Abstract
Neurological complications are frequently reported in an intensive care unit (ICU), as a manifestation of a critical systemic illness or of its treatment. On the specific setting of COVID-19 patients, peripheral nerve lesions can have a multiplicity of causes, such as post-infectious neuropathy, positioning-related neuropathy or iatrogeny. An unusual but potentially disabling complication of any peripheral nerve lesion is Complex Regional Pain Syndrome (CRPS). Although there have been no mechanistic studies assessing how SARS-CoV-2 might directly impact nociception, it is hypothesized that the systemic hyperinflammation seen in severe COVID-19 may contribute to peripheral and central neuronal sensitization, possibly increasing the risk of developing CRPS. This case report highlights the potential hazards and consequences of peripheral nerve injuries on an ICU setting in COVID-19 patients, as well as the importance of a multidisciplinary approach for an early diagnosis and treatment, which are directly related to a better prognosis.
Keywords: COVID-19, Critical care, Complex regional pain syndrome, Peripheral nerve injury, Case report
Highlights
-
•
In an intensive care unit, neurological complications are frequently encountered.
-
•
CRPS may occur after peripheral nerve injuries, leading to increased disability.
-
•
The systemic hyperinflammation of severe COVID-19 may contribute to neuronal sensitization.
-
•
Peripheral and central neuronal sensitization can lead to chronical and disproportionate pain.
-
•
A multidisciplinary approach is important in prompt diagnosis and treatment.
COVID-19; Critical care; Complex regional pain syndrome; Peripheral nerve injury; Case report.
1. Introduction
Neurological complications are frequently reported in an intensive care unit (ICU), as a manifestation of a critical systemic illness or of its treatment [1]. These complications may present during the ICU stay (stroke, anoxic-ischemic injuries, peripheral nerve injuries, delirium, seizures) or after ICU discharge (Post-traumatic Stress Disorder and depression) [1]. Specifically on COVID-19 patients, peripheral nerve lesions (PNL) most commonly occur as a manifestation of SARS-CoV-2 infection (post-infectious neuropathy), a sequela of COVID-19 critical illness (positioning-related neuropathy) or as consequence of a treatment (iatrogenic lesions) [2].
Peripheral nerve lesions usually follow a well-recognized clinical course, depending on lesions’ topography and severity, but if there is a superimposed development of Complex Regional Pain Syndrome (CRPS), a diverse array of clinical features may develop [3, 4]. This syndrome is characterized by regional pain, seemingly disproportionate to the usual course of any known lesion, usually associated with a distal pattern of abnormal sensory, motor, sudomotor, vasomotor and/or trophic findings [3]. It may be clinically diagnosed through the Budapest Criteria and its severity could be accessed using the CRPS Severity Score (CSS) [3, 5]. The present therapeutic standard-of-care is a multimodality approach including patient education, rehabilitation, psychological support, and pharmacological intervention [4].
2. Case report
We report a case of 35-year-old woman, right-handed, previously independent in all basic and instrumental daily-life activities (DLA), with history of congenital confluent pink spots located on the dorsal surface of the hands and forearms, asthma and morbid obesity. The patient was admitted to an Infectious Diseases-ICU due to acute respiratory distress syndrome (ARDS) 5 days after the diagnosis of SARS-CoV-2 infection. The progressive respiratory failure led to the need of respiratory and vasopressor support. Invasive mechanical ventilation was started on the 6th ICU Day and by that time an arterial line was placed on the left brachial artery by an experienced physician (through the antecubital fossa, guided by superficial anatomy references).
Twenty-four-hours later, the patient presented with a hematoma next to the line insertion site and absence of radial pulse. An upper-limb-doppler was requested, depicting a reduction of both radial and ulnar arteries flow. As so, the line was removed, and anticoagulation was started. A favorable clinical evolution was observed, and the patient was weaned from ventilation at the 12th ICU Day.
Ten days later, the neuro-motor examination revealed an asymmetric muscular strength impairment affecting mainly the left upper limb - according to Medical Research Council (MRC) scale, the score for the segments of the right upper and lower limbs strength was 4/5, also 4/5 for the left shoulder abduction and 3/5 for the left elbow flexion and extension, wrist flexion and extension and palmar prehension - with normal passive and active range of motion on all segments of the upper and lower limbs, normal deep tendon reflexes and muscular tonus, without any superficial sensorial alterations or complaints.
Due to the asymmetric muscular strength impairment, an electromyography was requested, depicting severe axonal lesion of the median nerve on the forearm. On nerve conduction studies, a significantly increased latency and decreased amplitude was reported on the left limb, both on the segments wrist-abductor pollicis brevis and elbow-wrist. On needle electromyography, signs of acute partial denervation on the left abductor pollicis brevis, first dorsal interosseous and flexor carpi radialis were identified. No other peripheral nerve injuries were identified.
An ultrasonographic evaluation was then performed, excluding the presence of hematomas on the nerve path or on other accessible plans on the superior left upper limb. Regarding pharmacological treatment, we highlight the absence of neurotoxic medications administered previously and during the hospital stay, as reported on Table 1. After sustained clinical stability, the patient was transferred to a rehabilitation facility.
