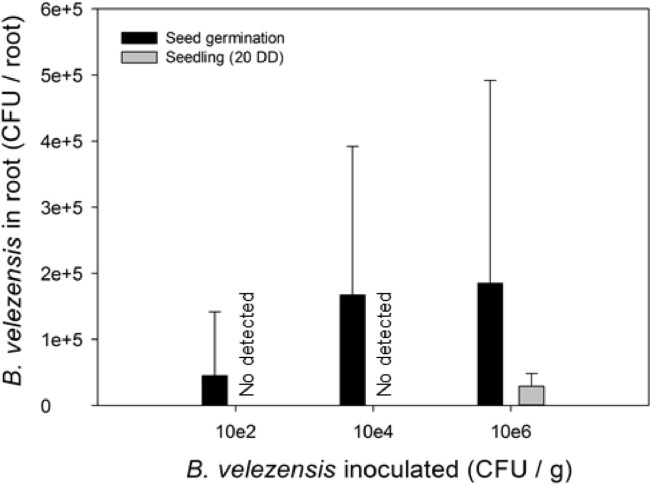
In addition to the in vitro test to evaluate the synthesis of related plant growth-promoting compounds, other criteria used to evaluate a strain as PGPB are the effect on seed germination and on the seedling growth. Then, the first objective of this work was to establish the application scenario of B. velezensis 83 to the growing media Peat Moss based (substrate) to evaluate the effect of the bacteria when it is present in germination or in the root of the tomato seedling. In the range of CFU/g substrate evaluated (102 – 106) no significant differences were found on the percentage of seed germination (supplemental Fig. S1a). It was observed that when the application was made from the germination of the seed, the treatment with a higher cell concentration of the bacteria inoculated to the substrate caused a delay in the development of the seedling (observed at 10 Days After Sowing, DAS). The germinated seeds in the condition of the treatment with 106 CFU/g substrate barely showed the leaves of the cotyledons at 7 DAS, while at lower concentrations of B. velezensis 83 (104 and 102 CFU/g substrate), the emergence of the first true leaf in the tomato seedlings was observed and presented similar appearance to the seedlings without application of B. velezensis 83 (control). The first true leaf was first observed in seedlings treated with 104, 102 CFU/g substrate and also in the control seedlings after 7 days. For seedlings treated with 106 CFU/g substrate the first true leaf was observed after 10 days (supplemental Fig. S1b). A strong negative effect on root biomass was observed even at low bacterial dose (102 CFU/g substrate) while the negative effect on shoot biomass was only evident at high bacterial dose (106 CFU/g substrate). In contrast, to apply 106, 104 or 102 CFU/g substrate to the seedlings had no effect on root and shoot growth (Fig. 1). Using a qPCR-based method developed specifically for the detection of B. velezensis 83 DNA (Supplemental material Fig. S2), the population established in each seedling root with 28 days of growth was quantified. It was found that B. velezensis 83 population was established in the range of 5 × 104-2 × 105 CFU/root when it was present from the seed germination stage, while when B. velezensis 83 was applied to 20 DD seedlings, the bacterium was detected at 3 × 104 CFU/root only in the seedlings treated with 106 CFU/g substrate (Fig. 2), no statistical differences were found among treatments.
Fig. 1.
Effect on root and shoot biomass of tomato Frodo seedlings (28 days of growth) of B. velezensis 83 inoculation to the substrate (using a concentration of 102, 104 or 106 CFU/g substrate). Control: substrate without inoculation of bacteria. a)B. velezensis 83 substrate inoculation in seed germination stage and b)B. velezensis 83 substrate inoculation in seedling (20 DD) stage. Different letters mean significant differences according to ANOVA and Tukey α= 0.05.
Fig. 2.
Population of B. velezensis 83 on seedling root (28 days of growth) detected by qPCR. Different letters mean significant differences according to ANOVA and Tukey α= 0.05.
The seed germination is affected by several factors, but mainly by plant hormones such as abscisic acid (ABA), ethylene, gibberellins, auxins (i.e. IAA), cytokinins and brassinosteroids (Miransari and Smith, 2014). It has been observed (Wagi and Ahmed, 2019) that strains have different capacity to produce auxins depending on the composition of the culture medium, the strains Bacillus cereus (So3II) and B. subtilis (Mt3b) showed different production of IAA depending on availability of tryptophan (IAA precursor) in the growth media. On the other hand, production of auxins by beneficial microorganisms promotes the interaction with the plant, as well as the jasmonic acid dependent plant resistance, which affects the expression of genes involved in auxins synthesis and transport (influx and efflux carriers) (Tsukanova et al., 2017). Also, it has been reported (Pérez-Flores et al., 2017) that VOC such as acetoine emitted by Bacillus methylotrophicus M4-96 affected the auxins genes expression and promoted the primary root growth and lateral root formation in A. thaliana. It has been observed (Asari et al., 2016) that in a medium containing A. thaliana root exudate, the VOC of B. amyloliquefaciens UCMB5113 increased 3-fold the plant biomass, regardless of the number of inoculated bacteria. However, without A. thaliana root exudate, the VOC of B. amyloliquefaciens UCMB5113 increased 2-fold the plant biomass and caused a negative effect on shoot biomass as the number of bacteria increased. Moreover, the crosstalk of auxins with the other plant hormones (i.e. cytokinin and ethylene) is complex and it affects several physiological processes in the plant at the same time (Liu et al., 2017a, Liu et al., 2017b). The plant growth promotion does not only depend on auxins production, but other factors could also be involved in the biostimulation of plant growth caused by a PGPB. In addition to IAA synthesis, phosphate solubilization, HCN, siderophore and NH3 production, the antifungal activity on phytopathogenic fungi and the mitigation abiotic stress, were the characteristics associated to the increased percentage of seed germination and seedling growth promoted by Bacillus strains inoculated in tomato (Ramavath et al., 2019). The biofilm formation (involving the synthesis of protein fibers (TasA) and exopolysaccharides (EPS) production by PGPB Bacillus strains) has been found as an important trait of B. amyloliquefaciens 54 in root colonization and to induce drought tolerance in tomato plants (Wang et al., 2019). The EPS production has been shown to be determinant for the capacity of B. velezensis FZB42 to biofilm formation and therefore for tomato root colonization (Al-Ali et al., 2018). Nevertheless, the exacerbated biofilm of B. velezensis FZ42 has also been observed negatively to affect the in vitro growth of A. thaliana seedlings (Balderas-Ruíz et al., 2020). On the other hand, the strains could have different capabilities for plant growth promotion and sometimes, also a decrease in some seedling growth parameters can be observed (i.e. shoot length and root dry weight) (Hernández-Pacheco et al., 2021). It has been suggested that plant growth promotion effect of B. velezensis FZB42 on Lemna minor was associated to auxin production compounds by the bacteria; however, only the diluted bacterial culture filtrates or the inoculation of low concentration of B. velezensis FZB42 (1 × 105 CFU) exhibited a plant growth promotion effect on in vitro cultured L. minor plantlets. The inoculation of high concentration of bacteria (1 × 107 CFU) had a negative effect on the plantlets growth (Idris et al., 2007). It is important to keep in mind that a PGPB can also exhibit a plant host specificity, as it has been shown with Aeromonas, Pseudomonas, Bacillus and Enterobacter strains isolates from tomato, only a positive growth promotion effect was observed in tomato plants, in contrast, when they were inoculated in groundnut, sorghum and chickpea had a negative or no growth promotion effect.
