Abstract
Background: Various articles show the high prevalence of sleep disorders and especially excessive daytime sleepiness (EDS) in patients with refractory epilepsy and the importance of personal and social burden of this complication on individuals. Considering the insufficient evidence to draw efficacy and safety of modafinil and methylphenidate to treat EDS in the patient with intractable seizures, we decided to compare the effect of methylphenidate and modafinil with the control group. It is hoped that this study will pave the way for further studies. Methods: This study is a clinical trial (IRCT20171030037093N22) (URL: https://www.irct.ir/trial/42485). The study population was patients with refractory epilepsy referred to the neurology clinic of Al-Zahra Hospital, Isfahan, Iran, from 2019 to 2020. The patients were randomly divided into three groups. The first group was treated with methylphenidate, the second group was treated with modafinil, and the third group was not received any medication such as modafinil and methylphenidate. Methylphenidate dosage was 10-20 mg/day. The patients were treated with modafinil at a dose of 200-600 mg/day. EPWORTH sleepiness scale (ESS) and Total Sleep Time (TST) were calculated before and 8 weeks after the intervention for the patients. Results: 47 patients were included and divided into 3 groups, methylphenidate (10 males and 9 females), modafinil (7 males and 13 females), and control (4 males and 4 females). There was no significant difference among the groups based on ESS before and after intervention and TST after the intervention (P>0.05), but the mean of TST was significantly lower in the control group than in methylphenidate and modafinil groups before the intervention (P=0.003). The change of ESS and TST before compared to after intervention in the methylphenidate and modafinil group were significant (P<0.001), but the changes of ESS and TST in the control group were not significant (P>0.05). The frequency of complications (P=0.74) and outcomes (P=0.07) were similar in both groups. Conclusion: Modafinil and methylphenidate are two effective and safe drugs to increase the quality of sleep in the patients. Additionally, ESS and TST scores are better in the patients who used modafinil and methylphenidate.
Keywords: Modafinil, methylphenidate, excessive daytime sleepiness, quality of life
Introduction
One of the most common complaints in patients with refractory epilepsy is sleep problems, and especially excessive daytime sleepiness (EDS). An EPWORTH sleepiness scale (ESS) >10 was reported in 18 to 47% of epileptic patients and 12 to 17% controls with a trend for higher ESS score in the patients with intractable seizure [1-6]. Various studies showed that about 43% of American adults have EDS which interferes with their daily activities and reduces their daily functioning [2,7-11]. In treating EDS, people with refractory epilepsy should exercise extreme caution and use the medications which do not decrease the seizure threshold and do not interfere with other antiepileptic drugs [12-14]. Methylphenidate or Ritalin is one of the drugs used to treat Attention Deficit Hyperactivity Disorder (ADHD), and its mechanism of action is the central nervous system or central nervous system (CNS) stimulation [15-18]. Methylphenidate is also used to treat narcolepsy, a condition in which a person suddenly has a sleep attack which has no control over their sleep condition [19,20]. In various studies, this drug had good effects on drowsiness and narcolepsy, but it is recommended to perform behavioral therapy and social and educational therapies along with this treatment [19]. Another drug used to treat sleep disorders, especially narcolepsy, is modafinil. It is a central chemical stimulant used to treat severe daily drowsiness associated with narcoleptic syndrome, sleep apnea, and sleep shift disorders [21]. The mechanism of modafinil action is unknown. It is not sympathomimetic and may increase dopamine levels in the brain by binding to the dopamine and reabsorbing dopamine [22]. There are various studies investigating modafinil effect on sleep disorders, especially EDS. In a study by Black et al., using modafinil in the treatment of EDS was confirmed, and it was stated that this drug is very effective and has few side effects [23]. Considering the importance of sleep disorders and especially EDS in the patients with refractory epilepsy [24] and personal and social burden of this complication on individuals, and there were different and insufficient results in another study for the effect of modafinil and methylphenidate on EDS, we decided to compare the effect of methylphenidate and modafinil with control. The daily drowsiness of the patients with refractory epilepsy was assessed. It is hoped that this study will pave the way for further studies.
