Abstract
Background and Objectives
The rate of infarct core progression in patients with acute ischemic stroke is variable and affects outcome of reperfusion therapy. We evaluated the hypoperfusion index (HI) to estimate the initial rate of core progression in patients with medium vessel occlusion (MeVO) compared to large vessel occlusion (LVO) stroke and within a larger time frame since stroke onset.
Methods
Core progression was assessed in 106 patients with acute stroke and CT perfusion. Using reperfusion trial core time criteria, fast progressors had core >70 mL within 6 hours of stroke onset and slow progressors had core ≤70 mL, mismatch ≥15 mL, and mismatch to core ratio ≥1.8 within 6 to 24 hours. The relationship between HI and infarct core progression (core/time) was examined using receiver operating characteristics to determine optimal HI cutoff. The HI cutoff was then tested in the overall cohort, compared between MeVO and LVO, and evaluated in patients up to 24 hours from stroke onset to differentiate fast from slow rate of core progression. HI threshold was assessed in a second independent cohort of 110 patients with acute ischemic stroke.
Results
In 106 patients with acute stroke, 6.6% were fast progressors, 27.4% were slow progressors, and 66% were not classified as fast or slow progressor by reperfusion trial core time criteria. HI >0.5 was associated with fast progression and able to distinguish fast from slow progressors (area under the curve [AUC] 0.94; 95% confidence interval [CI] 0.80–0.99). In MeVO (n = 26) HI >0.5 had a core progression of 0.30 mL/min compared to 0.03 mL/min for HI ≤0.5 (p < 0.001). In LVO (n = 80), HI >0.5 had a core progression of 0.26 mL/min compared to 0.02 mL/min for HI ≤0.5 (p < 0.001). In patients not classified as fast or slow progressor by reperfusion trial criteria, those with HI >0.5 had progression rate of 0.21 mL/min compared to 0.03 mL/min for those with HI ≤0.5 (p < 0.001). Validation in a second cohort of patients with acute ischemic stroke (n = 110; MeVO = 42, LVO = 68) yielded similar results for HI >0.5 to distinguish fast and slow core progression with an AUC of 0.84 (95% CI 0.72–0.97).
Discussion
HI can differentiate fast from slow core progression in MeVO and LVO within the first 24 hours of acute ischemic stroke. Consideration of core progression rate at time of stroke evaluation may have implications in the selection of patients with MeVO and LVO stroke for reperfusion therapy that warrant further study.
When cerebral artery occlusion occurs, the supplied brain region becomes ischemic. Without restored blood flow, the ischemic brain shifts to permanent infarction (core) over time. The rate at which infarct progression occurs varies among patients with acute ischemic stroke.1,2 In some, core progression is fast, over a few hours, whereas in others, it is slower, over many hours or even days. The rate of core progression is important; it provides an indication of time remaining to salvage ischemic brain tissue by reperfusion therapy.1-7 Accurate assessment of core progression may be useful clinically to aid in decisions of acute stroke transport, reperfusion therapy, and potentially neuroprotection strategy.1,2,8-12
The initial rate of core progression depends on several factors, including collaterals and their ability to maintain adequate oxygenation and nutrient supply to the brain during arterial occlusion.1,2,13,14 However, predicting collateral failure remains a challenge.13-15 Core progression can be estimated by core size on CT perfusion (CTP) relative to the time from stroke onset. Patients who develop a large core within the first few hours of stroke onset are fast progressors, whereas those with a small core and large areas of tissue at risk in the late window are slow progressors.1,2,16-19 In many patients, a clear designation as a fast or slow progressor is difficult to ascribe.1,2,8-11,18-20 Repeat perfusion imaging can assess core growth over time, but this often is not feasible and can introduce unnecessary delays.8,20 Furthermore, a progression rate that considers only core to time ratio fails to consider remaining tissue at risk. The hypoperfusion index (HI) is a tool to estimate stroke progression using CTP imaging. It was first described in 2008,21 with subsequent modifications to time-to-peak concentration thresholds, as a method to estimate the transition of ischemic brain tissue to infarction. HI is now preferably calculated as the ratio of time to peak concentration at >10 seconds divided by time to peak concentration at >6 seconds.22 Prior reports support its potential to estimate expected degree of core evolution based on severity of delay in blood flow to the brain. This is in contrast to the core divided by time measure of infarct progression, which only estimates brain tissue that has already infarcted.21,22
Reperfusion stroke trials have commonly estimated core progression using core volume relative to time, with fast progressors having core >70 mL within 6 hours of stroke onset and slow progressors having core ≤70 mL, mismatch ≥15 mL, and mismatch to core ratio ≥1.8 in 6–24 hours.3-7,16 However, by this definition of core progression, a subset of patients remains where the rate of core progression is not classified and HI may provide information.23 Additional evidence is needed to assess the role of HI in ischemic stroke and the relationship to core progression. To date, HI has been mostly studied in patients with large vessel occlusion (LVO) and focused on the first 6–8 hours of stroke onset.4,5 However, treatment windows for acute stroke interventions have been extended up to 24 hours.19,22,24,25 Moreover, a significant proportion of patients with stroke having medium vessel occlusion (MeVO; occlusion of the M2 segment of the middle cerebral artery) are increasingly being considered for possible endovascular therapy.26 In this study, we provide evidence regarding the performance of HI to assess initial rate of core progression in patients with MeVO compared to LVO within 24 hours of stroke onset.
