Abstract
Granulocyte colony-stimulating factors (G-CSFs) have been used post hematopoietic stem cell transplant (HSCT) for earlier neutrophil engraftment. The use of G-CSFs, and their effect on other post-HSCT outcomes remains debatable. In this systematic review and meta-analysis, we searched PubMed, Embase, Cochrane library, Google Scholar, and IndMed using a predefined search strategy. We included randomized controlled trials (RCTs) and non-randomized studies (NRSs) reporting data on G-CSF administration post-HSCT, published in the English language from their inception until Jan 31, 2021. The primary outcome of this systematic review and meta-analysis was to evaluate the time to neutrophil engraftment (NE). The secondary outcomes were probability of NE, time to platelet engraftment (PE), the incidence of graft-versus-host disease (GVHD), duration of hospital stay (HS), and overall survival (OS). The review is registered with PROSPERO (CRD42020206989). Fourteen studies were extracted (n=9850), of which five were RCTs, and nine were NRSs. As per Egger’s test, publication bias was not present for any outcome. After meta-analysis, we found that the duration of NE favouring G-CSF arm from RCTs was -0.94 days (SMD) [(95% CI: -1.38, -0.51); I2=35%], and from NRSs -1.2 days (SMD) [(95% CI: -1.43, -0.96); I2=74%]. For the outcome of GVHD, the relative risks (RR) of incidence for chronic GVHD and overall GVHD were not significant for the RCTs, and these were 1.11 (RR) [(95% CI: 1.00, 1.22); I2=43%] and 1.10 (RR) [(95% CI: 1.03, 1.18); I2=48%], respectively for NRSs. There was no difference in the incidence of GVHD (acute or chronic) in both arms. No significant difference was found between the two arms for the outcomes of PE, HS, and OS. For NE, there was a marginal benefit of around one day with the use of G-CSF. The use of G-CSF did not alter time to PE, the incidence of GVHD, HS, and OS in both arms.
Keywords: G-CSF, allogeneic, stem cell transplantation, neutrophil engraftment, platelet engraftment, graft-versus-host disease, hospital stay, overall survival
Introduction
Granulocyte colony-stimulating factors (G-CSFs) are used in hematopoietic stem cell transplant (HSCT) post-infusion of the hematopoietic stem cells (HSCs) to accelerate the recovery of neutrophils. There is evidence to support that use of G-CSF could shorten the duration of hospital stay [1]. Both G-CSF and granulocyte monocyte-colony stimulating factor (GM-CSF) were used post HSCT for accelerating neutrophil recovery in the past, but it is G-CSF that is predominantly used these days [2]. There is evidence that the use of G-CSF post HSCT may be associated with an increased risk of graft versus host disease (GVHD) [3]. Whether the reduction in the duration and severity of neutropenia post HSCT translates into a better overall survival, betterment in quality of life, or a reduction in costs of treatment is open to question. A systematic review and meta-analysis conducted by Dekker et al. (2006) showed that post HSCT, colony-stimulating factors (CSFs) reduced the risk of documented infections (relative risk [RR] 0.87; 95% CI, 0.76 to 1.00; P=.05) and duration of parenteral antibiotics (weighted mean difference, -1.39 days, 95% CI, -2.56 to -0.22; P=.02) but did not reduce infection-related mortality (TRM) (RR, 0.76; 95% CI, 0.41 to 1.44; P=0.4). CSFs did not increase grade 2 to 4 acute GVHD (RR, 1.03; 95% CI, 0.81 to 1.31; P=.8) or TRM (RR, 1.00; 95% CI, 0.78 to 1.29; P=.98). This analysis by Dekker et al. was detailed, but it was an analysis only of randomized controlled trials (RCTs) published up to 2006 [4]. This study did not analyze the evidence from non-randomized studies (NRSs).
Further, it included many studies that used GM-CSF, a colony-stimulating factor that is no longer used for the given indication. Their analysis included studies on both autologous and allogeneic HSCT. Subsequently, a review published by Trivedi et al. in 2009 [1] also described the use of G-CSF in both autologous and allogeneic settings. The authors did not perform a meta-analysis of the studies. They reported that post HSCT, G-CSF is commonly used to enhance stem cell engraftment to minimize the morbidity and mortality associated with prolonged neutropenia. They concluded that the use of G-CSF given after allogeneic transplantation has been shown to be reasonably safe and effective in reducing the time to neutrophil engraftment by 1-2 days in most studies; However, there is no consensus on the optimal use of G-CSF after high-dose chemotherapy followed by HSCT [1]. Subsequent to these reviews, more studies have been published which have tried to address this issue.
The aim of our study was to conduct an updated systematic review and meta-analysis to revisit the status of evidence regarding the use of G-CSF post hematopoietic stem cell transplant. Our objective was to determine the impact of G-CSF versus the comparator (either placebo or no drug) on the outcomes of neutrophil engraftment (NE), platelet engraftment (PE), acute graft versus host disease (ac GVHD) grade II-IV, chronic graft versus host disease (ch GVHD), duration of hospital stay (HS), and overall survival (OS) post allogeneic HSCT.
Methods
Overview
This study was exempted from approval of our Institute Ethics Committee (IEC). We used the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) statement for reporting systematic reviews and meta-analyses as a guide for this study [5] (Appendix 1). The protocol for the review was registered with the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42020206989) [6].
Search strategy and selection criteria
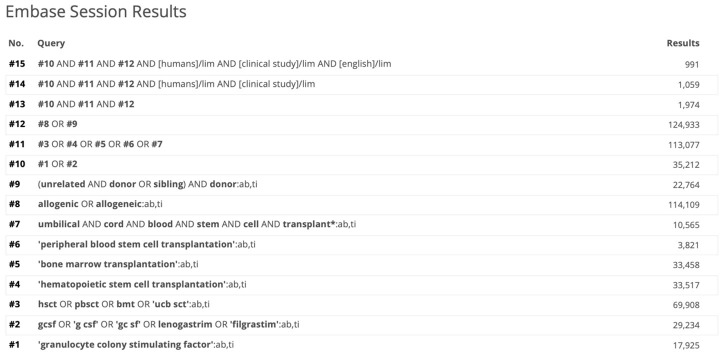
We searched the databases of PubMed, Embase, Cochrane library, Google Scholar, and IndMed using a predefined search strategy (Appendix 2-1, 2-2 and 2-3). We searched relevant articles from their inception till Jan 31, 2021. Hand-searching of the references of relevant articles was done. Further, we also did a hand-search of the abstracts published in the EBMT, SIOP, and ASBMT meetings from 2001 to 2020. We searched the publications published in the English language only. The search was carried out independently by two investigators (AKG and JPM). Any discrepancy was resolved by the third author (PH).
Eligibility criteria
Studies that had reported the use of G-CSF post allogeneic HSCT and had compared the outcomes with patients in which either no G-CSF or placebo was used were included. There were no restrictions based on age, disease, type of allogeneic HSCT, source of HSC, type of conditioning regimen, and GVHD prophylaxis. The meta-analysis was done for the following predefined clinical outcomes: time to NE and probability of NE, time to PE, ac GVHD gr II-IV, ch GVHD, HS, and OS. The characteristics of the studies in terms of age, conditioning, type of donor, source of HSC, the dose of stem cells, and GVHD prophylaxis used were also noted.
Data extraction and risk of bias assessment
We designed a data collection form in Microsoft Excel [7] to extract and enter the relevant data-fields from the selected full-text studies. The data collection sheet included the author’s information, year of publication, patient numbers, type of conditioning, stem cell source, type of donor, stem cell dose, data on NE, PE, ac GVHD, ch GVHD, duration of HS, and OS. The Newcastle Ottawa Scale (NOS) [8], modified for cohort studies, was used to assess the quality of NRSs included in this review [9]. Using NOS criteria, quality assessment was performed by using nine items allocated to three main categories: selection (score 4), comparability (score 2), and outcomes assessment (score 3). The interpretation of the scores were as follows: Good quality: 3 or 4 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome/exposure domain; Fair quality: 2 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome/exposure domain; Poor quality: 0 or 1 star in selection domain OR 0 stars in comparability domain OR 0 or 1 stars in outcome/exposure domain.
For the RCTs, we used version 2 of the Cochrane risk-of-bias tool (ROB2) and analyzed the risk of bias (ROB) for all the outcomes [10]. The assessment was done for the domains of the randomization process, deviations from intended interventions, missing outcome data, measurement of outcome, and selection of reported results. The final assessment for each study was graded as low risk, some concerns, or high risk.
Outcomes
The primary outcome of this systematic review and meta-analysis was to determine the time to NE. The secondary outcomes were probability of NE, time to PE, ac GVHD gr II-IV, ch GVHD, HS, and OS.
