Abstract
Objectives
To evaluate the effectiveness and tolerability of clobazam as an adjunctive treatment for adults with drug-resistant epilepsy.
Methods
We performed a single-center, retrospective chart review of patients aged ≥18 years with drug-resistant epilepsy who started clobazam between 2010 and 2018. Included patients had outpatient visits both before and ≥1 month after clobazam initiation. Epilepsy classification, seizure frequency before and after clobazam, duration of clobazam treatment, and adverse effects were analyzed.
Results
A total of 417 patients met the inclusion criteria. Mean age was 37.5 years, and 54% of patients were female. Patients were on a mean of 2.4 antiepileptic drugs at the time of initiation of clobazam. Epilepsy types were focal (56.8%), Lennox-Gastaut syndrome (LGS) (21.1%), generalized (15.1%), and unclassified (7.0%). At the first follow-up visit ≥1 month after clobazam initiation, 50.3% of patients had >50% reduction in seizure frequency, and 20.5% were seizure free. Of the initial cohort, 17.1% were followed >1 year and were seizure free at last follow-up. Response rates did not differ between different epilepsy classifications. Fifty-one percent of patients experienced ≥1 side effect, most commonly lethargy/fatigue (30.7%) or mood changes (10.8%). A total of 178 (42.6%) patients discontinued clobazam, most commonly due to adverse effects (55%).
Conclusions
Clobazam is effective and safe as a long-term adjunctive therapy for adults with drug-resistant epilepsy; efficacy in off-label use is similar to that in LGS.
Classification of Evidence
This study provides Class IV evidence that clobazam is an effective treatment for adults with drug-resistant epilepsy, independent of epilepsy classification.
Approximately one-third of patients with epilepsy have refractory disease, defined as continued seizures after trials of 2 antiepileptic drugs (AEDs) at therapeutic doses.1,2 Unfortunately, only 4% of patients with refractory epilepsy can expect seizure freedom with an additional AED.1 Clobazam, a long-acting benzodiazepine, is a medication that may prove to be both efficacious and tolerable in this treatment-resistant group. Clobazam has prolonged activity due to its active metabolite, N-desmethylclobazam, and, unlike typical 1,4-benzodiazepines, is a 1,5-benzodiazepine conferring increased specificity for the GABAA receptors with a2 rather than a1 subunits.3,4 As the a1 receptor is largely responsible for sedating effects of benzodiazepines, clobazam is therefore less sedating; this increased specificity has also been proposed to contribute to the lesser degree of tolerance seen with clobazam than with other benzodiazepines.5,6 Clobazam was approved by the United States Food and Drug Administration (FDA) in 2011 for treatment of Lennox-Gastaut syndrome (LGS).7 Since that time, there has been increasing off-label use of clobazam in adults with refractory epilepsy and anecdotal evidence that it may be more effective than other AEDs. Small studies performed outside of the United States have collected preliminary data that clobazam could be promising as adjunctive therapy.8,9
We report the results of a retrospective chart review at a single US academic epilepsy center characterizing the efficacy and tolerability of clobazam as an adjunctive agent for adults with drug-refractory epilepsy.
Methods
Patients
We conducted a single-center retrospective cohort study, which was approved by the Institutional Review Board of the University of Pennsylvania. Using Penn Data Store, the electronic medical records for all patients with outpatient visits at the Penn Epilepsy Center were queried for the presence of clobazam or Onfi in the medication history or active medication list. Inclusion criteria were a diagnosis of epilepsy of any type, age 18 years or older, at least 1 prescription for clobazam between January 1, 2010, and February 28, 2018, and at least 1 follow-up visit ≥1 month after clobazam initiation. Follow-up information was gathered through May 1, 2019. Patients were excluded if information in the clinical records was incomplete, if clobazam was not initiated despite being prescribed, if clobazam was initiated before establishing care at the Penn Epilepsy Center or before the study period, if the subject was not on standard daily dosing, if the subject underwent curative surgery, or if the subject had not yet had a follow-up visit after initiation of clobazam.
Patient charts were reviewed systematically for demographic and clinical data including age, sex, age at epilepsy onset, age at clobazam initiation, epilepsy etiology, seizure semiology, and seizure frequency as well as AED regimen, history of device implantation or surgical intervention, clobazam titration schedule, highest total daily dose, start date and length of use, reason for discontinuation, and treatment emergent adverse effects. Seizures were classified by mechanism of onset per International League Against Epilepsy guidelines.