Methods
Study Participants
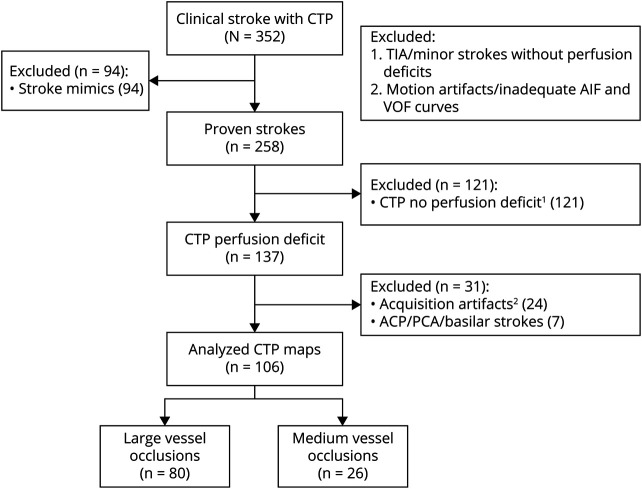
Consecutive patients with acute ischemic stroke assessed at the University of Alberta Hospital (Edmonton, Canada) between March and November 2019 were screened for this prospective observational cohort study (Figure 1). Inclusion criteria comprised age over 18 years, ischemic stroke either with LVO (internal carotid artery [ICA] and M1) or MeVO (proximal and distal M2 middle cerebral artery occlusion), and acute brain imaging with noncontrast CT head, CT angiogram head and neck, and CTP. Patients had to be within 24 hours of symptom onset and have premorbid modified Rankin Scale score of 2 or less. Patients excluded from the study were those with stroke mimics, TIA or posterior circulation strokes, perfusion maps of inadequate quality due to technical or other artifacts, or CTP nondetectable (minor) strokes. A first cohort of 106 patients with acute ischemic stroke was prospectively recruited from March to November 2019 who met study criteria. A second validation cohort of 110 patients with acute ischemic stroke were recruited from December 2019 to April 2020 and analyzed to confirm the results.
Figure 1. Flow Diagram for Patient Screening and Inclusion.
ACA = anterior cerebral artery; AIF = arterial input function; CTP = CT perfusion; PCA = posterior cerebral artery; VOF = venous output function.
Clinical Assessment
Patients were assessed using a standardized protocol for acute stroke evaluation. Ischemic stroke diagnosis required infarct on brain imaging (CT head or MRI diffusion sequence) and consistent clinical syndrome as assessed by a board-certified stroke neurologist. National Institutes of Health Stroke Scale (NIHSS) score was determined by a stroke neurologist on admission as part of the initial stroke evaluation. Demographics, clinical characteristics, and NIHSS were recorded at baseline for all patients in REDCap.
Image Acquisition and Postprocessing
Noncontrast CT head, followed by CT angiogram (CTA) head and neck and CTP head, were acquired as part of standardized clinical care pathway for acute stroke (eMethods, links.lww.com/WNL/B568). Alberta Stroke Program Early CT Score (ASPECTS) was recorded using the noncontrast CT head and dichotomized using a score of ≤7 or higher (scores range from 0 to 10, with higher scores indicating a smaller infarct core). LVO and MeVO were determined by CTA. CTP images were postprocessed using US Food and Drug Administration–approved RAPID (rapid processing of perfusion and diffusion; iSchemiaView) software for estimation of core, mismatch, and mismatch ratio, which were automatically calculated as part of acute stroke assessment. Images compatible with stroke syndromes and of adequate quality were included in analysis. The perfusion deficit volume was defined using time to peak concentration >6 seconds. Core was diagnosed if the relative cerebral blood flow was <30% of that in normal tissue. Mismatch was defined as tissue within the time to peak concentration >6 seconds deficit that was not the ischemic core (cerebral blood flow >30%).27 Mismatch ratio was calculated by dividing total perfusion deficit volume by core volume. Hypoperfusion index was generated by RAPID software based on the ratio of time to peak concentration >10 seconds divided by time to peak concentration >6 seconds.22 Core to time ratio was manually calculated postimaging to estimate rate of core progression by dividing core volume by time since stroke onset or last known well. In patients treated with endovascular therapy, recanalization status was determined by posttreatment conventional angiography scored by modified Thrombolysis in Cerebral Infarction rating by a board-certified neuroradiologist (eMethods).