Data-analysis and statistical methods
All meta-analyses were performed using R and dependencies, and the random-effects model was considered for pooling the effect size. Categorical data were compared using the Mantel-Haenszel method with fixed effect measures that produced relative risk (RR) with 95% confidence intervals (95% CIs). Continuous data were compared using inverse variance with random effect measures with data presented as standardized mean difference (SMD) with 95% CIs. As per the recommendation of the Cochrane guidebook for meta-analysis, we conducted separate analyses for the RCTs and the NRSs for each of the following outcomes: the time (in days) to achieve NE, the probability of NE, time (in days) to achieve PE, GVHD, HS, and OS. For GVHD, we also performed a sub-group analysis based on the acute or chronic nature of the GVHD. The heterogeneity of the research results was determined by the calculation of a I2 statistic and p-value. If the 95% CI of outcomes for RR did not include 1, or for SMD did not include zero, then we concluded that the outcomes were statistically significant.
Between studies, heterogeneity was quantified by Higgin’s & Thompson’s I2 value; an I2 of >50% was investigated. The investigation initially checked for errors due to data entry. To further explore the heterogeneity, we identified studies that were an outlier if that study’s CI did not overlap with the CI of the pooled effect, i.e., checking for extremely large effect sizes or extremely small effect sizes. We generated the pooled effect size again after the removal of those studies by updating the metanalysis. Outlier studies were removed. Since I2 is affected by study size, we also calculated the prediction interval to determine the range within which a potential future study might be present.
Publication bias was looked for by assessing asymmetry around pooled effect size in the funnel plot, visually; statistical assessment of the asymmetry was done using Egger’s test of the intercept (Appendix 3), wherein a significant result (P<0.05) indicated the presence of substantial asymmetry of the funnel plot. The risk of bias was plotted for the RCTs using the excel workbook of ROB2 [10].
Results
Search results
A total of 3236 abstracts were screened, and 60 abstracts were selected whose full texts were evaluated. After the review of the full texts, 14 studies (20 comparison groups; described below) were included for the final analysis. The flowchart of the studies is shown in Figure 1.
Figure 1.
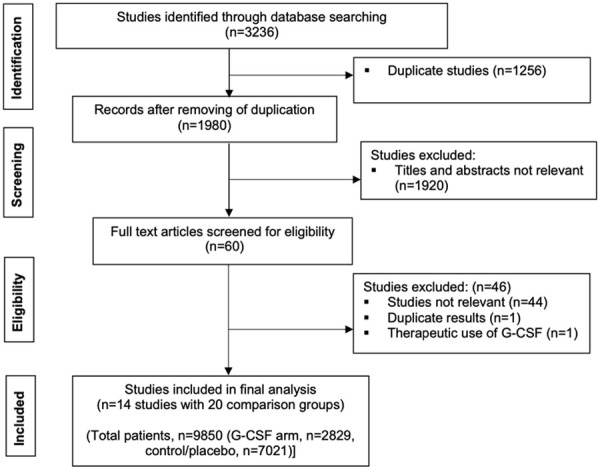
Flow diagram showing inclusion and exclusion of studies (G-CSF-granulocyte colony stimulating factor).
Description of the studies
Amongst the studies selected, 5 were RCTs (6 comparison groups) [11-16] and 9 NRSs (14 comparison groups) [2,3,17-23]. A few of these studies have compared various subgroups who either got G-CSF (G-CSF arm) or placebo/no G-CSF (control arm) post-infusion of HSC, and the data were presented in these studies as per the subgroups; we decided to analyze them as separate studies/groups. Thus, out of 5 RCTs, we had six groups for comparison [11-16], and out of 9 NRSs, we had 14 groups (total 20 comparison groups) [2,3,17-23].
Creation of comparison groups within studies
The study by Dallorso et al. had two groups-matched family donor (MFD) (n=53) and matched unrelated donor (MUD) (n=38), so it was analyzed as two studies; Dallorso-A and Dallorso-B [14]. Similarly, the study by Schriber et al., was divided into two groups based on the GVHD prophylaxis used as Schriber-A [cyclosporine (CSA)/methotrexate (MTX)/prednisolone (PSE)] [n=17 (G-CSF) vs n=27 (control)] and Schriber-B [cyclosporine (CSA)/prednisolone (PSE)] [n=13 (G-CSF) vs n=25 (control)] studies [17]. The studies by Ringden et al. [20] were analysed as Ringden-A (BM) [n=501 (G-CSF) vs n=1288 (control)] and Ringden-B (PB) [n=175 (G-CSF) vs n=259 (control)] studies. The study by Khoury et al. [21] was analysed as three studies: Khoury-A (HLA-identical sibling bone marrow) [n=282 (G-CSF) vs n=1153 (control)], Khoury-B (HLA-identical sibling peripheral blood) [n=216 (G-CSF) vs n=393 (control)] and Khoury-C (Matched unrelated bone marrow) [n=270 (G-CSF) vs n=405 (control)]. The analysis of the study by George et al. [23] was done in two groups (based on type of donor): George-A [MFD: n=313 (G-CSF) vs n=1174 (control)] and George-B [MUD: n=417 (G-CSF) vs n=1658 (control)] studies. As the study by Bishop et al. and Joshi et al. had the same set of patients, we analyzed them as a single group [11,12]. The summary of the creation of groups is depicted in Table 1.
Table 1.
Summary of creation of comparison groups
| Comparison groups’ summary | |||||
|---|---|---|---|---|---|
|
| |||||
| Studies | Basis of grouping | Groups | Total (n) | G-CSF (n) | Control/placebo (n) |
| Dallorso et al., 2002 [14] | Type of donor | MFD: Dallorso-A | 53 | 23 | 30 |
| MUD: Dallorso-B | 38 | 20 | 18 | ||
| Schriber et al., 1994 [17] | Type of GVHD prophylaxis | CSA/MTX/PSE: Schriber-A | 44 | 17 | 27 |
| CSA/PSE: Schriber-B | 38 | 13 | 25 | ||
| Ringdén et al., 2004 [20] | Source of stem cells | BM: Ringden-A | 1789 | 501 | 1288 |
| PB: Ringden-B | 434 | 175 | 259 | ||
| Khoury et al., 2006 [21] | Type of donor and source of stem cells | HLA-identical sibling BM: Khoury-A | 1435 | 282 | 1153 |
| HLA-identical sibling PB: Khoury-B | 609 | 216 | 393 | ||
| Matched unrelated BM: Khoury-C | 675 | 270 | 405 | ||
| George et al., 2020 [23] | Type of donor | MFD: George-A | 1487 | 313 | 1174 |
| MUD: George-B | 2075 | 417 | 1658 | ||
PB: Peripheral blood; BM: Bone marrow; MFD: Matched family donor; MUD: Matched unrelated donor; CDA: Cyclosporine; PSE: Prednisolone; MTX: Methotrexate.
A total of 9850 patients were included in these studies (G-CSF arm, n=2829 and control/placebo arm, n=7021). Among the 20 comparison groups (14 studies), 9 were adult groups [11-13,16-18,22,23], eight groups included adult and pediatric patients [2,15,19-21], two groups included exclusively pediatric patients [14], and there was no mention of the age in one group [3]. Characteristics of all the included studies in the meta-analysis are provided in Table 2.
Table 2.
