Abstract
There are special considerations when treating anastomotic leak after restorative proctocolectomy and ileal pouch–anal anastomosis. The epidemiology, risk factors, anatomic considerations, diagnosis and management, as well as the short- and long-term consequences to the patient are unique to this patent population. Additionally, there are specific concerns such as “tip of the J” leaks, transanal management of anastomotic leak/presacral sinus, functional outcomes after leak, and considerations of redo pouch procedures.
Keywords: ulcerative colitis, Crohn's colitis, familial adenomatous polyposis, ileoanal pouch, AL, surgery, complications
In the 21st century, ileal pouch–anal anastomosis (IPAA) is the ideal treatment for most patients who require surgical management of ulcerative colitis (UC), familial adenomatous polyposis (FAP), and selected cases of isolated Crohn's colitis without ileitis or perianal disease. First described in 1978 by Parks and Nicholls at St. Marks Hospital in London, this procedure restores intestinal continuity and offers patients an excellent quality of life. 1 The operation carries significant risks, most concerning being 5 to 15% anastomotic leak (AL), owing to multiple staple lines and an anastomosis between the thin-walled ileum and anal canal. 2
Epidemiology
Little population-based data exist on the topic of AL following IPAA, the vast majority of the literature is single institution case series, and reported rates of AL after IPAA varies widely from 5 to 19%. 2 AL may lead to pelvic sepsis, fistula formation, IPAA stricture, and pouch failure. Lian et al evaluated 1,965 IPAA procedures and found an AL rate of 5% and fistula formation of 7%. 3 In another large series from Germany with 494 patients, the overall incidence of pouch-related septic complications (PRSCs) was 19.2%. In a 24-year analysis of pediatric patients with FAP undergoing IPAA, Kennedy et al found a low rate of AL or pelvic abscess in their patient cohort (7.4%). 4
Risk Factors
Sahami et al looked specifically at inflammatory bowel disease (IBD) patients undergoing IPAA and found that body mass index (BMI) > 25 kg/m 2 , American Society of Anesthesiologist (ASA) score > 2, a disease duration of >5 years, and a concurrent administration of tumor necrosis factor inhibitors (TNFi's) and steroids were all independent risk factors for AL. 5 Manilich et al performed prognostic modeling of preoperative risk factors for pouch failure from 3,754 IPAA procedures and identified Crohn's disease (CD), diabetes, handsewn (HS) anastomosis, and older age as the strongest risk factors for pouch failure, a leading cause of which is postoperative pelvic sepsis. 6
Ulcerative Colitis versus Familial Adenomatous Polyposis
The rate of AL has been shown to be significantly different between IPAA surgery for UC and FAP; the rate of PRSC in UC was 23.4% in UC and only 9.4% in FAP ( p = 0.001). There was also a significant difference in the timing of presentation of PRSC. In FAP, 90% (17/19) of PRSC presented within the first year of follow-up, whereas only 63% (71/112) of PRSC occurred within the first year in UC patients. 7 Development of AL in UC patients greater than 1 year after surgery raises increasing concern for development of CD. Patients with UC have been shown to be at greater risk of pelvic sepsis within the first 4 months after surgery, compared with those with FAP, likely due to poor wound healing associated with immunosuppressants as well as comorbid malnutrition and anemia from severe disease activity. 7 8 Independent risk factors for PRSC in UC were systemic corticosteroids before surgery, patients younger than 50 years, greater than grade III proctitis, or a preoperative hemoglobin of less than 10 g/L. Patients taking prednisone-equivalent systemic steroids of >40 mg/day had a significantly greater risk of PRSC compared with patients taking <40 mg/day. For patients with FAP, the only independent risk factors identifiable in the same analysis were anastomotic tension and age >50 years. 7 Preoperative steroid use has been consistently demonstrated as an independent risk factor for anastomotic related complications after IPAA surgery. 9 Recently Ritter et al showed that any postoperative steroid taper was independently associated with an increased risk of pelvic sepsis (odds ratio [OR] = 2.3; confidence interval [CI]: 1.06–5.1; p = 0.04), as was total proctocolectomy (OR = 2.2; CI: 1.01–4.7; p = 0.05) at the time of the pouch. 9
Biologic Therapy
Whether or not biologic therapy before surgery is associated with increased complications after surgery remains highly controversial. Previously, Kulaylat et al found that TNFi agents were associated with increased postoperative complications if used within 90 days of surgery, although it is difficult to isolate from the data whether TNFi's per se was an independent risk factor or simply a surrogate for severity of disease, a notion that many subscribe to given the lack of a consistent signal for increased risk. 10 Recently, the National Surgical Quality Improvement Program's IBD Collaborative did not find that biologic exposure was associated with an increase in postoperative infections or surgical site infections on multivariate analysis. However, on univariate subgroup analysis of patients undergoing proctectomy biologic exposure was associated with increased leaks after proctetomy. 11 Other studies support this view of increased infectious or total postoperative complications in patients receiving TNFi's preoperatively. 12 13 14 This data, however, are contradicted by numerous other studies showing no difference in IPAA AL rates in patients receiving TNFi's, 15 16 17 18 19 20 21 22 23 24 and multiple meta-analyses have also yielded conflicting results. 25 26 27 28 The bulk of the data does not support this association, and many surgeons will operate when the next dose is due to be given, when serum blood levels are at their nadir. 29
Role of Staging Ileal Pouch–Anal Anastomosis Surgery
The role of proximal diversion in preventing ALs in IPAA is also controversial. There is conflicting data regarding whether proximal diversion decreases the total AL rate, although it is generally accepted that proximal diversion will decrease the consequences of AL by reduced pelvic contamination if AL occurs. 30 31 The modified two-stage procedure consists of a subtotal colectomy with end ileostomy, followed by completion proctectomy and IPAA as a second procedure without a diverting ileostomy. Zittan et al examined AL rates between a two-stage and modified two-stage procedures in a cohort of 460 UC patients and found the modified two-stage procedure had a lower rate of AL following IPAA (4.6 vs. 15.7%). 32 Similarly, Samples et al demonstrated no difference in leak rates in their cohort of 248 UC patients who underwent a classic two-stage versus a modified two-stage procedure. 33 They noted that patients undergoing a modified two-stage procedure had a higher risk profile with more recent steroid and biologic use at the time of their first operation, thus were more likely to have an subtotal colectomy; thus the AL rates between groups were similar, and the higher risk was mitigated by delayed pouch construction.
