Abstract
Background
Traditional Chinese medicine Smilax is the rhizome of liliaceous plant Smilax china L., which is used to treat pelvic inflammatory disease and anxieties.
Purpose
To investigate the mechanism of anti-inflammatory activity of the extract from Smilax china L. (ES).
Methods
The components of ES were identified by UPLC-QTOF-MS/MS. The anti-inflammatory activities were evaluated in xylene-induced ear oedema and egg white-induced plantar swelling test. Cell viability was examined by CCK-8 assay. The inflammatory mediators, proinflammatory cytokines, and MAPK and NF-κB signals in LPS-stimulated THP-1 cells were determined using ELISA, real-time PCR, and Western blot, respectively.
Results
20 compounds of ES were confirmed by comparing with the reference substance. ES displayed more prominent anti-inflammatory activity than the positive control “Jin Gang Teng” capsule in the in vivo acute inflammatory model. ES suppressed the expression of PGE2 and 6-Keot-PGF1α, and the ratio of IC50 (COX-1)/IC50 (COX-2) of ES was 3.15, which indicated that ES could selectively inhibit COX-2. ES dose-dependently (12.5, 25, and 50 mg/L) decreased the production and mRNA levels of proinflammatory cytokines IL-1β, IL-6, and TNF-α. Furthermore, ES significantly decreased LPS-induced phosphorylation of p38, JNK, ERK1/2, and p65, inhibiting the expression of IKKα and the degradation of IκBα.
Conclusion
The results suggested that ES could selectively inhibit the activity of COX-2, and the anti-inflammatory effect of ES was associated with the inhibition of IL-1β, IL-6, and TNF-α via negative regulation of MAPK and NF-κB signaling pathways in LPS-induced THP-1 cells.
1. Introduction
Inflammation is the first response occurring after damage or infection, which was regulated by many inflammatory mediators, including cyclooxygenase-2 (COX-2), prostaglandin E2 (PGE2), and proinflammatory cytokines (IL-1β, IL-6, and TNF-α) [1–3]. It has been generally accepted that the excessive production of proinflammatory cytokines and mediators due to monocytes and macrophages activation plays an important role in the progression of many inflammatory disease, such as rheumatic arthritis, chronic bronchitis, colitis, and glomerulonephritis [4]. During the inflammatory condition, monocytes and macrophages are activated; they secrete massive proinflammatory cytokines to regulate immune responses. Simultaneously, a large amount of PGE2 is generated by the inducible proteins COX-2, which induces the body's response to pain and inflammation [5].
LPS-induced THP-1 cells have been widely used as an in vitro model because THP-1 which is a human leukemia monocytic cell line can be stimulated by LPS to trigger the activation of multiple inflammatory signals such as mitogen-activated protein kinases (MAPKs) and nuclear transcription factor kappa-B (NF-κB) [6, 7]. MAPKs containing p38 MAPK, ERK, and JNK have important functions on regulation of cell differentiation, cell growth, and cellular response to cytokines in the immune system [8]. NF-κB signaling pathway plays a crucial role in regulating inflammation through transcription of COX and cytokine genes [9]. NF-κB is normally located in the cytoplasm with its inhibitor IκBα. However, LPS stimulation induces phosphorylation of IκBα leading to translocation of NF-κB into the nucleus where it activates transcription [10]. The activation of MAPK and NF-κB triggers the expression of genes encoding downstream inflammatory mediators and eventually causes inflammatory actions [11]. Smilax china L., also known as “Jin Gang Teng,” belongs to Liliaceae family. As a commonly used traditional Chinese medicine, the herb has been collected in the Chinese Pharmacopoeia (2015 version) and generally used to treat pelvic inflammatory disease, adnexitis, lump, and other diseases of gynecology clinically [12]. The efficacy of Smilax china L. has been declared on anti-inflammation [13], antinociception [14], and anticancer [15]. “Jin Gang Teng” capsule made from water extract of the herb has a good effect on gynecological inflammation, and its annual sales exceed 100 million. But the extraction rate of active components in water extract is low, and the mechanism of action is not entirely clear. The herb contains many chemical ingredients such as flavonoids, saponins, stilbene glycosides, polyphenols, etc. Previous research results showed that the flavonoids, saponins, and polyphenols of the herb are active ingredients inhibiting inflammation [16]. Our research group prepared extract using ethanol extraction and macroporous adsorption resin purification and improved the extraction and concentration rate of chemical compositions including flavonoids, saponins, tannins, etc. Although it has been reported that the herb can decrease the production of PGE2 and inhibit COX-2 activity [13], its anti-inflammatory mechanisms as well as the associated signaling pathways have not yet been fully illuminated. Therefore, in the present study, the components of ES were identified by UPLC-QTOF-MS/MS, and anti-inflammatory mechanisms of ES were investigated in LPS-induced THP-1 cells.
2. Materials and Methods
2.1. Preparation of ES
The dried powdered rhizomes of Smilax china L. (100 g) were firstly treated with 800 ml 60% ethanol 2 hours and subsequently with 600 ml 60% ethanol 1 hour for two more times. The extracts of the three treatments were collected, concentrated under reduced pressure, and filtered to get the total extracts. 70 ml 3% gelatin solution was added to the total extracts and placed statically for 24 h and then filtered. Distilled water was added to get filtrate at the concentration of 0.2 g/ml, which was subjected to D101 macroporous adsorption resin (2 BV/h) and successively eluted with 3 BV distilled water (2 BV/h) until the Molisch reaction was negative and then successively eluted with 5 BV 70% ethanol (2 BV/h). The eluate of 70% ethanol was collected and dried by rotary evaporation to obtain ES.
2.2. Chromatography
ES (0.05 g) was sonicated in 25 mL of methanol for 45 min. Prior to injection, an adequate volume (2 mL) was passed through a 0.22 μm membrane filter. A UPLC-QTOF-MS/MS system (Aglient, USA) with ZORBAX RRHD SB C18 column (100 mm × 2.1 mm, 1.8 mm) was used to detect the main compounds of ES. The following conditions were used for detection: column temperature 40°C, flow rate 0.4 mL/min, detection wavelength 290 nm (0–25 min) and 370 nm (25–30 min), mobile phase (A) methanol: acetonitrile (5 : 1), (B) 0.1% formic acid, and elution program is shown in Table 1. The injection volume was 1 μL. Mass spectrometry was used with the following conditions: sheath gas velocity 8 L per min, temperature 320°C, voltage 3.0 kV, negative ion mode, and range of measurement m/z = 100–1000.
Table 1.
