Abstract
Background and Objectives:
Recent studies suggest that bacteria influence the pathogenesis of primary CRC, yet their role in recurrence after resection is largely unknown. We have discovered that collagenase-producing bacteria promote cancer recurrence in mice, and that antibiotic bowel decontamination decreases colonization of these same organisms in humans. We hypothesized that preoperative combined mechanical and oral antibiotic bowel preparation would improve disease-free survival (DFS) in patients undergoing surgery for CRC.
Methods:
We reviewed a cancer registry of patients treated for CRC at a tertiary center. Patients who received bowel preparation were compared to those that did not via a 1:1-propensity score matched for follow-up, age, sex, BMI, stage, location, chemoradiation, infection, anastomotic leak, and blood transfusion.
Results:
1,279 patients met inclusion criteria. Following propensity score matching, 264 patients receiving bowel prep were matched to 264 patients who did not. Kaplan-Meier estimates showed that patients who received bowel prep had a significantly improved 5-year DFS compared to those that did not (76.3% vs. 64.2%;p<0.01). Cox regression demonstrated that bowel prep was associated with improved DFS (HR 0.57, 95%CI 0.37–0.89;p< 0.01).
Conclusion:
Combined mechanical and oral antibiotic bowel preparation is independently associated with improved recurrence free-survival in patients undergoing surgery for CRC.
Keywords: colorectal cancer, recurrence, bowel prep, microbiome, decontamination
Synopsis for Table of Contents:
We reviewed a cancer registry to understand how a combined mechanical and oral antibiotic bowel preparation influences postoperative recurrence rates. We report that bowel prep is independently associated with improved 5-year recurrence free-survival in postoperative colorectal cancer patients.
INTRODUCTION
Colorectal cancer (CRC) is the third most common form of cancer in both men and women worldwide1. In the current treatment paradigm, surgical resection of the primary tumor is typically required for cure. Depending upon the clinical stage, patients are often prescribed preoperative neoadjuvant and/or postoperative adjuvant chemotherapy or radiation in order to decrease the risk of a postoperative recurrence. Yet, even when patients receive optimal treatment, nearly 40% of patients will develop either a local recurrence or distant metastasis following surgery; following the diagnosis of recurrence, median survival is approximately 32 months2,3.
While the pathogenesis of CRC is multifactorial, the intestinal microbiome has been suggested to play an increasingly important role in the initiation and progression of primary CRC. Yet, the influence of gut microbes on the development of CRC recurrence and metastasis following surgery remains unclear4. Recently, preclinical models have highlighted the potential for the collagenase-producing bacteria Enterococcus faecalis and Proteus mirabilis to promote recurrence of colorectal tumors following surgery in mice5. This may suggest that preoperative eradication of these organisms may be a novel strategy to prevent recurrence.
Bacterial decontamination of the colon with a combined mechanical bowel preparation and oral antibiotics is often prescribed prior to surgical resection in order to prevent postoperative infections. Strikingly, it has recently been demonstrated that in humans, a combined mechanical and oral antibiotic preparation significantly reduces the colonization of the collagenase-producing bacteria that were found to drive cancer recurrence in mice5. We therefore hypothesized that combined mechanical and oral antibiotic bowel preparation to decontaminate the gut can prevent postoperative cancer recurrence in patients undergoing surgical resection. The aim of this study was to determine the association between preoperative combined bowel preparation with disease-free survival following surgery for CRC.
MATERIAL AND METHODS
Study design and population.
We performed a retrospective cohort study using the University of Chicago Medicine Comprehensive Cancer Center Registry. This prospectively maintained database collects data on all patients treated for gastrointestinal malignancies at the University of Chicago Comprehensive Cancer Center, which includes patients seen at the University of Chicago Medicine and its affiliated network of community hospitals. All data is abstracted by certified tumor registrars through the National Cancer Registry Association.
The study population included patients that underwent surgical resection of a primary colon or rectal adenocarcinoma between 2005 – 2018. Patients were excluded if they were less than 18 years of age, if they previously had surgical resection for a colon or rectal malignancy, if the there was a combined resection of the CRC with another organ, if the resection was for palliative intent with known metastatic disease, or if their cancer stage was unknown. This study was approved by the University of Chicago Biological Sciences Institutional Review Board (18–1222).
Outcome measures.
The primary outcomes of interest were colorectal cancer recurrence and disease-free survival. Recurrence was collected as a binary variable from patients’ medical records by abstractors from the data registry. Disease-free survival was defined as the time between the date of first surgical resection and the date of recurrence or last clinical evaluation for recurrence. Overall survival was a secondary outcome measure and was defined as the time between the date of first surgical resection and the date of death or last clinical evaluation.
Variables and data collection.
The primary variable of interest in this study was preoperative administration of a combined mechanical and oral antibiotic bowel preparation and was abstracted by manual chart review of the electronic medical record. Combined bowel prep was defined as prescription for both a preoperative oral purgative such as GoLYTELY, MiraLAX, Suprep, and the antibiotics neomycin (1000mg TID) and metronidazole (250mg TID) on the day before surgery. Patients were only included if they had both a purgative and antibiotics; patients who received only mechanical prep or only antibiotics were excluded. This binary variable categorized patients as either having received a bowel preparation prior to surgery or not having received a bowel preparation prior to surgery.