Lavryk et al examined whether there was any difference in short-term and long-term consequences in IPAA surgery if a diverting loop ileostomy was omitted. 34 In a retrospective review of 4,031 patients who underwent IPAA from 1983 to 2014, there were 326 (9%) patients who developed pelvic sepsis with a diverting ileostomy and 31 (7%) patients who developed pelvic sepsis without a diverting ileostomy ( p = 0.17). Although a total of 48% of patients in the group without a diverting loop ileostomy (DLI) ultimately required diversion versus 12% in the diverted group, at 10-year follow-up there were no differences in pouch survival between groups. The authors concluded that omission of a DLI in selected patients who had pelvic sepsis after IPAA surgery did not increase pouch failure or adversely affect quality of life in the long term. 34 Other authors have found no difference in AL rates or pouch-related complications in a one-stage IPAA versus a two- or three-stage IPAA. 31 35 It is important to note, however, that in the modern era with increased use of biologic therapy and immunosuppression given to nearly all patients with UC who are medically refractory as the indication for surgery, a one-stage IPAA may place patients at increased risk of pouch-related complications and pelvic sepsis. 28 29 36 37 38 Other studies have found that diversion did not prevent septic complications in the event of an IPAA leak, and diversion in itself was associated with long-term complications. 39 40
Sutured versus Stapled Ileal Pouch–Anal Anastomosis
In the largest meta-analysis on the subject of HS versus stapled IPAA, Lovegrove et al showed no significant difference in the postoperative complications between the two groups comprising a total of 4,183 patients. 41 The AL rate was 6.9% overall: 8.8% in the HS group and 5.2% in the stapled group ( p = 0.42). Pelvic sepsis, often used as an surrogate for IPAA leak, occurred in 63 of 878 (7.2%) patients with an HS pouch anal anastomosis and in 50 of 1,063 (4.7%) patients with a stapled anastomoses ( p > 0.05). Given the rarity of leaks as an endpoint, these studies, despite their size, may have been underpowered. In a recent nonrandomized study from Japan, FAP patients who underwent IPAA had significantly fewer pouch-related complications, such as AL, pelvic abscess, vaginal fistula, and anal anastomotic stricture, in stapled IPAA compared with HS anastomosis. 42
The Cleveland Clinic analyzed their large cohort of patients who underwent an IPAA to determine if an AL after an HS versus stapled anastomosis made a difference in patient outcomes. Of the 175 patients who had an AL after IPAA, 141 were stapled (5.5%) and 34 were HS (7.2%; p = 0.14). Interestingly, they found that functional results at 5 years were better after a leak from a stapled anastomosis compared with an HS anastomosis, specifically with a lower incontinence rate and lower nocturnal seepage rates, likely owing to the height of the anastomosis. 3
Other Risk Factors
Surgeon experience is important: low-volume surgeons are more likely to have pouch-related complications, particularly pelvic sepsis, in patients undergoing laparoscopic IPAA surgery. 43 Stapler size has also been examined as an independent predictor of multiple pouch-related outcomes but was not shown to be associated with an increased rate of leak. 44 Laparoscopic surgery, compared with open was not associated with increased pouch-related complications. 45 46 In a meta-analysis of short- and long-term outcomes of J, W, and S ileal reservoirs for restorative proctocolectomy, Lovegrove et al found that all three reservoirs had similar complication rates with no significant difference in the incidence of early postoperative complications between groups. 47
Malnutrition is associated with adverse outcomes. Preoperative hypoalbuminemia was associated with adverse outcomes in IPAA surgery and was an independent predictor of AL in a 405 patient cohort. 48 Certainly, it is known that IPAA construction in patients with undiagnosed CD have a worse outcome with increased rates of pouch failure as shown by Melton et al. 49 In a systemic review and meta-analysis, Pellino et al found a five-fold higher risk of pouch failure and a two-fold higher risk of strictures in CD after IPAA compared with UC. 50 Reese et al performed a systematic review comparing postoperative adverse events and functional outcomes after IPAA surgery in patients with CD and found a significantly higher rate of anastomotic strictures and pouch failure. 51
Anatomy of a Leak
There are several potential areas for an AL after IPAA surgery ( Fig. 1 ). The location of an AL after IPAA originates from areas of vulnerability within the pouch. The oversewn blind end of distal ileum (i.e., “tip of the J,” an area of relative ischemia), the circular staple or HS suture line of the PAA is an area of tension, and the linear suture or staple lines on the pouch body itself with crossing staple lines at the so called “jag.” 2 Heuschen et al examined the anatomical location of IPAA leaks and after exclusion of CD demonstrated that 76% of pelvic sepsis events were secondary to ALs, and the most common site was isolated PAA (56%), followed by pouch vaginal (13%) and proximal pouch (7%). 7
Fig. 1.
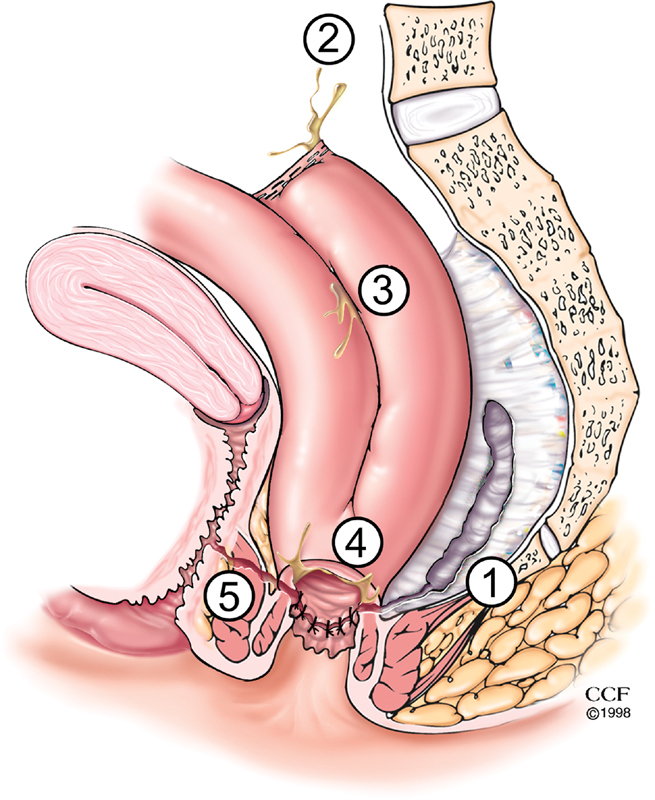
AL anatomy. 1. Presacral sinus, 2. tip of the J-pouch leak, 3. staple line leak along the J-pouch body, 4. leak from the IPAA, and 5. pouch vaginal fistula. AL, anastomotic leak; IPAA, ileal pouch–anal anastomosis.