All seizure types were included in the analyses. Study data were collected and managed using REDCap (Research Electronic Data Capture) tools hosted at the University of Pennsylvania.10
Standard Protocol Approvals, Registrations, and Patient Consents
This study was performed under a protocol approved by the University of Pennsylvania Internal Review Board (protocol number 827513).
Definitions
Clobazam start date was defined as the date of the first clobazam prescription with doses defined in mg/d. The clobazam dose for each patient was defined as the highest daily dose recorded within the observation period. Clobazam stop date was defined as the day the patient or caregiver was given a weaning schedule for drug discontinuation; this could have been done in person at an appointment or via telephone or online messaging contact with a provider. Reasons for discontinuation (efficacy, tolerability, or both) were based on notations in the electronic medical record. Observations were censored on May 1, 2019. Follow-up time was time from initiation of clobazam to either clobazam stop date or censor date. Baseline seizure frequency was collected from the office visit note on the day the patient initiated clobazam or the prior office visit closest to that date if clobazam was not first prescribed at an office visit. For the first follow-up visit at least 1 month after starting clobazam time point, patients were classified as seizure free if they had not seized since starting clobazam. For the final documented appointment at least 1 year after starting clobazam time point, patients were classified as seizure free if average seizure frequency was reported as <1 per year. Change in seizure frequency for between time points was calculated for duration of treatment. Tolerance was defined as either a shift to a higher category of seizure frequency (i.e., monthly to weekly) while on a stable clobazam dose or a 40% increase in clobazam dose to maintain the same seizure frequency after an initial reduction in seizure frequency on starting clobazam.
Statistical Analyses
The statistical analyses in this study were performed in Excel and R. Baseline and demographic statistics were summarized using descriptive statistics. Student t tests and Pearson χ2 tests were performed to compare groups. The Wilcoxon rank-sum test was performed to assess seizure frequency at the final follow-up visit compared with baseline for all patients still on clobazam.
Data Availability
Deidentified data not published within this article are available and will be shared on request from any qualified investigator.
Results
Characteristics of the Cohort
With the initial query of the electronic medical record, 659 patients were identified who had clobazam listed as a previous or current medication. A total of 242 patients were excluded; 89 were prescribed clobazam before establishment of care at the Penn Epilepsy Center, 26 were not followed by the Penn Epilepsy Center, 32 never filled the clobazam prescription, 94 did not have or had not yet had follow-up after starting clobazam, 6 were on clobazam since before age 18 years, 4 were not on a standard daily clobazam dosing (e.g., catamenial dosing), 4 had curative surgery, and 5 had incomplete information in the electronic medical record.
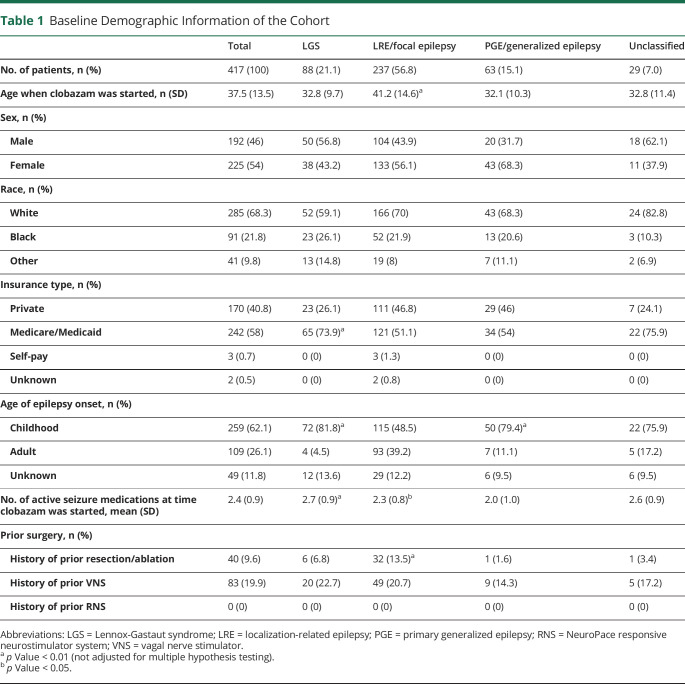
After the initial exclusion criteria were applied, there were 417 patients in the cohort whose characteristics are summarized in table 1. In addition, there were 25 patients who did not have clearly documented baseline seizure frequencies and were thus excluded from seizure frequency reporting but included in retention data. Three patients had unknown clobazam start and/or stop dates and were excluded from retention data but included in seizure frequency reporting. The initial cohort included 192 males and 225 females. Age at epilepsy onset was primarily in childhood, defined as less than 18 years (62.1%). Median age at clobazam initiation was 35 years (range 18–83 years).