Characteristics of all the included studies in meta-analysis
| Study | Year | Type of study | Total patients | Population | G-CSF (n) | Control/placebo (n) | Stem cell source | Conditioning | Type of donor | Dose of G-CSF | Route of G-CSF | Day of G-CSF initiation | MNC (×108/kg)/CD34 (×106/kg) (G-CSF arm) | MNC (×108/kg)/CD34 (×106/kg) (control/placebo arm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bishop/Joshi [11,12] | 2000/2003 | RCT | 50 | A | 26 | 24 | PB | MA | MFD | 10 | SC | 0 | MNC (range): 8.09 (2.95-15.77) | MNC (range): 8.05 (5.38-14.94) |
| CD34 (range): 10.36 (4.02-20.32) | CD34 (range): 8.12 (2.2.5-26.17) | |||||||||||||
| Przepiorka [13] | 2001 | RCT | 42 | A | 21 | 21 | PB | MA | Matched donor | 10 | SC | 1 | MNC (range): 5.8 (2.3-19.1) | 8.3 (2.8-23.8) |
| CD34 (range): 4.8 (2.2-20.5) | CD43 (range): 4.3 (1.9-16.2) | |||||||||||||
| Dallorso-A [14] | 2002 | RCT | 53 | P | 23 | 30 | BM | MFD | 10 | IV | 5 | - | - | |
| Dallorso-B [14] | 2002 | RCT | 38 | P | 20 | 18 | BM | MUD | 10 | IV | 5 | - | - | |
| Ernst [15] | 2008 | RCT | 51 | A, P | 25 | 26 | BM | MA | MFD | 5 | IV | 0 | MNC: >2 | MNC: >2 |
| Ben Othma [16] | 2018 | RCT | 145 | A | 69 | 76 | BM | MA | MFD | 5 | IV | 7 | MNC (range): 2.2 (1-3.87) | MNC (range): 1.8 (0.7-4.16) |
| Schriber-A [17] | 1994 | NRS | 44 | A | 17 | 27 | BM | MA | MFD | - | - | - | ||
| Schriber-B [17] | 1994 | NRS | 38 | A | 13 | 25 | BM | MA | MFD | - | - | - | ||
| Berger [18] | 1999 | NRS | 47 | A | 22 | 25 | BM | MA | MUD | 5 | IV | 1 | MNC (range): 2.75 (1.53-7.5) | MNC (range): 2.5 (1.2-4.25) |
| Ozcan [19] | 2001 | NRS | 56 | A, P | 28 | 28 | PB | MA | MFD | IV | 1 | MNC (range): 7.7 (2.71-29.3) | MNC (range): 6.4 (3.1-38.2) | |
| CD34 (range): 6.4 (2.99-30.8) | CD34 (range): 6.5 (2.1-18.9) | |||||||||||||
| Remberger [20] | 2003 | NRS | 155 | A, P | 66 | 89 | BM, PB | MA | MFD | 5 | IV | 10 | MNC (range): 5.1 (0.8-25.6) | MNC (range): 2.4 (0.7-10.7) |
| Ringdén-A [20] | 2004 | NRS | 1789 | A, P | 501 | 1288 | BM | MFD | - | - | 4 (0-14) | MNC (range): 2.92 (0.02-90) | MNC (range): 2.75 (0.15-73) | |
| CD34 (range): 3.52 (1-82.2) | CD34 (range): 3.5 (1.04-82) | |||||||||||||
| Ringdén-B [20] | 2004 | NRS | 434 | A, P | 175 | 259 | PB | MFD | - | - | 7 (0-14) | MNC (range): 8.4 (0.5-42) | MNC (range): 9.55 (0.5-83) | |
| CD34 (range): 5.7 (1.5-83.5) | CD34 (range): 6.1 (1-70) | |||||||||||||
| Khoury-A [21] | 2006 | NRS | 1435 | A, P | 282 | 1153 | BM | MA | MFD | - | - | 5 (0-7) | - | - |
| Khoury-B [21] | 2006 | NRS | 609 | A, P | 216 | 393 | PB | MA | MFD | - | - | 3 (0-7) | - | - |
| Khoury-C [21] | 2006 | NRS | 675 | A, P | 270 | 405 | BM | MA | MUD | - | - | 6 (0-7) | - | - |
| Ringden [3] | 2010 | NRS | 465 | - | 260 | 205 | BM, PB | MA, RIC | MFD, MUD | - | - | - | MNC (range): 3.8 (0.2-59.3) | MNC (range): 3.2 (0.6-63.8) |
| Singh [22] | 2020 | NRS | 162 | A | 65 | 97 | PB | MA, RIC | MFD, MUD | 5 | - | - | CD34 (range): 6.9 (2.1, 22.5) | CD34 (range): 7.1 (2.1, 25.6) |
| George-A [23] | 2020 | NRS | 1487 | A | 313 | 1174 | PB | MA, RIC | MFD | - | -- | - | - | |
| George-B [23] | 2020 | NRS | 2075 | A | 417 | 1658 | PB | MA, RIC | MUD | - | - | - | - |
RCT: Randomised controlled trial, NRS: Non-randomised study; A: Adult; P: Pediatric; PB: Peripheral blood; BM: Bone marrow; MA: Myeloablative; RIC: Reduced intensity conditioning; MFD: Matched family donor; MUD: Matched unrelated donor; SC: Subcutaneous; IV: Intravenous; MNC: Multinucleated cells.
In eight of the groups, peripheral blood (PB) was used as an HSC source [11-13,19-23], and in 10, it was bone marrow (BM) [14-18]. In two groups, both PB and BM were employed as an HSC source [2,3].
The type of conditioning used was mentioned in 16 groups, whereas it was not mentioned for four [14,20]. It was myeloablative (MA) in 12 groups [2,11-13,15-19,21], and a combination of MA and reduced-intensity conditioning (RIC) in 4 groups [3,22,23]. In 13 groups, a matched family donor (MFD) was the HSC donor [2,3,11,12,14-17,19-21,23]. A matched unrelated donor (MUD) was used in 4 studies [14,18,21,23] and a combination of MUD and MFD in 2 studies [3,22]. The dose of G-CSF varied between the groups compared, and in the ones that mentioned it, the dose was 5 mcg/kg/day for five [2,15,16,18,22], and 10 mcg/kg/day for four [11-14]. The day of G-CSF initiation post the infusion of HSC had a range from 0 days to 10 days. The route of GSCF used was intravenous in seven [14-16], and it was subcutaneous (SC) in two comparison groups [11-13], whereas the route was not mentioned in the rest.
Quality assessment of the studies
The risk of bias (ROB) for the outcome of NE is given in Figure 2. All the RCTs had a low risk of bias for the above outcome [11-16]. For the other outcomes, none of the studies were adjudged to have a high risk of bias. The methodological quality of the NRSs using the Newcastle-Ottawa Scale (NOS) for all outcomes is given in Table 3.
Figure 2.
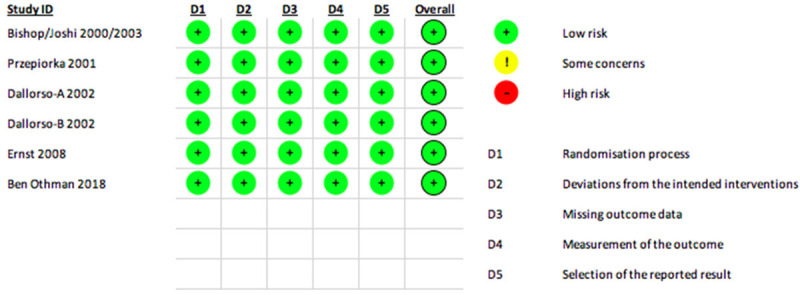
Risk of bias (ROB) assessment of the included studies in meta-analysis for the outcome of Neutrophil engraftment.
Table 3.
The methodological quality of the non-randomised studies (NRSs) using the Newcastle-Ottawa Scale (NOS)
| The Newcastle-Ottawa Scale (NOS) of NRS studies | ||
|---|---|---|
|
| ||
| Study | Outcomes assessed | Remarks |
| Schriber (1994) [17] | NE, ac GVHD II-IV | Poor quality |
| Berger (1999) [18] | All outcomes | Good quality |
| Ozcan (2001) [19] | All outcomes | Good quality |
| Remberger (2003) [2] | All outcomes | Good quality |
| Ringdén (2004) [20] | NE, ac GVHD II-IV, ch GVHD, OS | Good quality for NE, Poor for others |
| Khoury (2006) [21] | All outcomes except PE | Good quality |
| Ringden (2010) [3] | All outcomes | Good quality |
| Singh (2021) [22] | All outcomes | Good quality |
| George (2020) [23] | NE, HS | Good quality |
NE: Neutrophil engraftment; ac GVHD: Acute graft vs host disease; ch GVHD: Chronic graft vs host disease; PE: Platelet engraftment; OS: Overall survival, HS: Hospital stay.
Time to achieving neutrophil engraftment
The meta-analysis of all the 6 eligible comparison groups (n=379) [11-16] showed that the use of G-CSF had an advantage for neutrophil engraftment [SMD: -1.46 days (95% CI: -2.32, -0.60); I2=92%] (Figure 3). After removal of the 3 outlier studies [14,16], we included 3 RCTs for the final meta-analysis [11-13,15]. (Supplementary Figures 1, 2, 3) and found a time to NE -0.94 days (SMD) [(95% CI: -1.38, -0.51); I2=35%]; favoring the G-CSF arm and the heterogeneity decreased substantially at this stage.
Figure 3.
