ABSTRACT
Rabies, caused by rabies virus (RABV), is fatal to both humans and animals around the world. Effective clinical therapy for rabies has not been achieved, and vaccination is the most effective means of preventing and controlling rabies. Although different vaccines, such as live attenuated and inactivated vaccines, can induce different immune responses, different expressions of pattern recognition receptors (PRRs) also cause diverse immune responses. Toll-like receptor 4 (TLR4) is a pivotal PRR that induces cytokine production and bridges innate and adaptive immunity. Importantly, TLR4 recognizes various virus-derived pathogen-associated molecular patterns (PAMPs) and virus-induced damage-associated molecular patterns (DAMPs), usually leading to the activation of immune cells. However, the role of TLR4 in the humoral immune response induced by RABV has not yet been revealed. Based on TLR4-deficient (TLR4−/−) and wild-type (WT) mouse models, we report that TLR4-dependent recruitment of the conventional type 2 dendritic cells (CD8α− CD11b+ cDC2) into secondary lymph organs (SLOs) is critical for antigen presentation. cDC2-initiated differentiation of follicular helper T (Tfh) cells promotes the proliferation of germinal center (GC) B cells, the formation of GCs, and the production of plasma cells (PCs), all of which contribute to the production of RABV-specific IgG and virus-neutralizing antibodies (VNAs). Collectively, our work demonstrates that TLR4 is necessary for the recruitment of cDC2 and for the induction of RABV-induced humoral immunity, which is regulated by the cDC2-Tfh-GC B axis.
IMPORTANCE Vaccination is the most efficient method to prevent rabies. TLR4, a well-known immune sensor, plays a critical role in initiating innate immune response. Here, we found that TLR4-deficient (TLR4−/−) mice suppressed the induction of humoral immune response after immunization with rabies virus (RABV), including reduced production of VNAs and RABV-specific IgG compared to that occurred in wild-type (WT) mice. As a consequence, TLR4−/− mice exhibited higher mortality than that of WT mice after challenge with virulent RABV. Importantly, further investigation found that TLR4 signaling promoted the recruitment of cDC2 (CD8α+ CD11b−), a subset of cDCs known to induce CD4+ T-cell immunity through their MHC-II presentation machinery. Our results imply that TLR4 is indispensable for an efficient humoral response to rabies vaccine, which provides new insight into the development of novel rabies vaccines.
KEYWORDS: TLR4, humoral immunity, rabies virus, cDC2, lymph organs
INTRODUCTION
Rabies has caused fear and illness in humans and animals for thousands of years and is responsible for 59,000 human fatalities annually in recent years (1, 2). Rabies is caused by rabies virus (RABV), which is a member of the Lyssavirus genus within the Rhabdoviridae family. RABV infects the central nervous system (CNS), and causes serious nerve-related symptoms, including muscle spasms, paralysis, and encephalitis. Once symptoms occur, rabies is usually fatal (3, 4). Thus, prevention and control of rabies through vaccination of both humans and animals remains a priority. Various rabies vaccines have been developed for different applications, such as live attenuated vaccines for wildlife and inactivated vaccines for human and pets, and application of these vaccines in rabies control and elimination programs has achieved great success (5–8).
Toll-like receptors (TLRs) are evolutionarily conserved pattern recognition receptors (PRRs) belonging to a group of type I transmembrane proteins that recognize various microbial structural motifs called pathogen-associated molecular patterns (PAMPs) and various endogenous ligands known as damage-associated-molecular-patterns (DAMPs) (9). As a pivotal PRR, TLR4 recognizes various virus-derived PAMPs and virus-induced DAMPs, and this usually leads to the activation of immune cells (10, 11). Lipopolysaccharide (LPS), derived from the outer membrane of Gram-negative bacteria, is a well-known PAMP recognized by TLR4. LPS-detoxified TLR4-ligand monophosphoryl lipid A (MPLA) is an FDA-approved adjuvant for vaccines, and studies have shown that MPLA can promote the efficacy of rabies vaccines (12, 13). RABV induces the expression and accumulation of heat shock protein 70 kDa (Hsp70) in Negri body-like structures, where viral transcription and replication take place (14). Cellular heparan sulfate (HS) supports RABV adhesion and the subsequent entry of the virus into target cells. An interaction between RABV glycoprotein and HS has been proved (15). Overexpression of high-mobility group box 1 protein (HMGB1) promoted the activation of dendritic cells (DCs) and leads to a higher level of RABV-specific virus-neutralizing antibodies (VNA) (16). Previous studies found that several DAMPs, namely Hsp70, HS and HMGB1, were involved in the activation of TLR4-dependent pathways (11, 17) and infection by RNA viruses (18). Both directly virus-derived PAMPs and indirectly virus-induced DAMPs can manipulate TLR4 signaling to restore immune cell homeostasis when pathogen-induced immune responses occur.
Classical DCs (cDCs) are specialized antigen (Ag)-processing cells that efficiently present endogenous and exogenous Ags in the major histocompatibility complex (MHC) to other immune cells, thus forming a critical interface between innate and adaptive immunity. Mouse cDCs have traditionally been classified into two groups, the conventional type 1 dendritic cells (cDC1; CD8α+ CD11b−) and the conventional type 2 dendritic cells (cDC2; CD8α− CD11b+). cDC1 are functionally specialized in that they cross-present exogenous Ags on MHC-I molecules to CD8+ T cells (19). cDC2 are superior in taking up exogenous antigens and are particularly efficient at processing antigen for presentation on MHC-II and induce superior CD4+ T-cell proliferation compared to that induced by cDC1 (20, 21). In the present study, TLR4−/−and wild-type (WT) mice immunized with rabies vaccines and the induction of humoral immune response were compared. We found that TLR4 signaling modulates the balance between cDC1 and cDC2. TLR4-dependent recruitment of cDC2 promotes follicular helper T (Tfh) differentiation, leading to activation of germinal center cells (GCs) and proliferation of GC B and plasma cells (PCs). TLR4-dependent initiation of the cDC2-Tfh-GC B-cell axis is critical for the humoral immune response induced by rabies vaccines.
RESULTS
TLR4, but not TLR2, promotes the humoral immunity induced by RABV.
It is well known that RABV-specific virus-neutralizing antibodies (VNAs) interfere with the binding of RABV virions to receptors and viral fusion with host cells, thereby preventing viral infection (22, 23). To investigate the role of TLR4 in the RABV-induced humoral immune response, WT and TLR4−/−mice were immunized with a live RABV vaccine strain. LBNSE, and the levels of VNAs and RABV-specific IgG in the serum of mice were measured. In TLR4−/− mice, live RABV induced a decrease in VNA (Fig. 1A) and RABV-specific IgG (Fig. 1B) levels. To determine whether these humoral immune responses can protect WT and TLR4−/− mice against virulent RABV infection, at 6 weeks after immunization, mice were intracranially (i.c.) challenged with 50× the 50% lethal dose (LD50) of virulent RABV strain CVS-24. The results showed that approximately 50% of the WT mice but only 18% of the TLR4−/− mice survived RABV challenge (Fig. 1C). As a control, TLR2 deficiency had no impact on rabies vaccine-induced humoral immune response, including on VNA (Fig. 1D) and RABV-specific IgG (Fig. 1E) levels. Furthermore, no obvious difference in survival ratio between the WT and the TLR2−/− mice was observed (Fig. 1F). These results suggest that TLR4 deficiency, but not TLR2 deficiency, impairs the humoral immune response induced by live RABV, indicating that the TLR4 signaling pathway is critical for RABV-induced humoral immunity.
FIG 1.
Toll-like receptor 4 (TLR4) deficiency impairs rabies virus (RABV)-induced humoral immune response and decreases protection against virulent RABV. (A to F) Wild-type (WT), TLR4−/−, and TLR2−/− mice (n = 10 to 12/group) were intramuscularly (i.m.) immunized with live RABV (1 × 107 fluorescent focus units [FFU]/mouse). (A and D) Values of virus-neutralizing antibodies (VNAs) in TLR4−/− mice (A) and TLR2−/− (D) mice at the indicated time points. (B and E) Values of RABV-specific IgG in TLR4−/− mice (B) and TLR2−/− (E) mice. (C and F) Survival curves of TLR4−/− mice (C) and TLR2−/− mice (F) intracranially (i.c.) challenged with 50× the 50% lethal dose (LD50) of CVS-24 at 6 weeks after immunization. Statistical data are presented as mean ± standard error of the mean (SEM). Asterisks indicate significant differences between the indicated experimental groups; *, P < 0.05; **, P < 0.01; ns, not significant.
Maturation of RABV-stimulated APCs is TLR4 independent in vitro or in vivo.
