Abstract
Despite advances in rectal cancer surgery, anastomotic leakage (AL) remains a common complication with a significant impact on patient recovery, health care costs, and oncologic outcomes. The spectrum of clinical severity associated with AL is broad, and treatment options are diverse with highly variable practices across the colorectal community. To be effective, the treatment must match not only the patient's current status but also the type of leak, the surgeon's skill, and the resources available. In this chapter, we will review the current and emergent treatment modalities for AL after rectal cancer surgery.
Keywords: colorectal surgery, rectal cancer, anastomotic leak, endosponge, postoperative complication
Anastomotic leakage (AL) is one of the most dreaded complications in rectal cancer surgery, with both acute and long-term consequences for the patient. AL leads to increased morbidity, impaired functional outcomes, and potential oncological compromise. 1 2 3 4 Furthermore, the economic burden to health care systems is high, with increased postoperative complications, reinterventions, intensive care usage, lengthened hospital stay and readmissions. 5
The major risk factors for AL are known and summarized in Table 1 . The more distal the anastomosis, the higher the likelihood of failure, making the risk of AL for a distal rectal cancer nearly fivefold higher risk than an anastomotic leak after resection of colon cancer. 4 There is also increased awareness and improvements in surgical technique, technology, and perioperative care all aimed to reduce the incidence of AL. Despite all of this, we continue to struggle with the occurrence and management of this complication. Anastomotic leaks rates after rectal cancer surgery vary from 6 to 30%, depending on risk factors. 6 A lack of standardized definition for AL across the literature also affects the wide variance in reported incidence of AL after rectal cancer surgery. Large registry studies found leakage rates of 10.8% in Germany, 11.9% in Sweden, and 15.7% in the TaTME-registry. 7 8 9 Interestingly, when using clear definitions and re-checking data from medical charts, leakage rates are much higher. This was demonstrated in a large cross-sectional SNAPSHOT study where leakage rates at 30 days increased from 8.2 to 13.4% after chart review and in the Swedish registry where a total of 29% false-negative leaks were found. 8 10 Furthermore, in the Dutch SNAPSHOT study, one-third of the AL was diagnosed beyond 30 days and leak rates increased to 20% with long-term follow-up. 10 These findings demonstrate the enormous scope of the problem, but also the need for a uniform definition of AL and adequate follow-up time to obtain “real life” data.
Table 1. Risk factors for anastomotic leak.
| Preoperative considerations | Intraoperative considerations |
| Non-modifiable risk factors | Increased risk |
| Male gender Distal rectal anastomosis Tumor size >3 cm Advanced tumor stage Metastatic disease History of radiotherapy ASA >2 Diabetes mellitus Pulmonary disease Vascular disease Emergency surgery Past Smoker (>40 pack year history) |
Intraoperative contamination Operative duration >4 h Inotropes Blood loss Blood transfusion |
| Modifiable | Reduced risk |
| Current smoker Obesity Alcohol excess Corticosteroids Bevacizumab Malnutrition/hypoalbuminemia |
Preoperative antibiotics intravenous and mechanical and oral bowel prep |
It is paramount to identify and treat leaks early to minimize the potential short and long-term sequalae. The high prevalence of AL demands a structured and evidence-based approach for definition, diagnosis, and management of these leaks. However, literature on effective treatment is scarce, and consensus among surgeons/institutions is lacking, as are international guidelines. As we work toward a uniform definition and better understanding of how risk factors lead to AL, a comprehensive knowledge of management options is critical.
Defining Anastomotic Leak
The first step toward developing effective treatment strategies is a clear definition on AL. The definition for anastomotic leaks from the Association of Surgeons of Great Britain and Ireland is, “a leak of luminal contents from a surgical join between two hollow viscera.” Radiographic demonstration of a large collection of free fluid, extravasation of contrast material, or a perianastomotic fluid collection is an undisputed clinical definition of AL. 11 A recent Delphi study reached consensus on using the International Study Group of Rectal Cancer definition: “A defect of the intestinal wall integrity at the colorectal or colo-anal anastomotic site leading to a communication between the intra- and extraluminal compartments” combined with a grading system. 12 13
Diagnosing Anastomotic Leak
The most severe consequence of an anastomotic leak is overwhelming sepsis resulting in mortality. Delay in the recognition and intervention of a significant AL contributes directly to increased patient mortality. 14 Thus, it is imperative that surgeons use an appropriate level of suspicion for investigating and identifying anastomotic leaks before the patients' condition begins to deteriorate. Early diagnosis is also pivotal to facilitate optimal chances for healing before chronic inflammation causes further damage to an already scarred and fibrotic area from previous surgery and possible neoadjuvant treatment. Early diagnosis furthermore increases the ability for a re-laparoscopy should abdominal access be required in patients previously operated with a minimal invasive approach. 15 Diagnosis of AL is not always straight forward, particularly in those patients that have a primary diverting stoma. In addition to clinical symptoms of pain, fever, tachycardia, and ileus, serial C-reactive protein measurements have proven to be a good predictor of AL as early as postoperative day 3. 16 Any suspicion of AL should prompt a computed tomography (CT) scan. A recent systematic review on the value of CT scanning in the diagnosis of AL after colorectal surgery showed that the specificity to diagnose AL is high (>84%) but with a relatively low sensitivity (68–71%). 17 However, a CT scan offers the added advantage of defining the anatomy to allow for management planning. Intravenous contrast can be very helpful in identifying abscesses, while retrograde contrast enema can help identify air–fluid levels in proximity of the anastomosis and active contrast extravasation, the most sensitive and specific sign of AL. 18 The sensitivity and specificity of contrast enema for clinically significant abnormalities are 79.9 and 95.4%, respectively. 19 Endoscopic evaluation of the anastomosis can be considered in case of a negative CT scan if clinical suspicion remains. Rigid proctoscopy/anoscopy or endoscopy may determine the exact location and extent of the anastomotic dehiscence. In our unit and others, endoscopic evaluation 2-weeks postoperative is standard in defunctioned patients to assess anastomotic integrity, even if they are asymptomatic. This allows relatively early detection of these asymptomatic leaks of defunctioned anastomosis, and thus early management.