Clinical Presentation and Diagnosis
AL after IPAA has various presentations, ranging from asymptomatic radiologic findings, to postoperative pelvic sepsis, or rarely generalized peritonitis. An important concept, especially in diverted patients, is that subtle deviations in the expected postoperative course may be indicative of an AL. Leak presentation depends on the degree of anastomotic separation, the amount of pelvic contamination and whether the patient was diverted proximally. An anastomotic dehiscence of > 50% in an undiverted patient will present very differently to a small leak in a patient with a diverting loop ileostomy who may only present with an asymptomatic presacral sinus on contrast enema. It is our practice that any patient in the postoperative phase who has a fever or unexplained leukocytosis should be regarded as pelvic sepsis until proven otherwise. In diverted patients, a small AL may be clinically silent and only present when the patient is under evaluation for diverting loop ileostomy reversal, or after ileostomy reversal. Detecting ALs and treating early and appropriately is one of the most challenging aspects of pouch surgery. If an AL is suspected, a gentle and careful digital examination may reveal an anastomotic defect or localized tenderness over a fluctuant, bulging mass. 2 Biomarkers have been studied to help the aid with early diagnosis of AL after colorectal surgery. An elevated c-reactive protein (CRP) has been validated worldwide to be associated with ALs; however, few have looked at this in the setting of IPAA. 52 Clark et al from Australia evaluated drain fluid amylase in postoperative IPAA patients and found high levels to correlate with AL in a small cohort of patients. 53
Imaging
Cross-sectional imaging with intravenous and rectal contrast computerized tomography (CT) or magnetic resonance imaging (MRI) is the mainstay of detection of pelvic sepsis in the acute postoperative phase. 54 Fluoroscopic pouchography with water-soluble rectal contrast should use a “Christmas-tree” catheter instead of balloon-tipped catheter, as the balloon may occlude the leak and result in a false-negative test result. Sossenheimer et al found that abnormal pouchography before ileostomy takedown was associated with delayed takedown operation, not unexpectedly increased risk of pouch-related complications and increased risk of and shorter time to pouch failure. 55 These contrast enema studies may be performed to identify the presence and origin of a leak; however, CT has been shown to be significantly more sensitive than fluoroscopy in identifying abscesses and may also aid in diagnosing other causes for the patient's symptoms as well. 54 56 When possible, intravenous, oral, and pouch contrast should be used to identify AL. While not all ALs demonstrate extravasation of oral or pouch contrast, there may be abscesses that manifest as extraluminal fluid collections with air–fluid levels, well-defined enhancing walls, or a mass effect with displacement of pouch anteriorly. 54 On axial images, these fluid collections are often in the presacral area and can contain gas bubbles and layering, and presacral soft-tissue thickening may also be seen. An AL may also have more subtle radiological findings such as a poorly defined fluid collection intercalated within the ileal mesentery with irregular margins. If an MRI is used, extraluminal fluid collections have high signal intensity on T2-weighted MRI images and low signal intensity with rim enhancement on T1-gadolinium-enhanced MRI ( Fig. 2 ). A chronic presacral sinus or collection from an AL can cause osteomyelitis that may also be seen on MRI showing marrow edema, cortical destruction, and surrounding enhancement. 54 Irrespective of the modality used to image postoperatively, a close working relationship with a gastrointestinal (GI) radiologist is essential, and surgeons should review the images alongside their radiology colleagues to aid with interpretation as even experienced radiologists benefit from a multidisciplinary approach to radiographic interpretation of abnormal pouch anatomy.
Fig. 2.
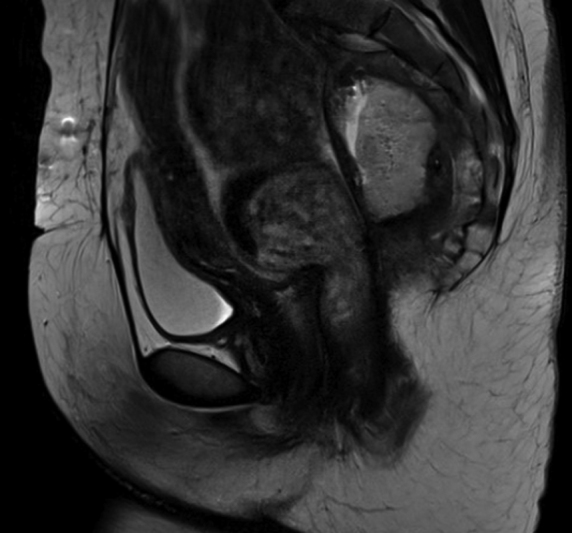
Presacral abscess. MRI image of a 10-cm presacral abscess with connection to the pouch body as demonstrated by extravasation of contrast. MRI, magnetic resonance imaging.
Management
Consequences of the AL can range from a chronic presacral sinus to uncontrolled sepsis. The degree of systemic impact, the location of the leak, the length of the sinus, and the extent of anastomotic disruption will determine the timing of leak presentation and the type and intervention required. Deciding on the degree of intervention required to heal the pouch AL is challenging. For uncontrolled leaks which present in undiverted patients, or after stoma closure, initial AL management follows the principles of sepsis management: resuscitation, antibiotics, and source control. Source control typically requires drainage procedures; either drains placed during operative washout or under radiologic guidance, transabdominal, or transanal. Management and interventions should be directed by a surgeon experienced in managing pouch-related complications. Potential interventions include nonoperative nothing by mouth (NPO) with TPN, local procedural interventions by radiology or gastroenterology, fecal diversion, surgical control of the leak or sinus (drainage, local repair, transanal fistulotomy, or endosponge therapy), and ultimately pouch revision or excision. 57 58 Antibiotic therapy should have gram negative and anaerobic coverage to cover gastrointestinal flora. Cultures can be performed with drainage procedures and antibiotics can be tailored to sensitivities if predominant organism or antimicrobial resistance is identified.
Operative Diversion for Ileal Pouch–Anal Anastomosis Anastomotic Leak
Diversion is usually the first step in patients who present without diversion or after reversal, as an uncontrolled AL can be devastating. If symptoms are minimal, local therapy may be attempted without diversion; however, operative fecal diversion with a loop ileostomy is standard practice. Among IPAA patients with AL who did not have initial diversion, Lavryk et al demonstrated that 48% of patients required subsequent diversion. 34 The condition of the patient and the location and size of the AL will determine the need for fecal diversion. For patients who have fecal contamination despite diversion, due to reentry of ileal contents into the distal limb, bedside oversewing of the efferent limb is recommended. Patients need a good understanding that the process of healing an AL may require many months and repeated interventions. Throughout this process, the patient and practitioner need to decide together whether adequate quality of life may be achieved with pouch salvage techniques or whether the best option is either a permanent stoma or pouch revision or excision. 58 59 Success of local interventions or pouch revision will then determine the ability to reverse the diverting ileostomy. Careful consideration of the loop of intestine used for the diverting ileostomy is important to allow for potential use of the diversion site as the apex of a future neo-IPAA; this is typically 20-cm proximal to the pouch inlet. 60
Transanal Management of Abscess or Sinus Tract
Local management of a pouch abscess and subsequent persistent presacral sinus or fistula tract requires serial procedures over months ( Fig. 3 ). MRI with rectal contrast (MR pouchogram or pouchography) allows high-resolution imaging to delineate the tract and local anatomy including assessment for osteomyelitis. Deep abscesses and tracts originating from the IPAA will often require transanal debridement and drainage. Adequate drainage is the most important intervention to resolve pouch-related sepsis. Transanal drainage with a mushroom catheter that is serially downsized may be very effective in draining and closing the cavity ( Fig. 4 ). For larger cavities, CT-guided drainage and transanal drainage are both effective; however, CT-guided drainage does carry a risk of complex suprasphincteric fistula formation along the drain tract. 61 Use of an endosponge, limited to the acute setting, to gradually close the cavity may speed recovery but is labor intensive for patient and surgeon alike. 62 If a residual sinus tract is short and close to the pouch, sinusotomy may be effective. This may be performed using several techniques. Operative sinusotomy is accomplished by inserting a clamp into the sinus and using cautery to fillet open the track, but depending on the anatomy of the anastomosis and the length of the sinus, an electrosurgical device or laparoscopic stapler can be insinuated into the sinus to open the tract ( Fig. 5 ). Alternatively, this may be performed endoscopic needle knife procedures as popularized by Lan and Shen. In a series of 109 patients who underwent serial endoscopic needle knife sinusotomy, Lan and Shen demonstrated complete healing in 54% of patients and 18% partial healing after a median of 2 years. However, sinus recurrence occurred in 25% of patients who had complete healing. 63
Fig. 3.