The plant growth promotion effect of B. velezensis 83 has been observed in A. thaliana as the increase of root and shoot biomass, and in maize as an increase of the plant height and root biomass (Balderas-Ruíz et al., 2020). Nevertheless, in the present work, it was not observed that B. velezensis 83 inoculation increased the root or shoot biomass for the tomato (Frodo variety) with different treatments (concentration) applied to substrate in the seed germination stage or to the substrate of growing seedling stage. However, the high concentration of bacteria inoculated in the substrate, caused a negative effect on the development of shoot and root of tomato seedlings as it has been observed in other plant-PGPB interaction models (Idris et al., 2007; Vaikuntapu et al., 2014; Balderas-Ruíz et al., 2020). Tomato exudates are mainly composed of sugars and organic acids, the latter being of higher concentration in the exudates, both types of compounds increase as the plant grows. The major organic acids are represented by citric, succinic and malic acids, while fructose and glucose are the major sugars (Kamilova et al., 2006). It has been reported (Tan et al., 2013) that the chemoattractant property of tomato exudates is positively correlated to exudates concentrations, the malic acid was one of the main compounds that promoted chemotaxis, swarming and establishment of B. amyloliquefaciens T-5 in tomato root. Additionally, the concentration of malic acid could be higher in roots of 4-day-old seedlings than in those of 21-day-old seedlings (Kamilova et al., 2006). On the other hand, it has been observed (Tan et al., 2013) an increase of up to an order of magnitude in the population level (CFU/g root) of B. amyloliquefaciens T-5 in tomato roots between 7- and 14-days post-inoculation of PGPR when malic acid was present in the rhizosphere of tomato plants. Therefore, it is probable that B. velezensis 83 establishment in the roots seedlings when the bacteria was present from the beginning of seed germination was associated to the differential concentration of organic acids (as malic acid) in tomato root. B. velezensis 83 has been isolated from mango tree foliage, the strain has the capability to form robust biofilms, and, in addition, genes to produce IAA, siderophore, phytase, acetoine/2,3-butanediol are present in its genome, as well as several genes involved in carbohydrate metabolism and plant cell wall degradation, which allows to B. velezensis 83 to stablish a benefic plant-bacteria interaction (Balderas-Ruíz et al., 2020). Considering the results obtained in this work, the next hypothesis is proposed: for inoculation on seed gemination stage 1) B. velezensis 83 produced a plant growth promoter compound (plant hormone or VOC) that affected the seed germination stage then all the plant growth or 2) B. velezensis 83 inoculated in high concentration quickly established the biofilm (involving cells and EPS) and affected the root development, therefore the nutrients uptake. More investigation is being done to study this phenomenon.
Effect of the application of B. velezensis 83 in tomato plants grown in greenhouse
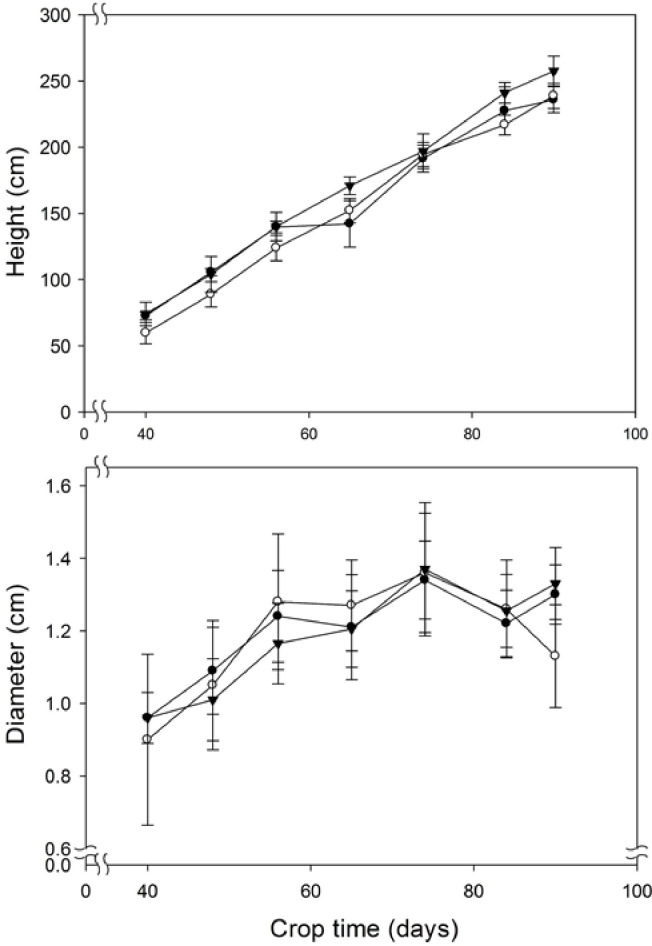
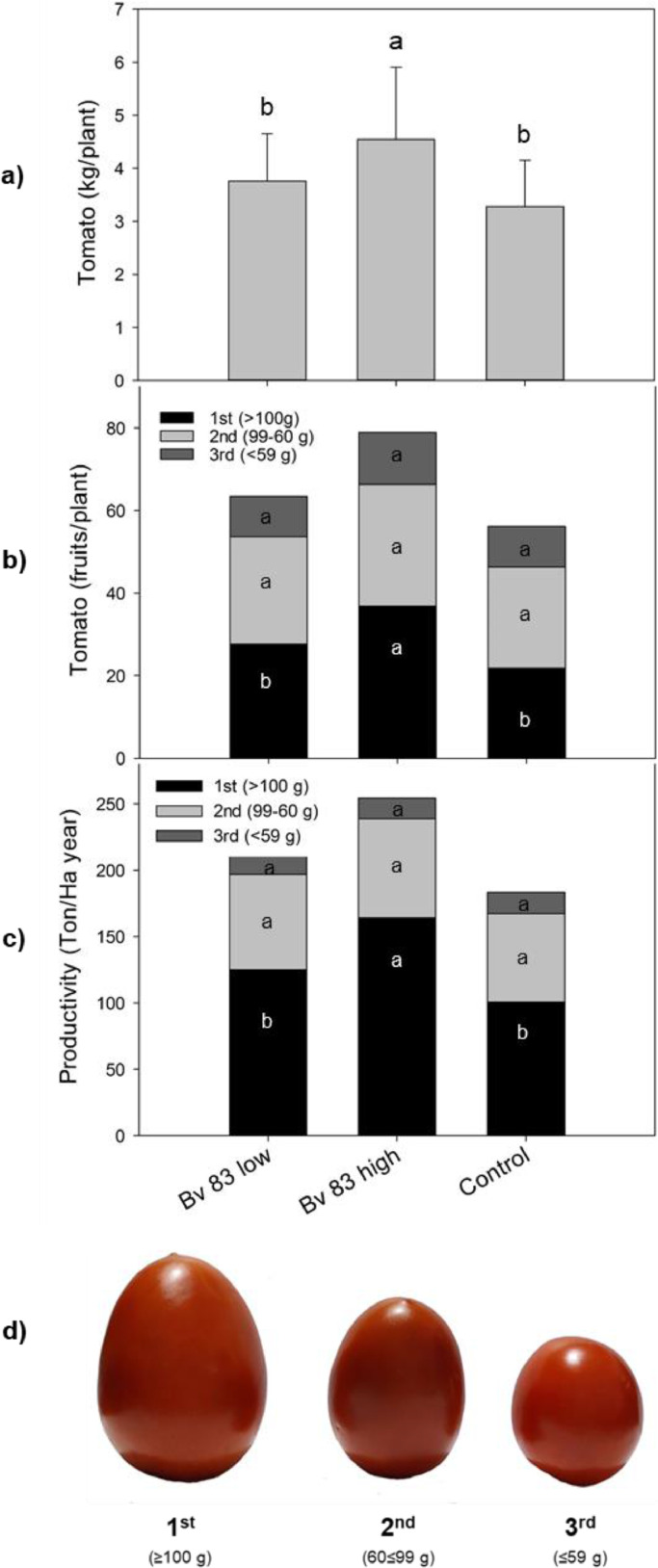
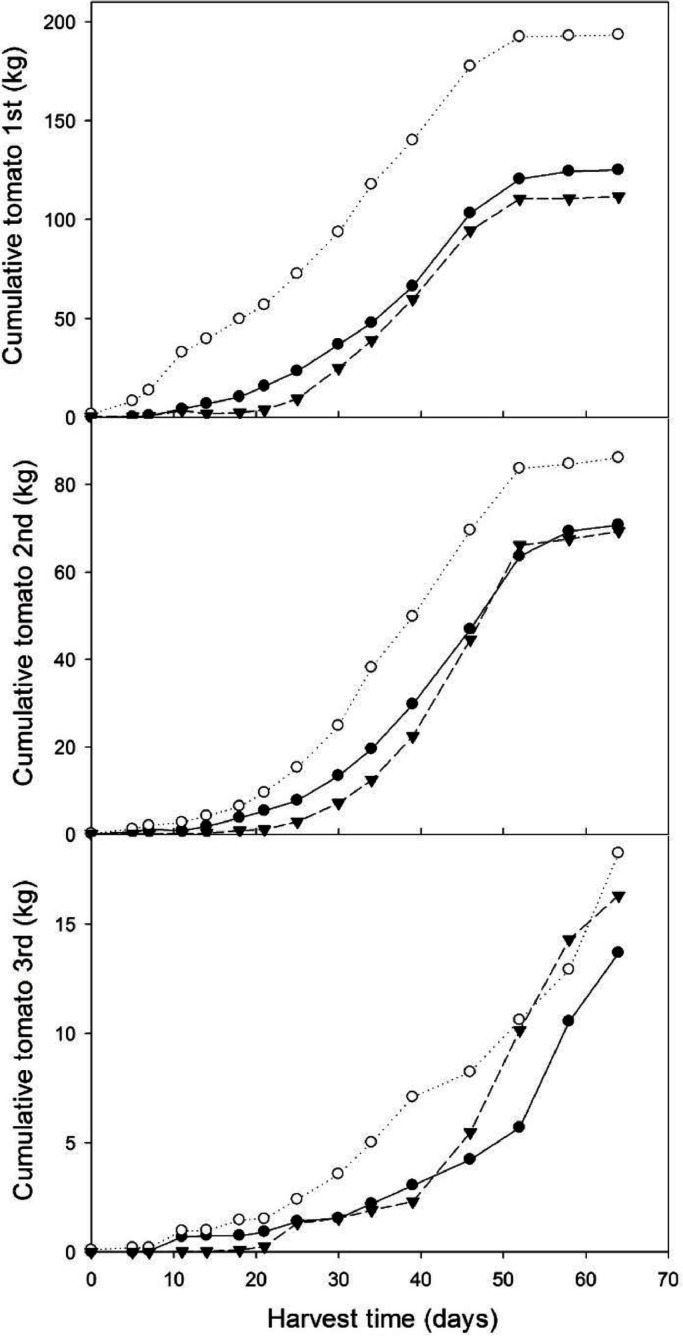
