Abstract
Objective
Oxidative stress-mediated inflammatory events involve in the progress of several diseases such as asthma, cancers, and multiple sclerosis. Auraptene (AU), a natural prenyloxycoumarin, possesses numerous pharmacological activities. Here, the anti-inflammatory effects of AU were investigated in lipoteichoic acid- (LTA-) induced macrophage cells (RAW 264.7).
Methods
The expression of cyclooxygenase (COX-2), tumor necrosis factor (TNF-α), interleukin-1β (IL-1β), and inducible nitric oxide synthase (iNOS) and the phosphorylation of extracellular signal-regulated kinase (ERK) 1/2, p38 MAPK, c-Jun N-terminal kinase (JNK), heme oxygenase (HO-1), p65, and IκBα were all identified by western blotting assay. The level of nitric oxide (NO) was measured by spectrometer analysis. The nuclear translocation of p65 nuclear factor kappa B (NF-κB) was assessed by the confocal microscopic staining method. Native polyacrylamide gel electrophoresis was performed to perceive the activity of antioxidant enzyme catalase (CAT).
Results
AU expressively reduced NO production and COX-2, TNF-α, IL-1 β, and iNOS expression in LTA-stimulated cells. AU at higher concentration (10 µM) inhibited ERK and JNK, but not p38 phosphorylation induced by LTA. Moreover, AU blocked IκB and p65 phosphorylation, and p65 nuclear translocation. However, AU pretreatment was not effective on antioxidant HO-1 expression, CAT activity, and reduced glutathione (GSH, a nonenzymatic antioxidant), in LTA-induced RAW 264.7 cells.
Conclusion
The findings of this study advocate that AU shows anti-inflammatory effects via reducing NF-κB/MAPKs signaling pathways.
1. Introduction
Various chemicals and pathogens considered as harmful stimuli produce inflammation, which is a protective response of our body. Inflammation can be classified as acute and chronic, which induces pain and tissue injuries. Rapid onset and short duration of action can be noticed in the acute form, which is facilitated by the excretion of numerous cytokines including interleukin-1 (IL-1), IL-6, IL-11, IL-8, and tumor necrosis factor-alpha (TNF-α) [1, 2]. Nevertheless, in chronic inflammation, persistence of the inflammatory reactions could induce the migration of lymphocytes and macrophages to the damaged tissues [3]. Chronic inflammatory responses have been associated with the progression of various diseases such as asthma, arthritis, and neurodegenerative disorders [4]. Studies have established the involvement of several mediators including prostaglandin E2 (PGE2) in inflammatory events. Various symptoms including bone metabolism, wound healing, kidney function, blood vessel, and the immune responses have been associated with PGE2 secretion [5]. Cyclooxygenase (COX-2) protein can be expressed in response to physical, chemical, and biological stimulation [6]. The production of PGE2 can be augmented by COX-2, which denotes a central step in the events of inflammation.
Oxidative stress is known to be induced by elevated reactive oxygen species (ROS) and nitric oxide (NO) or reduced antioxidant enzymes catalase (CAT) and superoxide dismutase (SOD) and nonenzymatic glutathione (GSH) [7, 8]. Studies have indicated that oxidative stress plays a major role in the progress of inflammatory diseases [9]. The major component of Gram-positive bacteria, lipoteichoic acid (LTA), induces pathogenesis of sepsis [10] and lung injury by producing inflammatory reactions [11]. Therefore, examining the mechanisms that control LTA-stimulated cell activation is important for the analysis and treatment of lung inflammatory diseases. This bacterial component stimulates the release of IL-1β, IL-6, and TNF-α [12]. LTA induces TNF-α and IL-6 expressions by inducing the phosphorylation of ERK1/2 in macrophages, and it also activates nuclear translocation of nuclear factor- (NF-) κB from the cytoplasm [13]. It has been proposed that various plant-based natural components have reported to have anti-inflammatory effects through suppressing inflammation-associated mediators and enhancing antioxidant defense molecules.
Auraptene, a geranyloxyl moiety of C-7 (7-geranyloxycoumarin), is a promising and most rich natural prenyloxycoumarin compound [14]. Plants of the Rutaceae family are the highest source of auraptene, and it is also the most general component of citrus fruits. Hence, citrus species are the major natural source of auraptene [14]. Several exciting pharmacological activities have been reported for this bioactive phytochemical such as antioxidant [15], anti-inflammatory [16], antimicrobial [17], antigenotoxic [18], neuroprotective [19], and immunomodulatory properties [20]. Murakami et al. [16] had well discussed the effect of auraptene in inflammation-mediated carcinogenesis. A study specified that dietary supplementation of auraptene in mice diminishes pulmonary metastasis of B16BL6 melanoma cells and prevents the growth of metastatic tumors in the lungs via inducing apoptosis [21]. In addition, auraptene showed promising effects of wound healing through inhibiting the secretion of inflammatory mediators in vitro, including IL-6 and IL-8 [22]. Hence, this study aimed to assess the anti-inflammatory mechanism of auraptene against LTA-stimulation in RAW 264.7 cells.
2. Materials and Methods
2.1. Materials
RAW 264.7 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA, TIB-71). Auraptene (AU, >98%, Figure 1(a)) was purchased from ChemFaces Biochem, Wuhan, Hubei, China. Sigma (St Louis, MO, USA) supplied potassium ferricyanide, ferric chloride, and dimethyl sulfoxide (DMSO). Santa Cruz Biotechnology (Dallas, TX, USA) supplied anti-iNOS and COX-2 polyclonal antibodies (pAb). We purchased antibodies against TNF-α, phospho-p38 MAPK Thr180/Tyr182, phospho-c-JNK (Thr183/Tyr185), phospho-p44/p42 ERK (Thr202/Tyr204), phospho-IκBα Ser32/36, and phospho-NF-κB p65 (Ser536) pAbs from Cell Signaling (Beverly, MA, USA). Anti-IL-1β and anti-HO-1 pAbs were purchased from BioVision (Milpitas, CA, USA) and Enzo (Farmingdale, New York, USA), respectively. The antibody against α-tubulin was purchased from NeoMarkers (Fremont, CA, USA). AU was dissolved in 0.1% DMSO.
Figure 1.
