Abstract
Viral respiratory tract infections cause significant morbidity in bone marrow transplant (BMT) patients. Speed and sensitivity of the FilmArray™ Respiratory Panel (FA-RP) can improve care but may prompt inappropriate testing. Studies describing FA-RP use in pediatric BMT patients are limited; we investigated FA-RP use, results, and clinical management to evaluate clinical significance of testing in pediatric BMT patients. Retrospective analysis of 671 respiratory specimens from 204 unique BMT patients between 01/01/2016 and 01/01/2019 was performed. Age, underlying diagnoses, FA-RP result, reason for FA-RP, and symptoms were abstracted. FA-RP impact on antimicrobial management, scheduled procedures, infection control measures, and hospital admission/discharge were investigated. Impacts of repeat testing were evaluated. Two hundred sixty-nine out of 671 specimens (40%) tested positive; human rhinovirus/enterovirus (hRV/hEV) was the most common (161/269, 60%). The primary reason for FA-RP was URI symptoms (402/671, 60%) with 54% testing positive. One hundred twenty-two out of 671 (18.2%) specimens were from asymptomatic patients; 14 (11.4%) tested positive. FA-RP informed antiviral initiation in 7/19 (36.8%), 7/8 (87.5%), and 5/30 (16.7%) of RSV, influenza, and human parainfluenza cases, respectively. In 11 cases, FA-RP informed azithromycin and ceftriaxone initiation, continuation, or discontinuation. BMT was delayed for three positives (two RSV, one hRV/hEV). In 22 instances, negative FA-RP cleared patients for BMT. In 70% of cases, repeats offered no new clinical information; all negative-to-positive cases had new or worsening respiratory symptoms. FA-RP was ordered on symptomatic and asymptomatic patients, provided rapid diagnosis in > 50% of symptomatic patients, and informed infection control measures for all inpatients and antiviral initiation in > 80% of influenza cases.
Keywords: Pediatric respiratory viral infections, Bone marrow transplant patients, Pediatric infectious diseases, Syndromic testing
Introduction
Respiratory viruses are leading causes of upper and lower respiratory infections worldwide [1–3]. In immunocompetent hosts, illness is generally self-limiting, while immunocompromised patients are at increased risk for severe, life-threating infections [4–6]. Adult and pediatric bone marrow transplant (BMT) recipients are particularly vulnerable, and respiratory viruses can cause significant morbidity and mortality due to prolonged immunosuppression [4, 7, 8]. BMT patients often suffer from weakened humoral and T cell-mediated immunity, which greatly impairs antiviral immune response [5]. Additionally, BMT recipients show higher rates of progression from upper respiratory infections (URI) to lower respiratory infections (LRI) [8]. Further complicating matters, symptoms, and clinical presentations of respiratory viral illness are rarely pathogen-specific, resulting in a large differential diagnosis often including bacterial agents [2, 9]. Before widespread adoption of molecular testing, clinicians faced long turnaround times and difficult empirical treatment decisions when treating potential viral respiratory infection. The development and implementation of rapid molecular diagnostics have significantly improved detection and identification by detecting multiple targets at once, increasing sensitivity, reducing time to positivity, and subsequently decreasing time to clinical intervention [10]. Previous reports evaluating clinical impacts of rapid respiratory viral panel tests (RVPs) on patient outcomes have shown RVPs can decrease length of stay in the intensive care unit (ICU), duration of antibiotic use, and duration of isolation precautions, while other studies have reported no significant effects on antibiotic management [10, 11]. Furthermore, previous studies highlight inherent value for both clinician and patient “knowing” the diagnosis and halting potential unnecessary workup, testing, and/or treatments [12].
The FilmArray® Respiratory Panel (FA-RP) (BioFire® Diagnostics, Salt Lake City, UT) is one sample-to-answer, multiplexed assay approved by the US Food and Drug Administration (FDA) for simultaneous detection of 17 viral respiratory pathogens and subtypes from nasopharyngeal swabs in approximately 1 h. Targets include human adenovirus (hAdV); human coronavirus 229E (hCoV-229E); human coronavirus HKU1 (hCoV-HKU1); human coronavirus NL63 (hCoV-NL63); human coronavirus OC43 (hCoV-OC43); human metapneumovirus (hMPV); human rhinovirus/enterovirus (hRV/hEV); influenza A virus (IAV) including differentiation between hemagglutinin (HA) sequences of IAV H1, pandemic IAV 2009 H1, and IAV H3; influenza B virus (IBV); parainfluenza virus 1 (PIV-1); parainfluenza virus 2 (PIV-2); parainfluenza virus 3 (PIV-3); parainfluenza virus 4 (PIV-4); respiratory syncytial virus A (RSV-A); and respiratory syncytial virus B (RSV-B). FA-RP can also detect Bordetella pertussis, Chlamydophila pneumoniae, and Mycoplasma pneumoniae; bacterial targets will not be discussed as we require confirmatory testing in our laboratory. Simplified workflows, high degree of accuracy, and speed have allowed implementation in a wide range of clinical settings. However, convenience and sensitivity present a double-edged sword: rapid turnaround times can improve clinical management, but gratuitous testing can increase burden on the laboratory and contribute to rising healthcare costs.
Previous RVP clinical impact studies have primarily focused on both immunocompetent and/or immunocompromised adult patient populations, and data on usage and clinical impact of RVP testing solely for immunocompromised pediatric patient populations is somewhat lacking [5, 13–15]. To this point, recommendations published in 2020 by the Infectious Disease Society of America (IDSA) Diagnostics Committee mentioned challenges including small sample sizes and few overall studies specifically assessing impact of molecular testing for respiratory tract infections within immunocompromised patients as a limitation for the development of specific clinical guidance [16]. Here, we performed retrospective analysis of a pediatric BMT population at a quaternary care, free-standing pediatric medical center in an effort to evaluate the clinical utility and impact of FA-RP, as well as to inform potential quality improvement policies and procedures to optimize FA-RP usage in our institution.