The elution program.
| t/min | A : B |
|---|---|
| 0 ⟶ 5 | 12 : 88 ⟶ 15 : 85 |
| 5 ⟶ 12 | 15 : 85 ⟶ 24 : 76 |
| 12 ⟶ 15 | 24 : 76 |
| 15 ⟶ 18 | 24 : 76 ⟶ 30 : 70 |
| 18 ⟶ 20 | 30 : 70 |
| 20 ⟶ 26 | 30 : 70 ⟶ 42 : 58 |
| 26 ⟶ 28 | 42 : 58 ⟶ 60 : 40 |
| 28 ⟶ 35 | 60 : 40 ⟶ 5 : 95 |
| 35 ⟶ 37 | 5 : 95 |
2.3. In Vivo Anti-Inflammatory Activity Determination
The animal experiments were complied with the Guide for the Care and Use of Laboratory Animals (NIH Publication no. 85–23, revised 1996) and approved by the Committee of Hubei University of Chinese Medicine for Institutional Animal Care and Use. Female Wistar rats (200 ± 20 g) and female Kunming mice (18 ± 2 g) were purchased from the Hubei experimental animal center (China, License no. SCXK (E) 2008–0005). They were housed in animal rooms under standard condition of light and temperature with a 12 h/12 h light/dark cycle.
The positive control “Jin Gang Teng” capsules (JGT) were purchased from Hubei Furen Pharmaceutical Co., Ltd. (Batch no. 090329).
2.3.1. Xylene-Induced Ear Oedema
Forty female Kunming mice were randomly divided into four groups: model group (saline), JGT group (the extract at the dose equalling to 13.5 g herb/kg), and two doses of ES (the extract at the doses equalling to 13.5 and 27 g herb/kg). Four groups were administered orally once a day for 4 days. 1 h after the last administration, the left ear was coated with 0.1 mL xylene in all mice and the right ear was considered as control. After 15 min, the animals were sacrificed, the ears were cut off and weighed. The swelling degree of ear was calculated using formula: degree of era oedema (%) = (the weight of the left ear − the weight of the right ear)/the weight of the right ear × 100%; inhibiting rate of inflammation (%) = mean of model group − mean of test group.
2.3.2. Egg White-Induced Plantar Swelling
Forty female Wistar rats were randomly divided into four groups: model group (saline), JGT group (the extract at the dose equalling to 10 g herb/kg), and two doses of ES (the extract at the doses equalling to 10 and 20 g herb/kg). Four groups were administered orally once a day for 14 days. On the 14th day, the volume of left hind paw of rats was measured. 1 h after the last administration, 0.05 mL of freshly prepared 10% fresh egg white aqueous solution was injected subcutaneously into the left hind paw of rats in each group. After 6 h, the volume of left hind paw was measured and the swelling rate of paw was calculated using formula: degree of plantar swelling (%) = (the volume of left hind paw − original volume of left hind paw)/original volume of left hind paw × 100%; inhibiting rate of inflammation (%) = mean of model group − mean of test group.
2.4. Cell Culture
THP-1 human monocytic cells from China Center for Type Culture Collection (CCTCC, Wuhan, China) were cultured in RPMI 1640 medium (Hyclone, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, USA), 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were incubated in an incubator at 37°C with 95% air and 5% CO2.
2.5. Cell Viability
Cell viability was examined using cell counting kit (CCK-8) (Engreen Biosystem, BJ, China). Briefly, THP-1 cells were seeded into 96-well plates at a density of 2 × 105 cells/mL. ES was added to the cells at the indicated concentrations (0, 6.25, 12.5, 25, 50, 100, 200, and 400 μg/mL) and incubated at 37°C for 24 h. An amount of 10 μL CCK-8 reagent was added to each well and incubated at 37°C for 4 h. Absorbance was measured at 450 nm using Model 680 microplate reader (Bio-Rad, CA, USA).
2.6. Determination of 6-Keot-PGF1α and PGE2 in LPS-Induced THP-1 Cells
THP-1 cells were seeded in 96-well plates at a density of 2 × 105 cells/mL. Cells were pretreated with LPS (1 μg/mL) for 12 h, then incubated with ES (6.25, 12.5, 25, 50, 100 μg/mL) for 12 h, and finally added with AA (10 μmol/L) for 30 min. Supernatant was collected and analyzed for cytokines production by using ELISA kits (Elabscience Biotechnology Co., Ltd., Wuhan, China) according to the manufacturer's protocols. Indomethacin (National Institutes for Food and Drug Control) and meloxicam (National Institutes for Food and Drug Control) were positive control. 6-Keot-PGF1α was measured as an indicator of COX-1 activity, while PGE2 was measured as an indicator of COX-2 activity. The inhibition percentage of 6-Keot-PGF1α and PGE2 was calculated and the half-maximal inhibitory concentration (IC50) values were determined by regression analysis.
2.7. Determination of IL-1β, IL-6, and TNF-α in LPS-Induced THP-1 Cells
THP-1 cells were seeded in 96-well plates at 2 × 105 cells/mL. The cells were treated with LPS (1 μg/mL) and ES (0, 6.25, 25, and 50 μg/mL) for 12 h. Supernatant was collected and analyzed for cytokines production by using ELISA kits (Elabscience Biotechnology Co., Ltd., Wuhan, China) according to the manufacturer's protocols.
2.8. Real-Time PCR
Total RNA was extracted by Trizol RNA isolation reagent (Invitrogen). The cDNA was generated using the First-Strand cDNA Synthesis Kit (TOYOBO). The synthesized cDNA was amplified by using SYBR® Premix Ex Taq™ (Takara) and quantitative real-time PCR assays were carried out in a Bio-Rad CFX96 touch q-PCR system (Bio-Rad, USA). The primer sequences (Invitrogen Biotechnology Co., Ltd., China) used for IL-1β, IL-6, TNF-α, and GAPDH are shown in Table 2. The PCR reaction consisted of denaturation at 95°C for 1 min, followed by 40 cycles of 15 s at 95°C, 20 s at 58°C, and 20 s at 72°C. All signals were normalized with GAPDH and the mRNA relative expression was calculated according to the methods of 2−△△Ct (where △△Ct = △Ct sample − △Ct reference).
Table 2.
qRT-PCR primer sequences.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| IL-1β | 5′-AAACAGATGAAGTGCTCCTTCCAGG-3′ | 5′-TGGAAGAACACCACTTGTTGCTCCA-3′ |
| IL-6 | 5′-AGTGAGGAACAAGCCAGAGC-3′ | 5′-GCATTTGTGGTTGGGTCAG-3′ |
| TNF-α | 5′-TTCCTCAGCCTCTTCTCCTT-3′ | 5′-GCTACAGGCTTGTCACTCGG-3′ |
| GAPDH | 5′-CCATGTTCGTCATGGGTGTGAACCA-3′ | 5′-GCCAGTAGAGGCAGGGATGATGTTC-3′ |
2.9. Western Blot
THP-1 cells were seeded in 6-well plates at 1.5 × 106 cells/mL. The cells were pretreated with or without LPS (1 μg/mL) for 1 h and then treated with ES (0, 12.5, 25, and 50 μg/mL) for 1 h. After incubation, the cells were harvested by centrifugation (1500 rpm, 5 min). Proteins were extracted by RIPA lysis Kit (Beyotime) containing phenylmethanesulfonyl fluoride (PMSF). Protein concentration in the supernatant was measured using BCA protein assay kit (Takara, Japan). Equal amounts of proteins were loaded on 10% SDS-polyacrylamide gel and transferred onto nitrocellulose membranes (Amersham Biosciences, UK). After blocking in Tris-buffered saline with 0.05% Tween 20 (TBST) containing 5% nonfat milk for 1.5 h, the membranes were incubated with primary antibodies (Cell Signaling Technology) at 4°C overnight. After washing, the membranes were incubated with HRP-conjugated IgG antibodies (Cell Signaling Technology) for 1 h. Membranes were developed using ECL Western blotting detection reagent (Bio-Rad). The band intensity was measured using the FluorChem 8000 system.