Fig. 3 shows plant growth (height and stem diameter) of the plant as a function of B. velezensis 83 inoculum (CFU/plant). The height of the plants linearly increased in the period going from 30 to 90 days of growth after transplanting the seedlings, the diameter of the plants increased until day 74. In both growth parameters, no significant differences were found between the treatments and the control plants. In contrast, a strong plant growth promotion effect of B. velezensis 83 was observed on the fruit productivity (Fig. 4). With a high bacterial inoculum (1 × 108 CFU/plant) treatment, the plants produced 4.5 kg/plant (12.7 kg/m2) while production in control plants only achieved 3.3 kg/plant (9.2 kg/m2) (Fig. 4a). In such conditions, the plants produced an average of 79 fruits/plant while control plants produced only 63 fruits/plant (Fig. 4b), and no significant differences in tomato production were found when the plants were inoculated with a low concentration (1 × 106 CFU/plant) treatment. Nevertheless, more interesting was the fact that high inoculum of B. velezensis 83 not only increased tomato productivity but also the quality of the fruits. While in control plants 27 first quality fruits/plant were obtained with the B. velezensis 83 treated plants (high inoculum) it was possible to obtain 37 first quality fruits, which represents an increase of 69% of the number of first quality fruit. Tomato production with the treatment B. velezensis 83 in high concentration was an estimate of 254 tons/Ha•year (Fig. 4c 4) and the differences were significant with respect to the other treatments. With the high concentration substrate treatment, it was possible to produce 164 tons/Ha•year of first quality tomato (≥100 g/fruit), which represented the 64% of the total production/year, while with the low concentration substrate treated (211 tons/Ha•year) and control (184 tons/Ha•year) plants produced less than 125 and 101 tons/Ha•year of first quality fruits, respectively. These results represent an increase of 38% in the productivity of tomato plants as well as a 19% increase in the quantity of first quality fruits. Additionally, it was observed that with a high concentration substrate treatment, the cumulated production of 1st quality tomato fruits was always higher compared to other treatments (Fig. 5), in the first eighteen days of harvest time, the harvest of first quality tomato fruits was about 13.6 kg while the low concentration substrate treatment and the control were only less than 1.0 kg. The cumulated production (kg) of 2nd quality tomato fruits also was higher than the low concentration substrate treatment and the control, and the 3rd quality tomato fruits was very similar for all the treatments; however, as showed before (Fig. 5), there was not significant differences in the tomato productivity. These results have shown the significant beneficial impact of B. velezensis 83 inoculation on tomato fruit productivity and quality, which, in turn, represents an economical benefit to the producers. The CUP of the greenhouse grown tomato with the B. velezensis 83 the high concentration substrate treated was of 0.3 USD/kg, while with a low concentration treatment or the control the CUP were 0.37 USD/kg and 0.4 USD/kg, respectively. Using these calculations it was estimated that the profability was 65% for greenhouse grown tomato with the B. velezensis 83 high concentration treatment and 25% for tomato control.