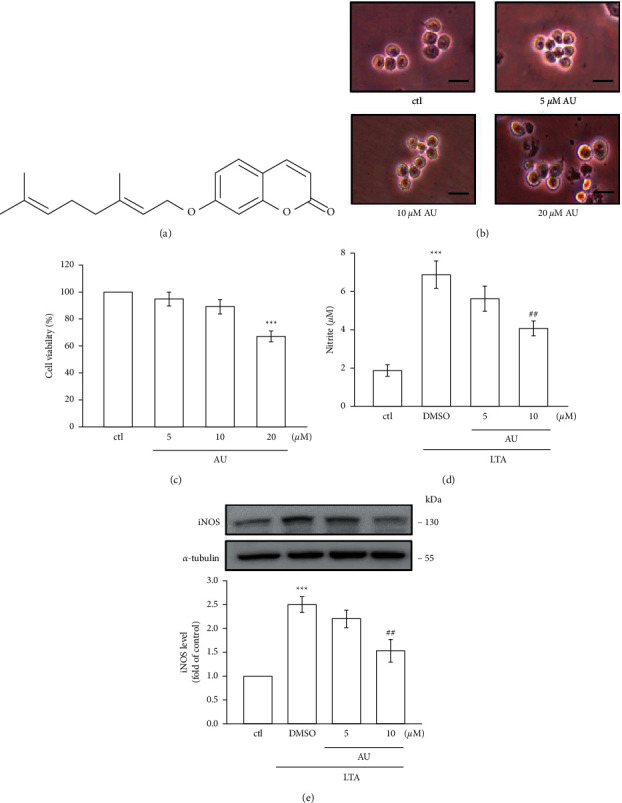
Chemical structure of auraptene (AU) and the effects of AU on morphology and cell viability and on LTA-induced NO production and iNOS expression in RAW 264.7 cells. (a) Chemical structure of AU. (b), (c) Cells were pretreated with AU (5, 10, or 20 μM) for 24. Cell viabilities were determined by the MTT assay. Scale bar = 25 μm. (d), (e) Cells were untreated or pretreated with AU (5 and 10 μM) for 30 min prior to stimulation with LTA (5 μg/ml) for 24 h. Control cells were not treated with LTA or AU. NO was measured using the Griess reaction assay. iNOS expression was detected using western blotting assay. The values shown are the means ± S.E.M. of four independent experiments. ∗∗∗P < 0.001 vs. the control cells; ##P < 0.01 vs. LTA-stimulated cells.
2.2. Cell Viability and Morphology of RAW Cells
RAW 264.7 cells were cultivated in Dulbecco's Modified Eagle's Medium (DMEM) at 37°C under 5% CO2 and 95% air. At a concentration of 1 × 105 cells/well, they were pretreated with AU (5–20 μM) for 24 h. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay was used to measure cell viability in which 5 mg/mL of MTT working solution was added to the culture medium. The formation of crystals was digested by suing 300 µl of DMSO. The formula of absorbance of treated cells/absorbance of control cells × 100% is used to measure the cell viability index.
2.3. Measurement of NO Production
To estimate the level of NO, AU at 5 and 10 μM was added to cells with or without LTA (5 μg/ml) for 24 h in the medium. Briefly, a 100 µl equal volume of culture suspension and Griess reagent was mixed and incubated for 10 min. NO levels were estimated by quantifying nitrite levels by an MRX absorbance reader with the optical density at 550 nm.
2.4. Immunoblotting Assay
The equal amount (50 µg) of proteins from 6 × 105 cells were run on 12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) gels. The separated proteins were transferred to polyvinylidene difluoride (PVDF) membranes and then blocked using 5% skim milk for 40 min. After blocking, the membrane was titrated with different primary antibodies of targeted proteins for 2 h and consequently incubated with anti-rabbit IgG or sheep anti-mouse IgG for 1 h. The intensity of protein bands was measured by using the Biolight Windows Application, V2000.01 (Bio-Profil, Vilber Lourmat, France) software.
2.5. Confocal Microscopy Assay
Cells were seeded at 5 × 104/well, cultured on cover slips, and treated by AU (10 μM) for 30 min and then triggered by LTA (5 μg/ml) for 1 h. Coverslips were successively fixed with 4% paraformaldehyde for 10 min at 37°C, double washed using PBS, incubated with 0.1% Triton X-100 for 10 min, and then, blocked with 5% BSA for 1 h. Besides, the primary p65 antibody was added over the coverslips at 4°C overnight, and then, secondary goat anti-rabbit IgG antibody was incubated for 1 h at 37°C. 4,6-Diamidino-2 phenylindole (DAPI) was used to stain nuclei in cells. The location of nuclear translocation of p65 was spotted by using the Leica TCS SP5 confocal spectral microscope imaging system (Mannheim, Germany).
2.6. Detection of Antioxidant Enzyme Catalase (CAT)
According to the method defined by Woodbury et al. [23], a native polyacrylamide gel electrophoresis (NATIVE-PAGE) was run to spot the relative banding patterns of antioxidant enzyme catalase (CAT). To this analysis, unlike normal SDS-PAGE, the running buffers and protein samples did not heat and omit SDS. The equal amounts of 50 μg proteins were run in 8% PAGE.
2.7. Statistical Analysis
The results are presented as mean ± standard error (S. E. M). The statistical difference among the groups was determined using one-way analysis of variance (ANOVA). Statistical alterations were detected significant. The P value of the Student–Newman–Keuls test was regarded as P < 0.05.
3. Results
3.1. AU Did Not Affect the Viability and Morphology of RAW 264.7 Cells
Cell morphology and viability were studied to evaluate the toxic effect of AU in RAW 264.7 cells. Among the tested concentrations of 5, 10, and 20 μM AU in RAW cells for 24 h, 5 and 10 μM did not affect cell morphology as well as viability (Figures 1(b) and 1(c)), respectively. However, AU at 20 μM significantly affected the morphology and viability of RAW cells. Thus, AU at feasible concentrations of 5 and 10 μM were used for the subsequent investigation.
