Abstract
Regulated cyclin-dependent kinase (CDK) levels and activities are critical for the proper progression of the cell division cycle. p12DOC-1 is a growth suppressor isolated from normal keratinocytes. We report that p12DOC-1 associates with CDK2. More specifically, p12DOC-1 associates with the monomeric nonphosphorylated form of CDK2 (p33CDK2). Ectopic expression of p12DOC-1 resulted in decreased cellular CDK2 and reduced CDK2-associated kinase activities and was accompanied by a shift in the cell cycle positions of p12DOC-1 transfectants (↑ G1 and ↓ S). The p12DOC-1-mediated decrease of CDK2 was prevented if the p12DOC-1 transfectants were grown in the presence of the proteosome inhibitor clasto-lactacystin β-lactone, suggesting that p12DOC-1 may target CDK2 for proteolysis. A CDK2 binding mutant was created and was found to revert p12DOC-1-mediated, CDK2-associated cell cycle phenotypes. These data support p12DOC-1 as a specific CDK2-associated protein that negatively regulates CDK2 activities by sequestering the monomeric pool of CDK2 and/or targets CDK2 for proteolysis, reducing the active pool of CDK2.
Cell cycle inhibitors of the p16INK4a and p21WAF1/CIP1/CAP20 families exert their effects by negatively regulating cyclin and cyclin-dependent kinase (CDK) complex formation and kinase activities (10, 14). While the p16INK4a family is specific for CDK4 and CDK6, and the p21WAF1/CIP1/CAP20 family of CDK inhibitors is universal for CDKs, there is no known specific inhibitor for CDK2. CDK2, when complexed with cyclins E and A, is implicated in G1/S transition, DNA replication, and progression through the DNA synthesis phase (6, 7, 9).
p12DOC-1 is a growth suppressor identified and isolated from normal keratinocytes (12). It is a highly conserved cellular gene. Our laboratory (12, 13) and others (4, 5) have cloned p12DOC-1 cDNA from human, mouse, and hamster. The full-length human and mouse p12DOC-1 cDNAs are 1.6 kb and 1.2 kb, respectively. Human p12DOC-1 has one additional amino acid at residue 19, which corresponds to an alanine, and differs from the mouse and hamster p12DOC-1 at only two other amino acid residues (Ala → Thr at residue 8 and Gly → Ser at residue 100). Human and rodent p12DOC-1 polypeptides have 97% identity, and the mouse and hamster p12DOC-1 protein sequences are identical. Human p12DOC-1 is a 115-amino-acid peptide with a molecular mass of 12.4 kDa (pI, 9.62).
Ectopic expression of p12DOC-1 in keratinocytes is associated with increased doubling time, suggestive of a growth suppressor function (11). These observations prompted us to examine if p12DOC-1 interacts with regulatory proteins in the cell division cycle. We report that p12DOC-1 associates with CDK2. Data are presented to support the role of p12DOC-1 as a specifically CDK2-associated protein, which, when overexpressed, negatively regulates CDK2-associated kinase activities and cell cycle phenotypes.
MATERIALS AND METHODS
Cell culture and transfections.
Transfection of human 293 cells was performed using Lipofectamine Plus (Life Technologies, Inc., Rockville, Md.) according to the manufacturer's protocol. Cells were harvested 12 to 48 h posttransfection for respective experiments. Transfections were done in 60- or 100-mm dishes in triplicate. Each experiment was repeated at least three times.
Site-directed mutagenesis.
Mutations of the amino acid sequence from positions 103 to 111 of p12DOC-1 were introduced using the QuickChange site-directed mutagenesis system (Stratagene, La Jolla, Calif.). Sequences were confirmed by automated DNA sequencing.
In vitro, cellular, and endogenous p12DOC-1 and CDK2 association assays.
For p12DOC-1 in vitro binding experiments, glutathione S-transferase (GST)–p12DOC-1 fusion protein was prepared from Escherichia coli. Cellular lysates from HeLa, 293, and A431 cells were prepared in ice-cold 0.5% NP-40 lysis buffer. GST or GST-p12DOC-1 (1 μg) was incubated with cell lysates (200 μg) for 2 h at 4°C in 500 μl of lysis buffer. Beads were washed four times with lysis buffer, loaded onto a sodium dodecyl sulfate (SDS)–10 or 12% polyacrylamide gel and blotted onto polyvinylidene difluoride (PVDF) membranes. The anti-CDK2 antibody (C18520 Clone 55; Transduction Laboratories, San Diego, Calif.) was used for immunodetection.
For p12DOC-1 and CDK2 cellular binding assays, 293 cells were transfected with pFLAG-DOC-1-wt (or pFLAG-DOC-1-A3) (cloned into pFLAG-CMV-2; Eastman Kodak Co., New Haven, Conn.) and wild-type CDK2 using Lipofectamine Plus (Life Technologies). Forty-eight hours posttransfection, cells were lysed in 0.5% NP-40 lysis buffer. The cell lysate (300 μg) was incubated with anti-FLAG (1 μg) (M5; Sigma Chemicals, St. Louis, Mo.) and anti-CDK2 antibodies (1 μg) (C18520 Clone 55; Transduction Laboratories, Lexington, Ky.) for 1 h at 4°C and then incubated with protein A/G agarose beads (Santa Cruz Biotechnology, Santa Cruz, Calif.) for 2 h at 4°C. Immunoprecipitated complexes were loaded onto 12 or 15% polyacrylamide gel and blotted onto polyvinylidene difluoride membranes. Anti-p12DOC-1 (Ab3) and anti-CDK2 antibodies (C18520 Clone 55; Transduction Laboratories) were used for immunodetection.