Fig. 3.
Effect of bioestimulation (B. velezensis 83) on the growth of tomato plants in the greenhouse. a) stem diameter (mm) and b) height (cm). Bv 83 low (●): B. velezensis 83 in 1 × 106 CFU/plant, Bv 83 high (○): B. velezensis 83 in 1 × 108 CFU/plant, and control (▼): plants without application of bacteria in substrate.
Fig. 4.
Effect of bioestimulation (B. velezensis 83) on the productivity of the tomato crop in the greenhouse depending on the treatment. Bv 83 low: B. velezensis 83 in 1 × 106 CFU/plant, Bv 83 high: B. velezensis 83 in 1 × 108 CFU/plant, and control: plants without application of bacteria in substrate. a) tomato production (Kg/plant). b) Number of tomato fruits/plant produced by quality category depending on the treatment. c) Tomato productivity (tons/Ha•year) and fruit quality. d) Fruit quality: 1st ≥100 g, 2nd 60≤99 g and 3rd ≤59 g. Different letters mean significant differences according to ANOVA and Tukey α= 0.05.
Fig. 5.
Cumulative tomato production (Kg) in the greenhouse depending on the treatment. Bv 83 low (●): B. velezensis 83 in 1 × 106 CFU/plant, Bv 83 high (○): B. velezensis 83 in 1 × 108 CFU/plant, and control (▼): plants without application of bacteria in substrate. Fruit quality: 1st ≥100 g, 2nd 60≤99 g and 3rd ≤59 g. Different letters mean significant differences according to ANOVA and Tukey α= 0.05.
It has been proposed (Basu et al., 2021) that an ideal PGPB should have the following characteristics: to be highly rhizosphere-competent and eco-friendly, to colonize the plant roots in significant numbers upon inoculation, to promote plant growth, to exhibit a broad spectrum of action (for biological control), to be compatible with other bacteria in the rhizosphere, as well as to be tolerant of physicochemical factors like heat, desiccation, radiations, and oxidants, also, it should demonstrate better competitive skills over the existing rhizobacterial communities. The use of Bacillus spp. inoculation has been extensively investigated due to the positive effect on crop production, this is in part because some strains help to the plants to cope with the biotic and abiotic stress; therefore, they are used as a sustainable choice against the use of agrochemicals. Unfortunately, few studies have evaluated the effect of bioinoculants on crop productivity. Factors as physiological growth stage of Bacillus sp. (planktonic cells or biofilm cells) and the nutrients (as Fe or iron) availability in the nutrient solution for greenhouse-grown tomato, have been shown to affect several plant growth parameters (height, root dry weight, shoot dry weight, root length, leaf area, number of leaves) and tomato fruit production (Ricci et al., 2019). The growth promotion activity of different species of PGPB was compared (Hernández-Pacheco et al., 2021) in Mexican husk tomato plants (Physalis ixocarpa) and in some interactions of PGPB-plant, the growth promotion effect was observed as an increased primary length root and increased number of secondary roots. However, these parameters not necessarily were associated with the typical increase in root weight or the increased stem length, even though a positive effect can be found in the total fresh weight of the plant. It has been reported (Akram et al., 2015) that Bacillus fortis or B. subtilis increased the root and shoot biomass of three different varieties (Fine Star, Río Grande, Red Power) of tomato plants, which increased tomato fruit production. The results were associated with the production of auxins, siderophore, phosphate solubilization. B. subtilis strain inoculated in tomato plants (Pishchik et al., 2018) increased the productivity of tomato variety Licurich and Moldova by 24% and 21%, respectively; the results were associated with the increase in the chlorophyll content in the plant, as well as a higher height and biomass of the plant, which promoted the higher production of fruits/plant and fruits of higher weight. The effect of individual inoculation of Bacillus pumilus, Pseudomonas putida, B. amyloliquefaciens or Bacillus mojavensis increased the biomass as well as the water content in the root (which favors the mobility of nutrients), which resulted in an increased fruit production of tomato fruits (between 39% and 18% more), with a higher content of macro and micronutrients (He et al., 2019). Therefore, it is likely that B. velezensis 83 displayed several growth promotion traits (related to the nutrient uptake) to influence the tomato production and fruit quality.
