Abstract
Background
After the global spread of SARS-CoV-2, research has highlighted several aspects of the pandemic, focusing on clinical features and risk factors associated with infection and disease severity. However, emerging results on the role of smoking in SARS-CoV-2 infection susceptibility or COVID-19 outcomes are conflicting, and their robustness remains uncertain.
Objective
In this context, this study aims at quantifying the proportion of SARS-CoV-2 antibody seroprevalence, studying the changes in antibody levels over time, and analyzing the association between the biochemically verified smoking status and SARS-CoV-2 infection.
Methods
The research design involves a 6-month prospective cohort study with a serial sampling of the same individuals. Each participant will be surveyed about their demographics and COVID-19–related information, and blood sampling will be collected upon recruitment and at specified follow-up time points (ie, after 8 and 24 weeks). Blood samples will be screened for the presence of SARS-CoV-2–specific antibodies and serum cotinine, being the latter of the principal metabolite of nicotine, which will be used to assess participants’ smoking status.
Results
The study is ongoing. It aims to find a higher antibody prevalence in individuals at high risk for viral exposure (ie, health care personnel) and to refine current estimates on the association between smoking status and SARS-CoV-2/COVID-19.
Conclusions
The added value of this research is that the current smoking status of the population to be studied will be biochemically verified to avoid the bias associated with self-reported smoking status. As such, the results from this survey may provide an actionable metric to study the role of smoking in SARS-CoV-2 infection and COVID-19 outcomes, and therefore to implement the most appropriate public health measures to control the pandemic. Results may also serve as a reference for future clinical research, and the methodology could be exploited in public health sectors and policies.
International Registered Report Identifier (IRRID)
DERR1-10.2196/32285
Keywords: antibody persistence, cotinine, COVID-19, SARS-CoV-2, seroprevalence, smoking impact, smoking status
Introduction
Overview
SARS-CoV-2 is the novel coronavirus strain that was first reported as a cluster of viral pneumonia cases of unknown etiology in Wuhan, the capital city of Hubei Province in China, on December 31, 2019. The spreading of SARS-CoV-2 reached pandemic proportion in March 2020, and as of April 2021, more than 141 million cases had been confirmed, and more than 3 million fatalities had occurred worldwide [1].
In late February 2020, the first nonimported cases of COVID-19 were identified in Italy. Since then, SARS-CoV-2 spread rapidly to the community, as reported by national health authorities [2]. On May 23, 2020, at the time of project proposal design, the Italian Ministry of Health reported that, of the 229,327 people who had contracted the virus, 57,752 were still positive, of whom 8695 (15%) were hospitalized with symptoms; 572 (1.0%) were admitted in intensive care units (ICUs); and the remaining 48,485 (84%) were self-isolating at home; 32,735 (14.3%) had died, and 138,840 (60.5%) healed on a total of 2,164,426 tested cases [3]. As of April 2021, 3,870,131 cases and 116,927 deaths have been recorded in the country [4].
Since the beginning of the pandemic, the global scientific community was engaged in intense research efforts to understand all aspects of this public health crisis, from the clinical features of the disease and risk factors for adverse outcome, the patterns of viral spread in the population, the role of asymptomatic or subclinical cases in human-to-human transmission, and the serological response. The clinical features of COVID-19 range from a self-limited flu-like syndrome to progressive lung involvement with respiratory failure and widespread systemic effects [5-7]. Epidemiological surveillance has primarily focused on hospitalized patients with severe disease, and as such, the full spectrum of the disease, including the extent and proportion of mild or asymptomatic infections, is less clear. Evidence suggests that asymptomatic or oligosymptomatic infection is not uncommon [8-11]. A recent review reported that at least one third of SARS-CoV-2 infections are asymptomatic and that almost three quarters of persons who are asymptomatic at the time of a positive polymerase chain reaction (PCR) test result will remain asymptomatic [12]. Remarkably, sequelae of previous viral pneumonia have been reported in chest computed tomography scans of asymptomatic individuals [13], and SARS-CoV-2 transmission from asymptomatic cases to others has been documented [13,14].
In this frame, a seroprevalence study is an ideal attempt for measuring the true infection rates in general or specific populations [11,15,16]. In Italy, a nationwide survey was conducted by the National Institute of Statistics, reporting an IgG seroprevalence of 2.5% [17]. However, there is limited use of seroprevalence studies to retrospectively identify potential predictors of infection susceptibility and disease severity, both in the general population and in specific subgroups (eg, smokers, pediatric populations, or older adults).
Several risk factors for severe COVID-19 have been identified, including cardiovascular disease, diabetes, obesity, and chronic obstructive pulmonary disease [18-21]. Intuitively, one important additional risk factor is expected to be cigarette smoking. Smokers have a higher risk for developing viral and bacterial respiratory infections [22-24], being five times more likely to have influenza and twice more likely to develop pneumonia [25].
However, the role of smoking in SARS-CoV-2 infection susceptibility and COVID-19 outcomes is still unclear. Although there appears to be a higher risk for ICU admission and adverse outcomes [26-28], it has been reported that the prevalence of smoking among hospitalized COVID-19 patients is far lower than would be expected based on population smoking prevalence [29-32]. These findings were initially derived from Chinese case series, and although it is possible that the prevalence of smokers in the Chinese case series may be underrepresented due to inaccurate recording of their smoking status, similar findings have been reported in France [30], Germany [33], Italy [34], and the United States [32,35].
