Abstract
Vitamin D3, as an indispensable and fat-soluble micronutrient, plays an important role in the health of humans and animals. At present, studies are focusing on the calcium absorption and immunoregulation function of vitamin D3; this study was aimed at exploring the antioxidative stress ability of vitamin D3 on diquat-induced intestinal dysfunction of ICR mice and the underlying mechanism. The results showed that oral gavage of vitamin D3 daily significantly improved the body weight gain and immune organ index and significantly reverted the abnormal changes of ALT, AST, SOD, GSH-Px, T-AOC, and MDA in the serum and jejunum induced by diquat. The addition of vitamin D3 also significantly reduced the concentration of DAO, D-LA, and certain proinflammatory cytokines in serum. Moreover, vitamin D3 improved the pathological morphology of the duodenum, jejunum, colon, liver, and kidney tissues, and it also largely attenuated the degree of inflammatory infiltration of macrophages and cell apoptotic index of jejunal epithelial tissue induced by diquat. The results demonstrated that vitamin D3 significantly recovered the intestinal barrier injury by enhancing the expression of mucins and tight junction proteins in the jejunum. In addition, the results indicated that vitamin D3 could significantly reduce the phosphorylation level of NF-κB (p65) and enhance the expression of Nrf2 and HO-1 in the jejunum compared with the diquat-induced group. This study suggested that oral administration of vitamin D3 can protect mice against oxidative damage by inhibiting the phosphorylation level of NF-κB (p65) and activating Nrf2-related signaling pathways.
1. Introduction
The active form of vitamin D3 (VitD3) is 1α,25-(OH)2-D3, which forms 25-hydroxyvitamin D3 under the catalysis of 25-hydroxylase in the liver and further forms 1α,25-(OH)2-D3 through the action of 1-α hydroxylase in the kidney [1]. VitD3 is a fat-soluble vitamin type that plays an important role in many physiological processes related to human health; the lack of VitD3 would inhibit the absorption of calcium and phosphorus in the small intestine and cause the reduction of bone mineral density, which increases the risk of fracture [2]. Apart from the nutritional effects, the immunoregulation function of VitD3 has also been widely reported. VitD3 has a high affinity for the nuclear vitamin D receptor (VDR), which could initiate the operation of vitamin D response elements and upregulate the transcriptional activity. Through the VDR-related pathway, VitD3 could be involved in regulating the innate and adaptive immune systems efficiently [3]. The confirmed evidence had demonstrated that VitD3 could effectively enhance the expression level of human LL-37 antimicrobial peptide in several cell types, which contributes to the anti-infective and antibacterial functions [4, 5]. VDR- and VitD3-related metabolic enzymes are broadly expressed among macrophagocytes, dendritic cells (DC), and activated lymphocytes, which further verifies the immunomodulatory effects of VitD3 [6]. VitD3 could reduce the secretion of M1-type proinflammatory cytokines and promote the polarization type from M1 to M2 [7]. The addition of VitD3 to mice with ulcerative colitis could ameliorate the inflammatory status by reducing the infiltration of macrophages [8]. It is also reported that VitD3 could downregulate the expression of MHC II, costimulatory molecules, and IL-12 to inhibit the differentiation and maturation of DC, finally blocking the activation of T lymphocytes [9]. As to the effects of VitD3 on lymphocytes, it was reported that VitD3 could suppress the proliferation of Th1 lymphocytes and reduce the secretion of proinflammatory cytokines such as IL-2, IL-6, and IL-17, while it could enhance the activity of Th2 lymphocytes and promote the expression of IL-4 and IL-10 [10]. Pretreatment of VitD3 on B lymphocytes could decrease the activation degree of CD40L and further reduce the production of T lymphocyte-originated proinflammatory cytokines [11].
The occurrence of oxidative stress (OS) in the body indicates the overproduction of reactive oxygen species (ROS) and will cause many diseases [12]. For instance, oxidative stress could destroy the structure of the intestine and lead to the dysfunction of the intestinal barrier. As a consequence, the inflammatory status will aggravate both the intestine and the whole circulatory system. The ROS leaked from the intestine may transfer to other parenchymal organs such as the liver, kidney, and spleen through blood, which could attack these organs and result in dysfunction [13]. Diquat (DQ), as an oxidant, is an ideal inducer to establish animal models of oxidative stress and has been widely used in different animals to duplicate the oxidative damage models successfully [14–16]. DQ could utilize molecular oxygen to produce a large amount of superoxide anion free radicals and attack the mitochondria to generate excess ROS [17], which could in turn cause oxidative damage and inflammation of the body.
The nutritional and anti-infective effects of VitD3 have been reported; however, there are few studies on the antioxidant stress function and the possible mechanism of VitD3. Therefore, this study was conducted to evaluate the protective effect of vitamin D3 on diquat-induced oxidative damage in the ICR mouse model; the NF-κB/Nrf2/HO-1 signaling pathway was also investigated to explore the underlying mechanism of vitamin D3 exerting antioxidant effects.
2. Materials and Methods
2.1. Reagents
The active form of VitD3 (1α,25-(OH)2-D3) (Catalog number V8070) was purchased from Solarbio (Beijing, China) with a purity ≥ 99%. Diquat (Catalog number D101258) was purchased from Aladdin (Shanghai, China). The kits to determine the activity or concentration of aspartate aminotransferase (AST) (Catalog number MK5778A), alanine aminotransferase (ALT) (Catalog number MK5727A), superoxide dismutase (SOD) (Catalog number MK3125A), glutathione peroxidase (GSH-Px) (Catalog number MK5870A), total antioxidant capacity (T-AOC) (Catalog number MK4580A), and malondialdehyde (MDA) (Catalog number MK5892A) in the serum and jejunum were purchased from Boster (Wuhan, China). The kits to determine the activity of diamine oxidase (DAO) (Catalog number A088-2-1) and the concentration of D-lactic acid (D-LA) (Catalog number H263) in serum were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The kits to detect the concentration of TNF-α (Catalog number EK0527), IL-1β (Catalog number EK0394), IL-6 (Catalog number EK0411), and IL-10 (Catalog number EK0417) in serum were purchased from Boster (Wuhan, China). The anti-F4/80 antibody (Catalog number sc-377009) used for immunohistochemical analysis was purchased from Santa Cruz (Northern California, USA). The TUNEL kit (Catalog number 11684809910) used for in situ apoptosis degree determination was purchased from Roche (Basel, Switzerland). The anti-ZO-1 antibody (Catalog number ab221547) used for immunofluorescence analysis was purchased from Abcam (Cambridge, England). The protein extraction kit (Catalog number KGP2100) and butyl cyanoacrylate (BCA) kit (Catalog number KGP902) used for extracting and determining the concentration of protein were purchased from KeyGen (Nanjing, China). The primary antibodies used for determining the protein expression level of NF-κB (p65) (Catalog number A00284-3), NF-κB (p-p65) (Catalog number BM5404), Nrf2 (Catalog number PB9290), HO-1 (Catalog number BM4010), and GAPDH (Catalog number A00227) were purchased from Boster (Wuhan, China). The TRITC-conjugated goat anti-rabbit IgG for ZO-1 (Catalog number 111-026-047) and HRP-conjugated rabbit anti-goat IgG (Catalog number MBS584630) were purchased from JIR (Scottsdale, USA). The related primers used for real-time PCR analysis were synthesized by Sangon Biotech (Shanghai, China). The TRIzol reagent (Catalog number 15596018) used for RNA isolation was purchased from Invitrogen (Carlsbad, USA). The M-MuLV reverse transcriptase kit (Catalog number 18057018) was purchased from Fermentas (Glen BURNIE, USA). The SYBR Premix Ex Taq Kit (Catalog number RR420A) was purchased from Takara Biotechnology (Shiga, Japan).
