Abstract
Objective
To determine the frequency of multiple spinal CSF leaks in a recent group of patients with spontaneous intracranial hypotension (SIH) who were investigated with digital subtraction myelography (DSM).
Methods
This observational study was conducted using data from a prospectively maintained data base of patients who meet the International Classification of Headache Disorders, third edition, criteria for SIH. The patient population consisted of a consecutive group of 745 patients with SIH who underwent DSM between March 2009 and February 2020. Based on the results of DSM, participants were classified according to the type and number of spinal CSF leaks.
Results
Among 398 patients with SIH and extradural CSF on spinal imaging, multiplicity of CSF leaks was observed in none of 291 patients with type 1a ventral leaks and in 4 (6.2%) of 65 patients with type 1b (postero-) lateral leaks. Among 97 patients with SIH from spinal CSF-venous fistulas (type 3 leaks) who did not have extradural CSF on spinal imaging, 9 patients (9.3%) had multiple fistulas (p < 0.0001 for comparison between groups). Type 3 and type 1a or 1b CSF leaks coexisted in an additional 5 patients.
Conclusions
Among patients with SIH, multiplicity of CSF leaks was observed radiographically in none of the patients with ventral leaks, in 6% of patients with lateral leaks, and in 9% of patients with CSF-venous fistulas. These results suggest that patients with SIH can be reassured that the occurrence of multiple CSF leaks is negligible to uncommon at most, depending on the type of CSF leak.

Spontaneous intracranial hypotension (SIH) is an increasingly recognized cause of headaches, although an initial misdiagnosis remains common.1-5 An orthostatic headache, i.e., a headache that is exacerbated by the upright position, is the prototypical manifestation of SIH, but the positional component often becomes less recognizable with the passage of time.1-5 The headache can be in any location, with the occipital/suboccipital region being most commonly involved. SIH may also cause a variety of other more or less serious neurologic manifestations such as hearing loss, dizziness, diplopia, nuchal rigidity, symptoms of behavioral variant frontotemporal dementia, coma, bibrachial amyotrophy, ventral spinal cord herniation, and superficial siderosis.1-5 Most, but not all, patients with SIH have an abnormal brain MRI showing one or more of the following features: subdural fluid collections, enhancement of the pachymeninges, engorgement of venous structures, pituitary enlargement, and sagging of the brain (mnemonic, SEEPS2).1-5 A spontaneous spinal CSF leak is the underlying cause in the great majority of, if not all, cases of SIH.1-6 Exact knowledge about the type and location of the spinal CSF leak is generally not necessary early in the disease process because most patients recover with conservative methods or with one or more nondirected epidural blood patches (Figure 1).1-6
Figure 1. Simplified Management Algorithm for Treatment and Imaging of Spontaneous Intracranial Hypotension (SIH).
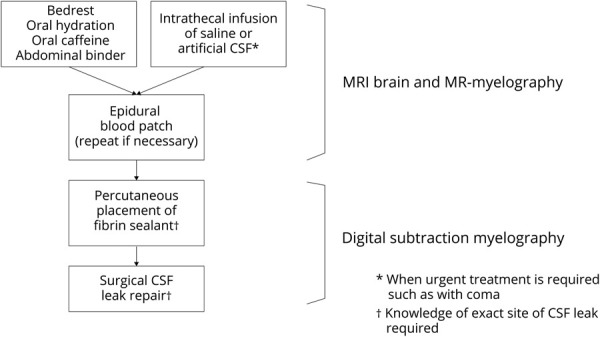
Brain and spine imaging is used to diagnose SIH. Knowledge about the exact location of the underlying spinal CSF leak is necessary only for directed treatment with, for example, percutaneous placement of fibrin sealant, or for surgical repair, and we use digital subtraction myelography for CSF leak localization. MR = magnetic resonance.
Multiple spinal CSF leaks are often diagnosed in patients with SIH, but this has been based mostly on the interpretation of conventional spinal imaging such as CT myelography, MRI, or radionuclide cisternography.7-10 These conventional tests have important limitations in localizing the exact source of a spontaneous spinal CSF leak. More recently developed sophisticated imaging techniques such as digital subtraction myelography (DSM) have allowed for the identification of the exact type and location of the spinal CSF leak.11
A comprehensive study of the multiplicity of spontaneous spinal CSF leaks has not been performed. We therefore reviewed the frequency of multiple spinal CSF leaks in a recently evaluated group of patients who all underwent DSM.