It is not clear how far the underrepresentation of smokers among COVID-19 inpatients reflects problems with poor reporting of the smoking status. Given the challenging circumstances of the pandemic, recall or reporting bias cannot be excluded. The possibility for inaccurate recording, false reporting, or underreporting of the smoking status due to the challenging situations at wards/ICUs with work overloads and operating in a persistent state of emergency should not be underestimated. Improving the quality of clinical and behavioral data mandates the need for an accurate and dedicated recording of the smoking status. Alternatively, population-level data collected outside of hospital settings are required. Another important limitation is that most of the observations were unadjusted for smoking-related comorbidities, which are known to be associated with higher risk for an adverse outcome in patients with COVID-19 [18]. Lack of adjustment for relevant confounders means it is not possible to disentangle the effect of smoking. Addressing all these limitations is important for evaluating clinical risk, developing clear public health messages, and identifying targets for intervention.
Research Objectives
Surveillance of antibody seropositivity in a population can allow inferences to be made about the cumulative incidence of infection in the population. Additionally, little is currently known about antibody kinetics. Asymptomatic infected persons may clear the virus more quickly than do symptomatic patients, and antibody titers in the former are likely to be lower, if they seroconvert at all, than in infected symptomatic patients [11,36]. Furthermore, understanding the association between smoking and SARS-CoV-2 infection susceptibility or COVID-19 outcomes is generally limited and of poor quality.
To summarize, there is a need for robust population-based evidence on the association of smoking with SARS-CoV-2 infection and COVID-19 outcomes, adjusting for potential confounding variables (eg, sociodemographic characteristics, key worker status, and comorbid health conditions), and a population seroprevalence study could be useful for this goal. The following key research questions will be addressed:
Does smoking increase susceptibility to SARS-CoV-2 infection?
Does smoking increase susceptibility to COVID-19 outcomes?
Does smoking affect the serological response after SARS-CoV-2 infection?
Research Proposal
We propose a 6-month prospective study using in combination a random population sample (taken from residents of the town of Troina; the town with the highest prevalence of positive SARS-CoV-2 cases in Sicily at the time of drafting the protocol in March-May 2020) and a convenience sample (taken from staff of the Troina’s main health care establishment, reported to have high infectious levels in the same time period) to investigate the prevalence of past infection, as determined by seropositivity (anti–SARS-CoV-2–specific IgG by enzyme-linked immunosorbent assay [ELISA]). The biochemically verified smoking status of the study population (ie, serum cotinine) will be correlated with serological data and COVID-19 outcomes (ie, clinical symptoms and hospitalization).
Study Aim
Epidemiological exposure data and venous blood (for measurements of anti–SARS-CoV-2–specific IgG and serum cotinine levels) will be systematically collected. Demographic, medical history, and epidemiological exposure data will be recorded from specifically designed questionnaires (for COVID-19 outcomes, relevant comorbidities, and smoking status) and shared rapidly in a format that can be easily aggregated, tabulated, and analyzed across many different regional (or national and international) settings for timely estimates of COVID-19 virus infection and its immunologic response rates according to the smoking status, and to inform public health responses and policy decisions.
Methods
Specific Aims
In summary, the main objectives of the study will be to:
Quantify the proportion of SARS-CoV-2–infected people (by serology assessment of anti–SARS-CoV-2 IgG levels) in a random population sample at baseline
Quantify the proportion of SARS-CoV-2–infected people (by serology assessment of anti–SARS-CoV-2 IgG levels) in a convenience sample (hospital staff) at baseline
Quantify the proportion of participants with asymptomatic SARS-CoV-2 infection
Quantify the proportion of mildly-to-moderately symptomatic (management at home) COVID-19 cases among all participants
Quantify the proportion of severely symptomatic (management at hospital) COVID-19 cases among all participants
Quantify the proportion of biochemically verified (by serum cotinine) current, former, and never smokers among all participants (random population sample + convenience sample)
-
Quantify the proportion and analyze the association between smoking status (current, former, and never smokers) and:
SARS-CoV-2 infection (asymptomatic and symptomatic combined)
Asymptomatic SARS-CoV-2 infection
Mild-to-moderate COVID-19 (management at home)
Severe COVID-19 (hospitalization needed)
Record relevant clinical confounders known to be associated with COVID-19 outcomes
Compare anti–SARS-CoV-2 IgG levels at baseline among current, former, and never smokers
Monitor and compare anti–SARS-CoV-2 IgG levels changes from baseline to 2 months and 6 months among current, former, and never smokers.
Study Design
This study will investigate the association between biochemically verified smoking status of the study population and serological data as well as COVID-19 outcomes (ie, clinical symptoms and hospitalization). The research design involves a 6-month prospective multiple cohort study with serial sampling of the same individuals each time. Sampling will be commenced in July and repeated at 8 weeks (Figure 1). A final follow-up visit will be carried out at 24 weeks.
Figure 1.