For protein preparations from human lung tissue, snap-frozen tissues were rapidly homogenized in 5 volumes of 1% NP-40 lysis buffer (1% NP-40, 150 mM NaCl, 50 mM Tris [pH 7.4], 1 mM sodium vanadate, 25 μg of aprotinin/ml, and 25 μg of leupeptin/ml). Tissues were obtained from the Massachusetts General Hospital Tumor Bank.
Gel filtration chromatography.
Normal human lung homogenates or HaCaT cell lysate (0.5 ml; 2.5 mg of total protein) was applied to a Protein PAK300sw gel filtration high-pressure liquid chromatography column (Waters). Elution was achieved with an isocratic flow of 50 mM Tris-HCl, pH 7.4, containing 150 mM NaCl, 50 mM NaF, and 2 mM EDTA at a flow rate of 0.45 ml/min. Fractions of 0.45 ml were collected and subjected to Western blotting analyses for p12DOC-1 and CDK2. The column was calibrated with protein standards of known molecular weights (catalase, aldolase, bovine serum albumin, ovalbumin, and chymotrypsin).
CDK2 kinase assays.
For kinase assays, cell lysates were prepared from 293 cells transfected for 48 h with pFLAG, pFLAG-DOC-1-wt, or pFLAG-DOC-1-A3. Immunoprecipitations were performed with antibodies specific to CDK2 (M2; Santa Cruz Biotechnology), cyclin A (BF683; Santa Cruz Biotechnology) and cyclin E (M20; Santa Cruz Biotechnology) and protein A/G agarose beads. The immune complexes were washed four times with kinase buffer (50 mM Tris [pH 7.4], 0.1 mM EDTA, 1 mM dithiothreitol) and resuspended in a final volume of 10 μl of kinase buffer. The kinase reactions included 500 ng of histone H1 or pRBc (the carboxyl-terminal fragment of pRB), 5 μM ATP, 10 mM MgCl2, and 10 μCi of [γ-32P]ATP and were incubated for 15 min at 37°C. At the end of the reaction, 10 μl of 2× SDS sample buffer was added, and proteins were loaded onto SDS–10% polyacrylamide gels. The gels were stained with Coomassie blue prior to autoradiography.
RESULTS
p12DOC-1 associates with CDK2.
We examined if the p12DOC-1-mediated growth suppression is due to its association with cyclins and CDKs. In vitro binding experiments were performed using a GST-p12DOC-1 fusion protein and human cellular lysates, followed by SDS-polyacrylamide gel electrophoresis (PAGE) and immunoblotting. The bound complexes were examined for the presence of cyclins and CDKs. Of the candidates surveyed (CDC2, CDK2, CDK4, CDK6, cyclin A, cyclin B, cyclin D1, cyclin D3, and cyclin E), GST-p12DOC-1 was found to associate with CDK2 (Fig. 1).
FIG. 1.
GST-p12DOC-1 associates with CDK2 in cell lysates. (A) CDK2 immunoblot showing cellular CDK2 from HeLa, 293, and A431 cells associating with the GST-p12DOC-1 fusion protein. Lanes 1, 4, and 7, GST control; lanes 2, 5, and 8, GST-p12DOC-1; lanes 3, 6, and 9, input lysate at 0.1×. (B) CDK2 immunoblot showing that GST-p12DOC-1 associates with the 34-kDa forms of CDK2. Lane 1, GST control; lanes 2 and 3, GST-p12DOC-1 (1 and 2 μg, respectively); lane 4, immunoprecipitation of CDK2. (C) Phosphotyrosine immunoblot showing that the p34CDK2 that associates with GST-p12DOC-1 does not contain phosphotyrosine residues (4G10; Upstate Biotechnology). The samples for panels B and C were run on long SDS-PAGE gels to resolve the 33- and 34-kDa CDK2 bands.
Figure 1A shows that the GST-p12DOC-1 fusion protein can associate with CDK2 in different human cell lysates. Similar experiments with CDC2, CDK4, and CDK6 showed that although the cellular levels of these proteins are similar, there is no association, indicating that the association of p12DOC-1 to CDK2 is specific (data not shown). Neither cyclin E nor cyclin A was detected in the bound GST-p12DOC-1–CDK2 complexes, suggesting that p12DOC-1 either associates with the monomeric form of CDK2 or has displaced the cognate cyclin (data not shown). CDK2 electrophoretically migrates as a doublet (HeLa and 293) or triplet (A431) on SDS-PAGE gels (Fig. 1B). Gu et al. have demonstrated that phosphorylation affects the electrophoretic mobility of CDK2 (6). The 34-kDa CDK2 band (p34CDK2) represents the monomeric nonphosphorylated and Tyr15-phosphorylated (by WEE1) forms of CDK2. The 33-kDa band (p33CDK2) represents forms of CDK2 that have been phosphorylated by CAK at amino acid residue Thr160. Figure 1B shows that p12DOC-1 associates predominantly with p34CDK2. To ascertain whether p12DOC-1 associates with the WEE1-Tyr15-phosphorylated or the monomeric nonphosphorylated form of p34CDK2, GST-p12DOC-1–CDK2-bound complexes were immunoblotted for the presence of phosphotyrosine. While phosphorylated tyrosine was detected in both the immunoprecipitated 33- and 34-kDa CDK2 bands (Fig. 1C, lane 4), the p34CDK2 associated with GST-p12DOC-1 did not contain detectable phosphorylated tyrosine (Fig. 1C, lanes 2 and 3), suggesting that p12DOC-1 associates with the monomeric nonphosphorylated form of CDK2.