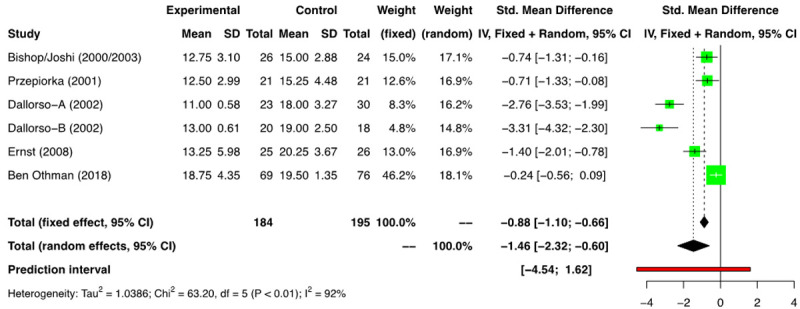
Forest plot of RCTs for neutrophil engraftment (NE).
In the meta-analysis of the 13 NRS comparison groups (n=9471) [2,3,17-23] we found a time to NE of -1.68 days (SMD) [(95% CI: -2.66, -0.71); I2=100%] favouring the G-CSF arm (Figure 4). However, owing to high heterogeneity, a stepwise outlier study removal was performed. The final analysis involving 7 NRSs (Supplementary Figures 4, 5, 6) showed the time to NE of -1.2 days (SMD) [(95% CI: -1.43, -0.96); I2=74%] favoring the G-CSF arm (Supplementary Figure 6).
Figure 4.
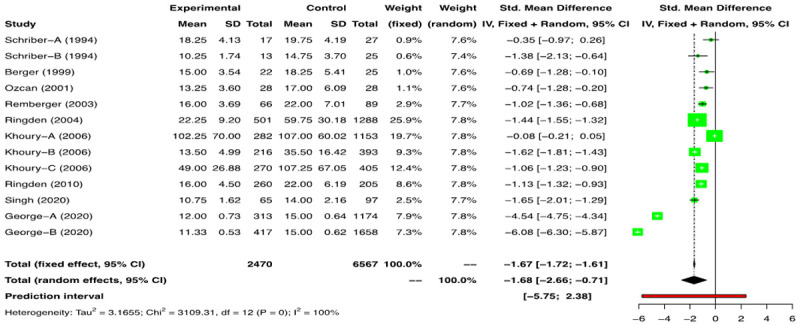
Forest plots of non-randomized studies (NRSs) for neutrophil engraftment (NE).
We found that G-CSF was associated with a statistically significant earlier recovery of neutrophils; however, this benefit was only 1.2 days for NRSs and 0.94 days for RCTs.
Probability of successfully achieving neutrophil engraftment
We analyzed the 8 NRSs groups and one RCT that reported the outcome. The meta-analysis of the NRSs groups [3,18,21-23] showed a risk ratio (RR) of 1.1 [(95% CI: 0.99, 1.01); I2=0] (Figure 5), and the single RCT which reported the above outcome had a RR of 1.04 (95% CI: 0.98, 1.1) [16] (Figure 6). The use of G-CSF did not alter the probability of achieving NE.
Figure 5.
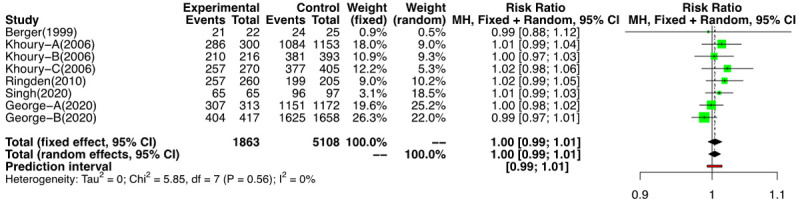
Forest plot of probability of non-randomized studies (NRSs) for neutrophil engraftment (NE).
Figure 6.

Forest plot of probability of RCT for neutrophil engraftment (NE).
Time to achieve platelet engraftment
Data in relation to the time taken to achieve platelet engraftment was heterogenous for the available studies. As the definitions of PE differed amongst the studies, we included those studies that provided the data on time taken to reach an unsupported platelet count of >20000/mm3. Seven comparison groups reported the above outcome [11-13,16-19]. In the meta-analysis of the 3 RCTs [11-13,16] (n=237) a time to PE was 0.10 days (SMD) [(95% CI: -0.44, 0.64); I2=72%] was found between the two groups (Figure 7). A meta-analysis of the four NRSs (n=185) showed the time to PE was 0.03 days (SMD) [(95% CI: -0.79, 0.86); I2=86%] between the two groups (Figure 8). The analysis did not find a difference in time to PE between the G-CSF and non-G-CSF arms.
Figure 7.
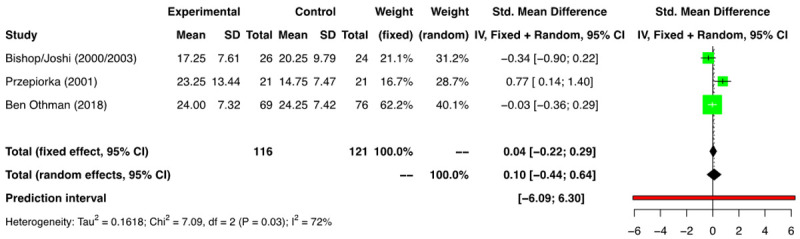
Forest plot of RCTs for platelet engraftment (PE).
Figure 8.
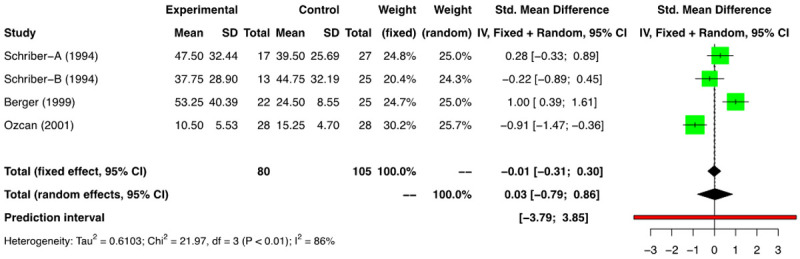
Forest plot of non-randomized studies (NRSs) for platelet engraftment (PE).
Acute GVHD grade II-IV
Out of a total of 18 comparison groups that reported on acute GVHD grade II-IV (n=6288), 6 were RCTs (n=377) [11-16], and 12 were NRSs (n=5909) [2,3,17-22]. The meta-analysis of the RCTs for the above outcome showed a RR of 0.91 [(95% CI: 0.71, 1.17); I2=0%] (Figure 9). The analysis of the NRSs revealed a RR of 1.15 [(95% CI: 0.95, 1.39); I2=60%] (Figure 10). Therefore, the use of G-CSF was not associated with an increased risk of acute GVHD grade II-IV. Funnel plots of RCTs and NRSs for the above outcome are provided in Supplementary Figures 7 and 8.
Figure 9.
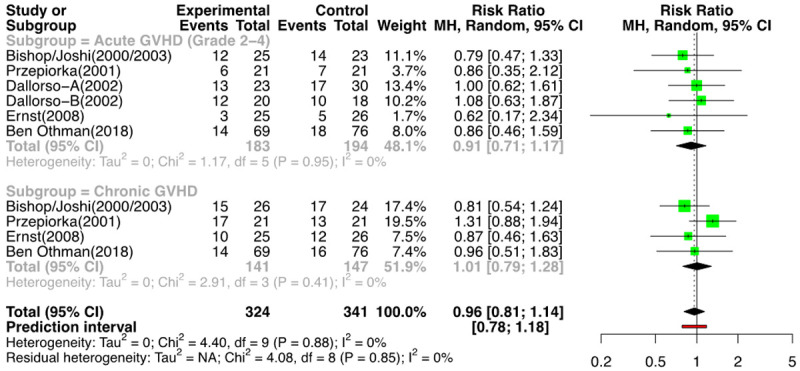
Forest plot of RCTs for GVHD.
Figure 10.
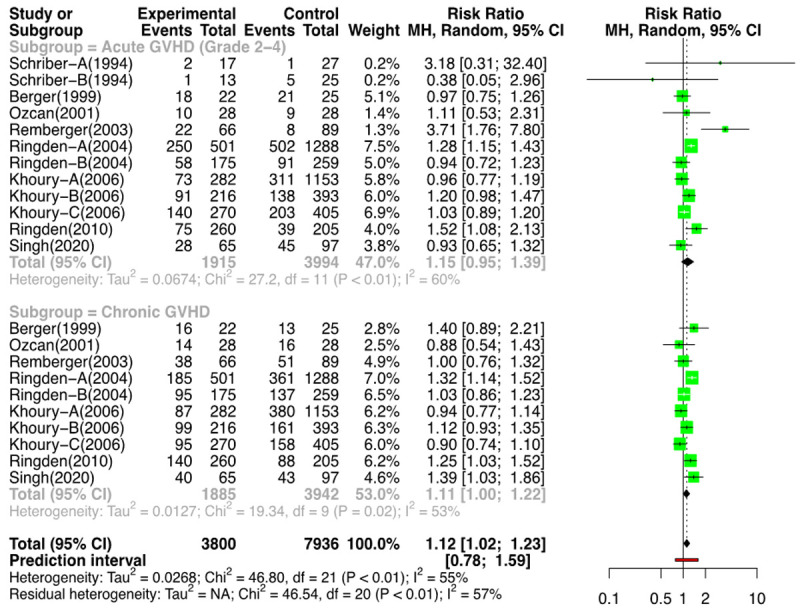
Forest plot non-randomized studies (NRSs) for GVHD.
