Abstract
In L6 muscle cells expressing wild-type human insulin receptors (L6hIR), insulin induced protein kinase Cα (PKCα) and β activities. The expression of kinase-deficient IR mutants abolished insulin stimulation of these PKC isoforms, indicating that receptor kinase is necessary for PKC activation by insulin. In L6hIR cells, inhibition of insulin receptor substrate 1 (IRS-1) expression caused a 90% decrease in insulin-induced PKCα and -β activation and blocked insulin stimulation of mitogen-activated protein kinase (MAPK) and DNA synthesis. Blocking PKCβ with either antisense oligonucleotide or the specific inhibitor LY379196 decreased the effects of insulin on MAPK activity and DNA synthesis by >80% but did not affect epidermal growth factor (EGF)- and serum-stimulated mitogenesis. In contrast, blocking c-Ras with lovastatin or the use of the L61,S186 dominant negative Ras mutant inhibited insulin-stimulated MAPK activity and DNA synthesis by only about 30% but completely blocked the effect of EGF. PKCβ block did not affect Ras activity but almost completely inhibited insulin-induced Raf kinase activation and coprecipitation with PKCβ. Finally, blocking PKCα expression by antisense oligonucleotide constitutively increased MAPK activity and DNA synthesis, with little effect on their insulin sensitivity. We make the following conclusions. (i) The tyrosine kinase activity of the IR is necessary for insulin activation of PKCα and -β. (ii) IRS-1 phosphorylation is necessary for insulin activation of these PKCs in the L6 cells. (iii) In these cells, PKCβ plays a unique Ras-independent role in mediating insulin but not EGF or other growth factor mitogenic signals.
Insulin induces mitogenic and metabolic effects in most cell types (54). At the proximal level, phosphorylation of the insulin receptor substrates (IRSs) represents one of the earliest events involved in the eliciting these effects (54). In particular, the ubiquitous insulin receptor substrate 1 (IRS-1) protein appears to play a major role in transduction of insulin proliferative effects (2, 28, 51). IRS-1 possesses multiple tyrosine phosphorylation residues, which allow it to interact with other signaling molecules containing SH2 domains and conveying mitogenic signals further downstream (49, 54). These include phosphatidylinsitol 3-kinase (PI 3-kinase) regulatory subunits (1, 30) and Grb2 (43, 44), an adapter protein which interacts with the exchange factor Son of Sevenless (SOS) and induces Ras activation. Ras functions as an activator of Raf which, in turn, activates mitogen-activated protein kinase kinase (MAPKK) and enables phosphorylation and stimulation of MAPK (19). These events have been shown to be necessary for cell proliferation to occur in response to insulin in most cell types (6, 33). However, there is also evidence that insulin signal can be conveyed into the MAPK pathway independently of Ras (11).
Protein kinase C (PKC) comprises a multigene family that encodes at least 12 distinct isoforms differing in catalytic and regulatory properties (21, 26, 32). These PKC isoforms can be divided into three subgroups based on cofactor requirements: (i) conventional PKCs (such as α, β, and γ) that are dependent on Ca2+ and diacylglycerol (DAG) for activity, (ii) novel PKCs (δ, ɛ, η, and θ) that are not dependent on Ca2+ but are activated by DAG, and (iii) atypical PKCs (ζ, λ, and ι) that are not dependent on Ca2+ and are not stimulated by DAG (20). PKC plays a pivotal role in controlling numerous cellular functions, including cell proliferation (26). PKC-mediated signaling systems have been shown to be activated following the stimulation of cell surface receptors by several growth factors such as epidermal growth factor (EGF) and platelet-derived growth factor (34, 35). Binding to these growth factor receptors leads to phospholipase activation and production of DAG (36) which, in turn, binds and activates PKC (32). PKCs may then provoke the activation of c-Ras and the Raf-MAPKK-MAPK pathway (24, 25, 41, 45). Also, DAG-regulated PKC may activate Raf independently of Ras (10, 24, 25).
Insulin has been shown to activate phospholipases (23, 46) and to stimulate the activity of several distinct PKC isoforms (3, 7, 18), but whether the activation of specific PKC isoforms is relevant to insulin mitogenic signal remains unknown. Also, the proximal events in insulin signaling leading to PKC activation are unclear. In the present report, we have addressed these issues by analyzing insulin mitogenic signaling in the L6 muscle cells. We have obtained evidence that PKCβ activation plays a major role in mediating insulin-dependent DNA synthesis in these cells, bypassing Ras and through the Raf-MAPKK-MAPK pathway.
MATERIALS AND METHODS
Materials.
Media, sera, and antibiotics for cell culture were from Life Technologies, Inc. (Grand Island, N.Y.). The Lipofectamine reagent, rabbit polyclonal antibodies directed against specific PKC isoforms, and the PKC assay system (catalog no. 13161-013) were purchased from Life Technologies, Inc. Recombinant PKCα, -β, -δ, and -ζ proteins, the PKCδ peptide substrate, and PKCɛ pseudosubstrate were from Calbiochem-Novabiochem (La Jolla, Calif.). Polyclonal IR antibodies were from Oncogene Science (Manhasset, N.Y.), and p42/p44 MAPK and Raf-1 antibodies were from Transduction Laboratories, Inc. (Lexington, Ky.). Phosphorylated MAP kinase antibodies were from New England Biolabs (Beverly, Mass.). The IRS-1 ribozyme (IRS-1 rib) and control ribozyme (c-rib) were generous gifts of M. Quon (National Institutes of Health, Bethesda, Md.) (37). Phosphorothioate PKCα and PKCβ antisense and control oligonucleotides have been previously described (12, 42) and were synthesized from PRIMM s.r.l. (Milan, Italy). The LY379196 inhibitor was a generous gift from Eli Lilly Co. (Indianapolis, Ind.) and has been described previously (4, 7, 22). PD98059 was purchased from ICN Biomedicals Inc. (Aurora, Ohio). Protein electrophoresis reagents were from Bio-Rad (Richmond, Va.), and Western blotting and enhanced chemiluminescence (ECL) reagents were from Amersham (Arlington Heights, Ill.). All other chemicals were from Sigma (St. Louis, Mo.).
Cell culture and transfection.
The L6 muscle cells were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum, 10,000 U of penicillin per ml, 10,000 μg of streptomycin per ml, and 2% l-glutamine, in a humidified CO2 incubator by the method of Caruso et al. (12). Transient transfection of IRS-1 rib and c-rib, the phosphorothioate oligonucleotides, and of the L61,S186 dominant negative Ras mutant (40) was performed by the Lipofectamine method according to the manufacturer's instruction. For these studies, 50 to 80% confluent cells were washed twice with Optimem and incubated for 8 h with 12 μg of IRS-1 rib or antisense oligonucleotide and 45 μl of Lipofectamine. The medium was then replaced with DMEM with 10% fetal calf serum, and cells were incubated for 15 h before being assayed.
Western blot analysis and immunoprecipitation procedure.
For these studies, the cells were solubilized in lysis buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 10 mM EDTA, 10 mM Na4P2O7, 2 mM Na3VO4, 100 mM NaF, 10% glycerol, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 10 μg of aprotinin per ml) for 2 h at 4°C. Lysates were centrifuged at 5,000 × g for 20 min and assayed by the method of Miele et al. (28). Briefly, solubilized proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to 0.45-μm-pore-size Immobilon-P membranes (Millipore, Bedford, Mass.). Upon incubation with the primary and secondary antibodies, immunoreactive bands were detected by ECL according to the manufacturer's instructions. Immunoprecipitation of specific PKC isoforms, IRS-1 or -2, MAPK, Raf-1, and IRs were accomplished as previously described (28).
PKC assay.
PKC activity was measured as previously described (18). Briefly, for these assays, cells were solubilized in extraction buffer (20 mM Tris [pH 7.5], 0.5 mM EDTA, 0.5 mM EGTA, 25 μg of aprotinin per ml, 25 μg of leupeptin) and then clarified by centrifugation at 5,000 × g for 20 min. Supernatants were further centrifuged at 60,000 × g for 2 h, and pellets were solubilized with extraction buffer containing 0.5% Triton X-100. Soluble pellets were supplemented with the lipid activators (10 mM phorbol 12-myristate 13-acetate (PMA), 0.28 mg of phosphatidylserine per ml, and 4 mg of dioleine per ml [final concentrations given]), and phosphorylation reactions were initiated by addition of the substrate solution, which contains 50 μM acetylated myelin basic protein (residues 4 to 14) [Ac-MBP(4-14)], 20 μM ATP, 1 mM CaCl2, 20 mM MgCl2, 4 mM Tris (pH 7.5), and 10 μCi of (3,000 Ci/mmol) [γ-32P]ATP per ml (final concentrations given). The reaction mixtures were incubated for 10 min at room temperature, rapidly cooled on ice, and spotted on phosphocellulose discs. Disc-bound radioactivity was quantitated by liquid scintillation counting. Activity of the specific PKC isoforms was assayed as described above but using precipitates with specific antibodies (12). Determinations of PKCδ and -ζ activities using the Ac-MBP(4-14) peptide as the substrate or the H-Arg-Phe-Ala-Val-Arg-Asp-Met-Arg-Gln-Thr-Val-Ala-Val-Gly-Val-Ile-Lys-Ala-Val-Asp-Lys-Lys-OH peptide (specific for PKCδ) or the pseudosubstrate region of PKCɛ (specific for PKCζ) provided consistent results.