Methods
This study was approved by our Medical Center's Institutional Review Board. Since January 1, 2001, all patients with SIH evaluated by us at Cedars-Sinai Medical Center in Los Angeles, California, have been enrolled prospectively in a registry. Using this registry, we reviewed the medical records and radiographic studies of a group of consecutive patients with SIH who underwent DSM at our institution. The diagnosis of SIH was based on the criteria of the International Classification of Headache Disorders, third edition,12 with minor modifications. These criteria require objective evidence of SIH, consisting of brain MRI showing stigmata of SIH (i.e., pachymeningeal enhancement, brain sagging, or subdural fluid collections), spinal imaging showing a CSF leak (i.e., the presence of extradural CSF or a CSF-venous fistula), or low CSF opening pressure (i.e., <6.0 cm H2O). The modification consists of also including patients who do not have headaches but whose symptoms are best explained by SIH. The type of underlying spinal CSF leak was classified according to a previously published classification system.11 Briefly, type 1 CSF leaks are caused by a dural tear located ventral to the spinal cord (type 1a) or (postero-) lateral to the spinal cord (type 1b). Type 2 CSF leaks are associated with simple (type 2a) or complex (type 2b [dural ectasia]) meningeal diverticula. Type 3 CSF leaks are CSF-venous fistulas (Figure 2). Type 4 CSF leaks are of indeterminate origin.
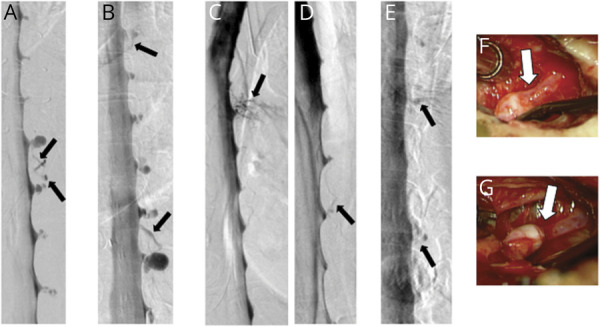
Figure 2. Classification of Spontaneous Spinal CSF Leaks in Spontaneous Intracranial Hypotension.
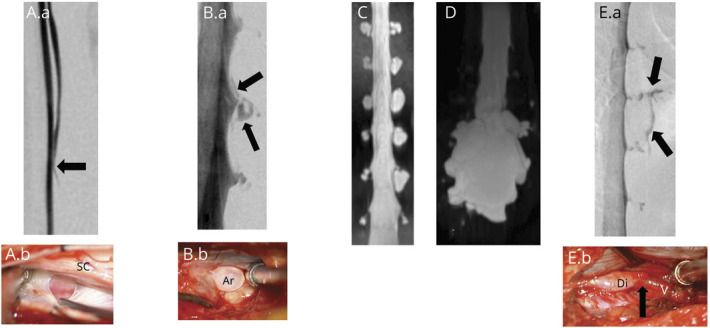
Type 1a CSF leaks are due to a ventral dural tear. Prone digital subtraction myelography (DSM) (A.a) showing location of the ventral leak (arrow) and intraoperative photograph ([A.b] posterior intradural view) showing ventral dural defect partially obscured by the spinal cord. Type 1b CSF leaks are due to a (postero)lateral dural tear. Lateral decubitus DSM (B.a) showing location of the lateral leak (arrows) and intraoperative photograph ([B.b] posterior extradural view) showing tear of the lateral thecal sac near the take-off of the nerve root sleeve with arachnoid (Ar) billowing out through the dural defect. Type 2a CSF leaks are associated with simple meningeal diverticula (C) and type 2b with dural ectasia (D). Type 3 CSF leaks are due to a CSF-venous fistula. Lateral decubitus DSM (E.a) showing location of the CSF venous fistula (arrows) and intraoperative photograph ([E.b] posterior intradural view) showing a small meningeal diverticulum (Di) transitioning into the vein (V) at the fistulous site (arrow).11
During the period of investigation, our imaging protocol consisted of universal brain MRI and MR myelography (heavily T2-weighted MRI without intrathecal gadolinium) for all patients, unless magnetic resonance was contraindicated. In general, DSM was reserved for patients who failed epidural blood patching. Conventional CT myelography has not been part of our imaging protocol since the routine use of DSM. A previously described DSM technique was used13 with some minor modifications, as published previously.14,15 Briefly, DSM is performed under general endotracheal anesthesia with deep paralysis and suspended respiration for maximal detail and temporal resolution. Patients are positioned in the prone or lateral decubitus position in a biplane angiography suite, with tilt table capability. Pillows or foam padding are placed to optimize cervicothoracic alignment. A fluoroscopically guided lumbar puncture is performed at the L2-3 level with a 22-gauge needle. An opening pressure is obtained at this time. Then, accurate needle position is confirmed with an injection of 0.5 mL of Omnipaque. Patients are then further positioned based on the area of interest, tilting the table to achieve contrast flow to the cervicothoracic spine. Finally, contrast is injected manually 1 mL per second with suspended respiration for 40–100 seconds while acquiring biplane subtraction images at 2 frames per seconds.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by our Medical Center's Institutional Review Board (Pro00049126).