2.2. Animal Experiments
The animal experiment was approved by the Animal Welfare and Ethics Committee of Hainan University (permit number: HNUAUCC-2020-00068) and conducted according to the National Institutes of Health guidelines for the care and treatment process of experimental animals strictly. Forty male ICR mice aged 7 weeks (24 ± 2 g) were purchased from the Experimental Animal Center of Hainan Medical School and raised for a one-week adaptation period. At the beginning of the formal experimental period, the mice were allotted to four groups (n = 10) randomly, as shown in the schematic diagram (Figure 1(b)): control (CK) group, VitD3 addition alone (VD) group, diquat-induced model (Di) group, and VitD3 addition+diquat (VD+Di) group. Firstly, the mice in the VD and VD+Di groups were administrated 100 ng VitD3 daily (1 mg VitD3 was dissolved in 1 mL 0.2% ethanol, and 2 μL VitD3 solution was taken into 1 mL PBS to make the final concentration of VitD3 as 2 ng/μL) through oral gavage. The other groups were given the same volume of PBS. On days 1 and 4, the Di and VD+Di groups were administered 10 mg diquat per kg B.W. suspended in 200 μL volume of PBS through intraperitoneal injection. The other groups were injected with the same volume of PBS. Ad libitum feeding and drinking were assured during the experimental period. The average body weight gain of each group was recorded daily.
Figure 1.
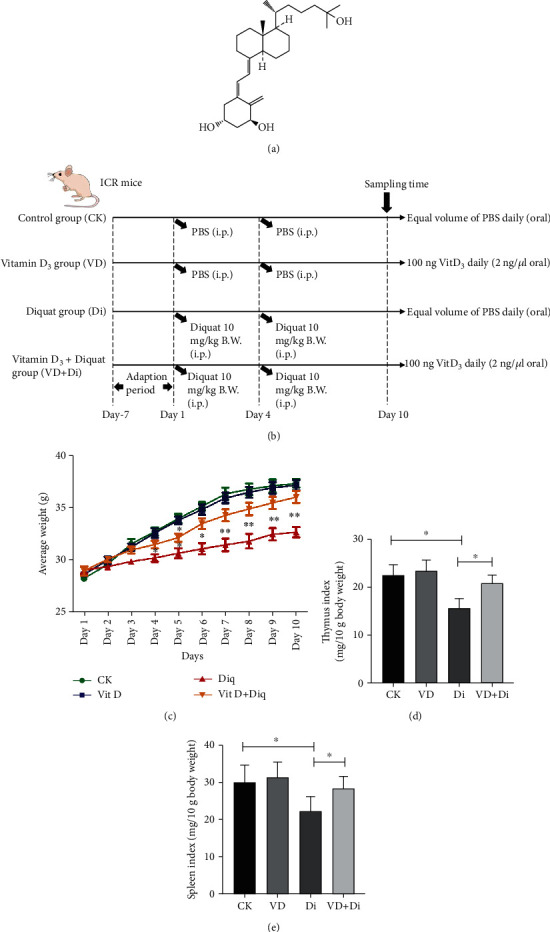
(a) The molecular structure of 1α,25-(OH)2-D3. (b) The experimental design and schematic diagram of treatments to each group. (c) Average body weight change of each group. (d) Thymus index and (e) spleen index of each group. All data were presented as mean ± SEM (n = 10). ∗P < 0.05, ∗∗P < 0.01.
2.3. Sample Collection
On day 10, mice were sacrificed under the status of anesthesia through pentobarbital sodium injection. The blood was collected by breaking the posterior orbital venous plexus. The serum of each group was collected by centrifuging at 3500 rpm for 5 min under the temperature of 4°C. A part of the duodenum, jejunum, colon, liver, and kidney tissues was cut out and fixed with 4% paraformaldehyde immediately to prepare the paraffin section for further analysis. Another part of the jejunum was used as the tissue homogenate to determine the activity of antioxidase and the concentration of protein involved with antioxidant regulation. The residual jejunum tissue was frozen into liquid nitrogen immediately and then transferred to a -80°C refrigerator for further analysis of the relative expression level of the corresponding mRNA.
2.4. Determination of the Immune Organ Index
Before the execution of the mice, the individual weight of mice in each group was recorded, and the corresponding spleen and thymus were isolated and weighed without blood on organ surfaces (using absorbent paper to dry the surface blood). The immune organ index (IOX) was calculated using the following formula: IOX = weight of the immune organ (mg)/body weight (g) × 10.
2.5. Detection of the Oxidative Damage Index and Inflammatory Cytokines in Serum
The activities of ALT, AST, and DAO in serum were determined through an ELISA kit. The concentrations of D-LA, IL-1β, IL-6, TNF-α, and IL-10 in serum were determined using an ELISA kit. All the assays were carried out according to the manufacturer's instructions. To introduce briefly, the diluted samples were added to the appointed hole in the ELISA plate and mixed with the corresponding HRP-marked antibody. After the reaction lasted for 50 minutes, the reaction fluid in the ELISA plate was discarded and washed with PBS 5 times. Then, the substrate was added in order and reacted for 20 minutes under 37°C without light. Finally, the terminating fluid was added to stop the reaction and the OD value was recorded under the wavelength of 450 nm by a microplate reader (SpectraMax M5, Molecular Devices, USA).
2.6. Determination of Antioxidative Enzymes in the Serum and Jejunum
The activities of SOD, GSH-Px, and T-AOC in the serum and jejunum tissues were determined by an ELISA kit. The concentration of MDA in the serum and jejunum was analyzed by an ELISA kit. All the assays were carried out according to the manufacturer's instructions.
2.7. Evaluation of Morphology in the Duodenum, Jejunum, Colon, Liver, and Kidney
The middle site of the duodenum, jejunum, and colon and the representative part of the liver and kidney were separated to carry out hematoxylin and eosin (H&E) staining. Briefly, the samples taken out from 4% paraformaldehyde solution were embedded in paraffin and cut into slices. Then, the deparaffinized slices were stained with hematoxylin and eosin in turn. The morphological characteristics were observed using a Leica NEWDM 4500BR microscope (Leica, Frankfurt, Germany) under different magnifications.