Materials and methods
Study design
The ethical committee approved this clinical trial study of Isfahan University of Medical Sciences (IR.MUI.MED.REC.1398.327), and also the protocol of current study was registered in the Iranian Registry of Clinical Trials (IRCT20171030037093N22). The study population was the patients with refractory epilepsy referred to the neurology clinic of Al-Zahra Hospital, Isfahan, Iran, from 2019 to 2020 with complaints of excessive daytime sleepiness. Inclusion criteria included patients with refractory epilepsy diagnosed with EPWORTH sleepiness scale (ESS) >9 by a neurologist and agreed to participate in the study. Moreover, exclusion criteria included patients with allergic to methylphenidate and modafinil or with a history of head trauma, diagnosis of other neurological diseases such as dementia, stroke, thyroid disease, substance abuse, liver or kidney failure, heart problems, and psychiatric problems. Furthermore, the patients who showed severe side effects during the study did not adhere to the treatment regularly or were not followed up during the study were excluded. First, the patients referred to the hospital clinic due to refractory epilepsy and complained of EDS were screened by neurologists, and the patients who met the inclusion criteria were included. ESS form diagnosed EDS, and all patients had informed consent to participate in the study.
EPWORTH sleepiness scale is a short questionnaire to assess daily drowsiness. ESS questionnaire has 8 questions to ask the patient about the possibility of drowsiness in daily activities (not necessarily daily), including sitting and reading books, watching TV, sitting in a public place without movement, and special activities as a traveler. Staying in a car for more than an hour, lying down and resting in the evening, sitting and resting after lunch and in the car behind the traffic, and each of these types of activities were based on the amount of drowsiness or confusion. Each question is scored between 0-3, and the total score is between 0-24. The scoring from 0 to 9 is normal, and more than 9 are considered EDS and require more specialized examinations. ESS questionnaire was filled by the patients before and after the intervention. The reality and validity of ESS were described in previous studies [25].
Total sleep time
Total sleep time [26] was the total sleep time of patient during 24 h. TST is included rapid eye movement (REM) [21], and non-rapid eye movement (NREM) sleeps duration and is calculated based on minute or hour. TST (=REM+NREM) was calculated before and after the intervention.
Study protocol
The patients were randomly divided into three groups. The first group was treated with methylphenidate, the second group was treated with modafinil, and the third group did not receive any medication such as modafinil and methylphenidate. The dosage of methylphenidate was 10-20 mg/day. The patients were treated with modafinil at a dose of 200-600 mg/day.
The patients were reevaluated by the EPWORTH sleepiness scale form [27] 8 weeks after the treatment period, and their recovery was assessed. Recovery of the patient after treatment was considered based on increasing ESS and TST scores (-2≤) without any complication, and the patients who recovered but had complications were considered partially recovered. Furthermore, the patients who not had any change of TST or ESS were considered not recovered.
Moreover, the complications of drugs were collected and evaluated after the intervention, the complications were included neurological such as headache, anxiety and increasing or decreasing sleep duration, and gastrointestinal complications.
Statistics
After collecting the study data, they were entered into SPSS version 24 and analyzed. The data were analyzed with chi-square, paired t-test, one-way ANOVA. Therefore, data were presented as mean ± SD and frequency and percentage. P-value less than 0.05 was considered significant.
Results
Baseline variables
A total of 47 patients were included and divided into methylphenidate (10 males and 9 females), modafinil (7 males and 13 females), and the control (4 males and 4 females) groups. There was no significant difference among the groups based on age, the duration of disease, and the frequency of seizures in months (P>0.05), but the number of used drugs in the control group was significantly lower than that in modafinil and methylphenidate groups (P=0.03).
TST, ESS, complications and outcomes
There was no significant difference among the groups based on ESS before and after intervention, and TST after intervention (P>0.05), but the mean of TST before intervention in the control group was significantly lower than that in methylphenidate and modafinil groups (P=0.003). The change of ESS and TST before compared to after intervention in the methylphenidate and modafinil were significant (P<0.001), but the change of ESS and TST in the control group was not significant (P>0.05) (Figures 1 and 2). The frequency of complications (P=0.74) and outcomes (P=0.07) were similar in both groups (Table 1).
Figure 1.