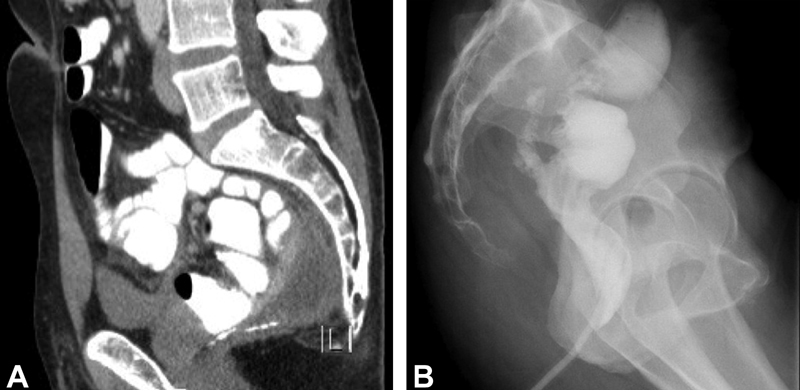
Presacral sinus. Presacral sinus demonstrated on ( A ) CT pelvis with rectal contrast extravasation from the IPAA and ( B ) water-soluble contrast enema after serial mushroom catheter downsizing and resolution of the abscess; note the persistent presacral soft-tissue thickening. CT, computed tomography; IPAA, ileal pouch–anal anastomosis.
Fig. 4.
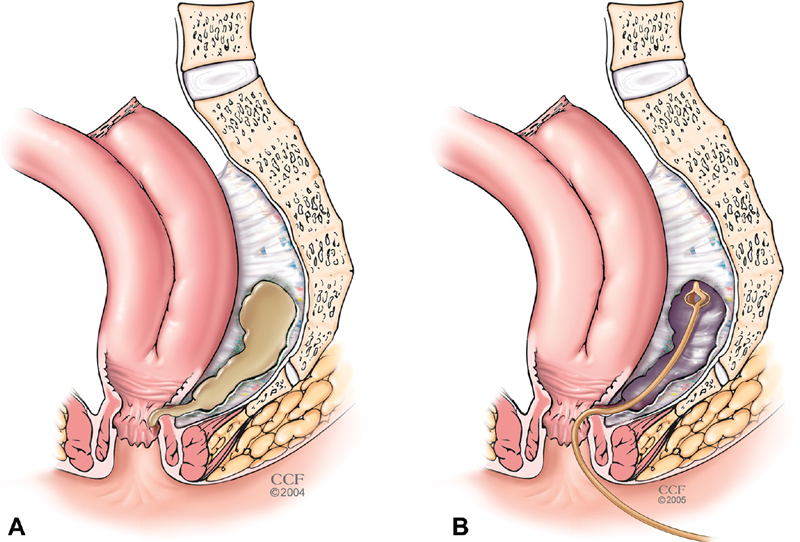
Mushroom catheter drainage. A presacral abscess ( A ) can be drained transanally with a mushroom catheter ( B ), healing is achieved with gradual shortening of the catheter, but residual fibrotic presacral soft-tissue thickening is common (panel C in Fig 5 ).
Fig. 5.
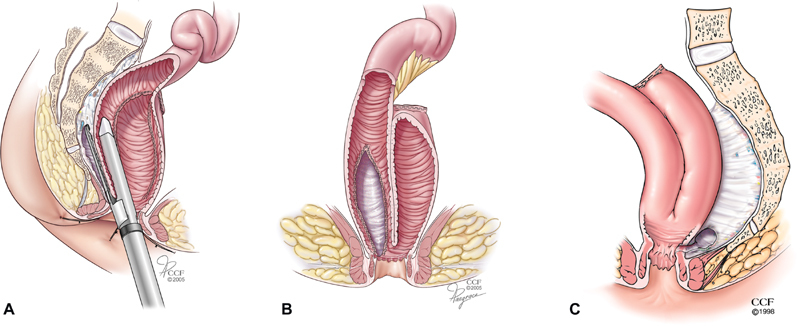
Stapled sinusotomy. In selected cases with a short-anal canal, an endoscopic linear stapler can be insinuated into the presacral sinus ( A ) and used to perform a stapled sinusotomy ( B ); residual fibrotic presacral soft-tissue thickening is common ( C ).
Pouch Vaginal Fistula
Pouch–vaginal fistula may result from either entrapment of the posterior wall of the vagina in the IPAA, from an anterior pouch–anal AL, or rarely from suppuration from a tip or J or pouch body leak. Anovaginal fistulae are typically related to CD of the anus. These fistulas are difficult to close, have high recurrence rates, and are one of the strongest predictors of pouch failure. Particularly for patients with late development of pouch complications (after 1 year), evaluation for CD is an important component of the workup for which biologic therapy will play a critical role prior to attempted surgical correction. 64 65 The rate of successful operative repair is historically less than 50%; however, in a retrospective series, Sapci et al found that 61% of local repair (ileal pouch advancement flap) and 69% of repeat IPAA construction were successful. 66
Tip of the J Leak
Leaks at the “tip of J” may also be difficult to treat but are often difficult to diagnose as well. Frequently, these leaks are occult, not seen on interval pouchogram and present clinically only after ileostomy reversal. Tip of the J leak often presents insidiously as an abscess on CT or MRI, extravasation on pouchogram, or a round defect on pouchoscopy. For those presenting with an abscess, requiring percutaneous drainage, fistulous connection with the J-pouch is often demonstrated with a drain study ( Fig. 6 ). If systemic symptoms are not controlled with drainage, patients often require rediversion. Concurrent fluoroscopy and guide wire localization aid with difficult to identify defects; we have also used injection of dilute methylene blue via the percutaneous drain to identify the source of the leak during pouchoscopy/exam under anesthesia (EUA). When a defect is visible on pouchoscopy, endoscopic closure may be attempted; successful closure with use of over the scope closure devices is reported as high as 66%. 67 Both local revisions of the pouch (HS closure or stapled revision) have been shown to have good functional and quality of life results ( Fig. 7 ). 68 The majority of patients will require rediversion and operative restapling of the tip of the J, and a minority will require a redo pouch procedure. Very small tip of the J leaks may be managed conservatively with a prolonged period of diversion and serial pouchograms. In a forthcoming retrospective cohort of 73 patients from Cleveland Clinic, the overall success rate in resolving the tip of the J leak was 86.3%; of the few ALs that were able to be managed nonoperatively, all healed, while 86.3% of those that required operative intervention healed (unpublished data). To avoid this complication, at the time of pouch construction, the terminal ileum may be transected with a transverse stapler at a 45-degree angle, so the tip of the J is closer to the mesenteric blood supply to decrease the risk of ischemia to this at risk area ( Fig. 7 ).
Fig. 6.