3.2. LTA-Induced NO Production and iNOS Were Inhibited by AU
Griess reaction was applied to measure the level of NO production in AU pretreated LTA-induced RAW 264.7 cells. Systemic inflammatory events have been reported to induce a proinflammatory mediator NO [24]. A rate‐limiting enzyme, inducible nitric oxide synthase (iNOS), regulates the production of NO [25]. To examine if AU inhibits NO production via the modulation of iNOS expression, the expression of iNOS was detected as shown in Figure 1(e). Figures 1(d) and 1(e) show that, at a high concentration of 10 μM, AU significantly inhibited the LTA‐induced production of NO and its enzyme iNOS expression (control: 1 ± 0, DMSO: 2.5 ± 0.2, 5 μM: 2.2 ± 0.2, 10 μM: 1.5 ± 0.2) in RAW 264.7 cells. This result apprehends that the inhibition of iNOS expression by AU may be involved in the inhibition of LTA‐induced NO production.
3.3. AU Inhibited LTA-Induced IL-1β, TNF-α, and COX-2 Expressions
LTA stimulated the levels of COX-2 (2.1 ± 0.3, P < 0.01), IL-1β (3.1 ± 0.3, P < 0.001), and TNF-α (3.3 ± 0.4, P < 0.001) dramatically compared to the nonstimulated control RAW cells (Figures 2(a)–2(d)). In contrast, AU at 5 and 10 μM distinctly alleviated COX-2 (5 μM: 1.5 ± 0.2, 10 μM: 1.1 ± 0.2), IL-1β (5 μM: 1.9 ± 0.3, 10 μM: 0.7 ± 0.1), and TNF-α (5 μM: 1.7 ± 0.3, 10 μM: 0.9 ± 0.2) induced by LTA. Moreover, AU more prominently inhibited IL-1β and TNF-α (Figures 2(c) and 2(d)).
Figure 2.
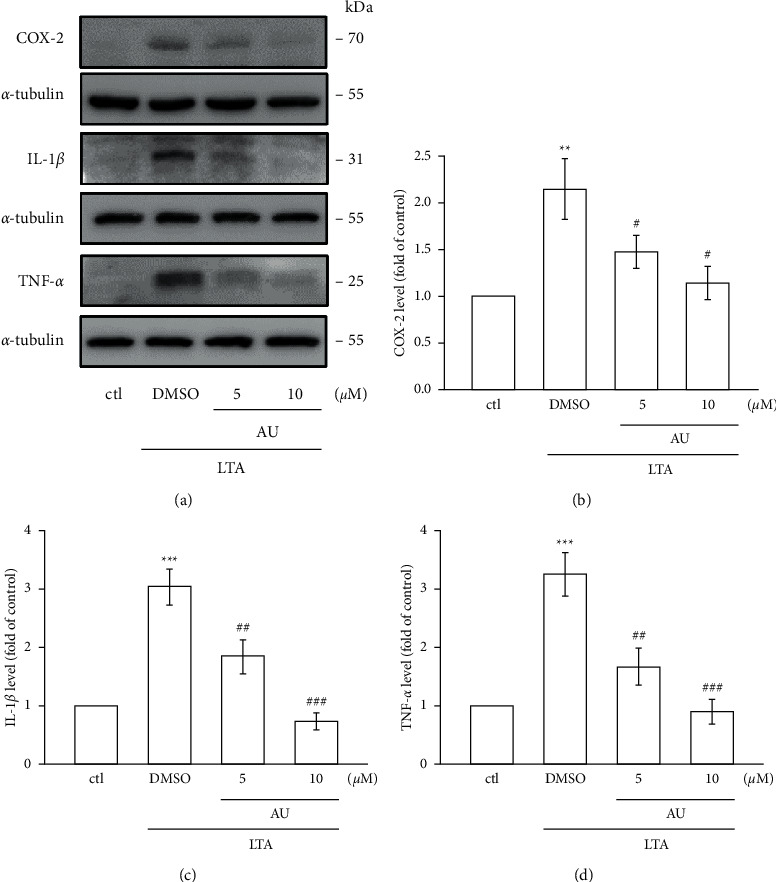
Effects of AU on the LTA-induced expression COX-2, IL-1β, and TNF-α in RAW 264.7 macrophages. (a)–(d) Cells were untreated or pretreated with AU (5 and 10 μM) for 30 min and then stimulated with LTA (5 μg/ml) for 24 h. COX-2, IL-1β, and TNF-α were detected as described in Section 2. The values shown are the means ± S.E.M. of four independent experiments. ∗∗P < 0.01 and ∗∗∗P < 0.001 vs. the control cells; #P < 0.05, ##P < 0.01, and ###P < 0.001 vs. LTA-stimulated cells.
3.4. AU Inhibits ERK1/2 and JNK1/2, But Not p38 MAPK Phosphorylation
We examined the effect of AU on LTA‐induced mitogen-activated protein kinases (MAPKs), since several studies have shown that these molecules actively involve on inflammation‐related events. Figure 3 shows the elevated phosphorylation of ERK1/2 (3.1 ± 0.5), JNK1/2 (3.2 ± 0.3), and p38 MAPK (3.2 ± 0.3) in LTA-induced RAW cells compared to control cells. However, AU at a higher concentration of 10 µM significantly diminished the LTA-induced phosphorylation of JNK1/2 (1.9 ± 0.2), and it concentration-dependently inhibited the ERK1/2 phosphorylation (5 μM: 1.8 ± 0.4, 10 μM: 1.4 ± 0.2); however, it is not effective on p38 (5 μM: 2.9 ± 0.4, 10 μM: 2.8 ± 0.2). These outcomes designated that AU reveals its inhibitory effects in LTA-induced inflammatory events in RAW 264.7 cells via suppressing ERK1/2 and JNK1/2 signaling cascade.
Figure 3.