2.10. Statistical Analysis
The results were presented as means ± SD. Statistical significance was determined by ANOVA and Student's t-test using SPSS software (IBM SPSS Statistics version 19.0, IBM Company). Values of P < 0.05 were considered as statistically significant.
3. Results
3.1. UPLC-QTOF-MS/MS Analysis of ES
58 compounds from ES were identified by UPLC-QTOF-MS/MS, 47 compounds were deduced by secondary mass spectrometry information, and 20 compounds were confirmed by comparing with the reference substance. The total ion chromatogram (TIC) is shown in Figure 1.
Figure 1.
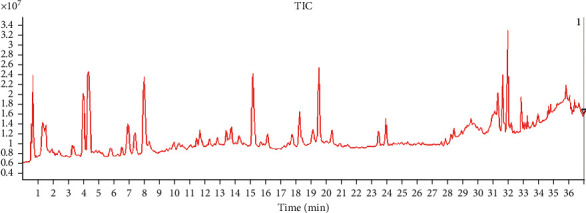
Total ion chromatogram (TIC) of ES detected by UPLC-QTOF-MS/MS.
The UPLC-QTOF-MS/MS information and structures of 20 compounds are shown in Figure 2 and Table 3, respectively.
Figure 2.
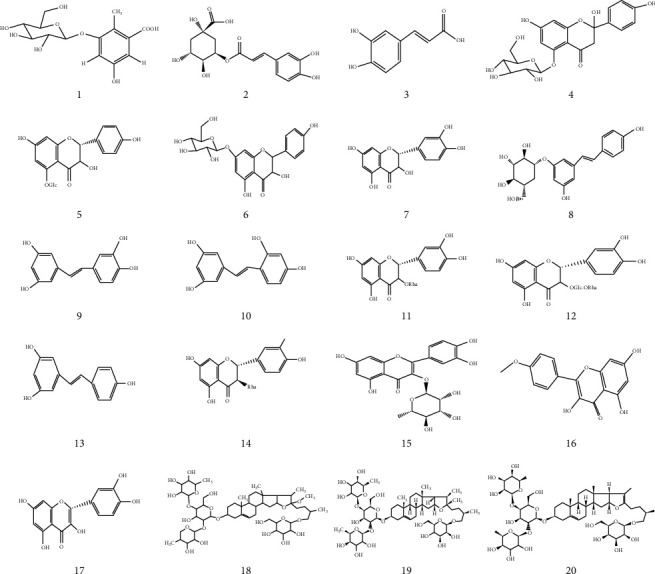
The structures of 20 compounds.
Table 3.
UPLC-QTOF-MS/MS information of 20 compounds.
| No. | t/min | [M-H]-1 (m/z) | Fragment ion (m/z) | Formula | Name |
|---|---|---|---|---|---|
| 1 | 4.004 | 329.0896 | 269, 191, 167 | C14H18O9 | 2-Methyl-5-hydroxybenzoic acid-3-O-β-D-Glucoside |
| 2 | 4.378 | 353.0908 | 191, 161, 241 | C16H18O | Chlorogenic acid |
| 3 | 4.920 | 179.0350 | 171, 135 | C9H8O4 | Caffeic acid |
| 4 | 5.827 | 449.1109 | 287, 259, 153 | C21H22O11 | 2,7,4-Trihydroxydihydroflavone-5-O-β-D-glucoside |
| 5 | 6.845 | 449.1124 | 287, 269, 153 | C21H22O11 | 7,4-Dihydroxydihydroflavonol-5-O-β-D-glucoside |
| 6 | 8.041 | 449.1138 | 287, 269, 259 | C21H22O11 | 7,4′-Dihydroxydihydroflavonol-7-O-β-D-glucoside |
| 7 | 11.076 | 303.0528 | 285, 177, 153 | C15H12O7 | Taxifolin |
| 8 | 11.806 | 389.1274 | 242, 227 | C20H22O8 | Polydatin |
| 9 | 12.806 | 243.0671 | 174, 159, 130 | C14H12O4 | Piceatannol |
| 10 | 13.689 | 243.0672 | 201, 174, 130 | C14H12O4 | Oxyresveratrol |
| 11 | 15.248 | 449.1119 | 303, 285, 241 | C21H22O11 | Astilbin |
| 12 | 17.284 | 609.1505 | 484, 301, 220 | C27H30O16 | Rutin |
| 13 | 17.759 | 227.0718 | 185, 164, 143 | C14H12O3 | Resveratrol |
| 14 | 19.438 | 433.1172 | 369, 269, 225 | C21H22O10 | Engeletin |
| 15 | 20.354 | 447.0956 | 301, 255, 179 | C21H21O11 | Quercitrin |
| 16 | 28.393 | 285.0408 | 218, 195, 151 | C15H10O6 | Kaempferide |
| 17 | 30.886 | 301.0359 | 254, 186, 118 | C15H10O7 | Quercetin |
| 18 | 30.970 | 1061.5500 | 1107, 915, 641 | C52H86O22 | Methylprotodioscin |
| 19 | 31.021 | 1047.5370 | 1093, 901, 597 | C51H84O22 | Protodioscin |
| 20 | 31.928 | 1029.5290 | 1075, 541, 883 | C51H82O21 | Pseudoprotodioscin |
3.2. In Vivo Anti-Inflammatory Activity
Applying of xylene-induced ear oedema in mice (Figure 3(a)), indicating acute inflammation. ES (the extract at the doses equalling to 13.5 and 27 g herb/kg) and JGT (the extract at the dose equalling to 13.5 g herb/kg) inhibited ear oedema with inhibiting rates of 9.70, 13.77, and 7.74%, respectively (Figure 3(a)).
Figure 3.
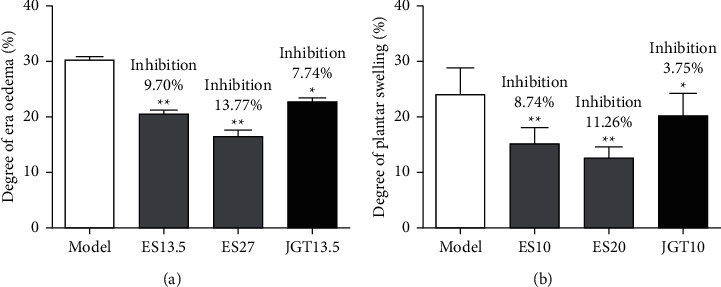
In vivo anti-inflammatory activity of ES. ES and JGT capsule inhibited xylene-induced ear oedema in mice (a) and egg white-induced plantar swelling in rats (b). Saline (model group), ES (the extract at the doses equalling to 13.5 and 27 g/kg in mice, 10 and 20 g/kg in rats), and JGT (the extract at the dose equalling to 13.5 g/kg in mice, 10 g/kg in rats) were orally administered, respectively. The mouse ear oedema and rat plantar swelling were induced, and the inhibitory activities were calculated as described in Materials and Methods, respectively. Data are presented as means ± SD (n = 10). ∗P < 0.05 and ∗∗P < 0.01 versus the model group.
