Highlights
-
•
Analyzed the cutC and cutD gene expression and their TMA production levels.
-
•
Bioinformatic analysis of cutC and cutD proteins showed conserved regions.
-
•
Analysis of cutC protein showed conserved choline binding active site residues.
-
•
TMA levels not only depend on cutC and cutD genes other factors are also involved.
Keywords: PCR Primers, Trimethylamine (TMA), Choline TMA-lyase (cutC) gene, Gut microbiota, Bioinformatics
Abstract
Recent studies revealed that some intestinal microorganisms anaerobically convert choline to trimethylamine (TMA) by choline TMA-lyase (cutC). TMA is further oxidized to trimethylamine-N-oxide (TMAO), by the liver enzyme flavin-dependent monooxygenase 3 (FMO3). TMA in the serum is correlated with the risk of cardiovascular disease and some other diseases in human. The objective of this study is to study the expression levels of cutC and its activating enzyme (cutD) gene for these microorganisms and their association with TMA production. In this study, we collected 20 TMA producing bacteria strains representing 20 species, and designed primers to evaluate their gene expression levels by reverse transcription quantitative PCR (RT-qPCR). In addition, TMA production was analyzed by UPLC-MS/MS. Results showed that gene expression levels of most individual strains were different when compared with the gene expression level of their glyceraldehyde-3 phosphate dehydrogenase (GAPDH) gene and the TMA production level of gut bacteria may not correlate with their cutC/cutD gene expression levels. Bioinformatic analysis of the CutC protein showed conserved choline binding site residues; cutD showed conserved S-adenosylmethionine (SAM) and two CX2-CX2-CX3 motifs. The present study reports that the TMA production level may not only depend on cutC/cutD gene expression. Other factors may need to be investigated.
Graphical abstract
Abbriviations: cutC, choline TMA-lyase; cutD, choline TMA-lyase activating enzyme; TMA, trimethylamine; TMAO, trimethylamine-N-oxide; FMO3, flavin-dependent monooxygenase 3; UPLC, ultra-performance liquid chromatography; SAM, S-adenosylmethionine; CVD, cardiovascular disease; cut, choline utilization; GAPDH, Glyceraldehyde 3-phosphate dehydrogenase
1. Introduction
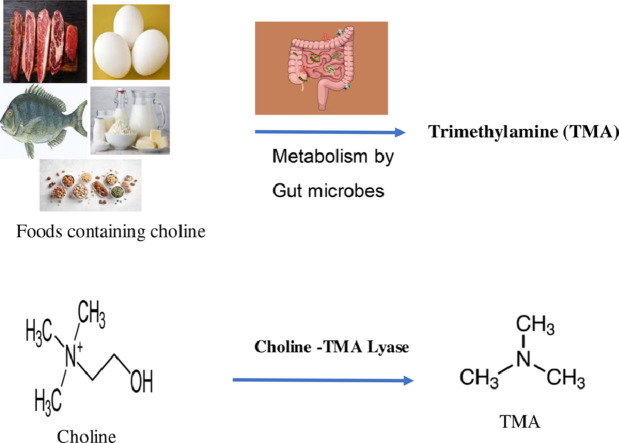
The human gut flora consists of more than 1000 microbial species, which play major roles in metabolism, immune function, digestion, bioactivation of nutrients and vitamins (Tremaroli and Backhed, 2012). Some gut microbes can metabolize the dietary components such as choline, phosphatidylcholine and carnitine and utilize them as growth substances (Koeth et al., 2013; Tang et al., 2013; Wang et al., 2011; Zeisel et al., 1989). Choline is one of the essential nutrients for many biological activities in human life, such as cell membrane function, methyl transfer events, and neurotransmission (Zeisel and da Costa, 2009). Intestinal microbes utilize choline as a carbon and energy source which generates the metabolite trimethylamine (TMA). Previous studies have shown that gut microbes anaerobically convert choline to trimethylamine and acetaldehyde, involving an initial C–N bond-cleavage (Hayward and Stadtman, 1959; Koeth et al., 2013; Zhang et al., 1999) in both animal models and humans. TMA produced by gut microbes reaches the liver rapidly via the portal circulation, further oxidized to odorless trimethylamine-N-oxide (TMAO) by the liver enzyme flavin-dependent monooxygenase 3 (FMO3) (Baker and Chaykin, 1962; Krueger and Williams, 2005; Shih et al., 2015). Dietary sources including red meat, fish, poultry and eggs are rich in choline (Zeisel and da Costa, 2009). Various dietary precursors including choline, lecithin, l- carnitine or TMAO (trimethylamine-N-oxide) are metabolized to TMA by the gut microbiota (Koeth et al., 2013; Miller et al., 2014; Zhang et al., 1999). In addition, reports also revealed that TMA generating gut microbes and host metabolic interaction are linked to multiple human diseases including atherosclerosis, cardiovascular disease (CVD), inherited metabolic disorder including trimethylaminuria (fish-malodor syndrome), nonalcoholic fatty liver disease (NAFLD), chronic kidney diseases and colorectal cancer (Bae et al., 2014; Christodoulou, 2012; Dumas et al., 2006; Mendelsohn and Larrick, 2013; Tang and Hazen, 2014; Tang et al., 2015, 2013, Wang et al., 2011).
Regarding the enzymes and genes for TMA production, studies with the choline-degrading sulfate reducing bacterium Desulfovibrio desulfuricans discovered the genes responsible for choline metabolism named choline utilization (cut) gene cluster (Craciun and Balskus, 2012). Transcriptional and biochemical analysis shows that the cut cluster consists of 19 open reading frames (ORFs) i.e., cutC, cutD, eight putative bacterial microcompartment (BMC) shell proteins (cutAEGKLNQR), two predicted coenzyme A (CoA)-acylating aldehyde oxidoreductases (cutB and cutF), a phosphotransacetylase (cutH), a putative chaperonin (cutI), an alcohol dehydrogenase (cutO), and a Ras-like GTPase (cutS) (Martinez-del Campo et al., 2015). Recent studies identified a novel microcompartment involved in choline metabolism (Herring et al., 2018; Jameson et al., 2016). This cluster includes cutC (Choline TMA-Lyase activ ity) homologous to glycyl radical enzyme (GRE); cutD which encodes a glycyl radical-activating protein. cutC and cutD are responsible for the initial C − N bond cleavage of choline that generates TMA and acetaldehyde (Craciun and Balskus, 2012; Craciun et al., 2014; Thibodeaux and van der Donk, 2012). Bioinformatics analysis revealed that the cut cluster was widely and discontinuously distributed in many human gut bacteria. The majority of the cutC gene containing strains are gastrointestinal tract isolates, cutC is also found in strains isolated from the urogenital tract, airways, and oral cavity; (Craciun and Balskus, 2012).
In this study we attempted to evaluate the gene expression level of cutC and cutD and their TMA production potential for 20 TMA producing strains belonging to 20 species of the major phyla found in the human gut, Escherichia coli, Escherichia fergusonii, Proteus mirabilis, Klebsiella pneumoniae subsp. pneumoniae, Providencia rettgeri, Providencia alcalifaciens, Providencia rustigianii, Clostridium sporogenes, Clostridium tetani, Klebsiella pneumoniae subsp. rhinoscleromatis, Klebsiella variicola, Klebsiella oxytoca, Anaerococcus hydrogenalis, Anaerococcus vaginalis, Anaerococcus tetradius, Hungatella hathewayi, Vibrio furnissii, Olsenella uli, Proteus penneri and Yokenella regensburgi. This study focuses on the cutC and cutD gene expression in different TMA producing species and the association of these gene expression levels with the TMA productivity of these bacteria. For this study, since accurate enumeration of the cell counts for each TMA bacteria strain is important to obtain the right conclusion, we used the gene expression level of a house keeping gene, i.e., Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene, for comparison, which has been generally used for internal control, i.e., to indicate the bacterial counts (Kozera and Rapacz, 2013). In addition, since many intestinal factors such as microbial population, host genotype, diet composition and gut environmental factors etc., all may affect the TMA production (Romano et al., 2015), this study was performed under in vitro conditions to eliminate the effects from these factors. So far, although TMA or TMAO production of different TMA producing strains have been reported (Craciun and Balskus, 2012), the association of cutC and cutD gene expression level with their TMA production level remains unknown.