Once an AL is confirmed, it is assured that the patient is nursed in a unit appropriate to their level of systemic illness and need for monitoring. Recommended time limits for intervention in the treatment of AL are determined by the severity of sepsis. In the absence of organ dysfunction, surgical or radiological intervention to achieve source control should be undertaken as soon as possible (where this is required), but always within 18 hours of diagnosis. If sepsis is complicated by evidence of organ dysfunction, source control should be achieved as soon as possible, but always within 6 hours of diagnosis. Septic shock should prompt immediate source control, and always within 3 hours to avoid preventable death.
Treatment Options
The spectrum of clinical severity associated with AL is broad, with presentations ranging from a small-contained leak without sepsis in a patient with a defunctioning stoma, to a life-threatening surgical emergency with profound peritonitis and septic shock. The surgeon must decide if an intervention is needed, and if so, if the anastomosis is salvageable or not. Due to this wide range, there has been a myriad of methods and techniques used by surgeons to manage AL. To be effective, the management must consider the current state of the patient, the patient's fitness or reserve, the extent of the anastomotic disruption, vitality of the colonic conduit, the surgeon's skill, and the resources available.
The International Study Group of Rectal Cancer grading system defines the management of colorectal anastomotic leaks. 13 Grade A AL may be managed expectantly. Grade B leakage requires active therapeutic intervention but does not necessarily require reoperation. Antibiotics and percutaneous drainage of fluid collections are the most common nonoperative interventions. Grade C AL requires relaparotomy to control life-threatening sepsis. The traditional operation with takedown of the anastomosis and end colostomy may be appropriate, but washout with drain placement and diverting loop ileostomy may also be appropriate.
Antibiotics
When an AL is suspected the immediate initiation of empiric use of antibiotics is the key. Antibiotic treatment alone is a reasonable option for small anastomotic defects without any associated collection amenable for transanal or percutaneous drainage. The treatment should be broad-spectrum with activity against both aerobic and anaerobic organisms to treat postoperative intra-abdominal infections. In addition, the microbiological profiles and resistance of the key pathogens causing intra-abdominal infection locally should be considered. 20 However, few studies have assessed the patterns of antibiotic treatment and resistance of pathogen profiles in patients who had AL after rectal cancer surgery. Accordingly, few AL patients have cultures sent of their pathogens to guide the antibiotic treatment regimen. One study from Korea examined nearly 1,000 patients undergoing elective surgery for the treatment of primary colorectal cancer, finding 4.2% experienced AL, and only 53.7% had culture studies for intra-abdominal fluid or abscess performed. 21 To determine the bacteria present in an AL, the CIAOW (Complicated intra-abdominal infections worldwide observational study) collected data from 1,898 patients in 68 medical institutions worldwide, finding the top pathogens from intraabdominal infections to be Escherichia coli (35.7%), Enterococcus (12.9%), Klebsiella (10.5%), Pseudomonas aeruginosa (5.1%), and Enterobacter spp . (4.1%). 22 In the Korean study, 11 total separate pathogens were identified, and approximately two kinds of bacteria were found per patient. In agreement with the CIAOW, E. coli and Enterococcus spp . (26.8% each) were the most frequently identified, followed by P. aeruginosa (12.2%). Of note, 12.1% contained “ESKAPE” antibiotic-resistant bacterial pathogens ( Enterococcus faecium , Staphylococcus aureus , Klebsiella pneumoniae , Acinetobacter baumannii , P. aeruginosa , and Enterobacter species ), with methicillin-resistant S. aureus (MRSA) the most common. The most common empiric antibiotic regimens are the penicillin/β-lactamase combination piperacillin/tazobactam (with or without metronidazole), a third-generation cephalosporin with metronidazole, a second-generation cephalosporin, a first-generation cephalosporin with metronidazole, and the carbapenem meropenem.
Local Drainage
Image-guided and transanal drainages have emerged as alternatives for managing grade B anastomotic leak with promising results. 23 24 25 In appropriate patients, image-guided percutaneous drainage is an attractive alternative to reoperation because of decreased morbidity and length of hospital stay. The decision to proceed with percutaneous drainage is based on the presence of a safe radiographic “window,” the availability of an experienced radiologist, the homogeneity of the fluid collection, and an abscess of at least 2 to 3 cm. There are many routes that can be followed to reach the abscess via the anterior abdominal wall or a transgluteal approach. Percutaneous CT-guided drainage is demonstrated to be a safe and effective alternative to surgery showing a very low complication rate. 26 27 The procedure is usually performed using local anesthesia and mild sedation under noninvasive hemodynamic monitoring. 23 28 The risk of forming a fistula tract, and infection alongside the drain tract needs to be take into account, particular in patients with AL after neoadjuvant radiotherapy.
