Abstract
Objective
Pediatric dystonic storm is an underrecognized entity. We aimed to evaluate the profiles of children presenting with dystonic storm in a referral hospital. Management schema and treatment responsiveness of this uncommonly reported entity were analyzed.
Methods
Retrospective review of all children (up to 18 years) hospitalized with dystonic storm over 39 months in the aforementioned facility.
Results
Twenty-three children whose ages ranged from 2 years 2 months to 14 years 4 months years (median: 6 years 11 months) (males: 13, females: 11) presented with dystonic storm. The annual incidence was 0.4 per 1,000 fresh admissions with an event rate of 0.9 per 1,000 for all admissions. All had Dystonia Severity Action Plan grades 4/5 with identifiable trigger in 13 (50%). Underlying dystonic disorder preexisted in 10 (43.4%); 8 of these had cerebral palsy. Polypharmacotherapy with >4 drugs out of trihexyphenidyl, tetrabenazine, clonazepam, gabapentin, levodopa-carbidopa, trichlorophos, and melatonin was needed. Supportive care and adequate sedation helped in symptom control. All children were managed with midazolam infusion over 2–10 days (median: 5 days). Mechanical ventilation was resorted to in 6 children (3–22 days). Vecuronium and propofol were used in 3/23 (13%) and 4/23 (17%) children, respectively. Deep brain stimulation was curative in 1 child. Hospitalization ranged from 5 to 31 (median: 11) days. Although there were no deaths, rhabdomyolysis was noted in 1 child. Postdischarge, 6 (26%) children relapsed.
Conclusions
Dystonic storm is a medical emergency mandating aggressive multimodal management. Supportive care, antidystonic drugs, and early elective ventilation alongside adequate sedation with benzodiazepines ameliorate complications. Relapses of dystonic storm are not uncommon.

Dystonic storm, also known as status dystonicus and dystonic crisis, is an emergency movement disorder.1 It remains underdiagnosed and underreported.2,3 It remains a highly distressing condition for parents of affected children in the absence of an effective and timely treatment. Management of dystonic storm demands multimodal approach in an intensive care setup with close monitoring for complications.4 No consensus guideline for management of this medical emergency exists. The present study seeks to estimate the burden of this entity among pediatric hospitalizations and analyze the clinical profile, management schema, and response variables of this vexing entity.
Methods
This study occurred in a quaternary care level armed forces medical services superspecialty hospital, which forms the final referral node for a network of medical facilities spread all over India. The hospital information system was searched for a diagnosis of dystonia/dystonic disorder/dystonic storm/status dystonicus from January 2017 till March 2020 with a filter of below 18 years of age. Medical records of all these children were retrieved and analyzed. Any child younger than 18 years admitted with an episode of dystonic storm was included in the study. Dystonic storm was defined as a severe, hyperkinetic movement disorder, which is life threatening and mandates emergency management.1,4 Clinical features were tabulated along with management strategies and treatment responsiveness. Data capture and statistical analysis were performed using Microsoft Excel.
Standard Protocol Approvals, Registrations, and Patient Consents
a) Approval by an ethical standards committee on human experimentation (institutional or regional) for any experiments using human participants was not obtained, as the study was a retrospective one and did not involve human experimentation in any manner.
b) Institutional ethical committee approval was obtained for the study.
c) Authorization has been obtained for disclosure (consent to disclose) of any recognizable persons in any information that may be published in the Journal, in derivative works by the AAN, or on the Journal's website.
Data Availability
Individual deidentified participant data pertaining to the demographic variables, baseline drugs, therapies administered, and outcomes shall be shared.