2. Materials and methods
2.1. Bacterial strains and growth conditions
Bacterial strains used in this study were obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ, Braunschweig, Germany), American Type Culture Collection (ATCC, Manassas, VA, USA) and Bioresource Collection and Research Center (BCRC, Hsinchu, Taiwan). Species, strain numbers and sources as well as culture media used for the growth of each bacteria strain were listed in Table 1. All strains were maintained at −80 °C in 50% glycerol stock. 200 μL of each frozen culture was inoculated into 3 mL of freshly prepared sterile medium and incubated at 37 °C for 18 – 24 hrs. 200 μL of active broth culture was re-inoculated into 5 mL sterile broth and incubated for 18 – 24 hrs at 37 °C for serial dilution, bacteria plate counting or DNA preparation. Nutrient broth (NB) used in this study was purchased from Difco™ (Becton, Dickinson and Company, Sparks, MD). Tryptic soy broth (TSB) and agar were purchased from Acumedia (Neogen, Lansing, MI, USA). Ten-fold serial dilutions of bacterial culture were made and 100 μL each of the dilutions were plated on agar plates (1.5% (w/v)) and colony-forming units per mL (CFU/mL) were counted. Choline-chloride (≥ 99%) was purchased from Sigma-Aldrich Chemie GmbH (Riedstrasse 2 D-89,555 Steinheim). d9 – TMA (cat no. T795807) chemicals were purchased from Toronto Research Chemicals (TRC, Ontario, Canada).
Table 1.
List of bacterial strains used in the present study.
| Strains | Strain No.a | Mediumb | Source |
|---|---|---|---|
| Proteus mirabilis | ATCC 7002; BCRC10725 | NB | urine of patient with kidney stones |
| Klebsiella pneumoniae subsp. pneumoniae | ATCC 33495; BCRC 10694 | NB | human urinary tract |
| Escherichia fergusonii | ATCC 35469; BCRC 15582 | NB | feces of 1-year-old boy |
| Escherichia coli | ATCC 43888; BCRC 15374 | TSB | human feces |
| Clostridium sporogenes | ATCC 7955; DSM 767; BCRC 10943 | TSB | – |
| Clostridium tetani | ATCC 19406; BCRC 80185 | TSB | – |
| Providencia alcalifaciens | ATCC 9886; DSM 30120; BCRC 13995 | NB | human feces |
| Providencia rettgeri | ATCC 31052; BCRC 12624 | NB | – |
| Providencia rustigianii | ATCC 33673; DSM 4541; BCRC 13997 | NB | human feces |
| Anaerococcus hydrogenalis | DSM 7454; ATCC 49630; BCRC 80846 | TSB | human feces |
| Anaerococcus vaginalis | DSM 7457; ATCC 51170; BCRC 80848 | TSB | ovarian abscess |
| Anaerococcus tetradius | DSM 2951; ATCC 35098; BCRC 80847 | TSB | human vagina |
| Hungatella hathewayi | DSM 13479; BCRC 80852 | TSB | human feces |
| Yokenella regensburgi | DSM 5079; ATCC 35313; BCRC 80857 | NB | human wrist wound |
| Vibrio furnissii | DSM 19622; ATCC 35016; BCRC 80856 | NB | human feces |
| Olsenella uli | DSM 7084; ATCC 49627; BCRC 80854 | TSB | human gingival crevice |
| Klebsiella pneumoniae subsp. rhinoscleromatis | ATCC 13884; DSM 16231; BCRC 17593 | NB | Nose of a patient, Sumatra |
| Klebsiella variicola | DSM 15968; ATCC BAA-830; BCRC 80853 | NB | – |
| Klebsiella oxytoca | ATCC 29516; BCRC 17136 | NB | – |
| Proteus penneri | DSM 4544; ATCC 33519; BCRC 80855 | NB | urine |
ATCC: American Type Culture Collection (Virginia); DSM: Deutsche Sammlung von Mikroorganismen und Zellkulturen(Germany); BCRC: Bioresource Collection and Research Center (Taiwan).
Nutrient broth (NB); Tryptone Soya Broth (TSB).
2.2. DNA extraction
Bacterial genomic DNA was prepared using the Viogene DNA/RNA extraction Kit Miniprep System (Viogene Laboratories, Taipei, Taiwan) according to manufacturer's instructions with minor modifications. Bacterial strains were grown in the appropriate medium under the conditions as described earlier. 1 mL of broth culture (1 – 3 × 108 CFU/mL) was centrifuged at 12,000 × g for 5 min. The cell pellet was washed twice with 1 mL of sterilized distilled water, and pelleted (12,000 × g for 5 min). The pellet was resuspended in 170 μL double deionized water, followed by addition of 30 μL lysozyme (2 mg/mL, Sigma). The mixture was incubated at 37 °C for 5 – 6 hrs followed by adjusting the volume to 500 μL with Extraction buffer (Viogene) and 20 μL proteinase K (20 mg/mL, Merck), and incubation at 60 °C for 18 hrs (or overnight). After incubation at 70 °C for another 30 min, total DNA was precipitated with 90% ethanol and extracted according to the manufacturer's manual (Viogene DNA/RNA extraction Kit). Afterwards, genomic DNA was eluted with 40 μL double deionized water and then stored at −20 °C until further use. DNA quality was check by gel electrophoresis using a 1.5% agarose gel.
2.3. Primer design
Nucleotide sequences coding for cutC, cutD and GAPDH gene of the TMA producing bacteria were retrieved from Genbank (http://www.ncbi.nlm.nih.gov/nuccore/) and European nucleotide archive (http://www.ebi.ac.uk/ena) files under the Accession Numbers specified in Table 2. Multiple sequence alignments were used for cutC and cutD gene sequence comparison. Based on the homologous sequence, PCR primers were designed using Primer3 (http://bioinfo.ut.ee/primer3–0.4.0/). Specificity was checked using Primer- BLAST (http://www.ncbi.nlm.nih.gov/ tools/primer-blast/). The sequences of the primers used and their appropriate annealing temperatures are shown in Table 2. The 25 μL final volume of the PCR mixture consisted of 200 μM of each deoxynucleoside triphosphate (PRO tech Technology Enterprise Co., Ltd., Taipei, Taiwan), 1 X PCR Buffer (PRO tech Technology Ent. Co.), 0.2 μM of each primer, 0.6 units of Prozyme (PRO tech Technology Ent. Co.) and 2 μL of each target DNA (150 – 200 ng). All PCR amplifications were performed in an Applied Biosystems 2720 thermal cycler. The thermal cycling conditions were as follows: initial cycle at 94 °C for 7 min followed by 35 cycles of 94 °C for 30 s, 54 °C to 58 °C for 30 s, 72 °C for 30 s and final cycle at 72 °C for 5 min. PCR products were analyzed by electrophoresis in 2% agarose gels, stained with ethidium bromide and visualized under ultraviolet light.
Table 2.