2.8. Analysis of the Inflammatory Infiltration of Macrophages in the Jejunum Tissue
The middle part of the jejunum was isolated for immunohistochemical analysis of macrophage infiltration by a specific antigen marker of F4/80. The sections embedding in paraffin were deparaffinized and rehydrated with distilled water. 1% w/v BSA was applied to block the nonspecific binding sites for 30 min, and the anti-F4/80 antibody was incubated overnight at 4°C with the section at a dilution of 1 : 100. Further, the sections were washed with PBS and treated with HRP-conjugated rabbit anti-goat IgG at the ratio of 1 : 1000, incubated for another 1 h at 4°C, and washed with PBS.
2.9. Determination of the Apoptotic Level of the Jejunal Epithelium
The same part of the jejunum was used for the analysis of the apoptosis degree of the jejunal epithelium. To introduce briefly, the samples were deparaffinized and xylene was used to increase the transparency of slices. TdT and dUTP were mixed at a ratio of 1 : 9 and incubated with the slices for 60 min at 37°C. The endogenous peroxidase was blocked, and the slices were allowed to dry naturally. The slices were then covered with converter peroxidase and incubated for another 37°C for 30 min. DAB was added to the slices, and distilled water was used to stop color development. The cell nucleus was then stained using hematoxylin as a counterstain, and the slices were dehydrated and mounted.
2.10. Detection of the In Situ Expression Level of ZO-1 in the Jejunum Tissue
To determine the expression abundance of ZO-1 in the jejunum, the jejunum tissues kept in 4% paraformaldehyde were prepared as paraffin sections for immunofluorescence analysis. To introduce briefly, the slices were deparaffinized and antigen retrieval was carried out. Further, the slices were incubated with 3% hydrogen dioxide in the darkroom and then incubated with a primary antibody specific for ZO-1 at the dilution ratio of 1 : 200. Finally, TRITC-conjugated goat anti-rabbit IgG for ZO-1 was incubated with the slices for 1 h in the darkness and DAPI was then used to stain the nucleus directly. Images were taken under a Leica fluorescence microscope (Leica, Frankfurt, Germany).
2.11. Analysis of the mRNA Expression Level of Mucins and Tight Junction Proteins in the Jejunum
The residual jejunum tissues preserved in the -80°C refrigerator were taken out, and RNA samples were extracted using the TRIzol reagent. cDNA synthesis was conducted by the M-MuLV reverse transcriptase kit. The relative mRNA expression level of each gene was determined by the real-time PCR disposing process using the SYBR Premix Ex Taq Kit under the ABI StepOne Plus Real-Time PCR System (Applied Biosystems, CA, USA). Data was analyzed based on the comparative threshold cycle (Ct) method and normalized to the housekeeping gene GAPDH. The corresponding primers used in the experiment are listed in Table 1; the relative mRNA expression levels of mucins (Mucin-1, Mucin-2) and tight junction genes (ZO-1, ZO-2, Claudin-1, and Occludin) were determined on jejunum samples.
Table 1.
The primers and sequences of qPCR.
| Gene | Product size (bp) | Sequence (5′⟶3′) | Accession number (5′⟶3′) |
|---|---|---|---|
| GAPDH | 212 | F: GAGAAACCTGCCAAGTATGATGAC | NM_008084.3 |
| ZO-1 | 198 | R: TAGCCGTATTCATTGTCATACCAG F: TCATCCCAAATAAGAACAGAGC R: GAAGAACAACCCTTTCATAAGC |
XM_006540786.1 |
| ZO-2 | 269 | F: GCTTTGGTGTGGACCAAGAT R: TCCATTATGGGTTTGCATGA |
XM_006526909.1 |
| Claudin-1 | 110 | F: GCTGGGTCATCCTGGCTTCT R: CCTGAGCGGTCACGATGTTGTC |
NM_016674.4 |
| Occludin | 86 | F: CTTTGGCTACGGAGGTGGCTAT R: CTTTGGCTGCTCTTGGGTCTG |
NM_006517566.1 |
| Mucin-1 | 147 | F: TGGATTGTTTCTGCAGATTTT R: CCTGACCTGAACTTGATGCT |
NM_013605.2 |
| Mucin-2 | 134 | F: CCCAGAAGGGACTGTGTATG R: TGCAGACACACTGCTCACA |
NM_023566.3 |
2.12. Detection of the Protein Expression Level of NF-κB (p65)- and Nrf2-Related Proteins in the Jejunum
The protein extraction kit was used to isolate the total protein from the jejunum, and the concentration of protein was determined by the BCA assay kit. The protein expression levels of NF-κB (p65), NF-κB (p-p65), Nrf2, HO-1, and GAPDH were evaluated by western blot analysis according to the previous method reported [18]. The density of protein bands was visualized by the ECL substrate kit (Bio-Rad, CA, USA) through Champchemi 500 Plus (Saizhi, Beijing, China) and quantified using the ImageJ analyzer software.
2.13. Statistical Analysis
Multiple comparison tests were carried out by one-way analysis of variance with GraphPad prism (version 7.0, San Diego, USA). The value of P < 0.05 (∗) was considered significant, and the value of P < 0.01 (∗∗) was considered highly significant. Results were expressed as mean ± standard error (SEM).
3. Results
3.1. The Effects of VitD3 on the Growth Performance and Immune Organ Index Induced by Diquat
As shown in Figure 1(c), oral gavage of VitD3 alone did not affect the average weight during the experimental period. Compared with the CK group, the average weight of the Di group was significantly lower (P < 0.05) since day 4. From day 7 to day 10, the average weight between the Di group and the CK group showed a highly significant difference (P < 0.01). Meanwhile, the average weight of the VD+Di group showed a normal level except on day 5, which presented a significant difference (P < 0.05) compared with the CK group. As shown in Figures 1(d) and 1(e), the thymus index and spleen index of the Di group were significantly lower than that of both the CK and VD+Di groups (P < 0.05), which indicated the protective effect of VitD3 to immune organs.
3.2. The Effects of VitD3 on the Oxidative Damage Index Challenged by Diquat
As shown in Figures 2(a) and 2(b), the activities of ALT and AST in serum of the Di group showed a highly significant difference compared with those of the CK group (P < 0.01). Compared with the Di group, the activities of ALT and AST in serum of the VD+Di group were significantly lower (P < 0.05), and there remained a significant difference between the CK and VD+Di groups (P < 0.05). As shown in Figure 3(a), the activity of DAO in serum of the Di group was significantly higher than that of both the CK and VD+Di groups (P < 0.05). As to the concentration of D-LA in serum (Figure 3(b)), the Di group showed a highly significant difference compared with the CK group (P < 0.01), and the VD+Di group showed a significantly lower level than the Di group (P < 0.05). There remained a significant difference between the CK and VD+Di groups (P < 0.05).
Figure 2.