Several lines of evidence suggest that TLR4 signaling is critical for the maturation of antigen-presenting cells (APCs), including DCs and B cells, when these APCs were activated by pathogens (24). In response to viral infection, DCs act as the major APCs that initiate the downstream adaptive immunity. To analyze the impact of TLR4 on DC maturation in vitro, primary bone marrow-derived dendritic cells (BMDCs) derived from WT and TLR4−/− mice were cultured, and the expression of CD80, CD86, and MHC-II on the cell surface — markers for maturing DCs — was measured. A well-described ligand of TLR4, LPS induced significant upregulation of CD80, CD86, and MHC-II molecules in WT BMDCs but failed to do so in TLR4−/− BMDCs. In contrast, the expression of CD80, CD86, and MHC-II on TLR4−/− BMDCs was comparable to that on WT BMDCs post live RABV infection (Fig. 2A to C).
FIG 2.
TLR4 deficiency does not affect the maturation of dendritic cells (DCs) or B cells after RABV stimulation. (A to F) Bone marrow-derived dendritic cells (BMDCs) and splenic B cells obtained from WT and TLR4−/− mice were cultured and stimulated with live RABV or lipopolysaccharide (LPS) or with Dulbecco’s modified Eagle’s medium (DMEM) as the control group (n = 3 or 4/group). At 24 h after stimulation, all cells were stained with antibodies that recognize specific markers, including CD11c for BMDCs and B220 for splenic B cells. (A and D) Gating strategies for detection of activated BMDCs (A) and splenic B cells (D) expressing CD80, CD86, or MHC-II. (B and E) Representative flow cytometry plots of activated BMDCs (B) and splenic B cells (E). Percentages of activated BMDCs (C) and splenic B cells (F). (G to J) WT and TLR4−/− mice (n = 5/group) were immunized i.m. with live RABV (1 × 107 FFU/mouse). At 3 dpi, all mice were euthanized, their inguinal lymph nodes (LNs) were collected and dispersed, and single-cell suspensions were prepared and stained with antibodies that specifically recognize activated classical DCs (cDCs). (G) Gating strategies for the detection of CD11c+ CD80+ cDC, CD11c+ CD86+ cDC, and CD11c+ MHC-II+ cDC within LNs. (H) Representative flow cytometry plots of activated cDCs within LNs. Percentage of activated cDCs within LNs between WT mice and TLR4−/− mice (I) or between the vaccinated mice and naive mice (J). Data are presented as mean ± standard deviation (SD). Asterisks indicate significant differences between the groups; **, P < 0.01; ***, P < 0.001; ns, not significant.
RABV-infected B cells, as APCs for helper T cells, are responsible for the induction of early protective T cell-dependent B-cell responses (25, 26). Thus, we cultured primary splenic B cells in vitro and performed an experiment similar to that we performed with primary BMDCs. Only upregulation of CD86 and MHC-II was observed in splenic B cells after stimulation. As expected, splenic B cells exhibited a similar trend to that observed in BMDCs upon stimulation with live RABV, LPS, or Dulbecco’s modified Eagle’s medium (DMEM) (Fig. 2D to F).
Previous studies have shown that cDCs are critical for the establishment of optimal immune responses after RABV immunization (23, 27). To further investigate the role of TLR4 deficiency in the maturation of cDCs, WT and TLR4−/− mice were immunized with live RABV. At 3 days postinfection (dpi), the expression of CD80, CD86, and MHC-II on cDCs from the inguinal lymph nodes (LNs) was analyzed by flow cytometry. Surprisingly, no difference was observed between WT and TLR4−/− mice in the expression of CD80, CD86, or MHC-II by cDCs (Fig. 2G to J). These results suggest that LPS-induced maturation of APCs depends on TLR4 signaling, while RABV-induced maturation of APCs does not.
Both TLR4-specific ligand and RABV induce the upregulation of cDC2 in spleen.
Our previous studies showed that MPLA, a TLR4-specific ligand derived from lipooligosaccharide (LOS), promoted the maturation of BMDCs and cDCs in LNs (12). Although pathogen-induced maturation of cDCs is the first step in the progression of antigen presentation, the second step, migration of antigen peptide-bearing cDCs from peripheral tissues into lymph organs, also plays a critical role in antigen presentation. In humans and mice, cDCs can be classified into cDC1, which have a preferential capacity for antigen cross-presentation via MHC-I molecules to CD8+ T cells, and cDC2, which display a bias for presentation to CD4+ T cells via MHC-II molecules (28). Thus, after groups of mice were separately immunized with DMEM, live RABV, inactivated RABV, or MPLA, we analyzed the composition of cDCs, including that of cDC1 and cDC2 in spleens. The gating strategies used for identifying cDC1 (CD8α+ CD11b−)/cDC2 (CD8α− CD11b+) are shown in Fig. 3A. The results presented in Fig. 3B to D showed that live RABV and MPLA significantly increased the number of cDC2 but not cDC1 in the spleens; in contrast, inactivated RABV failed to cause any changes in the balance between cDC1 and cDC2.
FIG 3.
Both TLR4 and live RABV promote accumulation of cDC2 in lymph organs of mice. (A to D) Groups of WT mice (n = 4 or 5/group) were immunized i.m. with live RABV (1 × 107 FFU/mouse), inactivated RABV (1 × 107 FFU/mouse), monophosphoryl lipid A (MPLA; 20 μg/mouse), or DMEM. At 3 dpi, the spleens were collected and dispersed, and single-cell suspensions were separated into cDC1 (CD8α+ CD11b−) and cDC2 (CD8α− CD11b+) by gating with CD3− CD19− CD11c+ MHC-II+ antibodies. (A) Gating strategies for detection of cDC1/cDC2. (B) Representative flow cytometry plots of cDC1/cDC2 within spleens. Percentages of (C) cDC2 and (D) cDC1. (E to J) WT and TLR4−/− mice (n = 3 to 5/group) were immunized i.m. with live RABV (1 × 107 FFU/mouse), inactivated RABV (1 × 107 FFU/mouse), or DMEM. At 3 days postinfection (dpi), the inguinal LNs and spleens of mice were collected. cDC1 and cDC2 were analyzed by flow cytometry. (E and H) Representative flow cytometry plots of cDC1/cDC2 within LNs (E) and spleens (H). (F, G, I, and J) Percentages of cDC2 in LNs (F) and spleens (I) and of cDC1 in LNs (G) and spleens (J). Data are presented as mean ± SEM. Asterisks indicate significant differences between the groups; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant.
TLR4 is indispensable for RABV-induced upregulation of cDC2 in lymph organs.
Our data showed that both TLR4-specific ligand and live RABV could induce the upregulation of cDC2 in spleens. To investigate whether TLR4 deficiency affects the composition of cDCs in lymph organs from mice immunized with rabies vaccine, WT and TLR4−/− mice were immunized with live or inactivated RABV. At 3 dpi, cDC1 and cDC2 within secondary lymphoid organs (SLOs), including inguinal LNs and spleens, were analyzed by flow cytometry. Representative plots showing the numbers of cDC1 and cDC2 within inguinal LNs and spleens are presented in Fig. 3E and H, respectively.
Indeed, in the steady-state (control group), no differences were observed between WT and TLR4-deficient mice in the numbers of cDC1 and cDC2 in SLOs. Interestingly, in the inflammatory state, live RABV activated fewer cDC2 (Fig. 3F and I) but more cDC1 (Fig. 3G and J) in the SLOs of TLR4−/− mice than in those of WT mice. Consistent with earlier experimental results, the numbers of cDC1 and cDC2 were not altered by inactivated RABV. These data indicate that the absence of TLR4 causes reduced number of cDC2, but not cDC1, in lymph organs from mice immunized with live RABV.
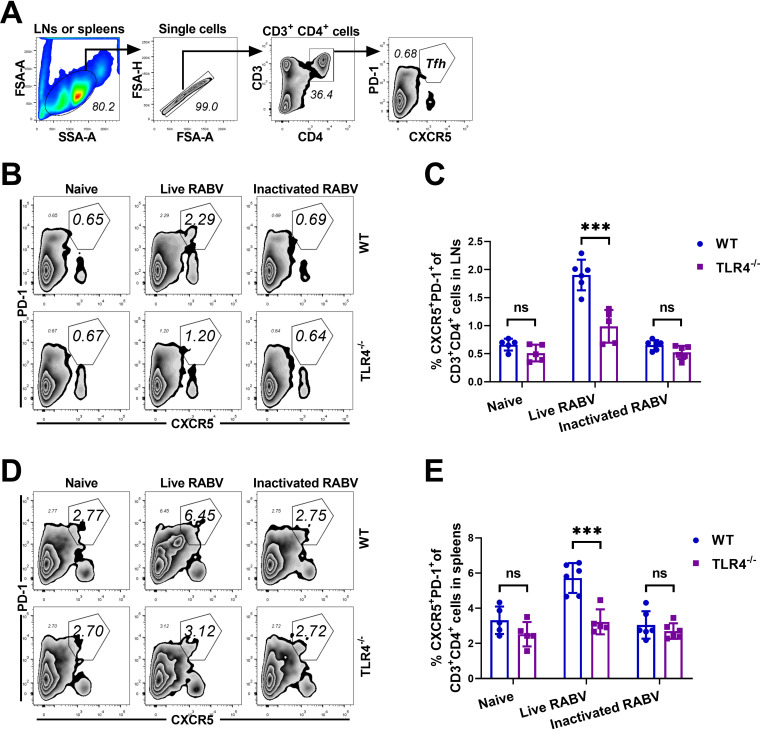
TLR4 promotes generation of Tfh cells in lymph organs after RABV immunization.