Table 1.
Pharmacological treatment administered to the patient.
| Before hospitalization | During Hospitalization | After discharge |
|---|---|---|
| Desogestrel 0.075 mcg/day |
Bronchodilators Ipratropium Bromide, 80 mcg; 4/day; 28 days Salbutamol, 100 mcg; 3/day; 30 days |
Desogestrel 0.075 mcg /day |
| Fluticasone/Salmeterol 250 mcg/50 mcg 1 inhalation on demand | Fluticasone/Salmeterol 250 mcg/50 mcg 1 inhalation on demand | |
|
Corticosteroids Dexamethasone, 7.5mg; 1/day; 10 days Prednisolone, 20mg; 1/day; 2 days |
||
|
Antivirals Oseltamivir, 75mg; 2/day; 5 days | ||
|
Non-steroidal anti-inflammatory drugs Ketorolac, 30mg; 1/day; 4 days Diclofenac, 75mg; 1/day; 4 days Ibuprofen, 400mg; 3/day; 2 days Parecoxib, 40mg; 1/day; 4 days | ||
|
Anti-thrombotic Aspirine, 100mg; 1/day; 10 days Enoxaparine, 60mg, 1/day, 5 days; 80mg, 3/day; 15 days; 120mg; 1/day; 5 days | ||
|
Antibiotics Ampicilin, 2000mg; 2/day; 3 days Ceftriaxone, 1000mg; 1/day; 6 days Piperacillin/tazobactam, 4000 + 500mg, 1/day; 5 days Vancomicine, 1000mg; 2/day; 5 days | ||
|
Proton pump inhibitors Pantoprazole, 40mg; 1/day; 19 days | ||
|
Antiemetics Ondansetron, 8mg; 1/day; 6 days Metoclopramide, 10mg; 3/day; 6 days | ||
|
Diuretics Furosemide 40mg; 2/day; 2 days -> 20mg; 1/day; 16 days Spironolactone 50mg; 1/day; 3 days | ||
|
Laxatives Bisacodyl, 10mg; 3/day; 4 days Lactulose, 15ml; 3/day; 5 days | ||
|
Neuromuscular block Rocuronium bromide, 7 days | ||
|
Sedative-Hypnotic Propofol, 11 days Fentanil, 13 days Dexmedetomidine, 6 days Zolpidem, 10mg; 1/day; 2 days | ||
|
Vasopressor support Noradrenaline, 7 days |
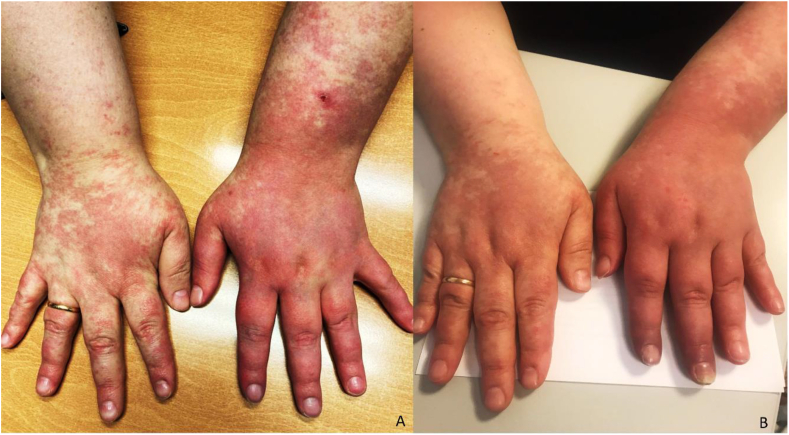
On the clinical appointment three months after discharge, the patient presented with moderate pain of the left hand (Numeric Scale of Pain: 4/10), pinprick hyperesthesia on the dorsal surface of the hand, regional temperature and skin color asymmetry (with increased regional temperature and redness on the affected hand), trophic changes (absence of nails growth and altered skin texture - thickness), hand edema and a “pointing finger” deformity, maintaining the muscular strength impairment with predominant involvement of the distal left upper limb (Figure 1A). These complains impacted the performance of some DLA, namely driving, shopping and manual tasks requiring fine motor control (e.g. preparing food, management of financial matters and medication). Due to these clinical manifestations, and in accordance with Budapest Criteria, CRPS was diagnosed, with a severity score of 14 according to CSS (Table 1).
Figure 1.
A: Clinical presentation on the first appointment. B: Clinical presentation on the second appointment, after multimodality therapeutic intervention.
A multimodality therapeutic approach was started, including patient education, rehabilitation and pharmacologic intervention. The patient education was performed by a Neurologist and a Physical Medicine and Rehabilitation doctor, in accordance with the European Federation of Pain recommendations [4]. The rehabilitation approach included twice-a-week one-hour sessions of occupational therapy with the following techniques: contrast baths, joint mobilization of the hand and wrist, manual isometric muscular strengthening of intrinsic and extrinsic hand muscles, mirror visual feedback therapy, fine motor control reeducation and analgesic massage. The pharmacologic intervention consisted of a 2-week cycle of ibuprofen 400mg three-times a day.