Chronic GVHD
Fourteen groups (n=6115); 4 RCTs (n=288) [11-13,15,16] and 10 NRSs (n=5827) [2,3,18-21,23] reported the outcomes for ch GVHD. On meta-analysis for the risk of ch GVHD, for the RCTs, the RR was 1.01 [(95% CI: 0.79; 1.28); I2=0%] (Figure 9), and for the NRSs, it was 1.11 [(95% CI: 1.00; 1.22); I2=53%] favoring the control arm (i.e., less incidence of ch GVHD with the use of G-CSF) (Figure 10). The forest plot of NRSs for GVHD (without outliers) is provided in Supplementary Figure 9.
Overall GVHD
When we analyzed the overall probability to cause GVHD (acute gr II to IV or chronic), the chance of any GVHD happening in the control/placebo arm compared to the G-CSF arm, there was a RR=0.96 [(95% CI: 0.81, 1.14); I2=0%] for the RCTs (Figure 9) and RR=1.12 [(95% CI: 1.02, 1.23); I2=57%] in the NRSs. It can be concluded that there was no increased risk of acute GVHD (gr II to IV) or ch GVHD with the use of G-CSF. Although the RR for acute grade II-IV GVHD, chronic GVHD, and overall GVHD incidence, after removing the outlier NRSs, was 1.10, 1.11, and 1.10, respectively, the lower limit of the confidence intervals in all was touching the line of no difference (Supplementary Figure 9).
Duration of hospital stay
Out of 14 comparison groups: 5 RCTs (n=379) [11-16] and 9 NRSs (n=7110) [2,3,17-23] reported data in relation to HS. Amongst the RCTs, two groups [11,12,16] provided only the median duration without range, so we conducted a meta-analysis of remaining RCTs [13-15] and found a HS of -1.04 days (SMD) [(95% CI: -1.91, -0.17); I2=86%], favoring the G-CSF arm (Figure 11). On excluding the outlier study we got a difference of-0.64 days (SMD) [(95% CI: -1.30; 0.03); I2=74%] (Supplementary Figure 12). The analysis of 9 NRSs, revealed a difference in HS of -0.11 day (SMD) [(95% CI: -0.96; 0.73); I2=100%] between the two arms (Figure 12) and on exclusion of the outliers this value was 0.28 day (SMD) [(95% CI: -0.16; 0.73); I2=92%] (Supplementary Figure 15). There was no difference in the duration of hospitalization between the two arms. The funnel plots for RCTs and NRSs are provided in Supplementary Figures 10, 11, 13 and 14.
Figure 11.
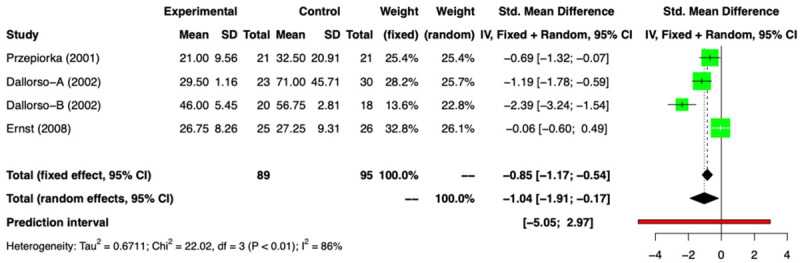
Forest plot of RCTs for Hospital stay (HS).
Figure 12.
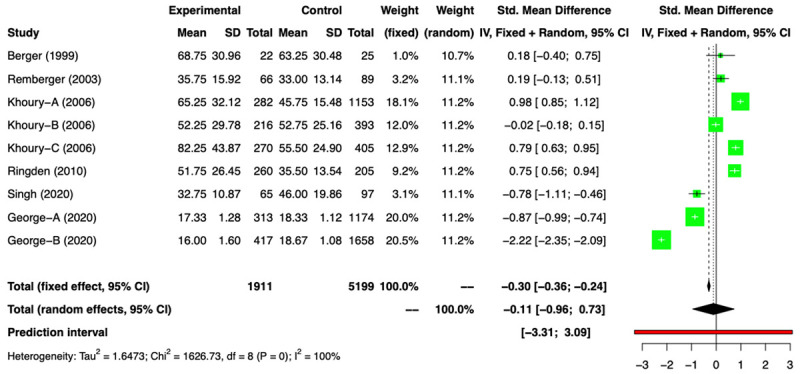
Forest plot of non-randomized studies (NRSs) for Hospital stay (HS).
Overall survival
Data on OS was listed in 4 RCTs (n=2880) [11-16] and a meta-analysis of these showed a RR of 1.06 [(95% CI: 0.90; 1.26); I2=0%] (Figure 13). Amongst the NRSs, we performed a meta-analysis of 6 groups that reported on OS (n=2946) [2,3,18-20], and a RR of 0.98 [(95% CI: 0.86; 1.13); I2=63%] was found (Figure 14). No difference in OS was noted between the two arms.
Figure 13.
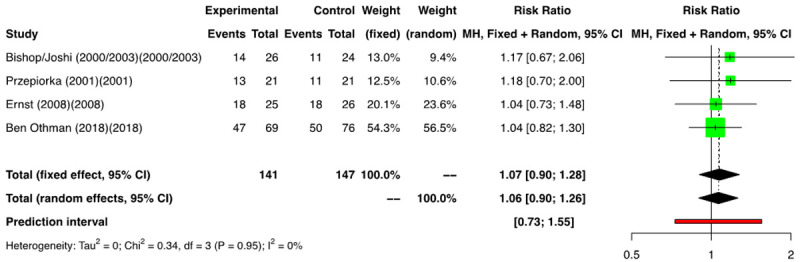
Forest plot of RCTs for overall survival (OS).
Figure 14.
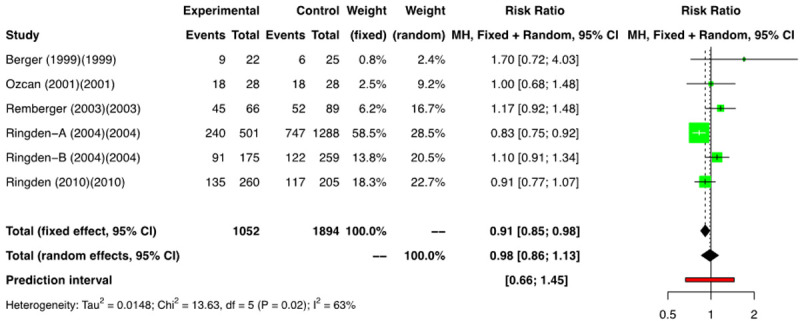
Forest plot of non-randomized studies (NRSs) for overall survival (OS).
The summary of outcomes in G-CSF vs control/placebo groups is described in Table 4.
Table 4.
Summary of outcomes in G-CSF versus control/placebo groups
| Outcomes of G-CSF versus control/placebo groups | |||||
|---|---|---|---|---|---|
|
| |||||
| Outcome | No of comparison groups | Effect | 95% CI | P | I2 |
| Neutrophil engraftment | |||||
| RCTs | 3* | -0.94 (SMD) | -1.38; -0.51 | 0.21 | 35% |
| NRSs | 7* | -1.20 (SMD) | -1.43; -0.96 | <0.01 | 74% |
| Platelet engraftment | |||||
| RCTs | 3* | -0.10 (SMD) | -0.44; -0.64 | 0.03 | 72% |
| NRSs | 4* | 0.03 (SMD) | -0.79; 0.86 | <0.01 | 86% |
| ac GVHD II-IV | |||||
| RCTs | 6 | 0.91 (RR) | 0.71; 1.17 | 0.85 | 0% |
| NRSs | 11* | 1.10 (RR) | 1.00; 1.22 | 0.06 | 43% |
| ch GVHD | |||||
| RCTs | 4 | 1.01 (RR) | 0.79; 1.28 | 0.88 | 0% |
| NRSs | 10* | 1.11 (RR) | 1.00; 1.22 | 0.02 | 53% |
| Hospital stay | |||||
| RCTs | 3* | -0.64 (SMD) | -1.30; 0.03 | 0.02 | 74% |
| NRSs | 4* | 0.28 (SMD) | -0.16; 0.73 | <0.01 | 92% |
| Overall survival | |||||
| RCTs | 4 | 1.06 (RR) | 0.90; 1.26 | 0.95 | 0% |
| NRSs | 6 | 0.98 (RR) | 0.86, 1.13 | 0.02 | 63% |
After excluding outliers (the values that are not marked with * did not have outliers in the analysis);
SMD: Standard mean difference; RCT: Randomized controlled trial; NRS: Non-randomized studies.