Primers used in this study.
| Species | Acc no | Cut C primers | Tm | Product size (bp) | Reference |
|---|---|---|---|---|---|
| Escherichia coli | EFJ62362 | F: AGCGAACTGGGAGCGAAATA R: TACGACCACGGTTGAGGACA |
56 |
421 | This study |
| Escherichia fergusonii | CAQ89502 | '' | '' | ||
| Proteus mirabilis | EEI47333 | F: CTGGCAGAACGTTTAGTTTCA R: TGGATTACCTTCCATTGCG |
58 | 492 | This study |
| Proteus penneri | EEG87333 | '' | '' | ||
| Klebsiella pneumoniae subsp. pneumoniae | ACI10981.1 | F: TCAAGTCGGTCAGCAAGATGAA R: CCGTACGGCTGATGATCTCGTC |
58 | 300 | This study |
| Klebsiella pneumoniae subsp. rhinoscleromatis | EEW38822 | '' | '' | ||
| Klebsiella variicola | ADC60394 | '' | '' | ||
| Klebsiella oxytoca | EHT04374 | ———–G———- ————-A——— |
'' | ||
| Providencia rettgeri | EFE54165 | F: CAGGGCTGATTTTCTCTGGT R: GAATTAAAGTTATGCACCA |
54 | 475 | This study |
| Providencia alcalifaciens | EEB46441 | '' | '' | ||
| Providencia rustigianii | EFB72255 | '' | '' | ||
| Clostridium sporogenes | EDU36695 | F: TCGTGAAGCAGGAGTATGGG R: GTCAACACGTCCTATAGACATACC |
58 | 460 | This study |
| Clostridium tetani | AAO36007 | '' | '' | ||
| Anaerococcus hydrogenalis | EEB36265 | F: GAGTAAGCGTAGAAGATGCTAGAG R: GAAAGTGTTCCATGGCAAAGTC |
56 | 673 | This study |
| Anaerococcus vaginalis | EEU12078 | '' | '' | ||
| Anaerococcus tetradius | EEI82584 | '' | '' | ||
| Clostridium hathewayi | EFC99034 | F: GATGTGGATCACCAGCGAGG R: GCATCATCTTGATGTGGGCG |
58 | 284 | This study |
| Yokenella regensburgi | EHM44496 | F: TGGGTGCGTGATGAACTTGA R: TCCATGCTCAGTGTGTCGAG |
58 | 317 | This study |
| Vibrio furnissii | ADT85554 | F: ACGGTAGAAAGCACAAGCGA R: TGAACGACCTTCCCAGAACG |
54 | 408 | This study |
| Olsenella uli | ADK67430 | F: AGCGAGATGATGTGGGTCAC R: AGCCCATCATGCAGTAGTCG |
56 | 343 | This study |
2.4. Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
All TMA producing bacteria were cultured under anaerobic conditions with 60 mM choline under their optimal conditions using anerobic jar (Mitsubishi™ AnaeroPack 7.0 L Rectangular Jar,) and MGC anaero pack (Mitsubishi Gas Chemical Company, Inc., Tokyo, Japan). RNA was extracted from 108 CFU/mL of 18 – 24 hrs culture (cultured until cells reached stationary phase) using PureLink™ RNA Mini Kit (Ambion life technologies, Invitrogen Life Technologies, Carlsbad, CA, USA). cDNA synthesis was carried out with reverse transcription kit (SuperScriptIII First-Strand Synthesis System, Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacture's protocol. The cDNA was stored at −20 °C until analysis. The quantitative real time polymerase chain reaction (q-PCR) was carried out in an ABI 7500 real-time detection system (Applied Biosystems, Foster, CA, USA) by using the KAPA SYBR FAST qPCR kit (KAPA Biosystems, Woburn, MA, USA) including SYBR Green Master mix and ROX reference dye, according to the manufacturer's instructions. The final 20 μL volume of the real- time PCR mixture consisted of 10 μL of 2 X KAPA SYBR FAST master mix, 2 μL of cDNA and 0.25 mM of each of the forward and reverse primers to amplify cutC, cutD and GAPDH genes. Using the following parameters: denaturation for 7 min at 94 °C, followed by 35 cycles of 94 °C for 30 s, 54 °C to 58 °C for 30 s, 72 °C for 30 s and final extension at 72 °C for 5 min. Primer sequences used in this study and the real-time PCR conditions are listed in Table 2. For each bacterium species, PCR products were confirmed by agarose gel electrophoresis to yield a unique and distinct band. Gene expression levels were compared with the GAPDH expression which, in general, was used as the internal control of viable cell counts (Livak and Schmittgen, 2001). The expression levels of cutC and cutD genes were shown by delta Ct values.
2.5. UPLC/MS/MS and quantitation of TMA production from choline
Fifty micro liters of overnight cultured bacterial cells (~108 CFU/mL) in stationary phase were inoculated into 1 mL of medium supplemented with 60 mM Choline-chloride (Craciun and Balskus, 2012), medium was filter sterilized before inoculation. All bacteria strains were incubated anaerobically for 72 hrs at 37 °C using anaerobic jar and anaeropack (MGC, Tokyo, Japan). The viable bacteria counts for all bacteria were 108 CFU/mL in stationary phase after 72 hrs. Supernatant was collected by centrifugation at 4 °C and filtered through a 13 – mm, 0.22 – μm pore-size filter (Millipore). Samples were kept in screw capped glass vials (Agilent Technologies, Palo Alto, CA, USA; part number 5182–0714) and stored at −80 °C until analysis using UPLC-MS/MS after derivatization using ethyl bromo‑acetate.
2.5.1. Sample preparation
Derivatization of TMA was carried out using ethyl bromo-acetate according to published methods with minor modifications (Lee et al., 2010). Briefly, to 25 μL of samples, 10 μL of internal standard (IS/D9 - TMA /1 ppm) was added. Afterwards, 30 μL of ethyl bromo-acetate (Sigma Aldrich Merck) (20 mg/mL in acetonitrile) and 1 μL of ammonia solution (26%) were added. This mixture was incubated at room temperature for 30 min in the dark. The reaction was stopped by adding 1 mL of acetonitrile (ACN) (50% ACN in 0.025% formic acid). The mixture was directly diluted with 100% ACN. The mixture was centrifuged for 3 min at 13,000 rpm then 10 μL of supernatant sample were injected and analyzed using Ultra performance liquid chromatography - tandem mass spectrometry (UPLC/MS/MS).
2.5.2. UPLC/MS/MS conditions and analysis
Quantification of TMA was conducted using a Waters ACQUITY UPLC system with Xevo TQ MS (mass spectrometer) (Johnson, 2008). Chromatographic separation was achieved on an ACQUITY UPLC BEH HILIC Column (17 um, 2.1 mm x 10 cm, Waters). The mobile phase consisted of (A) 95% ACN with 0.1% formic acid and 5% 10 mM ammonium formate buffer in water (B) 50 / 50 (v/v) ACN with 0.1% formic acid and 10 mM ammonium formate in H2O. The flow rate was maintained at 0.1 mL/min, column temperature of 30 °C. The gradient elution program was as follows: A (100%) – B (0%) (5 min); A (25%) – B (75%) (12.8 min); A (100%) – B (0%) (13 min) and A (100%) – B (0%) (14 min). MS experiments were conducted using a Waters Xevo TQ MS (Waters, Milford, MA, USA). Samples were ionized using electrospray ionization (ESI +) in positive ion mode and the precursor ion pairs operated in multiple reaction monitoring (MRM) mode were: m/z 146 → 118 for TMA and m/z 146.1 → 59 for d9-TMA with a capillary voltage of 3.0 kV, a source temperature of 150 °C, desolvation gas temperature of 500 °C and desolvation gas flow rate of 1000 L/hr. Argon was used as collision gas with flow set at 0.17 mL/min. The cone voltage was static at 5 V and the collision energy was 15 eV. Peak identification was performed using the MassLynx 4.1 SCN810 software package. The method validation including precision, accuracy, and detection limit was performed according to European union commission decision 2002/657/EC (European Commisiion, 2002). 5 ppm TMA was prepared to determine the precision and accuracy, after three repeated results on different days, the recovery rates are 91%, 82.7%, and 98.3%, respectively, with a variance of 8.61%. The results are in compliance with the 2002/657/EC specification. The limit of detection (LOD) corresponded to 1 ppm, the lowest TMA concentration of each standard sample. The experimental results show that the Signal/Noise is greater than 10. This method was used for routine inspection of quality control of the samples. We assayed new calibration curves for each experiment using a single measure at each calibrant concentration.