Reperfusion Trial Core Time Progression Criteria
Based on prior reports and reperfusion trial core time criteria, fast progressors were defined as strokes with LVO or MeVO and core >70 mL within 6 hours (early tier) of onset. Patients with LVO or MeVO and core ≤70 mL, mismatch ≥15 mL, and mismatch-to-core ratio ≥1.8 in 6–24 hours (late tier) were defined as slow progressors.3-7,16 Unclassified patients were all other patients with stroke not meeting the fast or slow progressor definitions. These definitions were based on thresholds used in acute stroke reperfusion trials: Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials (HERMES), Tenecteplase in Stroke Patients Between 4.5 and 24 Hours (TIMELESS), Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke 3 (DEFUSE 3), and DWI or CTP Assessment with Clinical Mismatch in the Triage of Wake-Up and Late Presenting Strokes Undergoing Neurointervention with Trevo (DAWN).3-5,7,16
Core Progression
Core progression was determined using core to time ratio, dichotomized by > or ≤0.1 mL/min. We defined “fast rate of core progression” as patients with initial rate of core progression >0.1 mL/min no matter the time from stroke onset and “slow rate of core progression” as ≤0.1 mL/min. This was derived from previous publications estimating core progression as core to time ratio, to separate fast from slow rate of progression.22,23
Statistical Analysis
We reported descriptive statistics including frequency (%), mean (SD), and median (interquartile range [IQR]) to summarize characteristics. Characteristics of fast and slow progressors (classified group) and the group not meeting reperfusion trial core time criteria (unclassified group) were compared by analysis of variance, Kruskal-Wallis rank, Pearson χ2, or Fisher exact test, as appropriate. Normality of data was assessed by Shapiro-Wilk test. Data analysis was conducted using STATA 16.0 (StataCorp LLC). Demographic and baseline characteristics of dichotomized HI between different groups (LVO and MeVO, early and late groups) were compared using Mann-Whitney test, Pearson χ2, Fisher exact test, and t test where appropriate. Statistical tests were 2-sided and were considered significant with p < 0.05.
Receiver operating characteristic (ROC) analysis was used to assess the ability of HI to differentiate fast and slow rate of core progression in the patients classified by reperfusion trial core time criteria. ROC analysis was used to assess the optimal HI cutoff to correctly classify patients as fast or slow progressors to optimize specificity, sensitivity, and likelihood ratio.
The performance of HI cutoff was assessed using ROC in the overall cohort, in MeVO and LVO groups, in early and late tiers, and in the group not classified by reperfusion trial core time criteria. Findings in the first cohort were examined in a second cohort.
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the Health Ethics Committee of the University of Alberta (Pro00066577). Written informed consent was received from all study participants.
Data Availability
Anonymized data not published within this article will be made available on request to any qualified investigator.
Results
Baseline Patient Characteristics
Among 352 patients undergoing CTP during the study period, 258 had a confirmed diagnosis of ischemic stroke. Perfusion deficits were present in 137 patients. We excluded 24 cases due to imaging artifacts and 7 due to non-ICA/M1/M2 strokes (Figure 1). Comparison of baseline characteristics between screened out and recruited patients is shown in eTable 1 (links.lww.com/WNL/B555). For the overall cohort, the median CTP core volume was 9 mL (IQR 0–37), the mismatch volume was 81 mL (IQR 40–113), the mismatch ratio was 3.2 (IQR 2.2–9.7), the HI was 0.4 (IQR 0.2–0.6), and the baseline NIHSS was 14 (IQR 10–20). There were 60 patients (56.6%) within the first 6 hours of stroke onset and 46 (44.4%) within 6–24 hours.
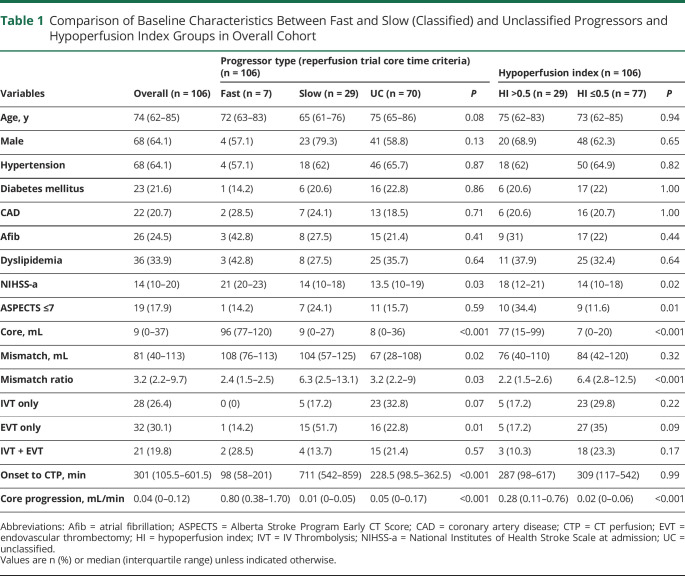
Fast progression was present in 6.6% (7/106) of patients, slow progression in 27.4% (29/106), and 66% (70/106) were unclassified (Table 1 and Figure 2). There were no significant differences in age, sex, or medical comorbidities between patients with fast compared to slow infarct core progression (Table 1 and eTable 2, links.lww.com/WNL/B556). Baseline median NIHSS was higher in patients with fast progression at 21 (IQR 20–23) compared to those with slow progression at 14 (IQR 10–18) (p = 0.03). Median core volume was larger in patients with fast progression at 96 mL (IQR 77–120) compared to 9 mL (IQR 0–27) in patients with slow progression (p < 0.001). Patients with fast progression had lower median mismatch ratio at 2.4 (IQR 1.5–2.5) compared to those with slow progression at 6.3 (IQR 2.5–13.1) (p = 0.03). All patients with fast progression (n = 7 [100%]) had HI >0.5, whereas only 3 (10.3%) slow progressors had HI >0.5 (p < 0.001). ASPECTS was not significantly different between fast and slow progressors (p = 0.59).