Other variables such as demographics, cancer related data, and other risk factors for recurrence and poor survival were abstracted from the data registry. Demographics evaluated included age (continuous), sex (binary: male/female), race (categorical: White/Black/Asian/Other), emergent operation (binary: yes/no), and body mass index (BMI) (categorical: underweight/normal/overweight/obese/unknown). Cancer characteristics evaluated were location (binary: colon/rectum), pathological stage (categorical: stage 1/2/3/4), neoadjuvant or adjuvant chemotherapy (binary: yes/no), and neoadjuvant or adjuvant radiation (binary: yes/no) and postoperative follow-up (categorical: 1 year/2 years/3 years/4 years/5 years/≥ 6 years). Additional risk factors for the development of cancer recurrence that were evaluated included whether patients received a blood transfusion (binary: yes/no), and development of 30 day postoperative infection (SSI) (binary: yes/no). Because of the association of anastomotic leak with recurrence, coupled with the fact that preoperative bowel preparation is associated with decreased incidence of anastomotic leak, anastomotic dehiscence was abstracted by manual chart review. Leak was defined as either dehiscence on reoperation or extravasation of contrast on a barium enema on CT imaging. Fluid adjacent to an anastomosis but without evidence of extravasation was abstracted as an SSI.
Statistical analysis.
To evaluate the association of administration of a combined preoperative bowel with recurrence and disease-free survival, a propensity score matching method was utilized to control for confounding variables. A multivariable logistic regression model was constructed to evaluate associations between receiving a combined preoperative bowel preparation with the aforementioned patient, disease, operation, and process-level factors. Using this logistic regression, a propensity score was calculated which represented each patient’s predicted probability of receiving a preoperative bowel preparation. Patients were then matched based on propensity scores using a 1:1 nearest neighbor match, within 0.005 standard deviations of the propensity score, without replacement. Balance between matched cohorts was assessed using paired statistical tests including the Wilcoxon signed rank test for non-parametric continuous variables, the McNemar Chi-square test for binary variables, and the McNemar-Brokar Chi-square test for categorical variables with >2 groups. A Cox proportional hazards regression model was created to evaluate hazard ratios for time to recurrence using the matched cohorts. Kaplan-Meier disease-free and overall survival curves between patients who received a combined preoperative bowel preparation and matched patients who did not receive a preoperative bowel preparation were constructed and compared using log-rank tests. All statistical analyses were performed using Stata 16.1 (College Station, TX).
RESULTS
Between 2005 – 2018, 1,279 adult patients underwent surgical resection for a colon or rectal malignancy during the study period and were included in the study. The baseline characteristics of the entire cohort are presented in Table 1. The mean age of the cohort was 61 years of age and ranged from 18 to 95 years of age. 53.6% of the cohort was male and the cohort was predominately White (66.8%) followed by Black (28.7%) and Asian (2.9%). 70.8% of patients underwent surgery for colon cancer whereas 29.2% underwent surgery for rectal cancer and there was a wide distribution of patients between stages 1, 2, 3, and 4 (19.9%, 25.8%, 30.2%, and 24.1%, respectively). Cancer recurrence occurred in 256 patients (20%) while the overall mortality rate was 28.2% (n=361).
Table 1:
Demographics of the entire cohort (n=1279)
| Characteristic | N (%) |
|---|---|
| Age | |
| Min-Max (years) | 15–95 |
| Mean (years ±SD) | 60.7 ±13.6 |
| Gender | |
| Male | 685 (53.6%) |
| Female | 594 (46.4%) |
| Race | |
| White | 855 (66.8%) |
| Black | 367 (28.7%) |
| Asian | 37 (2.9%) |
| Other | 20 (1.6%) |
| BMI | |
| < 18.5 | 63 (4.9%) |
| 18.5 – 24.9 | 394 (30.8%) |
| 25 – 29.9 | 371 (29.0%) |
| >30 | 309 (24.2%) |
| Unknown | 142 (11.1%) |
| Stage | |
| Stage 1 | 255 (19.9%) |
| Stage 2 | 330 (25.8%) |
| Stage 3 | 386 (30.2%) |
| Stage 4 | 308 (24.1%) |
| Site | |
| Colon | 905 (70.8%) |
| Rectum | 374 (29.2%) |
| Emergent Surgery | |
| No | 1269 (99.2) |
| Yes | 10 (0.8) |
| Anastomotic Leak | |
| No | 1213 (94.8%) |
| Yes | 66 (5.2%) |
| SSI | |
| No | 1092 (85.4%) |
| Yes | 187 (14.6%) |
| Bowel Prep | |
| No | 949 (74.2%) |
| Yes | 330 (25.8%) |
| Radiation | |
| No | 1024 (80.1%) |
| Yes | 255 (19.9%) |
| Chemotherapy | |
| No | 818 (64.0%) |
| Yes | 461 (36.0%) |
| Blood Transfusion | |
| No | 1137 (88.9%) |
| Yes | 142 (11.1%) |
| Years of Follow Up | |
| 1 year | 273 (21.3%) |
| 2 years | 224 (17.5%) |
| 3 years | 213 (16.7%) |
| 4 years | 147 (11.5%) |
| 5 years | 122 (9.5%) |
| ≥6 years | 200 (23.5%) |
SD = standard deviation
BMI = body mass index
SSI = surgical site infection
Of this entire cohort, 74.2% (n=949) of the patients did not receive a combined mechanical and oral antibiotic bowel preparation whereas 25.8% (n=330) did. Prior to propensity matching, patients that received bowel preperation were older, had a higher BMI, and received adjuvant chemoradiation less often (Table 2). Following propensity score matching for age, sex, race, BMI, cancer stage, cancer location, emergent surgery status, SSI, anastomotic leak, neoadjuvant or adjuvant chemoradiation, blood transfusion, and length of follow-up, 264 patients who received a combined mechanical and oral antibiotic bowel preparation were paired with 264 patients who did not receive a preoperative combined bowel preparation. After propensity matching, there were no significant differences between the matched groups using paired statistical tests (Table 3). Mean follow-up for the entire cohort of patients was 40 months and there was no difference in follow-up between patients whom received a preoperative combined bowel preparation verses those that did not.