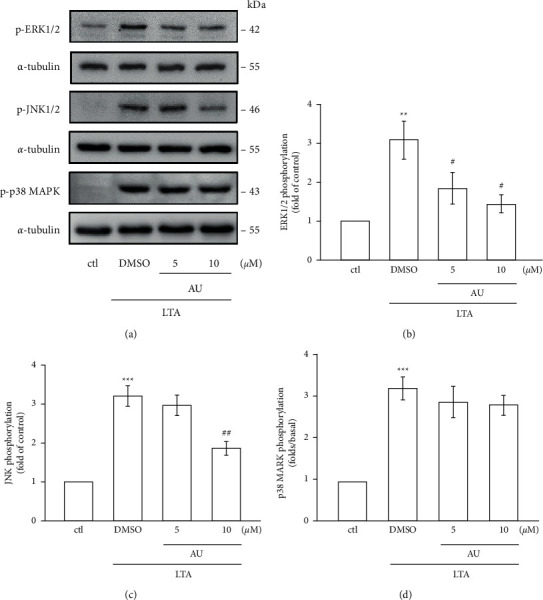
Effects of AU in LTA-induced phosphorylation of MAPKs in RAW 264.7 macrophages. (a) Cells were untreated or pretreated with AU (5 and 10 μM) for 30 min and were then stimulated with LTA (5 μg/ml) for 1 h. The specific pERK, pJNK, and p38 MAPK antibodies were used to detect these proteins. α-Tubulin was used as the internal control. (b)–(d) The statistical values shown are the means ± S.E.M. of four independent experiments. ∗∗P < 0.01 and ∗∗∗P < 0.001 vs. the control cells; #P < 0.05 and ##P < 0.01 vs. LTA-stimulated cells.
3.5. LTA‐Induced NF-κB Signaling Pathway Was Inhibited by AU
NF‐κB, a major transcription factor, is constantly inducing proinflammatory mediators and cytokines. This transcription factor translocates to the nucleus once it activates and binds with target DNA and then controls the activation of numerous inflammatory cytokines [25]. Here, the inhibitory effect of AU on NF‐κB signaling pathways was examined by investigating the phosphorylations of IκBα and p65 and also the nuclear translocation of p65 in LTA‐induced RAW cells. The results showed that AU reduced LTA‐induced IκBα (DMSO: 4.0 ± 0.7, 5 μM: 2.5 ± 0.5, and 10 μM: 1.4 ± 0.3) and p65 phosphorylation (DMSO: 3.5 ± 0.3, 5 μM: 2.7 ± 0.2, and 10 μM: 1.9 ± 0.2) (Figures 4(a) and 4(b)) and withdrew the nuclear translocation of p65 (Figure 4(c)). These results demonstrate that AU's anti-inflammatory effect in LTA-induced cells may probably be via inhibiting the NF‐κB signaling pathway.
Figure 4.
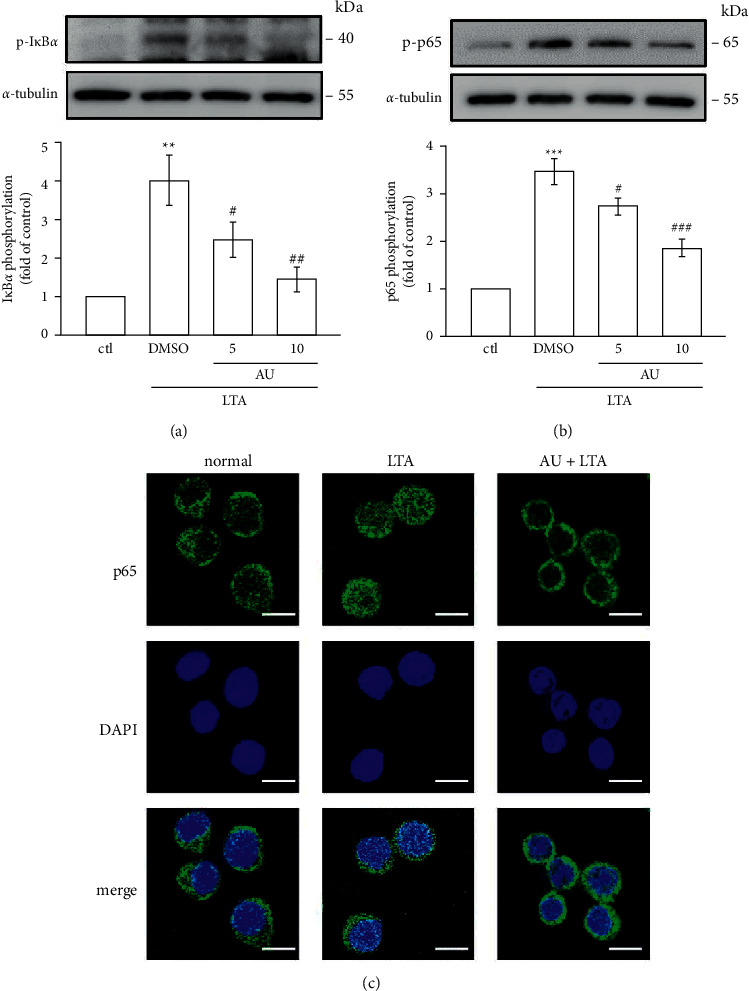
AU controls the NF-κB signaling pathway induced by LTA in RAW 264.7 macrophages. Cells were pretreated with AU (5 and 10 μM) for 30 min and were then stimulated with LTA (5 μg/ml) for 1 h. The phosphorylation of (a) IκBα and (b) p65 in LTA-induced RAW cells was detected as described in Section 2. (c) PTE inhibited LTA-induced p65 nuclear translocation. The values shown are the means ± S.E.M. of four independent experiments. ∗∗ < 0.01 and ∗∗∗ < 0.001 vs. the control cells; #P < 0.05, ##P < 0.01, and ###P < 0.001 vs. LTA-stimulated cells.
3.6. AU Enhances Antioxidant Defense Molecules
Oxidative stress occurs by the elevated levels of reactive oxygen species (ROS) and NO or reduced levels of antioxidant defense molecules, such as reduced glutathione (GSH), catalase (CAT), and superoxide dismutase (SOD) [7]. Numerous studies have established that oxidative stress could induce the progress of inflammatory diseases [26]. LTA stimulation in RAW cells has been demonstrated to decrease in the expression of HO-1 (1.7 ± 0.3), antioxidant enzyme catalase, and the nonenzymatic GSH (Figures 5(a)–5(c)). AU pretreatment was not effective on LTA-stimulated reduction of HO-1 (5 μM: 2.4 ± 0.4, 10 μM: 2.3 ± 0.3), CAT, and GSH in RAW cells. These results indicate that the antioxidant defense systems could not play a role in AU-mediated anti-inflammatory effects in LTA-stimulated RAW cells.