Table 1.
Comparison of Baseline Characteristics Between Fast and Slow (Classified) and Unclassified Progressors and Hypoperfusion Index Groups in Overall Cohort
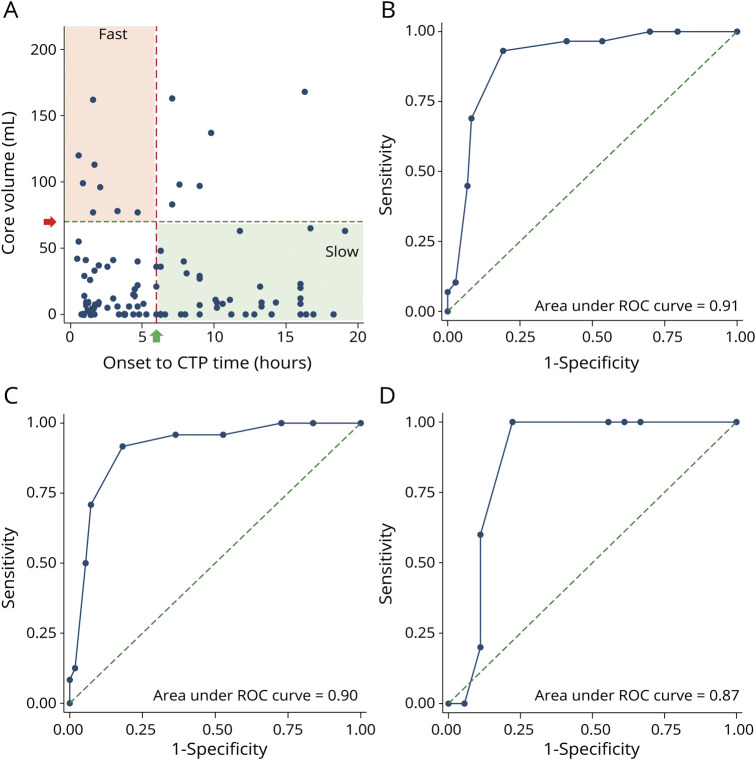
Figure 2. Patient Distribution by Core Volume to Time Since Stroke Onset and Receiver Operating Characteristics.
(A) Distribution of cohort by time since onset vs core volume. Onset to CT perfusion (CTP) time in hours (x-axis) plotted against core volume in mL (y-axis). Patients with stroke towards bottom left and top right remain unclassified by reperfusion trial core time criteria (see Methods). Bottom left area represents group of patients physicians may be especially interested in to differentiate fast from slow rate of core progression. (B) Receiver operating characteristic (ROC) curve for optimal hypoperfusion index (HI) cutoff (0.5) to distinguish fast and slow rate of core progression in entire cohort. (C) ROC curve for optimal HI cutoff (0.5) to distinguish fast and slow rate of core progression in large vessel occlusion stroke. (D) ROC curve for optimal HI cutoff (0.5) to distinguish fast and slow rate of core progression in medium vessel occlusion stroke.
HI Threshold to Distinguish Patients With Fast From Slow Core Progression
In patients with fast progression, the median HI was 0.6 (IQR 0.5–0.7), compared to those with slow core progression, where the median HI was 0.2 (IQR 0.1–0.4) (p < 0.001). The sensitivity and specificity for each HI threshold for patients meeting reperfusion trial core time criteria are shown in eTable 3 (links.lww.com/WNL/B557). An HI of 0.5 differentiated fast from slow progressors with 100% sensitivity, 89% specificity, and area under the curve (AUC) of 0.94 (95% confidence interval [CI] 0.80–0.99) (eFigure 1, links.lww.com/WNL/B566). In all 106 patients, the median core progression for those with HI ≤0.5 was 0.02 mL/min (IQR 0–0.06) compared to 0.28 mL/min (IQR 0.11–0.76) for HI >0.5 (p < 0.001). An HI of 0.5 differentiated fast from slow rate of core progression with an AUC of 0.91 (95% CI 0.83–0.96) (Figure 2). The sensitivity and specificity for each HI threshold for overall cohort are shown in eTable 4 (links.lww.com/WNL/B558). For patients within 6 hours of onset, the median core progression for HI ≤0.5 was 0.04 mL/min (IQR 0–0.09) compared to 0.66 mL/min (IQR 0.30–1.44) for HI >0.5 (p < 0.001) (AUC 0.90; 95% CI 0.79–0.96). For patients at 6–24 hours from stroke onset, the median core progression for HI ≤0.5 was 0.01 mL/min (IQR 0–0.03) compared to 0.17 mL/min (IQR 0.01–0.21) for HI >0.5 (p < 0.001) (AUC 0.94; 95% CI 0.80–0.98).