Biological control of B. velezensis 83 antagonism factor vs B. cinerea in tomato leaves and postharvest fruits
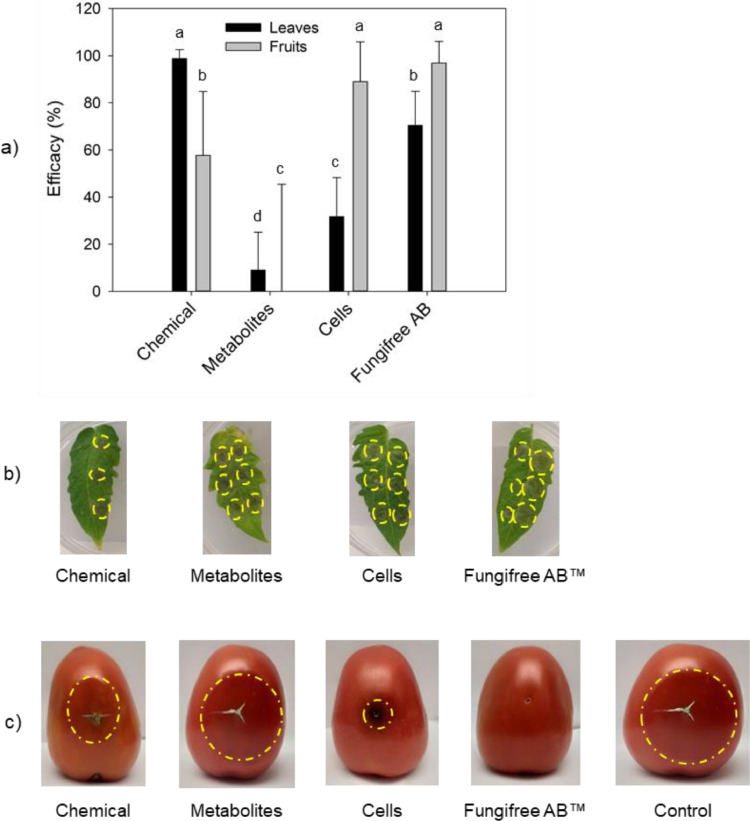
The antimicrobial activity of Bacillus spp metabolites has been extensively documented (Borriss, 2016; Fan et al., 2018; Fira, 2018; Keswani et al., 2020; Rabbee et al., 2019; Rabbee and Baek, 2020) and more than a dozen of bioformulations based on B. amyloliquefaciens (B. velezensis), B. subtilis, and B. pumilus are currently commercialized (Keswani et al., 2020). B. velezensis 83 is commercialized as a biofungicide (Fungifree AB™) for biological control of mango anthracnose (Balderas-Ruíz et al., 2020) and it is recommended to use in foliar application. It has been reported (Luna-Bulbarela et al., 2018) that Bacillomycin D (lipopeptide of the iturin family) produced by Bacillus sp 83 (now identified as B. velezensis 83), in concentrations above 19 μM affects spore germination and mycelial growth (in vitro tests) of C. gloeosporioides 09. Due to the characteristics of the production process of Fungifree AB™, the formulation contains two antagonism factors that are: spores (which turn to vegetative cells after germination), and metabolites (produced during the cultivation of the bacteria for spore production). Unravel which is the antagonism factor that has the main effect of biological control in the commercial product would allow us to optimize the production process of B. velezensis 83 and will be useful for designing biological control strategies. Fig. 6 shows the results of the biological control in vitro tests carried out with the different formulations of B. velezensis 83 antagonism factors against B. cinerea 05 infection in leaves and fruits. The efficacy of control was compared against a chemical control (Chlorotalonil). The highest control efficacy on B. cinerea 05 leaves infection was obtained with the chemical treatment (>95%). After this, the best treatment was Fungifree AB™ (>70%) followed by the cells (∼32%) and the least effective was the treatment with metabolites (<10%). The treatments containing cells (using Fungifree AB™ or cells-only formulation) were more effective than the treatment with metabolites only. On other hand, a higher control of B. cinerea 05 infected fruits was obtained with Fungifree AB™ and cell treatments (between 89% and 97%) and were statistically equal between them, followed by the chemical control (58%) while the metabolites had no control efficacy. In conclusion, in Fungifree AB™, the antagonism factor that showed the more relevant effect against B. cinerea 05 in tomato (leaves and fruits) were the cells (likely germinated spores). The high effectivity of fungal control in fruits, opens the possibility for a postharvest use of this biofungicide.
Fig. 6.
Effect of the treatment of B. velezensis 83 against B. cinerea applied preventively in leaves and fruits: a) B. velezensis 83 efficacy of control (%) of B. cinerea 05 infection, b) aspect of infection in leaves with different treatment and c) aspect of infection in leaves and tomato fruits with different treatment. Different letters mean significant differences according to ANOVA and Tukey α= 0.05.
The efficacy of control of the bacterial strains must be demonstrated in the plant system to which it is desired to protect, which can sometimes be complicated due to the lack of reproducibility of field tests. Therefore, the in vitro assays using biological tissues or postharvest fruits are used as alternative. In this way, the assays can be carried out in short time and in a reproducible way in contrast to the field tests. It has been shown (Toral et al., 2018) that B. velezensis XT1 CECT 8661 decreased the incidence of infection caused by B. cinerea in fruits of tomato, strawberry, and grapefruits by 50%, 12% and 100%, respectively. The use of vegetative cells of B. amyloliquefaciens RS-25 was more effective than the filtered (through 0.22 µm membranes) supernatant or the methanolic extracts (i.e. lipopeptides) isolated from the culture supernatant of the strain, to control B. cinerea infection in postharvest fruits (Chen et al., 2019). The control efficacy for B. cinerea infection in fruits of tomato, strawberry and grapefruits was associated with colonization, lipopeptides production (as surfactin, bacillomycin D and fengicin), as well as enzymes such as cellulase and protease, siderophores, and VOCs in vitro produced by B. amyloliquefaciens RS-25. Gao et al (2017) reported that B. velezensis ZSY-1 produced VOC as Pyrazine (2,5-dimethyl), benzothiazole, 4-chloro-3-methyl and phenol-2,4-bis (1,1-dimethylethyl), that achieved between 91-100% inhibition against B. cinerea using an in vitro test. All these works have associated the infection control of B. cinerea mainly by the Bacillus metabolites; however, these were produced in culture conditions favoring the production of antimicrobial metabolites or with extracts that contain them in a concentrated quantity. In this work, different results of control efficacy of the treatments between leaves and fruits were observed using the antagonism factors contained in the commercial product. It has been observed that the efficacy of the biological treatments with formulations containing B. velezensis 83 cells was higher in fruits than in leaves. In both cases, the formulation containing only metabolites had the least effective control. B. velezensis 83 genome contains the genes for antimicrobial metabolites (surfactin, bacillomicyn, fengicyn, bacillibactin, macrolactin, bacillaene, difficidin, amylocyclicin) implicated in the biological control of phytopathogens (Balderas-Ruíz et al., 2020). Therefore, in the field it is probable that B. velezensis 83 inhibits the growth of fungal phytopathogens exerting different mechanisms of antagonism due to in situ antimicrobial compounds production, competition by space and nutrients (biofilm formation) or also ISR (by i.e. VOC or surfactin production).