2.6. Bioinformatic analysis
2.6.1. Analysis of protein sequence and structure of cutC and cutD
The amino acid sequences were retrieved from National Center for Biotechnology Information (NCBI (https://www.ncbi.nlm.nih.gov/protein/)) and pairwise alignment has been conducted using Basic Local Alignment Search Tool (BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi)) (Altschul et al., 1990). Multiple sequence alignment was performed using Clustal omega (https://www.ebi.ac.uk/Tools/msa/clustalo/) (Madeira et al., 2019) and trimmed manually and visualized in Jalview (Waterhouse et al., 2009). Protein structure was edited and visualized in Swiss-PDB viewer (SPDBV) (Guex and Peitsch, 1997).
3. Results
3.1. Detection of choline TMA-lyase (CutC) and its activating enzyme (CutD)
Twenty strains of intestinal TMA bacteria collected from BCRC and DSMZ were listed in Table 1. cutC, cutD and GAPDH gene sequences for each bacteria species were retrieved from Genbank NCBI. Based on the conserved regions, primer sets targeted to the cutC and cutD gene for all these 20 bacteria species were designed. Sequences of primers and sizes of amplified products for each bacterium were listed in Table 2. All these primers were 100% complementary to their target gene sequences. Some primer sets were shared by two or more bacteria species belonging to the same genus or different genera. For example Escherichia coli and Escherichia fergusonii shared the same cutC and GAPDH primers; whereas Escherichia coli, Escherichia fergusonii, Klebsiella pneumoniae subsp. pneumoniae, Klebsiella pneumoniae subsp. rhinoscleromatis, Klebsiella variicola, Klebsiella oxytoca shared the same cutD gene primers (Table 2.2) due to sequence homology among some TMA bacteria species.
Table 2.2.
Primers used in this study.
| Organism | Acc no | CutD primers | Tm | Sizebp | Ref |
|---|---|---|---|---|---|
| Klebsiella pneumoniae subsp. rhinoscleromatis | NZ_GG703531.1 | EK-DF: AGTCCTGCATGATGATCTCCATC EK -DR........: AACTGCGGCGACTGCGTCA '' '' '' '' '' '' |
58 | 202 | This study |
| Klebsiella variicola | NZ_CP010523.1 | ||||
| Klebsiella oxytoca | NZ_KQ088036.1/ NZ_CP011597.1 | ||||
| Klebsiella pneumoniae subsp. pneumoniae | |||||
| Escherichia fergusonii | NC_011740.1 | ||||
| Escherichia coli | CP012631.1 | ||||
| Clostridium sporogenes | NZ_CP011663.1 | Cl-DF: CCACCTAATGTAACTCCACC Cl-DR........: GGAGATGGAATAAGAACTT |
359 | This study | |
| Clostridium tetani | NZ_JRGJ01000020.1 | 58 | |||
| Providencia rustigianii | NZ_GG703818.1 | PvD-F: ACCAAGCTGGTGATAAGGTA PvD-R: GACATTATGGGAAAAGATGTCAC '' '' |
54 | 489 | This study |
| Providencia alcalifaciens | NZ_ABXW01000042.1 | ||||
| Providencia rettgeri | |||||
| Proteus mirabilis | EEI47332.1 | Pr-DF: AGGCTGTAATATCCGCTGCC Pr-DR........: TACGGCGGACATTCTCATTAC '' |
54 | 554 | This study |
| Proteus penneri | EEG87332.1 | ||||
| Anaerococcus hydrogenalis | EEB36264.1 | Ah-DF: CAACGTCCATGATGGTCCAG Ah-DR........: AATTGGCATCCTTACCCTAACT |
56 | 457 637 |
This study This study |
| Anaerococcus tetradius | EEI82585.1 | '' |
|||
| Anaerococcus vaginalis | EEU12077.1 | Ah-DF: CAACGTCCATGATGGTCCAG Ah-DR........: AATTGGCATTCTTACCCTAACT |
56 | ||
| Hungatella hathewayi | ENY95336.1 | HD-F: TATGCTAAAGGGGATCAACG HD-R: GTGACAGGGAACTGATACTC |
56 | 237 | This study |
| Yokenella regensburgi | EHM44497.1 | Y-DF: AATGATGATCAACACCGCCG Y-DR........: TCGTTGAAGCCGCGAATAAG |
58 | 231 | This study |
| Vibrio furnissii | ADT85553.1 | V-DF: CCGAAATGAAGGGACGTATT V-DR........: CATGACTCACCATGACTTGA |
54 | 159 | This study |
| Olsenella uli | ADK67431.1 | Oul-DF: TTGAGAACGGATACAACGTC Oul-DR........: CATCCTTGATCTCGTAGTCG |
56 | 201 | This study |
Table 2.1.
Primers used in this study.
| Organism | Acc no | GAPDH Primers | Tm | Sizebp | Ref |
|---|---|---|---|---|---|
| Escherichia coli | CAR03139.2 | F: CCCGTCTCACAAAGACTGGC R: AGACGAACGGTCAGGTCAAC '' |
56 | 177 | This study |
| Escherichia fergusonii | M63367.1 | ||||
| Klebsiella pneumoniae subsp. pneumoniae | AAA25069.1 | F: GATGGCCCGTCTCACAAAGA R: ACGAACGGTCAGGTCAAC '' '' '' '' |
58 | ||
| Klebsiella pneumoniae subsp. rhinoscleromatis | CCI76472.1 | 180 | This study | ||
| Klebsiella oxytoca | AKL36813.1 | ||||
| Klebsiella variicola | AQL15641.1 | ||||
| Yokenella regensburgi | EHM47365.1 | ||||
| Proteus mirabilis | CAR43161.1 | F: TGACTGGTATGTCTTTCCGTG R: CAGAACGCCTTTCAGTTCGC '' '' '' '' |
58 | 140 | This study |
| Proteus penneri | |||||
| Providencia alcalifaciens | EEB45974.1 | ||||
| Providencia rustigianii | EFB73519.1 | ||||
| Providencia rettgeri | EFE55565.1 | ||||
| Clostridium tetani | AAO35015.1 | CLGF: CAGCACCAGCTAAAAATGAAGA CL-GR: CTGCTGCTCTTGCTCTTCTT '' |
58 | 246 | This study |
| Clostridium sporogenes | AKJ88346.1 | ||||
| Anaerococcus hydrogenalis | EEB36346.1 | F: AAGAGGTGGTAGAGCTGCAGCAC R: TTGTCATACCATGCAACAGT '' '' |
56 | ||
|
Anaerococcus vaginalis |
EEU12664.1 | 360 | This study | ||
| Anaerococcus tetradius | EEI83462.1 | ||||
| Clostridium hathewayi | CCZ63230.1 | F: CGGACCGCACAGAAAAGGCG R: GTGGAACCTGTCGGAACCG |
58 | 162 | This study |
| Vibrio furnissii | ABK58756.1 | F: TTGACGGTCCTTCTGCGAAA R: CAGTGTAGCCTAGTACGCCA |
54 | 271 | This study |
| Olsenella uli | ADK68376.1 | F: CTCCCTCACCAACCTCTACG R: CGAAGTACTTGATGGTGCGG |
56 | 295 | This study |
3.2. Expression level for genes of choline TMA-lyase and its activating enzyme
The expression level of cutC and cutD genes for each gut bacteria strain was analyzed by reverse transcription quantitative PCR (RT– qPCR). The cutC and cutD expression levels of each strain were compared with each other and with the GAPDH housekeeping gene of the same strain. The GAPDH gene has been commonly used as internal control i.e., to indicate the bacterial counts. Difference in gene expression in terms of delta Ct values are shown in Table 3. When Ct values of the cutC and cutD gene of each strain were compared with each other, five strains showed higher cutC gene expression and lower cutD expression. Six strains showed higher cutD gene expression but lower cutC gene expression (Table 3). Two stains showed nearly equivalent gene expression levels for both cutC and cutD genes, i.e., the strains of C. sporogenes and K. variicola (Table 3). Seven strains showed lower gene expression levels for both cutC and cutD genes. Additionally, it should be pointed out that except of strain C. sporogenes and K. variicola, the expression level of cutC and/or cutD for most TMA producing bacteria were not comparable with the gene expression level of their GAPDH gene which served as the internal control for viable cell count and for gene expression (Table 3).