Subcutaneous injection of egg white aqueous solution induced plantar swelling in rats (Figure 3(b)), indicating acute inflammation. ES (the extract at the doses equalling to 10 and 20 g herb/kg) and JGT (the extract at the dose equalling to 10 g herb/kg) inhibited plantar swelling with inhibiting rates of 8.74, 11.26, and 3.75%, respectively (Figure 3(b)).
3.3. Cytotoxicity of ES on THP-1 Cells
The effects of ES on THP-1 growth were examined by CCK-8 assay, and no cytotoxic effect was observed at 100 μg/mL concentration, while there was significant proliferation when the cells were treated with 200 and 400 μg/mL ES (Figure 4). Therefore, sample treatments between 6.25 and 100 μg/mL were used in the subsequent experiments.
Figure 4.
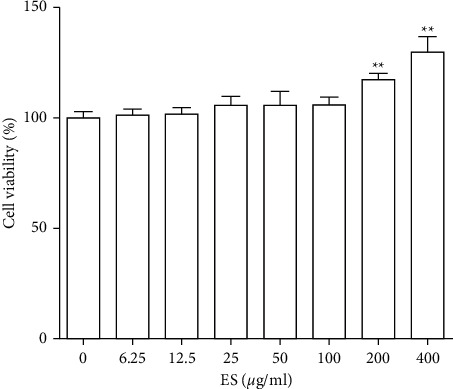
Effect of ES on the viability of THP-1 cells. Cells were treated with various concentrations (0, 6.25, 12.5, 25, 50, 100, 200, and 400 μg/mL) for 24 h and their viability was determined using a Cell Counting Kit (CCK-8). Data are presented as means ± SD (n = 3). ∗∗P < 0.01 versus the blank control group.
3.4. Selective Inhibitory Activity of COX-2
The PGE2 produced in LPS-induced THP-1 cells was quantified to determine COX-2 activity using a PGE2 standard curve, while the 6-Keot-PGF1α was quantified to determine COX-1 activity. The inhibitory activity of 6-Keot-PGF1α and PGE2 was expressed by the inhibition rate (Figure 5). IC50 was calculated, and IC50 (COX-1)/IC50 (COX-2) showed the selective inhibitory activity of COX-2.
Figure 5.
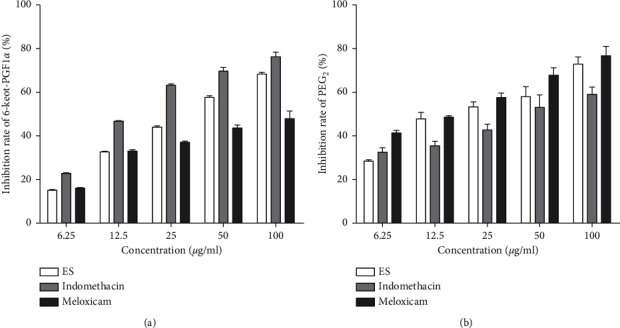
Effects of ES, indomethacin, and meloxicam on 6-Keot-PGF1α (a) and PGE2 (b) production in LPS-induced THP-1 cells. Cells were treated with ES, indomethacin, and meloxicam (6.25, 12.5, 25, 50, and 100 μg/mL) for 12 h after exposure to LPS (1 μg/mL) for 12 h. The supernatant was collected to ELISA kits. Data are presented as means ± SD (n = 3).
Treatment with ES reduced the expression of 6-Keot-PGF1α and PGE2 in a dose-dependent manner (Figure 5). Moreover, it showed that IC50 (COX-1)/IC50 (COX-2) of ES was 3.15, which was greater than 1 and close to the IC50 (COX-1)/IC50 (COX-2) of meloxicam (Figure 6 and Table 4). These results implied that ES had selective COX-2 inhibitory activity.
Figure 6.
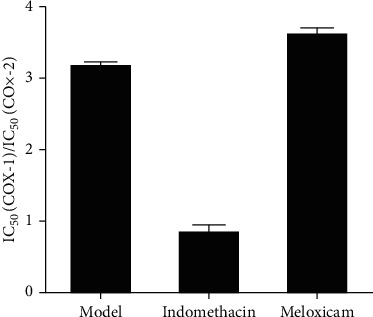
IC50 (COX-1)/IC50 (COX-2) of three drugs.
Table 4.
Selective COX-2 inhibitory activity of three drugs.
| Medicine | IC50 (COX-1) (mg/L) | IC50 (COX-2) (mg/L) | IC50 (COX-1)/IC50 (COX-2) |
|---|---|---|---|
| ES | 77.95 | 24.74 | 3.15 |
| Indomethacin | 37.86 | 45.91 | 0.82 |
| Meloxicam | 136.57 | 37.93 | 3.60 |
3.5. Effect of ES on the Expressions of Proinflammatory Cytokines IL-1β, IL-6, and TNF-α
ES dose-dependently decreased LPS-induced expression of IL-1β, IL-6, and TNF-α. Treatment with ES at the 12.5 μg/mL significantly decreased IL-1β, IL-6, and TNF-α to 27.25 ± 2.17 ng/L, 42.54 ± 2.68 ng/L, and 26.14 ± 2.93 ng/L, respectively (Figures 7(a)–7(c)). At the 50 μg/mL concentration of ES, the expressions of IL-1β, IL-6, and TNF-α were basically the same as those in the blank group. Likewise, ES significantly inhibited the expressions of IL-1β, IL-6, and TNF-α mRNA (Figures 7(d)–7(f)).
Figure 7.