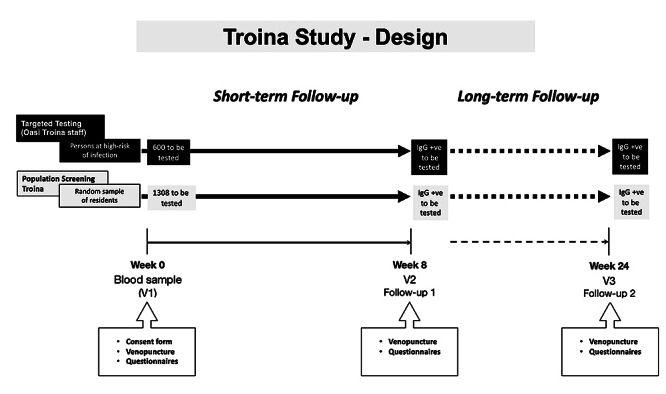
The Troina Study: design.
Study Population and Setting
Within the geographic scope of the study, high incidence of positive cases was identified in the general population of Troina, a town of around 9000 inhabitants in the province of Enna, in the center of Sicily. This town was hit hardest in terms of COVID-19 cases during the first wave in Italy [2-4], with hundreds of cases registered in the first epidemic weeks in March 2020 [37,38], and was declared a red zone on March 29, 2020, with the enforcement of lockdown retractions in that area [38]. The study population will consist of a population-based, age-stratified cohort in Troina that will be sampled through random selection of town residents. Identification and recruitment of participants will expand over different age groups to determine and compare age-specific attack rates. For logistic reasons, specimen and data collection will be performed at a single location, asking participants to travel to that location to participate in the study. Targeted testing will also be extended to a convenience sample consisting of about 600 staff members of Troina’s main health care establishment (high-risk individuals).
Eligibility Criteria
Any individual identified for recruitment, irrespective of age, can participate. Exclusion criteria will be refusal to provide informed consent or contraindication to venipuncture. Suspected or confirmed active/acute or prior SARS-CoV-2 infection should not be considered as an exclusion criterion for this investigation. Doing so would underestimate the extent of infection in the population. For individuals currently receiving medical care for COVID-19 infection, a family member or proxy may be used to complete the questionnaire on their behalf.
Smoking Status Definition
Current smokers will be defined as those who report that they smoke and have serum cotinine levels ≥20 ng/mL. Former smokers will be defined as those who report that they used to smoke in the past but not now and have serum cotinine levels <20 ng/mL. Never smokers will be defined as those who report that they never smoked in the past and have serum cotinine levels <20 ng/mL.
Data Collection
Each participant recruited into the study will be asked to complete a questionnaire that will record the following information: demographics, information about known COVID-19 and relevant clinical course, comorbidities, and smoking status.
Specimen Collection
A small amount of blood (10 mL) will be collected from each participant upon recruitment (T0) and at specified follow-up time points (T8wks, T24wks).
Specimen Transport and Biobanking
For each biological sample collected, the time of collection, the conditions for transportation, and the time of arrival at the study laboratory will be recorded. Specimens should reach the laboratory as soon as possible after collection. Serum should be separated from whole blood and stored at –20 °C or lower and shipped on dry ice. A biobanking facility will be established in Troina.
Sample Storage
Prior to testing, serum samples will be stored at –80 °C at the reference biobanking facility. It is recommended to aliquot samples prior to freezing to minimize freeze thaw cycles.
Serological Testing
Serologic assays of high sensitivity and specificity for SARS-CoV-2 have been recently validated and published. Serum samples will be screened for the presence of SARS-CoV-2–specific antibodies using a quantitative ELISA test for anti–SARS-CoV-2 IgG (Euroimmun, CND W0105040619) [39].
Serum samples will be stored at –80 °C until use, and the assay will be performed according to the manufacturer’s protocol. The neutralization capability, specificity, and sensitivity of the test have been thoroughly investigated and published together with the assay validation [40]. Reagent wells of the assays are coated with recombinant structural protein (S1 domain) of SARS-CoV-2. The optical density (OD) will be detected at 450 nm, and a ratio of the reading of each sample to the reading of the calibrator, included in the kit, will be calculated for each sample (OD ratio). The cutoff value for IgG OD ratio is 0.3. Following blocking, diluted serum (1:100 or 2-fold serially diluted for titers) will be added and incubated at 37 °C for 1 hour in the 96-well microtiter ELISA plates. Antigen-specific antibodies will be detected using peroxidase-labeled rabbit antihuman IgG and TMB as a substrate. The absorbance of each sample will be measured at 450 nm. Laboratory procedures involving sample manipulation must be carried out in a biosafety cabinet.