Insulin-mediated p21ras-GTP formation.
For the GTP overlay, 80% confluent L6 cells were serum and phosphate starved for 24 h and then exposed to 100 nM insulin for 15 min. Cell extracts were prepared, precipitated with Ras antibodies, and separated by SDS-PAGE. The gel was then soaked in 50 mM Tris-HCl (pH 7.5)–20% glycerol for 1 h and transferred onto nitrocellulose filters as described by Bucci et al. (9). After the transfer, the filters were rinsed twice for 10 min each time in a solution containing 50 mM NaH2PO4, 10 mM MgCl2, 2 mM dithiothreitol, and 0.3% Tween 20 (pH 7.5) and incubated with 2 μCi of [α-32P]GTP per ml for 2 h. After six 5-min washes, the filters were autoradiographed. Quantitation was achieved by laser densitometry.
MAPK, Raf-1 kinase, and PI 3-kinase activities.
Quantitation of MAPK and Raf-1 kinase activities was accomplished as described by Miele et al. (28). Briefly, cell lysates (200 μg of protein/assay) were immunoprecipitated for 18 h with either MAPK or Raf-1 antibodies and incubated with protein A-Sepharose for 2 h. Immobilized MAPK and Raf-1 kinases were washed three times with ice-cold TAT buffer (50 mM HEPES, [pH 7.5], 150 mM NaCl, 10 mM EDTA, 10 mM Na4P2O7, 2 mM Na3VO4, 100 mM NaF, 10% glycerol, 1% Triton X-100) and two more times with HNTGVa buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 2 mM Na3VO4, 10% glycerol, 10% Triton X-100) and then resuspended in HNTGVa buffer supplemented with 60 mM Mg acetate, 30 μM ATP, 6 mM dithiothreitol, 1 μg of MBP per ml, and 0.5 μCi of [γ-32P]ATP. Alternatively, Syntide 2 was also used as a Raf-1 substrate as described by Zou et al. (55). Upon incubation for 30 min at 25°C, reaction mixtures were spotted onto phosphocellulose discs and washed three times with 1% phosphoric acid and once more with ethanol. Disc-bound radioactivity was quantitated by liquid scintillation counting. For quantitation of PI 3-kinase activity, the cells were solubilized in TAN buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 10% glycerol, 1% Nonidet P-40, 10 mM EDTA, 10 mM Na4P2O7, 1 mM Na3VO4, 10 μg of aprotinin per ml, 10 μg of leupeptin per ml, 100 mM NaF, 1 mM phenylmethylsulfonyl fluoride). Aliquots of the lysates were precipitated with IRS-1 or IRS-2 antibodies, and PI 3-kinase activity was determined as described by Miele et al. (28).
Thymidine incorporation.
Cells were seeded in six-well plates. After 18 h, the cells were fed with DMEM supplemented with 0.25% bovine serum albumin. The cells were then maintained in this medium for an additional 16 h in the absence and presence of 100 nM insulin as indicated, followed by addition of 500 nCi of [3H]thymidine per ml. Four hours later, the cells were washed with ice-cold 0.9% NaCl and maintained in ice-cold 20% trichloroacetic acid for further 10 min. Cells were finally solubilized with 1 N NaOH, and radioactivity was quantitated by scintillation counting as described by Formisano et al. (17).
RESULTS
Insulin activation of PKC in L6 cells.
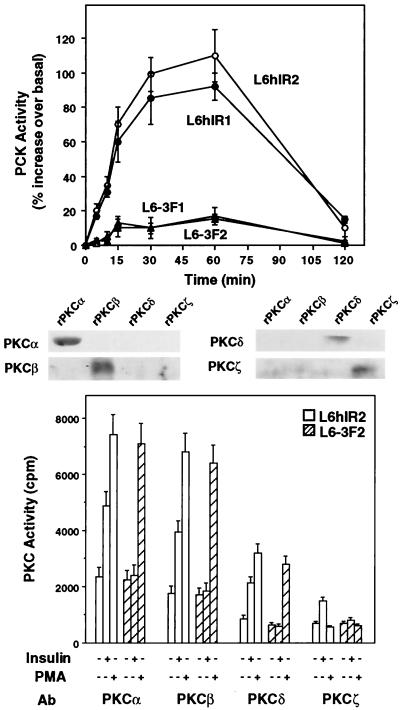
Insulin action on PKC activity was compared in L6 cells overexpressing either wild-type human IRs or a kinase-deficient IR mutant in which tyrosines in the regulatory loop (Tyr1146, Tyr1150, and Tyr1151) had been substituted with phenylalanines. Both the L6 cell clones expressing the wild-type human IRs (L6hIR1, 3.2 × 104 receptors/cell; L6hIR2, 3.7 × 104 receptors/cell) and those expressing the mutant receptors (L6-3F1, 3.8 × 104 receptors/cell; L6-3F2, 3.5 × 104 receptors/cell) have been previously reported (12). In L6hIR cells, insulin (100 nM) induced a time-dependent increase in membrane PKC activity (Fig. 1, top panel). This effect was reduced by 90% in the L6-3F cell clones. Also, exposure of the L6hIR2 cells to insulin increased by two- to threefold the activity of PKC in PKCα, -β, -δ, and -ζ immunoprecipitates (Fig. 1, bottom panel). Half-maximal insulin effect on each of these PKC isoforms was detected at 0.5 to 1 nM, and maximal effect was detected at 100 nM (data not shown). No insulin effect was measured for immunoprecipitates from the L6-3F2 cells. In contrast with the effect of insulin, 60 min of exposure to the phorbol ester PMA (1 μM) induced PKCα, -β, and -δ activities (but not PKCζ activity) in both L6hIR2 and L6-3F2 cells. Same results were obtained by comparing the L6hIR1 and L6-3F1 cell clones (data not shown).
FIG. 1.
Insulin effect on PKC activity in L6 cells. (Top) Cells expressing wild-type human IRs (L6hIR1 and L6hIR2 clones) or kinase-deficient IRs (L6-3F1 and L6-3F2 clones) were exposed to 100 nM insulin for the indicated times. The cells were then solubilized and assayed for PKC activity as described in Materials and Methods. PKC activity is plotted as percent increase above that measured in cells not exposed to insulin. Data points represent the means ± standard deviations of triplicate determinations in four independent experiments. (Bottom) L6hIR2 and L6-3F2 cells were stimulated with insulin for 30 min or PMA for 60 min, solubilized, and immunoprecipitated with isoform-specific PKC antibodies (Ab) (200 μg of protein/sample). PKC activity in the immunoprecipitates was assayed as described in Materials and Methods and is plotted as radioactivity incorporated in the Ac-MBP(4-14) substrate peptide. Bars represent the means ± standard deviations of duplicate determinations in four independent experiments. The specificity of PKC isoform antibodies was controlled by Western blotting recombinant PKCα, -β, -δ, and -ζ (rPKCα, -β, -δ, and -ζ) with the individual antibodies as shown.
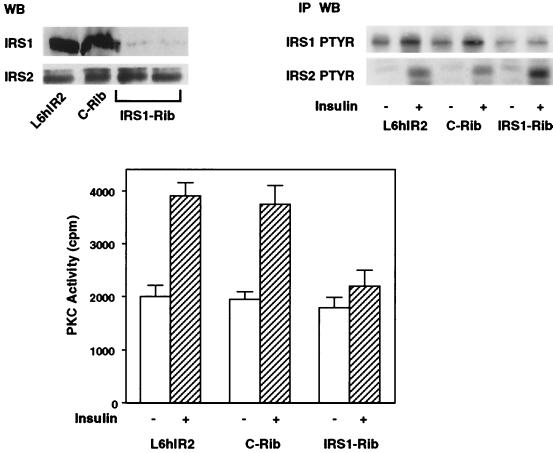
We next investigated the role of IRS-1, a major IRS, in mediating insulin activation of these PKC isoforms. To this end, we suppressed IRS-1 expression in L6hIR cells by transient transfection of an IRS-1 rib (37). This ribozyme reduced IRS-1 protein expression and insulin phosphorylation by >90% (P < 0.001) in these cells compared to the values for cells which were not transfected with the ribozyme (Fig. 2, top panel). No effects on IRS-1 expression and its phosphorylation by insulin were observed upon transfection of a c-rib. Interestingly, while not affecting the expression levels of IRS-2 protein, transfection of the IRS-1 rib increased IRS-2 phosphorylation by 50% (P < 0.001) compared to the values for both the nontransfected cells and those transfected with c-rib. In addition, insulin activation of PKC was inhibited by 80% (P < 0.001) in cells transfected with the IRS-1 rib compared to that in control cells (either cells not transfected with the ribozyme or cells transfected with c-rib) (Fig. 2, bottom panel). There was no change in insulin binding, IR expression, and autophosphorylation in the IRS-1 rib-transfected cells (data not shown).