Table 3.
Choline TMA-Lyase (Cut-C) and Choline TMA-Lyase activating enzyme (Cut-D) gene expression levels.
| Strains | cutD Cta value (Avg)b | GAPDH Cta value (Avg)b | cutC Cta value (Avg)b | cutD (Δ Ct)c | cutC (Δ Ct)c |
|---|---|---|---|---|---|
| Klebsiella pneumoniae subsp. pneumoniae | 22.46 | 25.12 | 21.4 | −2.66 | −3.72 |
| Proteus penneri | 22.99 | 23.35 | 33.27 | −0.36 | 9.92 |
| Anaerococcus vaginalis | 24.11 | 24.38 | 29.07 | −0.27 | 4.69 |
| Klebsiella variicola | 23.29 | 23.09 | 23.75 | 0.2 | 0.66 |
| Clostridium sporogenes | 27.45 | 27.21 | 27.03 | 0.24 | −0.18 |
| Yokenella regensburgi | 21.73 | 21.44 | 27.59 | 0.29 | 6.15 |
| Anaerococcus tetradius | 20.42 | 19.56 | 24.65 | 0.86 | 5.09 |
| Proteus mirabiliis | 23.3 | 21.57 | 30.2 | 1.73 | 8.63 |
| Providencia rettgeri | 22.38 | 20.6 | 16.75 | 1.78 | −3.85 |
| Klebsiella pneumoniae subsp. rhinoscleromatis | 25.25 | 23.39 | 21.39 | 1.86 | −2 |
| Klebsiella oxytoca | 23.39 | 21.2 | 20.97 | 2.19 | −0.23 |
| Anaerococcus hydrogenalis | 23.72 | 21.32 | 31.45 | 2.4 | 10.13 |
| Clostridium tetani | 28.99 | 26.25 | 28.47 | 2.74 | 2.22 |
| Providencia rustigianii | 24 | 19.9 | 18.83 | 4.1 | −1.07 |
| Escherichia fergusonii | 24.92 | 20.35 | 23.36 | 4.57 | 3.01 |
| Providencia alcalifaciens | 26.77 | 21.73 | 25.43 | 5.04 | 3.7 |
| Vibrio furnissii | 29.97 | 24.64 | 27.56 | 5.33 | 2.92 |
| Escherichia coli | 24.69 | 19.24 | 23.98 | 5.45 | 4.74 |
| Hungatella hathewayi | 28.3 | 21.3 | 26.23 | 7 | 4.93 |
| Olsenella uli | 29.15 | 21.23 | 23.08 | 7.92 | 1.85 |
ct cycle number to reach threshold.
Mean value for the gene expression of three runs.
Δ Ct = gene of interest (cutC/cutD) – housekeeping gene (GAPDH).
 .
.
3.3. TMA production
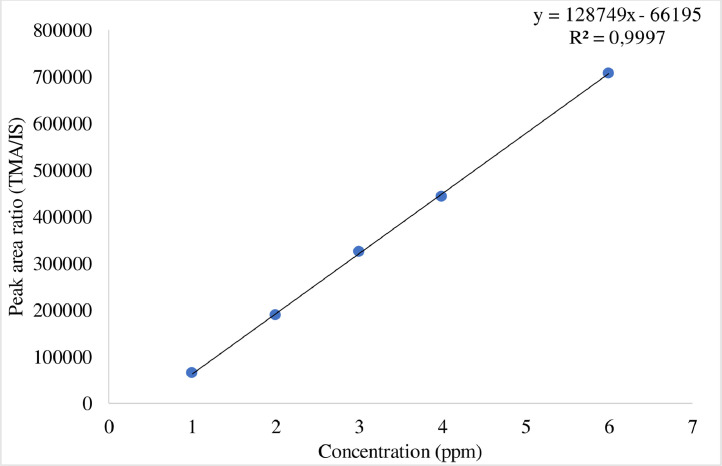
For TMA production, the cell-free supernatant was derivatized, diluted, and analyzed using UPLC-MS/MS according to the method described earlier. The calibration curve was plotted. For calibration standards, 25 μL of stock solution was prepared using 50% ACN containing 0.025% formic acid. The calibration standards were ranged from 1 to 6 ppm TMA. The calibration curve was constructed by plotting the peak area ratio of TMA to the internal standard concentration (TMA/IS) (Fig. 1). The linear matrix calibration curve had an R2 value of 0.99. All bacterial strains produce TMA except of Escherichia coli, Vibrio furnissii and Providencia rustigianii (Table 4).
Fig. 1.
Calibration curves for trimethylamine (TMA) by LC-MS/MS. IS: internal standard.
Table 4.
Trimethylamine (TMA) production.
| Strains | TMA ppma |
|---|---|
| Escherichia coli | < 1 |
| Escherichia fergusonii | 2718.30 |
| Klebsiella pneumoniae subsp. pneumoniae | 1439.10 |
| Klebsiella pneumoniae subsp. rhinoscleromatis | 369.26 |
| Klebsiella variicola | 1098.83 |
| Klebsiella oxytoca | 844.91 |
| Clostridium sporogenes | 8182.62 |
| Clostridium tetani | 1471.08 |
| Anaerococcus hydrogenalis | 4148.38 |
| Anaerococcus tetradius | 4765.02 |
| Anaerococcus vaginalis | 4125.42 |
| Hungatella hathewayi | 4157.40 |
| Yokenella regensburgi | 1567.02 |
| Vibrio furnissii | < 1 |
| Olsenella uli | 1982.76 |
| Providencia alcalifaciens | 5786.25 |
| Providencia rustigianii | < 1 |
| Providencia rettgeri | 6280.87 |
| Proteus mirabilis | 2398.50 |
| Proteus penneri | 844.91 |
PPM: parts per million.
The results revealed that strains with the higher gene expression levels of cutC did not necessarily mean that these strains produce higher quantity of TMA. For instance, K. pneumoniae subsp. rhinoscleromatis and K. oxytoca showed a higher cutC (Δ Ct: −2 and −0.2) gene expression level than its GAPDH expression level (Table 3), but their TMA levels were 369 and 844 ppm respectively (Table 4). For, C. sporogenes cutD and cutC gene expression levels were equivalent; i.e., Ct values were 27.45 and 27.03, respectively. However, this strain generated the highest TMA level from choline (8182.62 ppm, Table 4). E.coli, although its cell counts were higher based on the Ct value of GAPDH gene expression level (19.24), however, its cutD and cutC gene expression were lower when compared with its cell counts, and the TMA produced was less than 1 ppm only.