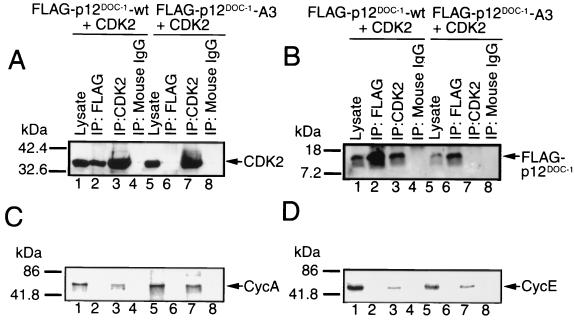
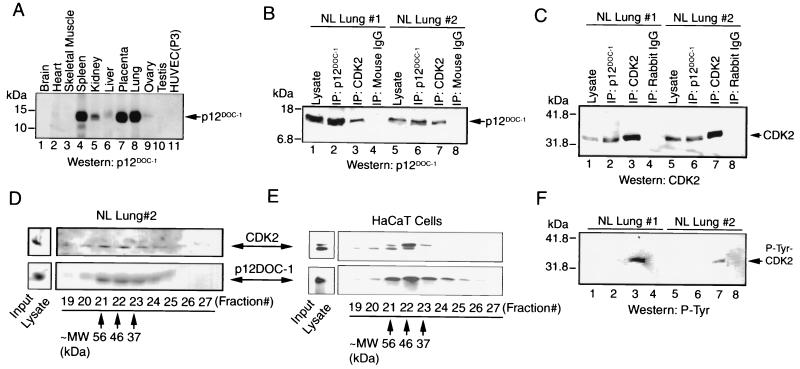
To examine if p12DOC-1 and CDK2 can interact in cells, a FLAG-tagged DOC-1 vector (pFLAG-DOC-1-wt) was cotransfected with pCDK2 into 293 cells. p12DOC-1 and CDK2 coprecipitated from the 293 cells (Fig. 2A and B, lanes 1 to 4). The immunoprecipitated p12DOC-1–CDK2 complexes were also examined for the presence of cyclins A and E, but they were not detected (Fig. 2C and D). To determine if a similar interaction can be detected for endogenously expressed p12DOC-1 and CDK2, a number of human tissues and cell types were examined for endogenous p12DOC-1 expression. p12DOC-1 was not detectable in tumor or immortalized cell lines (35 were examined). Immunoblotting lysates from a panel of normal human tissues revealed that p12DOC-1 is detectable in tissue from the spleen, kidney, placenta, and lung (Fig. 3A). Since lung tissue expresses the highest endogenous level of p12DOC-1, lysate preparations from two normal lungs were used to examine endogenous interactions of these proteins. Figure 3B and C showed that endogenous p12DOC-1 does interact with CDK2 in the lysate preparations from both lung tissue samples. Immunohistochemical staining of normal lung sections revealed that the majority of lung parenchymal cells coexpress p12DOC-1 and CDK2 (data not shown). To examine whether the CDK2 that coprecipitated with p12DOC-1 from the normal lung tissue is p34CDK2 and/or p33CDK2, the immunoblot shown in Fig. 3C was reprobed by the antiphosphotyrosine antibody 4G10. Figure 3F reveals that while immunoprecipitation of total CDK2 from the lung lysates contained tyrosine-phosphorylated forms (lanes 3 and 7), the CDK2 associated with p12DOC-1 was apparently not tyrosine phosphorylated (lanes 2 and 6), suggesting that p12DOC-1 predominantly associates with p33CDK2, the monomeric nonphosphorylated form. While the lack of detectable phosphotyrosine in the CDK2 associated with p12DOC-1 may reflect a smaller amount of CDK2 coimmunoprecipitated with p12DOC-1 (lanes 2 and 6), the data do support that endogenous p12DOC1 associates predominantly with the non-tyrosine-phosphorylated form of CDK2.
FIG. 2.
Association of p12DOC-1 and CDK2 in cells. (A and B) CDK2 and p12DOC-1 immunoblots showing the coprecipitation of p12DOC-1 with CDK2 in 293 cells cotransfected with pCDK2 and pFLAG-DOC-1-wt or pFLAG-DOC-1-A3. Lanes 1 and 5, lysate (30 μg); lanes 2 and 6, immunoprecipitation using anti-FLAG monoclonal antibody (M5; Sigma Chemicals); lanes 3 and 7, CDK2 immunoprecipitation using anti-CDK2 monoclonal antibody (C18520 Clone 55; Transduction Laboratories); lanes 4 and 8, negative control using nonimmune mouse immunoglobulin G for immunoprecipitation. (C and D) Reprobing of the same membranes shown in panels A and B for cyclins A and E, respectively.
FIG. 3.
Interaction of p12DOC-1 and CDK2 in vivo. (A) Immunoblot to detect p12DOC-1 in normal human tissue lysates. Thirty micrograms of tissue lysates was loaded onto each lane. HUVEC (P3) cells are third passage normal human umbilical endothelial cells. (B and C) Coprecipitation of p12DOC-1 with CDK2 in human lung lysates from two donors. Panel B shows a p12DOC-1 immunoblot using p12DOC-1 Ab3; panel C shows a CDK2 immunoblot using anti-CDK2 antibody (C18520 Clone 55; Transduction Laboratories). Lanes 1 and 5, input lysate (25 μg); lanes 2 and 6, p12DOC-1 immunoprecipitation; lanes 3 and 7, CDK2 immunoprecipitation; lanes 4 and 8, negative control using nonimmune mouse and rabbit immunoglobulin G for panels B and C, respectively. (D and E) Gel filtration chromatograph elution profiles of normal human lung lysate (#2) and HaCaT cells. Top panels show immunoblots for CDK2; bottom panels show immunoblots for p12DOC-1. Thirty micrograms of total proteins was used for the respective input lysate lanes. Approximate molecular sizes (MW) of fractions 21, 22, and 23 are calibrated against known molecular size standards. (F) Phosphotyrosine immunoblot of the same membrane used for panel C to show that the CDK2 coprecipitated with the endogenous p12DOC-1 detected in normal lung lysates was not tyrosine phosphorylated, suggesting that it is the monomeric nonphosphorylated p33CDK2.