Figure 5.
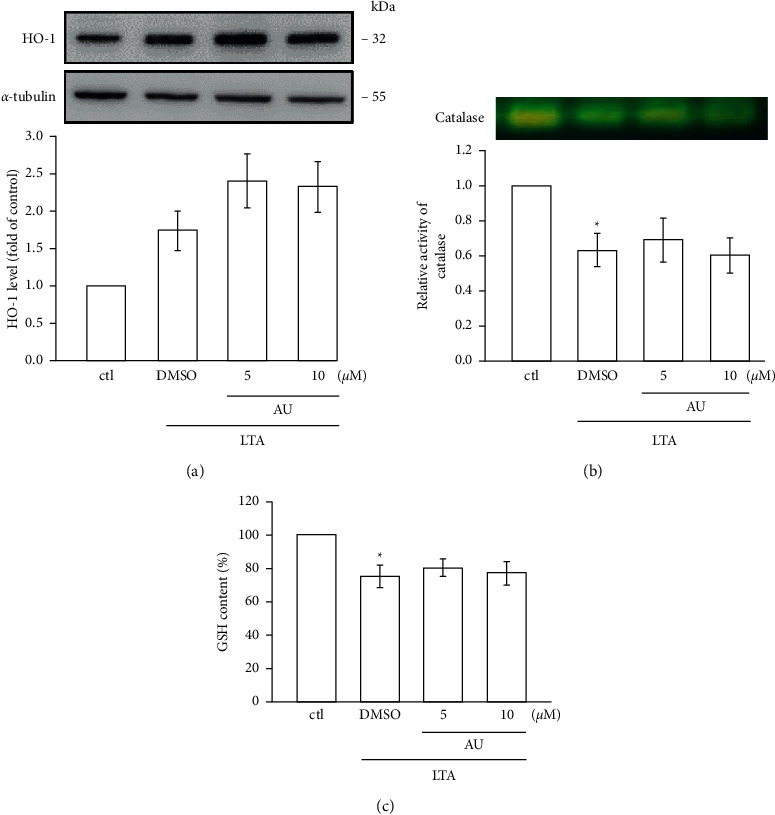
AU enhances antioxidant defense molecules in LTA-stimulated RAW cells. Cells were untreated or pretreated with AU (5 and 10 μM) for 30 min followed by LTA (5 μg/ml) for 24 h. The expression of HO-1 (a), catalase (CAT) activity (b), and glutathione (GSH) content (c) in LTA-induced RAW cells was determined using western blotting, native polyacrylamide gel electrophoresis (NATIVE-PAGE), and spectrophotometric analyses, respectively. The values shown are the means ± S.E.M. of four independent experiments. ∗P < 0.05 vs. the control cells.
4. Discussion
Auraptene (AU), a natural prenyloxycoumarin, is mostly present in citrus fruits. Auraptene (AU) possesses numerous pharmacological properties such as anticancer, antibacterial, antioxidant, and antiinflammatory [27]. Here, we found that auraptene (5 and 10 μM) did not display cytotoxicity in both control and LTA-stimulated RAW cells. Hence, the ideal concentrations of 5 and 10 μM of auraptene were used in this study. A study exposed that auraptene at concentrations of 5–40 μM had no cytotoxicity on murine lymphocytes [28]. Together, as revealed in the present study, anti-inflammatory and antioxidative effects of auraptene are not through its cytotoxicity. Moreover, this study found that anti-inflammatory effects of AU was facilitated via preventing the production of NO and its enzyme iNOS expression. Auraptene also inhibited the LTA-induced protein expression of IL‐1β and TNF‐α by inhibiting the mitogen activated protein kinases (MAPKs)/NF‐κB pathways.
As it is established, proinflammatory cytokines and mediators such as NO, IL-1β, IL-6, and TNF-α play a major role in the inflammatory process. Chronic inflammation has been reported to cause several diseases such as cancers, arthritis, and cardiovascular diseases [29]. A recent study specified that AU at 10–90 μM reduced the levels of IL-6 and TNF-α in phytohemagglutinin- (PHA-) stimulated human lymphocytes [30]. A previous study from these authors has also established that AU alleviates IL-4, IL-10, and interferon (IFN-γ) levels [29]. NO plays a role in the pathogenesis of several inflammatory disorders, and its production in activated macrophages via the rate-limiting enzyme iNOS induces several acute and chronic inflammatory conditions [31]. COX-2 is reported to be overexpressed during the course of LPS-induced inflammatory reaction [32]. Studies have described that the overexpression of iNOS and COX-2 stimulates the activation of NO and PGE2 in activated macrophages, respectively. Overproduction of such inflammatory mediators can result in chronic inflammatory diseases [33]. Here, we found that AU expressively and without causing cytotoxicity inhibits the level of NO in LTA-stimulated RAW 264.7 cells. The AU's inhibitory effect on LTA-induced NO production appears to involve the reduction of iNOS expression. Moreover, AU dramatically inhibited the LTA-induced expression of iNOS, COX-2, TNF‐α, and IL‐1β. Okuyama et al. [34] showed that AU suppressed the LPS-induced expression of COX-2, IL-1β, and TNF-α in astrocytes isolated from the cerebral cortex of ICR mice. Niu et al. found an inhibitory mechanism for AU via IL-2, IFN-γ, and IL-4 in lymphocytes isolated from C57BL/6 mice [28]. These results are consistent with our results and evident of the anti-inflammatory properties of AU.