Materials and methods
FA-RP workflow
Currently at Children’s Hospital Los Angeles (CHLA), the FA-RP is ordered at full discretion of the physician for suspected respiratory viral illness using institutional criteria of respiratory symptoms and admission as guidance when choosing to order FA-RP. Acceptable respiratory specimen types included nasopharyngeal (NP) swab in universal transport media, NP wash, NP aspirate, bronchoalveolar lavage (BAL), and tracheal aspirates. FA-RP testing is performed in the microbiology laboratory 24 h/day, 7 days/week. Full validation of the assay was conducted in the clinical virology laboratory prior to implementation for clinical use. Performance of FA-RP have also been reported in the literature with 80–100% sensitivity and 100% specificity [17, 18].
Data collection
Retrospective electronic medical record review was completed on all BMT patients for whom FA-RP was ordered over a 3-year period from 01/01/2016 to 01/01/2019. The following patient information was abstracted: (1) demographics such as age, gender, BMT diagnosis, location at time of order, and other underlying medical conditions; (2) FA-RP test result and specimen types; (3) symptoms at time of collection; (4) other infectious disease comorbidities present up to 2 weeks before the FA-RP test; (5) clinical rationale for the FA-RP based on documentation (e.g., presence of symptoms, pre-BMT screen, check for viral clearance); (6) measures of clinical management influenced by the FA-RP result including modifications in relevant antimicrobial management up to 48 h after FA-RP result, delays, or clearance for BMT and/or other procedures/treatments and modifications in droplet isolation precautions, if patient was admitted or discharged; and (7) incidence, rationale, and results of repeat testing within 7 and 14 days of initial test.
Epidemiology of viral respiratory infection in BMT cohort
FA-RP test results were abstracted. Total percent positive and total percent negative of total tested specimens were determined. Positives were further separated based on target(s) detected in both single and co-infection cases and compared to the total number of positives to capture epidemiology and incidence of viral infections in the BMT cohort detected by FA-RP.
Determination of factors to predict positivity
Clinical rationale for FA-RP order including symptomatology, screening, check for clearance, or others was used to group specimens based on these categories. Percent positive/negative in each group was calculated to determine what reason(s) for testing best predicted a positive FA-RP result. We defined the presence of URI symptoms as one or more of the following symptoms noted: rhinorrhea, cough, nasal congestion, airway congestion, throat pain, and/or sneezing. We defined the presence of LRI symptoms if one or more of the following symptoms were noted: tachypnea, increased work or breathing, and/or abnormal chest X-ray (CXR) including pleural effusion.
Antimicrobial management in accordance with FA-RP results
Given that these are medically complex BMT patients on numerous antimicrobials, each antimicrobial initiated or discontinued within 48 h of FA-RP result, as well as antimicrobial courses that didn’t change but overlapped with an FA-RP test, was abstracted based on patient antimicrobial summary charting. Additionally, the documented medical reason for each drug was evaluated first to generate a list of relevant antimicrobials in accordance with FA-RP result. Therefore, all antimicrobials being administered for reasons unrelated to FA-RP order and result such as pathogen prophylaxis, 48 h sepsis rule out, neutropenic fever, and/or treatment of other unrelated but active infections were removed from the analysis. Following generation of relevant antimicrobial list, the medical reason for initiation/escalation, continuation, or de-escalation/discontinuation management was further evaluated in accordance with FA-RP result in both FA-RP positive patients and FA-RP negative patients. Specific outcomes that were noted were positive FA-RP results that informed initiation/escalation of antivirals and subsequent de-escalation/discontinuation of empirical antibiotics/antifungals as well as negative FA-RP results that informed addition/escalation or continuation of antibiotics/antifungals.
Impact of FA-RP results on scheduled procedures
To determine the impact of FA-RP on scheduled BMT procedures, we evaluated when a BMT procedure was carried out ≤ 10 days after a positive FA-RP, incidences when BMT evaluated incidence procedure was delayed and for how long due to a positive FA-RP, and incidences when a negative FA-RP cleared an individual for a BMT procedure. The impact of FA-RP on clearing individuals for BMT procedure was evaluated by investigating FA-RP tests that were ordered as part of pre-BMT workup and indicated to have cleared a patient for BMT procedure. The same approaches were taken for determining impact of FA-RP on other scheduled procedures and chemotherapy treatments.
Repeat FA-RP testing
Patients that received repeat FA-RP testing were evaluated for two separate time blocks including ≤ 7 days between repeats and ≤ 14 days between repeats. For each set, incidences of concordance and discordance between initial and repeat testing were determined for FA-RP positive and negative tests. Furthermore, rationale for repeat testing was reviewed to understand circumstances surrounding multiple rounds of testing within a short timeframe. Additionally, impacts of repeat testing were evaluated including if a repeat result informed antimicrobial management, caused a delay in scheduled procedures/treatments, and/or informed clearance for procedures.
Statistical analyses
Proportions, percentages, and ratios based on totals were calculated using Microsoft® Excel version 16.42 to show incidence and compare values within the dataset where appropriate. Statistical tests such as the two-proportion z-test were employed using QuickCalcs GraphPad software to compare proportions to evaluate statistical significance between results obtained from FA-RP positive compared to the FA-RP negative group; p values < 0.05 were considered statistically significant.
Results
Patient demographics
FA-RP was performed on 671 respiratory specimens from 204 unique BMT patients spanning 01/01/2016 to 01/01/2019 ordered at physicians’ discretion. Nasopharyngeal (NP) swab was the most common specimen type (> 95%). The median patient age was 7 years (range 4 months to 24 years), and 126 (38%) were female. Most FA-RP orders originated from the inpatient BMT unit (61.7%) followed by outpatient BMT clinic (30.6%) (Table 1). Positive FA-RP results were found in 269 of 671 (40%) specimens from 136 patients. The most common targets detected were hRV/hEV (162/269, 60%), human parainfluenza viruses (HIPVs) 1–4 (30/269, 11%), and non-severe acute respiratory syndrome (non-SARS) hCoVs (24/269 (9%) (Table 2). Viral co-infections were detected in 19/269 (7.1%) cases from 17 patients, with hRV/hEV and hCoVs 8/19 (42%) most often seen together. Table 2 highlights targets and percentages detected.