FIG. 2.
PKC activity in L6 cells transfected with IRS-1 rib. (Top) L6hIR2 cells were transfected with either IRS1-Rib or c-Rib, solubilized, separated by SDS-PAGE, and blotted with IRS-1 or IRS-2 antibodies as indicated (left). WB, Western blot. Alternatively (right), the cells were exposed to 100 nM insulin for 5 min, and the cell lysates were precipitated with IRS-1 or IRS-2 antibodies, subjected to SDS-PAGE, and Western blotted with phosphotyrosine antibodies (PTYR). Blots were revealed by ECL as described in Materials and Methods. The autoradiographs shown are representative of six (left) and four (right) independent experiments. IP, immunoprecipitation. (Bottom) PKC activity was assayed in lysates from L6hIR cells either in the absence or upon transfection of c-rib or IRS1-Rib as described in the legend to Fig. 1. Bars represent the means ± standard deviations of triplicate determinations in four independent experiments.
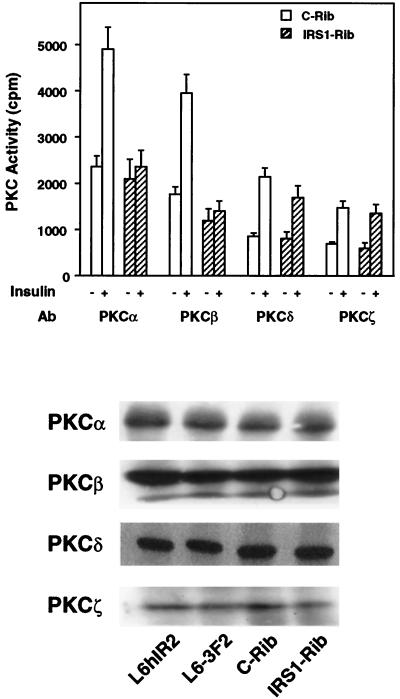
We also measured the activity of specific PKC isoforms in cells transfected with IRS-1 rib. As shown in Fig. 3 (top panel), basal PKC activity in precipitates of IRS-1 rib-transfected cells with antibodies to PKCα, -β, -δ, and -ζ was not significantly different from that of control cells. Insulin-activated PKCδ and -ζ activities were also comparable in the IRS-1 rib-transfected cells and in control cells. However, in cells transfected with IRS-1 rib, the insulin-dependent activities of PKCα and -β were specifically blocked. The expression levels of the individual PKC isoforms were not different in the cells which were transfected with the ribozymes (either IRS-1 rib or c-rib) and in those which were not (Fig. 3, bottom panel).
FIG. 3.
Activity of specific PKC isoforms in L6 cells transfected with IRS-1 rib. (Top) L6hIR2 cells transfected with either c-Rib or IRS1-Rib were stimulated with 100 nM insulin for 30 min, lysed, and precipitated with antibodies (Ab) to specific PKC isoforms as indicated. PKC activity was then assayed in the immunoprecipitates as described in Materials and Methods. Bars represent the means ± standard deviations of duplicate determinations in three independent experiments. (Bottom) For a control, lysates from the L6-3F2 cells and from the L6hIR2 cells (either those which were not transfected with ribozymes or those transfected with c-Rib or IRS1-Rib) were separated by SDS-PAGE and Western blotted with specific antibodies toward the PKC isoforms as indicated. Blots were revealed by ECL as described in Materials and Methods. The autoradiograph shown is representative of three independent experiments.
PKCβ action on MAPK.
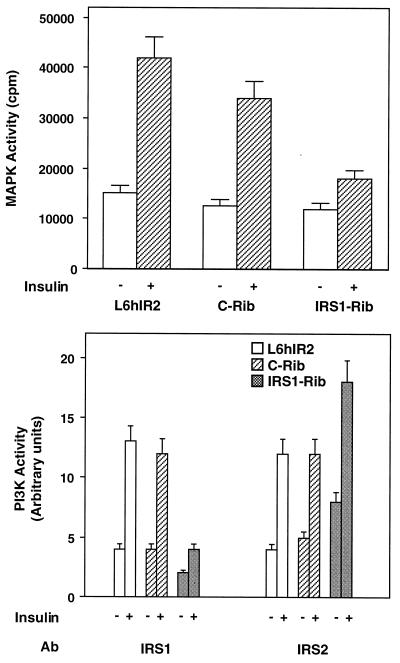
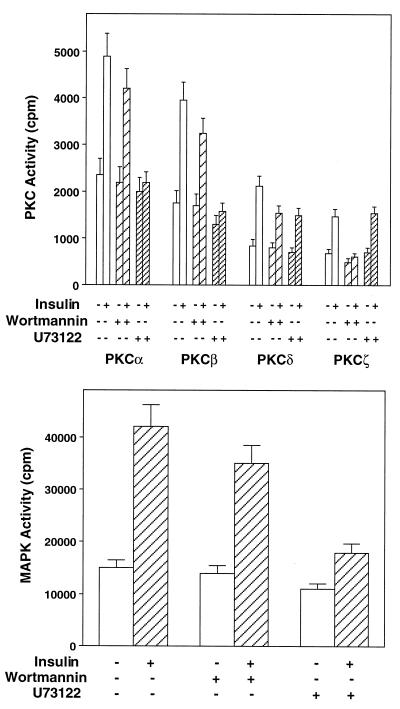
In muscle cells, the inhibition of insulin-dependent activation of PKCα and -β by IRS-1 rib was accompanied by an 80% inhibition (P < 0.001) of insulin-dependent MAPK activation (Fig. 4, top panel). As was the case for PKCα and -β, the control c-rib transfection was unable to inhibit MAPK activation. IRS-1-associated PI 3-kinase activity was barely detectable in cells transfected with IRS-1 rib compared to those transfected with c-rib and to those not transfected with the ribozymes (Fig. 4, middle panel). Consistent with the increased phosphorylation of IRS-2 in IRS-1 rib-transfected cells, IRS-2-associated PI 3-kinase activity was increased 60% (P < 0.001) compared to that in control cells (either cells transfected with c-rib or cells not transfected with the ribozyme) (Fig. 4, bottom panel). Interestingly, treatment of L6hIR cells with the PI 3-kinase inhibitor wortmannin (50 nM) inhibited insulin-stimulated PKCα and -β activities by only 20% (P < 0.05), which paralleled a similar inhibition of MAPK activation (Fig. 5). Wortmannin was much more effective on activation of PKCδ and -ζ (45 and 95% inhibition, respectively; P < 0.01). Unlike wortmannin, the phospholipase inhibitor U73122 almost completely blocked insulin activation of PKCα and -β (P < 0.001) and MAPK but inhibited PKCδ induction by only 45% (P < 0.001) and showed no effect on activation of PKCζ.
FIG. 4.
MAPK and PI 3-K activities in L6 cells transfected with IRS-1 rib. (Top) L6hIR2 cells, either those transfected with IRS1-Rib or c-Rib and those which were not transfected, were exposed to 100 nM insulin for 30 min at 37°C. Cell lysates (200 μg of protein/sample) were then precipitated with MAPK antibodies and assayed for MAPK activity as described in Materials and Methods. MAPK activity is plotted as radioactivity incorporated in the MBP MAPK substrate. (Bottom) For determination of PI 3-K activity, the cells were exposed to 100 nM insulin for 10 min at 37°C and 200 μg of solubilized proteins precipitated with IRS-1 or IRS-2 antibodies (Ab) as indicated. IRS-1- and IRS-2-associated PI 3-K was then assayed as described in Materials and Methods. Bars represent the means ± standard deviations of duplicate determinations in four (top) and five (bottom) independent experiments.
FIG. 5.
Effects of wortmannin and U73122 on PKC isoform and MAPK activities. (Top) L6hIR2 cells were incubated with either 50 μM wortmannin or 25 μM U73122 for 30 min and then further exposed to 100 nM insulin for 30 min as indicated. The cells were then solubilized, and lysates (200 μg of protein/sample) were precipitated with isoform-specific PKC antibodies (top) or MAPK antibodies (bottom). PKC and MAPK activities were then assayed in the immunoprecipitates as described in Materials and Methods. Bars represent the means ± standard deviations of duplicate determinations in four (top) and five (bottom) independent experiments.