The induction of inflammatory mediators involves the activation of multiple signal transduction pathways, including mitogen-activated protein kinases (MAPKs) such as p38, ERK, and JNK [35]. It is reported that blocking p38, ERK, and JNK MAPK pathways could decrease iNOS and COX-2 expression and TNF-α and IL-1β production in macrophage inflammation [36]. The MAPK/NF-κB signaling pathway was conveyed to play a vital role in the expression of TNF-α, IL-6, IL-1β, and COX-2 in many cell types [37]. Therefore, we examined the effect of AU on MAPK/NF-κB pathway activation. Niu et al. found esculin significantly inhibited the activation of the MAPK pathway in LPS-induced peritoneal macrophages [38]. Guo et al. found both degradation and phosphorylation of IκBα and activation of NF-κB p65 stimulated by LPS are significantly controlled by imperatorin in RAW 264.7 macrophages [39]. Our recent study found that pterostilbene, a natural substance of blueberry and an analog of resveratrol, significantly inhibited the NF-κB signaling pathway and ERK phosphorylation in RAW 264.7 cells [40]. Thus, it is proposed that coumarin derivatives may inhibit the MAPK/NF-κB signaling pathway in LPS-induced inflammatory reaction. The results of this study consistently showed that AU strongly reversed the LTA-induced phosphorylation of JNK and ERK and the nuclear translocation of the p65 subunit. The induction of NF-κB is controlled by IκB kinase (IKK) complex activation, and IKK phosphorylates IκBα and initiates ubiquitin-dependent IκBα degradation [41]. This process could lead NF-κB translocation to the nucleus, where it attaches to the promoter regions of the target gene and brings proinflammatory mediators such as iNOS, COX-2, TNF-α, and IL-6 [42]. The phosphorylation of IκB and p65 can be induced by LTA, and it also can induce p65 translocation from the cytoplasm to nuclei [13]. LTA binds with toll-like receptor (TLR2), which in turn activates NF‐κB and consequently translocated to nuclei from the cytoplasm [43]. Hence, these outcomes may propose that AU decreases LTA-induced inflammatory events in RAW cells via inhibiting the activation of JNK/ERK and NF‐κB pathways.
Activated oxygen (O2∗) radicals are metabolized to H2O and successively converted to H2O2 by superoxide dismutase enzymes (SOD) and then to H2O by glutathione peroxidase or to H2O2 and O2 by catalases (CAT) [44]. A previous study found that irisin, a molecule secreted from skeletal muscle in response to physical exercise, plays a regulatory role in an immune system activity and can protect the cell from free-radical-induced cellular oxidative damage by the activation of antioxidative mechanisms [45]. Furthermore, a rise in HO-1 expression was identified to exert both antioxidant and anti-inflammatory effects [44]. HO-1 plays an important role in the protection of oxidative stress in chronic disease [46]. Furthermore, HO-1 has been reported to inhibit various inflammatory responses to exhibit its cellular protective role. Several antioxidants can induce HO-1 expression to cope oxidative damage, and thus, compounds that can activate HO-1 expression may be favorable in the treatment of oxidative damage. A natural anti-inflammatory compound curcumin was found to increase the activity of CAT to protect RAW cells from LPS-induced ROS damages [47]. Reduction of reduced glutation (GSH) had reported to lead the progress of several diseases, as GSH inhibits oxidative stress-induced cell damage [48]. Therefore, we examined whether AU can involve the downstream mechanism via interaction with HO-1 to its antioxidative action. However, AU did not augment HO-1, CAT, and GSH, which postulates that antioxidant mechanisms may not associate to AU's anti-inflammatory role in LTA-induced RAW cells.
5. Conclusions
This study shows the anti-inflammatory effects of auraptene via diminishing iNOS, COX-2, IL-1β, and TNF-α expression in LTA-induced RAW 264.7 macrophages. The inhibitory property of AU is mediating at least in part via inhibiting NF-κB, along with the MAPK (JNK and ERK) pathway. Moreover, this study also found that AU's anti-inflammatory role was not depending on antioxidant mechanisms, as AU was not effective in HO-1, CAT, and GSH in the LTA-induced inflammatory RAW 264.7 cells.
Acknowledgments
This study was funded by grants from the Ministry of Science and Technology of Taiwan (MOST 107-2320-B-038-035-MY2 and MOST108-2320-B-038-031-MY3), Taipei Medical University (DP2-107-21121-N-02), Shin Kong Wu Ho-Su Memorial Hospital-Taipei Medical University (SKH-TMU-107-04), and Shin Kong Wu Ho-Su Memorial Hospital (2019SKHADR032 and 2021SKHAND005).
Data Availability
Data can be obtained from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
Chih-Hsuan Hsia, Thanasekaran Jayakumar, Wan-Jung Lu, and Joen-Rong Sheu authors are contributed equally in this work. CHH, TJ, and JRS designed work and wrote the paper. WJL, CWH, and CHH carried out the experiments. CHH, PSB, and WJL performed data analyses. WCH, MM, and YC provided interpretation. All authors approved for the final submission.
References
- 1.Tak P. P., Firestein G. S. NF-κB: a key role in inflammatory diseases. Journal of Clinical Investigation . 2001;107(1):7–11. doi: 10.1172/jci11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Askari V. R., Baradaran Rahimi V., Tabatabaee S. A., Shafiee-Nick R. Combination of Imipramine, a sphingomyelinase inhibitor, and β-caryophyllene improve their therapeutic effects on experimental autoimmune encephalomyelitis (EAE) International Immunopharmacology . 2019;77 doi: 10.1016/j.intimp.2019.105923.105923 [DOI] [PubMed] [Google Scholar]
- 3.Fu P., Birukova A. A., Xing J., et al. Amifostine reduces lung vascular permeability via suppression of inflammatory signalling. European Respiratory Journal . 2009;33(3):612–624. doi: 10.1183/09031936.00014808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Askari V. R., Shafiee-Nick R. The protective effects of β-caryophyllene on LPS-induced primary microglia M1/M2 imbalance: a mechanistic evaluation. Life Sciences . 2019;219(15):40–73. doi: 10.1016/j.lfs.2018.12.059. [DOI] [PubMed] [Google Scholar]