Table 1.
Summary of patient demographics and FA-RP panel ordering characteristics
| Total patients (n = 204) | |
|---|---|
| Demographics | |
| Female | 78 (38%) |
| Male | 126 (62%) |
| Median patient age | 7 years |
| Patient age range | 2 months to 24 years |
| Underlying diagnoses indicating BMT | |
| Leukemia/lymphoma | 121 (59.3%) |
| Other cancers | 41 (20.1%) |
| Immunodeficiencies | 32 (15.7%) |
| Aplastic anemia | 7 (3.4%) |
| Other inherited disorders | 3 (1.5%) |
| Respiratory specimen breakdown | |
| Patients with one specimen | 48 (23.5%) |
| Patients with ≥ two specimens | 156 (76.5%) |
| Total specimens (n = 671) | |
| Location at time of FA-RP order | |
| Inpatient, BMT unit | 414 (61.7%) |
| Outpatient BMT Clinic | 205 (30.6%) |
| Emergency room | 47 (7%) |
| Infusion center | 5 (0.7%) |
| RA-RP panel specimens | |
| Nasopharyngeal swab | 647 (96.4%) |
| Tracheal aspirate | 11 (1.6%) |
| Bronchoalveolar lavage | 8 (1.2%) |
| Nasopharyngeal wash | 5 (0.8%) |
| Total FA- RP positive | 269 (40%) |
Abbreviations: FA-RP, Film Array Respiratory Panel; BMT, bone marrow transplantation
Table 2.
FA-RP targets detected in positive cases
| Target | No. detected | % of all positive samples (n = 269) |
|---|---|---|
| hRV/hEV | 161 | 60 |
| PIVs | 30 | |
| PIV-1 | 5 | 1.9 |
| PIV-2 | 1 | 0.4 |
| PIV-3 | 23 | 8.5 |
| PIV-4 | 1 | 0.4 |
| Non-SARS hCoV | 24 | |
| hCoV OC43 | 14 | 5.1 |
| hCoV NL63 | 4 | 1.5 |
| hCoV 229E | 3 | 1.1 |
| hCoV HKU1 | 3 | 1.1 |
| RSV | 19 | 7.1 |
| hAdV | 5 | 1.9 |
| hMPV | 5 | 1.9 |
| Influenza A | 4 | 1.5 |
| Influenza B | 1 | 0.4 |
| Co-infections | 19 | |
| hAdV; hRV/hEV | 2 | 0.7 |
| hCoV HKU1; hRV/hEV | 2 | 0.7 |
| hCoV NL63; hRV/hEV | 2 | 0.7 |
| hCoV OC43; hRV/hEV | 2 | 0.7 |
| Influenza A; hRV/hEV | 2 | 0.7 |
| hAdV; PIV-4 | 1 | 0.4 |
| hAdV; hCoV OC43 | 1 | 0.4 |
| hCoV 229E; hRV/hEV | 1 | 0.4 |
| hCoV HKU1; hCoV OC43 | 1 | 0.4 |
| hCoV HKU1; Influenza B | 1 | 0.4 |
| hCoV HKU1; hMPV | 1 | 0.4 |
| hAdV; CoV NL53; hRV/hEV | 1 | 0.4 |
| hCoV OC43; PIV-4 | 1 | 0.4 |
| hMPV; hRV/hEV | 1 | 0.4 |
| PIV-3; hRV/hEV | 1 | 0.4 |
Abbreviations: FA-RP, Film Array Respiratory Panel; hRV/hEV, human rhinovirus/enterovirus; PIV, parainfluenza virus; hCoV, human coronavirus; RSV, respiratory syncytial virus; hAdV, human adenovirus; hMPV, human metapneumovirus; No., number
Clinical rationale for FA-RP order
The most common reason for FA-RP testing was upper respiratory infection (URI) symptoms with or without fever (402/671, 60%), of which, 218/402 (54.2%) tested positive (Table 3). hRV/hEV was most common followed by PIVs. Other reasons for testing included fever only, asymptomatic screening, lower respiratory tract (LRI) symptoms, and non-URI symptoms, i.e., nausea, diarrhea, ear pain, or malaise. A total of 138/671 (20.6%) specimens from 104 asymptomatic patients were tested for pre-procedural screening (BMT or other), general asymptomatic screening, or test of cure; 19/138 (13.8%) tested positive (Table 3). Of asymptomatic positives, hRV/hEV was detected in 18 specimens from 17 patients,and PIV-3 was detected in one patient. Those tested for “cure” of previous viral infection included 16 specimens from 15 previously positive and symptomatic patients, and a total of 5/16 (31.3%) remained hRV/hEV positive (Table 3).
Table 3.