MeVO vs LVO Stroke
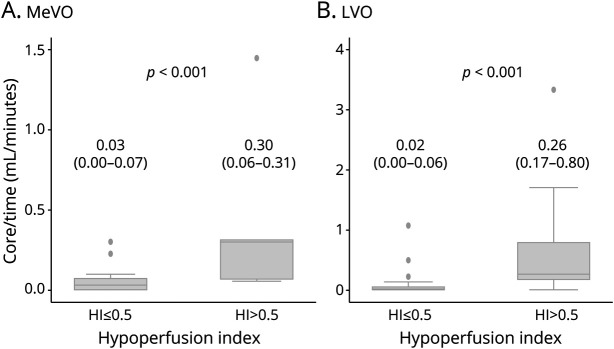
There were 26 patients with MeVO and 80 patients with LVO. Baseline characteristics were comparable between the MeVO and LVO groups (eTable 5, links.lww.com/WNL/B559). Within the MeVO and LVO stroke groups, baseline patient characteristics were not significantly different between those with HI ≤0.5 compared to those with HI >0.5 (eTable 6, links.lww.com/WNL/B560). In patients with MeVO stroke with HI ≤0.5, the median core progression was 0.03 mL/min (IQR 0–0.07) compared to 0.30 mL/min (IQR 0.06–0.31) for patients with HI >0.5 (p < 0.001) (Figure 3). In patients with LVO with HI ≤0.5, the median core progression was 0.02 mL/min (IQR 0–0.06) compared to 0.26 mL/min (IQR 0.17–0.80) for patients with HI >0.5 (p < 0.001) (Figure 3). For the MeVO and LVO patients, HI of 0.5 differentiated fast from slow rate of core progression with AUC of 0.87 (95% CI 0.82–0.96) and 0.90 (95% CI 0.66–0.97), respectively (Figure 2). Median onset to imaging time in patients with LVO (361.5, IQR 156–617 minutes) was slightly longer compared to those with MeVO (140.5, IQR 94–363 minutes). However, this was not statistically significant (eTable 5). The sensitivity and specificity for each HI threshold for MeVO and LVO patients are shown in eTable 7 (links.lww.com/WNL/B561).
Figure 3. Comparison of Rate of Core Progression Between Medium Vessel Occlusion and Large Vessel Occlusion Stroke in ≤0.5 and >0.5 Hypoperfusion Index Groups.
(A) Stroke patients with medium vessel occlusion (MeVO) and (B) stroke patients with large vessel occlusion (LVO).
Ability of HI to Identify Rate of Core Progression in Unclassified Stroke
There were 70 patients not meeting reperfusion trial core time criteria for fast or slow progressors (see Methods). In the unclassified group, those with HI >0.5 had faster median progression rate of 0.21 mL/min (IQR 0.06–0.38) compared to 0.03 mL/min (IQR 0–0.07) in those with HI ≤0.5 (p < 0.001). In MeVO in this cohort (n = 24), those with HI ≤0.5 had a median core progression of 0.01 mL/min (IQR 0–0.07) compared to those with HI >0.5, where the median core progression was 0.30 mL/min (IQR 0.06–0.31) (p = 0.03). In LVO (n = 46), those with HI ≤0.5 had a median core progression of 0.03 mL/min (IQR 0–0.07) compared to those with HI >0.5, where the median core progression was 0.20 mL/min (IQR 0.17–0.38) (p = 0.001). The HI was able to assess progression rate in 24 additional (24/26 = 92.3%) MeVO and 46 additional (46/80 = 57.5%) LVO cases (AUC 0.88, 95% CI 0.79–0.95) compared to reperfusion trial core time criteria for fast and slow progression described in the Methods (eFigure 1, links.lww.com/WNL/B566). The sensitivity and specificity for each HI threshold for unclassified stroke are shown in eTable 8 (links.lww.com/WNL/B562). When analyzed by time, unclassified patients within the first 6 hours of stroke onset with HI >0.5 had faster median progression rate of 0.31 mL/min (IQR 0.06–1.44) compared to 0.04 mL/min (IQR 0–0.09) in those with HI ≤0.5 (p = 0.001). In unclassified patients within 6–24 hours of stroke onset, those with HI >0.5 had median progression rate of 0.18 mL/min (IQR 0.11–0.22) compared to 0 mL/min (IQR 0–0.01) in those with HI ≤0.5 (p = 0.001) (eTable 9, links.lww.com/WNL/B563).
Validation
A second independent cohort of 110 patients (eFigure 2, links.lww.com/WNL/B567) was used to confirm the HI threshold of 0.5. The cohort had similar demographics to the first stroke cohort when divided by ≤0.5 and >0.5 HI groups (eTable 10, links.lww.com/WNL/B564). In the stroke patients with MeVO (n = 42), those with HI ≤0.5 had a median core progression of 0.01 mL/min (IQR 0–0.06) compared to those with HI >0.5 where the median core progression was 0.11 mL/min (IQR 0.04–0.26) (p = 0.001). In LVO cases (n = 68), those with HI ≤0.5 had a median core progression of 0.01 mL/min (IQR 0–0.04) compared to those with HI >0.5, where the median core progression was 0.11 mL/min (IQR 0.04–0.45) (p < 0.001). The sensitivity and specificity for each HI threshold in the second cohort are shown in eTable 11 (links.lww.com/WNL/B565). For the patients with MeVO and LVO stroke in the second cohort, HI of 0.5 differentiated fast from slow rate of core progression with AUC of 0.84 (95% CI 0.72–0.97) and 0.84 (95% CI 0.72–0.97), respectively (eFigure 2, links.lww.com/WNL/B567).
