Highlights
-
•
Computational biology.
-
•
Bacterial resistance.
-
•
Pseudomonas aeruginosa.
-
•
Gram-negative bacteria.
-
•
Polymyxin.
Keywords: Polymyxin, Computational biology, Bacterial resistance, Gram-negative bacteria
Abstract
Nowadays, clinical and scientific interest in antibiotics, as polymyxin, has increased due to the large number of reports of multiresistant Gram-negative bacteria, as Pseudomonas aeruginosa. The aim of this study was to investigate a related group of proteins for resistance to polymyxins, encoded by P. aeruginosa genome, through in silico analysis. The mobilized colistin resistance 1 (MCR1) protein from Escherichia coli was used for comparison. Similar sequences to the protein MCR1 in P. aeruginosa were analysed for physicochemical properties. 31 protein isoforms in P. aeruginosa (EptA) were found able to confer resistance to polymyxin showing protein lengths between 551 and 572 amino acids, with molecular mass values between 61.36 - 62. 80 kDa, isoelectric point between 6.10 to 7.17, instability index between 33.76 to 41.87, aliphatic index between 98.67 to 102.63 and the hydropathyindex between - 0.008 to 0.094. These proteins belong to the DUF1705 superfamily with bit-score values between 559.81 and 629.78. A high degree of similarity between EpTAs in P. aeruginosa was observed in relation to other proteins that confer resistance to polymyxins, present in Gram-negative bacteria species of clinical interest. Although, further studies are needed to identify the actual contribution of EptAs in P. aeruginosa species.
Introduction
Polymyxins are classified into five groups, named: A, B, C, D and E (Neiva et al., 2013). Although, currently only polymyxins B and E are commercially available (Pogue et al., 2017), or used in clinical interventions (Lorenzo et al., 2011), due to high toxicity of the other isoforms (Falagas and Kasiakou, 2005). This group of antibiotics was discovered in 1947 (Li et al., 2005), and polymyxin E was the first to be used in the clinic, in 1959 (Yu et al., 2015). Polymyxins were identified in Bacillus polimyxa strains (Stansly and Schlosser, 1947; Girardello and Gales, 2012), subspecies: colistinus Koyamae (Lorenzo et al., 2011). Polymyxins B and E have potent antimicrobial activity against several species of Gram-negative bacteria (Mendes and Burdmann, 2009; Carvalho and Cogo, 2014). Some hypotheses could explain the mechanism of polymyxins resistance acquired by P. aeruginosa: (I) Adaptive mechanism: Gradual adaptation, due to the presence of this antimicrobial component associated with the culture medium used in experimental settings or in clinical diagnosis (Zavascki et al., 2010).
Another possibility is the interference of this drug during active transport through the membrane, specifically in the lipid A portion of bacterial lipopolysaccharides (LPS) (Mendes and Burdmann, 2009) resulting in loss of OMPs (Outer Membrane Proteins) or reduction of interactions between polymyxin and the envelope (Moore et al., 1984); (II) Genetic mutation mechanism: Associated with increased levels of H1 protein (H1-T6SS), and cations replacement, minimizing the Mg2+ concentration and increasing the Ca2+concentration in cell membrane, reducing possible electrostatic interactions with polymyxin (Zhang et al., 2011; Fair and Tor, 2014; Morita et al., 2014). In 2010, it was shown that H1-T6SS in P. aeruginosa has three effector proteins, called Tse1–3 (type VI secretion exporters 1–3), where Tse1 and Tse3 can cleave peptidoglycan associated with the bacterial envelope (Hood et al., 2010), and this function of H1-T6SS is directly linked to antibiotic resistance in biofilms (Zhang et al., 2011).
Furthermore, absence of 2‑hydroxy-laureate and presence of 4-aminoarabinose in bacteria membrane and an increased A palmitate concentration in lipid A (Bonomo and Szabo, 2006), besides mutations in the PhoPQ and PmrAB systems that induces modified LPS operon (arn), resulting in addition to Lara4N (4-amino-4-deoxy-L-arabinose) in the lipid A portion of the LPS, could potentialize the resistance to polymyxins in P. aeruginosa (Schurek, 2009). Different modifications in lipid A may occur, including changes in the number of acyl chains and phosphoethanolamine addition (Tran et al., 2005; Herrera et al., 2010). Other possible resistance factors to polymyxins could be the induction of the pmrCAB operon. These genes comprise a three-component system: response regulator (pmrA), histidine kinase sensor (pmrB), and the protein that adds phosphoethanolamine to lipid A (pmrC).
Thus, polymyxins resistance could be related to increased expression of pmrC gene. In P. aeruginosa, N. meningitidis, S. enterica and S. plymuthica the product of the pmrC gene it is it is known as EptAs, whereas in the bacterium Escherichia coli it is known as MCR1, and Klebsiella pneumonia it is known as MCR1.9. In this context, P. aeruginosa, a Gram-negative bacterium, in the last decade has received much attention due to its capacity to cause serious infections in Brazilian hospitals (Lopes, 2009; Silva Filho and Silva, 2013; Bomfim and Knob, 2013). For this reason, this work aims to study the physicochemical properties of EptA proteins expressed in P. aeruginosa genomes deposited at the National Center for Biotechnology Information - NCBI (https://www.ncbi.nlm.nih.gov/) database, to improve the understanding of the mechanisms of resistance to polymyxins developed by this bacterial species.
Methods
Obtaining and analysing gene sequences
In this experimental approach, the National Center for Biotechnology Information - NCBI (https://www.ncbi.nlm.nih.gov/) database was initially evaluated to identify clusters that encode isoforms of the enzyme Lipid A phosphoethanolamine transferase (EptA) in P. aeruginosa genome. For this purpose, the EptA sequence (product of the mcr1 gene of E. coli, Access in GenBank: ASK04346.1) was used as bait to obtain target protein sequences from the Protein Blast tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Physicochemical characterization
To physicochemically characterize the EptAs isoforms present in different P. aeruginosa genomes we used: ProtParam server - Expasy for analysis of theoretical molecular weight (rM), isoelectric point (pI), instability index (II), aliphatic index (IA) and the grand average of hydropathicity index (GRAVY) (https://web.expasy.org/protparam/).