Discussion
The decision to use G-CSF post allogeneic HSCT is debatable. Multiple RCTs and NRSs have been published to evaluate their role in HSCT outcomes. Whereas most studies have demonstrated a benefit in the shortening of the duration of neutropenia, it remains a subject of contention whether this benefit is clinically meaningful and whether it translates into successful outcomes for other post HSCT parameters like HS and OS.
The review by Trivedi et al. showed that with the use of G-CSF post allogenic HSCT, a faster NE was achieved by 1-2 days. The evidence was more substantial with the use of PB HSC. There was no effect on the time to PE, the incidence of GVHD, HS, and OS with the use of G-CSF. They concluded that the use of G-CSF was safe in patients undergoing allogeneic HSCT, as long as they receive PB HSC. No substantial conclusion was made about the safety and efficacy of G-CSF with the use of BM HSC [1].
Dekker et al. in 2006 had published a systematic review and meta-analysis of the use of colony-stimulating factors after HSCT. The review included both autologous and allogeneic HSCT, and the authors studied the use of both G-CSF and GM-CSF. They concluded that CSFs were associated with a small reduction in the risk of documented infections but did not affect infection or treatment-related mortality. There was also no change in the incidence of GVHD with the use of G-CSF [4].
In our systematic review, we found that there was earlier engraftment of neutrophils with the use of G-CSF. Our results are consistent with the findings by Trivedi et al., who too found that G-CSF shortened the duration of neutropenia in autologous HSCT and reduced the time to engraftment in allogeneic HSCT by 1-2 days. A reduction in the risk of febrile neutropenia has also been documented by Wang et al. [24] in patients receiving myelosuppressive chemotherapy for those who received G-CSF. Cooper et al. also found a decrease in the FN risk in adult patients post-chemotherapy with the use of G-CSF [25]. The duration by which a faster NE was achieved is approximately one day with the use of G-CSF in our review (1.2 days for RCTs and 0.94 days for NRSs). We did not explore the clinical implication of this duration of earlier NE in terms of a cost and benefit analysis. The benefit of an earlier NE by one day in the G-CSF arm is debatable.
It is speculated that the use of G-CSF can lead to a delay in the engraftment of platelets due to a selective proliferation of myeloid progenitors [26]. Our analysis, however, did not find any difference in the time to PE with the use of G-CSF. This finding supports the level 1B evidence that administrations of CSFs do not delay PE post-HSCT [1].
Several NRSs have shown an increased incidence of GVHD with the use of CSFs [2,27]. An increased IL-6 level in the donor cells, when exposed to G-CSF, has been postulated as a cause for the increased GVHD. This finding has, however has been refuted in the meta-analysis by Dekker et al. and Ho et al. [4,28]. Similarly, Trivedi et al. reviewed 6 RCTs and found that the use of CSFs was not associated with an increase in the incidence of GVHD [1]. In our review, we found no increase in the risk of acute or chronic GVHD with the use of G-CSF. The RR for ac GVHD grade II-IV, ch GVHD, and overall GVHD incidence was 1.10, 1.11, and 1.10 respectively for NRSs (i.e., decreased risk with the use of G-CSF) (Supplementary Figure 9); however, the lower limit of the confidence intervals all the analyses was touching the line of no difference.
In our review and analysis, no difference in the duration of HS post HSCT was found between the two arms. In allogeneic HSCT, the duration of hospital stay has been found to be more with BMT than PBSC [2,13,18,21,29]. Both with PBSC and BMT, no significant difference has been found in the duration of HS with the use of G-CSF in both NRSs and RCTs [2,13,18,21,29]. Trivedi et al. too have concluded in their review that the benefit of early engraftment with G-CSF has not been shown to significantly decrease the length of hospital stay or improve the survival outcomes [1]. It is prudent to conclude that the early NE, just by approximately one day, demonstrated in our analysis would not translate into a meaningful reduction in the duration of HS.
Ringden et al. [20] have shown a higher TRM and decreased OS with the use of G-CSF in patients undergoing BM HSCT. This has, however, not been supported by the findings of many other studies, where the use of G-CSF post HSCT has not been shown to affect TRM and OS irrespective of the source of HSC [1]. The trend of G-CSF not affecting the important post-transplant outcomes seen in other reviews also reflects in the OS of our study. Both the analysis of the RCTs and NRSs did not show a survival difference between the two arms.
Authors view
The authors conclude that post HSCT use of G-CSF is associated with a minimal benefit in reducing the duration of neutropenia. Although a cost and benefit analysis was not performed in this review, it is unlikely that G-CSF administration would lead to clinical and financially relevant differences in outcomes. In the author’s own experience, in patients of juvenile myelomonocytic leukemia (JMML), where the authors group doesn’t use GCSF post-HSCT, the time to neutrophil engraftment is later but not significantly delayed in comparison to allogeneic HSCT where G-CSF is used.
Strengths
Our review was a robust systematic review and meta-analysis, which included both the NRSs and the RCTs that have tried to address the question. Previous analyses have included both G-CSF and GM-CSF, whereas G-CSF is the one mainly used for this indication nowadays. Our review focussed primarily on G-CSF and its use post-infusion of HSC in allogeneic HSCT. We conducted the meta-analysis of the NRSs and RCTs separately. Our review also comprehensively analyzed the important early (NE, PE, and ac GVHD), intermediate (hospital stay and ac GVHD), and late outcomes post HSCT (ch GVHD and OS).
Limitations
The limitations of our study were that we could not analyze all the outcomes post HSCT. We did not include the studies in languages other than English. The inclusion of a cost analysis would have made our study more comprehensive but couldn’t be done as most studies didn’t include it in their reporting. Based on donor type or stem cell source, subgroup analysis was not done as outcomes reported by the studies did not allow for the same.
Conclusions
In this systematic review of RCTs and NRSs evaluating the role of post-transplant G-CSF administration on HSCT-related outcomes, we found that there was a marginal benefit of approximately one day with the use of G-CSF for NE. The use of G-CSF did not alter time to PE, the incidence of GVHD, HS, and OS in both arms. The justification for G-CSF administration post-HSCT to achieve a benefit of one day for NE is debatable. The clinical and economic relevance is questionable.
Acknowledgements
We thank all the patients, surrogates, and researchers involved in the studies. We also thank Harshita Makkar for helping in reference management.
Appendix 1.
PRISMA 2009 Checklist
| Section/topic | # | Checklist item | Reported on page # |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 3 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 3 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). | 3, 4 |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. | 4 |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | 4 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 4 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | Appendix 2 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 4 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 4 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | 4 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 4, 5 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | 4, 5 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | 4, 5 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). | 4, 5, appendix 3 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | NA |
| RESULTS | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | 4 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. | Table 2 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | Figure 2 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | NA |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | Figures 4-15 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | Figure 2, Appendix 3 |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]). | NA |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers). | 6-8 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias). | 8 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | 9 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. | NA |
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097. For more information, visit: www.prisma-statement.org.
Appendix 2-1.