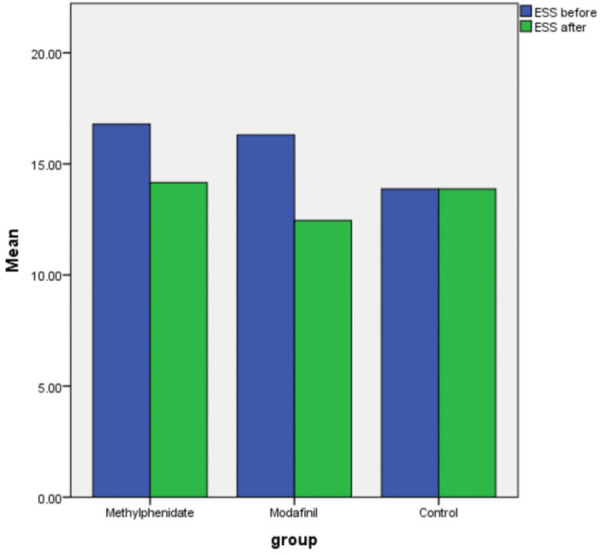
Changing ESS before and after intervention based on groups.
Figure 2.
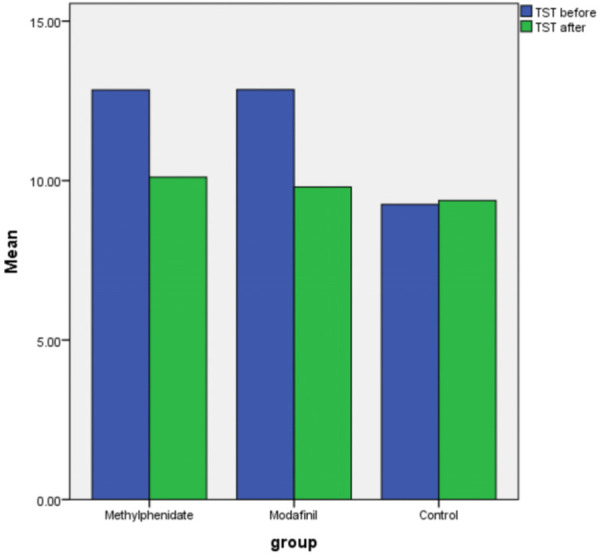
Changing TST before and after intervention based on groups.
Table 1.
Variables of study between study groups
| Variables | Methylphenidate | Modafinil | Control | P-value | |
|---|---|---|---|---|---|
| Gender (m/f) | 10/9 | 7/13 | 4/4 | 0.51* | |
| Age | 36.94±15.87 | 36.57±10.63 | 34.25±14.51 | 0.89** | |
| Duration of disease (m) | 19.01±12.89 | 20.90±10.84 | 23.37±14.62 | 0.69** | |
| Frequent of seizure in months | 5.31±3.93 | 3.60±0.99 | 4.01±1.51 | 0.13** | |
| Number of used drugs | 3.70±1.04 | 3.35±1.08 | 2.62±0.74 | 0.04** | |
| ESS | Before | 16.78±3.39 | 16.30±3.18 | 13.87±3.09 | 0.10** |
| After | 14.15±4.40 | 12.45±3.50 | 13.87±3.44 | 0.37** | |
| P*** | <0.001 | <0.001 | 0.98 | ||
| TST (h) | Before | 12.84±2.67 | 12.85±2.58 | 9.25±1.66 | 0.003** |
| After | 10.10±1.79 | 9.80±2.62 | 9.37±1.76 | 0.72** | |
| P*** | <0.001 | <0.001 | 0.75 | ||
| Complications | No | 15 (78.9%) | 13 (65%) | 6 (75%) | 0.74** |
| Yes | 4 (21.1%) | 6 (30%) | 2 (25%) | ||
| Outcomes | Recovered | 5 (26.3%) | 11 (55%) | 2 (25%) | 0.07* |
| Not recovered | 9 (47.4%) | 8 (40%) | 6 (75%) | ||
| Partial recovery | 5 (26.3%) | 1 (5%) | 0 | ||
ESS: EPWORTH sleepiness scale, TST: Total sleep time.
Chi Square test;
Independent T tes;
One-way ANOVA.