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Statistical Analysis
Baseline characteristics for all included participants and by classification groups are presented as percentages for categorical variables and mean (SD) values or median values and interquartile ranges (IQRs) for continuous variables. Nonparametric tests, χ2 tests, or Fisher exact tests were used to compare the distribution of those characteristics by group. Data analyses were performed with SAS software, version 9.4 (SAS Institute, Cary, NC).
Results
The patient population consisted of 745 patients with SIH who underwent DSM. The mean age of the 502 (67.4%) women and 243 (32.6%) men was 45.8 (SD 11.7) years (median [IQR], 46 years [38–55 years]; range, 9–85 years). Extradural CSF collections were present in 398 (53.4%) of the 745 patients. Among these 398 patients with an extradural CSF collection, 291 had a type 1a leak, 65 had a type 1b leak, 7 had a type 2a leak, 6 had a type 2b leak, none had a type 3 leak, and 29 had a type 4 leak. Extradural CSF collections were absent in 347 (46.6%) of the 745 patients. Among these 347 patients without an extradural CSF collection, 6 had a type 1a leak, 175 had a type 2a leak, 17 had a type 2b leak, 97 had a type 3 leak, and 52 had a type 4 leak.
Among the 291 patients with extradural CSF collections and a ventral (type 1a) leak, multiple simultaneous spinal CSF leaks at different sites were not visualized radiographically in any of the patients (0%, 95% confidence interval [CI]: 0.0–1.3). Among the 65 patients with extradural CSF collections and a posterolateral (type 1b) leak, multiple simultaneous spinal CSF leaks at different sites were visualized radiographically in 4 patients (6.2%, 95% CI: 1.7–15.0) (Figure 3). These multiple type 1b CSF leaks always were bilateral. Among the 13 patients with extradural CSF collections and a type 2 leak multiple simultaneous spinal CSF leaks at different sites were not visualized radiographically in any of the patients (0%, 95% CI: 0.0–24.7). Among the 97 patients with SIH without extradural CSF collections who had an identifiable CSF leak source, i.e., a CSF-venous fistula or type 3 CSF leak, multiple CSF-venous fistulas at different sites were visualized in 9 patients (9.3%, 95% CI: 4.3–16.9) (Figures 4–6) (p < 0.0001 for comparison between groups). In one of these patients with multiple spinal CSF-venous fistulas, the second fistula was determined to be a de novo fistula and was discovered 8 months after the initial CSF-venous fistula was surgically ligated (Figure 6). Two patients had bilateral CSF-venous fistulas, and 6 patients had multiple unilateral CSF-venous fistulas. The multiple unilateral CSF-venous fistulas were on the right in 3 patients and on the left in 3. Eight patients had 2 CSF-venous fistulas, and 1 patient had 4 CSF-venous fistulas (Figures 4–6).
Figure 3. Digital Subtraction Myelographies (DSMs) Demonstrating Bilateral Spontaneous Lateral (Type 1b) Dural Tears in 4 Patients.
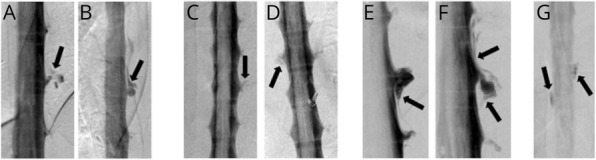
(A and B) Bilateral lateral decubitus DSMs in a patient with CSF leaks at left T10-11 and right T11-12 (arrows). (C and D) Prone DSMs in a patient with CSF leaks at left T9-10 and right T10-11 (arrows). (E and F) Bilateral lateral decubitus DSMs in a patient with bilateral CSF leaks at T10-11 (arrows). (G) Prone DSM in a patient with leaks at left T4-5 and right T3-4 (arrows).