Prediction of secondary structure and protein domain
Secondary structures of the EptA isoforms were predicted using the SopMa - Secondary Structure Prediction Method server (Geourjon and Deléage, 1995). For protein domain prediction all .fasta extension sequences were submitted to Conserved Domains Database (CDD) and Resources - NCBI - NIH server (Lu, 2020).
Phylogenetic analysis
The phylogenetic analysis was obtained from the alignment of EptA from P. aeruginosa sequences. For determination of the phylogenetic tree, the Neighbor Joining method (NJ) was used with pairwise deletion option and reliability index of 1000 replicas of bootstrap. The analysis was performed in the MEGA X Molecular Evolutionary Genetics Analysis – Phylogenetic (Kumar et al., 2018) software.
Results and discussions
P. aeruginosa is classified as a Gram-negative bacterium and are very versatile, capable of spreading on soils (Al-Saleh and Akbar, 2015), marshes (Teixeira et al., 2016), coastal marine habitats (Habbu et al., 2016), as well as plants and animals (Ellison et al., 2013). In this context, the easy environmental adaptation and dissemination have aroused the curiosity of many researchers around the world, resulting in several P. aeruginosa genomes being sequenced (genomes data were obtained from the National Center for Biotechnology Information - NCBI (https://www.ncbi.nlm.nih.gov/). The microorganism was found in many researches conducted in intensive care units (ICUs) (Basso et al., 2016). In Belém-PA, Brazil, infections caused by P. aeruginosa resulted in the second highest mortality rate in ICUs, only behind Mycobacterium tuberculosis species (Barros et al., 2016). Polymyxins B and E (colistin) are antibiotics that P. aeruginosa developed resistance against (Lorenzo et al., 2011; Barros et al., 2016; Lee et al., 2012), although the mechanism leading to it is not very clear (Stefani et al., 2017; Gutu, 2013).
On the other hand, in microorganisms as Acinetobacter baumannii, E. coli and Klebsiella pneumoniae this mechanism is well known, being a direct result of the mobile colistin resistance mcr1 gene (Olaitan et al., 2014; Chang et al., 2012; Liu, 2016). It is a common sense that the emergence of polymyxin-resistant strains could be associated with the use of colistin in agriculture and animal farms (Zheng et al., 2018; Economou and Gousia, 2015; Nguyen et al., 2016; Zheng et al., 2018). The Protein Blast server (https://blast.ncbi.nlm.nih.gov/Blast.cgi) was searched for sequences related to the mcr1 gene of E. coli present in genomes belonging to Pseudomonas genus. 101 protein sequences were identified, of which 80 were associated with P. aeruginosa, 4 with P. citronellolis, 4 with P. nitroreducens, 2 with P. alcaligenes, 2 with P. jinjuensis, and 9 other species, with all sequences belonging to Pseudomonas genus. Only the isoforms catalogued for the P. aeruginosa species were analysed, being distributed currently, among 18 known genomes, according to the identification of the rates (Table 1).
Table 1.
List of Gram-negative bacteria, Pseudomonas aeruginosa genomes, deposited at the National Center for Biotechnology Information - NCBI (https://www.ncbi.nlm.nih.gov/).
| Pseudomonas aeruginosa strains | |
|---|---|
| P. aeruginosa (taxid:287) | P. aeruginosa OS42 (taxid:1402581) |
| P. aeruginosa group (taxid:136841) | P. aeruginosa BWHPSA028 (taxid:1402528) |
| P. aeruginosa PAO1 (taxid:208964) | P. aeruginosa BL12 (taxid:1402553) |
| P. aeruginosa PAK (taxid:1009714) | P. aeruginosa PA7 (taxid:381754) |
| P. aeruginosa DSM 50071 (taxid:1123015) | P. aeruginosa BL04 (taxid:1402545) |
| P. aeruginosa E2 (taxid:1163395) | P. aeruginosa ATCC25324 (taxid:1163393) |
| P. aeruginosa str. PA17 (taxid:1333546) | P. aeruginosa VRFPA07 (taxid:1431713) |
| P. aeruginosa PA38182 (taxid:1407059) | P. aeruginosa UCBPP-PA14 (taxid:208963) |
| P. aeruginosa str. Stone130 (taxid:1125697) | P. aeruginosa BWHPSA037 (taxid:1402529) |
To avoid redundant data, all 80 EptA sequences from P. aeruginosa species were subjected to identity matrix analysis using the MEGA X software (Teixeira et al., 2016) resulting in only 59 unique sequences. Then, these sequences, were evaluated regarding the protein domain that they belong. After this evaluation, only 31 EptA isoforms remained, all belonging to the DUF1705 superfamily, similar to proteins encoded by E. coli (Stoesser et al., 2016), Salmonella enterica (Doumith, 2016), K. pneumonia (Di Pilato et al., 2016), Serratia plymuthica strain AS13 e Neisseria meningitides (Zhang et al., 2019), all Gram-negative bacteria. Some members of this superfamily are putative bacterial membrane proteins.
The number of amino acids presents in EptA isoforms of P. aeruginosa ranged from 551 to 572 amino acids (aa), and it was possible to recognize three groups based on the sequence size: (I) eleven isoforms formed by 551 aa; (II) a single isoform formed by 567 aa; and (III) two isoforms formed by 572 aa in length. The results obtained in this work in relation to the size of the sequences in P. aeruginosa EptA enzymes showed that it is larger than other proteins from E. coli MCR1, K. pneumoniae MCR1.9 and S. enterica MCR1.6 (GenBank ids: ASK04346.1, ARA74236.1 and WP_077248208.1, respectively) which has 541 aa (Compain et al., 2014), N. meningitidis EptA (GenBank id: ELL31702.1) which has 544 aa, and S. plymuthica EptA (GenBank id: KYG15235.1) which has 545 aa.