Discussion
HI was associated with rate of core progression in both MeVO and LVO stroke. HI >0.5 was able to distinguish fast from slow progressors up to 24 hours. In MeVO cases, HI >0.5 had a core progression of 0.30 mL/min, compared to 0.26 mL/min in LVO. Furthermore, HI was able to estimate rate of core progression in patients with otherwise unclassified progression by reperfusion trial core time criteria. The ability of HI to estimate core progression rates may have implications for the selection of patients for reperfusion therapy as discussed below.
Our study is supported by prior studies evaluating HI in acute ischemic stroke.19,24,25 In patients with LVO stroke, Olivot et al.19 found HI >0.4 to be associated with collateral failure on perfusion imaging and conventional angiography (AUC 0.73). Guenego et al.25 found a similar result in LVO strokes, reporting HI >0.4 to be associated with worse collaterals (sensitivity 79%, specificity 56%, AUC 0.70). In another study of 28 patients with LVO undergoing thrombectomy, HI of 0.5 or greater was related to core progression.22 In our study, HI >0.5 was associated with an increased rate of core progression in patients with LVO, consistent with prior cutoff.22
HI >0.5 also was associated with increased core progression rate in patients with MeVO (patients with occlusion of M2 branch of middle cerebral artery). MeVOs are an important group of patients with stroke to consider as they account for roughly 35%–40% of acute ischemic strokes.28,29 Which MeVO cases benefit from thrombectomy remains unclear.30,31 The rate of core progression estimated by HI warrants further evaluation to assess its potential in the selection of patients with MeVO for recanalization.29-33 Given that MeVO strokes tend to be smaller compared to LVO strokes, a rapid rate of core progression may have greater implications regarding timing of reperfusion by thrombectomy. MeVO may shift to a completed stroke faster than a LVO despite having similar progression rates.6,7,17 This will be of interest to examine in future thrombectomy treatment trials of MeVO. Our study also provides evidence of HI-based evaluation of core progression up to 24 hours from stroke onset, showing a similar performance in patients assessed in both early and late time windows. With an expansion of reperfusion treatment to longer time windows, this provides reassurance regarding HI evaluation of infarct progression over a range of treatment time windows.
The HI adds to the ability to assess core progression. Progression rate as assessed by reperfusion trial core time criteria leaves a large number of patients with stroke in an unclassified group. Furthermore, within the first 6 hours of stroke onset, only fast progressors can be reliably identified.1,2 Moreover, a small core at later time period may be a slow progressor or a completed small stroke.2,8,11,17 HI can circumvent the core and time restrictions laid by reperfusion trial core time criteria. By assessing time to peak concentration parameters (tissue likely to become infarcted), HI is able to provide an assessment of core progression rate in most patients with stroke in a manner that is less reliant on time from stroke onset (eFigure 2, links.lww.com/WNL/B567).22,25
The role HI may play in the management of acute ischemic stroke requires further evaluation. Given the ability of HI to assess core progression, patients with LVO or MeVO and low NIHSS may be of interest to assess.34-37 Potentially, patients with LVO or MeVO with low NIHSS and high HI may derive greater benefit from reperfusion, as they are more likely to experience core progression and infarct growth without treatment.22 HI may also have a role to guide decisions of late window thrombolysis and transport.6 Whether late window patients with stroke and high HI benefit from thrombolysis prior to transport for endovascular therapy evaluation will be of interest to explore. How HI assessment of core progression may contribute to decisions of reperfusion therapy and interfacility transport warrants further study.
Our study has a few limitations. First, posterior circulation strokes were not included in the study. The recruitment process may have introduced selection bias as it was an observational study, thus further evaluation in larger cohorts is required. However, patients not meeting study inclusion criteria had similar demographic and clinical characteristics as those studied. Furthermore, the ability of HI to assess core progression was confirmed in a second cohort, supporting the findings. Second, patients were recruited from a single tertiary referral center for stroke. Thus, a selection bias may exist toward patients being transferred for intervention. Brain imaging was performed at slightly longer time points from stroke onset in LVO compared to MeVO, although this was not significant. This trend was most likely related to interfacility transport of patients with LVO for thrombectomy. Third, we made the assumption that core growth starts at symptom onset and proceeds in a linear fashion over time. While consistent with prior studies, core growth is likely a dynamic process that is influenced by a range of factors such as blood pressure, recanalization, collateral failure, and tolerance of brain ischemia.15,38-40 However, HI has been shown to remain stable over a range of time frames since onset of stroke.22,25 Fourth, HI relies on CTP, which is not available at all centers where patients with stroke are assessed. This limits its use to centers where CTP can be performed, which may expand over time as data supporting the role of CTP in the management of stroke emerges. Automated processing of perfusion scans as performed by software programs such as RAPID are reducing barriers to widespread implementation of CTP.38 Finally, serial imaging over time is another method to assess core progression, which we did not perform. However, serial imaging is often not available in clinical practice and can add time delays to treatment. Future multicenter imaging studies monitoring infarct growth over time will be of value to further understand the dynamics of core growth in patients with acute ischemic stroke and how best to model it.