Results
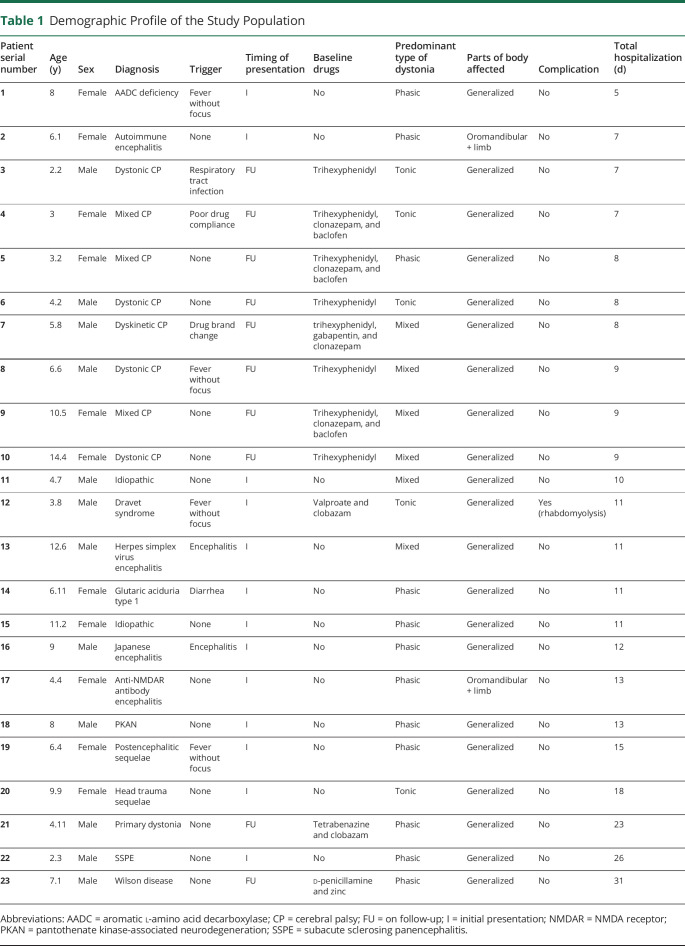
During the 39-month study period, 33 episodes of dystonic storm were identified among the 11,336 total pediatric admissions. These occurred among 23 children among the 16,632 children reporting to pediatric neurology out-patient department during this period and subsequently getting hospitalized. This leads to an annual incident rate of 0.4 per 1,000 fresh admissions below 18 years age and event rate of 0.9 per 1,000 per year for all admissions including recurrent hospitalizations. The demographic profile of the cases is presented in table 1. The ages of children ranged between 2 years 2 months to 14 years 4 months (median: 6 years 11 months). No significant difference in numbers of known dystonics on pediatric neurology follow-up vs those presenting de novo with dystonic storm was noted (n = 10 vs n = 13) (p = 0.38). There was no sex predilection (males: 13; females: 11; p = 0.56). The majority (19%) of children had Dystonia Severity Action Plan (DSAP) grade 4; 4 had DSAP grade 5 at admission. Children were worked up for etiology during their initial presentation as per prevailing standard guidelines. Neuroimaging was performed in all, whereas other investigations such as metabolic screening and genetic testing were ordered only if indicated. When children on follow-up were admitted with dystonic crisis, fresh investigations were ordered keeping in view possible impending metabolic derangements. Repeat neuroimaging was not performed.
Table 1.
Demographic Profile of the Study Population
About a third of these children had cerebral palsy (CP), whereas the rest had a multitude of conditions. Only 1 child had a primary genetically proven dystonia. The underlying condition in 1 child (serial no. 11, table 1) was not established despite extensive investigations.
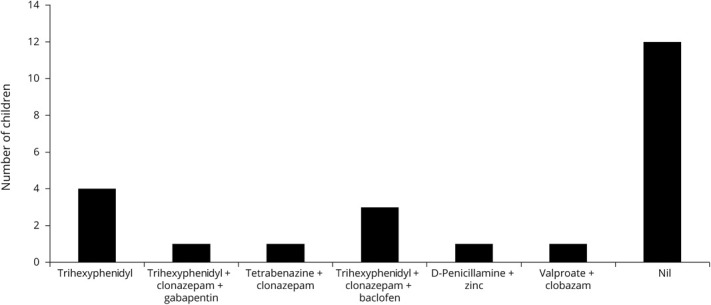
Dystonic storm occurred in 10/23 children (43%) while on antidystonic drug therapy (table 2, figure 1). A precipitating trigger was identifiable in 10/23 (43%) children (table 1). Precipitants included infectious trigger in 8 (fever without focus: 4; upper respiratory tract infection: 1; diarrhea: 1; encephalitis: 2), whereas 2 children had drug-related trigger (poor compliance: 1; change in drug brand: 1) (table 1). Most children (21/23; 91%) had generalized dystonia irrespective of underlying etiology. Predominant oromandibular and limb dystonia were noted in 2 children at presentation. Both of them had autoimmune encephalitis (anti-NMDA receptor antibody encephalitis: 1; seronegative autoimmune encephalitis: 1) (patient nos. 2 and 17, table 1). Mixed dystonia was the commonest, whereas predominant tonic dystonia and predominant phasic dystonia occurred in 5 and 6 children, respectively. Both children with autoimmune encephalitis had phasic dystonia. Concomitant cognitive regression with phasic dystonia along with EEG and CSF antimeasles antibody titers led to a diagnosis of subacute sclerosing panencephalitis (SSPE) in 1 case. All 3 children with underlying neurometabolic disorder (pantothenate kinase deficiency, Wilson disease, and glutaric aciduria type 1) had pronounced phasic dystonia.