- 5.Huang C.-j., Wu M.-C. Differential effects of foods traditionally regarded as “heating” and “cooling” on prostaglandin E2 production by a macrophage cell line. Journal of Biomedical Science . 2002;9(6):596–606. doi: 10.1007/bf02254987. [DOI] [PubMed] [Google Scholar]
- 6.Murakami A., Shigemori T., Ohigashi H. Zingiberaceous and citrus constituents, 1′-acetoxychavicol acetate, zerumbone, auraptene, and nobiletin, suppress lipopolysaccharide-induced cyclooxygenase-2 expression in RAW264.7 murine macrophages through different modes of action. Journal of Nutrition . 2005;135(12):2987s–2992s. doi: 10.1093/jn/135.12.2987s. [DOI] [PubMed] [Google Scholar]
- 7.Rahal A., Kumar A., Singh V., et al. Oxidative stress, prooxidants, and antioxidants: the interplay. BioMed Research International . 2014;2014:19. doi: 10.1155/2014/761264.761264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baradaran Rahim V., Khammar M. T., Rakhshandeh H., Samzadeh-Kermani A., Hosseini A., Askari V. R. Crocin protects cardiomyocytes against LPS-Induced inflammation. Pharmacological Reports . 2019;71(6):1228–1234. doi: 10.1016/j.pharep.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Khosravi M., Poursaleh A., Ghasempour G., Farhad S., Najafi M. The effects of oxidative stress on the development of atherosclerosis. Biological Chemistry . 2019;400(6):711–732. doi: 10.1515/hsz-2018-0397. [DOI] [PubMed] [Google Scholar]
- 10.Wang J. E., Dahle M. K., McDonald M., Foster S. J., Aasen A. O., Thiemermann C. Peptidoglycan and lipoteichoic acid in gram-positive bacterial sepsis: receptors, signal transduction, biological effects, and synergism. Shock . 2003;20(5):402–414. doi: 10.1097/01.shk.0000092268.01859.0d. [DOI] [PubMed] [Google Scholar]
- 11.Leemans J. C., Heikens M., van Kessel K. P. M., Florquin S., van der Poll T. Lipoteichoic acid and peptidoglycan from Staphylococcus aureus synergistically induce neutrophil influx into the lungs of mice. Clinical and Vaccine Immunology . 2003;10(5):950–953. doi: 10.1128/cdli.10.5.950-953.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenfield E. M., Beidelschies M. A., Tatro J. M., Goldberg V. M., Hise A. G. Bacterial pathogen-associated molecular patterns stimulate biological activity of orthopaedic wear particles by activating cognate Toll-like receptors. Journal of Biological Chemistry . 2010;285(42):32378–32384. doi: 10.1074/jbc.m110.136895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang H.-C., Lin K.-H., Tai Y.-T., Chen J.-T., Chen R.-M. Lipoteichoic acid-induced TNF-α and IL-6 gene expressions and oxidative stress production IN macrophages are suppressed BY ketamine through downregulating toll-like receptor 2-MEDIATED activation OF ERK1/2 and NFκB. Shock . 2010;33(5):485–492. doi: 10.1097/shk.0b013e3181c3cea5. [DOI] [PubMed] [Google Scholar]
- 14.Epifano F., Genovese S., Curini M. Auraptene: phytochemical and pharmacological properties. In: Matsumoto T., editor. Phytochemistry Research Progressed . New York, NY, USA: Nova Science Publishers Inc.; 2008. pp. 145–162. [Google Scholar]
- 15.Prince M., Li Y., Childers A., Itoh K., Yamamoto M., Kleiner H. E. Comparison of citrus coumarins on carcinogen-detoxifying enzymes in Nrf2 knockout mice. Toxicology Letters . 2009;185(3):180–186. doi: 10.1016/j.toxlet.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murakami A., Nakamura Y., Tanaka T. Suppression by citrus auraptene of phorbol ester- and endotoxin-induced inflammatory responses: role of attenuation of leukocyte activation. Carcinogenesis . 2000;21(10):1843–1850. doi: 10.1093/carcin/21.10.1843. [DOI] [PubMed] [Google Scholar]
- 17.Takeda K., Utsunomiya H., Kakiuchi S., et al. Citrus auraptene reduces Helicobacter pylori colonization of glandular stomach lesions in Mongolian gerbils. Journal of Oleo Science . 2007;56(5):253–260. doi: 10.5650/jos.56.253. [DOI] [PubMed] [Google Scholar]
- 18.Soltani F., Mosaffa F., Iranshahi M., et al. Auraptene from Ferula szowitsiana protects human peripheral lymphocytes against oxidative stress. Phytotherapy Research . 2010;24(1):85–89. doi: 10.1002/ptr.2874. [DOI] [PubMed] [Google Scholar]
- 19.Epifano F., Molinaro G., Genovese S., Ngomba R. T., Nicoletti F., Curini M. Neuroprotective effect of prenyloxycoumarins from edible vegetables. Neuroscience Letters . 2008;443(2):57–60. doi: 10.1016/j.neulet.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka T., Sugiura H., Inaba R. Immunomodulatory action of citrus auraptene on macrophage functions and cytokine production of lymphocytes in female BALB/c mice. Carcinogenesis . 1999;20(8):1471–1476. doi: 10.1093/carcin/20.8.1471. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka T., Kohno H., Murakami M. Suppressing effects of dietary supplementation of the organoselenium 1,4-henylenebis(methylene)selenocyanate and the Citrus antioxidant auraptene on lung metastasis of melanoma cells in mice. Cancer Research . 2000;14:3713–3716. [PubMed] [Google Scholar]
- 22.La V. D., Zhao L., Epifano F., Genovese S., Grenier D. Anti-inflammatory and wound healing potential of citrus auraptene. Journal of Medicinal Food . 2013;16(10):961–964. doi: 10.1089/jmf.2013.0029. [DOI] [PubMed] [Google Scholar]
- 23.Woodbury W., Stahmann A. K., Stahman M. A. An improved procedure using ferricyanide for detecting catalase isozymes. Analytical Biochemistry . 1971;44(1):301–305. doi: 10.1016/0003-2697(71)90375-7. [DOI] [PubMed] [Google Scholar]
- 24.Sharma J. N., Al-Omran A., Parvathy S. S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology . 2007;15(6):252–259. doi: 10.1007/s10787-007-0013-x. [DOI] [PubMed] [Google Scholar]
- 25.Li X., Stark G. R. NF-κB-dependent signaling pathways. Experimental Hematology . 2002;30(4):285–296. doi: 10.1016/s0301-472x(02)00777-4. [DOI] [PubMed] [Google Scholar]