Cotinine Assay
About 1mL of serum will be pipetted into 10 ml tubes and 100 ng/mL of Ortho-cotinine used as internal standard will be added. About 50 µL of 0.1 M aqueous sodium hydroxide solution will then be added to the culture tube followed by 325 µL of chloroform. The tube will be secured with cap and vortex mixed for ~3 minutes (using VX 2500 Multi Tube Vortex Mixer) and centrifuged for ~4 minutes (in Beckmann Allegra centrifuge) at 2500 rpm. Using a glass Pasteur Pipette, the top aqueous layer will be removed and discarded into the hazardous waste container and the organic layer will remain in the tube. About ~100 mg (0.1 g) of anhydrous sodium sulfate will be added to the organic layer and allowed to rest for ~3 minutes (which will allow the sodium sulfate to absorb any water that may be present in the organic layer). In the end, the clear organic layer (with no water) will be carefully removed (without disturbing the settled sodium sulfate), concentrated to ~100 µL vial insert and placed in a gas chromatography (GC) vial. The concentrated sample will be capped and arranged on to an auto sampler tray for GC injection. One microliter (µL) of each sample will be injected into HP-5 Capillary GC Column (0.32 mm ID, 25 m length, and 0.52 µm film thickness; bonded 5% phenyl; and 95% dimethylpolysiloxane) of GC–nitrogen phosphorous detector. The inlet temperature will be 250 ˚C at split-less mode. The initial oven temperature will be 70 ˚C with a 1-minute hold and then increased to 230 ˚C at the rate of 25 ˚C per minute. Every batch of samples will be run with 6 calibration levels (20, 50, 100, 200, 400, 600 ng/mL), 4 quality controls (20, 100, 400, 600 ng/mL), and 1 blank control for accurate quantification. The amount of cotinine will be reported in ng/mL. The limit of quantification of cotinine is 20 ng/mL.
Statistical Plan
Estimates of margin of error as a function of seroprevalence are low for 300 samples. We will be aiming for >1000 samples of a representative sample of the population by gender and age groups (0-17 years, 18-65 years, and 66 years and older).
For the enrollment of participants into the study, the following inclusion and exclusion criteria need to be fulfilled:
-
Inclusion in population criteria:
Aged into the aforementioned groups
Living in the town of interest
Willing to participate in the baseline and to be recontacted for follow-up waves
-
Exclusion from population criteria:
Persons belonging to the institutionalized population
-
Exclusion from sample (censoring) criteria:
Moved to another village during the study period
Those stating explicitly their wish not to be recontacted for this study
To correctly represent the population involved, the age groups of interest have been assigned to three different categories by gender in terms of population size. A corresponding targeted sample size for this study is specified for each category.
In the entire population, we estimated several sample sizes depending on the margin of error equal to 0.03, 0.04, or 0.05 for estimate proportions of the sampled population, as shown in Table 1.
Table 1.
The Troina Study: sample size calculation.
| Age groups and gender | Participants, n | Margin of error 3%a | Margin of error 4%b | Margin of error 5%c | |||||
| 0-17 years | |||||||||
|
|
Male | 694 | 100 | 47 | 29 | ||||
|
|
Female | 648 | 93 | 44 | 27 | ||||
|
|
Total | 1342 | 193 | 90 | 57 | ||||
| 18-65 years | |||||||||
|
|
Male | 2751 | 396 | 185 | 116 | ||||
|
|
Female | 2803 | 403 | 189 | 118 | ||||
|
|
Total | 5554 | 799 | 374 | 235 | ||||
| >65 years | |||||||||
|
|
Male | 961 | 138 | 65 | 41 | ||||
|
|
Female | 1237 | 178 | 83 | 52 | ||||
|
|
Total | 2198 | 316 | 148 | 93 | ||||
| Total | |||||||||
|
|
Male | 4406 | 634 | 297 | 186 | ||||
|
|
Female | 4688 | 674 | 316 | 198 | ||||
|
|
Total | 9094 | 1308 | 613 | 384 | ||||
a(N * 1308) / total (N=9094) = n.
b(N * 613) / total (N=9094) = n.
c(N * 384) / total (N=9094) = n.
We will draw a multilayered sample with a confidence level of 0.97% to minimize the margin of error to 3% and ensure the best reliability of the sample data. The planned total sample size comprises up to 1308 participants at the recruitment stage. The attrition rate is estimated at 10%.
Information will be collected in a standardized format according to the questionnaires and tools in the protocol. The data shared should include only the study identification number and not any personal identifiable information. We will report the following information:
The number of households and the number of individuals included
The age and sex of all individuals included
The antibody levels in the sample of all individuals included
The number of individuals with serologic evidence of COVID-19 virus infection (stratified by age)
The number of individuals with serologic evidence of COVID-19 virus infection who have reported symptoms
The number of individuals with known previous SARS-CoV-2 infection who were hospitalized or recovered at home
The number of individuals with serologic evidence of COVID-19 virus infection who are current, former, and never smokers (biochemically verified by serum cotinine assay)
Sociodemographic and baseline characteristics will be summarized for the Troina population, for the convenience sample, and for the total sample recruited. Categorical variables will be reported as numbers and proportions with 95% CIs. Between-group comparisons will be carried out using chi-square testing or Fisher exact test, as appropriate. Continuous variables will be reported as means and SDs, and as medians and IQRs. Between-group comparisons will be carried out using tests like analysis of variance, Mann-Whitney U test, or Wilcoxon signed rank test, as appropriate. The proportions of patients developing a positive PCR test for SARS-CoV-2 result over the course of the study will be reported as numbers and proportions with 95% CIs, separated by subgroups identifying those with symptomatic or asymptomatic infection. Data on change in antibody levels from baseline to follow-up will be presented for the whole recruited population. Statistical significance of change will be estimated using repeated-measures t testing. Correlation between smoking status and the risk of COVID-19 infection will be tested using a 3 × 2 chi-square test of independence. A P value ≤.05 will be considered to represent the threshold of statistical significance for all comparisons.