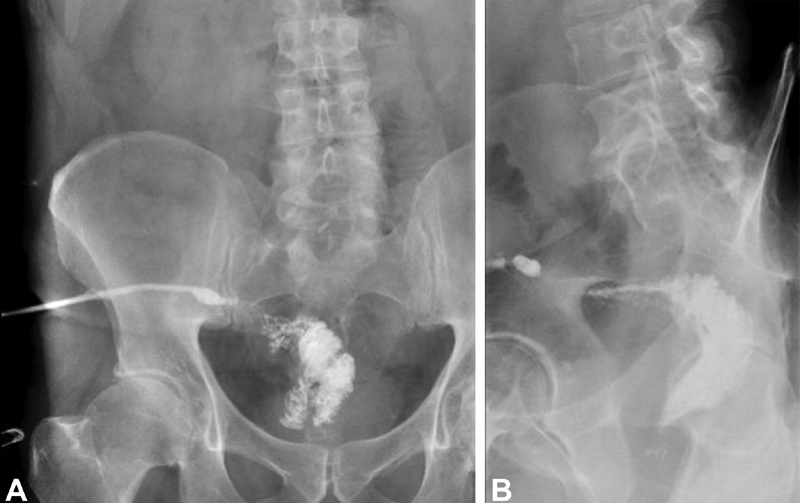
Tip of J fluoroscopic drain tube injection study demonstrating fistulous communication to the tip of the J; ( A ) anterior–posterior, ( B ) lateral.
Fig. 7.
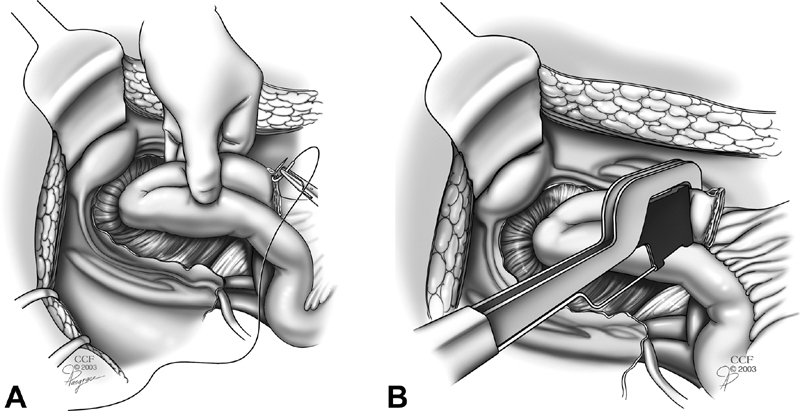
Revision the tip of the J by oversewing ( A ) or restapling ( B ) at 45 degrees to prevent relative ischemia to the tip of the J which is the farthest point from the mesentery.
Redo Ileal Pouch–Anal Anastomosis
Disconnection and reconnection, or pouch excision and neo-IPAA construction, may be indicated when patients are refractory to other methods of pouch salvage. Baixauli et al found that 82% of patients undergoing redo IPAA had a functioning pouch after a median of 32 months and survey results demonstrated good functional quality of life and patient satisfaction. 69 At a median of 7 years after redo or revision of IPAA, Remzi et al found that 20% of patients had recurrent IPAA failure. Redevelopment of pelvic sepsis after redo pouch was the strongest indicator of redo pouch failure. Those who retained their redo pouches at 7 years did have acceptable function and quality of life scores. 59
Consequences
Both short- and long-term consequences of AL after IPAA vary with the degree of leak and the ability to control it. Patients will present with symptoms ranging from malaise, discomfort, and leakage to septic shock causing multiorgan dysfunction. Long hospital stays and need for outpatient intravenous (IV) antibiotics are common. Due to the need for prolonged NPO status, prevention of malnutrition is paramount, often requiring inpatient TPN, potentially a central line and home TPN. About 6% of patients develop pelvic sepsis within 3 months of IPAA. Of these patients, 35% had AL, 37% formed fistulas, and 19% ultimately had pouch failure. Of those who retained their pouch, pouch sepsis was associated with worse functional outcomes, quality of life, and incontinence. 70 Frequently, patients require numerous procedures and may require a year or longer to heal the AL. Repeat pouch evaluation with pouchoscopy and imaging is important prior to closure of diverting ileostomy. Pouch AL likely impacts female fertility and fecundity; Gorgun et al demonstrated that initial IPAA construction reduces infertility rates regardless of approach, but a laparoscopic approach reduced the time required to conceive. 71 It was hypothesized that fewer adhesions associated with laparoscopic IPAA led to shorter time to pregnancy; however, no differences were found with patients who had postoperative complications or pouch revisions and the impact of pouch complications on fertility remained unclear.
AL after IPAA impacts pouch function. Pelvic sepsis is associated with higher pouch failure (19.5 vs. 4%). For those who retain their pouch, history of pouch sepsis was associated with worse pouch function, incontinence, and patient quality of life. 70 Commonly, chronic fibrosis results in a reigid, non-compliant pelvic floor, in which case it is unlikely that the original pouch nor a redo pouch will provide satisfactory function. For those patients who ultimately undergo pouch excision, IPAA-related sepsis was a predictor for perineal wound infections and persistent presacral sinus. 38
Conclusion
Restorative proctocolectomy and IPAA can provide significant quality of life benefit to patients who require colectomy. While this is a technically challenging operation, rates of major complications after IPAA have gradually decreased. AL is one of the feared complications and is challenging to manage. The leak rate after IPAA ranges from 5 to 15% and may be found at the IPAA, tip of the pouch, or staple lines of the body of the pouch. 2 72 AL may be asymptomatic and found on imaging studies done prior to ileostomy reversal or result in pelvic sepsis and require hospitalization. Interventions are tailored to the location of the leak and the systemic impact on the patient. Delayed ileostomy reversal or reestablishment of fecal diversion is frequently required to allow complete healing of the anastomotic disruption. ALs prolong a patient's recovery and return to normal function and may have long-term consequences. However, many if not most patients who develop ALs after IPAA will, with proper expert care, achieve healing and restoration of transanal defecation with an excellent quality of life.
Funding Statement
Funding None.
Conflict of Interest None declared.
Disclosures
SDH: consultant fees, Shionogi, Takeda, Guidepoint.