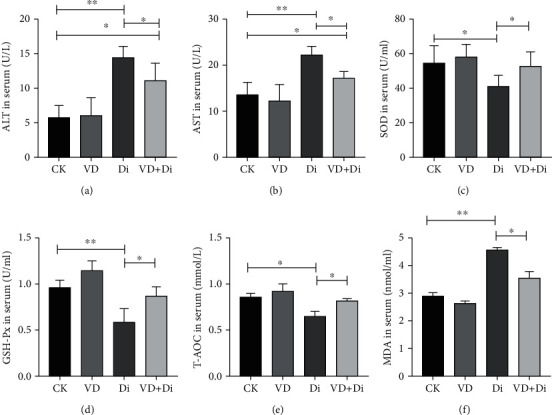
Effects of VitD3 on liver function and antioxidant capacity in mice. (a) ALT activity in serum. (b) AST activity in serum. (c) SOD activity in serum. (d) GSH-Px activity in serum. (e) T-AOC concentration in serum. (f) MDA concentration in serum. All data were presented as mean ± SEM (n = 10). ∗P < 0.05, ∗∗P < 0.01.
Figure 3.
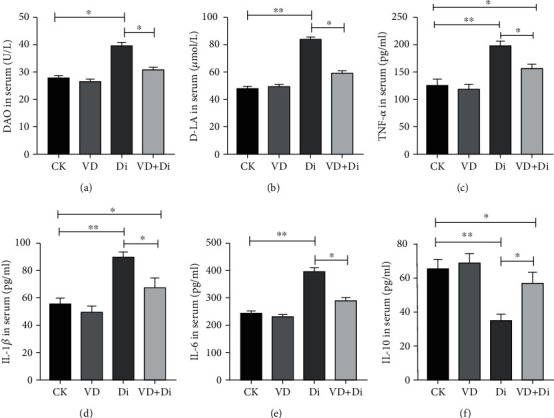
Effects of VitD3 on intestinal permeability and inflammatory cytokine secretion in serum of mice. (a) DAO activity in serum. (b) D-LA concentration in serum. (c) TNF-α concentration in serum. (d) IL-1β concentration in serum. (e) IL-6 concentration in serum. (f) IL-10 concentration in serum. All data were presented as mean ± SEM (n = 10). ∗P < 0.05, ∗∗P < 0.01.
3.3. The Effects of VitD3 on the Antioxidant-Related Index Challenged by Diquat
As shown in Figures 2(c) and 4(a), the activities of SOD in the serum and jejunum of the Di group were significantly lower than those of the CK and VD+Di groups (P < 0.05). The activities of GSH-Px in the serum and jejunum of the Di group (Figures 2(d) and 4(b)) showed a highly significant difference compared with those of the CK group (P < 0.01), while the activities of the VD+Di group were significantly higher than those of the Di group (P < 0.05). The concentration of T-AOC in the serum and jejunum of the Di group (Figures 2(e) and 4(c)) was significantly lower than that of the CK and VD+Di groups (P < 0.05), while the concentration of T-AOC in the jejunum remained significantly different between the CK and VD+Di groups (P < 0.05). The concentration of MDA in serum of the Di group was significantly higher than that of the VD+Di group (P < 0.05), while it showed a highly significant difference compared with the CK group (P < 0.01) (Figure 2(f)). The concentration of MDA in the jejunum of the Di group was significantly higher than that of the CK and VD+Di groups (P < 0.05) (Figure 4(d)).
Figure 4.
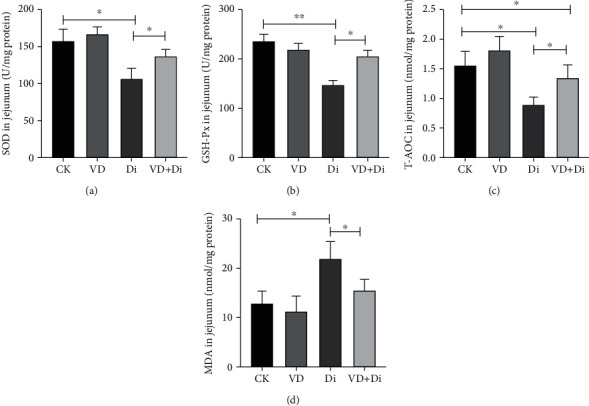
Effects of VitD3 on antioxidant capacity of the jejunum in mice. (a) SOD activity in the jejunum. (b) GSH-Px activity in the jejunum. (c) T-AOC concentration in the jejunum. (d) MDA concentration in the jejunum. All data were presented as mean ± SEM (n = 10). ∗P < 0.05, ∗∗P < 0.01.
3.4. The Effects of VitD3 on the Secretion of Inflammatory Cytokines in Serum Induced by Diquat
As shown in Figures 3(c)–3(e), the concentrations of TNF-α, IL-1β, and IL-6 in serum of the Di group showed a highly significant difference compared with those of the CK group (P < 0.01) and were significantly higher than those of the VD+Di group (P < 0.05), while the concentration of TNF-α and IL-1β in serum of the VD+Di group was still significantly higher than that of the CK group (P < 0.05). As shown in Figure 3(f), the concentration of IL-10 in serum of the Di group showed a highly significant difference compared with that of the CK group (P < 0.01) and was significantly lower than that of the VD+Di group (P < 0.05), while the concentration of IL-10 in the VD+Di group remained significantly lower than that in the CK group (P < 0.05).
3.5. The Effects of VitD3 on the Intestinal Morphology Challenged with Diquat
As shown in Figures 5(a)–5(c), diquat caused severe injuries to the mucosal architecture of the duodenum, jejunum, and colon. As indicated by the black arrows, the Di group had sparse intestinal villi, shedding and truncated intestinal epithelia in the jejunum and colon, and pathological edema in the submucosa of the duodenum, while the VitD3-treated group prominently reversed the pathological characteristics described above.
Figure 5.
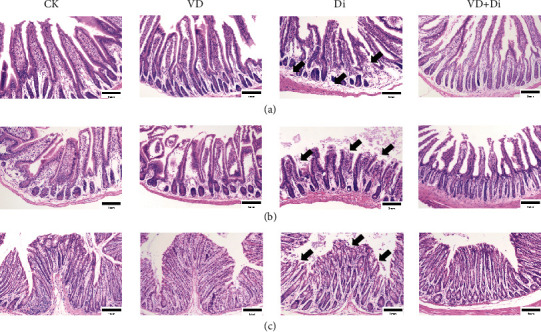
Effects of VitD3 on intestinal morphological characteristics. H&E staining of the (a) duodenum, (b) jejunum, and (c) colon tissues. The morphology of each segment was viewed at the magnification of 100x through a microscope.
3.6. The Effects of VitD3 on the Morphology of the Liver and Kidney Tissues Induced by Diquat
As shown in Figure 6(a), the hepatocytes were obviously vacuolated in the Di group, and the condensation of chromatin and nucleus swelling were also observed in the Di group (as indicated by the black arrows). Through the treatment of VitD3, the pathological features were largely improved. As shown in Figure 6(b), the Di group showed obvious degenerative changes in the tubules with exfoliated epithelia, pyknotic nuclei, and cystic dilatation (as indicated by the black arrows). The glomerulus was shrunken with wide Bowman's space (as indicated by the black double arrows), while minimal kidney tissue damage was observed in the VD+Di group.
Figure 6.