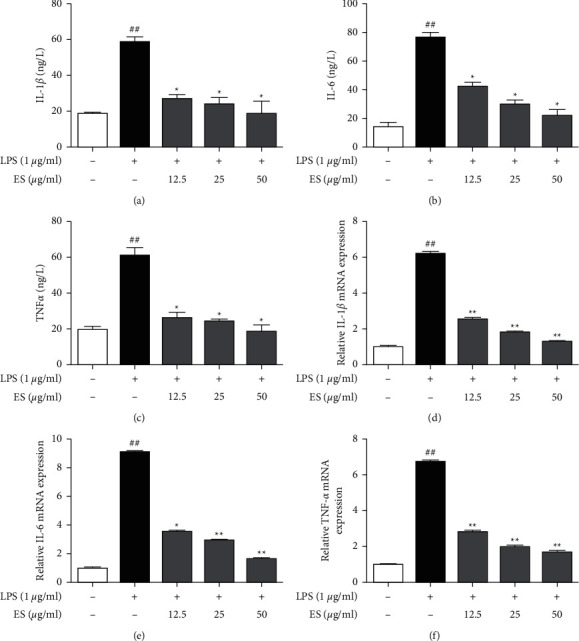
Effect of ES on proinflammatory cytokine production and gene expression in LPS-induced THP-1 cells. Cells were treated with LPS (1 μg/mL) and ES (12.5, 25, 50 μg/mL) for 12 h. The IL-1β, IL-6, and TNF-α contents were measured using ELISA kits (a–c). The mRNA expression levels of IL-1β, IL-6, and TNF-α were determined by qRT-PCR and normalized to the β-actin levels (d–f). Data are presented as means ± SD (n = 3). ##P < 0.01 versus the blank control group. ∗P < 0.05 and ∗∗P < 0.01 versus the LPS-treated group.
3.6. Effect of ES on MAPK Signaling Pathway in LPS-Induced THP-1 Cells
As presented in Figure 8, the phosphorylation levels of p38, JNK, and ERK1/2 were increased in THP-1 cells treated with LPS alone, and ES decreased LPS-induced phosphorylation of p38, JNK, and ERK1/2.
Figure 8.
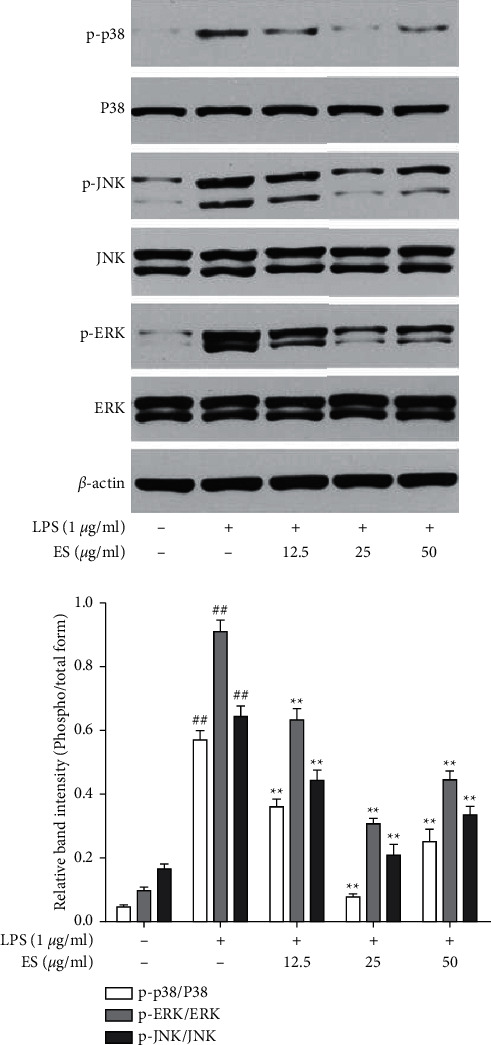
Effect of ES on MAPK signaling pathway in LPS-induced THP-1 cells. Cells were treated with ES (12.5, 25, and 50 μg/mL) for 1 h and then incubated with or without LPS (1 μg/mL) for 1 h. Protein expression was tested by western blot (##P < 0.01 versus the blank control group; ∗P < 0.05 and ∗∗P < 0.01 versus the LPS-treated group).
3.7. Effect of ES on NF-κB Signaling Pathway in LPS-Induced THP-1 Cells
To further explore the inhibiting effect of ES, its effect on NF-κB signaling pathway was investigated. The expression levels of IKKα, IκBα, and p-p65 were evaluated by Western blotting. As seen in Figure 9, ES suppressed LPS-induced expression of IKKα and degradation of IκBα and further inhibited phosphorylation of p65.
Figure 9.
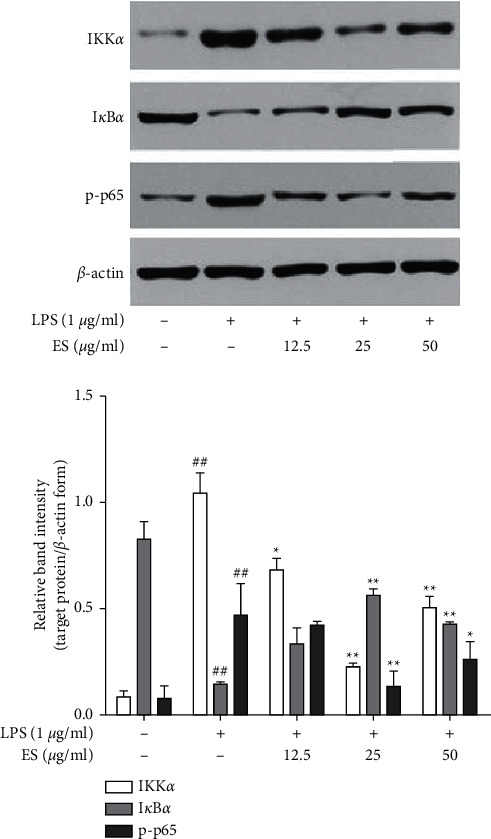
Effect of ES on the NF-κB signaling pathway in LPS-induced THP-1 cells. Cells were treated with ES (12.5, 25, and 50 μg/mL) for 1 h then incubated with or without LPS (1 μg/mL) for 1 h. Protein expression was tested by Western blot (##P < 0.01 versus the blank control group; ∗P < 0.05 and ∗∗P < 0.01 versus the LPS-treated group).
4. Discussion
The extract from Smilax china L. has been confirmed to have anti-inflammatory activities, which are likely related to flavonoids, saponins, and polyphenols. In this study, the activities of ES in equal doses were significantly better than the positive control “Jin Gang Teng” capsule in the in vivo acute inflammatory model. Furthermore, ES selectively inhibited LPS-induced overrelease of COX-2, as well as suppressed the transcription and expression of IL-1β, IL-6, and TNF-α in THP-1 cells. ES exerted this anti-inflammatory effect through negative regulating MAPK and NF-κB pathways.
Prostaglandins are important mediators of the body's response to pain and inflammation and are formed from essential fatty acids found in cell membranes. This reaction is catalysed by cyclooxygenase, a membrane-associated enzyme occurring in two isoforms: COX-1 and COX-2 [17]. The physiological prostaglandins are produced by catalytic COX-1, represented by PGI2, and PGI2 is very unstable in the body, which will be metabolized to stable 6-Keot-PGF1α. Therefore, the secretion of 6-Keot-PGF1α can reflect the activity of COX-1. The pathological prostaglandins are produced by catalytic COX-2, represented by PGE2, so the secretion of PGE2 can reflect the activity of COX-2 [18].