Table 2:
Unmatched bivariate associations for patients with and without preoperative combined bowel preparation (N=1279)
| Preoperative Combined Bowel Prep | |||
|---|---|---|---|
| Characteristic | |||
| Yes (n=330) | No (n=949) | P value | |
| Age | |||
| Mean | 62.9 ± 13.8 | 60.0 ± 13.4 | <0.001 a |
| Gender | |||
| Male | 174 (52.7) | 511 (53.9) | 0.726b |
| Female | 156 (47.3) | 438 (46.1) | |
| Race | |||
| White | 188 (57.0) | 667 (70.3) | <0.001 b |
| Black | 124 (37.6) | 243 (25.6) | |
| Asian | 9 (2.7) | 28 (2.9) | |
| Other | 9 (2.7) | 11 (1.2) | |
| BMI | |||
| < 18.5 | 19 (5.8) | 44 (4.6) | <0.001 b |
| 18.5 – 24.9 | 98 (29.7) | 296 (31.2) | |
| 25 – 29.9 | 100 (30.3) | 271 (28.6) | |
| >30 | 105 (31.8) | 204 (21.5) | |
| Unknown | 8 (2.4) | 134 (14.1) | |
| Stage | |||
| Stage 1 | 86 (26.1) | 169 (17.8) | <0.001 b |
| Stage 2 | 106 (32.1) | 224 (23.6) | |
| Stage 3 | 95 (28.8) | 291 (30.7) | |
| Stage 4 | 43 (13.0) | 265 (27.9) | |
| Site | |||
| Colon | 254 (77.0) | 651 (68.6) | <0.01 b |
| Rectum | 76 (23.0) | 298 (31.4) | |
| Emergent Surgery | |||
| No | 328 (99.4) | 941 (99.2) | 0.674b |
| Yes | 2 (0.6) | 8 (0.8) | |
| Anastomotic Leak | |||
| No | 314 (95.2) | 899 (94.7) | 0.766b |
| Yes | 16 (4.8) | 50 (5.3) | |
| SSI | |||
| No | 283 (85.8) | 809 (85.2) | 0.821b |
| Yes | 47 (14.2) | 140 (14.8) | |
| Radiation | |||
| No | 281 (85.2) | 743 (78.3) | <0.01 b |
| Yes | 48 (14.8) | 206 (21.7) | |
| Chemotherapy | |||
| No | 235 (71.2) | 583 (61.4) | <0.002 b |
| Yes | 95 (28.8) | 366 (38.6) | |
| Blood Transfusion | |||
| No | 267 (80.9) | 870 (91.7) | <0.001 b |
| Yes | 63 (19.1) | 79 (8.3) | |
| Years of Follow Up | |||
| 1 year | 112 (33.9) | 161 (17.0) | <0.001 b |
| 2 years | 58 (17.6) | 166 (17.5) | |
| 3 years | 69 (20.9) | 144 (15.2) | |
| 4 years | 43 (13.0) | 104 (10.9) | |
| 5 years | 26 (7.9) | 96 (10.1) | |
| ≥6 years | 22 (6.7) | 279 (29.3) | |
SD = standard deviation
BMI = body mass index
SSI = surgical site infection
P value determined using Mann-Whitney U test
P values determined using Chi-square test
Table 3:
Matched bivariate associations for patients with and without preoperative combined bowel preparation (N=528)
| Preoperative Combined Bowel Prep | |||
|---|---|---|---|
| Characteristic | |||
| Yes (n=264) | No (n=264) | P value | |
| Age | |||
| Mean | 61.8 ± 13.5 | 62.0 ± 13.5 | 0.767a |
| Gender | |||
| Male | 146 (55.3) | 149 (56.4) | 0.856b |
| Female | 118 (44.7) | 115 (43.6) | |
| Race | |||
| White | 166 (62.9) | 161 (61.0) | 0.875c |
| Black | 87 (32.9) | 88 (33.3) | |
| Asian | 7 (2.7) | 7 (2.7) | |
| Other | 4 (1.5) | 8 (3.0) | |
| BMI | |||
| < 18.5 | 15 (5.7) | 15 (5.7) | 0.953c |
| 18.5 – 24.9 | 85 (32.2) | 86 (32.6) | |
| 25 – 29.9 | 85 (32.2) | 83 (31.4) | |
| >30 | 71 (26.9) | 70 (26.5) | |
| Unknown | 8 (3.0) | 10 (3.8) | |
| Stage | |||
| Stage 1 | 63 (23.9) | 64 (24.2) | 0.869c |
| Stage 2 | 79 (29.9) | 78 (29.6) | |
| Stage 3 | 82 (31.1) | 81 (30.7) | |
| Stage 4 | 40 (15.1) | 41 (15.5) | |
| Site | |||
| Colon | 192 (72.7) | 197 (74.6) | 0.615b |
| Rectum | 72 (27.3) | 67 (25.4) | |
| Emergent Surgery | |||
| No | 262 (99.2) | 262 (99.2) | 1.0b |
| Yes | 2 (0.8) | 2 (0.8) | |
| Anastomotic Leak | |||
| No | 249 (94.3) | 246 (93.2) | 0.602b |
| Yes | 15 (5.7) | 18 (6.8) | |
| SSI | |||
| No | 228 (86.4) | 230 (87.1) | 1.0b |
| Yes | 36 (13.6) | 34 (12.9) | |
| Radiation | |||
| No | 221 (83.7) | 212 (80.3) | 0.286b |
| Yes | 43 (16.3) | 52 (19.7) | |
| Chemotherapy | |||
| No | 177 (67.1) | 179 (67.8) | 0.549b |
| Yes | 87 (32.9) | 85 (32.2) | |
| Blood Transfusion | |||