Discussion
The efficacy of Methylphenidate, modafinil on EDS was compared by assessing the ESS and TST score before and after the intervention. There are few studies comparing modafinil and methylphenidate on excessive daytime sleepiness, particularly in patients with epilepsy. The study reported by Adams et al. concluded that single dose of methylphenidate had an effect on caring cognitive deficit in epilepsy patients, and methylphenidate was a safe and effective treatment for cognition in the patients with epilepsy [28]. In review study single dose of methylphenidate affect attention deficits in adults with epilepsy, and methylphenidate was a possible treatment for attentional dysfunction in epilepsy [29]. Moreover, the methylphenidate was an effective and safe option to improve the quality of life in patients with epilepsy [30], but they didn’t report its effect on drowsiness. In our study, methylphenidate was a good and effective drug on EDS with minor complications and no change in seizure frequency. In a study conducted by Banerjee et al., Methylphenidate was mentioned as one of the safest and most effective treatments in EDS, and its effects were considered acceptable [31]. Our results confirmed that methylphenidate is an effective drug on EDS. Some studies on the effectiveness of methylphenidate and modafinil in EDS of Alzheimer and Parkinson patients proved their effectiveness with the acceptable side effects (19). But our study shows their effectiveness in a patient with intractable epilepsy.
In addition, Schmidt et al. compared methylphenidate and modafinil on negative emotion processing in healthy people, demonstrating modafinil has an acute effect on increasing brain activation in the limbic-cortical-striatal-palliadal-thalamic circuit and also amygdala responses. But the methylphenidate does not affect improving cognition, so modafinil’s cognitive enhancement effect has an adverse effect on emotion processing [32]. In our study, modafinil and methylphenidate were two effective drugs to decrease sleep and improve ESS in the patients. In the study by Jasinski, modafinil showed greater effect on sleep than methylphenidate with less facilitation of orthostatic tachycardia and less reduction of caloric intake [33]. Also, our data revealed that recovered patients in modafinil were higher than methylphenidate.
In a review study performed by Bonnet et al., 400 mg single dosage of modafinil was performed for patients with sleep loss [34]; In the study by Westcott that was performed on military personnel, modafinil was a wakefulness-promoting agent that improved cognitive performance and increased wakefulness among shift workers [35]. Modafinil was a more effective and useful drug in treating the patients with sleep and cognitive disorders [36].
Based on this study’s results, there were no significant differences between methylphenidate and modafinil with ESS and TST scores after the intervention, and there was no significant difference between methylphenidate and modafinil based on complications, but the frequency of recovered patients in modafinil group was higher than that in methylphenidate group.
In summary, modafinil and methylphenidate are two effective and safe drugs on increasing quality of sleep in the patients, and ESS and TST scores are better in the patients who used modafinil and methylphenidate. Besides, we need more study with a larger population and combining subjective and objective evaluation to confirm our results.
Disclosure of conflict of interest
None.
References
- 1.Ford ES, Cunningham TJ, Giles WH, Croft JB. Trends in insomnia and excessive daytime sleepiness among US adults from 2002 to 2012. Sleep Med. 2015;16:372–378. doi: 10.1016/j.sleep.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guilleminault C, Brooks SN. Excessive daytime sleepiness: a challenge for the practising neurologist. Brain. 2001;124:1482–1491. doi: 10.1093/brain/124.8.1482. [DOI] [PubMed] [Google Scholar]
- 3.Farrokhi M, Dabirzadeh M, Dastravan N, Etemadifar M, Ghadimi K, Saadatpour Z, Rezaei A. Mannose-binding lectin mediated complement pathway in autoimmune neurological disorders. Iran J Allergy Asthma Immunol. 2016;15:251–256. [PubMed] [Google Scholar]