References
- 1.Parks A G, Nicholls R J.Proctocolectomy without ileostomy for ulcerative colitis BMJ 19782(6130):85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Francone T D, Champagne B. Considerations and complications in patients undergoing ileal pouch anal anastomosis. Surg Clin North Am. 2013;93(01):107–143. doi: 10.1016/j.suc.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Lian L, Kiran R P, Remzi F H, Lavery I C, Fazio V W. Outcomes for patients developing anastomotic leak after ileal pouch-anal anastomosis: does a handsewn vs. stapled anastomosis matter? Dis Colon Rectum. 2009;52(03):387–393. doi: 10.1007/DCR.0b013e31819ad4f2. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy R D, Zarroug A E, Moir C R, Mao S A, El-Youssef M, Potter D D. Ileal pouch anal anastomosis in pediatric familial adenomatous polyposis: a 24-year review of operative technique and patient outcomes. J Pediatr Surg. 2014;49(09):1409–1412. doi: 10.1016/j.jpedsurg.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Sahami S, Bartels S AL, D'Hoore A. A multicentre evaluation of risk factors for anastomotic leakage after restorative proctocolectomy with ileal pouch-anal anastomosis for inflammatory bowel disease. J Crohn's Colitis. 2016;10(07):773–778. doi: 10.1093/ecco-jcc/jjv170. [DOI] [PubMed] [Google Scholar]
- 6.Manilich E, Remzi F H, Fazio V W, Church J M, Kiran R P. Prognostic modeling of preoperative risk factors of pouch failure. Dis Colon Rectum. 2012;55(04):393–399. doi: 10.1097/DCR.0b013e3182452594. [DOI] [PubMed] [Google Scholar]
- 7.Heuschen U A, Hinz U, Allemeyer E H. Risk factors for ileoanal J pouch-related septic complications in ulcerative colitis and familial adenomatous polyposis. Ann Surg. 2002;235(02):207–216. doi: 10.1097/00000658-200202000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heuschen U A, Allemeyer E H, Hinz U, Lucas M, Herfarth C, Heuschen G. Outcome after septic complications in J pouch procedures. Br J Surg. 2002;89(02):194–200. doi: 10.1046/j.0007-1323.2001.01983.x. [DOI] [PubMed] [Google Scholar]
- 9.Ritter K A, Burke J P, Stocchi L. Postoperative steroid taper is associated with pelvic sepsis after ileal pouch-anal anastomosis. Inflamm Bowel Dis. 2019;25(08):1383–1389. doi: 10.1093/ibd/izy388. [DOI] [PubMed] [Google Scholar]
- 10.Kulaylat A S, Kulaylat A N, Schaefer E W. Association of preoperative anti-tumor necrosis factor therapy with adverse postoperative outcomes in patients undergoing abdominal surgery for ulcerative colitis. JAMA Surg. 2017;152(08):e171538. doi: 10.1001/jamasurg.2017.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holubar S, Jai X, Hull T. OP25 Biologics before surgery for IBD: Are they associated with post-operative infectious outcomes? Results from the national surgical quality improvement programme inflammatory bowel disease collaborative. J Crohn's Colitis. 2020;14 01:S022–S023. [Google Scholar]
- 12.Eshuis E J, Al Saady R L, Stokkers P CF, Ponsioen C Y, Tanis P J, Bemelman W A. Previous infliximab therapy and postoperative complications after proctocolectomy with ileum pouch anal anastomosis. J Crohn's Colitis. 2013;7(02):142–149. doi: 10.1016/j.crohns.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Selvasekar C R, Cima R R, Larson D W.Effect of infliximab on short-term complications in patients undergoing operation for chronic ulcerative colitis J Am Coll Surg 200720405956–962., discussion 962–963 [DOI] [PubMed] [Google Scholar]
- 14.Mor I J, Vogel J D, da Luz Moreira A, Shen B, Hammel J, Remzi F H.Infliximab in ulcerative colitis is associated with an increased risk of postoperative complications after restorative proctocolectomy Dis Colon Rectum 200851081202–1207., discussion 1207–1210 [DOI] [PubMed] [Google Scholar]
- 15.Gainsbury M L, Chu D I, Howard L A. Preoperative infliximab is not associated with an increased risk of short-term postoperative complications after restorative proctocolectomy and ileal pouch-anal anastomosis. J Gastrointest Surg. 2011;15(03):397–403. doi: 10.1007/s11605-010-1385-6. [DOI] [PubMed] [Google Scholar]
- 16.Ferrante M, D'Hoore A, Vermeire S. Corticosteroids but not infliximab increase short-term postoperative infectious complications in patients with ulcerative colitis. Inflamm Bowel Dis. 2009;15(07):1062–1070. doi: 10.1002/ibd.20863. [DOI] [PubMed] [Google Scholar]
- 17.Krane M K, Allaix M E, Zoccali M. Preoperative infliximab therapy does not increase morbidity and mortality after laparoscopic resection for inflammatory bowel disease. Dis Colon Rectum. 2013;56(04):449–457. doi: 10.1097/DCR.0b013e3182759029. [DOI] [PubMed] [Google Scholar]
- 18.Lau C, Dubinsky M, Melmed G. The impact of preoperative serum anti-TNFα therapy levels on early postoperative outcomes in inflammatory bowel disease surgery. Ann Surg. 2015;261(03):487–496. doi: 10.1097/SLA.0000000000000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nørgård B M, Nielsen J, Qvist N, Gradel K O, de Muckadell O B, Kjeldsen J. Pre-operative use of anti-TNF-α agents and the risk of post-operative complications in patients with ulcerative colitis - a nationwide cohort study. Aliment Pharmacol Ther. 2012;35(11):1301–1309. doi: 10.1111/j.1365-2036.2012.05099.x. [DOI] [PubMed] [Google Scholar]
- 20.Schluender S J, Ippoliti A, Dubinsky M. Does infliximab influence surgical morbidity of ileal pouch-anal anastomosis in patients with ulcerative colitis? Dis Colon Rectum. 2007;50(11):1747–1753. doi: 10.1007/s10350-007-9008-3. [DOI] [PubMed] [Google Scholar]
- 21.Waterman M, Xu W, Dinani A. Preoperative biological therapy and short-term outcomes of abdominal surgery in patients with inflammatory bowel disease. Gut. 2013;62(03):387–394. doi: 10.1136/gutjnl-2011-301495. [DOI] [PubMed] [Google Scholar]
- 22.Kunitake H, Hodin R, Shellito P C, Sands B E, Korzenik J, Bordeianou L.Perioperative treatment with infliximab in patients with Crohn's disease and ulcerative colitis is not associated with an increased rate of postoperative complications J Gastrointest Surg 200812101730–1736., discussion 1736–1737 [DOI] [PubMed] [Google Scholar]
- 23.Bregnbak D, Mortensen C, Bendtsen F. Infliximab and complications after colectomy in patients with ulcerative colitis. J Crohn's Colitis. 2012;6(03):281–286. doi: 10.1016/j.crohns.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 24.Coquet-Reinier B, Berdah S V, Grimaud J-C. Preoperative infliximab treatment and postoperative complications after laparoscopic restorative proctocolectomy with ileal pouch-anal anastomosis: a case-matched study. Surg Endosc. 2010;24(08):1866–1871. doi: 10.1007/s00464-009-0861-0. [DOI] [PubMed] [Google Scholar]