Table 1.
Baseline Demographic Information of the Cohort
Epilepsy types were focal onset (56.8%), LGS (21.1%), generalized onset (15.1%), and unclassified (7.0%). Patients with LGS were on a higher number of AEDs at the time of clobazam initiation (2.7 vs 2.3, difference = 0.4, 95% confidence interval [CI] 0.2–0.7). They were also more likely to have childhood onset of epilepsy (OR 3.4, 95% CI 1.9–6.6) and were started on clobazam at a younger age (32.8 vs 38.7, difference = 5.9, 95% CI 3.4–8.5). Patients with generalized epilepsy were more likely to have childhood onset of epilepsy (OR 2.7, 95% CI 1.4–5.5) and were on a fewer number of AEDs at the time of clobazam initiation (2.0 vs 2.4, difference 0.4, 95% CI 0.2–0.7) (table 1).
The median clobazam dose after the initial titration period was 20 mg/d (mean 20.9 mg/d, SD = 13.7 mg/d, range 2.5–70 mg/d). Patients were taking a median of 2 other AEDs at the time of clobazam initiation (range 0–4). The median clobazam dose for patients who remained on the drug for at least 1 year was 30 mg/d (mean = 26.4 mg/d, SD = 14.3 mg/d, range 5–60 mg/d) Clobazam serum levels were not routinely assessed. Other baseline characteristics were similar between groups (table 1).
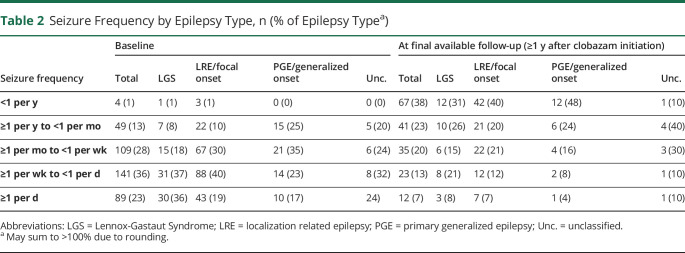
Baseline seizure frequency was collected for the entire cohort and separated by seizure type as outlined in table 2. Patients with LGS experienced the most severe seizure burden and were more likely to have frequency greater than once-daily seizures (OR 2.0, 95% CI 1.1–3.3) or once weekly (OR 2.1, 95% CI 1.2–3.7). Patients with primary generalized epilepsy (PGE) were less likely to have frequency greater than once weekly (OR 0.42, 95% CI 0.23–0.74) or once monthly (OR 0.39, 95% CI 0.19–0.81).
Table 2.
Seizure Frequency by Epilepsy Type, n (% of Epilepsy Typea)
Clobazam Efficacy
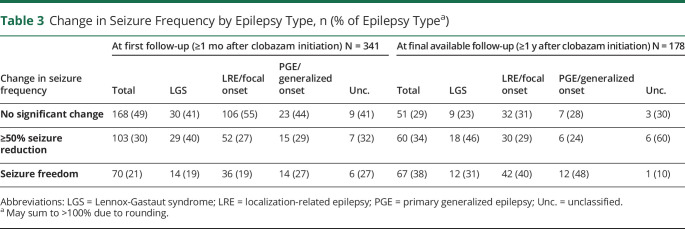
For the patients included in the seizure frequency arm of the study, 20.5% (95% CI 16.4%–25.3%, number needed to treat [NNT] = 5) reported seizure freedom at the first follow-up visit at least 1 month after starting clobazam. An additional 30.2% (95% CI 25.4%–35.4%, NNT = 3.3) experienced >50% reduction in their seizure frequency at this time point. At the final documented appointment in the study period, at least 1 year after starting clobazam, 178 patients (42.6%) remained on clobazam. Of the initial group (n = 417), 67 (19.6%, 95% CI 15.6%–24.4%, NNT = 5.1) were seizure free at the final available follow-up visit documented during the study period. Of the 70 patients who were seizure free at 1 month follow-up, 34 patients (49%, 95% CI 36.6%–60.7%) remained seizure free at 1-year follow-up.