| No | 223 (84.5) | 232 (87.9) | 0.423b |
| Yes | 41 (15.5) | 32 (12.1) | |
| Years of Follow Up | |||
| 1 year | 75 (28.4) | 73 (27.7) | 0.814c |
| 2 years | 54 (20.5) | 50 (18.9) | |
| 3 years | 53 (20.1) | 64 (24.2) | |
| 4 years | 37 (14.0) | 34 (12.9) | |
| 5 years | 23 (8.7) | 25 (9.5) | |
| ≥6 years | 22 (8.3) | 18 (6.8) | |
SD = standard deviation
BMI = body mass index
SSI = surgical site infection
P value determined by Wilcoxon signed rank test
P value determined by McNemar Chi-square test
P value determined by McNemar-Brokar Chi-square test
A Cox proportional hazards regression model was constructed using matched patients to evaluate time to cancer recurrence. After adjusting for age, sex, BMI, race, stage, cancer location, emergency surgery status, SSI, anastomotic leak, chemoradiation, blood transfusion and follow-up, results of the model found that receiving a combined mechanical and oral antibiotic bowel preparation was significantly associated with a lower hazard for recurrence (HR 0.57, 95% Confidence Interval [CI] 0.37–0.89). In addition, patients with lower cancer stage and those that were not prescribed chemoradiation had a significantly lower risk of recurrence (Table 4).
Table 4:
Cox Proportional Hazards Regression for Time to Cancer Recurrence in Propensity Score Matched Cohort (N=528)
| Characteristic | HR (95%CI) | P value |
|---|---|---|
| Bowel Prep | ||
| No | 1.0 | REF |
| Yes | 0.57 (0.37–0.89) | <0.05 |
| Age | 1.00 (0.98–1.02) | 0.906 |
| Gender | ||
| Male | 1.0 | REF |
| Female | 1.32 (0.85–2.03) | 0.212 |
| Race | ||
| White | 1.0 | REF |
| Black | 0.64 (0.38–1.07) | 0.089 |
| Asian | 0.15 (0.02–1.16) | 0.070 |
| Other | 2.16 (0.70–6.66) | 0.178 |
| BMI | ||
| < 18.5 | 1.19 (0.49–2.89) | 0.697 |
| 18.5 – 24.9 | 1.0 | REF |
| 25 – 29.9 | 0.49 (0.28–0.87) | <0.05 |
| >30 | 0.85 (0.50–1.44) | 0.540 |
| Unknown | 0.61 (0.14–2.66) | 0.511 |
| Stage | ||
| Stage 1 | 1.0 | REF |
| Stage 2 | 2.14 (1.01–2.89) | <0.05 |
| Stage 3 | 3.01 (1.43–6.28) | <0.05 |
| Stage 4 | 1.09 (0.43–2.80) | 0.854 |
| Site | ||
| Colon | 1.78 (1.06–2.99) | <0.05 |
| Rectum | 1.0 | REF |
| Emergent Surgery | ||
| No | 1.0 | REF |
| Yes | 5.34 (1.11–25.6) | <0.05 |
| Anastomotic Leak | ||
| No | 1.0 | REF |
| Yes | 1.05 (0.40–2.79) | 0.915 |
| SSI | ||
| No | 1.0 | REF |
| Yes | 0.46 (0.20–1.03) | 0.060 |
| Radiation | ||
| No | 1.0 | REF |
| Yes | 2.01 (1.24–3.26) | <0.01 |
| Chemotherapy | ||
| No | 1.0 | REF |
| Yes | 1.78 (1.11–2.85) | <0.05 |
| Blood Transfusion | ||
| No | 1.0 | REF |
| Yes | 0.74 (0.34–1.59) | 0.433 |
| Years of Follow Up | ||
| 1 year | 1.0 | REF |
| 2 years | 1.06 (0.33–3.42) | 0.917 |
| 3 years | 0.92 (0.29–2.90) | 0.890 |
| 4 years | 0.61 (0.18–2.07) | 0.430 |
| 5 years | 0.62 (0.17–2.24) | 0.463 |
| ≥6 years | 0.22 (0.05–0.95) | <0.05 |
BMI = body mass index
SSI = surgical site infection
Patients who received a preoperative combined bowel preparation had a median disease-free survival of 22 months and a 5-year estimated disease-free survival rate of 76% (95% CI 67%–83%) whereas patients who did not receive preoperative combined preparation had a median disease-free survival of 19.5 months and a 5-year estimated disease-free survival rate of 64% with (95% CI 55%–72%). Kaplan-Meier curves demonstrated that preoperative combined bowel preparation was associated with a significantly higher 5-year disease free survival (p<0.005) (Figure 1). There was no significant difference in overall survival between patients who received a preoperative combined bowel preparation and patients who did not (p=0.09) (Figure 2).