These results could be due to sequencing processes, or even location of EptA gene in a region of the P. aeruginosa genome difficult to sequence. These enzymes are still in process of evolution since the reports of polymyxins resistance in P. aeruginosa are very recent (Olaitan et al., 2014) for this reason the EptA isoform could currently have a smaller length than other microorganisms. The most frequent amino acids in P. aeruginosa EptAs belongs to the group of the aliphatic amino acids, including: leucine (13.6% to 17.1%), alanine (7.6% to 11.4%), glycine (6.7% to 7.9%), valine (5.2% to 7.8%) and the polar uncharged serine (5.8% to 6.6%), respectively. Moreover, amino acids in lower frequency are: the aromatic amino acid tryptophan (1.3% to 1.6%), the aliphatic amino acid methionine (1.7% to 2.2%), the polar uncharged amino acid cysteine (1.9% to 2.2%), and the positively charged amino acid histidine (1.7% to 2.4%).
When the EptAs in P. aeruginosa were compared with other microorganisms, a pattern of higher and lower frequency of amino acids was found, suggesting that these isoforms were derived from a single gene. When compared to the proteins MCR1 of E. coli, MCR1.9 of K. pneumoniae, and EptAs of S. enterica, N. meningitidis and S. plymuthica the same pattern repetition in relation amino acids frequency was found, with high rates of leucines, valines, alanines and serines. Only in the EptA isoforms from P. aeruginosa the glycine residues are among the five most frequent amino acids, being replaced by threonine (uncharged amino acid). In relation to the less frequent amino acids: methionine, cysteine and histidine, there is also repetition in the EptAs of these microorganisms (E. coli, K. pneumoniae, N. meningitidis, S. enterica, and S. plymuthica).
Only phenylalanine the fifth most frequent amino acid in EptA isoforms of P. aeruginosa, is replaced by tyrosine in the EptA of S. plymuthica, while the methionine amino acid, second most frequent in P. aeruginosa EptA, is replaced by glycine. Few differences in frequency of each amino acid may be directly associated with the different lengths of polypeptide chains that form these proteins in these microorganisms. The molecular mass (average) predicted for EptA isoforms of P. aeruginosa, showed variations from 61.36 to 62.80 kDa (Table 2). In this analysis two generic groups were observed based on their molecular masses (I) formed by 10 isoforms (32.26%, molecular mass approximately 61.5 kDa); (II) formed by 21 isoforms (67.74%, molecular mass approximately 62.5 kDa). In relation to the protein molecular masses of E. coli MCR1 (60,12 kDa), K. pneumoniae MCR1.9 (60.09 kDa) and EptAs of S. enterica (60.10 kDa), N. meningitidis (61.36 kDa) and S. plymuthica (61.64 kDa). This result may be due to the same reasons discussed above about the lengths of these proteins sequences. Although all these EptAs proteins belong to the DUF1705 superfamily (Lu, 2020), the isoforms expressed in P. aeruginosa, classified in group (I), are likely to be more active against polymyxins B and E, because their size is closer to the EptA of E. coli (Stoesser et al., 2016), S. enterica (Doumith, 2016), K. pneumonia (Di Pilato et al., 2016), S. plymuthica AS13 strain.
Table 2.
Identification of EptAs amino acid sequences encoded by the bacterium Pseudomonas aeruginosa based on access from the National Center for Biotechnology Information - NCBI (https://www.ncbi.nlm.nih.gov/). Domain analysis: Position-Specific Scoring Matrix (PSSM id), Domain (starting and ending amino acid), and Bit-score E-value were obtained after access to the Conserved Domain Database - CDD (https://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml). Physicochemical analysis: Molecular weight (rM), Theoretical (pI), Instability index (II), Aliphatic index (AI), and Grand average of hydropathicity (GRAVY) were obtained after access to the ProtParam tool (https://web.expasy.org/protparam/). All data revisited and checked on April 20th, 2021.
| GenBank id | Size (aa) | Domain |
Bit-score | rM (kDa) | pI | II | IA | GRAVY | |
|---|---|---|---|---|---|---|---|---|---|
| Start | end | ||||||||
| OKO10373.1 | 565 | 5 | 545 | 559.81 | 61.43 | 6.89 | 34.99 | 101.23 | 0.085 |
| WP_012076057.1 | 588 | 13 | 538 | 629.78 | 62.80 | 6.57 | 41.54 | 99.35 | 0.001 |
| WP_019485721.1 | 565 | 5 | 545 | 594.73 | 61.43 | 6.89 | 34.50 | 101.23 | 0.085 |
| WP_023081504.1 | 565 | 5 | 545 | 594.73 | 61.40 | 6.89 | 34.85 | 101.05 | 0.084 |
| WP_023118443.1 | 583 | 13 | 538 | 616.69 | 62.38 | 6.24 | 40.89 | 101.92 | 0.026 |
| WP_023120612.1 | 583 | 13 | 538 | 616.30 | 62.36 | 6.39 | 40.44 | 102.28 | 0.039 |
| WP_024914867.1 | 565 | 5 | 545 | 595.50 | 61.42 | 6.54 | 33.76 | 100.69 | 0.082 |
| WP_031694049.1 | 583 | 13 | 538 | 615.92 | 62.32 | 6.24 | 39.73 | 101.94 | 0.035 |
| WP_033962259.1 | 583 | 13 | 538 | 617.07 | 62.41 | 6.24 | 40.41 | 102.10 | 0.030 |
| WP_033981880.1 | 565 | 5 | 545 | 593.58 | 61.43 | 6.89 | 35.65 | 101.23 | 0.085 |
| WP_033997080.1 | 588 | 13 | 538 | 615.15 | 62.77 | 6.57 | 41.87 | 99.53 | 0.006 |
| WP_034015152.1 | 583 | 13 | 538 | 629.78 | 62.35 | 6.39 | 40.99 | 101.76 | 0.035 |
| WP_034038915.1 | 583 | 13 | 538 | 615.92 | 62.36 | 6.10 | 40.29 | 101.59 | 0.022 |
| WP_034053930.1 | 583 | 13 | 538 | 614.76 | 62.41 | 6.24 | 41.31 | 101.76 | 0.035 |
| WP_034070146.1 | 583 | 13 | 539 | 615.15 | 62.35 | 6.24 | 40.60 | 102.63 | 0.040 |
| WP_034072350.1 | 583 | 13 | 538 | 619.77 | 62.35 | 6.39 | 40.22 | 102.45 | 0.048 |
| WP_034075934.1 | 583 | 13 | 538 | 616.69 | 62.44 | 6.24 | 40.15 | 101.92 | 0.025 |