Clinical reason for FA-RP order and corresponding targets detected in positive cases
| Reason for FA-RP (n = 671) | No. specimens (% of total 671 tested in study) | No. positive per test reason (%) | % of total 269 positives in study | Breakdown of viral targets detected in positive cases (%) |
|---|---|---|---|---|
| URI symptoms | 319 (47.5) | 176 (55.2) | 65.4 |
hRV/hEV = 114 (64.8) PIVs = 17 (9.7) hCoVs = 17 (9.7) RSV = 6 (3.4) hAdV = 3 (1.7) hMPV = 3 (1.7) IVA = 1 (0.6) Co-infectionsa = 15 (8.5) |
| URI symptoms + fever | 83 (12.4) | 42 (50.6) | 15.6 |
hRV/hEV = 13 (31) PIVs = 6 (14.3) hCoVs = 4 (9.5) RSV = 10 (23.8) hADdV = 2 (4.8) hMPV = 2 (4.8) IVA = 3 (7.1) Co-infectionsb = 2 (4.8) |
| Fever only | 81 (12) | 19 (23.5) | 7.1 |
hRV/hEV = 12 (79) hCoVs = 3 (15.7) IVB = 1 Co-infectionsc = 3 (5.3) |
| Asymptomatic screening | 73 (10.9) | 9 (12.3) | 3.4 | hRV/hEV = 9 (100) |
| Per-procedural screening including pre-BMT | 49 (7.3) | 5 (10.2) | 2 |
hRV/hEV = 4 (80) PIV = 1 (20) |
| LRI symptoms | 42 (6.3) | 10 (23.8) | 3.7 |
hRV/hEV = 4 (40) PIV = 3 (30) RSV = 3 (30) |
| Asymptomatic, check for clearance after previous positive | 16 (2.4) | 5 (31.3) | 2 | hRV/hEV = 5 (100) |
| Non-URI symptom, i.e., nausea, diarrhea, ear pain, malaise | 5 (.8) | 1 (20) | .04 | PIV = 1 (100) |
| CXR pathology and respiratory symptoms | 3 (.4) | 2 (66.7) | .076 | PIV = 2 (100) |
Abbreviations: FA-RP, Film Array Respiratory Panel; URI, upper respiratory infection; LRI, lower respiratory infection; CXR, chest X-ray; No., number; BMT, bone marrow transplant; hRV/hEV, human rhinovirus/enterovirus; PIV, parainfluenza viruses; hCoV, human coronavirus; RSV, respiratory syncytial virus; hAdV, human adenovirus; hMPV, human metapneumovirus; IVA, influenza virus A; IVB, Influenza virus B
ahCoV_hRV/hEV (n = 6), hAdV_hRV/hEV (n = 2), hAdV_PIV (n = 1), hCoV_hCoV (n = 1), PIV_hRV/hEV (n = 1), hCoV_HPIV (n = 1), hAdV_hCoV (n = 1), hAdV_hCoV_hRV/hEV (n = 1), hCoV_IVB (n = 1)
bhCoV HKU1_hMPV, hMPV_hRV/hEV
cIVA_hRV/hEV (n = 2), hCoV NL63_hRV/hEV (n = 1)
Antimicrobial and antiviral management
FA-RP positives informed antiviral initiation of ribavirin with or without intravenous immunoglobulin (IVIG) or oseltamivir alone within 48 h in 19/56 (34%) patients who tested positive for RSV, IAV, IBV, PIV-1, or PIV-3 (Table 4). The average time to antiviral therapy initiation was 18.5 h from positive. Specifically, for IAV and IBV, the average time to oseltamivir initiation was 11.6 h. A total of 11/19 (58%) RSV, PIV-1, or PIV-3 positive patients received ribavirin with IVIG, while 1/19 (5.3%) received ribavirin alone. Most influenza positive patients 7/8 (87.5%) were prescribed oseltamivir (Table 4). A total of 37/56 (66%) patients that were positive for RSV, IAV, IBV, PIV-1, or PIV-3 were not prescribed antivirals (Table 4). No FA-RP negative patients received treatment with ribavirin, IVIG, or oseltamivir. Additionally, as expected, acyclovir, ganciclovir, valganciclovir, or foscarnet for viral prophylaxis or treatment of active herpes virus infection was unrelated to FA-RP.
Table 4.
Antiviral management in subset of FA-RP positive cases
| Antiviral treatment | |||||
|---|---|---|---|---|---|
| Target detected | Total positivea | Untreated (%) | Ribavirin (%) | Ribavirin + IVIG (%) | Oseltamivir (%) |
| RSV | 19 | 12 (63) | - | 7 (37) | - |
| Influenza A | 6 | 1 (16.7) | - | - | 5 (83.3) |
| Influenza B | 2 | 0 (0) | - | - | 2 (100) |
| PIV 1 | 5 | 2 (40) | 1 (20) | 2 (40) | - |
| PIV 3 | 24 | 22 (91.7) | - | 2 (8.3) | - |
aIncludes co-infection cases where the patient was positive for one of the targets listed in the table
Abbreviations: FA-RP, Film Array Respiratory Panel; PIVs, parainfluenza viruses; RSV, respiratory syncytial virus
Based on provider notes, 10/269 (3.7%) positive FA-RP results were temporally associated with continuation (4/10, 40%) or addition (6/10, 60%) of azithromycin within 48 h of result. The average time to addition was 17.4 h (range 11–23 h). In these cases, azithromycin was used for anti-inflammatory/immunomodulatory effects based on provider notes, and all patients had abnormal chest X-ray (CXR). Patients were positive for hRV/hEV (n = 3), hRSV (n = 3), PIV-3 (n = 2), PIV-1 (n = 1), or hCoV NL63 (n = 1). None showed evidence of co-infection and/or superimposed bacterial infection based on imaging, corresponding bacterial cultures, and/or molecular tests were negative. There were no instances where azithromycin was discontinued following a positive FA-RP. Azithromycin therapy was also noted in the FA-RP negative group including 17/402 (4.2%) instances in 14 patients. In 9 (53%) cases, azithromycin was initiated, and in 3 (17.6%) cases, the patient remained on previously prescribed course 3/17 (17.6%) due to abnormal CXR and/or concern for Mycoplasma pneumoniae. There was no documentation that the negative FA-RP informed these changes. To further elucidate azithromycin impacts, we compared proportions of FA-RP positive and negative patients where azithromycin therapy was continued or started. No statistically significant difference between the positive and negative groups was observed for addition of (p value 0.3) or continuing prescribed azithromycin (p value 0.6). Azithromycin was discontinued in 5/17 (29.4%) instances within 48 h of negative FA-RP result, but all were unrelated to FA-RP.