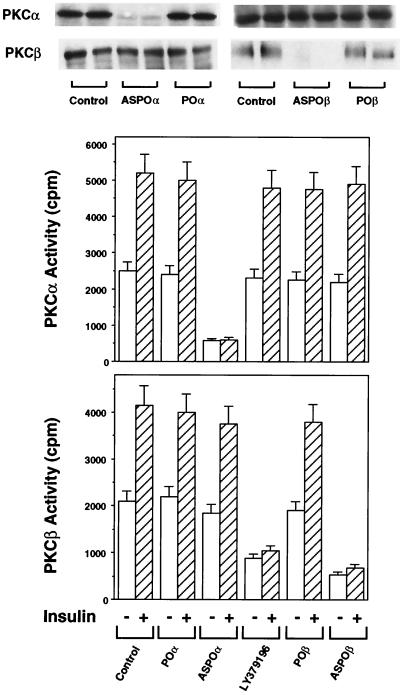
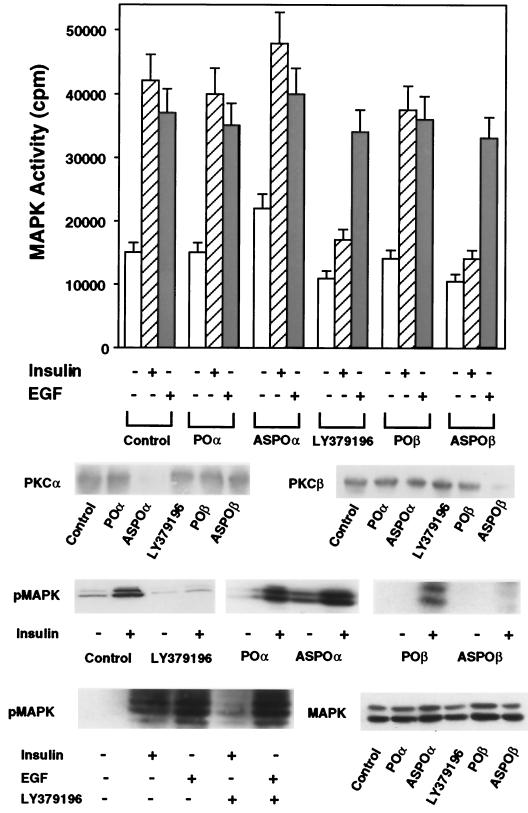
Since insulin stimulation of IRS-1 resulted in activation of PKCα and PKCβ and their induction by insulin paralleled that of MAPK, we hypothesized that one or both of these PKC isoforms may mediate the IRS-1-dependent activation of MAPK in L6 cells. To address this possibility, we used previously reported PKCα or PKCβ antisense oligonucleotides (12, 42) or LY379196, a specific inhibitor of PKCβ, to selectively inhibit the function of each of the two PKC isoforms. The specificity of LY379196 inhibition is shown by the 50% effective doses of LY379196 on the activity of PKC isoforms. The 50% effective doses (in micromolar) were as follows: 0.70 for PKCα, 0.04 for PKCβ, 0.75 for PKCδ, and 35 for PKCζ. Pretreatment of L6hIR2 cells with either PKCβ antisense oligonucleotide (ASPOβ) or LY379196 almost completely inhibited insulin-dependent PKCβ activity in immunoprecipitates from cell extracts, with no effect on PKCα (Fig. 6). The POβ control oligonucleotide showed no effect on PKCβ or PKCα activity. In addition, in PKCα immunoprecipitates derived from cells transfected with the PKCα antisense ASPOα (but not from cells transfected with the POα control oligonucleotide), insulin-stimulated PKCα activity was inhibited by >95%, while PKCβ remained unchanged. The block of PKCβ by either the antisense oligonucleotide or LY379196 was accompanied by almost complete inhibition of the insulin-stimulated (but not the EGF-stimulated) MAPK activity with only a small inhibition of basal MAPK activity (30% P < 0.001) (Fig. 7, top panel). In contrast, L6hIR2 cells transfected with the PKCα antisense oligonucleotide showed no significant change in insulin- or EGF-dependent MAPK activity compared to those measured in cells expressing the control oligonucleotide and in untreated cells. Transfection of the PKCα antisense oligonucleotide in L6hIR cells resulted in 40% increased non-insulin-dependent MAPK activity. Also, in cells transfected with the PKCα antisense oligonucleotide, basal MAPK phosphorylation was 70% higher than in cells transfected with the control oligonucleotide (Fig. 7, bottom panel). However, insulin stimulated MAPK phosphorylation by 10-fold, as in the control cells. In cells preincubated with LY379196 and cells treated with the PKCβ antisense oligonucleotide there was a marked reduction in insulin-stimulated phosphorylation of MAPK compared to a 10-fold stimulation in the untreated cells. As in the case of MAPK activity, MAPK phosphorylation in response to EGF was unaffected by PKCβ block. Neither PKCα block nor PKCβ block affected the expression levels of MAPK proteins. It appeared therefore that, in the muscle cells, PKCβ, but not PKCα, plays an important role in mediating IRS-1 activation of MAPK in response to insulin.
FIG. 6.
Blocking PKCα and PKCβ activities in L6 cells with antisense oligonucleotides or LY379196. L6hIR2 cells were preincubated with 50 nM LY379196 or transfected with either specific PKCα or PKCβ antisense oligonucleotides (ASPOα and ASPOβ, respectively). Oligonucleotides with the same base composition as the PKCα and -β antisense oligonucleotides but with random sequence (POα and POβ, respectively) were used for control. Cells were then exposed to 100 nM insulin for 30 min, solubilized, and precipitated with specific PKCα (middle) or PKCβ (bottom) antibodies. PKC activity in the immunoprecipitates was assayed as described in Materials and Methods and is plotted as radioactivity incorporated in the Ac-MBP(4-14) substrate. Bars represent the means ± standard deviations of duplicate determinations in three independent experiments. For control (top panel), lysates (300 μg of protein/assay) were subjected to SDS-PAGE and Western blotted with PKCα or PKCβ antibodies as indicated.
FIG. 7.
MAPK phosphorylation and activity in L6 cells upon blocking PKCα and -β. (Top) Antisense oligonucleotide and pharmacological blocking of PKCα or PKCβ in L6hIR2 cells was achieved as described in the legend to Fig. 5. The cells were then exposed to 100 nM insulin or EGF as indicated, solubilized, and precipitated with MAPK antibodies. MAPK activity was assayed in the immunoprecipitates as described in the legend to Fig. 4. Bars represent the means ± standard deviations of duplicate determinations in four independent experiments. For control (middle), lysates (300 μg of protein/assay) were subjected to SDS-PAGE and Western blotted with PKCα or PKCβ antibodies as indicated. Alternatively (bottom), proteins in the lysates were blotted with phosphorylated MAPK (pMAPK) or MAPK antibodies. The autoradiographs shown are representative of three (PKCα and PKCβ) or five (pMAPK and MAPK) independent Western blot determinations.
Raf activation by PKCβ.
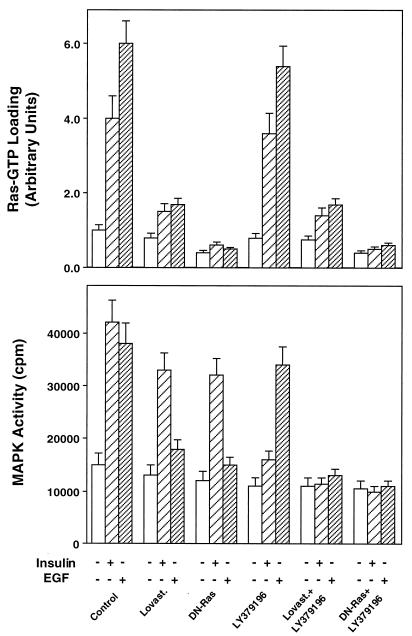
To elucidate the mechanism by which PKCβ may affect insulin action on MAPK, we inhibited insulin-dependent p21ras activation by transfection with the dominant negative mutant of Ras (L61,S186) or by preincubating the L6hIR2 cells with the HMG-coenzyme A-reductase inhibitor lovastatin (2 μg/ml). Both treatments blocked the abilities of insulin and EGF to stimulate p21ras-GTP loading (Fig. 8, top panel). In contrast, the LY379196 PKCβ inhibitor exhibited no effect on p21ras-GTP loading in response to insulin or EGF, both in the absence and presence of lovastatin or L61,S186 Ras. The lovastatin pretreatment and L61,S186 Ras expression inhibited insulin activation of MAPK by about 30% in these cells (P < 0.001) and EGF activation by almost 90% (Fig. 8, bottom panel). In contrast to these p21ras inhibitors, LY379196 decreased insulin-stimulated MAPK activity by >80% despite its complete lack of p21ras inhibitory action. EGF activation of MAPK was unaffected by LY379196. The inhibitory effects of insulin-stimulated MAPK activity by LY379196 and lovastatin or L61,S186 Ras were additive. Similar effects were observed by measuring MAPK phosphorylation instead of activity (data not shown). Thus, PKCβ seemed to control MAPK independently of Ras and at a step distal to Ras.