To provide additional evidence that endogenous p12DOC-1 associates with CDK2, normal lung lysates were subjected to gel filtration chromatography to determine if these proteins comigrate. Normal human lung homogenates were applied to a protein PAK300sw gel filtration high-pressure liquid chromatography column. Eluted fractions were immunoblotted for p12DOC-1 and CDK2. Figure 3D shows the elution profiles of p12DOC-1 and CDK2 from normal lung 2 (with the same preparation used for panels B and C), showing that the two proteins coelute from fractions 21, 22, and 23, corresponding to the apparent molecular masses of 56, 46, and 37 kDa, respectively, as calibrated with protein standards of known molecular weights (catalase, aldolase, bovine serum albumin, ovalbumin, and chymotrypsin). We have recently found that human keratinocyte HaCaT cells express detectable levels of endogenous p12DOC-1 and CDK2. Gel filtration chromatography was similarly performed using these cell lysates. Figure 3E shows the coelution of p12DOC-1 and CDK2 in fractions 21, 22, and 23. Note that p34CDK2 is the predominant form of CDK2 comigrating with p12DOC-1 in fractions 21 to 23. Some p33CDK2 apparently comigrates with p12DOC-1 as well, as a minor component. Thus, for both normal lung cells and the HaCaT cells, p12DOC-1 and CDK2 coelute in gel filtration. The elution profiles are very similar. The elution profile from the HaCaT cells further supports the comigration of p12DOC-1 with p34CDK2. In addition, in both lung cells and HaCaT cells, CDK2 was also detected in fractions 12 to 14 (data not shown), which may represent a high-molecular-mass (∼200-kDa) CDK2 complex with other CDK2 binding proteins. Cyclins A and E coelute with CDK2 in fractions 12 to 14.
Amino acids 109 to 111 of p12DOC-1 are critical for CDK2 association.
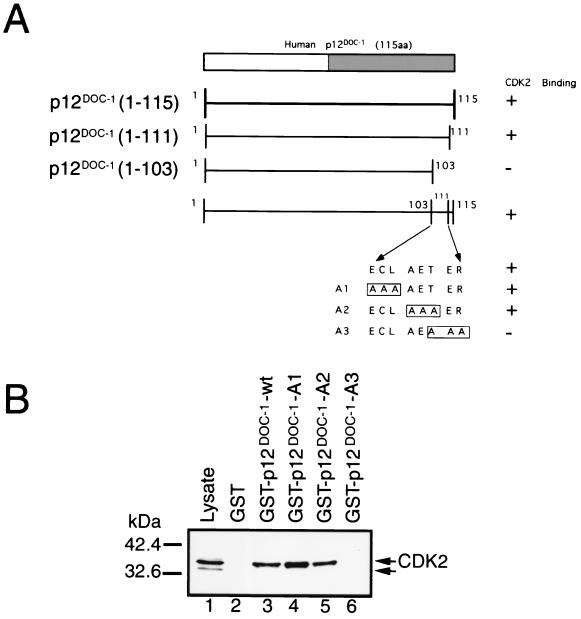
To verify the specificity of p12DOC-1's association with monomeric CDK2, we identified a domain in p12DOC-1 that is critical for its association with CDK2. Deleting the 12 amino acids [p12DOC-1(1–103)] from the C-terminal region negates CDK2 association, and deleting the last 4 amino acid residues [p12DOC-1(1–111)] retains CDK2 association (Fig. 4A). This thus identified the region from amino acid 103 to 111 as containing amino acids that are critical for association with CDK2. The eight amino acids in this region were mutagenized in sets of three (A1, A2, and A3). These mutants were expressed as GST fusion proteins and were used to determine their ability to associate with CDK2 in 293 cell lysate. Both A1 and A2, but not A3, mutants retained CDK2 association (Fig. 4B). The inability of the A3 mutant to interact with CDK2 was verified in cells by cotransfecting 293 cells with pCDK2 and either the FLAG-tagged-DOC-1 wild type (pFLAG-DOC-1-wt) or the A3 mutant (pFLAG-DOC-1-A3). Figure 2A and B show that the p12DOC-1-A3 mutant is expressed but did not interact with CDK2 (lanes 5 to 8).
FIG. 4.
Amino acids 109 to 111 are necessary for p12DOC-1's association with CDK2. (A) Schematic of the mutagenesis strategy. Mutants were created by the Stratagene QuickChange site-directed mutagenesis system. The gray box in the C terminus (amino acids 62 to 115) is a domain that is homologous to a domain in a C. elegans protein, Y43F4B.7, and a related protein, DOC-1R (15). (B) In vitro association of the A1, A2, and A3 mutants with monomeric CDK2 in 293 cell lysate. One microgram of GST or GST-p12DOC-1 wild-type or mutant protein was mixed with 200 μg of 293 cell lysate. The CDK2 immunoblot was done using anti-CDK2 antibody (C18520 Clone 55; Transduction Laboratories).
Ectopic expression of p12DOC-1 suppressed CDK2-associated kinase activities.