In one instance, ceftriaxone was prescribed prior to the FA-RP being ordered due to symptoms of URI and was subsequently discontinued within 5 h of positive RSV. All other instances of ceftriaxone initiation, continuation, or discontinuation (n = 46) in both FA-RP positive and negative groups were due to medical reasons unrelated to FA-RP. Changes to all other antibacterial and anti-fungal agents were due to reasons unrelated to FA-RP test result, e.g., prophylaxis, empirical neutropenic fever treatment, other infection, or end of antimicrobial course.
Changes in BMT procedure, other procedures, or chemotherapy courses due to FA-RP results
Of 671 total FA-RP tests, in 91 instances, FA-RP was performed ≤ 10 days prior to a scheduled BMT. In 88/91 (96.7%) instances, patients received their BMT as planned. This encompassed allogenic BMT (n = 45), CAR T cell therapy (n = 23), or autologous BMT (n = 20). A total of 30/88 (34%) were FA-RP positive, and the BMT procedure was completed within an average of 5.5 days of result. The majority, 21/30 (70%), were hRV/hEV positives. Additional targets not impacting BMT procedure are captured in Table 5. There were no instances of positive IAV, IBV, hMPV, or hAdV pre-BMT; therefore, these scenarios were not evaluated. The remaining instances where FA-RP preceded BMT ≤ 10 days included 58/88 (66%) from the FA-RP negative cohort; 22/58 (38%) were asymptomatic tested as part of a pre-BMT workup, and a negative result “cleared” them for BMT. There were three documented instances of BMT procedural delay related to positive FA-RP: two had CAR T cell therapy delayed for 10 days due to hRV/hEV and RSV, respectively, and the other had allogenic BMT postponed for 7 days due to RSV (Table 5).
Table 5.
Delay or continuation of BMT and other procedures in FA-RP positive patients
| Procedure | No. that continued with procedure (%) | No. positive tests where procedure continued (%) | Target detected (n) | No. with delayed procedure due to positive test | Target detected (n) | |
|---|---|---|---|---|---|---|
| BMT procedures (n = 91) | Allogenic BMT | 45 (49.5) | 9/45 (20) |
hRV/hEV (5) PIVs (2) hCoV OC43 (1) hCoV OC43_hRV/hEV (1) |
1 | RSV |
| Autologous BMT | 20 (22) | 10/20 (50) |
hRV/hEV (8) hCoV HKU1 (1) RSV (1) |
- | - | |
| CAR T cell | 23 (25.3) | 11/23 (47.8) |
hRV/hEV (8) PIVs (1) RSV (1) hCoV OC43_hRV/EV (1) |
2 |
hRV/hEV RSV |
|
| Other procedures (n = 16) | Chemotherapy | 4 (25) | 1/4 (25) | hRV/hEV (1) | 3 |
RV/EV (2) FluA (1) |
| Line or catheter placement under anesthesia | 8 (50) | 7/8 (87.5) |
hRV/hEV (6) PIVs (1) |
1 | RSV |
Abbreviations: FA-RP, Film Array Respiratory Panel; BMT, bone marrow transplant; No., number; hRV/hEV, human rhinovirus/enterovirus; PIVs, parainfluenza viruses; hCoV, human coronavirus; RSV, respiratory syncytial virus; BMA, bone marrow aspiration
For other procedures, we identified 16 instances where FA-RP was performed ≤ 10 days prior to a scheduled procedure and/or chemotherapy in our patient cohort. All tests were performed due to presence of URI symptoms. Of the 12 positives, four (33.3%) resulted in delay of a procedure and/or chemotherapy treatment (Table 5). It should be noted that per facility policy, procedures not performed under general anesthesia would not be delayed due to a positive FA-RP and therefore are not included. In the 8/12 (66.7%) instances where procedure or chemotherapy continued despite a positive test, 7/12 (58.3%) were positive for hRV/hEV (Table 5). There were only two instances where FA-RP was negative in the symptomatic cohort with a scheduled procedure or chemotherapy, and both were completed.
Other measures of clinical intervention
According to the facility’s protocol, all FA-RP positive patients with respiratory symptoms are placed on appropriate infection control precautions; therefore, FA-RP informed isolation in all 156 (100%) positive inpatient cases. Although de-isolation is not dependent on a negative test result, in the 16 cases where asymptomatic inpatients were tested for clearance after a previous FA-RP positive, 11 were repeat negative patients and de-isolated, and the other five tested positive again for hRV/hEV and were not de-isolated. A single IAV positive FA-RP informed discharge of a patient to complete oseltamivir therapy prior to suspended chemotherapy due to the IAV result. Regarding hospital admissions, 257 patients were tested from BMT outpatient clinic, infusion center, or the ED, and 50 (19.4%) patients were subsequently hospitalized. Of these, 10/50 (20%) were hospitalized due to respiratory symptoms with a positive FA-RP at the time of admission or shortly thereafter. Lastly, a single patient had delayed “catch-up immunization” vaccine administrations by 1 week due to PIV-1 infection.