Humoral immune response mediated by CD4+ T cells is important for RABV clearance (29, 30). Follicular helper T (Tfh) cells are specialized providers of T cells that help B cells and are essential for germinal center (GC) formation, GC B affinity maturation, the development of memory B cells, and production of the highest-affinity antibodies (31). cDCs efficiently prime Tfh cells, especially the cDC2 subset of these cells, in a process that plays an essential role in promoting the commitment of activated T cells to the Tfh lineage (31, 32). To investigate the role of TLR4 signaling in promoting Tfh cell differentiation, WT and TLR4−/− mice were immunized with live RABV, inactivated RABV, or DMEM. At 7 dpi, the inguinal LNs and spleens of the mice were harvested, and the Tfh cells in these organs were analyzed by flow cytometry. We gated on Tfh cells (CD3+ CD4+ CXCR5+ PD-1+) within inguinal LNs or spleens (Fig. 4A); representative plots of Tfh cells within inguinal LNs and spleens are shown in Fig. 4B and D, respectively.
FIG 4.
TLR4 deficiency impairs the generation of Tfh cells in lymph organs after rabies immunization. (A to E) WT and TLR4−/− mice (n = 5/group) were immunized i.m. with live RABV (1 × 107 FFU/mouse), inactivated RABV (1 × 107 FFU/mouse), or DMEM. At 7 dpi, these inguinal LNs and spleens of mice were collected and dispersed, and single-cell suspensions were stained with antibodies representing Tfh cells (CXCR5+ PD-1+) gated from CD3+ CD4+ cells. (A) Gating strategies for detection of Tfh cells. (B and D) Representative flow cytometry plots of Tfh within LNs (B) and spleens (D). (C and E) Percentages of Tfh cells within LNs (C) and spleens (E). Data are presented as mean ± SD. Asterisks indicate significant differences between the groups; ***, P < 0.001; ns, “not significant.
Previous studies have demonstrated that impaired cDC2 migration in DC-specific DOCK8−/− or IRF4−/− mice leads to reduced Tfh-cell priming and antibody development, whereas loss of CD103+ cDC1 in BATF3−/− mice does not (32). As expected, a significant decrease in the numbers of Tfh cells within inguinal LNs (Fig. 4C) and spleens (Fig. 4E) was observed in TLR4−/− mice immunized with live RABV compared to WT mice. In contrast, after immunization with inactivated RABV, no significant difference between WT and TLR4−/− mice in the percentage of Tfh cells was observed. While live RABV induced an inflammatory response, which was consistent with the increased number of cDC2 in SLOs, there was an associated increase in the percentage of Tfh cells, suggesting that TLR4 deficiency impairs the generation of Tfh cells in vivo and that cDC2 play an important role in promoting Tfh cell generation within SLOs.
TLR4 mediates the balance between Th1 and Th2 response after RABV immunization.
IgG2a are particularly potent in host defenses against viral infections due to their specific capacity to activate the complement system, bind to Fc receptors expressed on phagocytes, and induce Ab-dependent cell-mediated cytotoxicity (33, 34). To analyze the Ab isotypes produced after immunization with RABV, WT and TLR4−/− mice were immunized with live RABV, and the antibodies they produced were isotyped. As shown in Fig. 5A, TLR4−/− mice mounted strong IgG1 responses but failed to efficiently produce IgG2a upon immunization with live RABV. In mice, Th1-dependent gamma interferon (IFN-γ) induces the production of IgG2a, whereas the Th2 cytokine interleukin 4 (IL-4) stimulates the expression of IgG1; thus, immunoglobulins (Ig) of these specific isotypes are indicators of the underlying Th cell response (35).
FIG 5.
TLR4 deficiency impairs the generation of gamma interferon (IFN-γ)-secreting cells and the production of IgG2a after RABV immunization. (A) Live RABV-induced immunoglobulin (Ig) subsets in the serum of WT and TLR4−/− mice (n = 10/group) were measured, and the levels of RABV-specific IgG2a, IgG1, and IgG1/IgG2a were statistically analyzed. Data are presented as mean ± SEM. (B to E) WT and TLR4−/− mice (n = 5/group) were immunized i.m. with live RABV (1 × 107 FFU/mouse), inactivated RABV (1 × 107 FFU/mouse), or DMEM. At 7 dpi, all mice were euthanized, and their spleens were collected and cultured. (B and D) Representative plots of IFN-γ-secreting cells (B) and IL-4-secreting cells (D). (C and E) Results obtained for IFN-γ-secreting cells (C) and IL-4-secreting cells (E). Data are presented as mean ± SD. Asterisks indicate significant differences between the groups; *, P < 0.05; ***, P < 0.001. ns, not significant.
To further investigate the link between IgG isotypes and Th1/Th2 responses, WT and TLR4-deficient mice were immunized with live RABV, inactivated RABV, or DMEM. At 7 dpi, the spleens of the WT and TLR4−/− mice were harvested, the spleen cells were cultured, and the numbers of Th cells producing IFN-γ or IL-4 were assessed. Representative plots of IFN-γ-producing Th cells and IL-4-producing Th cells are presented in Fig. 5B and D, respectively.
As observed in enzyme-linked immunosorbent spot assay (ELISpot) experiments, TLR4−/− mice produced slightly fewer IFN-γ-producing Th cells (Fig. 5C) and slightly more IL-4-producing Th cells (Fig. 5E) than WT mice, although live RABV infection induced robust Th1 and Th2 responses in both WT and TLR4-deficient mice. Inactivated RABV and DMEM both failed to induce IFN-γ-producing Th cells and IL-4-producing Th cells in WT and TLR4−/− mice. The above-described data demonstrate that TLR4 signaling promotes the generation of IFN-γ-producing Th cells and the Th1 response while inhibiting the generation of IL-4-producing Th cells and the Th2 response.
TLR4 deficiency impairs the formation of GCs after RABV immunization.
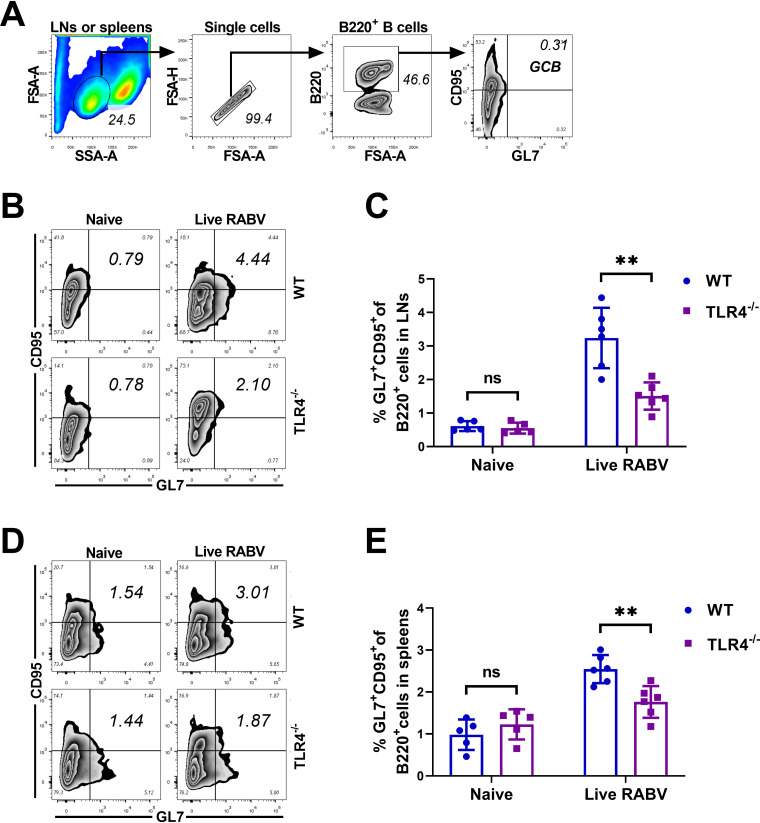
GCs are morphological structures in SLOs, including LNs and spleens, in which Ag-experienced B cells undergo diversification and affinity maturation; during this process, B cells proliferate with concurrent somatic hypermutation (SHM) of their immunoglobulin variable region (IgV) genes. High-affinity GC B cells survive with the help of signals provided by Tfh cells and return to the dark zone for further rounds of proliferation (36). The quality and size of GCs are directly regulated by Tfh cells, which in this way provide growth and differentiation signals to GC B cells. In our study, the effect of TLR4 signaling on GC formation was assessed after immunization of mice with live or inactivated RABV. Inguinal LNs were collected at 7 dpi, fixed, and sliced into 30-μm-thick sections for immunofluorescent staining using markers for B220, IgG, and GL7. As shown in Fig. 6A to C, significantly more GCs were observed in the inguinal LNs of WT mice than in the LNs of TLR4−/− mice after immunization with live RABV. Hence, TLR4 deficiency decreases the number of GCs induced in vivo by live RABV.