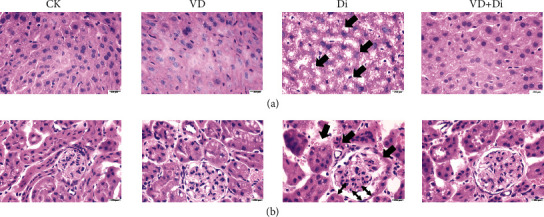
Effects of VitD3 on morphological characteristics of the liver and kidney. H&E staining of the (a) liver and (b) kidney. The morphology of each segment was viewed at the magnification of 100x through a microscope.
3.7. The Effects of VitD3 on the Macrophage Infiltration and Epithelial Cell Apoptotic Level of the Jejunum Tissue Challenged with Diquat
As shown in Figures 7(a) and 7(c), after the challenge of diquat, the infiltration of macrophages in the jejunum showed a highly significant difference between the CK and Di groups (P < 0.01), and the macrophage invasion index of the Di group was significantly higher than that of the VD+Di group (P < 0.05), while there remained a significant difference between the CK and VD+Di groups (P < 0.05). As shown in Figures 7(b) and 7(d), the cell apoptotic level of the jejunal epithelium in the Di group showed a highly significant difference compared with that in the CK group (P < 0.01), and the cell apoptotic index of the VD+Di group was significantly lower than that of the Di group (P < 0.05).
Figure 7.
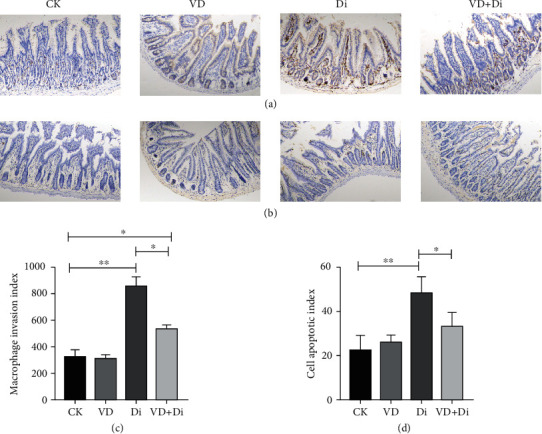
Effects of VitD3 on inflammatory infiltration of macrophages in the jejunum and cell apoptotic status in the jejunum. (a) Immunohistochemistry staining of macrophages (F4/80) in the jejunum tissue. (b) TUNEL staining of the jejunum tissue. All images were viewed at the magnification of 100x through a microscope. (c) Macrophage invasion index, which was calculated through collecting the integrated optical density (Image-Pro software) based on the positive reaction cells of at least six slices, and the average values were calculated and compared with each group. (d) Cell apoptotic index, which was calculated through collecting the integrated optical density (Image-Pro software) based on the positive reaction cells of at least six slices, and the average values were calculated and compared with each group. All data were presented as mean ± SEM (n = 6). ∗P < 0.05, ∗∗P < 0.01.
3.8. The Effects of VitD3 on the Expression Level of Intestinal Barrier Function-Related Genes in the Jejunum Induced by Diquat
As shown in Figure 8(a), the expression abundance of ZO-1 protein in the Di group was visibly lower than that in the other three groups. The relative expression level of intestinal barrier function-related genes was determined (Figures 8(b)–8(g)); the relative expression levels of Mucin-2, ZO-1, ZO-2, Claudin-1, and Occludin in the Di group showed a highly significant difference compared with those in the CK group (P < 0.01), while the relative expression level of Mucin-1 in the Di group was significantly lower than that in the CK group (P < 0.05). The relative expression levels of related genes above in the VD+Di group were significantly higher than those in the Di group consistently (P < 0.05). The expression levels of ZO-1, Claudin-1, and Occludin in the VD+Di group remained significantly lower than those in the CK group (P < 0.05).
Figure 8.
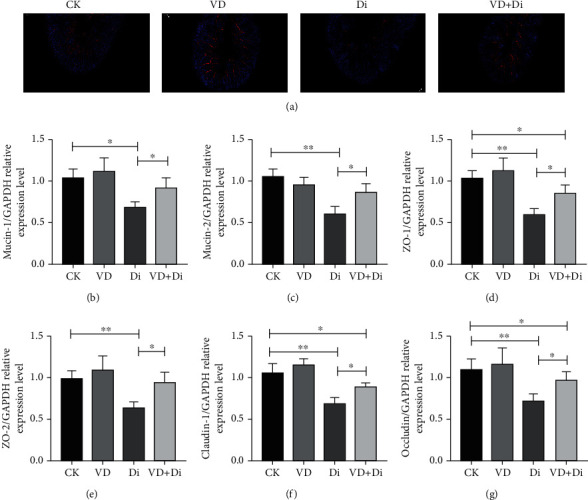
Effects of VitD3 on intestinal tight junction-related proteins and mucin expressions in the jejunum. (a) Immunofluorescence staining of ZO-1 protein in the jejunum tissue. The images were viewed at the magnification of 100x through a fluorescence microscope. Relative gene expression level of (b) Mucin-1, (c) Mucin-2, (d) ZO-1, (e) ZO-2, (f) Claudin-1, and (g) Occludin in the jejunum tissue. All data were presented as mean ± SEM (n = 6). ∗P < 0.05, ∗∗P < 0.01.
3.9. The Effects of VitD3 on the Inhibition of NF-κB (p-p65) and Upregulation of Nrf2-Related Pathways Induced by Diquat
As shown in Figures 9(a), 9(d), and 9(e), in the jejunum tissue, the phosphorylation level of NF-κB (p65) showed a highly significant difference compared with the CK group (P<0.01), while the treatment of VitD3 significantly alleviated this trend (P < 0.05). As indicated in Figure 9(b), the addition of VitD3 could significantly promote the expression of HO-1 compared with the Di group (P < 0.05). From the result of Figure 9(c), the expression level of Nrf2 in the Di group was significantly inhibited compared with that in the CK group (P < 0.05), while the treatment of VitD3 enhanced the expression level of Nrf2 significantly (P < 0.05). The result also demonstrated that the treatment of VitD3 alone could significantly promote the expression level of Nrf2 and HO-1 compared with the CK group (P < 0.01).
Figure 9.
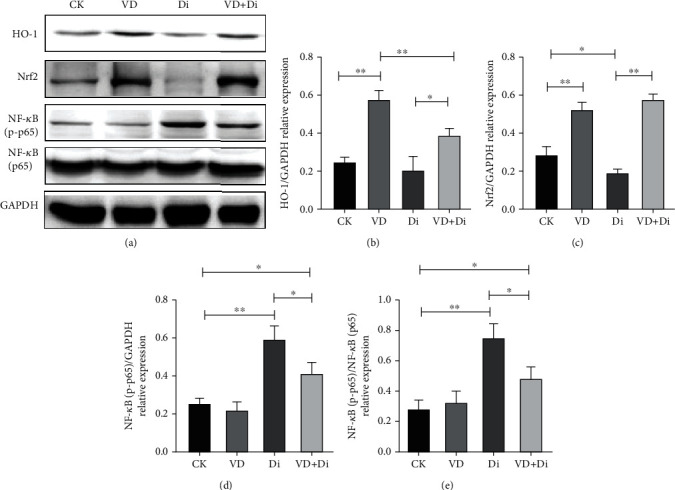
Effects of VitD3 on the Nrf2-mediated signaling pathway and phosphorylation level of NF-κB (p65) in the jejunum. (a) The expression levels of HO-1, Nrf2, NF-κB (p65), NF-κB (p-p65), and GAPDH proteins detected by western blot analysis. Quantitative analysis of the protein expression levels of (b) HO-1, (c) Nrf2, and (d, e) NF-κB (p-p65). All data were presented as mean ± SEM (n = 6). ∗P < 0.05, ∗∗P < 0.01.