| WP_034082976.1 | 588 | 13 | 538 | 615.53 | 62.76 | 6.57 | 41.87 | 98.85 | −0.003 |
| WP_043089441.1 | 583 | 13 | 538 | 616.69 | 62.49 | 6.10 | 40.30 | 101.92 | 0.020 |
| WP_044061861.1 | 565 | 5 | 545 | 595.89 | 61.45 | 6.89 | 34.85 | 101.48 | 0.089 |
| WP_054379545.1 | 583 | 13 | 539 | 616.69 | 62.34 | 6.24 | 40.94 | 102.45 | 0.043 |
| WP_057393034.1 | 565 | 5 | 545 | 593.96 | 61.42 | 7.17 | 33.76 | 101.05 | 0.083 |
| WP_058135045.1 | 588 | 13 | 538 | 627.86 | 62.78 | 6.57 | 41.87 | 98.67 | −0.008 |
| WP_058178590.1 | 583 | 13 | 538 | 617.84 | 62.33 | 6.24 | 40.56 | 101.94 | 0.035 |
| WP_083556735.1 | 565 | 5 | 545 | 601.28 | 61.51 | 7.17 | 35.20 | 101.40 | 0.082 |
| WP_086340251.1 | 565 | 5 | 545 | 593.58 | 61.38 | 6.89 | 34.70 | 100.53 | 0.076 |
| WP_086822489.1 | 583 | 13. | 538 | 616.69 | 62.35 | 6.10 | 41.84 | 101.76 | 0.030 |
| WP_088171235.1 | 565 | 5 | 545 | 595.12 | 61.43 | 6.89 | 34.50 | 101.76 | 0.094 |
| WP_093945147.1 | 583 | 13 | 538 | 616.69 | 62.35 | 6.39 | 40.40 | 101.41 | 0.028 |
| WP_096229894.1 | 565 | 5 | 592 | 592.81 | 61.36 | 7.17 | 34.35 | 101.05 | 0.090 |
| WP_096262676.1 | 565 | 5 | 545 | 594.35 | 61.42 | 7.17 | 35.30 | 101.05 | 0.084 |
The concentration of hydrogen ions in a solution where the ionization of the acid groups are equal to the ionization of the basic groups is known as the isoelectric point (Highberger, 1939). Data obtained on the isoelectric point (pI) of EptA isoforms of the microorganism P. aeruginosa demonstrated that 27 isoforms are acidic and only four are basic. Analyses performed with the proteins of other species demonstrated a pI of 6.31 for E. coli MCR1, and K. pneumoniae MCR1.9, 6.23 for S. enterica EptA, 6.67 for S. plymuthica EptA, that is, isoforms with acid pI, whereas the EptA belonging in N. meningitidis species feature pI of 7.57, that is, a pI basic. This observation is in perfect agreement with the EptA isoforms from P. aeruginosa, since 87.10% showed acidic pI and only 12.90% showed basic pI. This variation in isoelectric points presented by EptAs expressed in P. aeruginosa may be directly associated to its adaptive potential in different environments (Kumar et al., 2018; Teixeira et al., 2016; Al-Saleh and Akbar, 2015).
The instability index provides an estimate of how much a protein can remain stable during an experiment conducted in a test tube (Guruprasad et al., 1990). The instability index provides an inverse estimate of the metabolic stability of a protein (https://web.expasy.org/protparam/). All EptAs in P. aeruginosa sequences analysed in this study predicted instability index values ranging from 33.76 to 41.87, so all these isoforms are relatively stable analysis in test tubes (Table 2). The analysis of the instability index indicated values of 22.65 for E. coli MCR1 and S. enterica EptA protein (Doumith, 2016; Di Pilato et al., 2016), 21.78 for K. pneumoniae MCR1.9 (Zhang et al., 2019), 35.77 for N. meningitidis EptA and 41,11 for S. plymuthica EptA. Based on observed rates for P. aeruginosa EptA, the proteins from E. coli MCR1, K. pneumoniae MCR1.9, S. enterica and N. meningitidis EptAs are stable. The S. plymuthica species was the only one that presented unstable EptA. Similarly, in P. aeruginosa, two groups EptA were observed, the first group formed by 18 stables proteins (58.06%), and second by 13 unstable proteins (41.94%).
The protein aliphatic index is defined as the relative volume occupied by aliphatic side chains: alanine, valine, isoleucine and leucine (Ikai, 1980). It is generally considered a positive factor to increase protein thermostability, therefore, it demonstrates how much protein molecules are able to withstand heat denaturation. The results obtained on thermostability for the EptA isoforms of P. aeruginosa have shown values between 98.67 and 102.63 corroborating the previous data, indicating that many of these proteins are really stable (Ikai, 1980). When the aliphatic index of the other proteins was evaluated it presented values of 92.83 for E. coli MCR1 and S. enterica EptA, 93,03 for S. plymuthica EptA, 93.36 for K pneumoniae MCR1.9, and 96,21 for N. meningitidis EptA. Based on rates observed for P. aeruginosa EptA, the majority of the proteins are stable at 25 °C temperature as presented in the species E. coli, K. pneumoniae, S. enterica, and N. meningitidis. It is possible that P. aeruginosa EptA can tolerate higher temperatures because these proteins are important both in environmental adaptation as well in vivo febrile hosts infection (Ikai, 1980; Zhang et al., 2012; Chan et al., 2016).
The GRAVY value (hydrophobicity index) for a peptide or protein is calculated based on the sum of all amino acids hydropathy values, divided by the total number of residues present in the sequence (Oliveira et al., 2020). High positive values means that the amino acids located in the protein region are more hydrophobic. These scales are commonly used to predict the transmembrane alpha helices of membrane proteins (Kyte and Doolittle, 1982). GRAVY analysis from P. aeruginosa EptAs profile revealed that 29 isoforms (93.55%) are hydrophobic and 2 (6.45%) are hydrophilic (Table 2). This result indicates that mostly of these enzymes may be in transmembrane regions in the P. aeruginosa bacteria, while the other group of these enzymes must have differentiated their metabolic function. In other bacterial species, the evaluation of GRAVY indicated a value of 0,067 for S. enterica EptA, 0,018 for N. meningitidis EptA, 0.065 for E. coli MCR1, 0,067 for S. enterica EptA and 0.069 for K. pneumonia MCR1.9, all with hydrophobic profile.