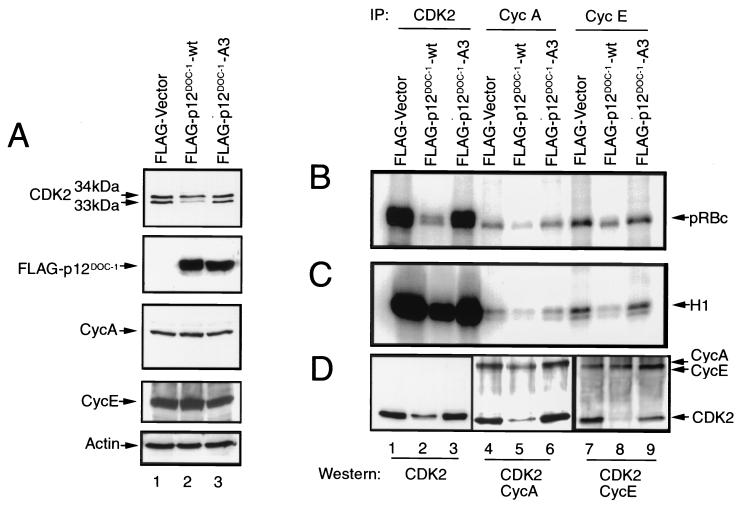
Cellular CDK2 kinase activity at any moment is largely a reflection of intracellular levels of cyclin E-associated CDK2 (G1/S) and cyclin A-associated CDK2 (S phase) (6–9). We evaluated the effect of ectopic p12DOC-1 expression on cellular CDK2 levels and CDK2-associated kinase activities in 293 cells. The p12DOC-1-A3 mutant was similarly ectopically expressed in parallel experiments to determine if any observed alteration in CDK2-mediated biochemical activities requires association with p12DOC-1. Figure 5A shows cellular levels of CDK2, FLAG-p12DOC-1-wt, FLAG-p12DOC-1-A3, cyclin A, cyclin E, and actin in control vector, p12DOC-1-wt, and p12DOC-1-A3 transfectants. Consistent with the in vitro biochemical data that p12DOC-1 associates predominantly with p34CDK2, the ratio of the p34CDK2 to the p33CDK2 forms of CDK2 is altered in the p12DOC-1 transfectants. In control vector transfectants, the ratio of p34CDK2 to p33CDK2 is ∼1:1, while in the p12DOC-1 transfectants, the ratio is ∼2:1. The ratio is restored to 1:1 in cells ectopically expressing the p12DOC-1-A3 mutant, indicating that the ability of p12DOC-1 to associate with CDK2 is necessary for the observed CDK2 alteration. The steady-state cellular level of CDK2 is reduced to about half in the 293 cells overexpressing p12DOC-1. The cellular levels of cyclins A and E are, however, similar in the control and p12DOC-1 (wild-type and A3) transfectants (Fig. 5A).
FIG. 5.
Ectopic expression of p12DOC-1 and CDK2 kinase activity in 293 cells. (A) Cellular levels of CDK2, FLAG-p12DOC-1-wt, FLAG-p12DOC-1-A3, cyclin A, cyclin E, and actin in control vector and p12DOC-1 transfectants. The samples were run on long SDS-PAGE gels to resolve the 33- and 34-kDa CDK2 bands. (B and C) In vitro phosphorylation using GST-pRBc and histone H1, respectively, as substrates. (D) CDK2, cyclin A, and cyclin E immunoblots to show intracellular levels of these proteins in p12DOC-1-wt (lanes 2, 5, and 8), p12DOC-1-A3 (lanes 3, 6, and 9), and control transfectants (lanes 1, 4, and 7). Lanes 1, 2, and 3, immunoblot for CDK2; lanes 4, 5, and 6, immunoblot for CDK2 and cyclin A; lanes 7, 8, and 9, immunoblot for CDK2 and cyclin E. Signals were quantified by exposing the probed membranes to a quantitative imaging system (Fluor-S MAX MultiImager; Bio-Rad). IP, immunoprecipitation.