FIG 6.
TLR4 deficiency impairs the formation of germinal centers (GCs) in LNs after RABV immunization. (A to C) WT and TLR4−/− mice (n = 5/group) were immunized i.m. with live RABV (1 × 107 FFU/mouse) or DMEM. At 7 dpi, all mice were euthanized, and their inguinal LNs were sliced into 30-μm-thick sections and used to analyze the formation of GCs. (A and B) Representative fluorescent images of tissue sections obtained from WT mice (A) and TLR4−/− mice (B). Blue, B220; green, IgG; red, GL7. Bar, 500 μm. (C) Number of GCs formed. Data are presented as mean ± SD. Asterisks indicate significant differences between the groups; *, P < 0.05; ns, not significant.
To evaluate the influence of TLR4 deficiency on the proliferation of GC B cells, WT and TLR4-deficient mice were immunized with live RABV or injected with DMEM. At 7 dpi, immune cells in the inguinal LNs or spleens of the animals were harvested and analyzed by flow cytometry (Fig. 7A) with gating on GC B cells (B220+ GL7+ CD95+). Representative plots for GC B cells from the inguinal LNs (Fig. 7B) and spleens (Fig. 7D) are shown. As shown in Fig. 7C and E, decreased numbers of GC B cells were observed in the inguinal LNs and spleens of TLR4-deficient mice immunized with live RABV. These data suggest that TLR4 deficiency impairs the formation of GCs and the proliferation of GC B cells post live RABV immunization.
FIG 7.
TLR4 deficiency impairs the proliferation of GC B cells after RABV immunization. (A to E) WT and TLR4−/−mice (n = 5/group) were immunized i.m. with live RABV (1 × 107 FFU/mouse) or DMEM. At 7 dpi, the inguinal LNs and spleens of mice were collected and dispersed, and single-cell suspensions were stained with antibodies that specifically recognize GC B cells (GL7+ CD95+) gated from B220+ cells. (A) Gating strategies for detection of GC B cells. (B and D) Representative flow cytometry plots of GC B cells within LNs (B) and spleens (D). (C and E) Statistical percentages of GC B cells within LNs (C) and spleens (E). Data are presented as mean ± SD. Asterisks indicate significant differences between the groups; **, P < 0.01; ns, not significant.
GC B cells can differentiate into either memory B cells or plasma cells (PCs), which secrete large quantities of antibodies in response to antigens. The GC reaction usually determines the number of PCs and the quantity of VNAs (37, 38). Therefore, the effect of TLR4 signaling on PC production in bone marrow (BM) was investigated after immunization with live RABV. As shown in Fig. 8A to C, the number of PCs in TLR4−/− mice decreased significantly compared to that in WT mice. Hence, TLR4 deficiency leads to a reduced production of PCs in mice immunized with live RABV vaccines.
FIG 8.
TLR4 deficiency impairs the proliferation of plasma cells after RABV immunization. (A to C) WT and TLR4−/− mice (n = 5/group) were immunized i.m. with live RABV (1 × 107 FFU/mouse) or DMEM. At 14 and 21 dpi, all mice were euthanized, and BM cells were collected from the femurs and tibias of the animals. (A) Gating strategies for detection of PCs (B220low CD138+). (B) Representative flow cytometry plots of PCs. (C) Statistical results obtained for PCs. (D) Diagram of the various immune components and their roles in inducing the cDC2-Tfh-GC B axis. Data are presented as mean ± SD. Asterisks indicate significant differences between the groups; *, P < 0.05; ns, not significant.
Together, our data demonstrate that in the presence of TLR4 live RABV immunization upregulates the recruitment of cDC2 in LNs by inducing some specific DAMPs in the local site. Then the recruitment of cDC2 facilitates the interaction between Tfh and GC B in lymphoid organs, and promotes the formation of GCs and generation of PCs, resulting in enhanced antibody production (Fig. 8D).
DISCUSSION
The production of VNAs relies on an efficient humoral immune response that is induced by activation of the cDC-Tfh-GC B-cell axis (23). Several lines of evidence suggest that TLR signaling is critically important for the maturation of DCs and the proliferation of T and B cells during immune responses (24). In particular, TLR4 signaling in both follicular dendritic cells (FDCs) and B cells contributes to the generation of memory B cells and plasma cells (PCs), which are important parts of humoral immune response (38, 39). Interestingly, in our mouse model, TLR4 deficiency, but not TLR2 deficiency (Fig. 1), was found to strongly impair the production of VNAs and RABV-specific IgG, as well as the protection induced by live RABV. As potent APCs for virus-derived antigens (Ags), cDCs play a critical role in linking innate and adaptive immunity (26). In the present study, TLR4 signaling in cDCs was further investigated to determine how this signaling contributes to RABV-induced humoral immune response. Indeed, cDCs, which are known to be true APCs that are widely distributed throughout the body, are responsible for processing and presenting Ags in vivo. However, live RABV-induced maturation of cDCs in LNs was not dependent on TLR4 signaling (Fig. 2G to J). In addition, both LPS and live RABV can induce the maturation of WT mouse-derived BMDCs and B cells in vitro, but only live RABV is capable of inducing the maturation of BMDCs and B cells derived from TLR4-deficient mice (Fig. 2A to F). Thus, RABV-induced maturation of APCs is TLR4 independent in vitro and in vivo. As reported in previous studies, BMDC activation and type I IFN production following RABV infection are independent of TLR3-MyD88 signaling, and DC activation relies on IFN-α/IFN-β receptor (IFNAR) signaling (40).
Recruitment of Ag-bearing DCs from peripheral tissues to LNs, a critical step in the maturing DC-initiated immune response, is directed by both the cell-bound chemokine CCL21 and the soluble chemokine CCL19. These chemokines form a gradient in each tissue that guides the tissue-resident DCs to move toward secondary lymphoid organs (SLOs) via lymphatic vessels (41). The expression of the cognate receptor CCR7 is crucial for the correct positioning of DCs and the initiation of specific immune responses (42). cDCs are distinguishable by their phenotypic markers, their anatomical distribution, and their immunological functions. cDC1 have a preferential capacity for Ag cross-presentation to CD8+ T cells via MHC-I molecules, whereas cDC2 have a bias for presentation to CD4+ T cells via MHC-II molecules (28). At the very early stages of the immune response, peripheral cDC2 carry the largest amount of Ag peptide-loaded MHC (pMHC), drain into SLOs, and prime naive CD4+ T cells (Tn); these activities lead to the development of humoral immunity (39). Our current study demonstrates that both MPLA (a modified TLR4-specific PAMP) and live RABV promote the number of cDC2, but not that of cDC1, in spleens. However, inactivated RABV did not disturb the balance between cDC2 and cDC1, suggesting that RABV itself did not contribute to cDC2 development (Fig. 3A to D). Therefore, the upregulation of cDC2 in lymphoid organs must involve endogenous TLR4-specific DAMPs that are released from infected or damaged cells upon RABV infection.
RABV-induced activation of DCs was independent of TLR4 signaling, but the quantity of cDC2 in SLOs reduced in TLR4−/− mice upon infection with live RABV. A study by Suzuki et al. (43) indicated that interferon regulatory factor 4 (IRF4) and IRF8 selectively played critical roles in the development of cDC2 and cDC1, respectively. Our data suggest that TLR4 deficiency does not affect the development of cDC2 or cDC1 (control groups in Fig. 3E to J) in the steady state. Under RABV-induced inflammatory conditions, TLR4 deficiency reduced the number of cDC2 in SLOs. Several lines of evidence have demonstrated that Tfh fate correlates with Ag doses and that pMHC-bearing cDC2 promote the differentiation of Tn into Tfh cells. As expected, we found that TLR4−/− mice had fewer Tfh cells (Fig. 4), GC B cells (Fig. 7), and PCs (Fig. 8) upon infection with live RABV than did WT mice. Hence, we propose the novel suggestion that the cDC2-Tfh-GC B-cell axis acts as an important pathway for RABV-induced humoral immunity.
Previous studies have shown that cDC1 are the major providers of IL-12 (44), which preferentially differentiates Th1 cells (45); however, our results suggest that TLR4 deficiency only slightly affects the balance between Th1 (IFN-γ-secreting) and Th2 (IL-4-secreting) cells (Fig. 5B to E). TLR4 signaling participates in regulating Th1/Th2 equilibrium in mice immunized with RABV. Both IFN-γ-dependent and IFN-γ-independent IgG2a class switching have been observed in vivo; IL-4 drives the switch to IgG1 by signaling through STAT6. The IgG2a isotype is particularly potent in host defenses against viral infections. Furthermore, in RABV-induced humoral immune response, Th1-biased response and IgG2a are critical for immune protection (34) and virus clearance from the central nervous system (CNS) (46). It is known that the class switch from IgM to IgG is dependent on the cognate interaction of B cells with Th cells. However, Jegerlehner et al. found that TLR9 expression in B cells was critical for class switch recombination to IgG2a (33). The data presented here suggest that TLR4 expression is critical for the class switch from IgM to IgG2a but not for the class switch to IgG1 (Fig. 5A) in RABV-immunized mice.