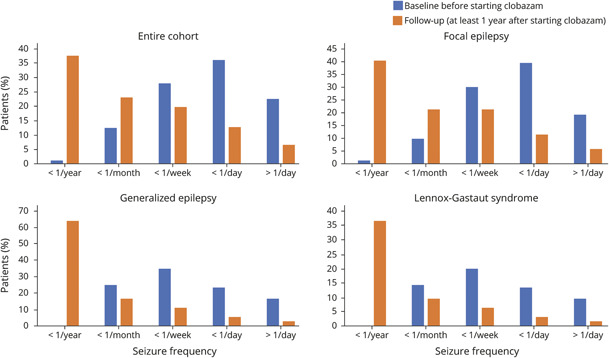
The median follow-up time was 2.1 years (interquartile range 1.1–4 years). The seizure frequency at the final follow-up visit was lower than it had been at baseline for all patients still on clobazam (W = 13,942, difference = 1.4 bins, 95% CI 1.2–1.6, Wilcoxon rank-sum test using frequency bins). These results held true for all subgroups including focal epilepsy (W = 4498, difference = 1.4 bins, 95% CI 1.2–1.7), PGE (W = 288, difference = 1.4 bins, 95% CI 0.8–1.9), and LGS (W = 659, difference = 1.5 bins, 95% CI 1.0–2.0) (figure 1). A subgroup analysis by epilepsy subgroup showed no statistically significant difference between epilepsy subgroups in clinical response to clobazam (tables 2 and 3).
Figure 1. Seizure Frequency Before and After Initiation of Clobazam.
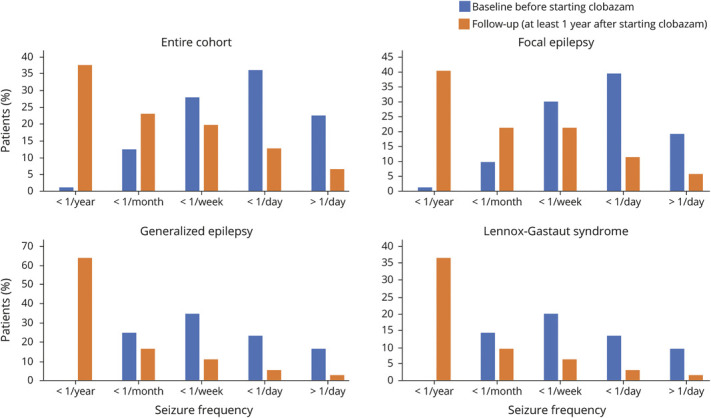
Seizure frequency at baseline (blue) and at final available follow-up appointment at least 1 year after clobazam initiation (orange).
Table 3.
Change in Seizure Frequency by Epilepsy Type, n (% of Epilepsy Typea)
Clobazam Tolerance
At the initial 1-month follow-up, 173 patients had experienced either a greater than 50% reduction in seizure frequency or achieved seizure freedom. Of these 173 patients, 20 patients (11.6%, 95% CI 7.4%–17.5%) required dose increase of greater than 40% to maintain their initial response to clobazam, and 16 patients (9.2%, 95% CI 5.5%–14.8%) experienced worsening seizure frequency. For those 34 patients who were seizure free at both 1-month and 1-year follow-up, 25 (73.5%, 95% CI 55.3%–86.5%) remained on the same or lower clobazam dose, and 9 (26.5%, 95% CI 13.5%–44.7%) required dose increase of greater than 40% to maintain seizure freedom. Of the 9 patients whose clobazam dose was increased, 7 patients were noted to have a dose increase of 5 mg. The median clobazam dose after the initial titration period at 1-month and 1-year follow-up was 10 mg/d (mean 16.8 mg/d, SD = 10.7 mg/d, range 5–40 mg/d) and 15 mg/d (mean 19.3 mg/d, SD = 11.2 mg/d, range 10–45 mg/d), respectively. Of the 70 patients who were seizure free at 1-month follow-up, 12 patients (17.1%) experienced recurrence of seizures, and 22 patients (31.4%) transitioned care or were lost to follow-up.