- 26.Singh A., Kukreti R., Saso L., Kukreti S. Oxidative stress: a key modulator in neurodegenerative diseases. Molecules . 2019;24(8):p. 1583. doi: 10.3390/molecules24081583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genovese S., Epifano F. Auraptene: a natural biologically active compound with multiple targets. Current Drug Targets . 2011;12(3):381–386. doi: 10.2174/138945011794815248. [DOI] [PubMed] [Google Scholar]
- 28.Niu X., Huang Z., Zhang L., Ren X., Wang J. Auraptene has the inhibitory property on murine T lymphocyte activation. European Journal of Pharmacology . 2015;750:8–13. doi: 10.1016/j.ejphar.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 29.Askari V. R., Baradaran Rahimi V., Rezaee S. A., Boskabady M. H. Auraptene regulates Th 1/Th 2/T Reg balances, NF-κB nuclear localization and nitric oxide production in normal and Th 2 provoked situations in human isolated lymphocytes. Phytomedicine . 2018;43:1–10. doi: 10.1016/j.phymed.2018.03.049. [DOI] [PubMed] [Google Scholar]
- 30.Askari V. R., Rahimi V. B., Zargarani R. Anti-oxidant and anti-inflammatory effects of auraptene on phytohemagglutinin (PHA)-induced inflammation in human lymphocytes. Pharmacological Reports . 2021;73:54–162. doi: 10.1007/s43440-020-00083-5. [DOI] [PubMed] [Google Scholar]
- 31.Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sciences . 2004;75(6):639–653. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 32.Lee J. K., Sayers B. C., Chun K.-S., et al. Multi-walled carbon nanotubes induce COX-2 and iNOS expression via MAP kinase-dependent and -independent mechanisms in mouse RAW264.7 macrophages. Particle and Fibre Toxicology . 2012;9(1):p. 14. doi: 10.1186/1743-8977-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao F., Chen L., Bi C., Zhang M., Jiao W., Yao X. In vitro anti-inflammatory effect of picrasmalignan A by the inhibition of iNOS and COX-2 expression in LPS-activated macrophage RAW 264.7 cells. Molecular Medicine Reports . 2013;8(5):1575–1579. doi: 10.3892/mmr.2013.1663. [DOI] [PubMed] [Google Scholar]
- 34.Okuyama S., Morita M., Kaji M., et al. Auraptene acts as an anti-inflammatory agent in the mouse brain. Molecules . 2015;20(11):20230–20239. doi: 10.3390/molecules201119691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim S. H., Smith C. J., Van Eldik L. J. Importance of MAPK pathways for microglial pro-inflammatory cytokine IL-1β production. Neurobiology of Aging . 2004;25(4):431–439. doi: 10.1016/s0197-4580(03)00126-x. [DOI] [PubMed] [Google Scholar]
- 36.Pearson G., Robinson F., Beers Gibson T., et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocrine Reviews . 2001;22(2):153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 37.Olajide O. A., Aderogba M. A., Fiebich B. L. Mechanisms of anti-inflammatory property of Anacardium occidentale stem bark: inhibition of NF-κB and MAPK signalling in the microglia. Journal of Ethnopharmacology . 2013;145(1):42–49. doi: 10.1016/j.jep.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 38.Niu X., Wang Y., Li W., et al. Esculin exhibited anti-inflammatory activities in vivo and regulated TNF-α and IL-6 production in LPS-stimulated mouse peritoneal macrophages in vitro through MAPK pathway. International Immunopharmacology . 2015;29(2):779–786. doi: 10.1016/j.intimp.2015.08.041. [DOI] [PubMed] [Google Scholar]
- 39.Guo W., Sun J., Jiang L., et al. Imperatorin attenuates LPS-induced inflammation by suppressing NF-κB and MAPKs activation in RAW 264.7 macrophages. Inflammation . 2012;35(6):1764–1772. doi: 10.1007/s10753-012-9495-9. [DOI] [PubMed] [Google Scholar]
- 40.Jayakumar T., Wu M. P., Sheu J. R. Involvement of antioxidant defenses and NF-κB/ERK signaling in anti-inflammatory effects of pterostilbene, a natural analogue of resveratrol. Applied Sciences . 2021;11:1–12. doi: 10.3390/app11104666. [DOI] [Google Scholar]
- 41.Oeckinghaus A., Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harbor Perspectives in Biology . 2009;1(4) doi: 10.1101/cshperspect.a000034.a000034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu T., Zhang L., Joo D., Sun S. C. NF-κB signaling in inflammation. Signal transduction and targeted therapy . 2017;2 doi: 10.1038/sigtrans.2017.23.17023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chuang C.-Y., Chen T.-L., Chen R.-M. Molecular mechanisms of lipopolysaccharide-caused induction of surfactant protein-A gene expression in human alveolar epithelial A549 cells. Toxicology Letters . 2009;191(2-3):132–139. doi: 10.1016/j.toxlet.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 44.Usluoğullari B., Usluogullari C. A., Balkan F., Orkmez M. Role of serum levels of irisin and oxidative stress markers in pregnant women with and without gestational diabetes. Gynecological Endocrinology: The Official Journal of the International Society of Gynecological Endocrinology . 2017;33(5):405–407. doi: 10.1080/09513590.2017.1284789. [DOI] [PubMed] [Google Scholar]
- 45.Mazur-Bialy A. I., Kozlowska K., Pochec E. Myokine irisin-induced protection against oxidative stress in vitro. Involvement of heme oxygenase-1 and antioxidazing enzymes superoxide dismutase-2 and glutathione peroxidase. Journal of Physiology and Pharmacology . 2018;69(1):117–125. doi: 10.26402/jpp.2018.1.13. [DOI] [PubMed] [Google Scholar]
- 46.Park B. G., Yoo C. I., Kim H. T., Kwon C. H., Kim Y. K. Role of mitogen-activated protein kinases in hydrogen peroxide-induced cell death in osteoblastic cells. Toxicology . 2005;215(1-2):115–125. doi: 10.1016/j.tox.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 47.Lin X., Bai D., Wei Z., et al. Curcumin attenuates oxidative stress in RAW264.7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS One . 2019;14(5) doi: 10.1371/journal.pone.0216711.e0216711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deponte M. The incomplete glutathione puzzle: just guessing at numbers and figures? Antioxidants and Redox Signaling . 2017;27(15):1130–1161. doi: 10.1089/ars.2017.7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be obtained from the corresponding author on reasonable request.