Whether TLR4 deficiency affects the expression of CCL19/CCL21 and CCR7 is still unknown, but several studies have reported that TLR4 activation promotes DC migration. For example, LPS, a natural TLR4 ligand, strongly increases monocyte migratory capacity in response to CCL19 in chemotaxis and transmigration assays, whereas TLR1, TLR2, and TLR9 agonists do not affect monocyte migratory capacity (45). However, molecules other than chemokines are also involved in DC trafficking, and several nonchemokine agonists released at inflammatory sites promote the recruitment of DCs or their precursors (47). Previous studies have reported that TLR4-related DAMPs are involved in immune responses after RABV infection (11, 17). For example, Bao et al. reported that endothelial heparan sulfate (HS) promotes chemokine presentation during the recruitment of lymphocytes and DCs to LNs (48), while Sasaki et al. reported that pathogenic strains of RABV (i.e., CVS, Nishigahara, and 1088) but not attenuated strains (HEP-Flurry and ERA) bind to HS (15). Dumitriu et al. indicated that the autocrine/paracrine release of high-mobility group box 1 protein (HMGB1) and the integrity of the HMGB1/RAGE pathway are required for the migratory function of DCs (49), while Wang et al. reported that HMGB1 improves RABV-induced humoral immunity through DC activation (16). In addition, upregulation of heat shock protein 70 (Hsp70) and its interaction with RABV nucleoprotein have been demonstrated by Lahaye and his colleagues (14), but the role of HSP70 in DC migration has not been elucidated. Collectively, RABV-induced DAMPs may be diverse, and thus far, no dedicated research has been conducted to investigate which specific DAMPs contribute to DC migration and how they regulate this process. Thus, further investigation is still needed to reveal this specific mechanism.
However, our findings did not distinguish the roles of TLR4 in cDC2, Tfh cells, GC B or other cell types. TLR4 deficiency in either cell type was sufficient to suppress humoral immune response after RABV immunization (50). Previous studies demonstrated that activation of TLR4 signaling in follicular dendritic cells (FDCs) strongly impacted somatic hypermutation (SHM) and the generation of Ig class-switched high-affinity PCs and memory B cells (39). TLR4 signaling in nonantigen-activated B cells also enhanced their trafficking to GC dark zones and the generation of memory B cells and PCs (38). TLR4 deficiency also directly impaired the expression of inducible costimulator (ICOS) on Tfh cells and Fas on GC B cells, further influencing the generation of Tfh cells and GC B cells. Here, we demonstrate that TLR4 deficiency has no impact on the generation of cDC2 but it impairs the recruitment of cDC2 into SLOs (51). Here, we demonstrate that TLR4 deficiency has no impact on the generation of cDC2 but that it impairs the recruitment of cDC2 into SLOs. Although the direct effect of TLR4 deficiency on Tfh and B cell function cannot be excluded, the decrease of cDC2 may impair the proliferation of Tfh and GC B cells in TLR4−/− mice after RABV immunization, which leads to a decline in RABV-specific VNAs, IgG, and IgG2a and incomplete protection against virulent RABV. In addition, TLR4 deficiency affects these inflammatory responses through MyD88- or TRIF-dependent signaling pathways, resulting in reducing release of proinflammatory cytokines (52). TLR4 deficiency also impairs macrophage bactericidal activity through the mechanical sensor Piezo (53). Hence, TLR4, employed as a PRR, is expressed on murine macrophage, endothelial cells, NK cells, and so on, and plays a critical role in the activation of innate immune response through recognition of diverse exogenous PAMPs and endogenous DAMPs (54).
In conclusion, we found that RABV-induced recruitment of cDC2 into SLOs is TLR4 dependent. As a consequence of the decreased number of cDC2, the differentiation of naive CD4+ T cells (Tn) cells into Tfh and Th1 cells and Tfh-directed GC reactions, including the proliferation of GC B cells and the production of PCs, were impaired. These results provide new knowledge of the role played by the TLR4 pathway in virus-induced humoral immunity and provide further insight into the mechanisms involved in DC functions in vivo.
MATERIALS AND METHODS
Viruses, cells, and animals.
The RABV vaccine strain LBNSE, carrying two mutants in the gene encoding the G protein at amino acid positions 194 and 333, was generated from the SAD-L16 cDNA clone as described previously. Lab-attenuated RABV strains CVS-24 and CVS-11 were propagated in suckling mouse brains. RABV titers and specific virus-neutralizing antibodies (VNAs) were titrated using BSR cells. BSR cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (catalog [cat.] no. 11965-092; Thermo Fisher Scientific, Inc., Waltham, MA) containing 10% or 2% (vol/vol) fetal bovine serum (FBS) (cat. no. 10099141; Thermo Fisher Scientific) and 100 IU/ml of penicillin/streptomycin (cat. no. C0222; Beyotime Biotechnology Co., Ltd., Shanghai, People’s Republic of China) and maintained at 37°C in an incubator with 5% CO2. Complete medium containing 10% FBS was used to feed and grow BSR cells, and incomplete medium containing 2% FBS was utilized to maintain BSR cells to amplify RABV.
C57BL/10 wild-type (WT) mice and TLR4 knockout (TLR4−/−) mice on a C57BL/10 background were purchased from GemPharmatech Co., Ltd. (Nanjing, Jiangsu, People’s Republic of China). Female C57BL/6 mice were obtained from the Center for Disease Control and Prevention of Hubei Province (Wuhan, Hubei, People’s Republic of China). All mice were housed in individually ventilated cages belonging to the Animal Facility at Huazhong Agricultural University (Wuhan, Hubei, People’s Republic of China). All of the mice used in this study were maintained in compliance with the recommendations set forth in the Regulations for the Administration of Affairs Concerning Experimental Animals made by the Ministry of Science and Technology of China. The experiments were conducted according to protocols that were approved by the Scientific Ethics Committee of Huazhong Agricultural University (permit number HZAUMO-2018-039).
Antibodies and reagents.
Antibodies directed against specific cell markers (directly labeled antibodies) were used to analyze immune cells by flow cytometry (12). Fluorescein isothiocyanate (FITC) anti-mouse CD11c antibody (cat. no. 117306), allophycocyanin (APC) anti-mouse CD80 antibody (cat. no.104714), phycoerythrin (PE) anti-mouse CD86 antibody (cat. no. 105008), and PE/Cy7 anti-mouse I-A/I-E (MHC-II) antibody (cat. no. 107630) were used to analyze the activation/maturation of BMDCs in vitro and conventional DCs in vivo. FITC anti-mouse CD4 antibody (cat. no. 100510), PE/CY7 anti-mouse CD3e antibody (cat. no. 100320), APC anti-mouse CD185 (CXCR5) antibody (cat. no. 145506), and PE anti-mouse CD279 (PD-1) antibody (cat. no. 135206) were utilized to analyze the numbers of Tfh cells. FITC anti-mouse/human CD45R/B220 antibody (cat. no. 103206), 647 anti-mouse/human GL7 antibody (cat. no. 144606), and PE anti-mouse CD95 antibody (cat. no. 554295) were applied to analyze the numbers of GC B cells, and FITC anti-mouse/human CD45R/B220 antibody (cat. no. 103206) and APC anti-mouse CD138 (Syndecan-1) antibody (cat. no. 142506) were used to analyze the numbers of PCs. APC anti-mouse CD3 antibody (cat. no. 100236), APC anti-mouse CD19 PE anti-mouse CD8α antibody (cat. no. 100707), BV421 anti-mouse/human antibody (cat. no. 101215), and CD11b BV421 M1/70 antibody (cat. no. 562605) were used to detect cDC1/cDC2 and CD8α− cDCs/CD8α+ cDCs. All of the antibodies used in this study were purchased from BioLegend, Inc. (San Diego, CA) or from BD Biosciences, Inc. (Franklin Lakes, NJ).
Viability staining solution (cat. no. 420404; BioLegend) was used to exclude dead cells. Lysis buffer (cat. no. 555899; BD Biosciences) was utilized to remove red cells. Monophosphoryl lipid A (MPLA, cat. no. vac-mpls) and ultrapure LPS-EB (cat. no. tlrl-3pelps) were purchased from Invivogen, Inc. (San Diego, CA). The β-propiolactone (BPL; cat. no. P9620) was purchased from Sigma-Aldrich Co., Ltd. (St. Louis, MO). The β-mercaptoethanol (BME; cat. no. 21985023) was purchased from Thermo Fisher Scientific, Inc.
Virus inactivation.