Fluid collections in close proximity of the anastomotic defect can be drained by an endoscopic or surgical transanal drainage. A small endoscopic or surgical drain (French Mallinckrodt or Mushroom catheter) is placed through the defect into the extraluminal fluid collection. 29 This allows the abscess to be flushed daily, and partly disrupts the barrier of the anal sphincter and as such minimizing the influx of mucus and debris. 30 This is especially effective in cases of small (<1 cm) defects with a draining sinus cavity in the pelvis. Placement of a transanal drain also allows for follow-up radiographic surveillance of the abscess cavity by the instillation of contrast through the drain. The drain may be removed when the cavity has decreased to the size of the drain. Successful resolution of the defect does not remove the risk of long-term complications associated with anastomotic leaks such as stricture formation, chronic sinus, and poor bowel function. However, these local drainage procedure can help to salvage a failed anastomosis. Several studies reported a salvage rate of the primary anastomosis between 50 and 92% in patients undergoing local drainage. However, the rate of definitive stoma is between 20 and 46%, still lower than after laparotomy for grade C leakage. 24 28 Continuing leakage of enteric contents or lack of clinical improvement should be treated with more aggressive interventions.
Both these “passive” treatment options for AL have some drawbacks. They are labor intensive, as drains need to properly managed and regularly flushed for optimal outcome. Both these approaches are also difficult to incorporate in a strict and well-defined pathway, as no clear guidance exist when they are best removed; i.e., based solely on output, contrast imaging, or biochemical markers. Too early removal, blockage of drains or inadequate drainage will lead to recurrence of sepsis. Challine et al reported that in one-third of patients a further transanal or percutaneous drainage was required after the removal of idex drain. 28 Finally, mucosal approximation and actual closure of de anastomotic defect are not obtained.
Stenting
The role of endoscopically placed self-expanding metal stents or covered stents has been described for the treatment of AL with promising results. 31 32 33 34 A stent is placed across the anastomotic defect to prevent contaminants from interfering with the healing of the defect and it remains in place for up to 2 months. Stent placement is usually combined with percutaneous drainage of the extraluminal abscess if present. With a low anastomosis, stent migration is a significant issue and most patients will spontaneously expel the stent. While the use of self-expanding metal stents remains investigative for AL, it is an option in patients with favorable anatomy. The current commercially available covered stents do not have a large enough diameter to minimize stent migration. Until appropriate stents are available, the risk of stent migration may be partially mitigated by the use of endoclips.
Endoscopic Application of Fibrin Glue and Clips
A recent review found more options for endoscopic treatment, but most studies are difficult to compare because of the small sample size and variety in treatments and patients. 35 Endoscopic injection with fibrin glue into the anastomotic cavity can be used to close the defect and is mostly used for closing small leaks (<5 mm) or fistulae. 36 37 It can be utilized as an addition to prior treatment, to close a small remaining defect. An anastomotic defect can be overcome by over the scope clipping (OTSC) and standard clips used for regular endoscopy to control bleeding or perforation can be used. 35 OTSC can be used in combination with other techniques (e.g., EVAC treatment) and is mainly suitable for small defects (<15 mm).
Endoscopic Vacuum Therapy
A relatively new treatment is endoscopic vacuum therapy (EVT) of the anastomotic defect and associated abscess cavity. This therapy consists of the placement of a polyurethane sponge via an introducer device in the abscess cavity which is connected to a suction system (Endo-SPONGE, B. Braun Medical B.V., Melsungen, Germany). 38 This allows continuous drainage of the abscess cavity, similar to a negative pressure vacuum system used for infected surgical wounds. The negative pressure increases local blood flow and the formation of healthy granulating tissue, while simultaneously reducing bacterial colonization and edema. The sponges can be tapered to fit the presacral cavity and are exchanged every 2 to 4 days to prevent ingrowth of the surrounding tissue into the endosponge. This treatment can be continued until the cavity has been completely filled with healthy tissue, or the cavity can be closed early with transanal sutures once the cavity appears clean. This modification of the technique, combining EVT with early surgical closure (ESC) was first introduced by our unit. 39 In our experience, a clean cavity is usually achieved after 2 to 4 sponge changes and the remaining anastomotic defect can then be closed by placing transanal sutures, if the afferent colon has not retracted. Depending on the level of the anastomosis, this can be performed either by using a transanal platform (e.g., GelPOINT Transanal Platform) for a higher anastomosis or directly transanally with aid of a Lonestar retractor system for a lower anastomosis. A small 6 to 8 Fr suction drain is left in the cavity to avoid a new abscess to form. This suction drain in the cavity is removed after 5 to 7 days and follow-up is performed by endoscopy and a CT-scan with rectal contrast ( Figs. 1 and 2 ). Detailed description on Endoscopic Vacuum Assisted Surgical Closure (EVASC) was published earlier by our group with a recent video vignette presenting the transanal closure technique. 40 41 A recent systematic review including 276 patients, that underwent EVT for AL, showed a healed anastomosis rate of 85.3% and stoma reversal rate of 75.9%. 42 However, this review is difficult to compare with other studies because of heterogeneity in patients (cancer, inflammatory bowel disease, familial adenomatous polyposis, etc.) and treatment (EVT alone or combined with transanal closure, fibrin glue, etc.). The CLEAN study, a prospective study which combined EVT and ESC for low rectal anastomoses, showed a healed anastomosis in 70% and restored continuity in 67%. 25 The healing rate increased when the treatment was started within 21 days after LAR, (73 vs. 67%) and continuity was restored in a higher proportion of patients (74 vs. 60%). 40 A recent retrospective cohort study ( n = 47) showed an overall success rate of 55% after EVT without ESC for AL. 43 However, also in this cohort significant better results were obtained if the treatment was started early; (72.4% success rate if the treatment started within 15 days after diagnosing AL compared with 27.8% in the late group). A recent retrospective study comparing EVT with ESC on indication, found similar healed anastomosis rates (80 vs. 90%) and restored continuity rates (70 vs. 70%), when started within 21 days or after 21 days. 44 In another retrospective study that compared EVT with conventional treatment, higher restored continuity rates were also achieved after EVT (96.7 vs. 65.9%). 45 However, the conventional group in this retrospective study included significantly more cancer patients and patients that underwent neoadjuvant radiotherapy. Nevertheless, EVT treatment seems also effective in patients who underwent preoperative neoadjuvant radiotherapy, but it is associated with larger cavities, longer duration of EVT, more endosponge changes, and longer time to closure of the leak. 43 Most patients are diverted prior to EVT to allow proper drainage and healing of the anastomosis, although it is possible to use EVT without a diverting stoma. 44
Fig. 1.