Figure 4. Digital Subtraction Myelographies (DSMs) Demonstrating Multiple Spontaneous CSF-Venous Fistulas (Type 3 Leaks) in 4 Patients.
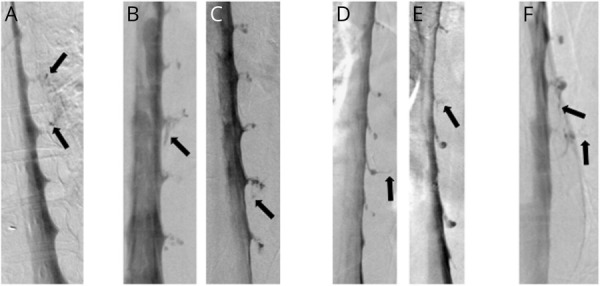
(A) Lateral decubitus DSM in a patient with CSF-venous fistulas (arrows) at right T7-8 and T8-9. (B and C) Bilateral lateral decubitus DSMs in a patient with CSF-venous fistulas (arrows) at left T10-11 and right T8-9. (D and E) Lateral decubitus DSMs in a patient with CSF-venous fistulas (arrows) at left T7-8 and T9-10. (F) Lateral decubitus DSM in a patient with CSF-venous fistulas (arrows) at right T4-5 and T5-6.
Figure 5. Digital Subtraction Myelographies (DSMs) Demonstrating Multiple Spontaneous CSF-Venous Fistulas (Type 3 Leaks) in 4 Patients.
(A) Lateral decubitus DSM in a patient with CSF-venous fistulas at left T8-9 and T9-10 (arrows). (B) Lateral decubitus DSM in a patient with CSF-venous fistulas at right T5-6 and T10-11 (arrows). (C and D) Bilateral lateral decubitus DSMs in a patient with CSF-venous fistulas at left T8-9 and right T5-6 (arrows). (E) Lateral decubitus DSM in a patient with CSF-venous fistulas at right T9-10 and T11-12 (arrows). (F and G) Corresponding intra-operative photographs confirming the presence of these 2 CSF-venous fistulas (arrows).
Figure 6. Digital Subtraction Myelographies (DSMs) Demonstrating 4 Spontaneous CSF-Venous Fistulas in 1 Patient, Including a De Novo Fistula.
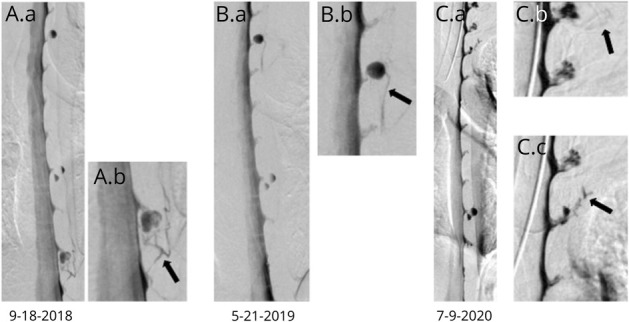
(A) Lateral decubitus DSM showing a left CSF-venous fistula at T10-11 (arrow). (B) Lateral decubitus DSM performed 8 months later now shows a left T-4-5 CSF-venous fistula (arrow) that was not present on the initial DSM despite excellent visualization of the associated meningeal diverticulum on the initial DSM. (C and D) Further lateral decubitus DSMs showing left C7-T1 (C) and T2-3 (D) CSF-venous fistulas. These levels were not as well visualized on the prior DSMs.
In 5 (1.3%) of the 398 patients with extradural CSF collections, DSM showed 2 different types of CSF leak originating from the same site. In all 6 patients, these were type 1 (a or b [ventral or posterolateral tear]) accompanied by type 3 (CSF-venous fistula) CSF leaks (Figure 7). For the purpose of this study, we consider the type 1 leak to be primary in these patients and the CSF-venous fistula to be a secondary phenomenon.
Figure 7. Coexistence of Type 1a (Ventral) or 1b (Posterolateral) CSF Leaks and CSF-Venous Fistulas (Type 3 CSF Leaks) in 5 Patients.