- 4.Rafiee Zadeh A, Ghadimi K, Mohammadi B, Hatamian H, Naghibi SN, Danaeiniya A. Effects of estrogen and progesterone on different immune cells related to multiple sclerosis. CJNS. 2018;4:83–90. [Google Scholar]
- 5.Najafi MR, Najafi MA, Shayan-Moghadam R, Saadatpour Z, Ghadimi K. Comparison of the efficacy of tegatard and tegretol as a monotherapy in patients with focal seizure with or without secondary generalization. Am J Clin Exp Immunol. 2020;9:58–63. [PMC free article] [PubMed] [Google Scholar]
- 6.Lehoczky L, Southworth AB, Martinez GZ, Belfort MA, Shamshirsaz AA, Shamshirsaz A, Sanz Cortes M, Nassr AA, Donepudi R, Whitehead WE, Johnson R, Meshinchi N, Espinoza J. Magnesium sulfate titration reduces maternal complications following fetoscopic closure of spina bifida. Prenat Diagn. 2021;41:983–988. doi: 10.1002/pd.5923. [DOI] [PubMed] [Google Scholar]
- 7.Mehvari J, Zare M, Andami R, Ghadimi K, Tabrizi N. Ictal and interictal electroencephalography of mesial and lateral temporal lobe epilepsy; a comparative study. CJNS. 2017;3:222–230. [Google Scholar]
- 8.Etemadifar M, Ghadimi M, Ghadimi K, Alsahebfosoul F. The serum amyloid β level in multiple sclerosis: a case-control study. CJNS. 2017;3:214–221. [Google Scholar]
- 9.Fahim M, Zadeh AR, Shoureshi P, Ghadimi K, Cheshmavar M, Sheikhinia N, Afzali M. Alcohol and multiple sclerosis: an immune system-based review. Int J Physiol Pathophysiol Pharmacol. 2020;12:58–69. [PMC free article] [PubMed] [Google Scholar]
- 10.Ashtari F, Madanian R, Shaygannejad V, Zarkesh SH, Ghadimi K. Serum levels of IL-6 and IL-17 in multiple sclerosis, neuromyelitis optica patients and healthy subjects. Int J Physiol Pathophysiol Pharmacol. 2019;11:267–273. [PMC free article] [PubMed] [Google Scholar]
- 11.Espinoza J, Shamshirsaz AA, Cortes MS, Pammi M, Nassr AA, Donepudi R, Whitehead WE, Castillo J, Johnson R, Meshinchi N. Two-port, exteriorized uterus, fetoscopic meningomyelocele closure has fewer adverse neonatal outcomes than open hysterotomy closure. Am J Obstet Gynecol. 2021;225:327.e1–327.e9. doi: 10.1016/j.ajog.2021.04.252. [DOI] [PubMed] [Google Scholar]
- 12.Tang F, Hartz A, Bauer B. Drug-resistant epilepsy: multiple hypotheses, few answers. Front Neurol. 2017;8:301. doi: 10.3389/fneur.2017.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shamshirsaz AA, Aalipour S, Stewart KA, Nassr AA, Yilmaz B, Erfani XH, Sundgren NC, Cortes MS, Donepudi RV, Lee TC. Perinatal characteristics and early childhood follow up after ex-utero intrapartum treatment for head and neck teratomas by prenatal diagnosis. Prenat Diagn. 2021;41:497–504. doi: 10.1002/pd.5894. [DOI] [PubMed] [Google Scholar]
- 14.Haddad S, Ghadimi K, Abrishamkar R, Asl NSM. Comparing laparoscopy and laparotomy procedures in the radical hysterectomy surgery for endometrial cancer: a basic review. Am J Transl Res. 2021;13:2456–2461. [PMC free article] [PubMed] [Google Scholar]
- 15.Morton WA, Stockton GG. Methylphenidate abuse and psychiatric side effects. Prim Care Companion J Clin Psychiatry. 2000;2:159–164. doi: 10.4088/pcc.v02n0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shakibaei F, Borhani M, Kahkeshani M, Ghadimi K. The effect of triphala lavender tablets on the treatment of children with attention deficit/hyperactivity disorder. JIMS. 2018;36:42–48. [Google Scholar]
- 17.Zadeh AR, Askari M, Azadani NN, Ataei A, Ghadimi K, Tavoosi N, Falahatian M. Mechanism and adverse effects of multiple sclerosis drugs: a review article. Part 1. Int J Physiol Pathophysiol Pharmacol. 2019;11:95–104. [PMC free article] [PubMed] [Google Scholar]
- 18.Zadeh AR, Ghadimi K, Ataei A, Askari M, Sheikhinia N, Tavoosi N, Falahatian M. Mechanism and adverse effects of multiple sclerosis drugs: a review article. Part 2. Int J Physiol Pathophysiol Pharmacol. 2019;11:105–114. [PMC free article] [PubMed] [Google Scholar]
- 19.Mitler MM, Shafor R, Hajdukovich R, Timms RM, Browman CP. Treatment of narcolepsy: objective studies on methylphenidate, pemoline, and protriptyline. Sleep. 1986;9:260–264. doi: 10.1093/sleep/9.1.260. [DOI] [PubMed] [Google Scholar]