Repeat testing
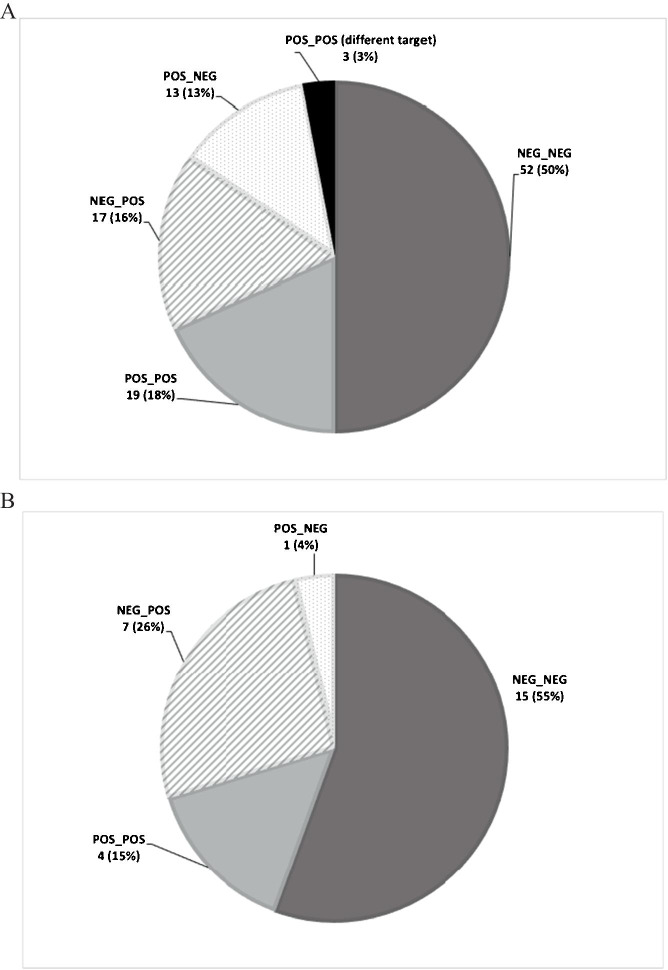
Of the 204 patients in our cohort, 66 (32%) were tested by FA-RP ≥ two times within a 14-day period, encompassing 189 specimens and 104 instances of repeat testing. A total of 18 patients (26.9%) had ≥ 3 specimens tested within a 14-day period. The majority of repeat testing (74/104, 71.2%) yielded the same result within 14 days (Fig. 1). Conflicting results were found in 30/104 (28.8%) repeats with positive-to-negative conversions (13, 12.5%) and negative-to-positive conversions (17, 16.3%) (Fig. 1). There were three positive-to-positive cases where a different target was detected upon repeat (Fig. 1). Rationale for the 104 repeats included new or continued URI symptoms with or without fever (77/104, 74%), worsening respiratory symptoms (11/104 10.6%), check for clearance after a previous positive (8/104, 7.7%), asymptomatic screen unrelated to a procedure (6/104, 5.8%), and pre-procedural screen based on patient account of previous cold-like symptoms (2/104, 1.9%). Data analyzed within a 7-day period showed similar results to analysis from the 14-day period (Fig. 1).
Fig. 1.
Repeat testing outcomes. Repeat testing completed within 14 days (A) and 7 days (B) of initial test revealed that greater than 70% of repeats offered no new clinical information, regardless of days between initial and repeat test. Overall, 17 instances of new viral detection were observed with targets including 12 human rhinovirus/enterovirus (hRV/EV), two parainfluenza virus 1 (PIV-1), one human coronavirus OC43 (hCoV-OC43), and one respiratory syncytial virus. There were three instances of new viral illness detection following a previous positive which included a human metapneumovirus (hMPV) to hCoV-OC43, a hRV/EV to hRV/EVh plu hCoV-OC43, and a hRV/EV to hMPV; all instances were seen in repeats completed past 7 days
There were two instances where negative-to-positive results informed escalation of antimicrobials within 48 h of repeat testing. In one case, an abnormal CXR 7 days after initial negative informed repeat testing which was PIV-1 positive, and the patient was placed on ribavirin and azithromycin within 42 h which is not standard for PIV-1 infections. In the second case, the patient tested negative despite having URI symptoms and then 8 days later clinically declined with oxygen desaturation to 85% and tested RSV positive. Patient was appropriately placed on ribavirin in combination with IVIG within 6 h, and azithromycin previously prescribed was continued.
For repeat tests between 7 and 14 days (i.e., 8–13 days after initial test), 140 specimens and 67 instances of retest were evaluated, and new clinical information was obtained in 20 (29.8%) cases when the retest result was different. Eleven out of 20 (55%) of cases were positive-to-negative conversions where the reason for retest was to test for clearance or persistence but not worsening URI symptoms. There were 9/20 (45%) negative-to-positive cases, and all patients had worsening respiratory symptoms. On average, repeat testing provided new information 10.5 days after initial test.
All 13 patients (100%) with positive-to-negative repeat tests performed ≤ 14 days later (which encompasses both the ≤ 14-day and ≤ 7-day groups) were switched from droplet isolation precautions to standard precautions. No other changes in clinical management were noted in the repeat testing group.
Discussion
Here, we investigated usage and clinical impacts of FA-RP testing ordered at full physician discretion in our pediatric BMT population to gauge current usage, investigate rationale for test ordering, and evaluate clinical effectiveness of testing. Based on our findings, most FA-RP testing is performed on BMT patients with respiratory symptoms, and in turn, respiratory symptomatology was the best predictor of test positivity. We report that a positive FA-RP ≤ 10 days preceding a scheduled BMT did not generally predict a delay in the BMT procedure with only three BMT procedural delays captured. In > 70% of the cases without procedural delays, patients were positive for hRV/hEV, which is often less severe than other respiratory viruses such as influenza or RSV [5]. It is important to appreciate that care of BMT patients is complex and decisions to delay BMT procedure is often a multifactorial decision that considers each individual patient’s clinical scenario. A positive FA-RP result may contribute to a small fraction of the decision-making process; BMT delay in the three cases documented here was in at least part due to FA-RP outcome based on provider notes.
Impact on antimicrobial therapy included antiviral and antibacterial management notably for the addition of ribavirin with or without IVIG, addition of oseltamivir, and addition or continuation of azithromycin. Azithromycin is well described to have anti-inflammatory and immunomodulatory actions beyond antibacterial effects, and it has been reported to aid in viral infection management in BMT patients [19]. Although azithromycin usage was impacted by FA-RP in certain cases, positive and negative cohorts showed no statistically significant difference in usage. No other antimicrobials documented, other than a single case of discontinued ceftriaxone, were impacted by FA-RP indicating the importance of other clinical reasons in driving antimicrobial treatment or prophylaxis irrespective of FA-RP. In many cases, there was no documented evidence that FA-RP altered clinical management. Further studies comparing FA-RP usage in healthy versus immunocompromised patients particularly in the pediatric population are warranted to provide an immunocompetent comparator group to evaluate differences in management across patient sub-populations and better elucidate other FA-RP impacts.