4. Discussion
The main source of bioactive vitamin D (VD) to humans originated from plant-derived ergosterol (VD2) and animal-derived cholecalciferol (VD3) or exposure of the skin under the ultraviolet (UV) light [19]. Nowadays, there are at least 1 billion people that are diagnosed with deficiency of VD around the world in the 21st century, and the trend is increasing by year [20]. More studies support the point that VD not only keeps the health of the skeleton through promoting the absorption of calcium [21] but also has important functions except nutritional effects, including regulating the immune system to exert anti-infective effects and improving the developmental process of noninfectious diseases [22, 23]. The antioxidant function of vitamin C has been widely reported; however, the antioxidant effects and possible mechanism of VitD3have few studies.
In this study, the diquat-induced mouse oxidative stress model was established to explore the effects of VitD3 on the damage induced by diquat. In the study, we found that the challenge of diquat could reduce the average growth speed of mice, while the addition of VitD3 eliminated this symptom. The spleen index and thymus index are important indicators reflecting the status of innate immunity [24]; the lower immune organ index in the diquat group indicated the suppression of the normal development of the immune function, which may have a negative influence on the growth performance. The addition of VitD3 reverted the trend to the normal status, which refers to the positive effects of VitD3 on the immune function of the body. ALT and AST are important transaminases that are mainly synthesized in the liver, and the damage of hepatocytes will lead to the release of ALT and AST into the blood [25]. In this study, the abnormal rising of ALT and AST in serum of the Di group reflected the injury of diquat to the liver function, and it was inferred that the possible cause was the occurrence of oxidative stress. Diquat is a typical reagent which could lead to the overproduction of ROS in the body [14, 26] and is usually used to induce the oxidative damage model in animals [27, 28]. In this study, we consistently found that the activities of the main antioxidant enzymes in the serum and jejunum tissues of the Di group were significantly lower than those of the CK group, while the concentration of MDA, which is positively associated with the oxidative stress status [29], was significantly higher than that of the CK group. As the addition of VitD3 could efficiently ameliorate the antioxidant function in the serum and jejunum, we deduced that VitD3 may have the potential to regulate certain target proteins related to the redox balance.
Nrf2 is a crucial protein playing a key role in cellular defense against oxidative stress as the transcription factor [30]. It could effectively enhance the expression of antioxidant-related genes through binding to the antioxidant response elements (ARE) [31, 32]. The downstream functional proteins include SOD, GSH-Px, GST, and HO-1. In this study, compared with the CK group, we found that VitD3 could prominently enhance the protein expression abundance of Nrf2 and HO-1, no matter whether added alone or added with the challenge of diquat. Combined with the results determined on the level of antioxidant enzymes above, it can be inferred that VitD3 could activate the expression of Nrf2 and in turn promote the synthesis of antioxidant-related proteins. As a consequence, the damage caused by oxidative stress to the liver and kidney could be largely attenuated. There is increasing evidence supporting the hypothesis that the crosstalk occurs between the NF-κB- and Nrf2-related pathways [33, 34]. NF-κB (p65) could interact with Keap1 to inhibit the activation of the Nrf2-ARE pathway [35], which was confirmed once again in our present study. Meanwhile, the activation of the phosphorylation level of NF-κB (p65) is positively associated with the secretion of proinflammatory cytokines such as IL-1β, IL-6, and TNF-α [36] and is negatively correlated with the secretion of anti-inflammatory cytokine IL-10 [37]. Our study further confirmed that the addition of VitD3 could distinctly lower the phosphorylation level of NF-κB (p65) compared with the Di group. As a consequence, the addition of VitD3 effectively reverted the concentration of inflammatory cytokines in serum to be close to the normal state. The results above suggested that VitD3 could activate Nrf2-related pathways and inhibit the phosphorylation of NF-κB (p65) to regulate the redox balance and inflammatory response simultaneously.
The occurrence of oxidative stress could directly damage the intestinal barrier function and cause dysfunction of digestion and absorption [38]. The abnormally high concentration of DAO and D-LA in serum always reflects the injury of intestinal barrier function and permeability [39]. In the study, the addition of VitD3 could effectively lower the concentration of DAO and D-LA in serum compared with the Di group, which suggested that VitD3 was involved in the improvement of intestinal barrier function. The description of the morphology of representative intestinal segments supported the point of view. As the jejunum is the main part which is responsible for the digestion and absorption of nutrients [40], keeping the healthy state of the intestinal barrier function of the jejunum tissue is crucial for normal growth performance. As tight junction proteins and mucins are the molecular basis of intestinal barrier structure [41], the expression level of the corresponding genes was determined. The results showed that diquat would destroy the structure of the intestinal barrier by inhibiting the expression level of the tight junction- and mucin-related genes, while the addition of VitD3 ameliorated the expression level of related genes above consistently. It is suggested that the amelioration of the imbalanced status of redox by VitD3 in the jejunum should be directly accounted for in this result.
The typical damage of oxidative stress to the intestine includes the invasion of inflammatory cells such as macrophages and neutrophils [42] and the increasing level of cell apoptosis in the intestinal epithelium [43]. In the study, we found that the diquat challenge caused serious infiltration of macrophages and the cell apoptosis degree in the intestinal epithelium of the Di group was visibly more severe than that of the CK group. The feeding of VitD3 reverted the pathological state to be close to the normal level. There may be two mechanisms that account for these results. One is the immunomodulatory function of VitD3 by inhibiting the phosphorylation level of NF-κB (p65), which in turn lowered the local inflammatory level in the jejunum tissues. The other reason may owe to the antioxidant function of VitD3, which was realized by promoting the expression of Nrf2 and downstream proteins associated with enhancing the antioxidant capacity of the body. As a consequence, superfluous reactive oxygen species could be eliminated timely and the induction of cell apoptosis could be largely avoided. The liver and kidney are essential organs ensuring the synthesis of the active form of VitD3 [44], and the diquat challenge could cause serious damage to these two organs [13, 16, 45]. In this study, we decided to add the active form of VitD3 directly. The external addition of VitD3 could effectively replenish the deficiency of endogenous synthesis of VitD3 caused by the diquat challenge. The results suggested that the addition of VitD3 could improve the typical pathological morphology of the liver and kidney, which would in turn promote the recovery of the corresponding biological functions.
5. Conclusions
In this study, we demonstrated that the exogenous supply of VitD3 could effectively alleviate diquat-induced oxidative stress and immune disorders. As a consequence, the intestinal barrier dysfunction and highly inflammatory status of the whole body were largely ameliorated. It was suggested that VitD3 may exert the function through activating the Nrf2-mediated signaling pathway while inhibiting the phosphorylation level of NF-κB (p65) (Figure 10). Our findings offer important evidence to support that the additional supply of VitD3 in daily life could efficiently prevent oxidative stress-induced diseases, especially for those infants and older people who are susceptible to oxidative stress attacks.