Figure 1: Propensity score matched Kaplan Meier disease-free survival curves for patients with and without preoperative combined bowel preparation.
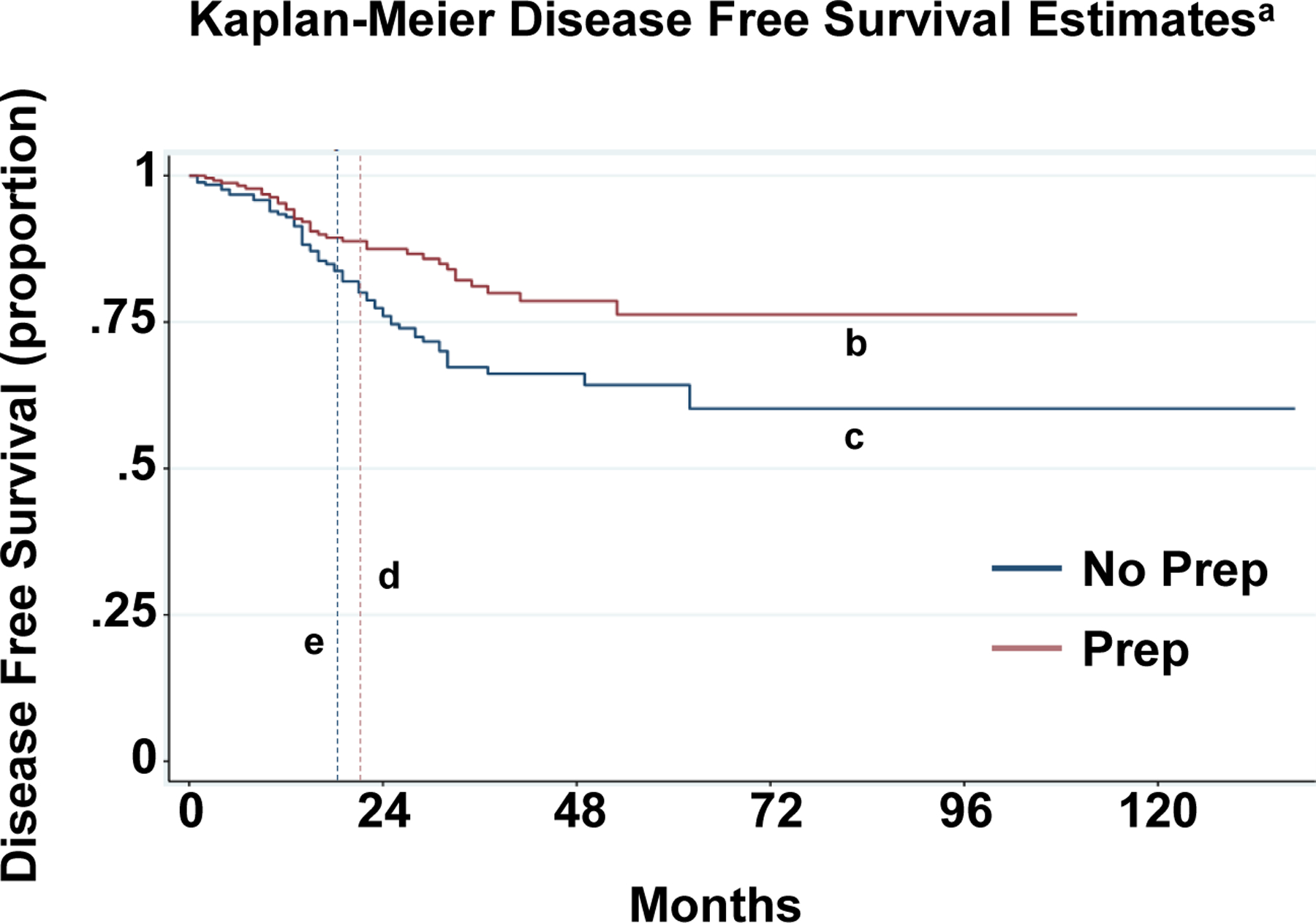
a Logrank test p<0.01
b 5-year DFS in Prep Patients= 76% with 95% CI 67%–83%
c 5-year DFS in No Prep Patients= 64% with 95% CI 55%–72%
d Median survival in Prep Patients= 22 months
e Median survival in No Prep Patients=19.5 months
Figure 2: Propensity score matched Kaplan Meier overall survival curves for patients with and without preoperative combined bowel preparation.