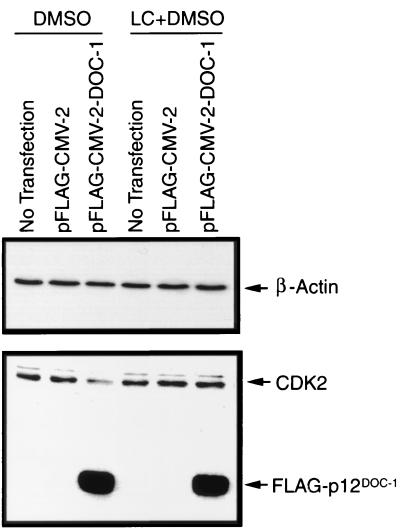
The cellular biochemical effect of ectopic p12DOC-1 expression on CDK2-associated kinase activities was examined by immunoprecipitating total CDK2, cyclin A-associated kinases (CDK2 and CDC2), and cyclin E-associated CDK2 from vector control, p12DOC-1-wt, and p12DOC-1-A3 transfectants. The immunoprecipitated complexes were used to phosphorylate known CDK2 substrates, pRBc (a carboxyl-terminal fragment of pRB) and histone H1, in separate reactions. Figure 5B shows that ectopic expression of p12DOC-1-wt reduces the CDK2-mediated phosphorylation of pRBc by approximately fivefold (lane 2). Similar reductions were observed for cyclin A-associated kinases (approximately threefold; lane 5) and cyclin E-associated CDK2 (approximately threefold; lane 8). Figure 5C shows parallel phosphorylation reactions with histone H1 as the substrate, demonstrating that the ectopic expression of p12DOC-1 also suppressed CDK2-associated histone H1 kinase activity (approximately threefold; lane 2). Similar suppressions were seen in cyclin A-associated histone H1 kinase (approximately twofold; lane 5) and cyclin E-associated histone H1 kinase activities (approximately threefold; lane 8). Ectopic expression of the p12DOC-1-A3 mutant showed kinase activity profiles similar to that of the control vector, indicating that association with p12DOC-1 is necessary for the observed alterations in CDK2-associated kinase activities (Fig. 5B and C, lanes 3, 6, and 9). These experiments have been independently repeated three times. Consistently we observed the relatively low levels of cyclin A- and cyclin E-associated CDK2 kinase activities in the 293 cells. Fig. 5D shows immunoblots to demonstrate the cellular levels of CDK2 alone and CDK2 associated with cyclins A and E in the transfectants. Cyclin A- and cyclin E-associated CDK2, however, are reduced by four- and fivefold, respectively (Fig. 5D, lanes 5 and 8). Steady-state cellular CDK2 levels are reduced by approximately twofold in p12DOC-1-wt transfectants (Fig. 5D, lane 2). These results suggest that ectopic expression of p12DOC-1-wt reduced CDK2-associated kinase activities, perhaps in part due to a decrease in CDK2 as a consequence of ectopic p12DOC-1-wt expression. This may suggest a role of p12DOC-1 in targeting CDK2 for proteolysis. The reduction of CDK2 coprecipitating with cyclins A and E further suggests that the association of p12DOC-1 with monomeric nonphosphorylated CDK2 may interfere or compete with binding of cyclins A and E to CDK2. It should be noted that the p12DOC-1-mediated suppression of CDK2-associated kinase activities is specific. In similar experiments involving immunoprecipitation of CDK4 and CDK6, ectopic expression of p12DOC-1 did not alter the phosphorylation pattern of pRBc or that of histone H1 (data not shown). To test the possibility that the p12DOC-1-mediated decrease in CDK2 levels took place through proteosome-dependent proteolysis, 293 cells were transfected in the presence or absence of the proteosome inhibitor clasto-lactacystin β-lactone. Figure 6 shows that the p12DOC-1-mediated proteolysis of CDK2 was averted when the transfectants were grown in the presence of the proteosome inhibitor (5 μM; 24 h).
FIG. 6.
p12DOC-1 targets CDK2 for proteolysis. 293 cells were transfected with the pFLAG vector or pFLAG-DOC-1 in the presence or absence of the proteosome inhibitor clasto-lactacystin β-lactone solubilized in dimethyl sulfoxide (5 μM) for 24 h. Top panel, immunoblot for β-actin to quantify proteins loaded. Bottom panel, immunoblot for CDK2 and FLAG-p12DOC-1.
Ectopic expression of p12DOC-1 suppresses CDK2-mediated cell cycle phenotypes.
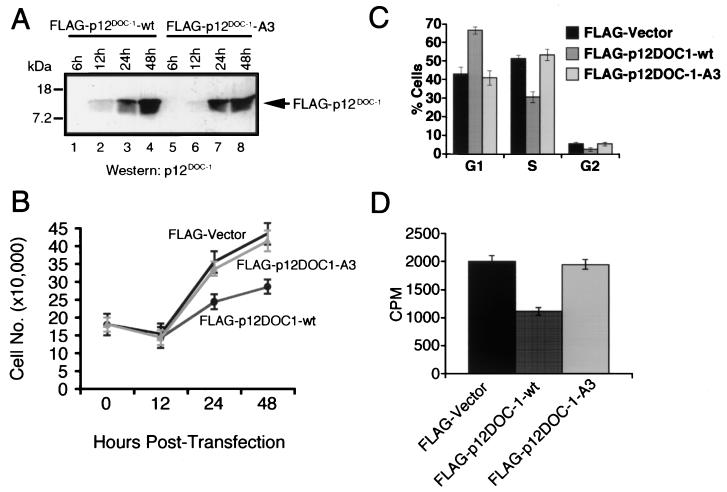
To gain insight into the potential role of p12DOC-1 with CDK2-mediated cell cycle phenotypes, we examined the effect of ectopic expression of p12DOC-1 in 293 cells. Our data predict that the effect of p12DOC-1's interaction with CDK2 will likely lead to the reduction of the intracellular pool of active CDK2, resulting in the negative regulation of CDK2-mediated cell cycle phenotypes (G1/S transition, S phase progression, and DNA replication). We indeed observed suppression of CDK2-mediated cell cycle phenotypes in cells ectopically expressing p12DOC-1 (Fig. 7). We consistently observed that ectopic p12DOC-1 expression in cells (Fig. 7A) was associated with growth suppression (Fig. 7B), changes in the cell cycle profile (↑ G1 and ↓ S) (P < 0.05) (Fig. 7C), and reduction of tritiated thymidine incorporation (P < 0.05) (Fig. 7D).
FIG. 7.
Effect of ectopic expression of p12DOC-1 on CDK2-mediated phenotypes in 293 cells. (A) Immunodetection of FLAG-p12DOC-1 expression in 293 cells. (B) Effect of p12DOC-1 ectopic expression on cell growth at 12, 24, and 48 h posttransfection. (C) Cell cycle positions at 48 h posttransfection. (D) Tritiated thymidine incorporation at 48 h posttransfection. For panels A and B, 293 cells were transfected with pFLAG vector or pFLAG-DOC-1 for the indicated time points and analyzed. For panels C and D, 293 cells were cotransfected with a neomycin expression vector (pcDNA3) and selected for 2 weeks in the presence of G418 at 400 μg/ml. Data are from three independent experiments. Transfection efficiency of 293 cells with Lipofectamine Plus (Life Technologies/Gibco-BRL, Grand Island, N.Y.) is ∼70%.