Data Collection Procedures
To accomplish the specific aims of the project, data will be stored in a database system after each completed collection step and transferred to central data management for further data processing and merging. Each participant will be assigned a unique identification code consisting of a number identifier that will be used for data merging. After data collection, data validation checks will be performed as agreed in a data validation plan, and data cleaning procedures will be used as applicable to ensure the best achievable quality of data for analysis purposes. Only the study staff working with the fieldwork provider will be able to identify the participants based on the identification codes. Only deidentified data will be transferred from the fieldwork provider to the central data management. All electronic data files will be kept on secure servers with backup processes in place. Personal data of participants must be strictly kept separately from study data and is accessed only by authorized staff for the purposes of the study conduct. Before starting data collection, the involved staff will receive training on the background and objectives of this study, on eligibility criteria, on the participant selection procedure, on ethical obligations, on completion and validation of the procedure, and on the data collection platform. Local fieldwork staff will be trained for each relevant data collection process and logistic-related procedure.
Eligible participants are informed about the study purpose, their requested tasks, time of involvement, data confidentiality, and data protection. Once they stated their willingness to participate, they will proceed with the screening and study enrollment. Enrolled participants have the right to stop their participation at any time without any penalty. Preferably, the reason for the premature end of participation will be recorded. Participants who drop out will not be replaced. Every effort will be made to protect participant confidentiality according to the General Data Protection Regulation.
Results
We expect to find a higher prevalence of antibodies in individuals at high risk for viral exposure (ie, health care personnel and other essential workers) according to previous evidence and to refine current estimates on the association between smoking status and SARS-CoV-2/COVID-19. A total of 1785 participants have been enrolled in the study. Data cleaning and analyses are ongoing.
Discussion
This project is the first population-based study that uses seroprevalence data and an objective assessment of the current smoking status to examine the association between smoking and SARS-CoV-2 infection susceptibility and severity. Additionally, the study will examine for the first time the magnitude of the seroconversion response in current smokers, compared to former and never smokers, and the changes in antibody titers over time according to the smoking status. Instead of focusing on hospitalized patients only, the study will also include participants who infected individuals who were either asymptomatic or had COVID-19 but were not hospitalized. It will also consider confounding factors in the association between smoking and SARS-CoV-2 that were not addressed in previous research. Finally, the results from this survey may serve as a reference to other contexts and provide an actionable metric to quantify and offer a clear overview of SARS-CoV-2 spread, and the methodology and findings can be exploited in public health research and policies to minimize the disease impact and implement the most appropriate prevention measures to protect susceptible populations.
Acknowledgments
The authors would like to thank Jas Mantero (European Centre for Disease Prevention and Control) and Mark Smolinski (Ending Pandemics) for the constructive feedback about the protocol, Alessia Visalli (Euroimmun Italia Srl) for the technical discussion about specificity and sensibility of anti–SARS-CoV-2 IgG, and Adrian Shalivari (Fullcro Srl, Roma, Italy) for the development of the electronic data capture system. The authors would also like to thank Concita Santoro, Annemarie Linder, the Mayor of Troina, Fabio Venezia, Chief of Staff of Protezione Civile, Alessandro Nasca, and all the volunteers of Protezione Civile for their help and assistance.
This investigator research protocol was sponsored by ECLAT SRL, a spin-off of the University of Catania, through a grant from the Foundation for a Smoke-Free World, a US nonprofit 501(c)(3) private foundation with a mission to end smoking in this generation. The foundation accepts charitable gifts from PMI Global Services Inc; under the Foundation’s Bylaws and Pledge Agreement with PMI Global Services Inc, the Foundation is independent from PMI Global Services Inc and the tobacco industry. The contents, selection, and presentation of facts, as well as any opinions expressed herein are the sole responsibility of the authors and under no circumstances shall be regarded as reflecting the positions of the Foundation for a Smoke-Free World, Inc. ECLAT SRL is a research-based company from the University of Catania that delivers solutions to global health problems with special emphasis on harm minimization and technological innovation.
Abbreviations
- ELISA
enzyme-linked immunosorbent assay
- GC
gas chromatography
- ICU
intensive care unit
- OD
optical density
- PCR
polymerase chain reaction
Footnotes
Authors' Contributions: RP conceived the study, contributed to its design, and drafted and finalized the manuscript. VT and GGC contributed to the design of the study, with particular input on analysis and interpretation of data. PF contributed to the design of the study and revised the manuscript. ACR contributed to the design of the study and will be involved in the project management to organize and coordinate the data collection process. SR contributed with input on analytical testing and will be involved in the project management to organize and coordinate the data collection process. DS contributed to the design of the study and will be involved in the project management and central coordination. F Caraci contributed to the design of the study and revised the manuscript. CR contributed to the design of the study and revised the manuscript. MT contributed with input on analytical testing. PZ contributed with input on analytical testing. JR contributed with input on analytical testing and revised the manuscript. MF contributed with input on analytical testing. JB contributed to the design of the study with particular input on analysis and interpretation of data. F Cibella contributed to the design of the study with particular input on analysis and interpretation of data. EI contributed to the design of the study and will be involved in the project management to organize and coordinate the data collection process. RF contributed to the design of the study and revised the manuscript.