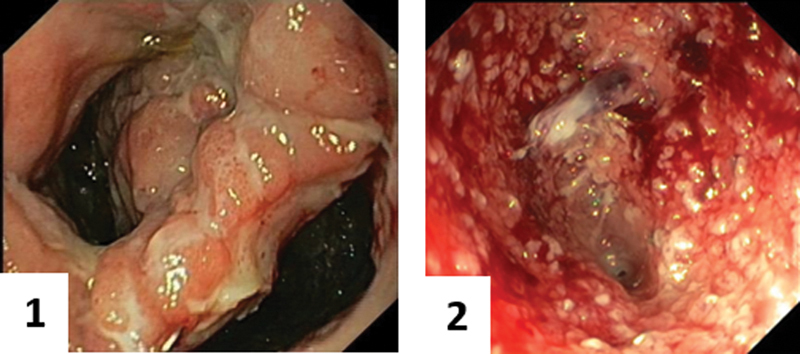
Before and after EVT-treatment. The cavity on the right appeared clean with granulation tissue and deemed suitable for ESC.
Fig. 2.

Transanal closure of the presacral cavity after prior EVT-treatment.
Endosponge treatment is a promising treatment and can increase anastomotic healing rates and improve restored continuity rates. When combined with ESC (EVASC), the presacral cavity previously cleaned by EVT can be closed and healing rates can increase even further. Finally, early start of EVT, when the neorectum is still pliable and unaffected by chronic inflammation, appears vital for optimal treatment of AL and optimize chances of restored continuity.
Surgical Intervention
Despite the increasing array of nonoperative options, surgical intervention still has an important role in the management of AL. Patients with sepsis and peritonitis should undergo immediate surgical intervention to obtain source control. Additionally, in patients who do not improve with nonoperative measures, surgical re-intervention needs to be considered. Source control with washout, adequate drainage, and fecal diversion, if not already present after the index surgery, are the main goals of surgical re-intervention for AL.
Worst case scenario, the surgeon may be forced to take the anastomosis down, and create an end colostomy. This leaves the patient with an unintended stoma that is unlikely to be reversed, particularly in those patients with a short rectal stump . Furthermore, removing the colonic conduit leaves an empty pelvis, that ideally requires filling to control pelvic sepsis. This can be achieved with a pedicled omentoplasty. However, when placed on an open rectal stump the risk for recurrent or uncontrolled sepsis is high in our experience as a tertiary referral center for chronic pelvic sepsis.
Ideally the anastomosis is preserved, with either direct repair of the anastomotic defect if accessible and if tissue quality allows or with adequate local drainage. The surgeon should use judgment if it is safe and feasible to repair the anastomosis. Attempts for an acute redo-anastomosis should not be undertaken in the presence of severe sepsis or septic shock, in patients with AL requiring inotropes, or in those with significant malnutrition and hypoalbuminemia. Diversion and passive drainage have a limited effectivity and still result in a chronic presacral sinus in half of all patients with AL at 1 year postoperatively. 10 Our proposed approach for these (chronic, i.e., sinus > 1 year postoperatively) presacral sinuses is depicted in Fig. 3 .
Fig. 3.
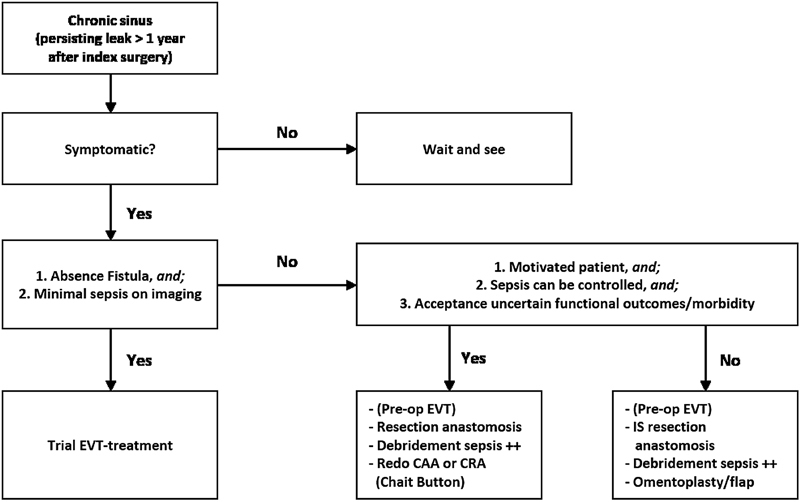
Treatment algorithm for a chronic presacral sinus (leak persisting > 1 year after index surgery).