Figure 10.
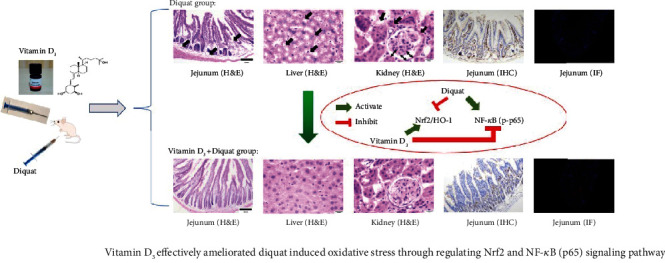
The conclusion of the effects of vitamin D3 on diquat-induced oxidative stress and the underlying mechanism.
Acknowledgments
This study was supported by the Natural Science Foundation of Hainan Province (320RC463), Research project of education and teaching reform in universities of Hainan province (Hnjg2020-23), and Research project of education and teaching reform of Hainan University (hdjy2026).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Ethical Approval
The animal experiment was approved by the Animal Welfare and Ethics Committee of Hainan University (permit number: HNUAUCC-2020-00068) and conducted according to the National Institutes of Health guidelines for the care and treatment process of experimental animals strictly.
Conflicts of Interest
The authors report there are no conflicts of interest to declare in this study.
Authors' Contributions
HZ and YL designed the whole experiment and wrote the paper. YL, XF, LG, CL, LC, and QW carried out the specific experiment. All authors listed have made a substantial and direct contribution to the work and approved it for publication.
References
- 1.Zhou A., Li L., Zhao G., et al. Vitamin D3 inhibits Helicobacter pylori infection by activating the VitD3/VDR-CAMP pathway in mice. Frontiers in Cellular and Infection Microbiology . 2020;10:p. 566730. doi: 10.3389/fcimb.2020.566730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adhikari R., White D., House J. D., Kim W. K. Effects of additional dosage of vitamin D3, vitamin D2, and 25-hydroxyvitamin D3 on calcium and phosphorus utilization, egg quality and bone mineralization in laying hens. Poultry Science . 2020;99(1):364–373. doi: 10.3382/ps/pez502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wobke T. K., Sorg B. L., Steinhilber D. Vitamin D in inflammatory diseases. Frontiers in Physiology . 2014;5:p. 244. doi: 10.3389/fphys.2014.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Svensson D., Nebel D., Nilsson B. O. Vitamin D3 modulates the innate immune response through regulation of the hCAP-18/LL-37 gene expression and cytokine production. Inflammation Research . 2016;65(1):25–32. doi: 10.1007/s00011-015-0884-z. [DOI] [PubMed] [Google Scholar]
- 5.Tada H., Shimizu T., Nagaoka I., Takada H. Vitamin D3 analog maxacalcitol (OCT) induces hCAP-18/LL-37 production in human oral epithelial cells. Biomedical Research . 2016;37(3):199–205. doi: 10.2220/biomedres.37.199. [DOI] [PubMed] [Google Scholar]
- 6.Borel P., Caillaud D., Cano N. J. Vitamin D bioavailability: state of the art. Critical Reviews in Food Science and Nutrition . 2015;55(9):1193–1205. doi: 10.1080/10408398.2012.688897. [DOI] [PubMed] [Google Scholar]
- 7.Heulens N., Korf H., Mathyssen C., et al. 1,25-Dihydroxyvitamin D modulates antibacterial and inflammatory response in human cigarette smoke-exposed macrophages. PLoS One . 2016;11(8, article e0160482) doi: 10.1371/journal.pone.0160482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu X., Zhu Y., Li C., et al. 1,25Dihydroxyvitamin D regulates macrophage polarization and ameliorates experimental inflammatory bowel disease by suppressing miR-125b. International Immunopharmacology . 2019;67:106–118. doi: 10.1016/j.intimp.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Aranow C. Vitamin D and the immune system. Journal of Investigative Medicine . 2011;59(6):881–886. doi: 10.231/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korf H., Wenes M., Stijlemans B., et al. 1,25-Dihydroxyvitamin D3 curtails the inflammatory and T cell stimulatory capacity of macrophages through an IL-10-dependent mechanism. Immunobiology . 2012;217(12):1292–1300. doi: 10.1016/j.imbio.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y., Chen H., Zeng X., Wang P., Li J., Wu W. Levamisole enhances immunity in ducklings vaccinated against Riemerella anatipestifer. Microbiology and Immunology . 2014;58(8):456–462. doi: 10.1111/1348-0421.12169. [DOI] [PubMed] [Google Scholar]
- 12.Cap M., Vachova L., Palkova Z. Reactive oxygen species in the signaling and adaptation of multicellular microbial communities. Oxidative Medicine and Cellular Longevity . 2012;2012 doi: 10.1155/2012/976753.976753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y. P., Gu Y. F., Zhao H. R., Zhou Y. M. Dietary squalene supplementation alleviates diquat-induced oxidative stress and liver damage of broiler chickens. Poultry Science . 2021;100(3):p. 100919. doi: 10.1016/j.psj.2020.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hao L., Cheng Y., Su W., et al. Pediococcus pentosaceus ZJUAF-4 relieves oxidative stress and restores the gut microbiota in diquat-induced intestinal injury. Applied Microbiology and Biotechnology . 2021;105(4):1657–1668. doi: 10.1007/s00253-021-11111-6. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H., Chen Y., Chen Y., et al. Comparison of the effects of resveratrol and its derivative pterostilbene on hepatic oxidative stress and mitochondrial dysfunction in piglets challenged with diquat. Food & Function . 2020;11(5):4202–4215. doi: 10.1039/d0fo00732c. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y., Chen Y., Zhang H., Wang T. Pterostilbene as a protective antioxidant attenuates diquat-induced liver injury and oxidative stress in 21-day-old broiler chickens. Poultry Science . 2020;99(6):3158–3167. doi: 10.1016/j.psj.2020.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nisar R., Hanson P. S., He L., Taylor R. W., Blain P. G., Morris C. M. Diquat causes caspase-independent cell death in SH-SY5Y cells by production of ROS independently of mitochondria. Archives of Toxicology . 2015;89(10):1811–1825. doi: 10.1007/s00204-015-1453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H., Xia X., Han F., et al. Cathelicidin-BF, a novel antimicrobial peptide from Bungarus fasciatus, attenuates disease in a dextran sulfate sodium model of colitis. Molecular pharmaceutics . 2015;12(5):1648–1661. doi: 10.1021/acs.molpharmaceut.5b00069. [DOI] [PubMed] [Google Scholar]
- 19.Christakos S., Dhawan P., Verstuyf A., Verlinden L., Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiological reviews . 2016;96(1):365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yegin Z., Fidan C., Kut A. Impact of vitamin D deficiency on cognitive functions in type 2 diabetic patients. Acta Endocrinologica . 2017;13(4):410–416. doi: 10.4183/aeb.2017.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daugaard S., Garde A. H., Hansen A. M., Vistisen H. T., Rejnmark L., Kolstad H. A. Indoor, outdoor, and night work and blood concentrations of vitamin D and parathyroid hormone. Scandinavian Journal of Work, Environment & Health . 2018;44(6):647–657. doi: 10.5271/sjweh.3745. [DOI] [PubMed] [Google Scholar]