Although, the hydrophilic P. aeruginosa EptAs role is not known, they are very similar to LptB protein, when the amino acid sequences are analysed, both are soluble proteins without transmembrane domain. According to Sperandeo et al., LptB was identified in an internal membrane complex in E. coli, forming a 140 kDa protein agglomerate, although no other proteins were detected (Sperandeo, 2007; Stenberg et al., 2005).
We suggest that hydrophobic and hydrophilic EptA in P. aeruginosa may interact in a similar way like LptA and LptB in E. coli and K. pneumoniae, together with a transmembrane partner unidentified yet, resulting in a membrane-associated complex required to transport deleterious molecules to this microorganism. On the other hand, the bacterial LPS modification, with the cations substitution present in the phosphate groups by L-Ara4N, neutralizing the lipids A charges, or PEtN modification, increases the net load from −1.5 to −1 (Nikaido, 2003). The L-Ara4N modification is the most effective of the two modifications due to the nature of the charge change. The net positive charge resulting from the modified LPS reduces its binding to polymyxins, leading to resistance (Olaitan et al., 2014). The composition of amino acid residues is directly associated with the structure and protein functions.
The secondary structures predicted for EptAs in P. aeruginosa using the SopMA server showed possible data grouped into two subgroups: (I) consisting of 20 isoforms (64.51%) with predominance of α-helices (44.15 - 47.68%), followed by loops (28.76 - 30.31%) and β-sheets (22.58 - 25.72%); (II) formed by 11 isoforms (35.49%) with predominance of loops (39.35 - 41.51%), followed by α-helices (30.75 - 34.18%) and β-sheets (26,12–28, 61%). When comparing the data obtained for EptAs from P. aeruginosa with MCR1 in E. coli, MCR1.9 in K. pneumoniae and EptAs in S. enterica, N. meningitidis and S. plymuthica, there is a greater proximity to the EptAs belonging to subgroup (I), since α-helices are predominant, followed by loops and β-sheets.
Although such differences can be observed in P. aeruginosa EptAs, this phenomenon could be explained due to variations observed in the polypeptides sequences, which could be associated with the thermostability shown by this isoforms (Ikai, 1980; Zhang et al., 2012), considering the combination of selective pressure factors that have acted on these proteins for decades (pressure, pH, temperature), besides the mutations that have accumulated. According to the analysis performed using Conserved Domains Database – CDD (Lu, 2020), all EptAs expressed by P. aeruginosa belong to the superfamily DUF1705 majority members of this family are putative bacterial membrane proteins, and this domain is found immediately in the N-terminal region, likewise all those used in this work as standard, included, MCR1 of E. coli, MCR1.9 of K. pneumoniae and EptAs of S. enterica, N. meningitidis and S. plymuthica.
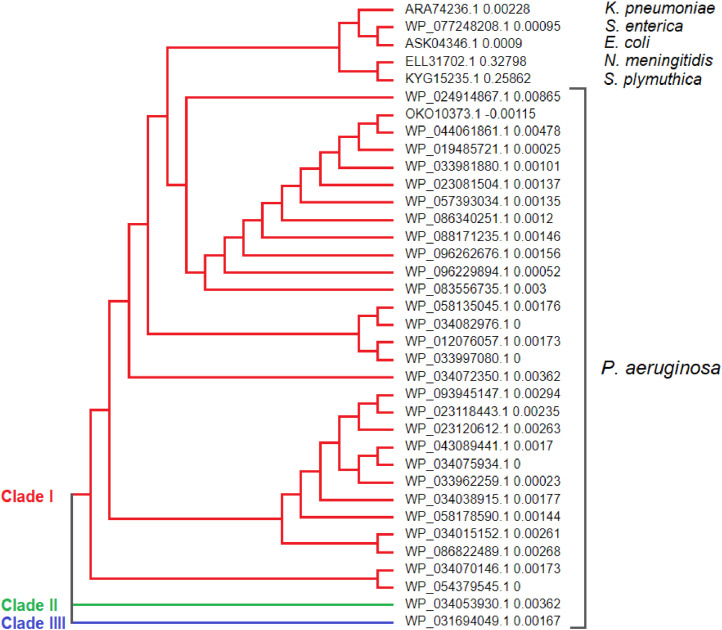
The alignment of the EptAs sequences was performed using the Molecular Evolutionary Genetics Analysis - MEGA X software. It revealed low amino acid variability along the polypeptide chains, however, these substitutions are equivalent, what means they belong to the same functional group (Data not shown). The largest consensus area is located between the amino acids 240 until the C-terminal region of these proteins. It is also possible to observe that the N-terminal region has a higher variation in these proteins. The dendogram was generated with support of the MEGA X software (Teixeira et al., 2016), with the aid of sequence alignment previously performed. It was shown that EptAs from P. aeruginosa formed two distinct clades as can be seen in Fig. 1: Clade I concentrates 93.55% of the identified isoforms. Interestingly, the MCR1 proteins of E. coli, MCR1.9 of K. pneumoniae and EptA of S. enterica were organized in the clade I, such result corroborates with the other results previously presented, strengthening other findings that predict the participation of these proteins in the mechanism of the resistance to polimixins by members from Enterobacteriaceae family (Nikaido, 2003; Alves and Behar, 2013; Andino and Hanning, 2015). In relation to, the clades II and III, both are formed by only one isoform (WP_034053930.1 and WP_031694049.1, respectively).
Fig. 1.
Cladogram generated by the Neighbor-Joining method employing alignment and multiple performance with Molecular Evolutionary Genetics Analysis software - MEGA X. Proteins belonging to the DUF1705 superfamily of P. aeruginosa and proteins responsible for polymyxin resistance in Gram-negative bacteria with clinical interest. Node values = bootstrap test (1000 pseudo-replicas).