Conclusion
In this work, B. velezensis 83 was applied to the substrate for tomato cultivation to evaluate the plant growth promotion effect of different concentrations of the bacteria in different stages of tomato development. The bacterial inoculation with B. velezensis 83 in seed germination stage or seedlings did not have a promotion effect or even had a negative effect over the plant growth, despite the wide range of bacterial concentration that was evaluated. In contrast, in plants grown in greenhouse, although there was no effect on plant growth, an evident stimulating effect over the quantity and the quality of fruits was observed when B. velezensis 83 was applied to the substrate in high concentration. The effect could be associated with several growth promotion traits as plant hormone production, VOC or biofilm formation capacity of B. velezensis 83. Overall, the results showed the potential of B. velezensis 83 to stimulate tomato production within the range expected for a greenhouse medium technology which is largely used by mexican tomato producers. Due to the yields and the quality of the fruits obtained, it was estimated that the profitability of the B. velezensis 83 treatment applied to the substrate (108 UFC/plant) was 2.5 times higher than the control. At the other hand, it has been shown that B. velezensis 83 cells (germinated spores) had the highest control efficiency to the infection of B. cinerea in leaves and postharvest fruit. Therefore, we conclude that spores are the main antagonism factor contained in the commercial product (Fungifree AB™). The high effectivity of fungal control in riped fruits, opens the possibility for a postharvest use of this biofungicide.
Author contributions
Karina A. Balderas-Ruíz: Methodology, Investigation, Formal analysis, Writing - Original Draft. Clara I. Gómez-Guerrero: Investigation, Formal analysis. Sergio Aranda-Ocampo: Conceptualization, Methodology, Visualization. Antonio M. Juárez: Conceptualization, Methodology, Formal analysis, Validation, Visualization and Software. Edibel Leyva: Conceptualization, Methodology, Investigation, Formal analysis, Resources and Supervision. Mauricio A. Trujillo-Roldán: Conceptualization, Methodology, Investigation, Formal analysis, Visualization, Supervision. Norma A. Valdez-Cruz: Conceptualization, Methodology, Investigation, Formal analysis, Visualization, Supervision. Enrique Galindo: Conceptualization, Supervision, Visualization, Funding acquisition. Leobardo Serrano-Carreón: Conceptualization, Visualization, Funding acquisition, Supervision. All authors contributed to the Writing - Review & Editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
KAB-R is a doctoral student from “Programa de Doctorado en Ciencias Bioquímicas”, at “Universidad Nacional Autónoma de México” (UNAM). We thank to Juan Adame Adame, Ramón González Bernal and Ernesto Salvador Ramírez Lugo for the excellent technical assistance to realize the greenhouse tomato crop. We thank the Institutional Program of the Instituto de Investigaciones Biomédicas-UNAM: “La producción de biomoléculas de interés biomédico en bacterias y hongos”. We thank Dussthon Llorente (CEO of Instrulite S. A. de C. V.) for his technical support.
Funding
This work was financed by the “Consejo Nacional de Ciencia y Tecnología” (CONACYT 247473). KABR acknowledge CONACyT for the PhD scholarship 361862.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.crmicr.2021.100076.
Contributor Information
Karina A. Balderas-Ruíz, Email: karina.balderas@ibt.unam.mx.
Clara I. Gómez-Guerrero, Email: clarago@gmail.com.
Mauricio A. Trujillo-Roldán, Email: maurotru@biomedicas.unam.mx.
Norma A. Valdez-Cruz, Email: adri@biomedicas.unam.mx.
Sergio Aranda-Ocampo, Email: saranda@colpos.mx.
Antonio M. Juárez, Email: amjuarez@icf.unam.mx.
Edibel Leyva, Email: eleyva@fira.gob.mx.
Enrique Galindo, Email: enrique.galindo@ibt.unam.mx.
Leobardo Serrano-Carreón, Email: leobardo.serrano@ibt.unam.mx.
Appendix. Supplementary materials
References
- Akram W., Anjum T., Ali B. Co-cultivation of tomato with two Bacillus strains: effects on growth and yield. J. Animal Plant Sci. 2015;25(6):1644–1651. [Google Scholar]
- Al-Ali A., Deravel J., Krier F., Béchet M., Ongena M., Jacques P. Biofilm formation is determinant in tomato rhizosphere colonization by Bacillus velezensis FZB42. Environ. Sci. Pollut. Res. Int. 2018;25(30):29910–29920. doi: 10.1007/s11356-017-0469-1. Epub 2017 Oct 23. PMID: 29063401. [DOI] [PubMed] [Google Scholar]
- Asari S., Matzén S., Petersen M.A., Bejai S., Meijer J. Multiple effects of Bacillus amyloliquefaciens volatile compounds: plant growth promotion and growth inhibition of phytopathogens. FEMS Microbiol. Ecol. 2016;92 doi: 10.1093/femsec/fiw070. fiw070-fiw070. [DOI] [PubMed] [Google Scholar]
- Asari S., Tarkowská D., Rolčík J., Novák O., Palmero D.V., Bejai S., Meijer J. Analysis of plant growth-promoting properties of Bacillus amyloliquefaciens UCMB5113 using Arabidopsis thaliana as host plant. Planta. 2017;245:15–30. doi: 10.1007/s00425-016-2580-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balderas-Ruíz K.A., Bustos P., Santamaria R.I., González V., Cristiano-Fajardo S.A., Barrera-Ortíz S., Mezo-Villalobos M., Aranda-Ocampo S., Guevara-García Á.A., Galindo E., Serrano-Carreón L. Bacillus velezensis 83 a bacterial strain from mango phyllosphere, useful for biological control and plant growth promotion. AMB Express. 2020;10(1):163. doi: 10.1186/s13568-020-01101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A., Prasad P., Das S.N., Kalam S., Sayyed R.Z., Reddy M.S., El Enshasy H. Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: recent developments, constraints, and prospects. Sustainability. 2021;13:1140. doi: 10.3390/su13031140. [DOI] [Google Scholar]
- Borriss R. In: Bacilli and Agrobiotechnology. Islam M., Rahman M., Pandey P., Jha C., Aeron A., editors. Springer; Cham: 2016. Phytostimulation and biocontrol by the plant-associated Bacillus amyloliquefaciens FZB42: an update. [DOI] [Google Scholar]
- Chen X., Wang Y., Gao Y., Gao T., Zhang D. Inhibitory abilities of Bacillus isolates and their culture filtrates against the gray mold caused by Botrytis cinerea on postharvest fruit. Plant Pathol. J. 2019;35(5):425–436. doi: 10.5423/PPJ.OA.03.2019.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Wang X., Ma Q., Bian L., Liu X., Xu Y., Zhang H., Shao J., Liu Y. Bacillus velezensis CLA178-induced systemic resistance of Rosa multiflora against crown gall disease. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.587667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chojnacka K. Innovative bio-products for agriculture. Open Chem. 2015;13:932–937. [Google Scholar]
- Erba D., Casiraghi M.C., Ribas-Agustí A., Cáceres R., Marfà O., Castellari M. Nutritional value of tomatoes (Solanum lycopersicum L.) grown in greenhouse by different agronomic techniques. J. Food Comp. Anal. 2013;31(2):245–251. [Google Scholar]
- Fan B., Blom J., Klenk H.P., Borriss R. Bacillus amyloliquefaciens, Bacillus velezensis, and Bacillus siamensis form an “operational group B. amyloliquefaciens” within the B. subtilis species complex. Front. Microbiol. 2017;8:1–15. doi: 10.3389/fmicb.2017.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan B., Wang C., Song X., Ding X., Wu L., Wu H., Gao X., Borriss R. Bacillus velezensis FZB42 in 2018: the gram-positive model strain for plant growth promotion and biocontrol. Front. Microbiol. 2018;9:2491. doi: 10.3389/fmicb.2018.02491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO, 2019. Crops. On line: 17/04/2021. In http://www.fao.org/faostat/en/#data/QC.