- 25.Narula N, Charleton D, Marshall J K. Meta-analysis: peri-operative anti-TNFα treatment and post-operative complications in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;37(11):1057–1064. doi: 10.1111/apt.12313. [DOI] [PubMed] [Google Scholar]
- 26.Yang Z, Wu Q, Wu K, Fan D. Meta-analysis: pre-operative infliximab treatment and short-term post-operative complications in patients with ulcerative colitis. Aliment Pharmacol Ther. 2010;31(04):486–492. doi: 10.1111/j.1365-2036.2009.04204.x. [DOI] [PubMed] [Google Scholar]
- 27.Yang Z, Wu Q, Wang F, Wu K, Fan D. Meta-analysis: effect of preoperative infliximab use on early postoperative complications in patients with ulcerative colitis undergoing abdominal surgery. Aliment Pharmacol Ther. 2012;36(10):922–928. doi: 10.1111/apt.12060. [DOI] [PubMed] [Google Scholar]
- 28.Selvaggi F, Pellino G, Canonico S, Sciaudone G. Effect of preoperative biologic drugs on complications and function after restorative proctocolectomy with primary ileal pouch formation: systematic review and meta-analysis. Inflamm Bowel Dis. 2015;21(01):79–92. doi: 10.1097/MIB.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 29.Holubar S D, Holder-Murray J, Flasar M, Lazarev M. Anti-tumor necrosis factor-α antibody therapy management before and after intestinal surgery for inflammatory bowel disease: a CCFA position paper. Inflamm Bowel Dis. 2015;21(11):2658–2672. doi: 10.1097/MIB.0000000000000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorfine S R, Fichera A, Harris M T, Bauer J J. Long-term results of salvage surgery for septic complications after restorative proctocolectomy: does fecal diversion improve outcome? Dis Colon Rectum. 2003;46(10):1339–1344. doi: 10.1007/s10350-004-6747-2. [DOI] [PubMed] [Google Scholar]
- 31.Weston-Petrides G K, Lovegrove R E, Tilney H S. Comparison of outcomes after restorative proctocolectomy with or without defunctioning ileostomy. Arch Surg. 2008;143(04):406–412. doi: 10.1001/archsurg.143.4.406. [DOI] [PubMed] [Google Scholar]
- 32.Zittan E, Wong-Chong N, Ma G W, McLeod R S, Silverberg M S, Cohen Z. Modified two-stage ileal pouch-anal anastomosis results in lower rate of anastomotic leak compared with traditional two-stage surgery for ulcerative colitis. J Crohn's Colitis. 2016;10(07):766–772. doi: 10.1093/ecco-jcc/jjw069. [DOI] [PubMed] [Google Scholar]
- 33.Samples J, Evans K, Chaumont N, Strassle P, Sadiq T, Koruda M. Variant two-stage ileal pouch-anal anastomosis: an innovative and effective alternative to standard resection in ulcerative colitis. J Am Coll Surg. 2017;224(04):557–563. doi: 10.1016/j.jamcollsurg.2016.12.049. [DOI] [PubMed] [Google Scholar]
- 34.Lavryk O A, Hull T L, Duraes L C. Outcomes of ileal pouch-anal anastomosis without primary diverting loop ileostomy if postoperative sepsis develops. Tech Coloproctol. 2018;22(01):37–44. doi: 10.1007/s10151-017-1737-2. [DOI] [PubMed] [Google Scholar]
- 35.Heuschen U A, Hinz U, Allemeyer E H, Lucas M, Heuschen G, Herfarth C. One- or two-stage procedure for restorative proctocolectomy: rationale for a surgical strategy in ulcerative colitis. Ann Surg. 2001;234(06):788–794. doi: 10.1097/00000658-200112000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lightner A L, Pemberton J H. The role of temporary fecal diversion. Clin Colon Rectal Surg. 2017;30(03):178–183. doi: 10.1055/s-0037-1598158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prudhomme M, Dehni N, Dozois R R, Tiret E, Parc R. Causes and outcomes of pouch excision after restorative proctocolectomy. Br J Surg. 2006;93(01):82–86. doi: 10.1002/bjs.5147. [DOI] [PubMed] [Google Scholar]
- 38.Maya A M, Boutros M, DaSilva G, Wexner S D. IPAA-related sepsis significantly increases morbidity of ileoanal pouch excision. Dis Colon Rectum. 2015;58(05):488–493. doi: 10.1097/DCR.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 39.Sahami S, Buskens C J, Fadok T Y. Defunctioning ileostomy is not associated with reduced leakage in proctocolectomy and ileal pouch anastomosis surgeries for IBD. J Crohn's Colitis. 2016;10(07):779–785. doi: 10.1093/ecco-jcc/jjv201. [DOI] [PubMed] [Google Scholar]
- 40.Widmar M, Munger J A, Mui A. Diverted versus undiverted restorative proctocolectomy for chronic ulcerative colitis: an analysis of long-term outcomes after pouch leak short title: outcomes after pouch leak. Int J Colorectal Dis. 2019;34(04):691–697. doi: 10.1007/s00384-019-03240-2. [DOI] [PubMed] [Google Scholar]
- 41.Lovegrove R E, Constantinides V A, Heriot A G. A comparison of hand-sewn versus stapled ileal pouch anal anastomosis (IPAA) following proctocolectomy: a meta-analysis of 4183 patients. Ann Surg. 2006;244(01):18–26. doi: 10.1097/01.sla.0000225031.15405.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konishi T, Ishida H, Ueno H. Postoperative complications after stapled and hand-sewn ileal pouch-anal anastomosis for familial adenomatous polyposis: a multicenter study. Ann Gastroenterol Surg. 2017;1(02):143–149. doi: 10.1002/ags3.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rencuzogullari A, Stocchi L, Costedio M, Gorgun E, Kessler H, Remzi F H. Characteristics of learning curve in minimally invasive ileal pouch-anal anastomosis in a single institution. Surg Endosc. 2017;31(03):1083–1092. doi: 10.1007/s00464-016-5068-6. [DOI] [PubMed] [Google Scholar]
- 44.Kirat H T, Kiran R P, Lian L, Remzi F H, Fazio V W. Influence of stapler size used at ileal pouch-anal anastomosis on anastomotic leak, stricture, long-term functional outcomes, and quality of life. Am J Surg. 2010;200(01):68–72. doi: 10.1016/j.amjsurg.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 45.Singh P, Bhangu A, Nicholls R J, Tekkis P. A systematic review and meta-analysis of laparoscopic vs open restorative proctocolectomy. Colorectal Dis. 2013;15(07):e340–e351. doi: 10.1111/codi.12231. [DOI] [PubMed] [Google Scholar]
- 46.Tilney H S, Lovegrove R E, Heriot A G. Comparison of short-term outcomes of laparoscopic vs open approaches to ileal pouch surgery. Int J Colorectal Dis. 2007;22(05):531–542. doi: 10.1007/s00384-006-0177-7. [DOI] [PubMed] [Google Scholar]