A redo procedure is a complex operation with a high morbidity and reintervention rate and patients with serious comorbidities might benefit from immediate intersphincteric resection. 46 47 EVAC treatment prior to redo-anastomosis can ensure a cleaner surgical field and a better recovery. The operation can be performed simultaneously in a two-team approach transabdominal and via the transanal route. The transanal approach is especially helpful to preserve any residual rectum for the new anastomosis. It also allows for a better view and access to a scared and inflamed pelvis. The standard approach entails a rectotomy distally to the previous anastomotic site followed by mobilization of the afferent colon if still present. After adequate mobilization of the afferent colon by the abdominal team, the colon is then pulled down the anal canal and a new anastomosis is either hand-sewn or created with a circular stapler, depending on the length of the distal cuff. The new anastomosis can also be created in a two-step approach. Temporary sutures are placed after pull-through and after 7 to 10 days the bowel is transected and the definitive anastomosis is created. Immediate pull-through shows better functional results, when compared with a two-step approach. 48
In a recent review, redo-anastomosis for either recurrent rectal cancer or chronic pelvic sepsis resulted in a successful restoration of bowel continuity in 79%, with a major complication rate of 16%. 49 The major complication rate appears low when compared with the individual cohort studies including only redo anastomosis for chronic pelvic sepsis. One retrospective study found a 41% leakage rate after redo surgery and restored continuity in 66% at end of follow-up. 46 AL after redo surgery was a risk factor for noncontinuity at the end of follow-up (OR 0.022). Another study showed a 40.6% morbidity rate and a restored continuity rate of 78.1%. 47 Redo anastomosis is often used to salvage the anastomosis after previous treatment has failed. A recent retrospective cohort study performed either transanal or radiological drainage in 54 patients with AL after rectal cancer surgery. 28 Drainage alone was successful in 50% and for patients that required an additional intervention, a redo-anastomosis was performed in 21 patients (39%). At the end of follow-up 80% of patients were stoma-free and 20% had an end-colostomy. Despite a relatively high restored continuity rate, many patients required major salvage surgery and lost their primary anastomosis.
When healing of the anastomosis does not seem plausible, complete dismantling of the anastomosis and creation of an end-colostomy can be performed. An intersphincteric resection of the rectal stump is preferred with thorough debridement of the chronic sinus, removal of any present fistulae, and filling of the pelvic cavity. To ensure adequate healing of the pelvis, well-vascularized tissue is needed, preferably from a nonirradiated area. This can be achieved by the creation of a myocutaneous flap (e.g., gracilis muscle flap) or by performing an omentoplasty. 50 51 Omentum is well-vascularized and improves angiogenesis and the immunological response. 52 53 Filling the presacral cavity also prevents prolapse of the small intestines into the cavity, decreasing the risk of new fistulae and obstruction. In patients with a chronic sinus, intersphincteric resection with filling of the anorectal cavity with omentum, wound healing can be achieved in 78% (88% when performed in a single setting). 51
Alternative approaches for a chronic sinus involve injection with fibrin glue after extensive curettage of the cavity or marsupialization of the sinus. 54 55 56 Marsupialization of the presacral space can be achieved using an endoscopic surgical stapler or by simple electrocautery and might be especially beneficial when the colonic wall is fibrotic and unlikely to heal.
Conclusion
Anastomotic leak after rectal cancer surgery remains a common complication with a great cost to patients and providers. Conventional treatment of AL consists of “divert and drain.” To control pelvic sepsis, a diverting stoma is created (if not created during primary resection) and any presacral abscess or fluid collection is drained. New techniques to treat AL after rectal cancer surgery show promising results, with improved anastomotic healing and restored continuity rates. Early diagnosis and proactive treatment are keys to preventing chronic complications from developing, preserving the primary anastomosis and preventing major salvage surgery. Literature on these effective treatment strategies remains scarce, despite the relatively high incidence of AL and its sequelae, and results from ongoing studies are eagerly awaited.
Funding Statement
Funding None.
Footnotes
Conflict of Interest None declared.