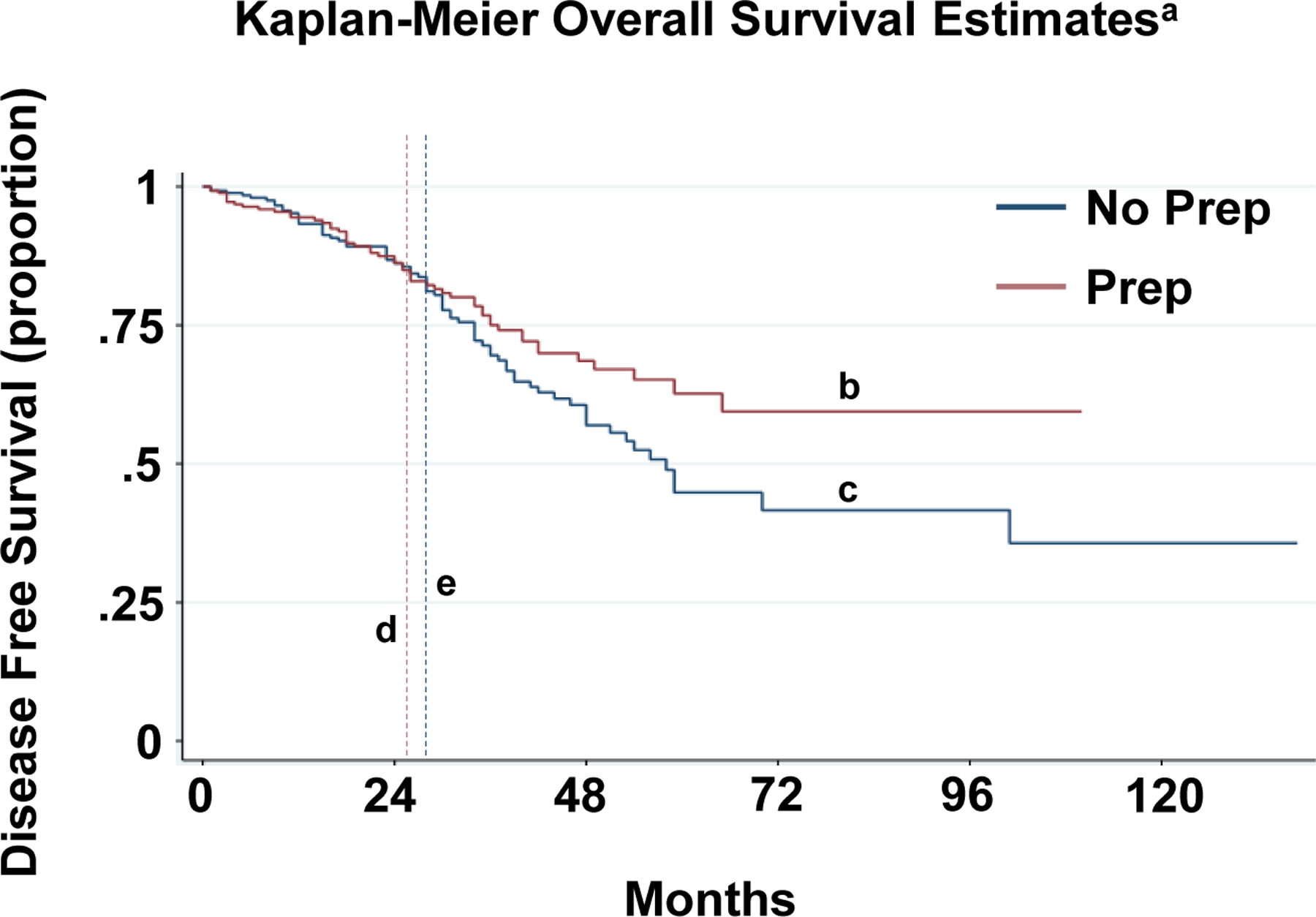
a Logrank test p=0.09
b 5-year Overall Survival in Prep Patients= 63% with 95% CI 52%–72%
c 5-year DFS in No Prep Patients= 45% with 95% CI 34%–55%
d Median survival in Prep Patients= 25 months
e Median survival in No Prep Patients=27 months
DISCUSSION
Surgical resection remains fundamental in acquiring long-term cure for CRC. Yet, even with appropriate use of adjuvant therapy, approximately 40% of patients will develop a postoperative recurrence. Since the mean survival of those patients who develop a postoperative recurrence is only 32 months, new strategies to prevent CRC recurrence following surgery are needed to improve overall CRC outcomes3. We found that patients whom received preoperative bacterial decontamination with a standard combined bowel preparation had a significantly lower risk of developing a postoperative cancer recurrence, and significantly longer disease-free survival compared to patients who did not receive preoperative bowel decontamination. These results suggest that the preoperative microbiome may play a critical and causative role in the development of postoperative recurrence, and that perioperative manipulation of the gut microbes may improve cancer outcomes.
Investigations over the past decade have highlighted the influence of the intestinal microbiome on the pathogenesis of primary gastrointestinal carcinoma. Bacterial communities that induce inflammation or generate radical oxygen species that can promote carcinogenesis are found to proliferate on tumor tissue6. Further, certain phylum such as Fusobacteria and class such as β-proteobacteria are significantly enriched on histologically high-grade tumors that have an increased risk of metastasis6. Strikingly, Lauke et al demonstrated that enhanced colonization of tumor tissues with Fusobacterium nucleatum and Bacteroides fragilis is associated with poor long-term oncologic outcomes such as decreased disease-free survival and increased cancer-specific mortality following surgical resection7. In addition to a growing list of organisms, these bacteria can directly or via microbiota-derived metabolites influence cancer signaling pathways that may trigger progression of CRC8,9.
Despite these recent discoveries, how intestinal bacteria can promote CRC recurrence following surgery remains to be elucidated. While the aim of CRC surgery is to acquire cure, it has been observed that surgery can accelerate residual disease or increase the establishment of new local or distal metastasis10. For decades it has been well-documented that in both murine models and human specimens, viable cancer cells are exfoliated from a primary gastrointestinal tumor and can bind to sites of injury such as healing anastomotic tissue11–13; yet, how the perioperative intestinal microenvironment shaped by surgery influences these cancer cells to develop into a postoperative recurrence is poorly understood. We have previously found that surgery significantly changes the composition and function of the perioperative intestinal microbiota, and therefore our group has recently investigated the role of bacteria in driving exfoliated cancer cells to promote postoperative tumors following surgery14,15.