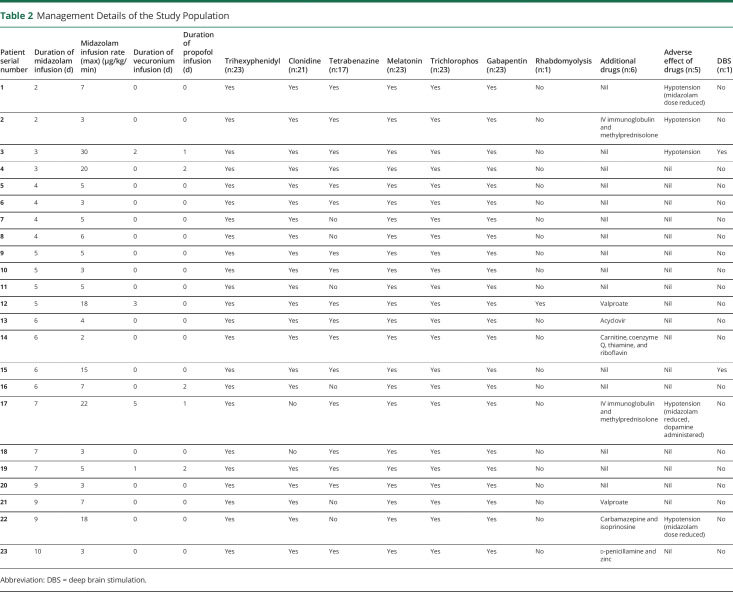
Table 2.
Management Details of the Study Population
Figure 1. Drugs Taken by the Children Before Initial Admission for Dystonic Storm.
Management included symptomatic pharmacotherapy and supportive care with adequate hydration, nutrition, and respiratory support (tables 1 and 2). Mechanical ventilation was resorted to in 6/23 (26%) children for variable duration ranging 3–22 days (figure 2). No ventilator-related complication was recorded. Rhabdomyolysis was documented in a male child with Dravet syndrome on day 3 of admission. Child was managed with adequate hydration, and he made an uneventful recovery by 24 hours.
Figure 2. Dot Plot Depicting Intervention(s) in Case of Dystonic Storm.
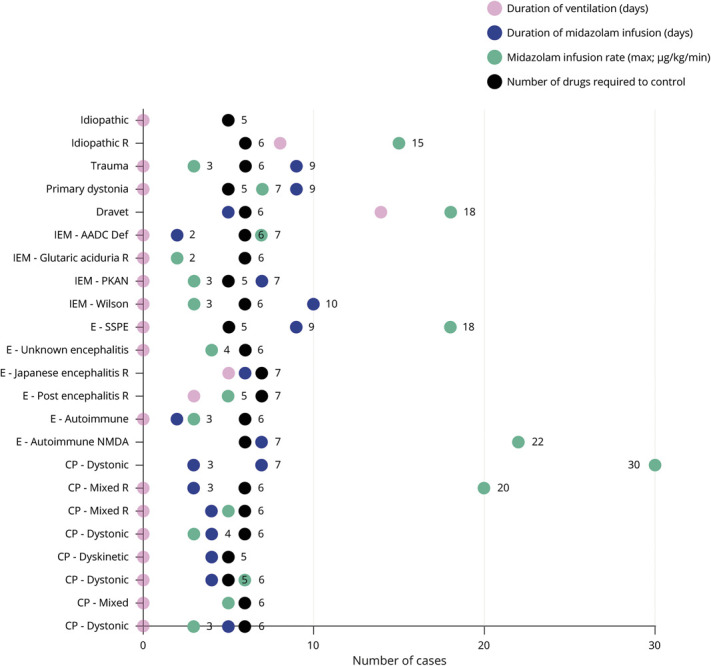
Numerical figures indicate number of cases. Suffix “R” in the diagnosis indicates that the particular case relapsed with dystonic storm at a later time point. AADC = aromatic l-amino acid decarboxylase; CP = cerebral palsy; IEM = inborn error of metabolism; PKAN = pantothenate kinase-associated neurodegeneration; SSPE = subacute sclerosing panencephalitis.