References
- 1.Lee S W, Gregory D, Cool C L. Clinical and economic burden of colorectal and bariatric anastomotic leaks. Surg Endosc. 2020;34(10):4374–4381. doi: 10.1007/s00464-019-07210-1. [DOI] [PubMed] [Google Scholar]
- 2.Kverneng Hultberg D, Svensson J, Jutesten H. The impact of anastomotic leakage on long-term function after anterior resection for rectal cancer. Dis Colon Rectum. 2020;63(05):619–628. doi: 10.1097/DCR.0000000000001613. [DOI] [PubMed] [Google Scholar]
- 3.Yang J, Chen Q, Jindou L, Cheng Y. The influence of anastomotic leakage for rectal cancer oncologic outcome: a systematic review and meta-analysis. J Surg Oncol. 2020;121(08):1283–1297. doi: 10.1002/jso.25921. [DOI] [PubMed] [Google Scholar]
- 4.Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg. 2011;253(05):890–899. doi: 10.1097/SLA.0b013e3182128929. [DOI] [PubMed] [Google Scholar]
- 5.La Regina D, Di Giuseppe M, Lucchelli M. Financial impact of anastomotic leakage in colorectal surgery. J Gastrointest Surg. 2019;23(03):580–586. doi: 10.1007/s11605-018-3954-z. [DOI] [PubMed] [Google Scholar]
- 6.Surgical Infection Study Group . Peel A L, Taylor E W. Proposed definitions for the audit of postoperative infection: a discussion paper. Ann R Coll Surg Engl. 1991;73(06):385–388. [PMC free article] [PubMed] [Google Scholar]
- 7.Lichthardt S, Wagner J, Löb S. Pathological complete response due to a prolonged time interval between preoperative chemoradiation and surgery in locally advanced rectal cancer: analysis from the German StuDoQ|Rectalcarcinoma registry. BMC Cancer. 2020;20(01):49. doi: 10.1186/s12885-020-6538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutegård M, Kverneng Hultberg D, Angenete E, Lydrup M L. Substantial underreporting of anastomotic leakage after anterior resection for rectal cancer in the Swedish Colorectal Cancer Registry. Acta Oncol. 2017;56(12):1741–1745. doi: 10.1080/0284186X.2017.1332423. [DOI] [PubMed] [Google Scholar]
- 9.International TaTME Registry Collaborative . Penna M, Hompes R, Arnold S. Incidence and risk factors for anastomotic failure in 1594 patients treated by transanal total mesorectal excision: results from the International TaTME Registry. Ann Surg. 2019;269(04):700–711. doi: 10.1097/SLA.0000000000002653. [DOI] [PubMed] [Google Scholar]
- 10.Dutch Snapshot Research Group . Borstlap W AA, Westerduin E, Aukema T S, Bemelman W A, Tanis P J. Anastomotic leakage and chronic presacral sinus formation after low anterior resection: results from a large cross-sectional study. Ann Surg. 2017;266(05):870–877. doi: 10.1097/SLA.0000000000002429. [DOI] [PubMed] [Google Scholar]
- 11.McDermott F D, Arora S, Smith J, Steele R JC, Carlson G L, Winter D C.(on behalf of the joint ASGBI/ACPGBI). Anastomotic leakage working group issues in professional practice. prevention, diagnosis and management of colorectal anastomotic leakageMarch 2016. Accessed December 2020 at:https://www.asgbi.org.uk/publications/professional-practice-booklets-members-only
- 12.van Helsdingen C P, Jongen A C, de Jonge W J, Bouvy N D, Derikx J P. Consensus on the definition of colorectal anastomotic leakage: a modified Delphi study. World J Gastroenterol. 2020;26(23):3293–3303. doi: 10.3748/wjg.v26.i23.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahbari N N, Weitz J, Hohenberger W. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. 2010;147(03):339–351. doi: 10.1016/j.surg.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy R, Jenkins I, Finan P J. Controversial topics in surgery: splenic flexure mobilisation for anterior resection performed for sigmoid and rectal cancer. Ann R Coll Surg Engl. 2008;90(08):638–642. doi: 10.1308/003588408X358774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vennix S, Bakker O J, Prins H A, Lips D J. Re-interventions following laparoscopic surgery for colorectal cancer: data from 818 individuals from the Dutch surgical colorectal audit. J Laparoendosc Adv Surg Tech A. 2014;24(11):751–755. doi: 10.1089/lap.2014.0385. [DOI] [PubMed] [Google Scholar]
- 16.Singh P P, Zeng I S, Srinivasa S, Lemanu D P, Connolly A B, Hill A G. Systematic review and meta-analysis of use of serum C-reactive protein levels to predict anastomotic leak after colorectal surgery. Br J Surg. 2014;101(04):339–346. doi: 10.1002/bjs.9354. [DOI] [PubMed] [Google Scholar]
- 17.Kornmann V N, Treskes N, Hoonhout L H, Bollen T L, van Ramshorst B, Boerma D. Systematic review on the value of CT scanning in the diagnosis of anastomotic leakage after colorectal surgery. Int J Colorectal Dis. 2013;28(04):437–445. doi: 10.1007/s00384-012-1623-3. [DOI] [PubMed] [Google Scholar]
- 18.Kauv P, Benadjaoud S, Curis E, Boulay-Coletta I, Loriau J, Zins M. Anastomotic leakage after colorectal surgery: diagnostic accuracy of CT. Eur Radiol. 2015;25(12):3543–3551. doi: 10.1007/s00330-015-3795-z. [DOI] [PubMed] [Google Scholar]