We have discovered that when mice undergo a colon resection that mimics what humans would undergo during resection, intraluminal tumor cells can migrate through the healing anastomosis to form a local tumor or liver metastasis identical to the pattern of recurrence seen in humans16. Strikingly these postoperative tumors only occur when mice are fed a high-fat, low-fiber Western diet, and when collagenase producing E. faecalis or P. mirabilis proliferate on anastomotic tissue. These organisms can bind to and activate components of the extracellular matrix such as matrix metalloproteinases (MMP) and the urokinase-plasminogen system, products of which are well-known to promote tumorigenesis15,17,18. Therefore, it is plausible that the microbiota that colonize the intestine perioperatively may create a unique microenvironmental niche filled with pro-tumorigenic products that induce exfoliated tumor cells to manifest postoperative recurrence. Consequently, preoperative decontamination of these microbes may represent a novel strategy in preventing recurrent disease.
To isolate the independent effect of preoperative bowel decontamination with a combined mechanical and oral antibiotic bowel preparation we used propensity matching to control for all of the variables that may influence the risk of recurrence such as tumor stage, tumor location, use of neoadjuvant and adjuvant chemoradiation, infections, blood transfusion, and anastomotic leak. To our knowledge, our study is the first to show that patients whom receive preoperative decontamination with a combined mechanical bowel prep and oral antibiotics have a significantly reduced risk for development of a postoperative cancer recurrence. Bowel prep significantly decreases the quantity of the microbiota immediately upon its implementation, with compositional and metabolomic changes that can persist for up to 14 days19,20. While the results have varied, bowel prep has consistently depleted organisms that have been associated with promoting colorectal carcinogenesis such as Peptostreptococcus spp. and Enterobacter spp21,22. Strikingly, our group has discovered that in humans, bowel prep significantly decreases colonization of those collagenolytic organisms that drove tumors in our mouse model of CRC recurrence5. In addition to its influence on the microbiome, the purgative component of the combined prep likely decreases the concentration of intraluminal exfoliated cells. In fact, while not standard practice in the United States, rectal irrigation on the day of surgery has been shown to decrease the number of intraluminal malignant cells and has been associated with decreased recurrence rates in some centers23,24. Because we only included patients whom were administered a combined prep, future studies will be necessary to define which component (purgative versus antibiotics) plays the dominant role in preventing postoperative recurrence.
Given that our investigation is a single-center observational study it has several limitations. First, preoperative bowel preparation with oral antibiotics was not routinely prescribed by all surgeons at our institution until 2015, and therefore our dataset is based upon historical controls within a single institution and there is concern for selection bias. Second, prior to propensity score matching, patients that did not receive a combined bowel preparation were more likely to receive neoadjuvant chemoradiotherapy and had significantly a longer follow-up, all of which could predict a higher rate of recurrence. While these variables were controlled for in the propensity score matching, it is possible that all potential biases were not included for in our model. While analysis of a national dataset could potentially limit these biases that result from a single center study, a database that contains both cancer outcomes (i.e. National Cancer Database) and prescription of bowel prep (i.e. National Surgical Quality Improvement Program) currently does not exist in the United States. Our findings therefore demand the creation of a multi-institutional national patient database that links both perioperative demographics, (i.e. bowel decontamination) with clinical cancer outcomes. Additionally, while there was a trend for improved overall survival in patients receiving a preoperative bowel prep, this was not statistically significant. Whether longer follow-up would result a significant difference in overall survival will need to be addressed in follow-up studies.
Overall, our study provides compelling evidence that local gastrointestinal bacteria may play a critical role in the development of recurrent colorectal cancer after surgical resection and may have important clinical implications. Our findings set the stage for follow-up basic and translation studies to provide novel insights into how preoperative decontamination influences the host-microbe-cancer interaction to prevent tumorigenesis. Analysis of each patient’s preoperative microbiome may inform individualized antimicrobials to rid the gut of bacteria that promote recurrence and reduce the mortality from colorectal cancer.
CONCLUSION
Analysis of a single institution prospectively collected cancer registry demonstrated that a combined mechanical and oral antibiotic bowel preparation is independently associated with improved 5-year recurrence free-survival in patients undergoing CRC.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Funding / Acknowledgments
There are no funding sources
Footnotes
This investigation was presented as an oral presentation at the 2020 Virtual American College of Surgeons Clinical Congress Annual Meeting
Disclosures: Authors of this manuscript report no disclosures.