- 20.Aria A, Forouharnejad K, Mortazavi M, Omidi A, Askari M, Ghadimi K, Mashinchi-Asl N. COVID 19 with neurological symptoms, rhabdomyolysis and brain death: a case report. Am J Clin Exp Immunol. 2020;9:114–117. [PMC free article] [PubMed] [Google Scholar]
- 21.Battleday RM, Brem AK. Modafinil for cognitive neuroenhancement in healthy non-sleep-deprived subjects: a systematic review. Eur Neuropsychopharmacol. 2015;25:1865–81. doi: 10.1016/j.euroneuro.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 22.Kaser M, Deakin JB, Michael A, Zapata C, Bansal R, Ryan D, Cormack F, Rowe JB, Sahakian BJ. Modafinil improves episodic memory and working memory cognition in patients with remitted depression: a double-blind, randomized, placebo-controlled study. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:115–122. doi: 10.1016/j.bpsc.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Black J, Swick T, Bogan R, Lai C, Carter LP. Impact of sodium oxybate, modafinil, and combination treatment on excessive daytime sleepiness in patients who have narcolepsy with or without cataplexy. Sleep Med. 2016;24:57–62. doi: 10.1016/j.sleep.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Gammino M, Zummo L, Bue AL, Urso L, Terruso V, Marrone O, Fierro B, Daniele O. Excessive daytime sleepiness and sleep disorders in a population of patients with epilepsy: a case-control study. J Epilepsy Res. 2016;6:79–86. doi: 10.14581/jer.16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Izci B, Ardic S, Firat H, Sahin A, Altinors M, Karacan I. Reliability and validity studies of the Turkish version of the epworth sleepiness scale. Sleep Breath. 2008;12:161–168. doi: 10.1007/s11325-007-0145-7. [DOI] [PubMed] [Google Scholar]
- 26.Linnemann RW, Friedman D, Altstein LL, Islam S, Bach KT, Georgiopoulos AM, Moskowitz SM, Yonker LM. Advance care planning experiences and preferences among people with cystic fibrosis. J Palliat Med. 2019;22:138–144. doi: 10.1089/jpm.2018.0262. [DOI] [PubMed] [Google Scholar]
- 27.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 28.Adams J, Alipio-Jocson V, Inoyama K, Bartlett V, Sandhu S, Oso J, Barry JJ, Loring DW, Meador K. Methylphenidate, cognition, and epilepsy: a double-blind, placebo-controlled, single-dose study. Neurol. 2017;88:470–476. doi: 10.1212/WNL.0000000000003564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leeman-Markowski BA, Adams J, Martin SP, Devinsky O, Meador KJ. Methylphenidate for attention problems in epilepsy patients: safety and efficacy. Epilepsy Behav. 2021;115:107627. doi: 10.1016/j.yebeh.2020.107627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adams J, Alipio-Jocson V, Inoyama K, Bartlett V, Sandhu S, Oso J, Barry JJ, Loring DW, Meador KJ. Methylphenidate, cognition, and epilepsy: a 1-month open-label trial. Epilepsia. 2017;58:2124–2132. doi: 10.1111/epi.13917. [DOI] [PubMed] [Google Scholar]
- 31.Banerjee D, Vitiello MV, Grunstein RR. Pharmacotherapy for excessive daytime sleepiness. Sleep Med Rev. 2004;8:339–354. doi: 10.1016/j.smrv.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt A, Müller F, Dolder PC, Schmid Y, Zanchi D, Egloff L, Liechti ME, Borgwardt S. Acute effects of methylphenidate, modafinil, and MDMA on negative emotion processing. Int J Neuropsychopharmacol. 2018;21:345–354. doi: 10.1093/ijnp/pyx112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jasinski DR. An evaluation of the abuse potential of modafinil using methylphenidate as a reference. J Psychopharmacol. 2000;14:53–60. doi: 10.1177/026988110001400107. [DOI] [PubMed] [Google Scholar]
- 34.Bonnet MH, Balkin TJ, Dinges DF, Roehrs T, Rogers NL, Wesensten NJ. The use of stimulants to modify performance during sleep loss: a review by the sleep deprivation and stimulant task force of the American academy of sleep medicine. Sleep. 2005;28:1163–1187. doi: 10.1093/sleep/28.9.1163. [DOI] [PubMed] [Google Scholar]
- 35.Westcott KJ. Modafinil, sleep deprivation, and cognitive function in military and medical settings. Mil Med. 2005;170:333–335. doi: 10.7205/milmed.170.4.333. [DOI] [PubMed] [Google Scholar]
- 36.Dubljević V, Ryan CJ. Cognitive enhancement with methylphenidate and modafinil: conceptual advances and societal implications. Neurosci Neuroeconomics. 2015;4:25–33. [Google Scholar]