Midazolam infusion was the cornerstone of management. A standardized institutional protocol was followed consisting of an early initiation of continuous midazolam infusion at admission titrated to achieve cessation of dystonia followed by tapering on achieving DSAP grade 3 and switch to an intermittent dosing schedule and subsequent substitution with oral clonazepam (figure 3). Midazolam infusion was continued for 2–10 days (median [interquartile range] 5 [4–7] days). The peak continuous infusion rate ranged from 2 to 30 μg/kg/min (median [interquartile range]: 5 μg/kg/min [3–14.5]) (table 1, figure 2). Hypotension occurred in 4 children, which necessitated tapering of the infusion in 3 children (13%) and addition of dopamine infusion 1 child. Inj. vecuronium was administered in 4 children (17%) for muscle relaxation. Propofol was used in 3 children (13%), which were tapered off by 36 hours (table 1). All children were simultaneously exhibited multiple antidystonia drugs (table 2). Injectable botulinum toxin was not exhibited to children with focal dystonia (patient nos. 2 and 17; table 1). These children had autoimmune encephalitis wherein evidence of injectable botulinum toxin therapy is sparse.
Figure 3. Algorithm for Dystonic Storm Management Followed in the Study Center.
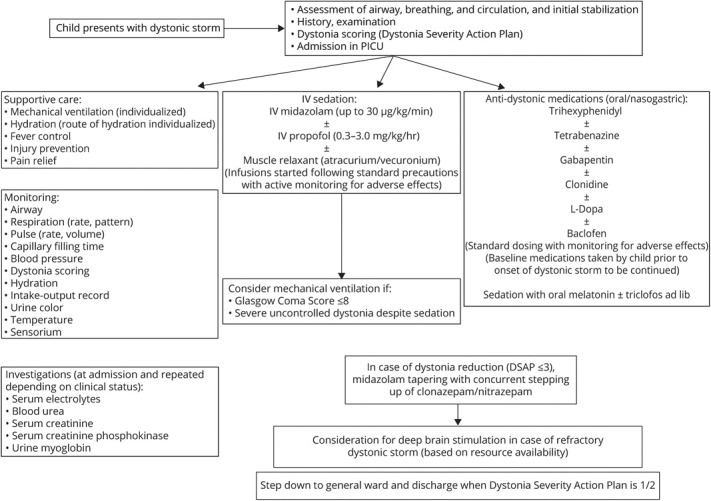
DSAP = Dystonia Severity Action Plan; PICU = pediatric intensive care unit.
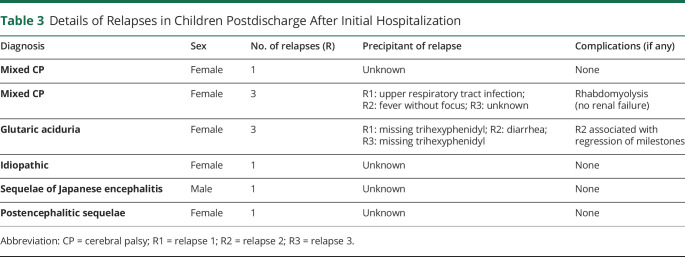
Fluctuations in dystonia were noted in every child, occasionally mandating increase in dosage of midazolam. Drugs were administered round the clock with adequate response, acceptability, and adverse effects. Two children of 21 (9%) exposed to clonidine had hypotension requiring discontinuation of drug. Exacerbations of dystonia were effectively controlled using intermittent dosing of melatonin/triclophos. This phenomenon was observed in every child even when on midazolam infusion while it was being tapered. Although midazolam was being tapered, children were also exhibited clonazepam/nitrazepam round the clock through oral route/nasogastric tube (figure 3), the dosing of which was guided based on symptom control and drug tolerance. Oral medications that were continued simultaneously included trihexyphenidyl, in certain cases depending on symptom responsiveness. As per the institutional protocol, trihexyphenidyl and gabapentin were exhibited universally, whereas tetrabenazine, gabapentin, clonidine, baclofen, and levodopa-carbidopa were prescribed sequentially based on the individual's symptom refractoriness. Levodopa-carbidopa and tetrabenazine were not administered simultaneously in view of antagonistic modes of action. However, baseline drugs, which a child was receiving before hospitalization, were continued in deference to the aforementioned protocol. Additional therapies were exhibited in children presenting with dystonic crisis due to defined etiologies (2 children with autoimmune encephalitis (IV immunoglobulin and methylprednisolone