The supernatants of LBNSE-infected BSR cells were harvested and centrifuged at 12,000 rpm to remove cell debris. β-Propiolactone (BPL) is widely used for the inactivation of viruses (DNA and RNA viruses) and modifies the structure of nucleic acids after reacting mainly with purine residues; thus, BPL was used to inactivate RABV as described previously (55). Briefly, the titrated LBNSE (1 × 108 fluorescent focus units [FFU]/ml) was inactivated by incubation in 0.25% (vol/vol) BPL at 4°C for 24 h, after which the residual BPL was hydrolyzed in a 37°C water bath for 2 h. The inactivated LBNSE containing 1 × 108 FFU/ml viral particles was used to immunize mice.
Viral titration.
RABV titer was measured through direct fluorescent antibody assays in BSR cells as described previously (56). In brief, 10-fold serially diluted RABV was first added to 96-well plates, using 8 wells for each virus gradient. Then, 2 × 104 BSR cells were added to each well, and the samples were incubated with serially diluted RABV at 37°C for 2 days in an incubator with 5% CO2. After incubation, the supernatant medium was discarded, and the adherent BSR cells were fixed with 80% ice-cold acetone at −20°C for 1 h. The cells were washed 3 times with phosphate-buffered saline (PBS) and stained with FITC-conjugated anti-RABV N protein antibodies for 1 h at 37°C. After 3 washes with PBS, the antigen-positive foci on the cells were counted under a IX51 fluorescence microscope (Olympus, Tokyo, Japan), and RABV titers were calculated as fluorescent focus units per milliliter (FFU/ml). All titer determinations were conducted in quadruplicate.
VNA measurement.
RABV-specific VNA titers were measured by the fluorescent antibody virus neutralization (FAVN) test in BSR cells, as described previously (23). Briefly, mouse serum was separated from whole blood and inactivated at 56°C for 30 min. Then, 100 μl of DMEM was added to each well of a 96-well plate, and 50 μl of inactivated serum was added to the wells in the first column and serially diluted 3-fold. After dilution, the serum was neutralized by addition of 100 FFU rabies challenge virus (CVS-11) to each well, followed by incubation of the plate at 37°C for 1 h. After incubation, BSR cells at 2 × 104 cells per well were added to neutralization medium in 96-well plates. After incubation for 72 h at 37°C in 5% CO2, the cells were fixed with 80% ice-cold acetone at −20°C for 30 min and stained with FITC-conjugated anti-RABV N protein antibodies at 37°C for 1 h. Fluorescence was observed using an Olympus IX51 fluorescence microscope. The fluorescence values of the measured serum samples were compared with those of a reference serum obtained from the National Institute for Biological Standards and Control (Hertfordshire, UK), and the results were normalized and quantified in international units per milliliter (IU/ml).
Preparation of BMDCs.
BMDCs were differentiated and harvested as described previously (40). Briefly, C57BL/6 mice were euthanized, and bone marrow (BM) cells were obtained from the tibias and femurs of the animals. The BM cells were filtered through 40-μm plastic mesh and resuspended in complete medium containing RPMI 1640 (cat. no. 72400047; Thermo Fisher Scientific), 100 IU/ml of penicillin/streptomycin, and 10% heat-inactivated FBS. Subsequently, 2 ml of complete medium containing 1 × 106 BM cells, 20 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF) (cat. no. 315-03-20; PeproTech Inc., Rocky Hill, NJ), and 10 ng/ml IL-4 (cat. no. 214-14-20; PeproTech) was placed in one well of a 6-well plate. Half of the medium was removed on day 2, and fresh medium supplemented with GM-CSF (2 ×, 40 ng/ml) and IL-4 (2 ×, 20 ng/ml) was warmed to 37°C and added to the well. On day 6, the nonadherent cells in the culture supernatant and the semiadherent cells were collected. BMDCs were harvested, washed once in 10 ml of RPMI 1640 medium, and used in experiments.
Preparation of pan-B cells.
WT mice and TLR4−/− mice on a C57BL/10 background were euthanized, and their spleens were removed and dispersed by gentle grinding. Pan-B cells from spleens were purified negatively using a MojoSort mouse pan-B cell isolation kit (cat. no. 480052; BioLegend) according to the manufacturer’s instructions. Briefly, a single-cell suspension (1 × 108 cells) was placed in a fresh tube, and 100 μl of a biotin antibody cocktail containing anti-CD90.2, anti-CD11c, anti-Gr-1, anti-CD49b, and anti-TER-119/erythroid antibodies was added and mixed with the cells, followed by depletion with 100 μl of streptavidin nanobeads. Finally, the pan-B cell suspension (1 × 106 cells/ml) was cultured in RPMI 1640 medium supplemented with 10% heat-inactivated FBS, 100 IU/ml penicillin/streptomycin, and 50 μM β-mercaptoethanol (BME) (25).
Immunoglobulin isotyping.
After immunization of mice with live or inactivated LBNSE, the animals’ sera were harvested and inactivated at 56°C for 30 min. RABV-specific immunoglobulin (Ig), including total IgG, IgG1, and IgG2a in serum was determined by enzyme-linked immunosorbent assay (ELISA) as described previously (56). Briefly, coating buffer (CB; 5 mM Na2CO3, pH 9.6) containing gradient-purified RABV was added to ELISA plates (100 μl CB with 500 ng purified RABV per well). After overnight incubation at 4°C, the ELISA plates were washed three times with PBST (PBS containing 0.5% Tween 80 [wt/vol]) and blocked with PBST containing 5% (wt/vol) low-fat milk for 6 h at 4°C. The serum was then diluted in PBST containing 5% (wt/vol) low-fat milk at 1:8,000 for total IgG, 1:600 for IgG2a, and 1:100 for IgG1. A 100-μl aliquot of the diluted serum was then added to the plates, and the plates were incubated for 1.5 h at 37°C. The medium in the plates was then discarded, and the plates were washed three times with PBST and incubated for 1 h at 37°C with 100 μl (1:10,000) of horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (cat. no. BA1051; Boster Biological Technology Co., Ltd., Wuhan, Hubei, People’s Republic of China) or with 100 μl (1:2,000) of HRP-conjugated secondary antibodies included with the Immunoglobulin Isotyping kit (cat. no. BF06004; BioDragon Immune Technology Co., Ltd., Beijing, People’s Republic of China). After incubation, the plates were washed three times with PBST, and a chromogenic reaction with 100 μl of tetramethylbenzidine (TMB) substrate (cat. no. AR1104; Boster) was allowed to continue for 25 min before the addition of 50 μl of 2 M H2SO4. The optical density (450 nm) of the wells was then measured using a SpectraMax 190 spectrophotometer (Molecular Devices, Sunnyvale, CA).
Flow cytometry.
Immune cells in the LNs, spleens, and BMs of the animals were analyzed by flow cytometry (57). Briefly, mouse tissues were collected, and solid tissues were carefully ground in precooled PBS (pH 7.4); the cells were resuspended in PBS containing 0.2% (wt/vol) bovine serum albumin (BSA), transferred to a centrifuge tube through a 40-μm nylon filter, centrifuged, and washed with PBS containing 0.2% (wt/vol) BSA. Red blood cells were removed using lysis buffer. After two washes with PBS, the single-cell suspensions in PBS (containing 0.2% BSA [wt/vol]) were counted. In total, 1 × 106 cells were stained by flow cytometric antibody. After incubation for 30 min at 4°C, the cells were washed twice with PBS containing 0.2% (wt/vol) BSA. Finally, stained cells were analyzed using a BD FACSVerse instrument.
Immunofluorescence histochemistry.
LNs were collected and fixed with 4% paraformaldehyde for 24 h, dehydrated in 30% (wt/vol) sucrose, embedded, and frozen in OCT medium at −20°C and sliced into 30-μm-thick sections. Tissue sections were transferred to microplates and stained with Alexa Fluor 647 anti-mouse/human CD45R/B220 (cat. no. 103226; BioLegend), Alexa Fluor 488 goat anti-mouse IgG (cat. no. 405313; BioLegend), or anti-human/mouse GL7 (cat. no. 13-5902-81; eBioscience, Thermo Fisher Scientific) followed by incubation with Alexa Fluor 594 streptavidin (cat. no. 405240; BioLegend) for 1 h at 37°C in the dark. The tissue sections were then washed three times with PBST and placed on ultrathin glass slides for observation. Fluorescent images were captured on an Olympus BX53 fluorescence microscope.
ELISpot assay.
Commercial ELISpot kits were purchased from Dakewe Biotech Co., Ltd. (Shenzhen, People’s Republic of China). The mouse IFN-γ precoated ELISpot kit (cat. no. DKW22-2000-096) and the mouse IL-4 precoated ELISpot kit (cat. no. DKW22-2040-096) were used to measure mouse IFN-γ- and IL-4-secreting cells. Splenocytes were isolated at 7 dpi, seeded into a precoated ELISpot plate and cultured for 16 h at 37°C with 5% CO2. After incubation, the plates to be used to obtain IFN-γ and IL-4 plots were washed and processed according to the manufacturer’s protocol. After a color reaction, the plates were scanned, and IFN-γ and IL-4 plots were analyzed by the Mabtech IRIS FluoroSpot/ELISpot reader, using RAWspot technology for multiplexing at the single-cell level.