Although the S. plymuthica species are classified as an Enterobacteriaceae, in clinical settings, members belonging to the Serratia genus, generally cause infections in the respiratory system (Domingo et al., 1994), urinary tract (Jain et al., 2017) and can cause necrotic cellulitis (Mahlen, 2011), instead of infections in the gastrointestinal tract, as observed with other microorganisms in this family. In addition, small mammals, vegetables and water seem to be the natural environment of S. plymuthica species (Grimont and Grimont, 2006). These S. plymuthica characteristics may be the cause of their differentiated grouping in relation to other enterobacteria as E. coli, K. pneumoniae and S. enterica. In the same way as S. plymuthica, N. meningitidis, was classified in a group of its own, because this species presents different biology among the other species discussed in this study. N. meningitidis is a Gram-negative Diplococcus and humans are the only natural host (Souza and Gagliani, 2011), causing cerebrospinal meningitis and/or septicaemia (Coureuil et al., 2013).
Considering the cladogram, it is possible to identify the way of acquisition of antimicrobial resistance to polymyxins. P. aeruginosa appears to be the species that initially developed resistance to polymyxins, followed by enterobacteria K. pneumoniae, E. coli and S. enterica, and then N. meningitidis and S. plymuthica bacteria. Finally, P. aeruginosa through EtpA proteins began to resist the action of polymyxins.
Conclusion
In conclusion these results showed the high degree of similarity between EptA proteins of P. aeruginosa with MCR1 of E. coli, MCR1.9 of K. pneumoniae and EptA proteins of S. enterica, N. meningitidis and S. plymuthica, based on their physicochemical properties, we demonstrated 31 protein isoforms in P. aeruginosa (EptA) found in to 18 genomes from P. aeruginosa closely related to proteins known to provide resistance to polymyxin in other species of Gram-negative bacteria of clinical interest. EptAs from P. aeruginosa studied here, belonging to DUF1705 family as indicated by values bit-score between 559.81 and 629.78. In addition, these EptAs showing lengths between 551 and 572 amino acids, molecular mass values between 61.36 - 62. 80 kDa, isoelectric point between 6.10 to 7.17, instability index between 33.76 to 41.87, aliphatic index between 98.67 to 102.63 and the hydropathy index between - 0.008 to 0.094. However, further studies are needed to identify the actual biochemical contribution of EptAs in P. aeruginosa species.
CRediT authorship contribution statement
Cindy Magda Araújo dos Santos Freire: Conceptualization, Methodology, Data curation, Writing – original draft. Alessandro Taunay-Rodrigues: Visualization, Investigation. Michelangelo Bauwelz Gonzatti: Conceptualization, Methodology, Data curation, Writing – original draft. Fátima Morgana Pio Fonseca: Conceptualization, Methodology, Data curation, Writing – original draft. José Ednésio da Cruz Freire: Supervision, Validation, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Cindy Magda Araújo dos Santos Freire, Email: cindy_lab@hotmail.com.
Alessandro Taunay-Rodrigues, Email: taunay.ale@gmail.com.
Michelangelo Bauwelz Gonzatti, Email: gonzatti@unifesp.br.
Fátima Morgana Pio Fonseca, Email: fatimamorganapf@gmail.com.
José Ednésio da Cruz Freire, Email: jednesio@gmail.com.
References
- Al-Saleh E., Akbar A. Occurrence of Pseudomonas aeruginosa in Kuwait soil. Chemosphere. 2015;120:100–107. doi: 10.1016/j.chemosphere.2014.06.031. [DOI] [PubMed] [Google Scholar]
- Alves A.P., Behar P.R.P. Infecções hospitalares por enterobactérias produtoras de KPC em um hospital terciário do sul do Brasil. Rev. da AMRIGS. 2013;57(3):213–218. [Google Scholar]
- Andino A., Hanning I. Salmonella enterica: Survival, colonization, and virulence differences among serovars. Sci. World J. 2015;2015:1–16. doi: 10.1155/2015/520179. no. ID 520179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros L.L.dos S., Maia C.do S.F., Monteiro M.C. Fatores de risco associados ao agravamento de sepse em pacientes em Unidade de Terapia Intensiva. Cad. Saúde Coletiva. 2016;24(4):388–396. doi: 10.1590/1414-462x201600040091. [DOI] [Google Scholar]
- Basso M.E., Pulcinelli R.S.R., do C. Aquino A.R., Santos K.F. Prevalência de infecções bacterianas em pacientes internados em uma unidade de terapia intensiva (UTI),” Rev. Bras. Anál. Clín. 2016;48(4):383–388. doi: 10.21877/2448-3877.201600307. [DOI] [Google Scholar]
- Bomfim L.B., Knob A. Perfil epidemiológico das infecções causadas por Pseudomonas aeruginosa em um hospital privado no município de Guarapuava-PR. Rev. Saúde.Com. 2013;9(4):264–274. [Google Scholar]
- Bonomo R.A., Szabo D. Mechanisms of multidrug resistance in Acinetobacter species and Pseudomonas aeruginosa. Clin. Infect. Dis. 2006;43(SUPPL. 2):49–56. doi: 10.1086/504477. no. [DOI] [PubMed] [Google Scholar]
- Carvalho L.L., Cogo V.D.N. Resistência às polimixinas em bactérias Gram-negativas: Uma revisão microbiológica. Vis. Acad. 2014;15(1):119–129. [Google Scholar]
- Chan K., et al. Transcriptome analysis of Pseudomonas aeruginosa PAO1 grown at both body and elevated temperatures. PeerJ. 2016;4(e2223):1–19. doi: 10.7717/peerj.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K.C., et al. Clonal spread of multidrug-resistant Acinetobacter baumannii in eastern Taiwan. J. Microbiol. Immunol. Infect. 2012;45:37–42. doi: 10.1016/j.jmii.2011.09.019. [DOI] [PubMed] [Google Scholar]