The most common viruses detected in our BMT cohort were hRV/hEV, followed by PIV-3, non-SARS-hCoVs, and RSV. These findings align with other studies that show high prevalence of these viruses relative to others in both immunocompetent and immunocompromised patients [2, 8, 9]. Most patients tested by FA-RP had respiratory symptoms; however, a number of asymptomatic patients were tested either for general screening purposes, pre-procedurally, or test of cure after a previous positive. As FA-RP indication does not include patients with an absence of signs and symptom suspicious for a respiratory infection, the utilization of this test in asymptomatic patients is a deviation from the manufacturer’s recommendation.
Multiplex panels like the FA-RP are a double-edged sword in clinical microbiology laboratories. These tests are highly sensitive, fast, easy to use, and simultaneously test for multiple targets [2]. This allows providers to more effectively manage their patients than was previously possible with traditional methods or even singleplex, standalone molecular assays [10, 12]. However, these very same benefits can lead to overuse. Here, we report that repeat testing is not uncommon in BMT patients and > 70% do not offer any new clinical information when evaluated at both ≤ 7-day and ≤ 14-day repeat marks. A previous study in adults found that repeat respiratory panel testing had limited clinical utility [20]. However, given the lack of published literature, there are currently no national or international guidelines addressing best practices surrounding repeat syndromic respiratory panel testing. At CHLA, we employ a 7-day restriction on FA-RP re-orders, which was motivated by institutional specific laboratory stewardship initiatives. Our findings here could potentially inform institutional restriction extension to ~ 10 days without greatly impacting patient care based on results that repeats offered new information on average 10.5 days after the first test. Restriction removal could be informed by strict patient criteria that were shown in our findings to be associated with negative-to-positive results, including new or worsening respiratory symptoms or potential cluster investigation identified by infection prevention team. Additional studies are underway at our institution solely investigating repeat FA-RP testing across all patient populations to further develop potential guidance. Furthermore, multicenter studies evaluating implementation of such criteria would be beneficial to better understand the impact of such approaches and to potentially harmonize how institutions address issues surrounding repeat syndromic panel testing.
There are important limitations to this work. This is a single-center study in a quaternary care, pediatric medical center and may not reflect findings from other institutions. Next, the notable limitations of retrospective chart review present challenges in evaluating nuances of undocumented patient care, and therefore, relevant information could have been missed. Although it’s important to know how results from the microbiology laboratory inform care in our sickest patients, these patients are often complex, and the importance of “knowing” a diagnosis can provide the care team, the patient, and the patient’s family piece of mind and halt clinical workup(s), which are difficult to measure in retrospective studies. Future prospective studies that directly capture decision-making processes of the BMT provider are needed. Lastly, our study only reviewed data obtained from the FA-RP panel which is not the only multiplex respiratory viral panel offered at CHLA; we additionally offer the Cepheid GeneXpert® Xpert Xpress Flu/RSV test. Additional studies to address impacts of multiple respiratory panels employed at the same institution, including the nuances of algorithm/reflex testing between panels not captured here, are warranted. Furthermore, comparator studies evaluating the performance of various multiplex respiratory panels available from different manufacturers/vendors would further elucidate variability in panel-specific detection rate(s) and clinical management within the pediatric BMT population to better understand best approaches for this highly complex patient group.
In summary, we demonstrate that FA-RP provided rapid etiological diagnosis in over half of symptomatic patients tested. Additionally, FA-RP informed clinical course in most cases an antiviral initiation was available, aided providers in determining if a delay in BMT or other procedure/treatment was necessary, and informed isolation/de-isolation procedures in all tested patients. Furthermore, we show that testing asymptomatic patients and repeat testing are common for BMT patients and often do not offer new clinical information to drive management. Multiplex molecular respiratory panels certainly have their place in modern medicine, and studies like this one are important to understand how clinicians utilize testing and the impact of results on specific patient populations and corresponding changes in clinical management.
Author contribution
N/A.
Data availability
Data archiving is not mandatory but will be made available upon reasonable request.
Code availability
N/A.
Declarations
Ethics approval
This single-center study was conducted at Children’s Hospital Los Angeles (CHLA), a free-standing, quaternary care pediatric medical center, and was determined to be exempt by the CHLA Institutional Review Board (IRB). All BMT patients for whom the FA-RP was ordered between 01/01/2016 and 01/01/2019 were included in this study.
Consent to participate
N/A
Consent to publication
N/A
Conflict of interest
J. D. B has received research funding from BioFire Diagnostics for other studies not related to this work. All other authors have no conflicts of interest relevant to this article to disclose.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mimi R. Precit, Email: mprecit@kumc.edu
Jennifer Dien Bard, Email: jdienbard@chla.usc.edu.