Lateral (A) and antero-posterior (B) prone digital subtraction pyelograms (DSMs) showing a ventral CSF leak and associated CSF-venous fistula at T1-2 (arrows). C: Lateral prone DSM showing a ventral CSF leak and associated CSF-venous fistula at T11-12 (arrows). Lateral (D) and antero-posterior (E) prone DSMs showing a ventral CSF leak and associated CSF-venous fistula at T2-3 (arrows). Lateral (F) and antero-posterior (G) prone DSMs showing a ventral CSF leak and associated CSF-venous fistula at T1-2 (arrows). H: lateral decubitus DSM showing a lateral CSF leak and associated CSF-venous fistula at left T10-11 (arrows).
Discussion
In this study of 745 patients with SIH studied with DSM, we demonstrate that multiple spinal CSF leaks are not common and that the frequency of CSF leak multiplicity depends on the type of CSF leak. The radiographic diagnosis of spinal CSF leak has traditionally relied on the demonstration of extradural CSF collections, and it is from this patient population that most of our knowledge regarding the multiplicity of CSF leaks is derived. In the scientific literature, the presence of multiple CSF leaks is regularly mentioned. For example, in a 2006 review article authored by one of us, it is stated that multiple simultaneous CSF leaks are frequently demonstrated.2 However, in more recent reviews, the multiplicity of CSF leaks as a common finding among patients with SIH has been called into question.5 In reality, very few studies have specifically reported on the frequency of multiple CSF leaks. Using traditional imaging techniques such as CT myelography, intradural gadolinium-enhanced MRI, or radionuclide cisternography, the frequency of multiple spinal CSF leaks has been reported to be between 10% and 50%.7-10 In a systemic review and meta-analysis of SIH, spinal CSF leaks were reported to be multiple in 24% of patients.16 In a recent study using DSM, however, not a single instance of multiple CSF leaks was reported in 19 patients with SIH with extradural CSF collections due to ventral (type 1a) leaks (n = 15) or posterolateral (type 1b) leaks (n = 4).17 Similarly, using dynamic CT myelography, a single source of CSF leak was identified in all 48 patients with SIH with extradural CSF collections due to a ventral (type 1a) leak and in all 13 SIH patients with a posterolateral (type 1b) leak.18 In the present study, among 291 patients with SIH with extradural CSF collections caused by a ventral tear (type 1a leak), we were not able to demonstrate a single radiographically confirmed case of multiple simultaneous CSF leaks originating from different sites. However, we did find multiple (always bilateral) CSF leaks originating from different sites in 6% of 65 patients with SIH with extradural CSF collections caused by a posterolateral tear (type 1b leak).
The principal reason for the appearance of multiple spinal CSF leaks on conventional CT myelography, radionuclide cisternography, or MRI is that once CSF has escaped from a single dural defect into the epidural space, CSF can flow quite freely throughout the enlarged epidural space and can escape from the spinal canal through the neural foramina, especially at the cervicothoracic junction or through the porous connective tissue membrane at the C1-2 level. The appearance of such false localizing signs at the cervicothoracic junction19 and at the C1-2 level has been noted previously.20 These spinal CSF leaks that have the appearance of multiple simultaneous CSF leaks are associated with extensive extradural intraspinal CSF collections and can result from ventral or posterolateral tears or, rarely, from tears originating from meningeal diverticula. DSM has the unique capability of visualizing contrast dye within seconds of intrathecal injection and its escape into the epidural space through the dural tear.
The demonstration that multiple simultaneous spinal CSF leaks are at most a rare event among patients with SIH with extradural CSF on spinal imaging and are seen only in patients with posterolateral (type 1b) CSF leaks has important implications for our understanding of the pathophysiology of spontaneous spinal CSF leaks. Although a generalized connective tissue disorder is commonly suspected in patients with SIH,21-24 our study shows the importance of other, probably local, factors in the development of spontaneous spinal CSF leaks. One of these factors is the presence of a bony spicule. Such bony spicules have been reported as the cause of SIH since the 1990s,25 and it has become apparent that they can be detected in most patients with a ventral (type 1a) spinal CSF leak at the site of the dural tear, usually in the thoracic spine.26-30 Other local factors, for example in patients with (postero-) lateral dural tears (type 1b CSF leak), which generally are not associated with any bony pathology, remain to be discovered.