- Compain F., et al. Complete nucleotide sequence of two multidrug-resistant IncR plasmids from Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2014;58(7):4207–4210. doi: 10.1128/AAC.02773-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coureuil M., Join-Lambert O., Lécuyer H., Bourdoulous S., Marullo S., Nassif X. Pathogenesis of meningococcemia. Cold Spring Harb. Perspect. Med. 2013;3(a012393):1–14. doi: 10.1101/cshperspect.a012393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pilato V., Arena F., Tascini C., Cannatelli A., De Angelis H., Fortunato S. mcr-1.2, a new mcr variant carried on a transferable plasmid from a colistin-resistant KPC carbapenemase-producing Klebsiella pneumoniae strain of sequence type 512. Antimicrob. Agents Chemother. 2016;60(9):5612–5615. doi: 10.1128/AAC.01075-16. Address. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo D., Limia A., Alarcon T., Sanz J.C., Del Rey M.C., Lopez-Brea M. Nosocomial septicemia caused by Serratia plymuthica. J. Clin. Microbiol. 1994;32(2):575–577. doi: 10.1128/jcm.32.2.575-577.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doumith M., et al. Detection of the plasmid-mediated mcr-1 gene conferring colistin resistance in human and food isolates of Salmonella enterica and Escherichia coli in England and Wales. J. Antimicrob. Chemother. 2016;71(8):2300–2305. doi: 10.1093/jac/dkw093. [DOI] [PubMed] [Google Scholar]
- Economou V., Gousia P. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect. Drug Resist. 2015;8:49–61. doi: 10.2147/IDR.S55778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison M.L., Matthew J., Iiii F., Parrish W., Danell A.S., Pesci E.C. The transcriptional regulator Np20 Is the zinc uptake regulator in Pseudomonas aeruginosa. PLoS ONE. 2013;8(9):1–11. doi: 10.1371/journal.pone.0075389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair R.J., Tor Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Medicin. Chem. 2014;6:25–64. doi: 10.4137/PMC.S14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falagas M.E., Kasiakou S.K. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Rev. Anti-Infective Agents. 2005;40:1333–1341. doi: 10.1086/429323. [DOI] [PubMed] [Google Scholar]
- Geourjon C., Deléage G. Sopma: Significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Bioinformatics. 1995;11(6):681–684. doi: 10.1093/bioinformatics/11.6.681. [DOI] [PubMed] [Google Scholar]
- Girardello R., Gales A.C. Resistência às polimixinas: Velhos antibióticos, últimas opções terapêuticas. Rev. Epidemiol. Control. Infecção. 2012;2(2):66–69. doi: 10.17058/reci.v2i2.2504. [DOI] [Google Scholar]
- Grimont F., Grimont P.A.D. The genus Serratia. Prokaryotes. 2006;6:197–214. [Google Scholar]
- Guruprasad K., Reddy B.V.B., Pandit M.W. Correlation between stability of a protein and its dipeptide composition: A novel approach for predicting in vivo stability of a protein from its primary sequence. Protein Eng. 1990;4(2):155–161. doi: 10.1093/protein/4.2.155. [DOI] [PubMed] [Google Scholar]
- Gutu A.D., et al. Polymyxin resistance of Pseudomonas aeruginosa phoQ mutants is dependent on additional two-component regulatory systems. Antimicrob. Agents Chemother. 2013;57(5):2204–2215. doi: 10.1128/AAC.02353-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habbu P., Warad V., Shastri R., Madagundi S., Kulkarni V.H. Antimicrobial metabolites from marine microorganisms. Chin. J. Nat. Med. 2016;14(2):101–116. doi: 10.1016/S1875-5364(16)60003-1. [DOI] [PubMed] [Google Scholar]
- Herrera C.M., Hankins J.V., Trent M.S. Activation of PmrA inhibits LpxT-dependent phosphorylation of lipid A promoting resistance to antimicrobial peptides. Mol. Microbiol. 2010;76(6):1444–1460. doi: 10.1111/j.1365-2958.2010.07150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highberger J.H. The isoelectric point of collagen. J. Am. Chem. Soc. 1939;61(9):2302–2303. doi: 10.1021/ja01878a010. [DOI] [Google Scholar]
- Hood R.D., et al. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe. 2010;7(1):25–37. doi: 10.1016/j.chom.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikai A. Thermostability and aliphatic index of globular proteins. J. Biochem. 1980;88(6):1895–1898. [PubMed] [Google Scholar]
- Jain S., Arora S., Saha R., Kaur I.R. Serratia plymuthica: A community acquired uropathogen. Indian J. Med. Sci. 2017;69(1):31–32. doi: 10.18203/issn.0019-5359.IndianJMedSci20170488. [DOI] [Google Scholar]
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35(6):1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lee J.Y., Lim M.H., Heo S.T., Ko K.S. Repeated isolation of Pseudomonas aeruginosa isolates resistant to both polymyxins and carbapenems from 1 patient. Diagn. Microbiol. Infect. Dis. 2012;72(3):267–271. doi: 10.1016/j.diagmicrobio.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Li J., Nation R.L., Milne R.W., Turnidge J.D., Coulthard K. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int. J. Antimicrob. Agents. 2005;25(1):11–25. doi: 10.1016/j.ijantimicag.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Liu Y.Y., et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016;16(2):161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- Lopes H.V. O tratamento das infecções graves por Pseudomonas aeruginosa. Rev. Panam. Infectol. 2009;11(3):74–76. [Google Scholar]
- Lorenzo J.de J.C., Ramírez A.M., Muñoz Y.G. Polimixinas en la era de la multidrogorresistencia. Rev. Enfermedades Infecc. en Pediatr. 2011;25(98):66–70. [Google Scholar]
- Lu S., et al. CDD/SPARCLE: The conserved domain database in 2020. Nucl. Acids Res. 2020;48(D1):D265–D268. doi: 10.1093/nar/gkz991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlen S.D. Serratia infections: From military experiments to current practice. Clin. Microbiol. Rev. 2011;24(4):755–791. doi: 10.1128/CMR.00017-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes C.A.C., Burdmann E.A. Polimixinas: Revisão com ênfase na sua nefrotoxicidade. Rev. Assoc. Med. Bras. 2009;55(6):752–759. doi: 10.1590/s0104-42302009000600023. [DOI] [PubMed] [Google Scholar]