pulse followed by oral prednisolone), 1 child with Wilson disease (d-penicillamine and zinc), glutaric aciduria type 1 (treated with carnitine, coenzyme Q, and thiamine), herpes simplex encephalitis (IV acyclovir), and SSPE (carbamazepine and isoprinosine). All children were discharged after attaining DSAP grade 1. Duration of hospitalization ranged from 5 to 31 days (median: 11 days) (table 2). Children with CP needed a significantly lesser duration of midazolam infusion and had a significantly short period of hospitalization. On follow-up, 6 children had relapse of dystonic storm (table 3). All drug formulations were generic in nature and manufactured in India. The underlying illness in these children was varied. For instance, a child with CP diagnosed on the basis of clinical history and neuroimaging (serial no. 2, table 3) had multiple relapses. Investigations for genetic/metabolic etiologies were omitted in the aforementioned child in view of supportive clinical history and neuroimaging as per prevailing institutional policy. However, another child with relapsing dystonic crisis (serial no. 4, table 3) had undergone an extensive battery of investigations including whole-exome sequencing with no diagnostic yield. No child died during the study period.
Table 3.
Details of Relapses in Children Postdischarge After Initial Hospitalization
Discussion
Dystonic storm is infrequently reported in the pediatric literature. There are ∼100 case reports among all age groups with few large series.1 Actual incidence is likely to be higher owing to an underdiagnosis by most clinicians.1 Our prevalence estimates suggest that it is not an uncommon entity in a pediatric neurology service. Akin to the published literature, no sex predilection at presentation was noted by us.5
The number of children who presented initially with dystonic storm was similar to those who were already on follow-up for dystonia and had acute exacerbation. Above observation may possibly be attributed to the underlying etiologies. The majority of these (8/23) on follow-up for dystonia had CP, whereas 1 child each had Wilson disease and primary dystonia. Fasano et al.5 studied a total of 89 episodes of dystonic storm in 68 patients, wherein 37.8% of children with secondary dystonia had CP. CP comprised 35% of children who were on follow-up for dystonia in our series too.
An initial presentation with dystonic storm should prompt clinicians to actively look for underlying etiologies. Dystonic storm may be the initial presentation at varying ages. Children who presented de novo for the first time had varied etiologies including infective (encephalitis), immune-mediated (autoimmune encephalitis), neurotransmitter disorder, metabolic, and posttraumatic. In 1 child with SSPE with dystonic storm, the phasic dystonia was indicative of an epileptic event and responded to carbamazepine. Children presenting with autoimmune encephalitis and herpes simplex virus encephalitis also responded to specific treatment modalities.