- Fira D., Dimkic I., Berić T., Lozo J., Stanković S. Biological control of plant pathogens by. Bacillus species. J. Biotechnol. 2018 doi: 10.1016/j.jbiotec.2018.07.044. [DOI] [PubMed] [Google Scholar]
- Gao Z., Zhang B., Liu H., Han J., Zhang Y. Identification of endophytic Bacillus velezensis ZSY-1 strain and antifungal activity of its volatile compounds against Alternaria solani and Botrytis cinerea. Biol. Control. 2017;105:27–39. doi: 10.1016/j.biocontrol.2016.11.007. [DOI] [Google Scholar]
- He Y., Pantigoso H., Wu Z., Vivanco J. Co-inoculation of Bacillus sp. and Pseudomonas putida at different development stages acts as a biostimulant to promote growth, yield and nutrient uptake of tomato. J. Appl. Microbiol. 2019;127:196–207. doi: 10.1111/jam.14273. [DOI] [PubMed] [Google Scholar]
- Hemming S., Zwart F.d., Elings A., Petropoulou A., Righini I. Cherry tomato production in intelligent greenhouses—sensors and AI for control of climate, irrigation, crop yield, and quality. Sensors. 2020;20:6430. doi: 10.3390/s20226430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Pacheco C.E., Orozco-Mosqueda M.C., Flores A., Valencia-Cantero E., Santoyo G. Tissue-specific diversity of bacterial endophytes in Mexican husk tomato plants (Physalis ixocarpa Brot. ex Horm.), and screening for their multiple plant growth-promoting activities. Curr. Res. Microbial Sci. 2021;2 doi: 10.1016/j.crmicr.2021.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoagland D.R., Arnon D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. Circ. 1938 http://hdl.handle.net/2027/uc2.ark:/13960/t51g1sb8j [Google Scholar]
- Idris E.E., Iglesias D.J., Talon M., Borriss R. Tryptophan-dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol. Plant-Microbe Interact. 2007;20(6):619–626. doi: 10.1094/mpmi-20-6-0619. [DOI] [PubMed] [Google Scholar]
- Kamilova F., Kravchenko L.V., Shaposhnikov A.I., Azarova T., Makarova N., Lugtenberg B. Organic acids, sugars, and L-tryptophane in exudates of vegetables growing on stonewool and their effects on activities of rhizosphere bacteria. Mol. Plant-Microbe Interact. 2006;19(3):250–256. doi: 10.1094/MPMI-19-0250. [DOI] [PubMed] [Google Scholar]
- Keswani C., Singh H.B., García-Estrada C., Caradus J., He Y.W., Mezaache-Aichour S., Glare T.R., Borriss R., Sansinenea E. Antimicrobial secondary metabolites from agriculturally important bacteria as next-generation pesticides. App. Microbiol. Biotechnol. 2020;104(3):1013–1034. doi: 10.1007/s00253-019-10300-8. [DOI] [PubMed] [Google Scholar]
- Khatoon Z., Huang S., Rafique M., Fakhar A., Kamran M.A., Santoyo G. Unlocking the potential of plant growth-promoting rhizobacteria on soil health and the sustainability of agricultural systems. J. Environ. Manag. 2020;273 doi: 10.1016/j.jenvman.2020.111118. [DOI] [PubMed] [Google Scholar]
- Kumar A., Prakash A., Johri B.N. In: Bacteria in Agrobiology: Crop Ecosystems. Maheshwari DK, editor. Springer; Berlin Heidelberg: 2011. Bacillus as PGPR in crop ecosystem; pp. 37–59. [Google Scholar]
- Liu J., Moore S., Chen C., Lindsey K. Crosstalk complexities between auxin, cytokinin, and ethylene in Arabidopsis root development: from experiments to systems modeling, and back again. Mol. Plant. 2017;10(12):1480–1496. doi: 10.1016/j.molp.2017.11.002. [DOI] [PubMed] [Google Scholar]
- Liu S., Hao H., Lu X., Zhao X., Wang Y., Zhang Y., Xie Z., Wang R. Transcriptome profling of genes involved in induced systemic salt tolerance conferred by Bacillus amyloliquefaciens FZB42 in Arabidopsis thaliana. Sci. Rep. 2017;7(1):10795. doi: 10.1038/s41598-017-11308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna-Bulbarela A., Tinoco-Valencia R., Corzo G., Kazuma K., Konno K., Galindo E., Serrano-Carreón L. Effects of bacillomycin D homologues produced by Bacillus amyloliquefaciens 83 on growth and viability of Colletotrichum gloeosporioides at different physiological stages. Biol. Control. 2018;127:145–154. doi: 10.1016/j.biocontrol.2018.08.004. [DOI] [Google Scholar]
- Mangmang J.S., Deaker R., Rogers G. Optimal plant growth-promoting concentration of Azospirillum brasilense inoculated to cucumber, lettuce and tomato seeds varies between bacterial strains. Israel J. Plant Sci. 2015;62(3):145–152. [Google Scholar]
- Miransari O., Smith D.L. Plant hormones and seed germination. Environ. Exper. Botany. 2014;99:110–121. doi: 10.1016/j.envexpbot.2013.11.005. [DOI] [Google Scholar]
- Moore D., Dowhan D. Purification and concentration of DNA from aqueous solutions. Curr. Protocols Mol. Biol. 2002 doi: 10.1002/0471142727.mb0201as59. [DOI] [PubMed] [Google Scholar]