HI was able to estimate the initial rate of core progression in patients with MeVO and LVO acute ischemic stroke up to 24 hours after onset. Patients with HI >0.5 have a fast rate of infarct progression. Further evaluation of HI is needed to determine whether it could aid in the selection and management of patients with stroke treated with reperfusion therapy.
Glossary
- ASPECTS
Alberta Stroke Program Early CT Score
- AUC
area under the curve
- CI
confidence interval
- CTA
CT angiogram
- CTP
CT perfusion
- HI
hypoperfusion index
- ICA
internal carotid artery
- IQR
interquartile range
- LVO
large vessel occlusion
- MeVO
medium vessel occlusion
- NIHSS
National Institutes of Health Stroke Scale
- RAPID
rapid processing of perfusion and diffusion
- ROC
receiver operating characteristic
Appendix. Authors

Study Funding
The authors report no targeted funding.
Disclosure
A.Z. Nomani, J. Kamtchum-Tatuene, J.L. Rempel, T. Jeerakathil, I. Winship, K.A. Khan, B.H. Buck, and A. Shuaib report no disclosures relevant to the manuscript. G.C. Jickling reports receiving grant funding from the Canadian Institutes of Health Research, Heart and Stroke Foundation, Canadian Foundation for Innovation, NIH, and University Hospital Foundation. Go to Neurology.org/N for full disclosures.
References
- 1.Rocha M, Jovin TG. Fast versus slow progressors of infarct growth in large vessel occlusion stroke: clinical and research implications. Stroke. 2017;48(9):2621-2627. [DOI] [PubMed] [Google Scholar]
- 2.Rocha M, Desai SM, Jadhav AP, Jovin TG. Prevalence and temporal distribution of fast and slow progressors of infarct growth in large vessel occlusion stroke. Stroke. 2019;50(8):2238-2240. [DOI] [PubMed] [Google Scholar]
- 3.Campbell BCV, Majoie CBLM, Albers GW, et al. ; HERMES Collaborators. Penumbral imaging and functional outcome in patients with anterior circulation ischaemic stroke treated with endovascular thrombectomy versus medical therapy: a meta-analysis of individual patient-level data. Lancet Neurol. 2019;18(1):46-55. [DOI] [PubMed] [Google Scholar]
- 4.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21. [DOI] [PubMed] [Google Scholar]
- 6.Ma H, Campbell BCV, Parsons MW, et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med. 2019;380(19):1795-1803. [DOI] [PubMed] [Google Scholar]
- 7.Tenecteplase in stroke patients between 4.5 and 24 hours (TIMELESS). ClinicalTrials.gov identifier: NCT03785678. Accessed January 5, 2021. clinicaltrials.gov/ct2/show/NCT03785678
- 8.Broocks G, Rajput F, Hanning U, et al. Highest lesion growth rates in patients with hyperacute stroke. Stroke. 2019;50:189-192. [DOI] [PubMed] [Google Scholar]
- 9.Direct transfer to an endovascular center compared to transfer to the closest stroke center in acute stroke patients with suspected large vessel occlusion (RACECAT). ClinicalTrials.gov identifier: NCT02795962. Accessed January 7, 2021. clinicaltrials.gov/ct2/show/NCT02795962
- 10.Nie X, Pu Y, Zhang Z, Liu X, Duan W, Liu L. Futile recanalization after endovascular therapy in acute ischemic stroke. Biomed Res Int. 2018;2018:5879548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuentes B, Leciñana MA, Ximénez-Carrillo A, et al. Futile interhospital transfer for endovascular treatment in acute ischemic stroke: the Madrid Stroke Network experience. Stroke. 2015;46(8):2156-2161. [DOI] [PubMed] [Google Scholar]
- 12.Boulanger JM, Lindsay MP, Gubitz G, et al. Canadian stroke best practice recommendations for acute stroke management: prehospital, emergency department, and acute inpatient stroke care, 6th edition, update 2018. Int J Stroke. 2018;13(9):949-984. [DOI] [PubMed] [Google Scholar]
- 13.Shuaib A, Butcher K, Mohammad AA, Saqqur M, Liebeskind DS. Collateral blood vessels in acute ischaemic stroke: a potential therapeutic target. Lancet Neurol. 2011;10(10):909-921. [DOI] [PubMed] [Google Scholar]
- 14.Campbell BC, Christensen S, Tress BM, et al. Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J Cereb Blood Flow Metab. 2013;33(8):1168-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin L, Chen C, Tian H, et al. Perfusion computed tomography accurately quantifies collateral flow after acute ischemic stroke. Stroke. 2020;51(3):1006-1009. [DOI] [PubMed] [Google Scholar]
- 16.Goyal M, Menon BK, Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomized trials. Lancet. 2016;387(10029):1723-1731. [DOI] [PubMed] [Google Scholar]
- 17.Albers GW. Late window paradox. Stroke. 2018;49(3):768-771. [DOI] [PubMed] [Google Scholar]
- 18.Ospel JM, Holodinsky JK, Goyal M. Management of acute ischemic stroke due to large-vessel occlusion: JACC focus seminar. J Am Coll Cardiol. 2020;75(15):1832-1843. [DOI] [PubMed] [Google Scholar]