Clobazam Tolerability
Of the entire cohort of patients, 213 (51%) experienced at least 1 treatment-related adverse effect. The most common were lethargy and fatigue in 128 (30.7%), mood and behavioral changes in 45 (10.8%), dizziness and imbalance in 28 (6.7%), drooling in 9 (2.2%), and cognitive difficulties in 7 (e.g., memory and problem solving) (1.7%).
The probability of remaining on clobazam was 69.3% at 12 months. A total of 178 (42.7%) patients in the cohort discontinued clobazam during the study period. The most commonly cited reasons for discontinuation were treatment-related adverse effects (55%), poor efficacy (15%), and a combination of adverse effects and poor efficacy (21%). A majority of patients who discontinued clobazam did so soon after medication initiation. Of the group who discontinued clobazam, 53 (29.8%) discontinued in the first month after starting clobazam, and an additional 44 (24.7%) discontinued in the following 5 months (figure 2).
Figure 2. Percent of Patients of the Initial Cohort Remaining on Clobazam Over Time.
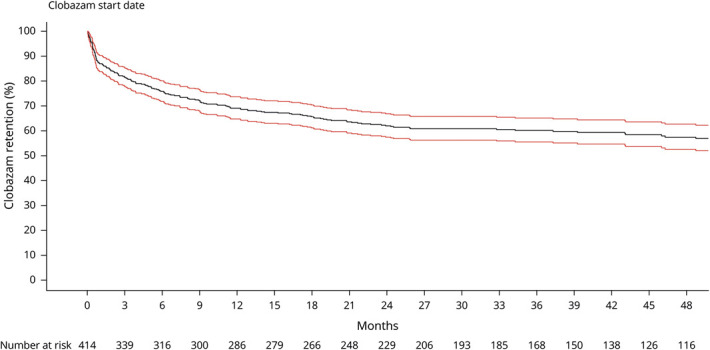
Kaplan-Meier curve for clobazam retention (solid line) with 95% confidence interval (red lines). Clobazam retention rates are approximately 80%, 70%, and 60% at the 3-month, 1-year, and 2-year marks, respectively. Few patients discontinued clobazam after the 2-year mark.
Discussion
The key observations from this open-label retrospective study are as follows: (1) an estimated 50.3% of adult patients with drug-refractory epilepsy experienced a ≥50% reduction in seizure frequency after adjunctive initiation of clobazam; (2) an estimated 17.1% achieved long-term seizure freedom; (3) there was no statistically significant difference in clobazam efficacy between focal-onset epilepsy, generalized-onset epilepsy, and LGS; and (4) clobazam was well tolerated with lethargy and fatigue and mood changes cited as the most common treatment-related adverse effects.
The efficacy and safety of clobazam as adjunctive therapy has previously been shown in patients aged 2–60 years with LGS. Clobazam was effective in reducing mean weekly drop seizure rates in these patients in a dose-dependent manner.7 It was approved by the FDA in 2011 in tablet and oral suspension form and in 2018 in oral film form for the adjunctive treatment of seizures associated with LGS in patients aged 2 years or older.11,12 This currently remains the only FDA-approved indication for use of clobazam in the United States, although clobazam is approved for adjunctive use in other seizure types in other countries including Canada, New Zealand, and Japan.13,14
It is important to note that the teratogenic risks of clobazam are uncertain, which is a concern for women of childbearing potential. One preliminary study raises concern that clobazam may have high malformation risks.15 In our study, 54% of patients were women, the majority of whom were of childbearing age. Patients with epilepsy who become pregnant should be encouraged to enroll in The North American Antiepileptic Drug Pregnancy Registry or the equivalent registry in their country of residence so that the risk of clobazam during pregnancy can be clarified.
Multiple other AEDs have been studied and approved by the FDA for adjunctive use in adults with refractory epilepsy. In randomized control trials comparing other AEDs to placebo, the frequency of a 50% responder rate in the intervention arms ranged from 20% to 43%. Seizure freedom rate in these trials typically approximated 5%,16–18 although trials of cenobamate have indicated higher seizure-free rates, notably 21% at the highest dose in the largest randomized trial to date.19 Although direct comparison is not possible in the absence of a placebo-controlled trial, our findings indicate that the addition of clobazam may result in high rates of seizure freedom (∼20%) in patients with intractable epilepsy.