Search strategy employed for PubMed
| 18 | ((((Allogenic) OR (Allogeneic)) AND (((((((hematopoietic stem cell transplant$) OR (bone marrow transplant$)) OR (HSCT)) OR (Peripheral blood Stem cell Transplant$)) OR (PBSCT)) OR (Stem Cell Transplant$)) OR (BMT))) NOT (Autologous)) AND (((((((Granulocyte colony stimulating Factor$) OR (G-CSF)) OR (GCSF)) OR (Filgrastim)) OR (Lenograstim)) OR (Biosimilar GCSF)) OR (Biosimilar$ Granulocyte Colony stimulating Factor$)) |
| 17 | ((((Allogenic) OR (Allogeneic)) AND (((((((hematopoietic stem cell transplant$) OR (bone marrow transplant$)) OR (HSCT)) OR (Peripheral blood Stem cell Transplant$)) OR (PBSCT)) OR (Stem Cell Transplant$)) OR (BMT))) NOT (Autologous)) AND (((((((Granulocyte colony stimulating Factor$) OR (G-CSF)) OR (GCSF)) OR (Filgrastim)) OR (Lenograstim)) OR (Biosimilar GCSF)) OR (Biosimilar$ Granulocyte Colony stimulating Factor$)) |
| 16 | ((((((Granulocyte colony stimulating Factor$) OR (G-CSF)) OR (GCSF)) OR (Filgrastim)) OR (Lenograstim)) OR (Biosimilar GCSF)) OR (Biosimilar$ Granulocyte Colony stimulating Factor$) |
| 15 | Biosimilar$ Granulocyte Colony stimulating Factor$ |
| 14 | Biosimilar GCSF |
| 13 | ((((Granulocyte colony stimulating Factor$) OR (G-CSF)) OR (GCSF)) OR (Filgrastim)) OR (Lenograstim) |
| 12 | (((Allogenic) OR (Allogeneic)) AND (((((((hematopoietic stem cell transplant$) OR (bone marrow transplant$)) OR (HSCT)) OR (Peripheral blood Stem cell Transplant$)) OR (PBSCT)) OR (Stem Cell Transplant$)) OR (BMT))) NOT (Autologous) |
| 11 | ((((((hematopoietic stem cell transplant$) OR (bone marrow transplant$)) OR (HSCT)) OR (Peripheral blood Stem cell Transplant$)) OR (PBSCT)) OR (Stem Cell Transplant$)) OR (BMT) |
| 10 | (Allogenic) OR (Allogeneic) |
| 9 | Allogeneic |
| 8 | Allogenic |
| 7 | BMT |
| 6 | Stem Cell Transplant$ |
| 5 | PBSCT |
| 4 | Peripheral blood Stem cell Transplant$ |
| 3 | HSCT |
| 2 | bone marrow transplant$ |
| 1 | hematopoietic stem cell transplant$ |
Appendix 2-2.
Search strategy employed for Embase
Appendix 2-3.
Search strategy employed for CENTRAL (Cochrane Controlled Trials Register)
| #1 | Granulocyte colony stimulating factor | 4234 |
| #2 | MeSH descriptor: [Granulocyte Colony-Stimulating Factor] explode all trees | 1494 |
| #3 | MeSH descriptor: [Hematopoietic Stem Cell Transplantation] explode all trees | 1370 |
| #4 | #2 AND #3 | 259 |
| Total | 259 |
Appendix 3.
Eggers Test
| Analysis | Eggers’ test of the intercept | Publication bias | |||
|---|---|---|---|---|---|
| NE duration | |||||
| NRS with outliers | intercept | 95% CI | t | p | No |
| -3.872 | -22.68 - 14.93 | -0.404 | 0.694 | ||
| Eggers’ test does not indicate the presence of funnel plot asymmetry. | |||||
| NRS with no outliers | intercept | 95% CI | t | p | No |
| 1.731 | -0.56 - 4.02 | 1.484 | 0.198 | ||
| Eggers’ test does not indicate the presence of funnel plot asymmetry. | |||||
| RCT with outliers | intercept | 95% CI | t | p | Yes |
| -8.323 | -12.11 - -4.53 | -4.302 | 0.013 | ||
| Eggers’ test indicates the presence of funnel plot asymmetry. | |||||
| RCT with no outliers | intercept | 95% CI | t | p | No |
| -9.712 | -59.48 - 40.05 | -0.383 | 0.767 | ||
| Eggers’ test does not indicate the presence of funnel plot asymmetry. | |||||
| NE probability | |||||
| NRS | intercept | 95% CI | t | p | No |
| 0.937 | -0.75 - 2.63 | 1.088 | 0.318 | ||
| Eggers’ test does not indicate the presence of funnel plot asymmetry. | |||||
| RCT - only one study | - not done - - | ||||
| GVHD | |||||
| RCT | intercept | 95% CI | t | p | No |
| -1.108 | -2.53 - 0.31 | -1.533 | 0.163 | ||
| Eggers’ test does not indicate the presence of funnel plot asymmetry. | |||||
| NRS | intercept | 95% CI | t | p | No |
| -0.058 | -1.4 - 1.29 | -0.084 | 0.934 | ||
| Eggers’ test does not indicate the presence of funnel plot asymmetry. | |||||
| Survival | |||||
| NRS | intercept | 95% CI | t | p | No |
| 2.389 | 0.53 - 4.25 | 2.522 | 0.0652 | ||
| Eggers’ test does not indicate the presence of funnel plot asymmetry. | |||||
| RCT | intercept | 95% CI | t | p | No |
| 0.754 | 0.31 - 1.2 | 3.302 | 0.0807 | ||
| Eggers’ test does not indicate the presence of funnel plot asymmetry. | |||||
| Hospital stay | |||||
| NRS with outliers | intercept | 95% CI | t | p | No |
| 7.727 | -18.76 - 34.22 | 0.572 | 0.585 | ||
| Eggers’ test does not indicate the presence of funnel plot asymmetry. | |||||
| RCT with outliers | intercept | 95% CI | t | p | No |
| -13.629 | -23.4 - -3.86 | -2.734 | 0.112 | ||
| Eggers’ test does not indicate the presence of funnel plot asymmetry. | |||||
| NRS with no outliers | intercept | 95% CI | t | p | No |
| -0.013 | -10.76 - 10.73 | -0.002 | 0.998 | ||
| Eggers’ test does not indicate the presence of funnel plot asymmetry. | |||||
| RCT with no outliers | intercept | 95% CI | t | p | No |
| -19.899 | -64.11 - 24.31 | -0.882 | 0.5397882 | ||
| Eggers’ test does not indicate the presence of funnel plot asymmetry. | |||||
| Platelet | |||||
| NRS | intercept | 95% CI | t | p | No |
| 14.605 | -27.66 - 56.87 | 0.677 | 0.568 | ||
| Eggers’ test does not indicate the presence of funnel plot asymmetry. | |||||
| RCT | intercept | 95% CI | t | p | No |
| 2.161 | -7.26 - 11.59 | 0.449 | 0.7311 | ||
| Eggers’ test does not indicate the presence of funnel plot asymmetry. | |||||
Disclosure of conflict of interest
None.
Abbreviations
- ASBMT
American Society for Blood and Marrow Transplantation
- BM
Bone marrow
- BMT
Bone marrow transplantation
- BMT
Bone marrow transplantation
- CI
Confidence interval
- EBMT
European Society for Blood and Marrow Transplantation
- GVHD
Graft-versus-host disease
- G-CSF
Granulocyte colony-stimulating factor
- GM-CSF
Granulocyte monocyte-colony stimulating factor
- HSC
Hematopoietic stem cell
- HSCT
Hematopoietic stem cell transplant
- HS
Hospital stay
- IV
Intravenous
- MA
Myeloablative
- NE
Neutrophil engraftment
- MFD
Matched family donor
- MUD
Matched unrelated donor
- NRSs
Non-randomized studies
- NOS
Newcastle Ottawa Scale
- OS
Overall survival
- PB
Peripheral blood
- PE
Platelet engraftment
- RCT
Randomized controlled trial
- RIC
Reduced-intensity conditioning
- ROB
Risk-of-bias
- RR
Relative risk
- SMD
Standardized mean difference
- SC
Subcutaneous
- SIOP
International Society of Pediatric Oncology
- TRM
Treatment-related mortality
Supporting Information
References
- 1.Trivedi M, Martinez S, Corringham S, Medley K, Ball ED. Optimal use of G-CSF administration after hematopoietic SCT. Bone Marrow Transplant. 2009;43:895–908. doi: 10.1038/bmt.2009.75. [DOI] [PubMed] [Google Scholar]
- 2.Remberger M, Naseh N, Aschan J, Barkholt L, LeBlanc K, Svennberg P, Ringden O. G-CSF given after haematopoietic stem cell transplantation using HLA-identical sibling donors is associated to a higher incidence of acute GVHD II-IV. Bone Marrow Transplant. 2003;32:217–223. doi: 10.1038/sj.bmt.1704108. [DOI] [PubMed] [Google Scholar]
- 3.Ringden O, Hassan Z, Karlsson H, Olsson R, Omazic B, Mattsson J, Remberger M. Granulocyte colony-stimulating factor induced acute and chronic graft-versus-host disease. Transplantation. 2010;90:1022–1029. doi: 10.1097/TP.0b013e3181f585c7. [DOI] [PubMed] [Google Scholar]
- 4.Dekker A, Bulley S, Beyene J, Dupuis LL, Doyle JJ, Sung L. Meta-analysis of randomized controlled trials of prophylactic granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor after autologous and allogeneic stem cell transplantation. J. Clin. Oncol. 2006;24:5207–5215. doi: 10.1200/JCO.2006.06.1663. [DOI] [PubMed] [Google Scholar]
- 5.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta A, Meena JP, Pandey RM. Efficacy and safety of granulocyte colony-stimulating factor in patients post-allogeneic hematopoietic stem cell transplant-a systematic review and meta-analysis. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020206989.