Statistical analysis.
We used Prism 8.4 (GraphPad Software, Inc., San Diego, CA) to perform the statistical analysis. The results are expressed as the mean ± standard deviation (SD) or mean ± standard error of the mean (SEM). For analyses of survival data, the log-rank test was used. The significance of the differences between groups was evaluated by one-way analysis of variance followed by Tukey’s post hoc test or Student’s t test. P values of ≤0.05 (*), ≤0.01 (**), and ≤0.001 (***) between the groups were regarded as significant, highly significant, and very highly significant, respectively.
ACKNOWLEDGMENTS
This work was supported by the National Program for Key Research Projects of China (grant 2016YFD0500400 to Ling Zhao) and by the National Natural Science Foundation of China (grants 31522057 and 31372419 to Ling Zhao and grants 31720103917 and 31330078 to Zhen F. Fu).
The funders had no role in study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
Contributor Information
Ling Zhao, Email: zling604@yahoo.com.
Bryan R. G. Williams, Hudson Institute of Medical Research
REFERENCES
- 1.Robardet E, Bosnjak D, Englund L, Demetriou P, Martin PR, Cliquet F. 2019. Zero endemic cases of wildlife rabies (classical rabies virus, RABV) in the European Union by 2020: an achievable goal. Trop Med Infect Dis 4:124. 10.3390/tropicalmed4040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonnaud EM, Troupin C, Dacheux L, Holmes EC, Monchatre-Leroy E, Tanguy M, Bouchier C, Cliquet F, Barrat J, Bourhy H. 2019. Comparison of intra- and inter-host genetic diversity in rabies virus during experimental cross-species transmission. PLoS Pathog 15:e1007799. 10.1371/journal.ppat.1007799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang S, Lv F, Yuan Y, Fan C, Li J, Sun W, Hu J. 2019. Whole-brain mapping of monosynaptic afferent inputs to cortical CRH neurons. Front Neurosci 13:565. 10.3389/fnins.2019.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahrlund-Richter S, Xuan Y, van Lunteren JA, Kim H, Ortiz C, Pollak Dorocic I, Meletis K, Carlen M. 2019. A whole-brain atlas of monosynaptic input targeting four different cell types in the medial prefrontal cortex of the mouse. Nat Neurosci 22:657–668. 10.1038/s41593-019-0354-y. [DOI] [PubMed] [Google Scholar]
- 5.Bingham J, Schumacher CL, Hill FW, Aubert A. 1999. Efficacy of SAG-2 oral rabies vaccine in two species of jackal (Canis adustus and Canis mesomelas). Vaccine 17:551–558. 10.1016/S0264-410X(98)00233-3. [DOI] [PubMed] [Google Scholar]
- 6.Willet M, Kurup D, Papaneri A, Wirblich C, Hooper JW, Kwilas SA, Keshwara R, Hudacek A, Beilfuss S, Rudolph G, Pommerening E, Vos A, Neubert A, Jahrling P, Blaney JE, Johnson RF, Schnell MJ. 2015. Preclinical development of inactivated rabies virus-based polyvalent vaccine against rabies and filoviruses. J Infect Dis 212:S414–S424. 10.1093/infdis/jiv251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esposito JJ, Knight JC, Shaddock JH, Novembre FJ, Baer GM. 1988. Successful oral rabies vaccination of raccoons with raccoon poxvirus recombinants expressing rabies virus glycoprotein. Virology 165:313–316. 10.1016/0042-6822(88)90692-7. [DOI] [PubMed] [Google Scholar]
- 8.Blaney JE, Wirblich C, Papaneri AB, Johnson RF, Myers CJ, Juelich TL, Holbrook MR, Freiberg AN, Bernbaum JG, Jahrling PB, Paragas J, Schnell MJ. 2011. Inactivated or live-attenuated bivalent vaccines that confer protection against rabies and Ebola viruses. J Virol 85:10605–10616. 10.1128/JVI.00558-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu X, Lei C, Xia T, Zhong X, Yang Q, Shu HB. 2019. Regulation of TRIF-mediated innate immune response by K27-linked polyubiquitination and deubiquitination. Nat Commun 10:4115. 10.1038/s41467-019-12145-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Montfoort N, van der Aa E, van den Bosch A, Brouwers H, Vanwolleghem T, Janssen HLA, Javanbakht H, Buschow SI, Woltman AM. 2016. Hepatitis B virus surface antigen activates myeloid dendritic cells via a soluble CD14-dependent mechanism. J Virol 90:6187–6199. 10.1128/JVI.02903-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang HA, Hreggvidsdottir HS, Palmblad K, Wang HC, Ochani M, Li JH, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y, Akira S, Bierhaus A, Erlandsson-Harris H, Andersson U, Tracey KJ. 2010. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci USA 107:11942–11947. 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen C, Zhang C, Li R, Wang Z, Yuan Y, Li H, Fu Z, Zhou M, Zhao L. 2019. Monophosphoryl-lipid A (MPLA) is an efficacious adjuvant for inactivated rabies vaccines. Viruses 11:1118. 10.3390/v11121118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu X, Liu R, Zhu N. 2013. Enhancement of humoral and cellular immune responses by monophosphoryl lipid A (MPLA) as an adjuvant to the rabies vaccine in BALB/c mice. Immunobiology 218:1524–1528. 10.1016/j.imbio.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Lahaye X, Vidy A, Fouquet B, Blondel D. 2012. Hsp70 protein positively regulates rabies virus infection. J Virol 86:4743–4751. 10.1128/JVI.06501-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasaki M, Anindita PD, Ito N, Sugiyama M, Carr M, Fukuhara H, Ose T, Maenaka K, Takada A, Hall WW, Orba Y, Sawa H. 2018. The role of heparan sulfate proteoglycans as an attachment factor for rabies virus entry and infection. J Infect Dis 217:1740–1749. 10.1093/infdis/jiy081. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Liang Q, Zhang YJ, Yang J, Li MM, Wang KL, Cui M, Chen HC, Fu ZF, Zhao L, Zhou M. 2017. An optimized HMGB1 expressed by recombinant rabies virus enhances immunogenicity through activation of dendritic cells in mice. Oncotarget 8:83539–83554. 10.18632/oncotarget.18368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brennan TV, Lin L, Huang X, Cardona DM, Li Z, Dredge K, Chao NJ, Yang Y. 2012. Heparan sulfate, an endogenous TLR4 agonist, promotes acute GVHD after allogeneic stem cell transplantation. Blood 120:2899–2908. 10.1182/blood-2011-07-368720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taguwa S, Yeh MT, Rainbolt TK, Nayak A, Shao H, Gestwicki JE, Andino R, Frydman J. 2019. Zika virus dependence on host Hsp70 provides a protective strategy against infection and disease. Cell Rep 26:906–920.e3. 10.1016/j.celrep.2018.12.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.den Haan JMM, Lehar SM, Bevan MJ. 2000. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med 192:1685–1695. 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin C, Han JA, Koh H, Choi B, Cho Y, Jeong H, Ra JS, Sung PS, Shin EC, Ryu S, Do Y. 2015. CD8α− dendritic cells induce antigen-specific T follicular helper cells generating efficient humoral immune responses. Cell Rep 11:1929–1940. 10.1016/j.celrep.2015.05.042. [DOI] [PubMed] [Google Scholar]
- 21.Hilligan KL, Ronchese F. 2020. Antigen presentation by dendritic cells and their instruction of CD4+ T helper cell responses. Cell Mol Immunol 17:587–599. 10.1038/s41423-020-0465-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piccinotti S, Whelan SPJ. 2016. Rabies internalizes into primary peripheral neurons via clathrin coated pits and requires fusion at the cell body. PLoS Pathog 12:e1005753. 10.1371/journal.ppat.1005753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao L, Toriumi H, Wang HL, Kuang Y, Guo XF, Morimoto K, Fu ZF. 2010. Expression of MIP-1 alpha (CCL3) by a recombinant rabies virus enhances its immunogenicity by inducing innate immunity and recruiting dendritic cells and B cells. J Virol 84:9642–9648. 10.1128/JVI.00326-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medzhitov R. 2001. Toll-like receptors and innate immunity. Nat Rev Immunol 1:135–145. 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 25.Lytle AG, Norton JE, Jr, Dorfmeier CL, Shen S, McGettigan JP. 2013. B cell infection and activation by rabies virus-based vaccines. J Virol 87:9097–9110. 10.1128/JVI.00800-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belz G, Mount A, Masson F. 2009. Dendritic cells in viral infections. Handb Exp Pharmacol (188):51–77. 10.1007/978-3-540-71029-5_3. [DOI] [PubMed] [Google Scholar]