Our study suggests that development of tolerance to clobazam does not occur for the majority of patients who respond to and remain on the drug for greater than 1 month. A standard definition for tolerance has not been outlined in previous literature. Most prior studies have used relapse in seizure frequency and/or increase in drug dose to sustain treatment response to define tolerance. Prior studies, using a variety of definitions, reported estimates of development of tolerance ranging from 0% to 86%, with a mean of 36%.20 A retrospective chart review, which defined tolerance as a relapse in seizure frequency despite constant clobazam dose, found a tolerance rate of 50% in patients with initial seizure reduction of greater than 75%.21 A later open-label study defined tolerance as a dosage increase of 40% plus loss of response and found a rate of tolerance of 12%.6 We broadly considered either a relapse in seizure frequency or dose increase to represent tolerance; our study found development of tolerance at 1 year in approximately one-fourth of patients, a lower rate than most prior studies, and a rate of discontinuation that is highest in the first month after initiation of clobazam.
This study is subject to limitations of a retrospective chart review. Seizure count data to establish seizure frequency and seizure freedom were based on patient self-report, which is inherently unreliable. Challenges in accurately quantifying seizure types such as absence and myoclonic seizures in patients with LGE and generalized epilepsy may also lead to inaccuracies in calculating seizure frequency. In addition, the postintervention follow-up period was not consistent between patients as next appointments were made at different intervals based on the individual patient and provider. All patients included in the study were on multiple AEDs; other AEDs were being adjusted while patients were on clobazam, meaning that clobazam dose changes were not made in isolation. Formal cognitive assessments were not obtained, and thus, objective cognitive side effects are unknown. Last, there was no control group for direct comparison.
Future directions to evaluate the efficacy of clobazam would include a prospective study or randomized control trial in adults with refractory epilepsy. This would allow for clarification of the time to reduced seizure burden or seizure freedom and evaluation of efficacy and tolerability at different doses. Further statistical analyses of a larger cohort may elucidate whether clobazam is more or less effective at specific doses or when combined with specific AEDs.
Clobazam, a long-acting benzodiazepine, has been used for treatment of refractory epilepsy and anxiety for the past decade. In the United States, clobazam is FDA approved only for the treatment of seizures associated with LGS, a disabling pediatric-onset epilepsy syndrome. However, in recent years, there has been increasing off-label use of clobazam in adults with refractory epilepsy and anecdotal report that it may be more effective than other AEDs in this population. In this retrospective chart review, we found clobazam to be effective in achieving seizure freedom and reducing seizure burden in adults with drug-refractory epilepsy, independent of epilepsy type. The drug was well tolerated by this population. Clobazam is an efficacious and safe long-term option as adjunctive therapy for adults with drug-refractory epilepsy. This study provides Class IV evidence that clobazam is an effective treatment for adults with drug-resistant epilepsy, independent of epilepsy classification.
TAKE-HOME POINTS
→ Over 50% of adults with drug-refractory epilepsy treated with adjunctive clobazam had notable seizure reduction.
→ Of patients maintained on clobazam, 17% achieved long-term seizure freedom.
→ Clobazam was similarly effective across all epilepsy classifications.
→ Clobazam was well tolerated overall; lethargy, fatigue, and mood changes were the most common adverse effects.
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Study Funding
No targeted funding reported.