One of the most important untoward consequences of diagnosing multiple CSF leaks rather than a single source of CSF leakage is that treatment is directed at the wrong level or levels, making the treatment less successful and associated with increased risk. This is most commonly seen at the C1-2 level or cervicothoracic junction. Although such false localizing signs have been well described, treatments directed at such sites continue to be reported.31-35 In addition, the diagnosis of multiple spinal CSF leaks may result in a defeatist attitude both on the patient's and treating physician's part with regard to the chances of successful treatment of SIH and with regard to the risk of future CSF leaks.
A more recently discovered type of CSF leak, the spinal CSF-venous fistula or type 3 CSF leak, is a unique type of CSF leak because it is not associated with an extradural CSF collection. Spontaneous spinal CSF-venous fistulas were first described in 2014,36 and the importance of such fistulas in SIH has since been confirmed by several groups of investigators.14,15,37-40 These fistulas are not visible on routine CT myelography or MRI, and their detection requires more sophisticated imaging modalities such as dynamic CT myelography or DSM, explaining why their existence was not discovered until recently. We found that when DSM is performed in the lateral decubitus position, CSF-venous fistulas can be detected in about three-fourths of patients with SIH and a brain MRI showing the stigmata of SIH but no extradural CSF collection on spine imaging.15 In the present study, we were able to demonstrate multiplicity of CSF-venous fistulas in 9% of patients with such fistulas. In one of these patients, a second CSF-venous fistula was not visualized on the original DSM presenting good evidence that these fistulas are acquired and not congenital lesions. In prior studies, including our own, multiplicity of spinal CSF-venous fistulas was not reported except for Duvall et al.,39 who mention the possible existence of bilateral CSF-venous fistulas in their patients, although this could not be definitively confirmed.
We also were able to demonstrate CSF-venous fistulas at the site of the dural tear in a small minority (1.3%) of patients with SIH with extradural CSF collections. The fistula always was at or near the site of the dural tear. The exact pathogenesis of this association is not known, but we consider these CSF-venous fistulas to be secondary phenomena. The coexistence of these 2 types of CSF leaks could explain failure of treatment if only the primary dural tear is repaired.
Appendix. Authors

Limitations
It should be noted that the current study mostly represents a highly selected group of patients referred to a quaternary referral center for SIH and the generalizability of our findings is unknown. However, this if anything should have skewed the population toward patients with multiple CSF leaks, which would presumably be more difficult to treat, strengthening the main finding in our study. Another limitation is that it is possible that we have missed a number of patients with multiple simultaneous spinal CSF leaks in this study, and indeed, intraoperative observations have suggested the presence of separate leaks in a few of our patients with extradural CSF collections, but this was never corroborated radiographically.
Study Funding
No targeted funding reported.
Disclosure
The authors report no disclosures relevant to the manuscript. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
TAKE-HOME POINTS
→ Imaging to determine the type and location of the underlying spinal CSF leak is indicated in patients with SIH and persistent symptoms.
→ Multiple spinal CSF leaks are not common in patients with SIH, and their frequency depends on the type of CSF leak.
→ Multiple spinal CSF leaks were found in none of the patients with ventral type 1a CSF leaks, in about one-twentieth of patients with (postero-) lateral type 1b CSF leaks, and in about one-tenth of patients with type 3 CSF leaks, the CSF-venous fistulas.
→ The de novo appearance of spinal CSF-venous fistulas suggests that these are acquired lesions.