Fever without focus was the commonest trigger in our study. Other common trigger factors previously reported include gastroenteritis, medication adjustment, and constipation.1,6 About half of children in our study had a definite trigger, suggesting a possible relevance of avoiding/preventing exposure to a known precipitants. About one-third of cases of dystonic storm may occur unprovoked.6 Temporizing therapy, supportive care, and antidystonia measures are the pillars recommended in the management of dystonic storm.1 In the absence of a consensus statement, indication for mechanical ventilation needs to be based on institutional protocols along with objective dystonia grading scores. Based on our experience, we advocate an early and elective mechanical in dystonic storm to avoid bulbar, metabolic, and respiratory complication following high rates of sedation and anesthesia for control of the dystonia. Prevention of lung infection by following strict asepsis and critical care guidelines is vital.1,7 Varying underlying etiologies mandate case-based treatment of underlying etiology. Yet, we achieved dystonia management using a uniform protocol. DSAP score is a simple, reproducible, and objective tool with good delineation of dystonic crisis.8 This grading system in provides an objective benchmark for admission, management, and discharge of children. Other supportive care involves adequate hydration, nutrition pain relief, sedation, and airway protection.9
Drug therapy is reportedly effective in only upto 10% of cases of dystonic storm with midazolam being a key drug.5 Our experience too suggests that pharmacologic management with injectable midazolam along with other drugs is the initial modality of choice to manage dystonic storm. Although greater percentage of children in our study had symptom cessation with pharmacotherapy, both the cohorts are not comparable due to multiple confounders. Symptomatic pharmacotherapy is as crucial and effective as definitive therapy for underlying etiology.1,7,10 We suggest a combination polypharmacotherapy approach with multiple antidystonic drugs with differing mechanisms of action and benzodiazepines rather than a single agent uptitration strategy.1,7Trihexyphenidyl, tetrabenazine, and gabapentin were extensively used in our study. These drugs are commonly reported adjuncts for managing dystonic storm, although the percentage of usage is not reported in the literature.11,12 Adequate sedation forms one of the cornerstones of management of a dystonic crisis since sleep is proven to be beneficial in decreasing dystonia.1,13 Midazolam was used extensively in our cohort. This remains an effective sedative with short half-life, good cardiovascular profile with muscle relaxant properties, and an additional antidystonic effect. Efficacy is rapidly lost due to tolerance.1,6,9 In the absence of IV clonidine, oral clonidine was used orally as an add-on therapy in our study with minimal adverse effects. Use of clonidine by oral, enteral, or IV route has been previously reported for dystonic crisis in children.1,14 Alongside these agents, we relied on melatonin and triclophos for achieving adequate sedation. Nasogastric administration of these drugs was started even when child used to be on midazolam infusion. These medications had minimal adverse effects, were well tolerated in children, and enabled reduction of midazolam/clonazepam to which individual developed tolerance rapidly. The literature does not reveal use of these drugs specifically in dystonic crisis. Clonidine too has additional sedative effects.
Although deep brain stimulation (DBS) is a known effective modality for management of this entity, financial issues and requirement of experienced neurosurgery team may be challenges in DBS, especially in resource-constrained settings.5,15–17 In our study, drug therapy was successful in all cases. Globus pallidus interna-DBS was performed in 1 child after resolution of symptoms. The child remains under follow-up with dystonia grade DSAP 1–2. Intrathecal baclofen is also effective in treating dystonic storm in both primary and secondary dystonia.14,18 We did not prescribe this in view of the high cost and invasiveness entailed by this therapy.
Dystonic storm can be potentially fatal with multiple complications.19 Multidrug therapy, adequate supportive care, and close monitoring were the keys in prevention of complications in our study. In our study, mean durations of hospitalization of children with neurologic conditions other than CP were significantly longer than those with CP. There is a ground to study possible correlation between treatment refractoriness of dystonic storm and underlying etiology. Relapse is common in cases with dystonia who present with dystonic storm.7,17 Ensuring drug compliance, parental education, close follow-up, and early management of infections may be some strategies to minimize relapses. The simplistic model, sizeable patient population, and uniform protocolized drug usage policy are the strengths of the study. Possibility of referral bias and the retrospective study model are the inherent limitations of the study. The standard practices followed in the study center may not be practiced across all health care facilities due to nonavailability of pediatric neurology services and resource limitations, and hence, outcomes in the community setting may differ.
To conclude, in this retrospective, hospital-based study, fresh-onset dystonic storm occurred secondary to multiple varied etiologies with an identifiable trigger in only 50%. Children on follow-up for dystonic and mixed CP comprised a significant proportion of cases that had exacerbation of symptoms secondary to a triggering event. Objective DSAP scoring, titrated dosing of midazolam infusion, balanced approach to elective mechanical ventilation, and concurrent polypharmacotherapy were successful in safe management of this condition with minimal complications. Rates of rhabdomyolysis were insignificant. Adequate sedation helped in amelioration of symptoms and enabled early taper of benzodiazepines. Relapse occurred in 26% cases mostly due to preventable factors.
TAKE-HOME POINTS
→ Pediatric dystonic storm occurs secondary to multiple etiologies.
→ CP is the commonest etiology of pediatric dystonic storm.
→ The trigger of dystonic storm may be identified in only about 50% of children.
→ The core management tools of dystonic storm include meticulous assessment and monitoring, titrated polypharmacotherapy, adequate sedation, and balanced approach to elective mechanical ventilation.