FIG. 8.
Insulin-dependent Ras function in L6 cells. (Top) L6hIR2 cells were treated with lovastatin or transfected with the dominant negative L61,S186 Ras mutant and/or exposed to LY379196 as indicated. The cells were then stimulated with insulin or EGF (100 nM) and solubilized, and the Ras GTP loading was estimated by the GTP overlay assay as described in Materials and Methods. Overlay filters were autoradiographed, and GTP loading was quantitated by densitometry. (Bottom) The cell lysates were precipitated with MAPK antibody, and MAPK activity was assayed as described in the legend to Fig. 4. Bars represent the means ± standard deviations of duplicate determinations in four (top) and three (bottom) independent experiments.
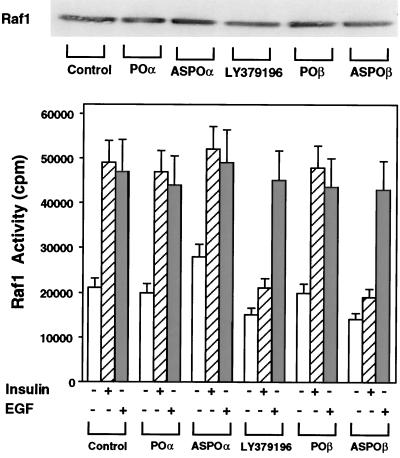
Previous work in different cell types showed that PKC may phosphorylate and activate Raf (24). We therefore sought to determine whether PKCβ may control Raf activity in L6 muscle cells. To this end, we first inhibited PKCβ activity in the cells with the antisense oligonucleotide or LY379196 and then measured phosphorylation of inactivated MEK by Raf immunoprecipitates. As shown in Fig. 9, there was little insulin-dependent Raf kinase activity in the immunoprecipitates from cells exposed to the PKCβ antisense oligonucleotide or LY379196. In the absence of the inhibitor, however, insulin caused a 2.5-fold increase in Raf kinase activity. Raf immunoprecipitates from cells transfected with the PKCα antisense oligonucleotide showed no significant difference in kinase activity compared to that in control cells. In contrast with the results with insulin, EGF stimulation of Raf was unaffected by PKCα or PKCβ block. Neither LY379196 nor any of the oligonucleotides affected Raf levels in the cells.
FIG. 9.
Insulin-dependent Raf-1 activation in L6 cells. L6hIR2 cells were treated with LY379196 or transfected with either specific PKCα or PKCβ antisense oligonucleotides (ASPOα and ASPOβ, respectively) or the control oligonucleotides specified in the legend to Fig. 5 (POα and POβ). The cells were then exposed to 100 nM insulin for 30 min, solubilized, and precipitated with Raf-1 antibodies (200 μg of protein/sample). The immunoprecipitates were assayed for Raf-1 kinase activity as described in Materials and Methods. Bars represent the means ± standard deviations of duplicate determinations in four independent experiments. For control (top panel), parallel precipitates were blotted with Raf-1 antibodies and revealed by ECL as described in Materials and Methods.
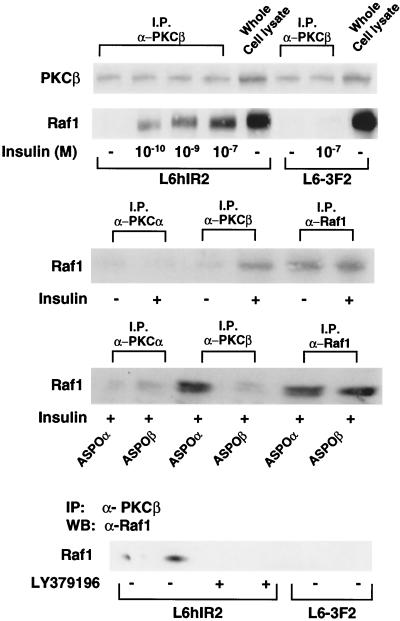
Interestingly, in L6hIR2 cells, PKCβ coprecipitated with Raf-1 (Fig. 10, top panel). This did not occur in cells expressing the kinase-deficient insulin receptor (L6-3F2). Very little Raf-1 was found in PKCα antibody precipitates from the L6hIR2 cells (Fig. 10, middle panel). Insulin increased PKCβ-Raf coprecipitation by >10-fold. The effect of insulin was dose dependent (Fig. 10, top panel), was not affected by transfection with PKCα antisense oligonucleotides, and did not occur after antisense oligonucleotide block of PKCβ or its inhibition with LY379196 (Fig. 10, bottom panels). These data suggested that, in response to insulin, PKCβ controls insulin stimulation of MAPK by direct activation of Raf-1.
FIG. 10.
Insulin-dependent PKCβ-Raf1 coprecipitation in L6 cells. (Top) L6hIR2 and L6-3F2 cells were exposed to the indicated concentrations of insulin for 30 min, solubilized, and precipitated (200 μg of protein/sample) with PKCβ antibodies as shown. Precipitates and whole-cell lysates from the two cell types were Western blotted with PKCβ antibodies, and filters were stripped and further probed with Raf-1 antibodies. For control (middle), L6hIR2 cells were stimulated with 100 nM insulin for 30 min, lysed, and precipitated with PKCα, PKCβ, or Raf-1 antibodies as indicated. Precipitates were Western blotted with Raf-1 antibodies. Alternatively (bottom), the cells were transfected with PKCα or PKCβ antisense oligonucleotides or treated with LY379196, stimulated with 100 nM insulin for 30 min, precipitated with PKCα, PKCβ, or Raf-1 antibodies, and blotted with Raf-1 antibodies. All filters were revealed by ECL as described in Materials and Methods. The autoradiographs shown are representative of three (top and middle) and three (bottom) independent experiments. Abbreviations: I.P. and IP, immunoprecipitation; α-PKCβ, antibody against PKCβ; WB, Western blotting.
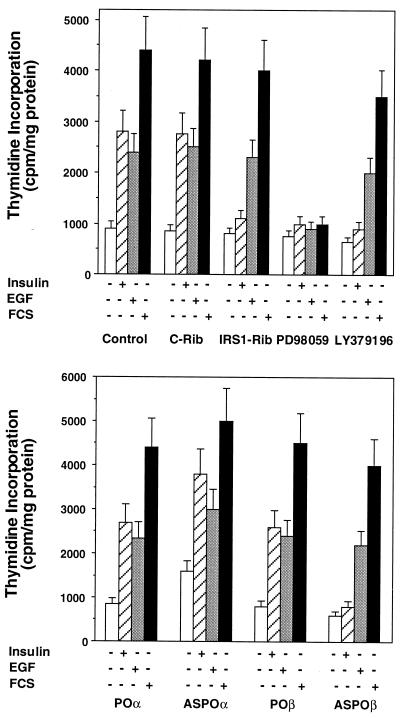
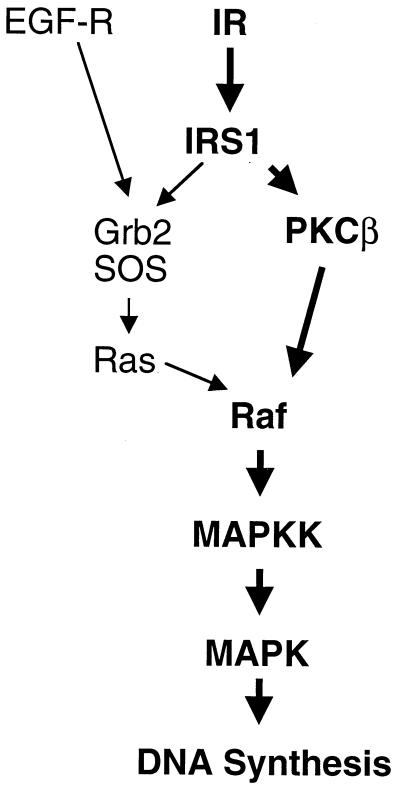
In L6hIR cells, inhibition of PKCβ with LY379196 was accompanied by a >90% inhibition of insulin-stimulated thymidine incorporation with only a slight decrease in basal incorporation (Fig. 11, top panel). The same was observed upon cell transfection with IRS-1 rib or cell treatment with the MAPK inhibitor PD98059 (50 μM). PD98059 also blocked EGF- and serum-stimulated thymidine incorporation in L6 cells. Unlike the results with insulin, however, EGF- and serum-induced thymidine incorporation were unaffected by both the PKCβ inhibitor and IRS-1 rib. Like the LY379196 PKCβ inhibitor, PKCβ antisense oligonucleotide specifically blocked insulin- but not EGF- or serum-induced thymidine incorporation in L6hIR cells (Fig. 11, bottom panel). In contrast, PKCα antisense oligonucleotide had no effect on thymidine incorporation, whether stimulated by insulin, EGF, or serum. This indicated that, in L6 cells, EGF and insulin mitogenic signals converged on MAPK through distinct pathways, the first via Ras and the second, bypassing Ras, through direct activation of Raf (Fig. 12).