- 22.Grant W. B., Lahore H., McDonnell S. L., et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients . 2020;12(4) doi: 10.3390/nu12061620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsutsui Y., Hays F. A. A link between Alzheimer's and type II diabetes mellitus? Ca+2-mediated signal control and protein localization. BioEssays . 2018;40(6, article e1700219) doi: 10.1002/bies.201700219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y., Xu A., Liu P., Li Z. Effects of Fuzhuan brick-tea water extract on mice infected with E. coli O157:H7. Nutrients . 2015;7(7):5309–5326. doi: 10.3390/nu7075218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pirimoglu B., Sade R., Polat G., Islek A., Kantarci M. Analysis of correlation between liver fat fraction and AST and ALT levels in overweight and obese children by using new magnetic resonance imaging technique. The Turkish Journal of Gastroenterology . 2020;31(2):156–162. doi: 10.5152/tjg.2020.18594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li M., Yuan D., Liu Y., Jin H., Tan B. Dietary puerarin supplementation alleviates oxidative stress in the small intestines of diquat-challenged piglets. Animals . 2020;10(4) doi: 10.3390/ani10040631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J., Zhang Y., Li Y., Yan H., Zhang H. L-Tryptophan enhances intestinal integrity in diquat-challenged piglets associated with improvement of redox status and mitochondrial function. Animals . 2019;9(5) doi: 10.3390/ani9050266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Y. Q., Yuan L., Gao H. B., Yao D. Q., Chen Q. S., Tian Y. P. Establishment and evaluation of acute diquat poisoning model in Wistar rats. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi . 2019;37(5):342–346. doi: 10.3760/cma.j.issn.1001-9391.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Verma M. K., Jaiswal A., Sharma P., Kumar P., Singh A. N. Oxidative stress and biomarker of TNF-alpha, MDA and FRAP in hypertension. Journal of medicine and life . 2019;12(3):253–259. doi: 10.25122/jml-2019-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alotaibi M. F., Al-Joufi F., Abou Seif H. S., et al. Umbelliferone inhibits spermatogenic defects and testicular injury in lead-intoxicated rats by suppressing oxidative stress and inflammation, and improving Nrf2/HO-1 signaling. Drug Design, Development and Therapy . 2020;14:4003–4019. doi: 10.2147/DDDT.S265636. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Liu J. J., Zhao G. X., He L. L., et al. Lycium barbarum polysaccharides inhibit ischemia/reperfusion-induced myocardial injury via the Nrf2 antioxidant pathway. Toxicology Reports . 2021;8:657–667. doi: 10.1016/j.toxrep.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang S., Huang X. L., Chen J., et al. Curcumin protects BEAS2B cells from PM2.5induced oxidative stress and inflammation by activating NRF2/antioxidant response element pathways. International Journal of Molecular Medicine . 2021;47(4) doi: 10.3892/ijmm.2021.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Z., Xiao J., Liu H., et al. Astaxanthin attenuates oxidative stress and immune impairment in D-galactose-induced aging in rats by activating the Nrf2/Keap1 pathway and suppressing the NF-kappaB pathway. Food & Function . 2020;11(9):8099–8111. doi: 10.1039/d0fo01663b. [DOI] [PubMed] [Google Scholar]
- 34.Hu B., Wei H., Song Y., et al. NF-kappaB and Keap1 interaction represses Nrf2-mediated antioxidant response in rabbit hemorrhagic disease virus infection. Journal of Virology . 2020;94(10) doi: 10.1128/JVI.00016-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu M., Li H., Liu Q., et al. Nuclear factor p65 interacts with Keap1 to repress the Nrf2-ARE pathway. Cellular Signalling . 2011;23(5):883–892. doi: 10.1016/j.cellsig.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 36.Ben-Neriah Y., Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nature Immunology . 2011;12(8):715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 37.Zhang D., Li N., Wang Y., et al. Methane ameliorates post-operative cognitive dysfunction by inhibiting microglia NF-kappaB/MAPKs pathway and promoting IL-10 expression in aged mice. International Immunopharmacology . 2019;71:52–60. doi: 10.1016/j.intimp.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Zhang H., Yan A., Liu X., et al. Melatonin ameliorates ochratoxin A induced liver inflammation, oxidative stress and mitophagy in mice involving in intestinal microbiota and restoring the intestinal barrier function. Journal of Hazardous Materials . 2021;407:p. 124489. doi: 10.1016/j.jhazmat.2020.124489. [DOI] [PubMed] [Google Scholar]
- 39.Chen Y., Miao L., Yao Y., et al. Dexmedetomidine ameliorate CLP-induced rat intestinal injury via inhibition of inflammation. Mediators of Inflammation . 2015;2015 doi: 10.1155/2015/918361.918361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takizawa Y., Tobe Y., Sakamoto N., Sakamoto J., Hayashi M. Sodium nitroprusside enhances absorption in the rat jejunum via the transcellular route. The Journal of Membrane Biology . 2020;253(3):221–228. doi: 10.1007/s00232-020-00118-1. [DOI] [PubMed] [Google Scholar]
- 41.He C., Deng J., Hu X., et al. Vitamin A inhibits the action of LPS on the intestinal epithelial barrier function and tight junction proteins. Food & Function . 2019;10(2):1235–1242. doi: 10.1039/c8fo01123k. [DOI] [PubMed] [Google Scholar]
- 42.Balkrishna A., Solleti S. K., Singh H., Sharma N., Varshney A. Withanolides from Withania somnifera ameliorate neutrophil infiltration in endotoxin-induced peritonitis by regulating oxidative stress and inflammatory cytokines. Planta Medica . 2021 doi: 10.1055/a-1438-2816. [DOI] [PubMed] [Google Scholar]
- 43.Baregamian N., Song J., Papaconstantinou J., Hawkins H. K., Evers B. M., Chung D. H. Intestinal mitochondrial apoptotic signaling is activated during oxidative stress. Pediatric Surgery International . 2011;27(8):871–877. doi: 10.1007/s00383-011-2880-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parizadeh S. M., Rezayi M., Jafarzadeh-Esfehani R., et al. Association of vitamin D status with liver and kidney disease: a systematic review of clinical trials, and cross-sectional and cohort studies. International Journal for Vitamin and Nutrition Research . 2021;91(1-2):175–187. doi: 10.1024/0300-9831/a000540. [DOI] [PubMed] [Google Scholar]
- 45.Xing J., Chu Z., Han D., et al. Lethal diquat poisoning manifesting as central pontine myelinolysis and acute kidney injury: a case report and literature review. The Journal of International Medical Research . 2020;48(7) doi: 10.1177/0300060520943824. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.