- Moore R.A., Chan L., Hancock R.E.W. Evidence for two distinct mechanisms of resistance to polymyxin B in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1984;26(4):539–545. doi: 10.1128/AAC.26.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Y., Tomida J., Kawamura Y. Responses of Pseudomonas aeruginosa to antimicrobials. Front. Microbiol. 2014;4(422):1–8. doi: 10.3389/fmicb.2013.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiva L.B.M., Fonseca C.D., Watanabe M., Vattimo M.F.F. Polimixina B: Efeito dose e tempo dependente na nefrotoxicidade in vitro. ACTA Paul. Enferm. 2013;26(1):57–62. doi: 10.1590/S0103-21002013000100010. [DOI] [Google Scholar]
- Nguyen N.T., et al. Use of colistin and other critical antimicrobials on pig and chicken farms in Southern Vietnam and its association with resistance in commensal Escherichia coli bacteria. Appl. Environ. Microbiol. 2016;82(13):3727–3735. doi: 10.1128/AEM.00337-16. Editor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Molecular basis of bacterial outer membrane permeability. Microbiol. Mol. Biol. Rev. 2003;67(4):593–656. doi: 10.1128/MMBR.67.4.593-656.2003. doi: 10.1128/MMBR.67.4.593–656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaitan A.O., Morand S., Rolain J.M. Mechanisms of polymyxin resistance: Acquired and intrinsic resistance in bacteria. Front. Microbiol. 2014;5:1–18. doi: 10.3389/fmicb.2014.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira C.de, Sales L.S., Freire J.E.da C., da C. Freire J.E. Modelagem Estrutural de uma Quitinase (GH19) de Melão e Análise de Docking Molecular com N-Acetil-β(1-4)-D-Glicosamina. Revista Arquivos Científicos (IMMES) 2020;3(1):162–171. doi: 10.5935/2595-4407/rac.immes.v3n1p162-171. [DOI] [Google Scholar]
- Pogue J.M., Ortwine J.K., Kaye K.S. Clinical considerations for optimal use of the polymyxins: A focus on agent selection and dosing. Clin. Microbiol. Infect. 2017;23(4):229–233. doi: 10.1016/j.cmi.2017.02.023. [DOI] [PubMed] [Google Scholar]
- Schurek K.N., et al. Involvement of pmrAB and phoPQ in polymyxin B adaptation and inducible resistance in non-cystic fibrosis clinical isolates of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2009;53(10):4345–4351. doi: 10.1128/AAC.01267-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva Filho L.V.R.F., Filho Silva S., et al. Pseudomonas aeruginosa infection in patients with cystic fibrosis: Scientific evidence regarding clinical impact, diagnosis, and treatment. J. Bras. Pneumol. 2013;39(4):495–512. doi: 10.1590/S1806-37132013000400015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza D.A.G., Gagliani L.H. Estudo retrospectivo da meningite meningocócica no estado de São Paulo. Rev. UNILUS Ensino e Pesqui. 2011;8(15):32–44. [Google Scholar]
- Sperandeo P., et al. Characterization of lptA and lptB, two essential genes implicated in lipopolysaccharide transport to the outer membrane of Escherichia coli. J. Bacteriol. 2007;189(1):244–253. doi: 10.1128/JB.01126-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansly P.G., Schlosser M.E. Studies on polymyxin: Isolation and identification of Bacillus polymyxa and differentiation of polymyxin from certain known antibiotics. J. Bacteriol. 1947;54(5):549–556. doi: 10.1128/jb.54.5.549-556.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani S., et al. Relevance of multidrug-resistant Pseudomonas aeruginosa infections in cystic fibrosis. Int. J. Med. Microbiol. 2017;307(6):353–362. doi: 10.1016/j.ijmm.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Stenberg F., et al. Protein complexes of the Escherichia coli cell envelope. J. Biol. Chem. 2005;280(41):34409–34419. doi: 10.1074/jbc.M506479200. [DOI] [PubMed] [Google Scholar]
- Stoesser N., Mathers A.J., Moore C.E., Day N.P.J., Crook D.W. Colistin resistance gene mcr-1 and pHNSHP45 plasmid in human isolates of Escherichia coli and Klebsiella pneumoniae. Lancet Infect. Dis. 2016;16:285–286. doi: 10.1016/S1473-3099(16)00010-4. [DOI] [PubMed] [Google Scholar]
- Teixeira P., Tacão M., Alves A., Henriques I. Antibiotic and metal resistance in a ST395 Pseudomonas aeruginosa environmental isolate: A genomics approach. Mar. Pollut. Bull. 2016;110(1):75–81. doi: 10.1016/j.marpolbul.2016.06.086. [DOI] [PubMed] [Google Scholar]
- Tran A.X., et al. Resistance to the antimicrobial peptide polymyxin requires myristoylation of Escherichia coli and Salmonella typhimurium lipid A. J. Biol. Chem. 2005;280(31):28186–28194. doi: 10.1074/jbc.M505020200. [DOI] [PubMed] [Google Scholar]
- Yu Z., Qin W., Lin J., Fang S., Qiu J. Antibacterial mechanisms of polymyxin and bacterial resistance. Biomed Res. Int. 2015;2015:1–11. doi: 10.1155/2015/679109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavascki A.P., Carvalhaes C.G., Picão R.C., Gales A.C. Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii: Resistance mechanisms and implications for therapy. Expert Rev. Anti. Infect. Ther. 2010;8(1):71–93. doi: 10.1586/eri.09.108. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wei W., Huang M., Umar Z., Feng Y. Definition of a family of nonmobile colistin resistance (NMCR-1) determinants suggests aquatic reservoirs for MCR-4. Adv. Sci. 2019;6(11) doi: 10.1002/advs.201900038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Hinz A.J., Nadeau J.P., Mah T.F. Pseudomonas aeruginosa tssC1 links type VI secretion and biofilm-specific antibiotic resistance. J. Bacteriol. 2011;193(19):5510–5513. doi: 10.1128/JB.00268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Smith J.C., Zhu Q., Guo Z., MacDonald N.E. A five-year review of Pseudomonas aeruginosa bacteremia in children hospitalized at a single center in southern China. Int. J. Infect. Dis. 2012;16(8):e628–e632. doi: 10.1016/j.ijid.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Zheng B., et al. Discovery and characterisation of an Escherichia coli ST206 strain producing NDM-5 and MCR-1 from a patient with acute diarrhoea in China. Int. J. Antimicrob. Agents. 2018;51(2):273–275. doi: 10.1016/j.ijantimicag.2017.09.005. [DOI] [PubMed] [Google Scholar]