3.4. Bioinformatic analysis
3.4.1. Pairwise alignment of cutC and cutD
The cutC gene cluster was characterized in Desulfovibrio desulfuricans (Craciun and Balskus, 2012), we compared this sequence with our 20 TMA proucing strains. All cutC and cutD protein sequences were downloaded and pairwise alignment with Desulfovibrio desulfuricans and Klebsiella pneumoniae was conducted using BlastP. The resulting alignment showed protein sequences were highly similar. The cutC gene cluster was originally characterized from Desulfovibrio desulfuricans, we performed multiple sequence analysis (MSA) of 20 strains with Desulfovibrio desulfuricans. The cutC alignment with Desulfovibrio desulfuricans identities ranged from 61% ~ 81%. Since protein structure of Klebsiella pneumoniae cutC was available, we also performed MSA of 20 species with Klebsiella pneumoniae. The result showed that the identities ranged from 63% ~ 99% (Table S1). CutD pairwise alignment was also performed, the results were shown in Table S2. BLAST hits having an identity between 44% ~ 76% to cutD from Desulfovibrio desulfuricans and from Klebsiella pneumoniae ranged from 43% ~ 99% (Table S2).
3.4.2. Multiple sequence alignment of cutC and its active site
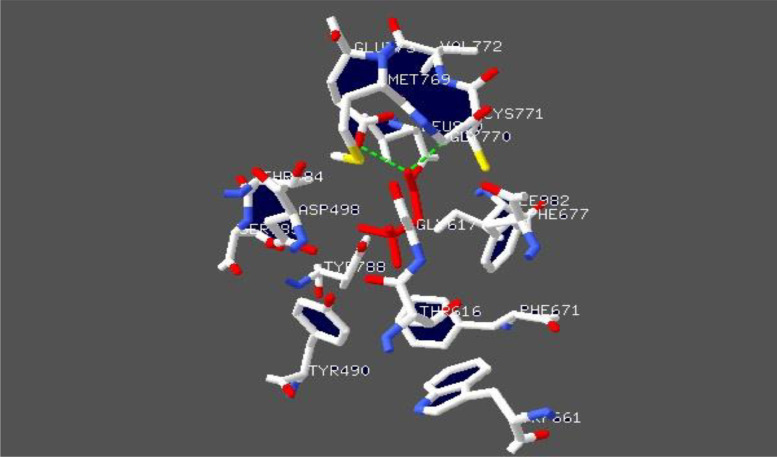
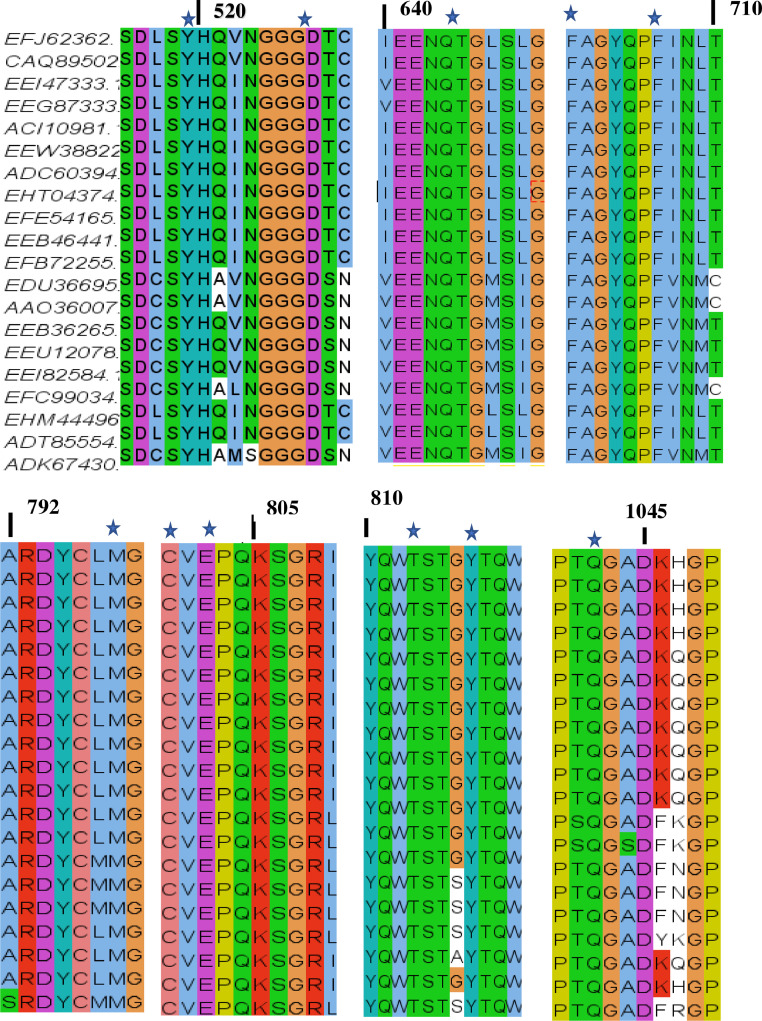
To study the conservation of the cutC protein, we performed BlastP search against Protein Data Bank (PDB) using default parameters. Protein template structure of Klebsiella pneumoniae (PDBID:5A0U) (Kalnins et al., 2015), was downloaded and visualized in SPDBV. Klebsiella pneumoniae 5A0U contains eight chains (ABCDEFGH). We used chain A to show the choline binding interactions. Active site residues were labelled Cys771, Gly770, Glu773, Leu 980, Thr784, Tyr788, Asp498, Tyr490, Phe677, Gly617, Thr 616, Val772, Met769, Ile982, Ser785, Phe 671, Trp661 and shown in Fig. 2. To confirm the presence of conserved active site residues sequences, we performed a MSA of the cutC gene using Clustal Omega. Results showed that all 20 species contain conserved active site residues (Fig. 3). We also observed that sequence was less similarly in N-terminal region than other region (Fig. S1).
Fig. 2.
The active site residues of choline trimethylamine-lyase (CutC) from Klebsiella pneumoniae (PDB 5A0U) (Kalnins et al., 2015). Choline is displayed in red color, Hydrogen bonds as green dashed lines. The image was visualized in SPDBV.
Fig. 3.
Multiple sequence alignment of choline TMA- lyase (CutC) proteins of Escherichia coli (EFJ62362); Escherichia fergusonii (CAQ89502); Proteus mirabilis (EEI47333); Proteus penneri (EEG87333); Klebsiella pneumoniae subsp. pneumoniae (ACI10981.1); Klebsiella pneumoniae subsp. rhinoscleromatis (EEW38822); Klebsiella variicola (ADC60394); Klebsiella oxytoca (EHT04374); Providencia rettgeri (EFE54165); Providencia alcalifaciens (EEB46441); Providencia rustigianii (EFB72255); Clostridium sporogenes (EDU36695); Clostridium tetani (AAO36007); Anaerococcus hydrogenalis (EEB36265); Anaerococcus vaginalis (EEU12078); Anaerococcus tetradius (EEI82584); Clostridium hathewayi (EFC99034); Yokenella regensburgi (EHM44496); Vibrio furnissii (ADT85554); Olsenella uli (ADK67430). Asterisks indicate conserved active site residues Providencia rustigianii (EFB 72255) numbering is shown. Sequences were visualized in Jalview.
3.4.3. Multiple sequence alignment of cutD
To understand the relationship between cutC and its activating protein cutD, we performed MSA of cutD of 20 TMA producing strains. Results were shown in Fig. S2. The alignment results showed that all strains possess conserved regions of glycyl radical enzyme (GRE) active motif, S-adenosylmethionine (SAM) binding motif and two CX2-CX2-CX3 motifs (Fig. S2). The C-terminal region has a low degree of similarity to the other regions (Fig. S2).