- 47.Lovegrove R E, Heriot A G, Constantinides V. Meta-analysis of short-term and long-term outcomes of J, W and S ileal reservoirs for restorative proctocolectomy. Colorectal Dis. 2007;9(04):310–320. doi: 10.1111/j.1463-1318.2006.01093.x. [DOI] [PubMed] [Google Scholar]
- 48.Nisar P J, Appau K A, Remzi F H, Kiran R P. Preoperative hypoalbuminemia is associated with adverse outcomes after ileoanal pouch surgery. Inflamm Bowel Dis. 2012;18(06):1034–1041. doi: 10.1002/ibd.21842. [DOI] [PubMed] [Google Scholar]
- 49.Melton G B, Fazio V W, Kiran R P. Long-term outcomes with ileal pouch-anal anastomosis and Crohn's disease: pouch retention and implications of delayed diagnosis. Ann Surg. 2008;248(04):608–616. doi: 10.1097/SLA.0b013e318187ed64. [DOI] [PubMed] [Google Scholar]
- 50.Pellino G, Vinci D, Signoriello G. Long-term bowel function and fate of the ileal pouch after restorative proctocolectomy in patients with Crohn's disease. A systematic review with meta-analysis and metaregression. J Crohn's Colitis. 2020;14(03):418–427. doi: 10.1093/ecco-jcc/jjz146. [DOI] [PubMed] [Google Scholar]
- 51.Reese G E, Lovegrove R E, Tilney H S. The effect of Crohn's disease on outcomes after restorative proctocolectomy. Dis Colon Rectum. 2007;50(02):239–250. doi: 10.1007/s10350-006-0777-x. [DOI] [PubMed] [Google Scholar]
- 52.Singh P P, Zeng I SL, Srinivasa S, Lemanu D P, Connolly A B, Hill A G. Systematic review and meta-analysis of use of serum C-reactive protein levels to predict anastomotic leak after colorectal surgery. Br J Surg. 2014;101(04):339–346. doi: 10.1002/bjs.9354. [DOI] [PubMed] [Google Scholar]
- 53.Clark D A, Cuda T, Riddell A, Radford-Smith G, Solomon M. Drain fluid amylase as a sensitive biomarker for the early detection of anastomotic leakage in ileal pouch surgery. Colorectal Dis. 2019;21(04):460–464. doi: 10.1111/codi.14536. [DOI] [PubMed] [Google Scholar]
- 54.Broder J C, Tkacz J N, Anderson S W, Soto J A, Gupta A. Ileal pouch-anal anastomosis surgery: imaging and intervention for post-operative complications. Radiographics. 2010;30(01):221–233. doi: 10.1148/rg.301095084. [DOI] [PubMed] [Google Scholar]
- 55.Sossenheimer P H, Glick L R, Dachman A H. Abnormal pouchogram predicts pouch failure even in asymptomatic patients. Dis Colon Rectum. 2019;62(04):463–469. doi: 10.1097/DCR.0000000000001285. [DOI] [PubMed] [Google Scholar]
- 56.Thoeni R F, Fell S C, Engelstad B, Schrock T B. Ileoanal pouches: comparison of CT, scintigraphy, and contrast enemas for diagnosing postsurgical complications. AJR Am J Roentgenol. 1990;154(01):73–78. doi: 10.2214/ajr.154.1.2104730. [DOI] [PubMed] [Google Scholar]
- 57.Holubar S D. Prevention, diagnosis, and treatment of complications of the IPAA for ulcerative colitis. Dis Colon Rectum. 2018;61(05):532–536. doi: 10.1097/DCR.0000000000001094. [DOI] [PubMed] [Google Scholar]
- 58.Holubar S D, Neary P, Aiello A. Ileal pouch revision vs excision: short-term (30-day) outcomes from the National Surgical Quality Improvement Program. Colorectal Dis. 2019;21(02):209–218. doi: 10.1111/codi.14476. [DOI] [PubMed] [Google Scholar]
- 59.Remzi F H, Aytac E, Ashburn J.Transabdominal redo ileal pouch surgery for failed restorative proctocolectomy: lessons learned over 500 patientsIn:Ann Surg 201526204675–682. [DOI] [PubMed] [Google Scholar]
- 60.Schwartzberg D M, Esen E, Remzi F H. Thoughtful ileostomy creation in patients undergoing redo IPAA. Dis Colon Rectum. 2020;63(01):117–120. doi: 10.1097/DCR.0000000000001535. [DOI] [PubMed] [Google Scholar]
- 61.Kirat H T, Remzi F H, Shen B, Kiran R P. Pelvic abscess associated with anastomotic leak in patients with ileal pouch-anal anastomosis (IPAA): transanastomotic or CT-guided drainage? Int J Colorectal Dis. 2011;26(11):1469–1474. doi: 10.1007/s00384-011-1272-y. [DOI] [PubMed] [Google Scholar]
- 62.Okkabaz N, Esen E, Schwartzberg D M, Remzi F H, Kirat H T. Hand-crafted endoluminal vacuum-assisted drainage for anastomotic leak after IPAA. Dis Colon Rectum. 2019;62(10):1259–1262. doi: 10.1097/DCR.0000000000001453. [DOI] [PubMed] [Google Scholar]
- 63.Lan N, Shen B. Endoscopic treatment of ileal pouch sinus. Inflamm Bowel Dis. 2018;24(07):1510–1519. doi: 10.1093/ibd/izy029. [DOI] [PubMed] [Google Scholar]
- 64.Nisar P J, Kiran R P, Shen B, Remzi F H, Fazio V W. Factors associated with ileoanal pouch failure in patients developing early or late pouch-related fistula. Dis Colon Rectum. 2011;54(04):446–453. doi: 10.1007/DCR.0b013e318206ea42. [DOI] [PubMed] [Google Scholar]
- 65.Sivathondan P C, Bloemendaal A, Travis S, Mortensen N, George B D. Management of pouch-vaginal fistulas – experience from our institution. Color Dis. 2020;22(04):439–444. doi: 10.1111/codi.14904. [DOI] [PubMed] [Google Scholar]
- 66.Sapci I, Akeel N, DeLeon M F, Stocchi L, Hull T. What is the best surgical treatment of pouch-vaginal fistulas? Dis Colon Rectum. 2019;62(05):595–599. doi: 10.1097/DCR.0000000000001313. [DOI] [PubMed] [Google Scholar]
- 67.Kochhar G S, Shen B. Endoscopic treatment of leak at the tip of the “J” ileal pouch. Endosc Int Open. 2017;5(01):E64–E66. doi: 10.1055/s-0042-121664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kirat H T, Kiran R P, Oncel M, Shen B, Fazio V W, Remzi F H. Management of leak from the tip of the “J” in ileal pouch-anal anastomosis. Dis Colon Rectum. 2011;54(04):454–459. doi: 10.1007/DCR.0b013e31820481be. [DOI] [PubMed] [Google Scholar]
- 69.Baixauli J, Delaney C P, Wu J S, Remzi F H, Lavery I C, Fazio V W. Functional outcome and quality of life after repeat ileal pouch-anal anastomosis for complications of ileoanal surgery. Dis Colon Rectum. 2004;47(01):2–11. doi: 10.1007/s10350-003-0003-z. [DOI] [PubMed] [Google Scholar]
- 70.Kiely J M, Fazio V W, Remzi F H, Shen B, Kiran R P. Pelvic sepsis after IPAA adversely affects function of the pouch and quality of life. Dis Colon Rectum. 2012;55(04):387–392. doi: 10.1097/DCR.0b013e318246418e. [DOI] [PubMed] [Google Scholar]
- 71.Gorgun E, Cengiz T B, Aytac E. Does laparoscopic ileal pouch-anal anastomosis reduce infertility compared with open approach? Surgery. 2019;166(04):670–677. doi: 10.1016/j.surg.2019.04.045. [DOI] [PubMed] [Google Scholar]
- 72.Sherman J, Greenstein A J, Greenstein A J. Ileal j pouch complications and surgical solutions: a review. Inflamm Bowel Dis. 2014;20(09):1678–1685. doi: 10.1097/MIB.0000000000000086. [DOI] [PubMed] [Google Scholar]