DISCUSSION
We have identified a novel biological partner of CDK2, p12DOC-1. The discovery was aided in part by the observation that overexpression of p12DOC-1 is associated with suppression of growth in mammalian cells. Using a GST-p12DOC-1 fusion protein, we have found it to interact with CDK2 in 293, HeLa, and A431 cells. The CDK2 that interacts with p12DOC-1 in 293 cells was found to be the monomeric nonphosphorylated form. Mutation analysis revealed that amino acids 109 to 111 of p12DOC-1 are critical for CDK2 association.
While the initial finding that p12DOC-1 interacts with CDK2 was made with the 293, HeLa, and A431 cells, none of these cells expressed detectable endogenous p12DOC-1. As indicated in Fig. 3A, the expression of p12DOC-1 is restricted to normal tissues. No tumor tissues or transformed cell lines have been found to express p12DOC-1 (35 were examined). Only recently have we found that the human epithelial cells HaCaT express detectable p12DOC-1. The lack of detectable p12DOC-1 in tumor cell lines and tissues is perhaps a reason why this low-molecular-weight CDK2-associated protein has not yet been found to interact with CDK2 by standard approaches, such as yeast hybrid screens or coprecipitation. In addition, it may be possible that the expression of p12DOC-1 is associated with growth inhibition, such as quiescence.
Overexpression of p12DOC-1 altered the cellular equilibrium of the inactive (p34) and active (p33) forms of CDK2, apparently by sequestering the inactive form and/or target CDK2 for proteolysis. This effect was nullified when the p12DOC-1-A3 mutant was transfected into the 293 cells. The amino acids 109 to 111 were mutated in this p12DOC-1-A3 mutant, which also ablated its binding to CDK2, suggesting that the association of p12DOC-1 with CDK2 increases the inactive form of CDK2. Our data support p12DOC-1's specific association with the monomeric nonphosphorylated form of CDK2. p12DOC-1 may act on the biochemical pathway of CDK2 activation at or before association with cyclins E and A. This idea is based on our data that neither cyclin E nor cyclin A was detected in the GST-p12DOC-1 cellular lysate or the immunoprecipitated FLAG-p12DOC-1–CDK2 complexes. In addition, p12DOC-1 has no effect on CDK2-associated kinase activities following association with cyclin E or A in vitro (data not shown). Finally, p12DOC-1 does not affect CDK2's downstream phosphorylation by WEE1 and CAK. WEE1 and CAK kinase activities are not affected in the presence of p12DOC-1 (data not shown). The net effect of p12DOC-1's interaction with CDK2 is therefore likely to be the reduction of the intracellular pool of active CDK2, leading to the suppression of CDK2-mediated cell cycle phenotypes (G1/S transition, S phase progression, and DNA replication).
Our data in Fig. 5 and 6 also support a role for p12DOC-1 in targeting p33CDK2 for proteolysis. Ectopic expression of p12DOC-1 in 293 cells causes a decrease of CDK2 through proteosome-dependent proteolysis (Fig. 6). These two mechanisms (sequestering p34CDK2 and targeting p33CDK2 for proteolysis), independently or in combination, can reduce cellular levels of p33CDK2 and the associated CDK2-mediated biological activities.
The p12DOC-1-A3 mutant was identified by its inability to retain CDK2 association in vitro. While amino acids 109 to 111 (TER) are of importance in mediating CDK2 association, there are likely to be additional sites in p12DOC-1 that are involved in CDK2 interaction. Chen et al. have recently aligned a number of cyclin-CDK-binding proteins (p45SKP2, E2F-1, E2F-2, E2F-3, p107, p130, p21, p27, and p57) and identified an “RxL” motif in the C terminus that is of importance in cyclin-CDK binding (1, 2). Conversion of the “RxL” motif of p27 to “AxA” abolishes its interaction with cyclin-CDK. It is interesting to note that in both p12DOC-1 and the related protein DOC-1R (15), there is an “RxL” motif seven amino acids N terminal to the identified “TER” CDK2 interaction site. The amino acid sequence in this region in p12DOC-1 is RGLVRECLAETERNAR, and that for DOC-1R is RALVRECLAETERNAR. This region resides in the C-terminal domains of both proteins, which share significant homology with a domain in a Caenorhabditis elegans protein, Y43F4B.7. We have mutated the RAL region in p12DOC-1 to AAA and found that the resultant p12DOC-1(R112A/L114A) mutant binds to CDK2 no differently than wild-type p12DOC-1 (data not shown), suggesting that this motif is likely not to be involved in cyclin-CDK2 association.
It will be of importance to know the region of CDK2 that p12DOC-1 binds to. While we have not yet mapped the p12DOC-1 interactive domains in CDK2, a set of nine CDK2 point mutants (for CDK2-30, point mutants V30A, A31F, and L32A; for CDK2-33, K33A; for CDK2-38, D38A and E40A; for CDK2-145, D145N; for CDK2-150, R150A, A151F, and F152A; for CDK2-159, Y159A and T160D; for CDK2-204, P204A, D206A, D208A, and D210A; for CDK2-217, R217A; and for CDK2-250, P250L) was obtained to determine whether any of these mutations will affect p12DOC-1 binding (3). Expressing p12DOC-1 and CDK2, wild type and mutants, by in vitro transcription and translations revealed that all nine CDK2 mutants and the wild type coprecipitated with p12DOC-1 (data not shown). Thus, these nine mutations do not affect p12DOC-1 binding. Efforts are in progress to use a conventional strategy similar to the one employed in this study to map the region in p12DOC-1 to which CDK2 binds.