- Olanrewaju O.S., Glick B.R., Babalola O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017;33:197. doi: 10.1007/s11274-017-2364-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan P., Cheema A., Paliyath G. Solanaceous fruits including tomato, eggplant, and peppers. Encycl. Food Health. 2016:24–32. doi: 10.1016/b978-0-12-384947-2.00696-6. [DOI] [Google Scholar]
- Pérez-Flores P., Valencia-Cantero E., Altamirano-Hernández J., Pelagio-Flores R., López-Bucio J., García-Juárez P., Macías-Rodríguez L. Bacillus methylotrophicus M4-96 isolated from maize (Zea mays) rhizoplane increases growth and auxin content in Arabidopsis thaliana via emission of volatiles. Protoplasma. 2017;254:2201–2213. doi: 10.1007/s00709-017-1109-9. [DOI] [PubMed] [Google Scholar]
- Pishchik V.N., Vorobyev N.I., Ostankova Y.V., Semenov A.V., Totolian A.A, Popov A.A., Khomyakov Y.V., Udalova O.R., Shibanov D.V., Vertebny V.E., Dubovitskaya V.I., Sviridova O.V., Walsh O.S., Shafian S. Impact of Bacillus subtilis on tomato plants growth and some biochemical characteristics under combined application with humic fertilizer. Int. J. Plant Soil Sci. 2018;22:1–12. doi: 10.9734/IJPSS/2018/41148. [DOI] [Google Scholar]
- Rabbee M.F., Ali M.S., Choi J., Hwang B.S., Jeong S.C., Baek K.H. Bacillus velezensis: a valuable member of bioactive molecules within plant microbiomes. Molecules. 2019;24(6):1046. doi: 10.3390/molecules24061046. PMID: 30884857; PMCID: PMC6470737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbee F.M., Baek K.H. Antimicrobial activities of lipopeptides and polyketides of Bacillus velezensis for agricultural applications. Molecules. 2020;25(21):4973. doi: 10.3390/molecules25214973. PMID: 33121115; PMCID: PMC7662345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RaboResearch. World Vegetable Map 2018. On line: 23/06/2021: https://research.rabobank.com/publicationservice/download/publication/token/4YFFJRVJFTLbjNUgedUZ.
- Ramavath K., Hameeda B., Reddy G. Enhancement of plant growth in tomato by inoculation with plant growth promoting Bacillus spp. World J. Agric. Res. 2019;7(2):69–75. [Google Scholar]
- Reddy S., Singh A.K., Masih H., Benjamin J.C., Ojha S.K., Ramteke P.W., Singla A. Effect of Azotobacter sp. and Azospirillum sp. on vegetative growth of Tomato (Lycopersicon esculentum) J. Pharmacog. Phytochem. 2018;7(4):2130–2137. [Google Scholar]
- Ricci E., Schwinghamer T., Fan D., Smith D.L., Gravel V. Growth promotion of greenhouse tomatoes with Pseudomonas sp. and Bacillus sp. biofilms and planktonic cells. App. Soil Ecol. 2019;138:61–68. doi: 10.1016/j.apsoil.2019.02.009. [DOI] [Google Scholar]
- SIIN . 2018. Sistema Nacional de Información e Integración de Mercados.http://www.economia-sniim.gob.mx/Precios_de_Frutas_y_Hortalizas.htm On line: 25/Junio/2018. [Google Scholar]
- Tan S., Yang C., Mei X., Shen S., Raza W., Shen Q., Xu Y. The effect of organic acids from tomato root exudates on rhizosphere colonization of Bacillus amyloliquefaciens T-5. App. Soil Ecol. 2013;64:15–22. doi: 10.1016/j.apsoil.2012.10.011. [DOI] [Google Scholar]
- Toral L., Rodríguez M., Béjar V., Sampedro I. Antifungal activity of lipopeptides from Bacillus XT1 CECT 8661 against Botrytis cinerea. Front. Microbiol. 2018;9:1315. doi: 10.3389/fmicb.2018.01315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo-Roldán M.A., Valdez-Cruz N.A., Gonzalez-Monterrubio C.F., Acevedo-Sánchez E.V., Martínez-Salinas C., García-Cabrera R.I., Gamboa-Suasnavart R.A., Marín-Palacio L.D., Villegas J., Blancas-Cabrera A. Scale-up from shake flasks to pilot-scale production of the plant growth-promoting bacterium Azospirillum brasilense for preparing a liquid inoculant formulation. App. Microbiol. Biotechnol. 2013;97(22):9665–9674. doi: 10.1007/s00253-013-5199-9. [DOI] [PubMed] [Google Scholar]
- Tsukanova K.A., Chebotar V.K., Meyer J.J.M., Bibikova T.N. Effect of plant growth-promoting rhizobacteria on plant hormone homeostasis. S. Afr. J. Bot. 2017;113:91–102. doi: 10.1016/j.sajb.2017.07.007. [DOI] [Google Scholar]
- USDA, 2021. Mexico: Tomato Annual (Report MX2021-0030). https://www.fas.usda.gov/data/mexico-tomato-annual-4. On line 17/09/21.
- Vaikuntapu P.R., Dutta S., Samudrala R.B., Rao V.R., Kalam S., Podile A.R. Preferential promotion of Lycopersicon esculentum (Tomato) growth by plant growth promoting bacteria associated with tomato. Indian J. Microbiol. 2014;54(4):403–412. doi: 10.1007/s12088-014-0470-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagi S., Ahmed A. Bacillus spp.: potent microfactories of bacterial IAA. PeerJ. 2019;7:e7258. doi: 10.7717/peerj.7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D.C., Jiang C.H., Zhang L.N., Chen L., Zhang X.Y., Guo J.H. Biofilms positively contribute to Bacillus amyloliquefaciens 54-induced drought tolerance in tomato plants. Int. J. Mol. Sci. 2019;20(24):6271. doi: 10.3390/ijms20246271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.