COX-2 is closely related to inflammation and its product PGE2 is an important inflammatory medium [19]. Classic epoxy enzyme inhibitors such as aspirin, indomethacin, and many other nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit both COX-1 and COX-2, which play anti-inflammatory effect accompanied by gastrointestinal and renal side effects [20]. COX-2 selective inhibitors such as meloxicam have a weak inhibitory activity of COX-1, consequently reducing the adverse reactions.
ES inhibited overproduction of 6-Keot-PGF1α and PGE2 induced by LPS, and the inhibition rate of PGE2 was greater than 6-Keot-PGF1α at the same concentration (Figure 5). Moreover, it showed that IC50 (COX-1)/IC50 (COX-2) of ES was 3.15, which was greater than 1 and close to the IC50 (COX-1)/IC50 (COX-2) of meloxicam (Figure 6). These results suggested that ES had selective COX-2 inhibitory activity; therefore, ES may be used as a potentially developable drug for inflammation diseases.
Proinflammatory cytokines such as IL-1β, IL-6, and TNF-α, which play crucial roles in the development of inflammatory diseases, are also involved in the innate immunity and autoimmune diseases [21]. Thus, blocking the effects of proinflammatory mediators offers an attractive therapeutic strategy. In this study, ES suppressed LPS-induced overproduction of IL-1β, IL-6, and TNF-α in a dose-dependent manner (Figures 7(a)–7(c)).
In THP-1 cells, LPS induces IL-1β, IL-6, and TNF-α transcription leading to overproduction of IL-1β, IL-6, and TNF-α; thus, the effects of ES on the expression of IL-1β, IL-6, and TNF-α mRNAs were further examined [22]. The present study revealed that ES significantly and dose-dependently suppressed the expression of IL-1β, IL-6, and TNF-α mRNAs in LPS-induced THP-1 cells (Figures 7(d)–7(f)). These results indicated that ES inhibited inflammation by regulating the gene transcription of proinflammatory cytokines.
Activation of MAPKs is known to be associated with the expression of multiple genes that together regulate the inflammatory processes. MAPK family with three major components p38, JNK, and ERK has been reported to play an important role in the upregulation of biosynthesis of IL-1β, IL-6, and TNF-α and transcriptional target enzymes (e.g., COX-2 and iNOS) [23]. Therefore, the effects of ES on LPS-induced phosphorylation of MAPKs in THP-1 cells were evaluated using Western blot analysis. As shown in Figure 8, the phosphorylation levels of p38, JNK, and ERK were increased in cells treated with LPS alone. Importantly, ES treatment significantly inhibited phosphorylation of p38, JNK, and ERK at all concentrations from 12.5 μg/mL to 50 μg/mL. These results revealed that the high activity of ES against the LPS-induced inflammatory stimuli was attributed to the inhibition of the phosphorylation of p38, JNK, and ERK, which may downregulate the levels of inflammatory mediators and cytokines in THP-1 cells. Interestingly, ES exhibited high activity at 25 μg/mL, a much better inhibition potential in comparison to the concentration of 50 μg/mL. The more pronounced effect at intermediate concentration than high concentration may be due to the multicomponent and multitarget synergistic effect, which is very common in traditional Chinese medicines [24, 25].
During the immune cells rest stage, NF-κB remains inactive as part of a complex with p65, p50, and IκBα. IκBα is an inhibitory protein that binds to the p50/p65 heterodimer in the cytoplasm. Upon stimulation by LPS or certain cytokines, IκBα is phosphorylated by the IκB kinase (IKK) and degraded, promoting the activation and phosphorylation of p65 subunit that allows the translocation of NF-κB into the nucleus and leads to the transcription of proinflammatory mediators [26]. Thus, the underlying inhibitory mechanism was then focused on the LPS-stimulated cellular NF-κB pathway and changes in the content and forms of IKK, IκB-α, and p65 were investigated. Western blot analysis indicated that NF-κB pathway was activated upon the addition of LPS with significant overexpression of IKKα and phosphorylated p65 protein as well as degradation of IκB-α (Figure 9). Moreover, there was observable inhibition of IKKα overproduction by ES at three concentrations. Simultaneously, ES treatment increased level of IκB-α and decreasd phosphorylated p65 (Figure 9). Because activation of IKK allows IκBα to be phosphorylated and ubiquitinated from p50/p65 complex, which leads to phosphorylation of p65, it is likely that ES downregulated expression of IKKα that resulted in the prevention of the degradation of IκB-α and phosphorylation of p65. In addition, this was consistent with the results of LPS-stimulated cellular MAPK pathway showing that ES was more effective at intermediate concentration on NF-κB regulated proteins. All these results implied that ES blocked the activation of the NF-κB pathway in LPS-induced THP-1 cells through inhibition of IKKα overexpression.
Based on UPLC-QTOF-MS/MS, 20 compounds from ES were explored and verified by the reference substance, which mainly were flavonoids, saponins, and tannins including taxifolin, astilbin, rutin, resveratrol, polydatin, oxyresveratrol, engeletin, quercitrin, quercetin, and methylprotodioscin. Many flavonoids, saponins, and tannins have been shown to participate in the regulation of inflammatory mediators and signal transduction pathways [27–29]. For example, resveratrol suppressed the expression of TNF-α, IL-6, and COX-2 through a decrease in the intracellular levels of ERK1/2, as well as activation of NF-κB in activated HMC-1 cells [30]. Polydatin effectively inhibited NO and PGE2 production and reduced iNOS and COX-2 expression at protein and transcriptional levels through NF-κB and MAPK pathways in LPS-induced RAW264.7 cells [31]. However, some compounds play an anti-inflammatory role by regulating specific targets. In previous studies, taxifolin suppressed expression of IL-1β and IL-6 mRNA in LPS-activated RAW264.7 cells, but not that of TNF-α [32]. Astilbin significantly suppressed production of NO and TNF-α, as well as mRNA expression of iNOS and TNF-α in LPS-induced RAW 264.7 cells, but did not affect IL-6 release or its mRNA expression [33]. Oxyresveratrol inhibited iNOS expression rather than iNOS enzyme activity through downregulation of NF-κB binding activity and significant inhibition of COX-2 activity [34]. Methylprotodioscin inhibited the activation of JNK and c-Jun while the activation of p38 MAPK, ERK, and NF-κB was not significantly affected [35]. Rutin protected spinal cord cells by reducing oxidative stress and inflammation and by decreasing the expression of proapoptotic proteins via inhibition of the p38 MAPK pathway [36]. The composition of ES is complex, and the targets and effects of each component are different. Thus, the anti-inflammatory effect of ES is likely achieved by multiple components and multiple targets.
5. Conclusions
In conclusion, ES prepared using ethanol extraction and macroporous adsorption resin purification mainly contained flavonoids, saponins, and tannins, and 20 compounds from ES were confirmed by comparing with the reference substance. ES inhibited the overproduction of 6-Keot-PGF1α and PGE2 induced by LPS and showed selective COX-2 inhibitory activity. ES decreased production and mRNA levels of proinflammatory cytokines (IL-1β, IL-6, and TNF-α). ES also blocked the activation of the MAPK and NF-κB pathways, which may be closely related to the inhibitory effects of ES on inflammatory mediators (Figure 10). Moreover, ES may exert anti-inflammatory effect through multiple components and multiple targets.