- 19.Habib K, Gupta A, White D, Mazari F AK, Wilson T R. Utility of contrast enema to assess anastomotic integrity and the natural history of radiological leaks after low rectal surgery: systematic review and meta-analysis. Int J Colorectal Dis. 2015;30(08):1007–1014. doi: 10.1007/s00384-015-2225-7. [DOI] [PubMed] [Google Scholar]
- 20.Solomkin J S, Mazuski J E, Bradley J S. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(02):133–164. doi: 10.1086/649554. [DOI] [PubMed] [Google Scholar]
- 21.Yang G, Kim C W, Lee S H. Patterns of antibiotics and pathogens for anastomotic leakage after colorectal cancer surgery. Korean J Clin Oncol. 2019;15(02):79–85. [Google Scholar]
- 22.Morrissey I, Hackel M, Badal R, Bouchillon S, Hawser S, Biedenbach D. A review of ten years of the Study for Monitoring Antimicrobial Resistance Trends (SMART) from 2002 to 2011. Pharmaceuticals (Basel) 2013;6(11):1335–1346. doi: 10.3390/ph6111335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Creavin B, Ryan E J, Kelly M E. Minimally invasive approaches to the management of anastomotic leakage following restorative rectal cancer resection. Colorectal Dis. 2019;21(12):1364–1371. doi: 10.1111/codi.14742. [DOI] [PubMed] [Google Scholar]
- 24.Blumetti J, Chaudhry V, Cintron J R. Management of anastomotic leak: lessons learned from a large colon and rectal surgery training program. World J Surg. 2014;38(04):985–991. doi: 10.1007/s00268-013-2340-y. [DOI] [PubMed] [Google Scholar]
- 25.Maggiori L, Bretagnol F, Lefèvre J H, Ferron M, Vicaut E, Panis Y. Conservative management is associated with a decreased risk of definitive stoma after anastomotic leakage complicating sphincter-saving resection for rectal cancer. Colorectal Dis. 2011;13(06):632–637. doi: 10.1111/j.1463-1318.2010.02252.x. [DOI] [PubMed] [Google Scholar]
- 26.Harisinghani M G, Gervais D A, Maher M M. Transgluteal approach for percutaneous drainage of deep pelvic abscesses: 154 cases. Radiology. 2003;228(03):701–705. doi: 10.1148/radiol.2283020924. [DOI] [PubMed] [Google Scholar]
- 27.Robert B, Chivot C, Rebibo L, Sabbagh C, Regimbeau J M, Yzet T. Percutaneous transgluteal drainage of pelvic abscesses in interventional radiology: a safe alternative to surgery. J Visc Surg. 2016;153(01):3–7. doi: 10.1016/j.jviscsurg.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Challine A, Lefèvre J H, Creavin B. Can a local drainage salvage a failed colorectal or coloanal anastomosis? A prospective cohort of 54 patients. Dis Colon Rectum. 2020;63(01):93–100. doi: 10.1097/DCR.0000000000001516. [DOI] [PubMed] [Google Scholar]
- 29.Sirois-Giguère E, Boulanger-Gobeil C, Bouchard A. Transanal drainage to treat anastomotic leaks after low anterior resection for rectal cancer: a valuable option. Dis Colon Rectum. 2013;56(05):586–592. doi: 10.1097/DCR.0b013e31827687a4. [DOI] [PubMed] [Google Scholar]
- 30.Shalaby M, Thabet W, Buonomo O. Transanal tube drainage as a conservative treatment for anastomotic leakage following a rectal resection. Ann Coloproctol. 2018;34(06):317–321. doi: 10.3393/ac.2017.10.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamazza A, Sterpetti A V, De Cesare A, Schillaci A, Antoniozzi A, Fiori E. Endoscopic placement of self-expanding stents in patients with symptomatic anastomotic leakage after colorectal resection for cancer: long-term results. Endoscopy. 2015;47(03):270–272. doi: 10.1055/s-0034-1391403. [DOI] [PubMed] [Google Scholar]
- 32.Pérez Roldán F, González Carro P, Villafáñez García M C. Usefulness of biodegradable polydioxanone stents in the treatment of postsurgical colorectal strictures and fistulas. Endoscopy. 2012;44(03):297–300. doi: 10.1055/s-0031-1291482. [DOI] [PubMed] [Google Scholar]
- 33.Lamazza A, Sterpetti A V, De Cesare A, Schillaci A, Antoniozzi A, Fiori E. Endoscopic placement of self-expanding stents in patients with symptomatic anastomotic leakage after colorectal resection for cancer: long-term results. Endoscopy. 2015;47(03):270–272. doi: 10.1055/s-0034-1391403. [DOI] [PubMed] [Google Scholar]
- 34.Abbas M A. Endoscopic management of acute colorectal anastomotic complications with temporary stent. JSLS. 2009;13(03):420–424. [PMC free article] [PubMed] [Google Scholar]
- 35.Chorti A, Stavrou G, Stelmach V. Endoscopic repair of anastomotic leakage after low anterior resection for rectal cancer: a systematic review. Asian J Endosc Surg. 2020;13(02):141–146. doi: 10.1111/ases.12733. [DOI] [PubMed] [Google Scholar]
- 36.Chopra S S, Mrak K, Hünerbein M. The effect of endoscopic treatment on healing of anastomotic leaks after anterior resection of rectal cancer. Surgery. 2009;145(02):182–188. doi: 10.1016/j.surg.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Lippert E, Klebl F H, Schweller F. Fibrin glue in the endoscopic treatment of fistulae and anastomotic leakages of the gastrointestinal tract. Int J Colorectal Dis. 2011;26(03):303–311. doi: 10.1007/s00384-010-1104-5. [DOI] [PubMed] [Google Scholar]