- 27.Wanjalla CN, Faul EJ, Gomme EA, Schnell MJ. 2010. Dendritic cells infected by recombinant rabies virus vaccine vector expressing HIV-1 Gag are immunogenic even in the presence of vector-specific immunity. Vaccine 29:130–140. 10.1016/j.vaccine.2010.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mildner A, Jung S. 2014. Development and function of dendritic cell subsets. Immunity 40:642–656. 10.1016/j.immuni.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 29.Perry LL, Lodmell DL. 1991. Role of CD4+ and CD8+ T cells in murine resistance to street rabies virus. J Virol 65:3429–3434. 10.1128/JVI.65.7.3429-3434.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tantawichien T, Jaijaroensup W, Khawplod P, Sitprija V. 2001. Failure of multiple-site intradermal postexposure rabies vaccination in patients with human immunodeficiency virus with low CD4+ T lymphocyte counts. Clin Infect Dis 33:E122—E124. 10.1086/324087. [DOI] [PubMed] [Google Scholar]
- 31.Crotty S. 2014. T follicular helper cell differentiation, function, and roles in disease. Immunity 41:529–542. 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krishnaswamy JK, Gowthaman U, Zhang B, Mattsson J, Szeponik L, Liu D, Wu R, White T, Calabro S, Xu L, Collet MA, Yurieva M, Alsen S, Fogelstrand P, Walter A, Heath WR, Mueller SN, Yrlid U, Williams A, Eisenbarth SC. 2017. Migratory CD11b+ conventional dendritic cells induce T follicular helper cell-dependent antibody responses. Sci Immunol 2:eaam9169. 10.1126/sciimmunol.aam9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jegerlehner A, Maurer P, Bessa J, Hinton HJ, Kopf M, Bachmann MF. 2007. TLR9 signaling in B cells determines class switch recombination to IgG2a. J Immunol 178:2415–2420. 10.4049/jimmunol.178.4.2415. [DOI] [PubMed] [Google Scholar]
- 34.Liu R, Wang J, Yang Y, Khan I, Zhu N. 2016. Rabies virus lipopeptide conjugated to a TLR7 agonist improves the magnitude and quality of the Th1-biased humoral immune response in mice. Virology 497:102–110. 10.1016/j.virol.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 35.Firacative C, Gressler AE, Schubert K, Schulze B, Müller U, Brombacher F, von Bergen M, Alber G. 2018. Identification of T helper (Th)1- and Th2-associated antigens of Cryptococcus neoformans in a murine model of pulmonary infection. Sci Rep 8:2681. 10.1038/s41598-018-21039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bannard O, Horton RM, Allen CD, An J, Nagasawa T, Cyster JG. 2013. Germinal center centroblasts transition to a centrocyte phenotype according to a timed program and depend on the dark zone for effective selection. Immunity 39:912–924. 10.1016/j.immuni.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith KM, Pottage L, Thomas ER, Leishman AJ, Doig TN, Xu D, Liew FY, Garside P. 2000. Th1 and Th2 CD4+ T cells provide help for B cell clonal expansion and antibody synthesis in a similar manner in vivo. J Immunol 165:3136–3144. 10.4049/jimmunol.165.6.3136. [DOI] [PubMed] [Google Scholar]
- 38.Hwang IY, Park C, Harrison K, Kehrl JH. 2009. TLR4 signaling augments B lymphocyte migration and overcomes the restriction that limits access to germinal center dark zones. J Exp Med 206:2641–2657. 10.1084/jem.20091982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garin A, Meyer-Hermann M, Contie M, Figge MT, Buatois V, Gunzer M, Toellner KM, Elson G, Kosco-Vilbois MH. 2010. Toll-like receptor 4 signaling by follicular dendritic cells is pivotal for germinal center onset and affinity maturation. Immunity 33:84–95. 10.1016/j.immuni.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Faul EJ, Wanjalla CN, Suthar MS, Gale M, Wirblich C, Schnell MJ. 2010. Rabies virus infection induces type I interferon production in an IPS-1 dependent manner while dendritic cell activation relies on IFNAR signaling. PLoS Pathog 6:e1001016. 10.1371/journal.ppat.1001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schumann K, Lämmermann T, Bruckner M, Legler DF, Polleux J, Spatz JP, Schuler G, Förster R, Lutz MB, Sorokin L, Sixt M. 2010. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity 32:703–713. 10.1016/j.immuni.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 42.Tiberio L, Del Prete A, Schioppa T, Sozio F, Bosisio D, Sozzani S. 2018. Chemokine and chemotactic signals in dendritic cell migration. Cell Mol Immunol 15:346–352. 10.1038/s41423-018-0005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suzuki S, Honma K, Matsuyama T, Suzuki K, Toriyama K, Akitoyo I, Yamamoto K, Suematsu T, Nakamura M, Yui K, Kumatori A. 2004. Critical roles of interferon regulatory factor 4 in CD11bhighCD8α− dendritic cell development. Proc Natl Acad Sci USA 101:8981–8986. 10.1073/pnas.0402139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Theisen DJ, Davidson JTt, Briseño CG, Gargaro M, Lauron EJ, Wang Q, Desai P, Durai V, Bagadia P, Brickner JR, Beatty WL, Virgin HW, Gillanders WE, Mosammaparast N, Diamond MS, Sibley LD, Yokoyama W, Schreiber RD, Murphy TL, Murphy KM. 2018. WDFY4 is required for cross-presentation in response to viral and tumor antigens. Science 362:694–699. 10.1126/science.aat5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trinchieri G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol 3:133–146. 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 46.Lebrun A, Garcia S, Li J, Kean RB, Hooper DC. 2017. Protection against CNS-targeted rabies virus infection is dependent upon type-1 immune mechanisms induced by live-attenuated rabies vaccines. TropicalMed 2:22. 10.3390/tropicalmed2030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sozzani S. 2005. Dendritic cell trafficking: more than just chemokines. Cytokine Growth Factor Rev 16:581–592. 10.1016/j.cytogfr.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 48.Bao X, Moseman EA, Saito H, Petryniak B, Petryanik B, Thiriot A, Hatakeyama S, Ito Y, Kawashima H, Yamaguchi Y, Lowe JB, von Andrian UH, Fukuda M. 2010. Endothelial heparan sulfate controls chemokine presentation in recruitment of lymphocytes and dendritic cells to lymph nodes. Immunity 33:817–829. 10.1016/j.immuni.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dumitriu IE, Bianchi ME, Bacci M, Manfredi AA, Rovere-Querini P. 2007. The secretion of HMGB1 is required for the migration of maturing dendritic cells. J Leukoc Biol 81:84–91. 10.1189/jlb.0306171. [DOI] [PubMed] [Google Scholar]
- 50.Zettel K, Korff S, Zamora R, Morelli AE, Darwiche S, Loughran PA, Elson G, Shang L, Salgado-Pires S, Scott MJ, Vodovotz Y, Billiar TR. 2017. Toll-Like receptor 4 on both myeloid cells and dendritic cells is required for systemic inflammation and organ damage after hemorrhagic shock with tissue trauma in mice. Front Immunol 8:1672. 10.3389/fimmu.2017.01672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li R, Liu J, Wu S, Zai X, Li Y, Yang Q, Hou L, Xu J, Chen W. 2018. Toll-like receptor 4 signalling regulates antibody response to adenoviral vector-based vaccines by imprinting germinal centre quality. Immunology 155:251–262. 10.1111/imm.12957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gaddis DE, Michalek SM, Katz J. 2011. TLR4 signaling via MyD88 and TRIF differentially shape the CD4+ T cell response to Porphyromonas gingivalis hemagglutinin B. J Immunol 186:5772–5783. 10.4049/jimmunol.1003192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geng J, Shi Y, Zhang J, Yang B, Wang P, Yuan W, Zhao H, Li J, Qin F, Hong L, Xie C, Deng X, Sun Y, Wu C, Chen L, Zhou D. 2021. TLR4 signalling via Piezo1 engages and enhances the macrophage mediated host response during bacterial infection. Nat Commun 12:3519. 10.1038/s41467-021-23683-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miura K, Ishioka M, Minami S, Horie Y, Ohshima S, Goto T, Ohnishi H. 2016. Toll-like receptor 4 on macrophage promotes the development of steatohepatitis-related hepatocellular carcinoma in mice. J Biol Chem 291:11504–11517. 10.1074/jbc.M115.709048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perrin P, Morgeaux S. 1995. Inactivation of DNA by beta-propiolactone. Biologicals 23:207–211. 10.1006/biol.1995.0034. [DOI] [PubMed] [Google Scholar]
- 56.Li Y, Zhou M, Luo Z, Zhang Y, Cui M, Chen H, Fu ZF, Zhao L. 2017. Overexpression of interleukin-7 extends the humoral immune response induced by rabies vaccination. J Virol 91:e02324-16. 10.1128/JVI.02324-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y, Wu Q, Zhou M, Luo Z, Lv L, Pei J, Wang C, Chai B, Sui B, Huang F, Fu ZF, Zhao L. 2020. Composition of the murine gut microbiome impacts humoral immunity induced by rabies vaccines. Clin Transl Med 10:e161. 10.1002/ctm2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]