→ Dystonic storm relapses mostly due to preventable factors.
Appendix. Authors
Study Funding
No targeted funding reported.
Disclosure
The authors report no relevant disclosures. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Allen NM, Lin JP, Lynch T, et al. Status dystonicus: a practice guide. Dev Med Child Neurol 2014;56:105–112. [DOI] [PubMed] [Google Scholar]
- 2.Arshad MF, Ahmad E, Biddanda AN, et al. Status dystonicus: a diagnosis delayed. BMJ Case Rep 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed R, Griffiths B, Lumsden DE. Dystonia in paediatric intensive care: a retrospective prevalence study. Arch Dis Child 2020;105:912–914. [DOI] [PubMed] [Google Scholar]
- 4.Tabbal SD. Childhood dystonias. Curr Treat Options Neurol 2015;17:339. [DOI] [PubMed] [Google Scholar]
- 5.Fasano A, Ricciardi L, Bentivoglio AR, et al. Status dystonicus: predictors of outcome and progression patterns of underlying disease. Mov Disord 2012;27:783–788. [DOI] [PubMed] [Google Scholar]
- 6.Grosso S, Verrotti A, Messina M, et al. Management of status dystonicus in children: cases report and review. Eur J Paediatr Neurol 2012;16:390–395. [DOI] [PubMed] [Google Scholar]
- 7.Mariotti P, Fasano A, Contarino MF, et al. Management of status dystonicus: our experience and review of the literature. Mov Disord 2007;22:963–968. [DOI] [PubMed] [Google Scholar]
- 8.Lumsden DE, Lundy C, Fairhurst C, et al. Dystonia severity action plan: a simple grading system for medical severity of status dystonicus and life-threatening dystonia. Dev Med Child Neurol 2013;55:671–672. [DOI] [PubMed] [Google Scholar]
- 9.Nirenberg MJ, Ford B. Dystonic storm. In: Frucht SJ, editor. Movement Disorder Emergencies: Diagnosis and Treatment, 2nd ed. New York: Springer; 2013:125–134. [Google Scholar]
- 10.Cloud LJ, Jinnah HA. Treatment strategies for dystonia. Expert Opin Pharmacother 2010;11:5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liow NY, Gimeno H, Lumsden DE, et al. Gabapentin can significantly improve dystonia severity and quality of life in children. Eur J Paediatr Neurol 2016;20:100–107. [DOI] [PubMed] [Google Scholar]
- 12.Paliwal VK, Gupta PK, Pradhan S. Gabapentin as a rescue drug in D-penicillamine-induced status dystonicus in patients with Wilson disease. Neurol India 2010;58:761–763. [DOI] [PubMed] [Google Scholar]
- 13.Hertenstein E, Tang NK, Bernstein CJ, et al. Sleep in patients with primary dystonia: a systematic review on the state of research and perspectives. Sleep Med Rev 2016;26:95–107. [DOI] [PubMed] [Google Scholar]
- 14.Kirkham FJ, Haywood P, Kashyape P, et al. Movement disorder emergencies in childhood. Eur J Paediatr Neurol 2011;15:390–404. [DOI] [PubMed] [Google Scholar]
- 15.Keen JR, Przekop A, Olaya JE, et al. Deep brain stimulation for the treatment of childhood dystonic cerebral palsy. J Neurosurg Pediatr 2014;14:585–593. [DOI] [PubMed] [Google Scholar]
- 16.Tsering D, Tochen L, Lavenstein B, et al. Considerations in deep brain stimulation (DBS) for pediatric secondary dystonia. Childs Nerv Syst 2017;33:631–637. [DOI] [PubMed] [Google Scholar]
- 17.Termsarasab P, Frucht SJ. Dystonic storm: a practical clinical and video review. J Clin Mov Disord 2017;28:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saleh C, Gonzalez V, Coubes P. Role of deep brain stimulation in the treatment of secondary dystonia-dyskinesia syndromes. Handb Clin Neurol 2013;116:189–208. [DOI] [PubMed] [Google Scholar]
- 19.Combe L, Abu-Arafeh I. Status dystonicus in children: early recognition and treatment prevent serious complications. Eur J Paediatr Neurol 2016;20:966–970. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual deidentified participant data pertaining to the demographic variables, baseline drugs, therapies administered, and outcomes shall be shared.