FIG. 11.
Thymidine incorporation in L6 cells following insulin, EGF, or serum exposure. L6hIR2 cells were transfected with the IRS-1 or the control ribozymes (IRS1-Rib or C-Rib, respectively) or pretreated with the MAPK or PKCβ inhibitors PD98059 and LY379196 (top). Alternatively, the cells were transfected with either the specific PKCα or PKCβ antisense oligonucleotide (ASPOα or ASPOβ, respectively) or the control oligonucleotides specified in the legend to Fig. 5 (POα and POβ). The cells were exposed to 100 nM insulin or to EGF or to 10% fetal calf serum (FCS) for 12 h, as indicated. [3H]thymidine incorporation into DNA was then assayed as described in Materials and Methods. Bars represent the means ± standard deviations of triplicate determinations in four independent experiments.
FIG. 12.
Schematic comparison of the mechanisms involved in insulin- and EGF-stimulated mitogenesis in L6 cells. Unlike the situation with EGF and other serum growth factors, insulin-induced MAPK activity in L6 cells appears to occur largely through Raf-1 rather than Ras activation. EGF-R, EGF receptor. The boldfaced pathway has been described for the first time in this paper.
DISCUSSION
Insulin activates PKC in a variety of cell types and tissues (3, 7, 18). In addition, certain PKC isoforms have been reported to play an important role in insulin action (13, 27, 47), regulation of IR kinase (5, 15) and intracellular sorting (18). However, the specific role of each individual PKC isoform in insulin signaling and the molecular mechanism causing PKC activation by insulin are still unclear. For instance, the role of PKCβ in insulin action has not been consistently reported (4, 7, 13, 48). In L6 skeletal muscle cells, we have shown that IR kinase activity is necessary to allow insulin activation of multiple PKC isoforms, including classical (α and β), novel (δ), and atypical (ζ) PKCs. In fact, mutant IRs featuring substitution of the regulatory tyrosines with phenylalanines simultaneously lose their abilities to undergo kinase activation in response to insulin (12, 29) and to transduce insulin activation of these different PKCs. In the muscle cells, however, the molecular mechanisms involved in receptor activation of the classical PKCs versus the other PKC isoforms appear to be distinct. Hence, we report that blocking IRS-1 expression with an IRS-1 ribozyme selectively impairs insulin activation of PKCα and -β without affecting PKCδ and -ζ. Whether insulin activation of PKCδ and -ζ is mediated by substrates other than IRS-1, such as IRS-2, and their specific role in insulin signaling, are presently under investigation in our laboratory.
We have also shown that, in L6 cells, the activation of PKCβ is necessary for insulin stimulation of MAPK function and for insulin proliferative responses. In fact, inhibition of PKCβ activity by either antisense oligonucleotide or the LY379196 specific agent (as well as the inhibition of IRS-1 expression) almost completely blocks the effects of insulin on MAPK and on DNA synthesis. The involvement of PKCβ in mitogenic signal transduction appears to be specific for the insulin mitogenic pathway, since neither the PKCβ antisense oligonucleotide nor LY379196 affected EGF- or serum-induced mitogenesis.
Brüning et al. (8) have recently reported that, in fibroblasts from animals with IRS-1 knockout, insulin-like growth factor I can still activate MAPK and induce DNA synthesis. However, the molecular mechanism remains elusive. The different roles of IRS-1 in transducing mitogenic signals in these fibroblasts and in the muscle cells may depend on diversity in the pathways used by insulin and insulin-like growth factor I for inducing cell proliferation. In addition, mitogenic signals may feature tissue specificity. In fact, while abundant in insulin-responsive tissues (7, 38) and necessary for insulin-induced mitogenesis in muscle cells (this study) and in mouse hepatocytes (data not shown), PKCβ is not even expressed in fibroblasts (20). This indicates diversity in the events responsible for insulin-induced mitogenesis in fibroblasts compared to insulin-responsive tissues. In addition, different cells may use different PKC isoforms to enter the MAPK pathway. Consistent with this, Sajan et al. (39) have recently reported that, in rat adipocytes, PKCζ plays a major role in insulin induction of MAPK activity. At variance, we report here that, in muscle cells, wortmannin elicits only slight inhibition of insulin-induced MAPK activity while completely blocking that of PKCζ.
Despite the relevance of PKCβ activation to insulin-regulated MAPK function, pharmacological inhibition of MAPK produced no effect on insulin-dependent PKCβ activity (data not shown). Thus, at least in the L6 muscle cells, activation of PKCβ by insulin seems to occur upstream of MAPK. Previous reports for 3T3-L1 adipocytes showed that PKCs may inhibit GTPase-activating proteins and activate Ras, conveying insulin signal through the MAPK cascade (41). In the L6 cells, however, selective block of Ras with lovastatin or a dominant negative Ras mutant causes only a slight inhibition of insulin-stimulated MAPK activation, which is an additive effect to that caused by the PKCβ inhibitor LY379196. This indicates that PKCβ activation of the MAPK pathway is largely independent of Ras in muscle cells. In fact, we showed that PKCβ inhibition almost completely blocked insulin activation of Raf-1 kinase in the L6 cells. Thus, in these cells, Raf-1 kinase represents at least one of the molecules conveying insulin mitogenic signals downstream of PKCβ. Most mitogenic signals initiated by tyrosine kinase receptors are believed to converge on MAPK mainly through direct Ras activation (19). However, Raf-1 kinase has been previously reported to be also phosphorylated and activated by PKCs in different cell types (24, 25, 45). In addition, Raf activation by PKC enables phorbol ester and vascular endothelial growth factor mitogenic signal transduction to MAPK independent of Ras (50, 52). Also, in 3T3-L1 adipocytes, insulin activates MAPK through an unknown mechanism independent of Ras (11). We now provide evidence that, in muscle cells, IRS-1-mediated PKCβ activation may directly activate Raf, thus inducing MAPK activity and the mitogenic pathway independently of Ras (Fig. 10). In addition, we provide evidence that PKCβ activation is necessary for driving its interaction with Raf. Hence, treatment of cells with the PKCβ inhibitor LY379196 prevents PKCβ-Raf coprecipitation similar to the antisense oligonucleotide block of PKCβ expression. To our knowledge, the data in the present report represent the first indication that insulin, unlike EGF and other serum growth factors, activates MAPK and mitogenesis mainly through PKCβ and largely bypasses Ras in insulin target tissues such as the muscle cells.
In agreement with the results of other investigators (16, 23, 46), the mechanism of IRS-1-dependent activation of PKCβ in L6 cells may involve insulin triggering of phospholipase activity which, in turn, activates PKCβ. In fact, we report that treatment of L6 cells with the phospholipase C inhibitor U73122 simultaneously blocks PKCβ and MAPK activation by insulin. In contrast, insulin-induced PI 3-kinase activation does not appear to be a major mechanism responsible for induction of PKCβ and mitogenesis in L6 cells. In fact, the PI 3-kinase inhibitor wortmannin elicits little effect on PKCβ activation in L6 cells, and in the same cells, insulin-stimulated PI 3-kinase activity persists in association with IRS-2 during treatment with IRS-1 ribozyme, a condition under which MAPK activity is inhibited.
Previous work in other laboratories has shown that, in different cell types, PKCβ overexpression results in increased serine/threonine phosphorylation of the IR with inhibition of its tyrosine kinase (5, 14). Based on these findings, it has been proposed that PKCβ regulates insulin signaling in cells at the receptor level. In the present report, we show that PKCβ enables insulin mitogenic signal, at least in part, by controlling Raf-1 kinase activation. Thus, these data identify PKCβ as a novel molecule in the insulin mitogenic pathway and indicate that PKCβ may be involved in insulin action both at the receptor and post-receptor levels.
Unlike PKCβ, blocking PKCα by antisense oligonucleotide expression in L6 cells increased basal MAPK phosphorylation and activity while not affecting insulin stimulation of MAPK. In L6 cells, PKCα is physically associated to the IR and phosphorylates the receptor on serine and threonine residues, inhibiting its kinase activity (12). We have previously shown that release of the PKCα inhibitory action on the receptor, either by inhibiting PKCα expression or by inducing its dissociation from the receptor, activates the receptor kinase and glucose uptake, independent of insulin (12). The block of PKCα by antisense oligonucleotide is likely to be responsible for the constitutive activation of the insulin mitogenic pathway as well. It appears from these data that multiple PKC isoforms are embedded in the insulin mitogenic and metabolic pathways controlling the flow of signals either at the receptor level or at more distal step in its signaling cascade.
ACKNOWLEDGMENTS
P. Formisano and F. Oriente contributed equally to this work.
This work was supported in part by the European Community (grant QLG1-CT-1999-00674), grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC), the Ministero dell' Università e della Ricerca Scientifica, and the C.N.R. Target Project on Biotechnology to F.B. The financial support of Telethon-Italy (grant 0896 to F.B.) is gratefully acknowledged. Matilde Caruso and Giovanni Vigliotta are recipients of fellowships of the Federazione Italiana per la Ricerca sul Cancro (FIRC).