Conflict of interest: The authors report no conflicts of interest.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Jeffery M, Hickey BE, Hider PN. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane database Syst Rev. 2007;(1):CD002200. doi: 10.1002/14651858.CD002200.pub2 [DOI] [PubMed] [Google Scholar]
- 3.Ryuk JP, Choi G-S, Park JS, et al. Predictive factors and the prognosis of recurrence of colorectal cancer within 2 years after curative resection. Ann Surg Treat Res. 2014;86(3):143–151. doi: 10.4174/astr.2014.86.3.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stakelum A, Zaborowski A, Collins D, Winter DC. The influence of the gastrointestinal microbiome on colorectal metastasis: a narrative review. Colorectal Dis. 2020;22(9):1101–1107. doi: 10.1111/codi.14930 [DOI] [PubMed] [Google Scholar]
- 5.Gaines S, van Praagh JB, Williamson AJ, et al. Western Diet Promotes Intestinal Colonization by Collagenolytic Microbes and Promotes Tumor Formation After Colorectal Surgery. Gastroenterology. October 2019. doi: 10.1053/j.gastro.2019.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinross J, Mirnezami R, Alexander J, et al. A prospective analysis of mucosal microbiome-metabonome interactions in colorectal cancer using a combined MAS 1HNMR and metataxonomic strategy. Sci Rep. 2017;7(1):8979. doi: 10.1038/s41598-017-08150-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lauka L, Reitano E, Carra MC, et al. Role of the intestinal microbiome in colorectal cancer surgery outcomes. World J Surg Oncol. 2019;17(1):204. doi: 10.1186/s12957-019-1754-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohseni AH, Taghinezhad-S S, Fu X. Gut microbiota-derived metabolites and colorectal cancer: New insights and updates. Microb Pathog. October 2020:104569. doi: 10.1016/j.micpath.2020.104569 [DOI] [PubMed] [Google Scholar]
- 9.Vigneswaran J, Shogan BD. The Role of the Intestinal Microbiome on Colorectal Cancer Pathogenesis and its Recurrence Following Surgery. J Gastrointest Surg. 2020;24(10):2349–2356. doi: 10.1007/s11605-020-04694-4 [DOI] [PubMed] [Google Scholar]
- 10.Tohme S, Simmons RL, Tsung A. Surgery for Cancer: A Trigger for Metastases. Cancer Res. 2017;77(7):1548–1552. doi: 10.1158/0008-5472.CAN-16-1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hale VL, Chen J, Johnson S, et al. Shifts in the Fecal Microbiota Associated with Adenomatous Polyps. Cancer Epidemiol Biomarkers Prev. 2017;26(1):85–94. doi: 10.1158/1055-9965.EPI-16-0337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kluger Y, Galili Y, Yossiphov J, Shnaper A, Goldman G, Rabau M. Model of implantation of tumor cells simulating recurrence in colonic anastomosis in mice. Dis Colon Rectum. 1998;41(12):1506–1510. http://www.ncbi.nlm.nih.gov/pubmed/9860330. [DOI] [PubMed] [Google Scholar]
- 13.Umpleby HC, Fermor B, Symes MO, Williamson RC. Viability of exfoliated colorectal carcinoma cells. Br J Surg. 1984;71(9):659–663. http://www.ncbi.nlm.nih.gov/pubmed/6478151. [DOI] [PubMed] [Google Scholar]
- 14.Shogan BD, Smith DP, Christley S, Gilbert JA, Zaborina O, Alverdy JC. Intestinal anastomotic injury alters spatially defined microbiome composition and function. Microbiome. 2014;2:35. doi: 10.1186/2049-2618-2-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shogan BD, Belogortseva N, Luong PM, et al. Collagen degradation and MMP9 activation by Enterococcus faecalis contribute to intestinal anastomotic leak. Sci Transl Med. 2015;7(286):286ra68. doi: 10.1126/scitranslmed.3010658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaines S, van Praagh JB, Williamson AJ, et al. Western Diet Promotes Intestinal Colonization by Collagenolytic Microbes and Promotes Tumor Formation Following Colorectal Surgery. Gastroenterology. October 2019. doi: 10.1053/j.gastro.2019.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobson RA, Wienholts K, Williamson AJ, et al. Enterococcus faecalis exploits the human fibrinolytic system to drive excess collagenolysis: implications in gut healing and identification of druggable targets. Am J Physiol Gastrointest Liver Physiol. 2020;318(1):G1–G9. doi: 10.1152/ajpgi.00236.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang JL, Seetoo D q, Wang Y, et al. Urokinase-type plasminogen activator and its receptor in colorectal cancer: independent prognostic factors of metastasis and cancer-specific survival and potential therapeutic targets. Int J cancer. 2000;89(5):431–439. doi: [DOI] [PubMed] [Google Scholar]
- 19.Nagata N, Tohya M, Fukuda S, et al. Effects of bowel preparation on the human gut microbiome and metabolome. Sci Rep. 2019;9(1):4042. doi: 10.1038/s41598-019-40182-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drago L, Toscano M, De Grandi R, Casini V, Pace F. Persisting changes of intestinal microbiota after bowel lavage and colonoscopy. Eur J Gastroenterol Hepatol. 2016;28(5):532–537. doi: 10.1097/MEG.0000000000000581 [DOI] [PubMed] [Google Scholar]
- 21.Watanabe M, Murakami M, Nakao K, Asahara T, Nomoto K, Tsunoda A. Randomized clinical trial of the influence of mechanical bowel preparation on faecal microflora in patients undergoing colonic cancer resection. Br J Surg. 2010;97(12):1791–1797. doi: 10.1002/bjs.7253 [DOI] [PubMed] [Google Scholar]
- 22.Long X, Wong CC, Tong L, et al. Peptostreptococcus anaerobius promotes colorectal carcinogenesis and modulates tumour immunity. Nat Microbiol. 2019;4(12):2319–2330. doi: 10.1038/s41564-019-0541-3 [DOI] [PubMed] [Google Scholar]
- 23.Sayfan J, Averbuch F, Koltun L, Benyamin N. Effect of rectal stump washout on the presence of free malignant cells in the rectum during anterior resection for rectal cancer. Dis Colon Rectum. 2000;43(12):1710–1712. doi: 10.1007/BF02236855 [DOI] [PubMed] [Google Scholar]
- 24.Okoshi K, Kono E, Tomizawa Y, Kinoshita K. Can rectal washout reduce anastomotic recurrence after anterior resection for rectal cancer? A review of the literature. Surg Today. 2020;50(7):644–649. doi: 10.1007/s00595-019-01825-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