While we have demonstrated interaction of endogenous p12DOC-1 and CDK2 in the normal lung lysates, the actual physiological relevance and contribution of p12DOC-1 to CDK2 biology is currently being studied. We have recently identified cellular models (such as the HaCaT cells) that will permit the biochemical and functional interactions of endogenous p12DOC-1 and CDK2 to be examined.
Our data support that p12DOC-1 is a specific CDK2-associated protein capable of negative regulation of CDK2-associated activities. Our data suggest that p12DOC-1 negatively regulates CDK2 through a mechanism that is different from that of the p16INK4a and p21WAF1/CIP1/CAP20 families of CDK inhibitors (11). It is likely that p12DOC-1 sequesters the monomeric form of CDK2 prior to its association with cyclins E and A and/or targets CDK2 for proteolysis. Studies are in progress to detail these mechanisms as well as the physiological relevance of the interactions between p12DOC-1 and CDK2 in cells. It is unclear what upstream signals regulate p12DOC-1. Perhaps regulatory signals in G1/S transition, S phase progression, and DNA replication may feed into this regulation pathway. It is intriguing to note that while the p21WAF1/CIP1/CAP20 family of CDK inhibitors is universal for CDKs and the p16INK4a family is specific for CDK4 and CDK6, p12DOC-1 may be a specific CDK2-associated protein that suppresses CDK2 activities.
ACKNOWLEDGMENTS
We thank David Morgan for the baculovirus containing CDK2, Edward Harlow for the baculoviruses containing CDK4 and CDK6, and Li-Huei Tsai for the expression plasmid containing CDK2. We also thank Karl Münger, Philip Hinds, and Yong Kim for critical reading of the manuscript.
This work was supported by NIH grants P01 DE12467 and R01 DE08680 (to D.T.W.W.), and R29 DE 11983 (to R.T.). H.O. is a Research Fellow of the Japan Society for the Promotion of Science (JSPS).
S.S. and H.O. contributed equally to this report.
REFERENCES
- 1.Chen J, Jackson P K, Kirschner M W, Dutta A. Separate domains of p21 involved in the inhibition of Cdk kinase and PCNA. Nature. 1995;374:386–388. doi: 10.1038/374386a0. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Saha P, Kornbluth S, Dynlacht B D, Dutta A. Cyclin-binding motifs are essential for the function of p21CIP1. Mol Cell Biol. 1996;16:4673–4682. doi: 10.1128/mcb.16.9.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen B A, Colas P, Brent R. An artificial cell-cycle inhibitor isolated from a combinatorial library. Proc Natl Acad Sci USA. 1998;95:14272–14277. doi: 10.1073/pnas.95.24.14272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daigo Y, Suzuki K, Maruyama O, Miyoshi Y, Yasuda T, Kabuto T, Imaoka S, Fujiwara T, Takahashi E, Fujino M A, Nakamura Y. Isolation, mapping and mutation analysis of a human cDNA homologous to the doc-1 gene of the Chinese hamster, a candidate tumor suppressor for oral cancer. Genes Chromosomes Cancer. 1997;20:204–207. [PubMed] [Google Scholar]
- 5.Gordon H M, Kucera G, Salvo R, Boss J M. Tumor necrosis factor induces genes involved in inflammation, cellular and tissue repair, and metabolism in murine fibroblasts. J Immunol. 1992;148:4021–4027. [PubMed] [Google Scholar]
- 6.Gu Y, Rosenblatt J, Morgan D O. Cell cycle regulation of CDK2 activity by phosphorylation of Thr160 and Tyr15. EMBO J. 1992;11:3995–4005. doi: 10.1002/j.1460-2075.1992.tb05493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan D O. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 8.Musunuru K, Hinds P W. Cell cycle regulators in cancer. New York, N.Y: Karger Landes Systems; 1997. [Google Scholar]
- 9.Rosenblatt J, Gu Y, Morgan D O. Human cyclin-dependent kinase 2 is activated during the S and G2 phases of the cell cycle and associates with cyclin A. Proc Natl Acad Sci USA. 1992;89:2824–2828. doi: 10.1073/pnas.89.7.2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serrano M, Hannon G J, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 11.Sherr C J. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 12.Todd R, McBride J, Tsuji T, Donoff R B, Nagai M, Chou M Y, Chiang T, Wong D T. Deleted in oral cancer-1 (doc-1), a novel oral tumor suppressor gene. FASEB J. 1995;9:1362–1370. doi: 10.1096/fasebj.9.13.7557027. [DOI] [PubMed] [Google Scholar]
- 13.Tsuji T, Duh F M, Latif F, Popescu N C, Zimonjic D B, McBride J, Matsuo K, Ohyama H, Todd R, Nagata E, Terakado N, Sasaki A, Matsumura T, Lerman M I, Wong D T W. Cloning, mapping, expression, function, and mutation analyses of the human ortholog of the hamster putative tumor suppressor gene doc-1. J Biol Chem. 1998;273:6704–6709. doi: 10.1074/jbc.273.12.6704. [DOI] [PubMed] [Google Scholar]
- 14.Xiong Y, Hannon G J, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Tsao H, Tsuji T, Minoshima S, McBride J, Majewski P, Todd R, Shimizu N, Wong D T, Housman D E, Haluska F G. Identification and mutation analysis of DOC-1R, a DOC-1 growth suppressor-related gene. Biochem Biophys Res Commun. 1999;255:59–63. doi: 10.1006/bbrc.1999.0148. [DOI] [PubMed] [Google Scholar]