We thank E. Consiglio for continuous support and advice during the course of this work. We also thank L. Beguinot (DIBIT, H.S. Raffaele, Milan, Italy) for advice and critical reading of the manuscript, M. Quon for providing the IRS-1 ribozyme, M. Bifulco for donating the dominant negative Ras cDNA, and D. Liguoro for the technical help.
REFERENCES
- 1.Antonetti D A, Algenstaedt P, Kahn C R. Insulin receptor substrate 1 binds two novel splice variants of the regulatory subunit of phosphatidylinositol 3-kinase in muscle and brain. Mol Cell Biol. 1996;16:2195–2203. doi: 10.1128/mcb.16.5.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araki E, Lipes M A, Patti M E, Bruning J C, Haag B, Johnson R S, Kahn C R. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature. 1994;372:186–190. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- 3.Bandyopadhyay G, Standaert M L, Zhao L, Yu B, Avignon A, Galloway L, Karnam P, Moscat J, Farese R V. Activation of protein kinase C (alpha, beta, and zeta) by insulin in 3T3/L1 cells. Transfection studies suggest a role for PKC-zeta in glucose transport. J Biol Chem. 1997;272:2551–2558. doi: 10.1074/jbc.272.4.2551. [DOI] [PubMed] [Google Scholar]
- 4.Bandyopadhyay G, Standaert M L, Galloway L, Moscat J, Farese R V. Evidence for involvement of protein kinase C (PKC)-ζ and noninvolvement of diacylglycerol-sensitive PKCs in insulin-stimulated glucose transport in L6 myotubes. Endocrinology. 1997;138:4721–4731. doi: 10.1210/endo.138.11.5473. [DOI] [PubMed] [Google Scholar]
- 5.Bossenmaier B, Mosthaf L, Mischak H, Ullrich A, Haring H U. Protein kinase C isoforms beta 1 and beta 2 inhibit the tyrosine kinase activity of the insulin receptor. Diabetologia. 1997;40:863–866. doi: 10.1007/s001250050761. [DOI] [PubMed] [Google Scholar]
- 6.Boulton T G, Nye S H, Robbins D J, Ip N Y, Radziejewska E, Morgenbesser S D, DePinho R A, Panayotatos N, Cobb M H, Yancopoulos G D. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991;65:663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- 7.Braiman L, Sheffi-Friedman L, Bak A, Tennenbaum T, Sampson S R. Tyrosine phosphorylation of specific protein kinase C isoenzymes participates in insulin stimulation of glucose transport in primary cultures of rat skeletal muscle. Diabetes. 1999;48:1922–1929. doi: 10.2337/diabetes.48.10.1922. [DOI] [PubMed] [Google Scholar]
- 8.Brüning J C, Winnay J, Cheatham B, Kahn C R. Differential signaling by insulin receptor substrate 1 (IRS-1) and IRS-2 in IRS-1-deficient cells. Mol Cell Biol. 1997;17:1513–1521. doi: 10.1128/mcb.17.3.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bucci C, Parton R G, Mather I H, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- 10.Cai H, Smola U, Wixler V, Eisenmann-Tappe I, Diaz-Meco M T, Moscat J, Rapp U, Cooper G M. Role of diacylglycerol-regulated protein kinase C isotypes in growth factor activation of the Raf-1 protein kinase. Mol Cell Biol. 1997;17:732–741. doi: 10.1128/mcb.17.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carel K, Kummer J L, Schubert C, Leitner W, Heidenreich K A, Draznin B. Insulin stimulates mitogen-activated protein kinase by a Ras-independent pathway in 3T3-L1 adipocytes. J Biol Chem. 1996;271:30625–30630. doi: 10.1074/jbc.271.48.30625. [DOI] [PubMed] [Google Scholar]
- 12.Caruso M, Miele C, Oriente F, Maitan A, Bifulco G, Andreozzi F, Condorelli G, Formisano P, Beguinot F. In L6 skeletal muscle cells, glucose induces cytosolic translocation of protein kinase C alfa and transactivates the insulin receptor kinase. J Biol Chem. 1999;274:28637–28644. doi: 10.1074/jbc.274.40.28637. [DOI] [PubMed] [Google Scholar]
- 13.Chalfant C E, Ohno S, Konno Y, Fisher A A, Bisnauth L D, Watson J E, Cooper D R. A carboxy-terminal deletion mutant of protein kinase C beta II inhibits insulin-stimulated 2-deoxyglucose uptake in L6 rat skeletal muscle cells. Mol Endocrinol. 1996;10:1273–1281. doi: 10.1210/mend.10.10.9121494. [DOI] [PubMed] [Google Scholar]
- 14.Chin J E, Dickens M, Tavare J M, Roth R A. Overexpression of protein kinase C isoenzymes alpha, beta I, gamma, and epsilon in cells overexpressing the insulin receptor. Effects on receptor phosphorylation and signaling. J Biol Chem. 1993;268:6338–6347. [PubMed] [Google Scholar]
- 15.Chin J E, Liu F, Roth R A. Activation of protein kinase C alpha inhibits insulin-stimulated tyrosine phosphorylation of insulin receptor substrate-1. Mol Endocrinol. 1994;8:51–58. doi: 10.1210/mend.8.1.7512195. [DOI] [PubMed] [Google Scholar]
- 16.Donchenko V, Zannetti A, Baldini P M. Insulin-stimulated hydrolysis of phosphatidylcholine by phospholipase C and phospholipase D in cultured rat hepatocytes. Biochim Biophys Acta. 1994;1222:492–500. doi: 10.1016/0167-4889(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 17.Formisano P, Sohn K J, Miele C, Di Finizio B, Petruzziello A, Riccardi G, Beguinot L, Beguinot F. Mutation in a conserved motif next to the insulin receptor key autophosphorylation sites deregulates kinase activity and impairs insulin action. J Biol Chem. 1993;268:5241–5248. [PubMed] [Google Scholar]
- 18.Formisano P, Oriente F, Miele C, Caruso M, Auricchio R, Vigliotta G, Condorelli G, Beguinot F. In NIH3T3 fibroblasts, insulin receptor interaction with specific protein kinase isoforms controls receptor intracellular routing. J Biol Chem. 1998;273:13197–13202. doi: 10.1074/jbc.273.21.13197. [DOI] [PubMed] [Google Scholar]
- 19.Garrington T P, Johnson G L. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol. 1999;11:211–218. doi: 10.1016/s0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- 20.Hug H, Sarre T F. Protein kinase C isoenzymes: divergence in signal transduction? Biochem J. 1993;291:329–343. doi: 10.1042/bj2910329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaken S. Protein kinase C isozymes and substrates. Curr Opin Cell Biol. 1996;8:168–173. doi: 10.1016/s0955-0674(96)80062-7. [DOI] [PubMed] [Google Scholar]
- 22.Jirousek M R, Gillig J R, Heath W F, Gonzalez C M, McDonald III J H, Neel D A, Rito C J, Stramm L E, Singh U, Melikan-Badalian A, Baevsky M, Ballas L M, Hall S E, Winneroski L L, Faul M M. ( S )-13-[ ( monomethylamino ) methyl ] -10,11,14,15-tetrahydro-4,9:16,21-dimetheno-1H,13H-dibenzo [ E,K ] pyrrolo- [ 3,4-H ] [ 1,4,13 ] oxadiza-cyclohexadecin-1,3(2H)-dione ( LY333531) and related analogues: isozyme selective inhibitors of protein kinase Cb (PKCb) J Med Chem. 1996;39:2664–2671. doi: 10.1021/jm950588y. [DOI] [PubMed] [Google Scholar]
- 23.Kayali A G, Eichhorn J, Haruta T, Morris A J, Nelson J G, Vollenweider P, Olefsky J M, Webster N J. Association of the insulin receptor with phospholipase C-gamma (PLCgamma) in 3T3-L1 adipocytes suggests a role for PLCgamma in metabolic signaling by insulin. J Biol Chem. 1998;273:13808–13818. doi: 10.1074/jbc.273.22.13808. [DOI] [PubMed] [Google Scholar]
- 24.Kolch W, Heidecker G, Kochs G, Hummel R, Vahidi H, Mischak H, Finkenzeller G, Marme D, Rapp U R. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993;364:249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- 25.Marais R, Light Y, Mason C, Paterson H, Olson M F, Marshall C J. Requirement of Ras-GTP-Raf complexes for activation of Raf-1 by protein kinase C. Science. 1998;280:109–112. doi: 10.1126/science.280.5360.109. . (Erratum, 280:987.) [DOI] [PubMed] [Google Scholar]