4. Discussion
Recent published papers showed that TMA but not TMAO is deleterious to circulatory system, it may be a toxin and a marker of cardiovascular risk (Ufnal, 2020; Jaworska et al., 2019). Previous study has indicated that gut microbial communities were differ from individual to individual (Human microbiome project consortium). The variations in the quantity of the TMA producing bacteria of human gut can affect the TMA and TMAO levels in them. (Falony et al., 2015; Romano et al., 2015)..Many gut bacterial phyla i.e. Firmicutes, Proteobacteria and Actinobacteria, possess a choline degradation pathway (cutC/cutD), except of Bacteroidetes, the most abundant genus in human stool (Craciun and Balskus, 2012; Falony et al., 2015). In this study, we made an effort to find the association of cutC/cutD gene expression levels with the TMA production levels in gut bacteria. 20 TMA producing strains were collected and performed in vitro studies, for both gene expression and TMA production levels with varying cell culture time. 18 – 24 hrs and 72 hrs cultures were used for gene expression and TMA production respectively, where they reached their stationary phases. NB and TSB media, having 60 mM choline were used for all strains to produce TMA (Romano et al., 2015). Our conditions were optimized according to the Craciun et al., 2012 and Romano et al., 2015; etc.
For the gene expression assay, degenerate cutC gene primers have been designed and used by Martinez-del Campo et al., 2015; However, these primers can not be useful for the quantification of the expression level of cutC and cutD genes, since primer–template mismatches would lead to inaccuracy in the measurement of the gene expression level or gene quantities (Ledeker and De Long, 2013). The CutC gene showed 61 – 83% of sequence homology across different species (Craciun and Balskus, 2012). Our bioinformatic analysis of CutC gene sequence also showed the homology of 61 – 81% with Desulfovibrio desulfuricans. As Klebsiella pneumoniae cutC protein structure is available (Kalnins et al., 2015), we performed MSA with TMA producing strains used in this study. Our in-silico study showed that 63 ~ 99% identity with Klebsiella pneumoniae. Based on the MSA, the primers for each bacterium were designed which would give accurate quantification of their gene expression level. Moreover, although the bacterial count of each strain has been adjusted to 108 CFU/mL, we used the GAPDH gene expression level as internal control to compare the gene expression level for cutC and cutD and to find the possible association of these gene expression levels with the TMA production level of each strain and also as reference gene to assure mRNA transcription (Kozera and Rapacz, 2013; Vogel and Marcotte, 2012). Even though cutD is the gene for activating the enzyme of cutC gene, it is interesting to note that high cutD gene expression does not necessarily mean to have higher cutC gene expression. For example, the Ct values for cutD gene were 23, and for cutC gene were 33 and 30 in two strains namely Proteus mirabilis and P. penneri (Table 3).
The whole cells of the TMA producing bacteria was used for TMA production study. Even though the strains belong to the same cluster their TMA production levels were different. For example, Proteus, Klebsiella and Providencia strains belong to the same type II cut cluster (Martinez-del Campo et al., 2015) but their TMA levels were different. It was reported that the expression of Cut gene cluster was controlled in organisms by two transcriptional regulatory proteins i.e. MerR and TetR (Martinez-del Campo et al., 2015). In addition, TMA production from choline by cutC gene was not only regulated by glycyl radical-activating protein (cutD), but also by various other factors such as the presence of S-adenosylmethionine (SAM) and transcriptional regulator (Craciun et al., 2014). In addition, studies identified a gut microbial pathway YeaW/X that produces TMA from various precursors i.e., γBB, L-carnitine, choline and betaine (Koeth et al., 2014; Zhu et al., 2014). Previous studies also showed that conserved active site residues and difference in the cutC gene (Martinez-del Campo et al., 2015; Bodea et al., 2016). In the current in silico study, cutD protein showed conserved domains and poor identical residues at c-terminal and cutC protein showed conserved active site residues and poor sequence similarity at N-terminal. In this study, we evaluated the gene expression levels for cutC and cutD and the quantity of TMA produced under the optimal growth conditions. To understand the quantity differences in gene expression and TMA production, isolation and quantitation of the Choline TMA-lyase from each of the strains and comparison of their activities may be needed.
To summarize, in this study, only one strain representing each species was used due to the difficulty in collecting them. It is not known that, if all strains of the same species would show the same gene expression level and TMA production level. Few limitations of our study include usage of single strain representing each species, utilization of in vitro conditions for gene expression and TMA production studies. The results may differ in the gut environment where thousands of bacteria interact with each other. However, the study and analysis reported here may be useful for further evaluation of the effect of different factors affecting the gene expressions of cutC/cutD and TMA production.
Funding information
This research work was supported by the Ministry of Science and Technology under the grant no. MOST-104–2320-B-241–002-MY2, MOST 103–2313-B241–001 and NSC 102–2632-B-241–001-MY3–3.
CRediT authorship contribution statement
Latha Ramireddy: Investigation, Methodology, Writing – original draft, Software. Hau-Yang Tsen: Supervision, Conceptualization, Funding acquisition, Writing – review & editing. Yu-Chen Chiang: Supervision. Chen Ying Hung: Conceptualization. Fu-Chih Chen: Investigation. Hsien- Tung Yen: Methodology, Validation.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
We would like to give our deep thanks to Ministry of Science and Technology, Taipei, Taiwan for supporting this work.
Biography
Hungatella hathewayi
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.crmicr.2021.100043.
Contributor Information
Latha Ramireddy, Email: latha.ramireddy@gmail.com.
Hau-Yang Tsen, Email: hytsen36@gmail.com.
Appendix. Supplementary materials
References
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bae S., Ulrich C.M., Neuhouser M.L., Malysheva O., Bailey L.B., Xiao L., Brown E.C., Cushing-Haugen K.L., Zheng Y., Cheng T.D., Miller J.W., Green R., Lane D.S., Beresford S.A.A., Caudill M.A. Plasma choline metabolites and colorectal cancer risk in the Women’s Health Initiative Observational Study. Cancer Res. 2014;74(24):7442–7452. doi: 10.1158/0008-5472.CAN-14-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J.R., Chaykin S. The biosynthesis of trimethylamine-N-oxide. J. Biol. Chem. 1962;237:1309–1313. doi: 10.1016/S0021-9258(18)60325-4. [DOI] [PubMed] [Google Scholar]
- Bodea S., Funk M.A., Balskus E.P.;., Drennan C.L. Molecular basis of C-N bond cleavage by the glycyl radical enzyme choline trimethylamine-lyase. Cell Chem. Biol. 2016;23(10):1206–1216. doi: 10.1016/j.chembiol.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulou J. Trimethylaminuria: an under-recognised and socially debilitating metabolic disorder. J. Paediatr. Child Health. 2012;48(3):E153–E155. doi: 10.1111/j.1440-1754.2010.01978.x. [DOI] [PubMed] [Google Scholar]
- Craciun S., Balskus E.P. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc. Natl. Acad. Sci. U S A. 2012;109(52):21307–21312. doi: 10.1073/pnas.1215689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craciun S., Marks J.A., Balskus E.P. Characterization of choline trimethylamine-lyase expands the chemistry of glycyl radical enzymes. ACS Chem. Biol. 2014;9(7):1408–1413. doi: 10.1021/cb500113p. [DOI] [PubMed] [Google Scholar]
- Dumas M.E., Barton R.H., Toye A., Cloarec O., Blancher C., Rothwell A., Fearnside J., Tatoud R., Blanc V., Lindon J.C., Mitchell S.C., Holmes E., McCarthy M.I., Scott J., Gauguier D., Nicholson J.K. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc. Natl. Acad. Sci. U S A. 2006;103(33):12511–12516. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commisiion Commission Decision of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results 2002/657/EC. Off. J. Eur. Commun. 2002;L221:8–36. [Google Scholar]