Disclosure
M. Gelfand receives clinical trial support from Aquestive, Biogen, Cerevel, Eisai, Epilepsy Foundation, Engage Therapeutics, Livanova, Otsuka, SK Life Science, and UCB. K.A. Davis serves on advisory boards for Eisai and Eton Pharmaceuticals. She receives research funding from Eisai. A. Jamil, N. Levinson, C.E. Hill, and P. Khankhanian have no financial relationships to disclose. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med 2000;342:314–319. [DOI] [PubMed] [Google Scholar]
- 2.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010;51:1069–1077. [DOI] [PubMed] [Google Scholar]
- 3.Gauthier AC, Mattson RH. Clobazam: a safe, efficacious, and newly rediscovered therapeutic for epilepsy. CNS Neurosci Ther 2015;21:543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jensen HS, Nichol K, Lee D, Ebert B. Clobazam and its active metabolite ndesmethylclobazam display significantly greater affinities for α2- versus α1-GABAA–receptor complexes. PLos One 2014;9:e88456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sankar R. GABA(A) receptor physiology and its relationship to the mechanism of action of the 1,5-benzodiazepine clobazam. CNS Drugs 2012;26:229–244. [DOI] [PubMed] [Google Scholar]
- 6.Gidal BE, Wechsler RT, Sankar R, et al. Deconstructing tolerance with clobazam: post hoc analyses from an open-label extension study. Neurology 2016;87:1806–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng YT, Conry JA, Drummond R, Stolle J, Weinberg MA; OV-1012 Study Investigators. Randomized, phase III study results of clobazam in Lennox-Gastaut syndrome. Neurology 2011;77:1473–1481. [DOI] [PubMed] [Google Scholar]
- 8.Koeppen D, Baruzzi A, Capozza M, et al. Clobazam in therapy-resistant patients with partial epilepsy: a double-blind placebo-controlled crossover study. Epilepsia 1987;28:495–506. [DOI] [PubMed] [Google Scholar]
- 9.Montenegro MA, Cendes F, Noronha AL, et al. Efficacy of clobazam as add-on therapy in patients with refractory partial epilepsy. Epilepsia 2001;42:539–542. [DOI] [PubMed] [Google Scholar]
- 10.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.PubChem. Clobazam. Available at: pubchem.ncbi.nlm.nih.gov/compound/2789. Accessed February 7, 2020.
- 12.Drugs@FDA: FDA-approved drugs. Available at: accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&Appl No=210833. Accessed February 7, 2020.
- 13.Shimizu H, Kawasaki J, Yuasa S, Tarao Y, Kumagai S, Kanemoto K. Use of clobazam for the treatment of refractory complex partial seizures. Seizure 2003;12:282–286. [DOI] [PubMed] [Google Scholar]
- 14.Larrieu JL, Lagueny A, Ferrer X, Julien J. Epilepsy with continuous discharges during slow-wave sleep. Treatment with clobazam [in French]. Rev Electroencephalogr Neurophysiol Clin 1986;16:383–394. [DOI] [PubMed] [Google Scholar]
- 15.Thomas SV, Jose M, Divakaran S, Sankara Sarma P. Malformation risk of antiepileptic drug exposure during pregnancy in women with epilepsy: results from a pregnancy registry in South India. Epilepsia 2017;58:274–281. [DOI] [PubMed] [Google Scholar]
- 16.Shorvon SD, Löwenthal A, Janz D, Bielen E, Loiseau P. Multicenter double-blind, randomized, placebo-controlled trial of levetiracetam as add-on therapy in patients with refractory partial seizures. European Levetiracetam Study Group. Epilepsia 2000;41:1179–1186. [DOI] [PubMed] [Google Scholar]
- 17.Chung S, Sperling MR, Biton V, et al. Lacosamide as adjunctive therapy for partial-onset seizures: a randomized controlled trial. Epilepsia 2010;51:958–967. [DOI] [PubMed] [Google Scholar]
- 18.Topiramate as add-on therapy: pooled analysis of randomized controlled trials in adults—Reife—2000—Epilepsia—Wiley Online Library. Available at: onlinelibrary.wiley.com/doi/abs/10.1111/j.1528-1157.2000.tb02175.x. Accessed February 10, 2020. [DOI] [PubMed]
- 19.Krauss GL, Klein P, Brandt C, et al. Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: a multicentre, double-blind, randomised, placebo-controlled, dose-response trial. Lancet Neurol 2020;19:38–48. [DOI] [PubMed] [Google Scholar]
- 20.Robertson MM. Current status of the 1,4- and 1,5-benzodiazepines in the treatment of epilepsy: the place of clobazam. Epilepsia 1986;27(suppl 1):S27–S41. [DOI] [PubMed] [Google Scholar]
- 21.Singh A, Guberman AH, Boisvert D. Clobazam in long-term epilepsy treatment: sustained responders versus those developing tolerance. Epilepsia 1995;36:798–803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified data not published within this article are available and will be shared on request from any qualified investigator.