- 26.Mellor H, Parker P J. The extended protein kinase C superfamily. Biochem J. 1998;332:281–292. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendez R, Kollmorgen G, White M F, Rhoads R E. Requirement of protein kinase Cζ for stimulation of protein synthesis by insulin. Mol Cell Biol. 1997;17:5184–5192. doi: 10.1128/mcb.17.9.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miele C, Caruso M, Calleja V, Auricchio R, Oriente F, Formisano P, Condorelli G, Cafieri A, Sawka-Verhelle D, Van Obberghen E, Beguinot F. Differential role of insulin receptor substrate (IRS)-1 and IRS-2 in L6 skeletal muscle cells expressing the Arg1152→Gln insulin receptor. J Biol Chem. 1999;274:3094–3102. doi: 10.1074/jbc.274.5.3094. [DOI] [PubMed] [Google Scholar]
- 29.Murakami M S, Rosen O M. The role of insulin receptor autophosphorylation in signal transduction. J Biol Chem. 1991;266:22653–22660. [PubMed] [Google Scholar]
- 30.Myers M G, Jr, Backer J M, Sun X J, Shoelson S, Hu P, Schlessinger J, Yoakim M, Schaffhausen B, White M F. IRS-1 activates phosphatidylinositol 3′-kinase by associating with src homology 2 domains of p85. Proc Natl Acad Sci USA. 1992;89:10350–10354. doi: 10.1073/pnas.89.21.10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myers M G, Jr, Wang L M, Sun X J, Zhang Y, Yenush L, Schlessinger J, Pierce J H, White M F. Role of IRS-1-GRB-2 complexes in insulin signaling. Mol Cell Biol. 1994;14:3577–3587. doi: 10.1128/mcb.14.6.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newton A C. Regulation of protein kinase C. Curr Opin Cell Biol. 1997;9:161–167. doi: 10.1016/s0955-0674(97)80058-0. [DOI] [PubMed] [Google Scholar]
- 33.Nishida E, Gotoh Y. The MAP kinase cascade is essential for diverse signal transduction pathways. Trends Biochem Sci. 1993;18:128–131. doi: 10.1016/0968-0004(93)90019-j. [DOI] [PubMed] [Google Scholar]
- 34.Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988;334:661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- 35.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 36.Noh D Y, Shin S H, Rhee S G. Phosphoinositide-specific phospholipase C and mitogenic signaling. Biochim Biophys Acta. 1995;1242:99–113. doi: 10.1016/0304-419x(95)00006-0. [DOI] [PubMed] [Google Scholar]
- 37.Quon M J, Butte A J, Zarnowski M J, Sesti G, Cushman S W, Taylor S I. Insulin receptor substrate 1 mediates the stimulatory effect of insulin on GLUT4 translocation in transfected rat adipose cells. J Biol Chem. 1994;269:27920–27924. [PubMed] [Google Scholar]
- 38.Rogue P, Labourdette G, Masmoudi A, Yoshida Y, Huang F L, Huang K P, Zwiller J, Vincendon G, Malviya A N. Rat liver nuclei protein kinase C is the isozyme type II. J Biol Chem. 1990;265:4161–4165. [PubMed] [Google Scholar]
- 39.Sajan M P, Standaert M L, Bandyopadhyay G, Quon M J, Burke T R, Farese R V. Protein kinase C-ζ and phosphoinositides-dependent protein kinase-1 are required for insulin-induced activation of ERK in rat adipocytes. J Biol Chem. 1999;274:30495–30500. doi: 10.1074/jbc.274.43.30495. [DOI] [PubMed] [Google Scholar]
- 40.Santillo M, Mondola P, Gioielli A, Serù R, Iossa S, Annella T, Vitale M, Bifulco M. Inhibitors of ras farnesylation revert the increased resistance to oxidative stress in K-ras transformed NIH 3T3 cells. Biochem Biophys Res Commun. 1996;229:739–745. doi: 10.1006/bbrc.1996.1874. [DOI] [PubMed] [Google Scholar]
- 41.Schubert C, Carel K, DePaolo D, Leitner W, Draznin B. Interactions of protein kinase C with insulin signaling. Influence on GAP and Sos activities. J Biol Chem. 1996;271:15311–15314. doi: 10.1074/jbc.271.26.15311. [DOI] [PubMed] [Google Scholar]
- 42.Simpson R U, O'Connel T D, Pan Q, Newhouse J, Somerman M J. Antisense oligonucleotides targeted against protein kinase C-beta and C-betaII block 1,25-(OH)2D3-induced differentiation. J Biol Chem. 1998;273:19587–19591. doi: 10.1074/jbc.273.31.19587. [DOI] [PubMed] [Google Scholar]
- 43.Skolnik E Y, Batzer A, Li N, Lee C H, Lowenstein E, Mohammadi M, Margolis B, Schlessinger J. The function of GRB2 in linking the insulin receptor to Ras signaling pathways. Science. 1993;260:1953–1955. doi: 10.1126/science.8316835. [DOI] [PubMed] [Google Scholar]
- 44.Skolnik E Y, Lee C H, Batzer A, Vicentini L M, Zhou M, Daly R, Myers Jr M J, Backer J M, Ullrich A, White M F, et al. The SH2/SH3 domain-containing protein GRB2 interacts with tyrosine-phosphorylated IRS1 and Shc: implications for insulin control of ras signalling. EMBO J. 1993;12:1929–1936. doi: 10.1002/j.1460-2075.1993.tb05842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sozeri O, Vollmer K, Liyanage M, Frith D, Kour G, Mark G E, Stabel S. Activation of the c-Raf protein kinase by protein kinase C phosphorylation. Oncogene. 1992;7:2259–2262. [PubMed] [Google Scholar]
- 46.Standaert M L, Bandyopadhyay G, Zhou X, Galloway L, Farese R V. Insulin stimulates phospholipase D-dependent phosphatidylcholine hydrolysis, Rho translocation, de novo phospholipid synthesis, and diacylglycerol/protein kinase C signaling in L6 myotubes. Endocrinology. 1996;137:3014–3020. doi: 10.1210/endo.137.7.8770926. [DOI] [PubMed] [Google Scholar]
- 47.Standaert M L, Galloway L, Karnam P, Bandyopadhyay G, Moscat J, Farese R V. Protein kinase C-zeta as a downstream effector of phosphatidylinositol 3-kinase during insulin stimulation in rat adipocytes. Potential role in glucose transport. J Biol Chem. 1997;272:30075–30082. doi: 10.1074/jbc.272.48.30075. [DOI] [PubMed] [Google Scholar]
- 48.Standaert M L, Bandyopadhyay G, Galloway L, Soto J, Ono Y, Kikkawa U, Farese R V, Leitges M. Effects of knockout of the protein kinase C β gene on glucose transport and glucose homeostasis. Endocrinology. 1999;140:4470–4477. doi: 10.1210/endo.140.10.7073. [DOI] [PubMed] [Google Scholar]
- 49.Sun X J, Crimmins D L, Myers Jr M G, Miralpeix M, White M F. Pleiotropic insulin signals are engaged by multisite phosphorylation of IRS-1. Mol Cell Biol. 1993;13:7418–7428. doi: 10.1128/mcb.13.12.7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takahashi T, Ueno H, Shibuya M. VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene. 1999;18:2221–2230. doi: 10.1038/sj.onc.1202527. [DOI] [PubMed] [Google Scholar]
- 51.Tamemoto H, Kadowaki T, Tobe K, Yagi T, Sakura H, Hayakawa T, Terauchi Y, Ueki K, Kaburagi Y, Satoh S, Sekihara H, Yoshioka S, Horikoshi H, Furuta Y, Ikawa Y, Kasuga M, Yazaki Y, Aizawa S. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature. 1994;372:182–186. doi: 10.1038/372182a0. [DOI] [PubMed] [Google Scholar]
- 52.Ueda Y, Hirai S, Osada S, Suzuki A, Mizuno K, Ohno S. Protein kinase C activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J Biol Chem. 1996;271:23512–23519. doi: 10.1074/jbc.271.38.23512. [DOI] [PubMed] [Google Scholar]
- 53.Wang Z, Glück S, Zhang L, Moran M F. Requirement for phospholipase C-γ1 enzymatic activity in growth factor-induced mitogenesis. Mol Cell Biol. 1998;18:590–597. doi: 10.1128/mcb.18.1.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.White M F. The insulin signalling system and the IRS proteins. Diabetologia. 1997;40(Suppl. 2):S2–S17. doi: 10.1007/s001250051387. [DOI] [PubMed] [Google Scholar]
- 55.Zou Y, Komuro I, Yamazaki T, Aikawa R, Kudoh S, Shiojima I, Hiroi Y, Mizuno T, Yazaki Y. Protein kinase C, but not tyrosine kinases or ras, plays a critical role in angiotensin II-induced activation of Raf-1 kinase and extracellular signal-regulated protein kinases in cardiac myocytes. J Biol Chem. 1996;271:33592–33597. doi: 10.1074/jbc.271.52.33592. [DOI] [PubMed] [Google Scholar]