References
- 1.Mokri B. Spontaneous cerebrospinal fluid leaks: from intracranial hypotension to cerebrospinal fluid hypovolemia—evolution of a concept. Mayo Clin Proc. 1999;74(11):1113-1123. [DOI] [PubMed] [Google Scholar]
- 2.Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. 2006;295(19):2286-2296. [DOI] [PubMed] [Google Scholar]
- 3.Rahman M, Bidari SS, Quisling RG, Friedman WA. Spontaneous intracranial hypotension: dilemmas in diagnosis. Neurosurgery. 2011;69(1):4-14. [DOI] [PubMed] [Google Scholar]
- 4.Mokri B. Spontaneous intracranial hypotension. Continuum (Minneap Minn). 2015;21(4 headache):1086-1108. [DOI] [PubMed] [Google Scholar]
- 5.Amrhein TJ, Kranz PG. Spontaneous intracranial hypotension: imaging in diagnosis and treatment. Radiol Clin North Am. 2019;57(2):439-451. [DOI] [PubMed] [Google Scholar]
- 6.Schievink WI, Schwartz MS, Maya MM, Moser FG, Rozen TD. Lack of causal association between spontaneous intracranial hypotension and cranial cerebrospinal fluid leaks. J Neurosurg. 2012;116(4):749-754. [DOI] [PubMed] [Google Scholar]
- 7.Cohen-Gadol AA, Mokri B, Piepgras DG, Meyer FB, Atkinson JLD. Surgical anatomy of dural defects in spontaneous spinal cerebrospinal fluid leaks. Neurosurgery. 2006;58(4 suppl 2):238-245. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K, Mima T, Akiba Y. Chronic subdural hematoma associated with spontaneous intracranial hypotension: therapeutic strategies and outcomes of 55 cases. Neurol Med Chir (Tokyo). 2016;56(2):69-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.So Y, Park JM, Lee PM, et al. Epidural blood patch for the treatment of spontaneous and iatrogenic orthostatic headache. Pain Physician. 2016;19(8):E1115-E1122. [PubMed] [Google Scholar]
- 10.Cebeci H, Bilgin C, Candan S, Demir AB, Hakyemez B. Spinal cerebrospinal fluid leakage in spontaneous intracranial hypotension: an intrathecal gadolinium enhanced MR-myelography study. J Belg Soc Radiol. 2020;104(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schievink WI, Maya MM, Jean-Pierre S, et al. A classification system of spontaneous spinal CSF leaks. Neurology. 2016;87(7):673-679. [DOI] [PubMed] [Google Scholar]
- 12.Headache Classification Committee of the International Headache Society (HIS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(9):1-211. [DOI] [PubMed] [Google Scholar]
- 13.Hoxworth JM, Trentman TL, Kotsenas AL, et al. The role of digital subtraction myelography in the diagnosis and localization of spontaneous spinal CSF leaks. AJR Am J Roentgenol. 2012;199(3):649-653. [DOI] [PubMed] [Google Scholar]
- 14.Schievink WI, Moser FG, Maya MM, Prasad RS. Digital subtraction myelography for the identification of spontaneous spinal CSF-venous fistulas. J Neurosurg Spine. 2016;24(6):960-964. [DOI] [PubMed] [Google Scholar]
- 15.Schievink WI, Maya MM, Moser FG, et al. Lateral decubitus digital subtraction myelography to identify spinal CSF-venous fistulas in spontaneous intracranial hypotension. J Neurosurg Spine. 2019:1-4. doi: 10.3171/2019.6.SPINE19487. [DOI] [PubMed] [Google Scholar]
- 16.D'Antona L, Merchan MAJ, Vassiliou A, et al. Clinical presentation, investigation findings, and treatment outcomes of spontaneous intracranial hypotension syndrome: a systematic review and meta-analysis. JAMA Neurol. 2021;78(3):329-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farb RI, Nicholson PJ, Peng PW, et al. Spontaneous intracranial hypotension: a systematic imaging approach for CSF leak localization and management based on MRI and digital subtraction myelography. AJNR Am J Neuroradiol. 2019;40(4):745-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobrocky T, Winklehner A, Breiding PS, et al. Spine MRI in spontaneous intracranial hypotension for CSF leak detection: nonsuperiority of intrathecal gadolinium to heavily T2-weighted fat-saturated sequences. AJNR Am J Neuroradiol. 2020;41(7):1309-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schievink WI, Maya MM, Chu RC, Moser FG. False localizing sign of cervico-thoracic CSF leak in spontaneous intracranial hypotension. Neurology. 2015;84(24):2445-2448. [DOI] [PubMed] [Google Scholar]
- 20.Schievink WI, Maya MM, Tourje J. False localizing sign of C1-2 cerebrospinal fluid leak in spontaneous intracranial hypotension. J Neurosurg. 2004;100(4):639-644. [DOI] [PubMed] [Google Scholar]