After eight weeks and fifteen rehabilitation sessions, there was a significative improvement on the patients' subjective complains and on objective measurements. Regarding subjective complains, the patient suffered paroxysmal pain less frequently, reported a subjective muscular strength increase that was confirmed on neuro-motor examination (scoring 4/5 on the MRC on the hand and wrist segments and 5/5 on the proximal segments of the left upper limb), maintaining the “pointing finger” deformity and an asymmetric vaso/sudomotor pattern. On objective measurements, the CSS was 7 points lower (Figure 1B), which represented as significant improvement in accordance with CSS smallest real difference value (4,9 points) [5]. Moreover, the patient was already able to perform all basic DLA and almost all instrumental DLA, including shopping, driving for small distances, managing financial matters and medication. Nonetheless, the patient still reported some disability on some steps of food preparation and heavy domestic work due to the lack of manual dexterity. Consequently, the therapeutic approach was further tailored, focusing specially on muscular strengthening and stretching, as well as on normalization of hand use and gesture reeducation, alongst with the prescription of ibuprofen on demand. Follow-up appointments were scheduled each 8–12 weeks to evaluate patients’ evolution and optimize therapeutic interventions.
3. Discussion
Irrespective of the admission diagnosis, PNL are not rare in the ICU setting, and can occur after intravenous or intra-arterial line placement or removal. The most frequently affected nerves are the superficial branch of the radial nerve, the medial and lateral antebrachial cutaneous nerves and the radial and ulnar dorsal sensory branches of the hand [1]. PNL may result from pressure neuropraxia secondary to fluid extravasation or hematoma near the cannulation site, from chemical damage from medications or it can be directly inflicted by the needle [1]. CRPS is an unusual (incidence of 0.82/100000 person-years) but potentially disabling complication of any PNL, typically classified as type 2 in this context [4]. Given the heterogeneous and labile nature of the syndrome, clinical presentations may differ substantially between patients and even for the same patient time; therefore, assessment, tracking of changes and therapeutic planning may be challenging in the setting of CRPS.
To our knowledge, this is the first report of CRPS after an iatrogenic nervous lesion on a critically ill COVID-19 patient. Although there have been no dedicated mechanistic studies at how the SARS-CoV-2 virus might directly impact nociception, the broad systemic hyperinflammation seen in severe COVID-19 may contribute to peripheral and central neuronal sensitization, leading to chronic and possibly disproportionate pain, which can be a possible explanation for the development of CRPS on this case [2].
4. Conclusions
This case highlights a potential hazard of arterial puncture of the brachial artery - peripheral nervous lesions -, even under controlled conditions, specifically on COVID-19 patients. Although unusual, CRPS may occur after a peripheral nerve injury, leading to potentially significant function losses. An effective multidisciplinary approach is extremely important to attain a prompt diagnosis and successful treatment. We highlight the need of an increased awareness of this syndrome and its diagnostic and therapeutic approaches, both on acute and subacute stages after an injury [6]. This knowledge is critically important, because early diagnosis and treatment are associated with better prognosis [4, 7].
Declarations
Author contribution statement
All authors listed have significantly contributed to the investigation, development and writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
No data was used for the research described in the article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Rubinos C., Ruland S. Neurologic complications in the intensive care unit. Curr. Neurol. Neurosci. Rep. 2016;16(6):57. doi: 10.1007/s11910-016-0651-8. [DOI] [PubMed] [Google Scholar]
- 2.McFarland A.J., Yousuf M.S., Shiers S., Price T.J. Neurobiology of SARS-CoV-2 interactions with the peripheral nervous system: implications for COVID-19 and pain. Pain Rep. 2021;6(1):e885. doi: 10.1097/PR9.0000000000000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harden N.R., Bruehl S., Perez R., et al. Validation of proposed diagnostic criteria (the "Budapest Criteria") for complex regional pain syndrome. Pain. 2010;150(2):268–274. doi: 10.1016/j.pain.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goebel A., Barker C., Birklein F., et al. Standards for the diagnosis and management of complex regional pain syndrome: results of a European Pain Federation task force. Eur. J. Pain. 2019;23(4):641–651. doi: 10.1002/ejp.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harden R.N., Maihofner C., Abousaad E., et al. A prospective, multisite, international validation of the complex regional pain syndrome severity score. Pain. 2017;158(8):1430–1436. doi: 10.1097/j.pain.0000000000000927. [DOI] [PubMed] [Google Scholar]
- 6.Miller C., Williams M., Heine P., Williamson E., O'Connell N. Current practice in the rehabilitation of complex regional pain syndrome: a survey of practitioners. Disabil. Rehabil. 2019;41(7):847–853. doi: 10.1080/09638288.2017.1407968. [DOI] [PubMed] [Google Scholar]
- 7.Storz C., Kraft E. Schmerz; (Berlin, Germany): 2021. [Occupational Therapy for Complex Regional Pain Syndrome] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.