- Falony G., Vieira-Silva S., Raes J. Microbiology meets big data: the case of gut microbiota-derived trimethylamine. Annu. Rev. Microbiol. 2015;69:305–321. doi: 10.1146/annurev-micro-091014-104422. [DOI] [PubMed] [Google Scholar]
- Guex N., Peitsch M.C. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18(15):2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- Hayward H.R., Stadtman T.C. Anaerobic degradation of choline. I. Fermentation of choline by an anaerobic, cytochrome-producing bacterium, Vibrio cholinicus n. sp. J. Bacteriol. 1959;78:557–561. doi: 10.1128/JB.78.4.557-561.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring T.I., Harris T.N., Chowdhury C., Mohanty S.K., Bobik T.A. A bacterial microcompartment is used for choline fermentation by Escherichia coli 536. J. Bacteriol. 2018;200(10) doi: 10.1128/JB.00764-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson E., Fu T., Brown I.R., Paszkiewicz K., Purdy K.J., Frank S., Chen Y. Anaerobic choline metabolism in microcompartments promotes growth and swarming of Proteus mirabilis. Environ. Microbiol. 2016;18(9):2886–2898. doi: 10.1111/1462-2920.13059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworska K., Hering D., Mosieniak G., Bielak-Zmijewska A., Pilz M., Konwerski M., Gasecka A., Kapłon-Cieślicka A., Filipiak K., Sikora E., Hołyst R., Ufnal M. TMA, A Forgotten Uremic Toxin, but Not TMAO, Is Involved in Cardiovascular Pathology. Toxins (Basel) 2019;11(9):490. doi: 10.3390/toxins11090490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D.W. A flow injection electrospray ionization tandem mass spectrometric method for the simultaneous measurement of trimethylamine and trimethylamine N-oxide in urine. J Mass Spectrom. 2008;43(4):495–499. doi: 10.1002/jms.1339. Apr. [DOI] [PubMed] [Google Scholar]
- Kalnins G., Kuka J., Grinberga S., Makrecka-Kuka M., Liepinsh E., Dambrova M., Tars K. Structure and Function of CutC Choline Lyase from Human Microbiota Bacterium Klebsiella pneumoniae. J. Biol. Chem. 2015;290(35):21732–21740. doi: 10.1074/jbc.M115.670471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeth R.A., Levison B.S., Culley M.K., Buffa J.A., Wang Z., Gregory J.C., Org E., Wu Y., Li L., Smith J.D., Tang W.H.W., DiDonato J.A., Lusis A.J., Hazen S.L. gamma-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 2014;20(5):799–812. doi: 10.1016/j.cmet.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeth R.A., Wang Z., Levison B.S., Buffa J.A., Org E., Sheehy B.T., Britt E.B., Fu X., Wu Y., Li L., Smith J.D., DiDonato J.A., Chen J., Li H., Wu G.D., Lewis J.D., Warrier M., Brown J.M., Krauss R.M., Tang W.H., Bushman F.D., Lusis A.J., Hazen S.L. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013;19(5):576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozera B., Rapacz M. Reference genes in real-time PCR. J. Appl. Genet. 2013;54(4):391–406. doi: 10.1007/s13353-013-0173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger S.K., Williams D.E. Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism. Pharmacol. Ther. 2005;106(3):357–387. doi: 10.1016/j.pharmthera.2005.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledeker B.M., De Long S.K. The effect of multiple primer-template mismatches on quantitative PCR accuracy and development of a multi-primer set assay for accurate quantification of pcrA gene sequence variants. J. Microbiol. Methods. 2013;94(3):224–231. doi: 10.1016/j.mimet.2013.06.013. [DOI] [PubMed] [Google Scholar]
- Lee S.-.K., Kim D.-.H., Jin C.-.B., Yoo H.-.H. Determination of Urinary Trimethylamine and Trimethylamine N-oxide by Liquid Chromatography-Tandem Mass Spectrometry Using Mixed-Mode Stationary Phases. B. Korean Chem. Soc. 2010;31(2):483–486. doi: 10.5012/bkcs.2010.31.02.483. [DOI] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Madeira F., Park Y.M., Lee J., Buso N., Gur T., Madhusoodanan N., Basutkar P., Tivey A.R.N., Potter S.C., Finn R.D., Lopez R. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019;47(W1):W636–W641. doi: 10.1093/nar/gkz268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-del Campo A., Bodea S., Hamer H.A., Marks J.A., Haiser H.J., Turnbaugh P.J., Balskus E.P. Characterization and detection of a widely distributed gene cluster that predicts anaerobic choline utilization by human gut bacteria. MBio. 2015;6(2) doi: 10.1128/mBio.00042-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn A.R., Larrick J.W. Dietary modification of the microbiome affects risk for cardiovascular disease. Rejuvenation Res. 2013;16(3):241–244. doi: 10.1089/rej.2013.1447. [DOI] [PubMed] [Google Scholar]
- Miller C.A., Corbin K.D., da Costa K.A., Zhang S., Zhao X., Galanko J.A., Blevins T., Bennett B.J., O'Connor A., Zeisel S.H. Effect of egg ingestion on trimethylamine-N-oxide production in humans: a randomized, controlled, dose-response study. Am. J. Clin. Nutr. 2014;100(3):778–786. doi: 10.3945/ajcn.114.087692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano K.A., Vivas E.I., Amador-Noguez D., Rey F.E. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. MBio. 2015;6(2):e02481. doi: 10.1128/mBio.02481-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih D.M., Wang Z., Lee R., Meng Y., Che N., Charugundla S., Qi H., Wu J., Pan C., Brown J.M., Vallim T., Bennett B.J., Graham M., Hazen S.L., Lusis A.J. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J. Lipid Res. 2015;56(1):22–37. doi: 10.1194/jlr.M051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W.H., Hazen S.L. The contributory role of gut microbiota in cardiovascular disease. J. Clin. Invest. 2014;124(10):4204–4211. doi: 10.1172/JCI72331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W.H., Wang Z., Kennedy D.J., Wu Y., Buffa J.A., Agatisa-Boyle B., Li X.S., Levison B.S., Hazen S.L. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 2015;116(3):448–455. doi: 10.1161/CIRCRESAHA.116.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W.H., Wang Z., Levison B.S., Koeth R.A., Britt E.B., Fu X., Wu Y., Hazen S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013;368(17):1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeaux C.J., van der Donk W.A. Converging on a mechanism for choline degradation. Proc. Natl. Acad. Sci. U S A. 2012;109(52):21184–21185. doi: 10.1073/pnas.1219534110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremaroli V., Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- Ufnal M. Trimethylamine, a toxic precursor of trimethylamine oxide, lost in medical databases. J. Nutr. 2020;150(2):419. doi: 10.1093/jn/nxz265. [DOI] [PubMed] [Google Scholar]
- Vogel C., Marcotte E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012;13(4):227–232. doi: 10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Klipfell E., Bennett B.J., Koeth R., Levison B.S., Dugar B., Feldstein A.E., Britt E.B., Fu X., Chung Y.M., Wu Y., Schauer P., Smith J.D., Allayee H., Tang W.H., DiDonato J.A., Lusis A.J., Hazen S.L. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse A.M., Procter J.B., Martin D.M., Clamp M., Barton G.J. Jalview Version 2–a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25(9):1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel S.H., da Costa K.A. Choline: an essential nutrient for public health. Nutr. Rev. 2009;67(11):615–623. doi: 10.1111/j.1753-4887.2009.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel S.H., daCosta K.A., Youssef M., Hensey S. Conversion of dietary choline to trimethylamine and dimethylamine in rats: dose-response relationship. J. Nutr. 1989;119(5):800–804. doi: 10.1093/jn/119.5.800. [DOI] [PubMed] [Google Scholar]
- Zhang A.Q., Mitchell S.C., Smith R.L. Dietary precursors of trimethylamine in man: a pilot study. Food Chem. Toxicol. 1999;37(5):515–520. doi: 10.1016/s0278-6915(99)00028-9. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Jameson E., Crosatti M., Schäfer H., Rajakumar K., Bugg T.D., Chen Y. Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proc Natl Acad Sci U S A. 2014;111(11):4268–4273. doi: 10.1073/pnas.1316569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.