Conflicts of Interest: RP is a full tenured professor of Internal Medicine at the University of Catania (Italy) and Medical Director of the Institute for Internal Medicine and Clinical Immunology at the same University. In relation to his recent work in the area of respiratory diseases, clinical immunology, and tobacco control, RP has received lecture fees and research funding from Pfizer, GlaxoSmithKline, CV Therapeutics, NeuroSearch A/S, Sandoz, MSD, Boehringer Ingelheim, Novartis, Duska Therapeutics, and Forest Laboratories. Lecture fees from a number of European EC industry and trade associations (including FIVAPE in France and FIESEL in Italy) were directly donated to vaper advocacy no-profit organizations. RP has also received grants from European Commission initiatives (U-BIOPRED and AIRPROM) and from the Integral Rheumatology & Immunology Specialists Network initiative. He has also served as a consultant for Pfizer; Global Health Alliance for Treatment of Tobacco Dependence; CV Therapeutics; Boehringer Ingelheim; Novartis; Duska Therapeutics; Electronic Cigarette Industry Trade Association, in the UK; Arbi Group Srl; Health Diplomats; and Sermo Inc. RP has served on the Medical and Scientific Advisory Board of Cordex Pharma, Inc; CV Therapeutics; Duska Therapeutics Inc; Pfizer; and PharmaCielo. RP is also founder of the Center for Tobacco Prevention and Treatment at the University of Catania and of the Center of Excellence for the Acceleration of Harm Reduction at the same university, which has received support from the Foundation for a Smoke-Free World to conduct 8 independent investigator-initiated research projects on harm reduction. RP is currently involved in a patent application concerning an app tracker for smoking behavior developed for ECLAT SRL. RP is also currently involved in the following pro bono activities: scientific advisor for Lega Italiana Anti Fumo (Italian for Italian Anti-Smoking League), the Consumer Advocates for Smoke-Free Alternatives, and the International Network of Nicotine Consumers Organizations; Chair of the European Technical Committee for Standardization on “Requirements and test methods for emissions of electronic cigarettes” (CEN/TC 437; WG4). JR receives research support from the Foundation for a Smoke-Free World, Philip Morris International, Altria, and JUUL Labs; consults with Revive Pharmaceuticals; and consults and has patent purchase agreements with Philip Morris International. SR does consulting work for ECLAT SRL. All other authors have no relevant conflicts of interest to declare in relation to this study.
References
- 1.World Health Organization WHO Coronavirus Disease (COVID-19) Dashboard. [2021-04-18]. https://covid19.who.int .
- 2.Istituto Superiore di Sanità Sorveglianza integrata COVID-19: i principali dati nazionali. EpiCentro. [2021-04-18]. https://www.epicentro.iss.it/coronavirus/sars-cov-2-sorveglianza-dati .
- 3.COVID-19 Italia Monitoraggio della situazione. Dipartimento Protezione Civile. [2021-04-18]. https://mappe.protezionecivile.gov.it/it/mappe-emergenze/mappe-coronavirus .
- 4.Balasco N, D'Alessandro V, Ferrara P, Smaldone G, Vitagliano L. Analysis of the time evolution of COVID-19 lethality during the first epidemic wave in Italy. Acta Biomed. 2021 May 12;92(2):e2021171. doi: 10.23750/abm.v92i2.11149. http://europepmc.org/abstract/MED/33988144 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. World Health Organization. [2020-04-18]. https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov .
- 6.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang F. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 Apr;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. http://europepmc.org/abstract/MED/32085846 .S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guzik T, Mohiddin S, Dimarco A, Patel V, Savvatis K, Marelli-Berg FM, Madhur MS, Tomaszewski M, Maffia P, D'Acquisto F, Nicklin SA, Marian AJ, Nosalski R, Murray EC, Guzik B, Berry C, Touyz RM, Kreutz R, Wang DW, Bhella D, Sagliocco O, Crea F, Thomson EC, McInnes IB. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020 Aug 01;116(10):1666–1687. doi: 10.1093/cvr/cvaa106. http://europepmc.org/abstract/MED/32352535 .5826160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu J, Ji P, Pang J, Zhong Z, Li H, He C, Zhang J, Zhao C. Clinical characteristics of 3062 COVID-19 patients: a meta-analysis. J Med Virol. 2020 Oct;92(10):1902–1914. doi: 10.1002/jmv.25884. http://europepmc.org/abstract/MED/32293716 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roxby AC, Greninger AL, Hatfield KM, Lynch JB, Dellit TH, James A, Taylor J, Page LC, Kimball A, Arons M, Schieve LA, Munanga A, Stone N, Jernigan JA, Reddy SC, Lewis J, Cohen SA, Jerome KR, Duchin JS, Neme S. Detection of SARS-CoV-2 among residents and staff members of an independent and assisted living community for older adults - Seattle, Washington, 2020. MMWR Morb Mortal Wkly Rep. 2020 Apr 10;69(14):416–418. doi: 10.15585/mmwr.mm6914e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Z, Song C, Xu C, Jin G, Chen Y, Xu X, Ma H, Chen W, Lin Y, Zheng Y, Wang J, Hu Z, Yi Y, Shen H. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020 May;63(5):706–711. doi: 10.1007/s11427-020-1661-4. http://europepmc.org/abstract/MED/32146694 .10.1007/s11427-020-1661-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Della Valle P, Fabbri M, Madotto F, Ferrara P, Cozzolino P, Calabretto E, D'Orso MI, Longhi E, Polosa R, Riva M, Mazzaglia G, Sommese C, Mantovani L, The Mustang-Occupation-Covid-Study Group Occupational exposure in the Lombardy Region (Italy) to SARS-CoV-2 infection: results from the MUSTANG-OCCUPATION-COVID-19 Study. Int J Environ Res Public Health. 2021 Mar 04;18(5):2567. doi: 10.3390/ijerph18052567. https://www.mdpi.com/resolver?pii=ijerph18052567 .ijerph18052567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oran DP, Topol EJ. The proportion of SARS-CoV-2 infections that are asymptomatic: a systematic review. Ann Intern Med. 2021 May;174(5):655–662. doi: 10.7326/M20-6976. https://www.acpjournals.org/doi/abs/10.7326/M20-6976?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, He Y, Tong J, Qin Y, Xie T, Li J, Li J, Xiang J, Cui Y, Higgs ES, Xiang J. Characterization of an asymptomatic cohort of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infected individuals outside of Wuhan, China. Clin Infect Dis. 2020 Nov 19;71(16):2132–2138. doi: 10.1093/cid/ciaa629. http://europepmc.org/abstract/MED/32442265 .5842166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang L, Zhang X, Zhang X, Wei Z, Zhang L, Xu J, Liang P, Xu Y, Zhang C, Xu A. Rapid asymptomatic transmission of COVID-19 during the incubation period demonstrating strong infectivity in a cluster of youngsters aged 16-23 years outside Wuhan and characteristics of young patients with COVID-19: A prospective contact-tracing study. J Infect. 2020 Jun;80(6):e1–e13. doi: 10.1016/j.jinf.2020.03.006. http://europepmc.org/abstract/MED/32283156 .S0163-4453(20)30117-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakurai A, Sasaki T, Kato S, Hayashi M, Tsuzuki S, Ishihara T, Iwata M, Morise Z, Doi Y. Natural history of asymptomatic SARS-CoV-2 infection. N Engl J Med. 2020 Aug 27;383(9):885–886. doi: 10.1056/NEJMc2013020. http://europepmc.org/abstract/MED/32530584 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hung IF, Cheng VC, Li X, Tam AR, Hung DL, Chiu KH, Yip CC, Cai J, Ho DT, Wong S, Leung SS, Chu M, Tang MO, Chen JH, Poon RW, Fung AY, Zhang RR, Yan EY, Chen L, Choi CY, Leung K, Chung TW, Lam SH, Lam TP, Chan JF, Chan K, Wu T, Ho P, Chan JW, Lau C, To KK, Yuen K. SARS-CoV-2 shedding and seroconversion among passengers quarantined after disembarking a cruise ship: a case series. Lancet Infect Dis. 2020 Sep;20(9):1051–1060. doi: 10.1016/S1473-3099(20)30364-9. http://europepmc.org/abstract/MED/32539986 .S1473-3099(20)30364-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Primi risultati dell'indagine di sieroprevalenza SARS-CoV-2. Istituto Nazionale di Statistica. [2021-04-18]. https://www.istat.it/it/files/2020/08/ReportPrimiRisultatiIndagineSiero.pdf .
- 18.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. http://europepmc.org/abstract/MED/32171076 .S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan W, Liang W, Zhao Y, Liang H, Chen Z, Li Y, Liu X, Chen R, Tang C, Wang T, Ou C, Li L, Chen P, Sang L, Wang W, Li J, Li C, Ou L, Cheng B, Xiong S, Ni Z, Xiang J, Hu Y, Liu L, Shan H, Lei C, Peng Y, Wei L, Liu Y, Hu Y, Peng P, Wang J, Liu J, Chen Z, Li G, Zheng Z, Qiu S, Luo J, Ye C, Zhu S, Cheng L, Ye F, Li S, Zheng J, Zhang N, Zhong N, He J, China Medical Treatment Expert Group for COVID-19 Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020 May;55(5):2000547. doi: 10.1183/13993003.00547-2020. http://erj.ersjournals.com/lookup/pmidlookup?view=long&pmid=32217650 .13993003.00547-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med. 2020 Jun 18;382(25):e102. doi: 10.1056/NEJMoa2007621. http://europepmc.org/abstract/MED/32356626 . [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Caci G, Albini A, Malerba M, Noonan DM, Pochetti P, Polosa R. COVID-19 and obesity: dangerous liaisons. J Clin Med. 2020 Aug 04;9(8):2511. doi: 10.3390/jcm9082511. https://www.mdpi.com/resolver?pii=jcm9082511 .jcm9082511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med. 2004 Nov 08;164(20):2206–16. doi: 10.1001/archinte.164.20.2206.164/20/2206 [DOI] [PubMed] [Google Scholar]
- 23.Stämpfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol. 2009 May;9(5):377–84. doi: 10.1038/nri2530.nri2530 [DOI] [PubMed] [Google Scholar]
- 24.Feldman C, Anderson R. Cigarette smoking and mechanisms of susceptibility to infections of the respiratory tract and other organ systems. J Infect. 2013 Sep;67(3):169–84. doi: 10.1016/j.jinf.2013.05.004.S0163-4453(13)00129-1 [DOI] [PubMed] [Google Scholar]
- 25.Hiding in plain sight: treating tobacco dependency in the NHS. Royal College of Physicians. 2018. [2021-04-18]. https://www.rcplondon.ac.uk/projects/outputs/hiding-plain-sight-treating-tobacco-dependency-nhs .