- 21.Schrijver I, Schievink WI, Godfrey M, Meyer FB, Francke U. Spontaneous spinal cerebrospinal fluid leaks and minor skeletal features of Marfan syndrome: a microfibrillopathy. J Neurosurg. 2002;96(3):483-489. [DOI] [PubMed] [Google Scholar]
- 22.Mokri B. Familial occurrence of spontaneous spinal CSF leaks: underlying connective tissue disorder. Headache. 2008;48(1):146-149. [DOI] [PubMed] [Google Scholar]
- 23.Liu FC, Fuh JL, Wang YF, Wang SJ. Connective tissue disorders in patients with spontaneous intracranial hypotension. Cephalalgia. 2011;31(6):691-695. [DOI] [PubMed] [Google Scholar]
- 24.Reinstein E, Pariani M, Bannykh S, Rimoin DL, Schievink WI. Connective tissue spectrum abnormalities associated with spontaneous cerebrospinal fluid leaks: a prospective study. Eur J Hum Genet. 2013;21(4):386-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vishteh AG, Schievink WI, Baskin JJ, Sonntag VK. Cervical bone spur presenting with spontaneous intracranial hypotension. Case report. J Neurosurg. 1998;89(3):483-484. [DOI] [PubMed] [Google Scholar]
- 26.Eross EJ, Dodick DW, Nelson KD, Bosch P, Lyons M. Orthostatic headache syndrome with CSF leak secondary to bony pathology of the cervical spine. Cephalalgia. 2002;22(6):439-443. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida H, Takai K, Taniguchi M. Leakage detection on CT myelography for targeted epidural blood patch in spontaneous cerebrospinal fluid leaks: calcified or ossified spinal lesions ventral to the thecal sac. J Neurosurg Spine. 2014;21(3):432-441. [DOI] [PubMed] [Google Scholar]
- 28.Beck J, Ulrich CT, Fung C, et al. Diskogenic microspurs as a major cause of intractable spontaneous intracranial hypotension. Neurology. 2016;87(12):1220-1226. [DOI] [PubMed] [Google Scholar]
- 29.Schievink WI, Ross L, Prasad RS, Maya MM. Vanishing calcification associated with a spontaneous ventral spinal cerebrospinal fluid leak. Cephalalgia. 2016;36(14):1366-1369. [DOI] [PubMed] [Google Scholar]
- 30.Rosebrock RE, Diehn FE, Luetmer PH, et al. Penetrating osseous spicules causing high-flow ventral CSF leaks in the setting of relatively low BMI: a preliminary study. Clin Neuroradiol. 2018;28(4):539-543. [DOI] [PubMed] [Google Scholar]
- 31.Wang E, Wang D. Successful treatment of spontaneous intracranial hypotension due to prominent cervical cerebrospinal fluid leak with cervical epidural blood patch. Pain Med. 2015;16(5):1013-1018. [DOI] [PubMed] [Google Scholar]
- 32.Falatko SR, Kelkar P, Setty P, Tong D, Soo TM. C1-C2 cryptic cerebrospinal fluid leak directly identified by pressurized radionuclide cisternography: case report and review of the literature. Surg Neurol Int. 2015;6:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Decramer T, Van Dyck-Lippens PJ, Franken TP, et al. A small leak will sink the brain: targeted C1-C2 patching. World Neurosurg. 2017;101:816.e1-816.e3. [DOI] [PubMed] [Google Scholar]
- 34.Enomoto N, Mure H, Okazaki T, et al. Posttraumatic cerebrospinal fluid leak associated with an upper cervical meningeal diverticulum. World Neurosurg. 2018;116:50-55. [DOI] [PubMed] [Google Scholar]
- 35.Akiba C, Bandai H, Yoshitaka I, et al. Cerebrospinal fluid leak presented with the C1-C2 sign caused by spinal canal stenosis: a case report. BMC Neurol. 2020;20:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schievink WI, Moser FG, Maya MM. CSF-venous fistula in spontaneous intracranial hypotension. Neurology. 2014;83(5):472-473. [DOI] [PubMed] [Google Scholar]
- 37.Kumar N, Diehn FE, Carr CM, et al. Spinal CSF venous fistula: a treatable etiology for CSF leaks in craniospinal hypovolemia. Neurology. 2016;86(24):2310-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kranz PG, Amrhein TJ, Gray L. CSF venous fistulas in spontaneous intracranial hypotension: imaging characteristics on dynamic and CT myelography. AJR Am J Roentgenol. 2017;209(6):1360-1366. [DOI] [PubMed] [Google Scholar]
- 39.Duvall JR, Robertson CE, Cutsforth-Gregory JK, et al. Headache due to spontaneous spinal cerebrospinal fluid leak secondary to cerebrospinal fluid-venous fistula: case series. Cephalalgia. 2019;39(14):1847-1854. [DOI] [PubMed] [Google Scholar]
- 40.Chazen JL, Robbins MS, Strauss SB, Schweitzer AD, Greenfield JP. MR myelography for the detection of CSF-venous fistulas. AJNR Am J Neuroradiol. 2020;41(5):938-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.