- 19.Olivot JM, Mlynash M, Inoue M, et al. Hypoperfusion intensity ratio predicts infarct progression and functional outcome in the DEFUSE 2 Cohort. Stroke. 2014;45(4):1018-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–e418. [DOI] [PubMed] [Google Scholar]
- 21.Bang OY, Saver JL, Alger JR, et al. Determinants of the distribution and severity of hypoperfusion in patients with ischemic stroke. Neurology. 2008;71(22):1804-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guenego A, Mlynash M, Christensen S, et al. Hypoperfusion ratio predicts infarct growth during transfer for thrombectomy. Ann Neurol. 2018;84(4):616-620. [DOI] [PubMed] [Google Scholar]
- 23.Seo WK, Liebeskind DS, Yoo B, et al. UCLA Penumbra Imaging Investigators. Predictors and functional outcomes of fast, intermediate, and slow progression among patients with acute ischemic stroke. Stroke. 2020;51(8):2553-2557. [DOI] [PubMed] [Google Scholar]
- 24.Guenego A, Marcellus DG, Martin BW, et al. Hypoperfusion intensity ratio is correlated with patient eligibility for thrombectomy. Stroke. 2019;50(4):917-922. [DOI] [PubMed] [Google Scholar]
- 25.Guenego A, Fahed R, Albers GW, et al. Hypoperfusion intensity ratio correlates with angiographic collaterals in acute ischaemic stroke with M1 occlusion. Eur J Neurol. 2020;27(5):864-870. [DOI] [PubMed] [Google Scholar]
- 26.Goyal M, Ospel JM, Menon BK, Hill MD. MeVO: the next frontier? J Neurointerv Surg. 2020;12(6):545-547. [DOI] [PubMed] [Google Scholar]
- 27.Straka M, Albers GW, Bammer R. Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging. 2010;32(5):1024-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang P, Zhang Y, Zhang L, et al. Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. 2020;382(21):1981-1993. [DOI] [PubMed] [Google Scholar]
- 29.Altenbernd J, Kuhnt O, Hennigs S, Hilker R, Loehr C. Frontline ADAPT therapy to treat patients with symptomatic M2 and M3 occlusions in acute ischemic stroke: initial experience with the Penumbra ACE and 3MAX reperfusion system. J Neurointerv Surg. 2018;10(5):434-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim YW, Son S, Kang DH, Hwang YH, Kim YS. Endovascular thrombectomy for M2 occlusions: comparison between forced arterial suction thrombectomy and stent retriever thrombectomy. J Neurointerv Surg. 2017;9(7):626-630. [DOI] [PubMed] [Google Scholar]
- 31.Dorn F, Lockau H, Stetefeld H, et al. Mechanical thrombectomy of m2-occlusion. J Stroke Cerebrovasc Dis. 2015;24(7):1465-1470. [DOI] [PubMed] [Google Scholar]
- 32.Grossberg JA, Rebello LC, Haussen DC, et al. Beyond large vessel occlusion strokes: distal occlusion thrombectomy. Stroke. 2018;49(7):1662-1668. [DOI] [PubMed] [Google Scholar]
- 33.Sarraj A, Sangha N, Hussain MS, et al. Endovascular therapy for acute ischemic stroke with occlusion of the middle cerebral artery M2 segment. JAMA Neurol. 2016;73(11):1291-1296. [DOI] [PubMed] [Google Scholar]
- 34.Endovascular therapy for low NIHSS ischemic strokes (ENDOLOW). ClinicalTrials.gov identifier: NCT04167527. Accessed January 10, 2021. clinicaltrials.gov/ct2/show/NCT04167527
- 35.Coutts SB, Dubuc V, Mandzia J, et al. Tenecteplase-tissue-type plasminogen activator evaluation for minor ischemic stroke with proven occlusion. Stroke. 2015;46(3):769-774. [DOI] [PubMed] [Google Scholar]
- 36.Griessenauer CJ, Medin C, Maingard J, et al. Endovascular mechanical thrombectomy in large-vessel occlusion ischemic stroke presenting with low National Institutes of Health Stroke Scale: systematic review and meta-analysis. World Neurosurg. 2018;110:263-269. [DOI] [PubMed] [Google Scholar]
- 37.Messer MP, Schönenberger S, Möhlenbruch MA, et al. Minor stroke syndromes in large-vessel occlusions: mechanical thrombectomy or thrombolysis only? AJNR Am J Neuroradiol. 2017;38(6):1177-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vagal A, Wintermark M, Nael K, et al. Automated CT perfusion imaging for acute ischemic stroke: pearls and pitfalls for real-world use. Neurology. 2019;93(20):888-898. [DOI] [PubMed] [Google Scholar]
- 39.Martins N, Aires A, Mendez B, et al. Ghost infarct core and admission computed tomography perfusion: redefining the role of neuroimaging in acute ischemic stroke. Interv Neurol. 2018;7(6):513-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rao VL, Mlynash M, Christensen S, et al. Collateral status contributes to differences between observed and predicted 24-h infarct volumes in DEFUSE 3. J Cereb Blood Flow Metab. 2020;40(10):1966-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available on request to any qualified investigator.