- 26.Vardavas C, Nikitara K. COVID-19 and smoking: a systematic review of the evidence. Tob Induc Dis. 2020;18:20. doi: 10.18332/tid/119324. http://europepmc.org/abstract/MED/32206052 .20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simons D, Shahab L, Brown J, Perski O. The association of smoking status with SARS-CoV-2 infection hospitalization and mortality from COVID-19: a living rapid evidence review. Qeios. 2020. [2021-04-18]. https://www.qeios.com/read/article/582 . [DOI] [PMC free article] [PubMed]
- 28.Liu W, Tao Z, Wang L, Yuan ML, Liu K, Zhou L, Wei S, Deng Y, Liu J, Liu HG, Yang M, Hu Y. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) 2020 May 05;133(9):1032–1038. doi: 10.1097/CM9.0000000000000775. http://europepmc.org/abstract/MED/32118640 .00029330-202005050-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19) Eur J Intern Med. 2020 May;75:107–108. doi: 10.1016/j.ejim.2020.03.014. http://europepmc.org/abstract/MED/32192856 .S0953-6205(20)30110-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyara M, Tubach F, Pourcher V, Morelot-Panzini C, Pernet J, Haroche J, Lebbah S, Morawiec E, Gorochov G, Caumes E, Hausfater P, Combes A, Similowski T, Amoura Z. Low incidence of daily active tobacco smoking in patients with symptomatic COVID-19. Qeios. 2021. [2021-04-18]. https://www.qeios.com/read/article/574 .
- 31.Farsalinos K, Barbouni A, Poulas K, Polosa R, Caponnetto P, Niaura R. Current smoking, former smoking, and adverse outcome among hospitalized COVID-19 patients: a systematic review and meta-analysis. Ther Adv Chronic Dis. 2020;11:2040622320935765. doi: 10.1177/2040622320935765. https://journals.sagepub.com/doi/10.1177/2040622320935765?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed .10.1177_2040622320935765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CDC COVID-19 Response Team Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 - United States, February 12-March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020 Apr 03;69(13):382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dreher M, Kersten A, Bickenbach J, Balfanz P, Hartmann B, Cornelissen C, Daher A, Stöhr R, Kleines M, Lemmen SW, Brokmann JC, Müller T, Müller-Wieland D, Marx G, Marx N. Charak- teristik von 50 hospitalisierten COVID-19-patienten mit und ohne ARDS. Dtsch Arztebl Int. 2020:271–278. doi: 10.3238/arztebl.2020.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaibazzi N, Tuttolomondo D, Guidorossi G, Botti A, Tedeschi A, Martini C, Mattioli M. Smoking prevalence is low in symptomatic patients admitted for COVID-19. medRxiv. doi: 10.1101/2020.05.05.20092015. Preprint posted online on June 13, 2020. [DOI] [Google Scholar]
- 35.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, Tobin KA, Cerfolio RJ, Francois F, Horwitz LI. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020 May 22;369:m1966. doi: 10.1136/bmj.m1966. http://www.bmj.com/lookup/pmidlookup?view=long&pmid=32444366 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Long Q, Tang X, Shi Q, Li Q, Deng H, Yuan J, Hu J, Xu W, Zhang Y, Lv F, Su K, Zhang F, Gong J, Wu B, Liu X, Li J, Qiu J, Chen J, Huang A. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020 Aug;26(8):1200–1204. doi: 10.1038/s41591-020-0965-6.10.1038/s41591-020-0965-6 [DOI] [PubMed] [Google Scholar]
- 37.Monitoraggio e mappatura dinamica dei contagi da COVID-19 in Sicilia. Regione Siciliana Dipartimento Protezione Civile. [2021-09-04]. https://www.protezionecivilesicilia.it/it/219-monitoraggio-e-mappatura-dinamica-dei-contagi-da-covid-19-nel-territorio-della-regione-sicilia.asp .
- 38.Ordinanza contingibile e urgente n. 12 del 29-03-2020. Regione Siciliana. [2021-09-04]. https://www.regione.sicilia.it/sites/default/files/2020-12/Ordinanza_n12_del_29032020.pdf .
- 39.Manufacturer protocol Anti-SARS-CoV-2 ELISA. EUROIMMUN Medizinische Labordiagnostika AG. [2021-09-04]. https://www.euroimmun.com .
- 40.Okba NM, Müller MA, Li W, Wang C, GeurtsvanKessel CH, Corman VM, Lamers MM, Sikkema RS, de Bruin E, Chandler FD, Yazdanpanah Y, Le Hingrat Q, Descamps D, Houhou-Fidouh N, Reusken CB, Bosch B, Drosten C, Koopmans MP, Haagmans BL. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg Infect Dis. 2020 Jul;26(7):1478–1488. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]