Figure 10.
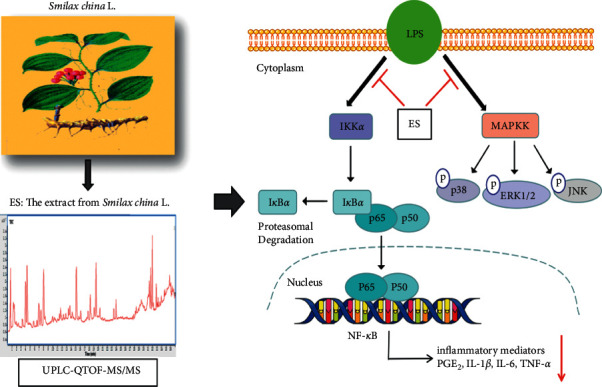
Graphical abstract. The ES was prepared using ethanol extraction and macroporous adsorption resin purification. The components of ES were identified by UPLC-QTOF-MS/MS. The anti-inflammatory effect of ES was associated with the inhibition of IL-1β, IL-6, and TNF-α via negative regulation of MAPK and NF-κB signaling pathways in LPS-induced THP-1 cells.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (no. 31370378).
Abbreviations
- ES:
The extract from Smilax china L.
- LPS:
Lipopolysaccharide
- UPLC-QTOF-MS/MS:
Ultra-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry
- JGT:
Jin Gang Teng
- COX-2:
Cyclooxygenase-2
- PGE2:
Prostaglandin E2
- NF-κB:
Nuclear factor kappa-B
- MAPKs:
Mitogen-activated protein kinases
- ERK:
Extracellular signal-regulated kinase
- JNK:
C-jun N-terminal kinase
- IL-1β:
Interleukin-1β
- IL-6:
Interleukin-6
- TNF-α:
Tumor necrosis factor
- IC50:
The half-maximal inhibitory concentration
- TIC:
Total ion chromatogram
- PGI2:
Prostaglandin I2.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Disclosure
Some research work in this manuscript has been presented as a poster in the 18th World Congress of Basic and Clinical Pharmacology (WCP2018).
Conflicts of Interest
The authors declare that they have no conflicts of interest regarding the publication of this paper.
Authors' Contributions
Siyi Jianga and Qiong Wei contributed equally to this work.
References
- 1.Choi R. J., Shin E. M., Jung H. A., Choi J. S., Kim Y. S. Inhibitory effects of kaurenoic acid from Aralia continentalis on LPS-induced inflammatory response in RAW264.7 macrophages. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology . 2011;18:677–682. doi: 10.1016/j.phymed.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Jiang F., Guan H. N., Liu D. Y., Wu X., Fan M. C., Han J. C. Flavonoids from sea buckthorn inhibit the lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages through the MAPK and NF-κB pathways. Food and Function . 2017;22:1313–1322. doi: 10.1039/c6fo01873d. [DOI] [PubMed] [Google Scholar]
- 3.Pham T. H., Kim M. S., Le M. Q., et al. Fargesin exerts anti-inflammatory effects in THP-1 monocytes by suppressing PKC-dependent AP-1 and NF-kB signaling. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology . 2017;24:96–103. doi: 10.1016/j.phymed.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Liung T., Lundberg S., Varasanyi M., et al. Rectal nitric oxide as biomarker in the treatment of inflammatory bowel disease: responders versus nonresponders. World Journal of Gastroenterology . 2006;112:3386–3392. doi: 10.3748/wjg.v12.i21.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeong D. H., Kim K. B., Kim M. J., Kang B. K., Ahn D. H. Skipjack tuna (Katsuwonus pelamis) eyeball oil exerts an anti-inflammatory effect by inhibiting NF-kappaB and MAPK activation in LPS-induced RAW 264.7 cells and croton oil-treated mice. International Immunopharmacology . 2016;40:50–56. doi: 10.1016/j.intimp.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Chanput W., Mes J. J., Wichers H. J. THP-1 cell line: an in vitro cell model for immune modulation approach. International Immunopharmacology . 2014;23:37–45. doi: 10.1016/j.intimp.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Sharif O., Bolshakov V. N., Raines S., Newham P., Perkins N. D. Transcriptional profiling of the LPS induced NF-kappaB response in macrophages. BMC Immunology . 2007;8:1–17. doi: 10.1186/1471-2172-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park H. H., Kim M. J., Li Y., et al. Britanin suppresses LPS-induced nitric oxide, PGE2 and cytokine production via NF-kappaB and MAPK inactivation in RAW 264.7 cells. International Immunopharmacology . 2013;15:296–302. doi: 10.1016/j.intimp.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Israf D. A., Tham C. L., Syahida A., et al. Atrovirinone inhibits proinflammatory mediator synthesis through disruption of NF-κB nuclear translocation and MAPK phosphorylation in the murine monocytic macrophage RAW 264.7. Phytomedicine : International Journal of Phytotherapy and Phytopharmacology . 2010;17:732–739. doi: 10.1016/j.phymed.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Cho E. J., An H. J., Shin J. S., et al. Roxatidine suppresses inflammatory responses via inhibition of NF-kappaB and p38 MAPK activation in LPS-induced RAW 264.7 macrophages. Journal of Cellular Biochemistry . 2011;112:3648–3659. doi: 10.1002/jcb.23294. [DOI] [PubMed] [Google Scholar]
- 11.Kang S. R., Park K. I., Park H. S., et al. Anti-inflammatory effect of flavonoids isolated from Korea Citrus aurantium L. on lipopolysaccharide-induced mouse macrophage RAW 264.7 cells by blocking of nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) signalling pathways. Food Chemistry . 2011;129:1721–1728. doi: 10.1016/j.foodchem.2011.06.039. [DOI] [Google Scholar]
- 12.Jiang S. Y., Song X. Y., Zhang D. D., et al. Spectrum-effect relationship between UPLC fingerprint of Smilax China and anti-pelvic inflammation in rats. China Journal of Chinese Materia Medica . 2019;44:3323–3329. doi: 10.19540/j.cnki.cjcmm.20190523.302. [DOI] [PubMed] [Google Scholar]
- 13.Li X., Chu L. L., Liu S. S., Zhang W. K., Lin L. Z., Zheng G. D. Smilax China L. flavonoid alleviates HFHS-induced inflammation by regulating the gut-liver axis in mice. Phytomedicine . 2021 doi: 10.1016/j.phymed.2021.153728. [DOI] [PubMed] [Google Scholar]
- 14.Li X., Yang L., Xu M., Qiao G., Zheng G. Smilax China L. polyphenols alleviates obesity and inflammation by modulating gut microbiota in high fat/high sucrose diet-fed C57BL/6J mice. Journal of Functional Foods . 2021;77 doi: 10.1016/j.jff.2020.104332.104332 [DOI] [Google Scholar]
- 15.Tettey C. O., Yang I. J., Shin H. M. Smilax China leaf extracts suppress pro-inflammatory adhesion response in human umbilical vein endothelial cells and proliferation of HeLa cells. Archives of Physiology and Biochemistry . 2018;126:287–291. doi: 10.1080/13813455.2018.1520262. [DOI] [PubMed] [Google Scholar]