- 38.Weidenhagen R, Gruetzner K U, Wiecken T, Spelsberg F, Jauch K W. Endoscopic vacuum-assisted closure of anastomotic leakage following anterior resection of the rectum: a new method. Surg Endosc. 2008;22(08):1818–1825. doi: 10.1007/s00464-007-9706-x. [DOI] [PubMed] [Google Scholar]
- 39.Gardenbroek T J, Musters G D, Buskens C J. Early reconstruction of the leaking ileal pouch-anal anastomosis: a novel solution to an old problem. Colorectal Dis. 2015;17(05):426–432. doi: 10.1111/codi.12867. [DOI] [PubMed] [Google Scholar]
- 40.Borstlap W AA, Musters G D, Stassen L PS. Vacuum-assisted early transanal closure of leaking low colorectal anastomoses: the CLEAN study. Surg Endosc. 2018;32(01):315–327. doi: 10.1007/s00464-017-5679-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Talboom K, van Kesteren J, Sonneveld D JA, Tanis P J, Bemelman W A, Hompes R. Early transanal closure after vacuum-assisted drainage for anastomotic leakage in rectal cancer surgery—a video vignette. Colorectal Dis. 2020;22(08):973–974. doi: 10.1111/codi.15032. [DOI] [PubMed] [Google Scholar]
- 42.Shalaby M, Emile S, Elfeki H, Sakr A, Wexner S D, Sileri P. Systematic review of endoluminal vacuum-assisted therapy as salvage treatment for rectal anastomotic leakage. BJS Open. 2018;3(02):153–160. doi: 10.1002/bjs5.50124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.on behalf the French GRECCAR group . Abdalla S, Cotte E, Epin A. Short-term and long-term outcome of endoluminal vacuum therapy for colorectal or coloanal anastomotic leakage: results of a nationwide multicenter cohort study from the French GRECCAR Group. Dis Colon Rectum. 2020;63(03):371–380. doi: 10.1097/DCR.0000000000001560. [DOI] [PubMed] [Google Scholar]
- 44.Huisman J F, van Westreenen H L, van der Wouden E J. Effectiveness of endosponge therapy for the management of presacral abscesses following rectal surgery. Tech Coloproctol. 2019;23(06):551–557. doi: 10.1007/s10151-019-02007-9. [DOI] [PubMed] [Google Scholar]
- 45.Kühn F, Janisch F, Schwandner F. Comparison between endoscopic vacuum therapy and conventional treatment for leakage after rectal resection. World J Surg. 2020;44(04):1277–1282. doi: 10.1007/s00268-019-05349-5. [DOI] [PubMed] [Google Scholar]
- 46.Westerduin E, Borstlap W AA, Musters G D. Redo coloanal anastomosis for anastomotic leakage after low anterior resection for rectal cancer: an analysis of 59 cases. Colorectal Dis. 2018;20(01):35–43. doi: 10.1111/codi.13844. [DOI] [PubMed] [Google Scholar]
- 47.Woo I T, Park J S, Choi G S, Park S Y, Kim H J, Park I K. Clinical outcomes of a redo for a failed colorectal or coloanal anastomosis. Ann Coloproctol. 2018;34(05):259–265. doi: 10.3393/ac.2018.05.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boullenois H, Lefevre J H, Creavin B. Long-term functional results and quality of life after redo rectal surgery: delayed versus immediate colo-anal anastomosis. Colorectal Dis. 2020;22(08):885–893. doi: 10.1111/codi.14983. [DOI] [PubMed] [Google Scholar]
- 49.Westerduin E, Klaver C EL, van Geloven A AW, Westerterp M, Bemelman W A, Tanis P J. Outcome after redo surgery for complicated colorectal and coloanal anastomosis: a systematic review. Dis Colon Rectum. 2018;61(08):988–998. doi: 10.1097/DCR.0000000000001129. [DOI] [PubMed] [Google Scholar]
- 50.Borel Rinkes I H, Wiggers T. Gracilis muscle flap in the treatment of persistent, infected pelvic necrosis. Eur J Surg. 1999;165(04):390–391. doi: 10.1080/110241599750006956. [DOI] [PubMed] [Google Scholar]
- 51.Musters G D, Borstlap W A, Bemelman W A, Buskens C J, Tanis P J. Intersphincteric completion proctectomy with omentoplasty for chronic presacral sinus after low anterior resection for rectal cancer. Colorectal Dis. 2016;18(02):147–154. doi: 10.1111/codi.13086. [DOI] [PubMed] [Google Scholar]
- 52.Goldsmith H S, Griffith A L, Kupferman A, Catsimpoolas N. Lipid angiogenic factor from omentum. JAMA. 1984;252(15):2034–2036. [PubMed] [Google Scholar]
- 53.Meza-Perez S, Randall T D. Immunological functions of the omentum. Trends Immunol. 2017;38(07):526–536. doi: 10.1016/j.it.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swain B T, Ellis C N. Fibrin glue treatment of low rectal and pouch-anal anastomotic sinuses. Dis Colon Rectum. 2004;47(02):253–255. doi: 10.1007/s10350-003-0040-7. [DOI] [PubMed] [Google Scholar]
- 55.Alsanea N, Alabbad S. Use of the endostapler for the treatment of non-healing sinus secondary to a dehisced colorectal anastomosis. Tech Coloproctol. 2010;14(03):249–251. doi: 10.1007/s10151-010-0600-5. [DOI] [PubMed] [Google Scholar]
- 56.Abild N, Bulut O, Nielsen C B. Endoscopic stapled marsupialisation of chronic presacral sinus following low anterior resection: a simple option in selected cases. Scand J Surg. 2012;101(04):307–310. doi: 10.1177/145749691210100416. [DOI] [PubMed] [Google Scholar]