References
- 1.Fendrick AM, Monto AS, Nightengale B, Sarnes M (2003) The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch Intern Med [cited 2020 3];163:487–94. https://jamanetwork.com/doi:10.1001/archinte.163.4.487 [DOI] [PubMed]
- 2.Leber AL, Everhart K, Daly JA, Hopper A, Harrington A, Schreckenberger P et al (2018) Multicenter evaluation of BioFire FilmArray Respiratory Panel 2 for detection of viruses and bacteria in nasopharyngeal swab samples. [cited 2020 3] [DOI] [PMC free article] [PubMed]
- 3.Johnston S, Holgate S (1996) Epidemiology of viral respiratory tract infections. In: Viral and other infections of the human respiratory tract. Springer Netherlands; [cited 2020 3]. pp 1–38. https://link.springer.com/chapter/10.1007/978-94-011-7930-0_1. 10.1007/978-94-011-7930-0_1
- 4.Boeckh M (2008) The challenge of respiratory virus infections in hematopoietic cell transplant recipients. Br J Haematol [cited 2020 3];143:455–67. https://pubmed.ncbi.nlm.nih.gov/18785968/. 10.1111/j.1365-2141.2008.07295.x [DOI] [PMC free article] [PubMed]
- 5.Hammond SP, Gagne LS, Stock SR, Marty FM, Gelman RS, Marasco WA et al (2012) Respiratory virus detection in immunocompromised patients with film array respiratory panel compared to conventional methods. J Clin Microbiol [cited 2020 3]; 50:3216–21. /pmc/articles/PMC3457462/?report=abstract. 10.1128/JCM.00538-12 [DOI] [PMC free article] [PubMed]
- 6.Pochon C, Voigt S (2019) Respiratory virus infections in hematopoietic cell transplant recipients. Front. Microbiol. [cited 2021 26];10. https://pubmed.ncbi.nlm.nih.gov/30687278/. 10.3389/fmicb.2018.03294 [DOI] [PMC free article] [PubMed]
- 7.Wendt CH, Weisdorf DJ, Jordan MC, Balfour HH, Hertz MI (1992) Parainfluenza virus respiratory infection after bone marrow transplantation. N Engl J Med [cited 2021 26];326:921–6. https://www.nejm.org/doi/full/10.1056/NEJM199204023261404. 10.1056/nejm199204023261404 [DOI] [PubMed]
- 8.Ison MG (2009) Respiratory syncytial virus and other respiratory viruses in the setting of bone marrow transplantation. Curr Opin Oncol [cited 2020 3];21:171–6. http://journals.lww.com/00001622-200903000-00014. 10.1097/CCO.0b013e328324bc1c [DOI] [PubMed]
- 9.Hassan IA, Chopra R, Swindell R, Mutton KJ (2003) Respiratory viral infections after bone marrow/peripheral stem-cell transplantation: the Christie Hospital experience. Bone Marrow Transplant. [cited 2020 3]; 32:73–7. https://www.nature.com/bmt. 10.1038/sj.bmt.1704048 [DOI] [PubMed]
- 10.Rogers BB, Shankar P, Jerris RC, Kotzbauer D, Anderson EJ, Watson JR et al (2015) Impact of a rapid respiratory panel test on patient outcomes. Arch Pathol Lab Med [cited 2020 3];139:636–41. https://pubmed.ncbi.nlm.nih.gov/25152311/. 10.5858/arpa.2014-0257-OA [DOI] [PubMed]
- 11.Martinez RM, Kay HE, Scicchitano LM, Wolk DM, Martinez RM (2010) Implementation of non-batched respiratory virus assay significantly impacts patient outcomes in the ICU laboratory method intervention: FilmArray Respiratory Pathogen Panel (BioFire Diagnostics)
- 12.Cawcutt KA, Fey PD, Kalil AC (2017) Respiratory pathogen panels in the hospital: good or unnecessary? Curr Opin Infect Dis [cited 2021 26];30:226–30. https://pubmed.ncbi.nlm.nih.gov/28118220/. 10.1097/QCO.0000000000000357 [DOI] [PubMed]
- 13.Azadeh N, Sakata KK, Saeed A, Mullon JJ, Grys TE, Limper AH et al (2018) Comparison of respiratory pathogen detection in upper versus lower respiratory tract samples using the BioFire FilmArray respiratory panel in the immunocompromised host. Can Respir J 2018.10.1155/2018/2685723 [DOI] [PMC free article] [PubMed]
- 14.MZ Kayser, B Seeliger, C Valtin, J Fuge, S Ziesing, T Welte et al (2021) Clinical decision making is improved by BioFire Pneumonia Plus in suspected lower respiratory tract infection after lung transplantation: Results of the prospective DBATE-IT* study. Transpl Infect Dis [cited 2021 18];e13725–e13725. https://europepmc.org/article/med/34542213. 10.1111/TID.13725 [DOI] [PubMed]
- 15.Couturier MR, Barney T, Alger G, Hymas WC, Stevenson JB, Hillyard D, et al. Evaluation of the FilmArray® Respiratory Panel for clinical use in a large children’s hospital. J Clin Lab Anal. 2013;27:148–154. doi: 10.1002/JCLA.21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanson KE, (IDSA) for the DC of the IDS of A, Azar MM, (IDSA) for the DC of the IDS of A, Banerjee R, (IDSA) for the DC of the IDS of A et al (2020) Molecular testing for acute respiratory tract infections: clinical and diagnostic recommendations from the IDSA’s diagnostics committee. Clin Infect Dis [cited 2021 18];71:2744–51. https://academic.oup.com/cid/article/71/10/2744/5830781. 10.1093/CID/CIAA508 [DOI] [PMC free article] [PubMed]
- 17.Ramanan P, Bryson AL, Binnicker MJ, Pritt BS, Patel R (2017) Syndromic panel-based testing in clinical microbiology. Clin Microbiol Rev [cited 2021 3];31. https://journals.asm.org/doi/abs/10.1128/CMR.00024-17. 10.1128/CMR.00024-17 [DOI] [PMC free article] [PubMed]
- 18.Babady NE (2013) The FilmArray® respiratory panel: an automated, broadly multiplexed molecular test for the rapid and accurate detection of respiratory pathogens. Expert Rev Mol Diagn [cited 2021 3];13:779. /pmc/articles/PMC7103684/. 10.1586/14737159.2013.848794 [DOI] [PMC free article] [PubMed]
- 19.Min JY, Jang YJ (2012) Macrolide therapy in respiratory viral infections. Mediators Inflamm. 2012.10.1155/2012/649570 [DOI] [PMC free article] [PubMed]
- 20.Qavi AJ, McMullen A, Burnham C-AD, Anderson NW (2020) Repeat molecular testing for respiratory pathogens: diagnostic gain or diminishing returns? J Appl Lab Med [cited 2021 20]; 5:897–907. https://academic.oup.com/jalm/article/5/5/897/5827483. 10.1093/jalm/jfaa029 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data archiving is not mandatory but will be made available upon reasonable request.
N/A.