- 7.Download Excel|Buy Microsoft Excel Spreadsheet Software [Internet]. [cited 2021 Mar 9] Available from: https://www.microsoft.com/en-in/microsoft-365/excel.
- 8.Luchini C, Stubbs B, Solmi M, Veronese N. Assessing the quality of studies in meta-analyses: advantages and limitations of the Newcastle Ottawa Scale. World J Metaanal. 2017;5:80–84. [Google Scholar]
- 9.Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, Perruolo E, Parati G ESH Working Group on CV Risk in Low Resource Settings. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PLoS One. 2016;11:e0147601. doi: 10.1371/journal.pone.0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.RoB 2: a revised Cochrane risk-of-bias tool for randomized trials [Internet]. [cited 2021 Mar 20] Available from: /bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials.
- 11.Bishop MR, Tarantolo SR, Geller RB, Lynch JC, Bierman PJ, Pavletic ZS, Vose JM, Kruse S, Dix SP, Morris ME, Armitage JO, Kessinger A. A randomized, double-blind trial of filgrastim (granulocyte colony-stimulating factor) versus placebo following allogeneic blood stem cell transplantation. Blood. 2000;96:80–85. [PubMed] [Google Scholar]
- 12.Joshi SS, Bishop MR, Lynch JC, Tarantolo SR, Abhyankar S, Bierman PJ, Vose JM, Geller RB, McGuirk J, Foran J, Bociek RG, Hadi A, Day SD, Armitage JO, Kessinger A, Pavletic ZS. Immunological and clinical effects of post-transplant G-CSF versus placebo in T-cell replete allogeneic blood transplant patients: results from a randomized double-blind study. Cytotherapy. 2003;5:542–552. doi: 10.1080/14653240310003648. [DOI] [PubMed] [Google Scholar]
- 13.Przepiorka D, Smith TL, Folloder J, Anderlini P, Chan KW, Koorbling M, Lichtiger B, Norfleet F, Champlin R. Controlled trial of filgrastim for acceleration of neutrophil recovery after allogeneic blood stem cell transplantation from human leukocyte antigen-matched related donors. Blood. 2001;97:3405–3410. doi: 10.1182/blood.v97.11.3405. [DOI] [PubMed] [Google Scholar]
- 14.Dallorso S, Rondelli R, Messina C, Pession A, Giorgiani G, Fagioli F, Locatelli F, Manzitti C, Balduzzi A, Prete A, Cesaro S, Lanino E, Dini G. Clinical benefits of granulocyte colony-stimulating factor therapy after hematopoietic stem cell transplant in children: results of a prospective randomized trial. Haematologica. 2002;87:1274–1280. [PubMed] [Google Scholar]
- 15.Ernst P, Bacigalupo A, Ringdén O, Ruutu T, Kolb HJ, Lawrinson S, Skacel T. A phase 3, randomized, placebo-controlled trial of filgrastim in patients with haematological malignancies undergoing matched-related allogeneic bone marrow transplantation. Arch Drug Inf. 2008;1:89–96. doi: 10.1111/j.1753-5174.2008.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Othman TB, Ghedira H, Abdejlil NB, Lakhal A, Torjemane L, Hamed LB, Hamida S, Zouari B, Ladeb S. Filgrastim following HLA-identical allogeneic bone marrow transplantation: long-term outcomes of a randomized trial. Biol Blood Marrow Transplant. 2018;24:2459–2465. doi: 10.1016/j.bbmt.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 17.Schriber JR, Chao NJ, Long GD, Negrin RS, Tierney DK, Kusnierz-Glaz C, Lucas KS, Blume KG. Granulocyte colony-stimulating factor after allogeneic bone marrow transplantation. Blood. 1994;84:1680–1684. [PubMed] [Google Scholar]
- 18.Berger C, Bertz H, Schmoor C, Behringer D, Potthoff K, Mertelsmann R, Finke J. Influence of recombinant human granulocyte colony-stimulating factor (filgrastim) on hematopoietic recovery and outcome following allogeneic bone marrow transplantation (BMT) from volunteer unrelated donors. Bone Marrow Transplant. 1999;23:983–990. doi: 10.1038/sj.bmt.1701746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozcan M, Ustun C, Akçağlayan E, Akan H, Arslan Ö, Ilhan O, Beksaç M, Gurman G, Demirer T, Arat M, Celebi H, Konuk N, Uysal A, Koç H. Recombinant human granulocyte colony-stimulating factor (rh-G-CSF) may accelerate hematopoietic recovery after HLA-identical sibling allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant. 2001;27:499–505. doi: 10.1038/sj.bmt.1702816. [DOI] [PubMed] [Google Scholar]
- 20.Ringden O, Labopin M, Gorin NC, Le Blanc K, Rocha V, Gluckman E, Reiffers J, Arcese W, Vossen JM, Jouet JP, Cordonnier C, Frassoni F. Treatment with granulocyte colony-stimulating factor after allogeneic bone marrow transplantation for acute leukemia increases the risk of graft-versus-host disease and death: a study from the Acute Leukemia Working Party of the European Group for blood and marrow transplantation. J. Clin. Oncol. 2004;22:416–423. doi: 10.1200/JCO.2004.06.102. [DOI] [PubMed] [Google Scholar]
- 21.Khoury HJ, Loberiza FR Jr, Ringdén O, Barrett AJ, Bolwell BJ, Cahn JY, Champlin RE, Gale RP, Hale GA, Urbano-Ispizua A, Martino R, McCarthy PL, Tiberghien P, Verdonck LF, Horowitz MM. Impact of posttransplantation G-CSF on outcomes of allogeneic hematopoietic stem cell transplantation. Blood. 2006;107:1712–1716. doi: 10.1182/blood-2005-07-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh V, Jang H, Kim S, Ayash L, Alavi A, Ratanatharathorn V, Uberti JP, Deol A. G-CSF use post peripheral blood stem cell transplant is associated with faster neutrophil engraftment, shorter hospital stay and increased incidence of chronic GVHD. Leuk Lymphoma. 2021;62:446–453. doi: 10.1080/10428194.2020.1827244. [DOI] [PubMed] [Google Scholar]
- 23.George G, Martin AS, Chhabra S, Eapen M. The effect of granulocyte colony-stimulating factor use on hospital length of stay after allogeneic hematopoietic cell transplantation: a retrospective multicenter cohort study. Biol Blood Marrow Transplant. 2020;26:2359–2364. doi: 10.1016/j.bbmt.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Baser O, Kutikova L, Page JH, Barron R. The impact of primary prophylaxis with granulocyte colony-stimulating factors on febrile neutropenia during chemotherapy: a systematic review and meta-analysis of randomized controlled trials. Support Care Cancer. 2015;23:3131–3140. doi: 10.1007/s00520-015-2686-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper KL, Madan J, Whyte S, Stevenson MD, Akehurst RL. Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer. 2011;11:404. doi: 10.1186/1471-2407-11-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ojeda E, Garcia-Bustos J, Aguado M, Arrieta R, Quevedo E, Yuste VJ, Canales M, Hernandez-Navarro F. A prospective randomized trial of granulocyte colony-stimulating factor therapy after autologous blood stem cell transplantation in adults. Bone Marrow Transplant. 1999;24:601–607. doi: 10.1038/sj.bmt.1701972. [DOI] [PubMed] [Google Scholar]
- 27.Eapen M, Horowitz MM, Klein JP, Champlin RE, Loberiza FR, Ringden O, Wagner JE. Higher mortality after allogeneic peripheral-blood transplantation compared with bone marrow in children and adolescents: the Histocompatibility and Alternate Stem Cell Source Working Committee of the International Bone Marrow Transplant Registry. J. Clin. Oncol. 2004;22:4872–4880. doi: 10.1200/JCO.2004.02.189. [DOI] [PubMed] [Google Scholar]
- 28.Ho VT, Mirza NQ, Junco Dd Dd, Okamura T, Przepiorka D. The effect of hematopoietic growth factors on the risk of graft-vs-host disease after allogeneic hematopoietic stem cell transplantation: a meta-analysis. Bone Marrow Transplant. 2003;32:771–775. doi: 10.1038/sj.bmt.1704228. [DOI] [PubMed] [Google Scholar]
- 29.Stinson TJ, Adams JR, Bishop MR, Kruse S, Tarantolo S, Bennet CL. Economic analysis of a phase III study of G-CSF vs placebo following allogeneic blood stem cell transplantation. Bone Marrow Transplant. 2000;26:663–666. doi: 10.1038/sj.bmt.1702579. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.