- 16.Zheng X. W., Zhang L., Zhang W. Y., et al. Two new xanthones, a new lignin, and twenty phenolic compounds from Smilax China and their NO production inhibitory activities. Journal of Asian Natural Products Research . 2019;22:509–520. doi: 10.1080/10286020.2019.1598395. [DOI] [PubMed] [Google Scholar]
- 17.Taylor J. L. S., van Staden J., Jäger A. K. COX-1 and COX-2 inhibitory activity in extracts prepared from Eucomis species, with further reference to extracts from E. autumnalis autumnalis. South African Journal of Botany . 2002;68:80–85. doi: 10.1016/S0254-6299(15)30446-4. [DOI] [Google Scholar]
- 18.Hu Y. F., Cheng G. F. Establishment of screening models for selective cyclooxygenase-2 inhibitors. Acta Pharmaceutica Sinica . 2000;35:343–346. doi: 10.16438/j.0513-4870.2000.05.006. [DOI] [Google Scholar]
- 19.de Leval X., Delarge J., Devel P., et al. Evaluation of classical NSAIDs and COX-2 selective inhibitors on purified ovine enzymes and human whole blood. Prostaglandins, leukotrienes, and essential fatty acids . 2001;64:211–216. doi: 10.1054/plef.2001.0262. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell J. A., Akarasereenont P., Thiemermann C., Flower R. J., Vane J. R. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proceedings of the National Academy of Sciences of the United States of America . 1993;90:11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li D., Chen J., Ye J., et al. Anti-inflammatory effect of the six compounds isolated from Nauclea officinalis Pierrc ex Pitard, and molecular mechanism of strictosamide via suppressing the NF-kappaB and MAPK signaling pathway in LPS-induced RAW 264.7 macrophages. Journal of Ethnopharmacology . 2017;196:66–74. doi: 10.1016/j.jep.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Yang E. J., Yim E. Y., Song G., Kim G. O., Hyun C. G. Inhibition of nitric oxide production in lipopolysaccharide-activated RAW 264.7 macrophages by Jeju plant extracts. Interdisciplinary Toxicology . 2009;2:245–249. doi: 10.2478/v10102-009-0022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Y., Kim S. C., Yu T., et al. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediators of Inflammation . 2014;2014 doi: 10.1155/2014/352371.352371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Q. H., Li B. T., Tu T. Compound traditional Chinese medicine in treatment of diabetes. China Journal of Chinese Materia Medica . 2019;44:1104–1109. doi: 10.19540/j.cnki.cjcmm.20181212.001. [DOI] [PubMed] [Google Scholar]
- 25.Weng X. G., Li Y. J., Chen Y., et al. Research initiative of new thought on “main effect” of TCM formulae--new thinking on mechanism of compound action and compatibility mechanism of Chinese herbal compound formulae. China Journal of Chinese Materia Medica . 2018;43:3782–3786. doi: 10.19540/j.cnki.cjcmm.20180522.001. [DOI] [PubMed] [Google Scholar]
- 26.Lo J. Y., Kamarudin M. N., Hamdi O. A., Awang K., Kadir H. A. Curcumenol isolated from Curcuma zedoaria suppresses Akt-mediated NF-kappaB activation and p38 MAPK signaling pathway in LPS-stimulated BV-2 microglial cells. Food and Function . 2015;6:3550–3559. doi: 10.1039/c5fo00607d. [DOI] [PubMed] [Google Scholar]
- 27.Ribeiro D., Freitas M., Lima J. L. F. C., Fernandes E. Proinflammatory pathways: the modulation by flavonoids. Medicinal Research Reviews . 2015;35:877–936. doi: 10.1002/med.21347. [DOI] [PubMed] [Google Scholar]
- 28.Tunon M., Garcia-Mediavilla M., Sanchez-Campos S., Gonzalez-Gallego J. Potential of flavonoids as anti-inflammatory agents: modulation of pro- inflammatory gene expression and signal transduction pathways. Current Drug Metabolism . 2009;10:256–271. doi: 10.2174/138920009787846369. [DOI] [PubMed] [Google Scholar]
- 29.Yao Y., Yang X., Shi Z., Ren G. Anti-inflammatory activity of saponins from Quinoa (Chenopodium quinoa Willd.) Seeds in lipopolysaccharide-stimulated RAW 264.7 macrophages cells. Journal of Food Science . 2014;79:H1018–H1023. doi: 10.1111/1750-3841.12425. [DOI] [PubMed] [Google Scholar]
- 30.Kang O. H., Jang H. J., Chae H. S., et al. Anti-inflammatory mechanisms of resveratrol in activated HMC-1 cells: pivotal roles of NF-kappaB and MAPK. Pharmacological Research . 2009;59:330–337. doi: 10.1016/j.phrs.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Lou T., Jiang W., Xu D., Chen T., Fu Y. Inhibitory effects of polydatin on lipopolysaccharide-stimulated RAW 264.7 cells. Inflammation . 2015;38:1213–1220. doi: 10.1007/s10753-014-0087-8. [DOI] [PubMed] [Google Scholar]
- 32.Rhee H., Endale M., Kamruzzaman S. M., et al. Taxifolin inhibited the nitric oxide production and expression of pro-inflammatory cytokine mRNA in lipopolysaccharide-stimulated RAW264.7 cells. Journal of Biomedical Science . 2008;14:147–155. [Google Scholar]
- 33.Lu C. L., Zhu Y. F., Hu M. M., et al. Optimization of astilbin extraction from the rhizome of Smilax glabra, and evaluation of its anti-inflammatory effect and probable underlying mechanism in lipopolysaccharide-induced RAW264.7 macrophages. Molecules . 2015;20:625–644. doi: 10.3390/molecules20010625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung K. O., Kim B. Y., Lee M. H., et al. In-vitro and in-vivo anti-inflammatory effect of oxyresveratrol from Morus alba L. Journal of Pharmacy and Pharmacology . 2003;55:1695–1700. doi: 10.1211/0022357022313. [DOI] [PubMed] [Google Scholar]
- 35.Lee J. H., Lim H. J., Lee C. W., et al. Methyl protodioscin from the roots of Asparagus cochinchinensis attenuates airway inflammation by inhibiting cytokine production. Evidence-Based Complementary and Alternative Medicine . 2015;2015 doi: 10.1155/2015/640846.640846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song H. L., Zhang X., Wang W. Z., et al. Neuroprotective mechanisms of rutin for spinal cord injury through anti-oxidation and anti-inflammation and inhibition of p38 mitogen activated protein kinase pathway. Neural Regeneration Research . 2018;13:128–134. doi: 10.4103/1673-5374.217349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


