Abstract
Background and objectives: Drug delivery by nebulization has become a crucial strategy for treating different respiratory and lung diseases. Emerging evidence implicates stem cell therapy as a promising tool in treating such conditions, not only by alleviating the related symptoms but by improving the prognosis. However, delivery of human peripheral blood-derived stem cells (hPBSCs) to the respiratory airways remains an innovative approach yet to be realized. This study is an analytic, translational, and in vitro research to assess the viability and morphological changes of identified cell populations in hPBSCs cocktail derived from COVID-19 patients. Methods and results: Peripheral blood (PB) samples were obtained from patients enrolled in the SENTAD-COVID Study (ClinicalTrials.gov Reference: NCT04473170). hPBSCs cocktails (n=15) were provided by the Cells Processing Laboratory of Abu Dhabi Stem Cells Center, and were nebulized by three different methods of nebulization: compressor (jet), ultrasonic, and mesh. Our results reported that nucleated CD45dim cell count was significantly lower after the three nebulization methods, but nucleated CD45- cells show a significant decrease only after mesh nebulization. Mesh-nebulized samples had a significant reduction in viability of both CD45dim and CD45- cells. Conclusions: This study provides evidence that stem cells derived from PB of COVID-19 patients can be nebulized without substantial loss of cell viability, cell count, and morphological changes using the compressor nebulization. Therefore, we recommend compressor nebulizers as the preferable procedure for hPBSCs delivery to the respiratory airways in further clinical settings.
Keywords: Peripheral blood stem cells, cell survival, nebulizers, COVID-19, lung diseases
Introduction
Acute and chronic lung diseases persistently rank among the most fatal diseases across developed countries [1], and this burden is predicted to grow due to the recent COVID-19 pandemic. Increasing attention has been focused on using stem cells as a novel therapeutic approach for patients suffering from several damaging and untreatable lung diseases. Unlike pharmacological therapies developed to alleviate the symptoms, stem-cell-based therapy can positively affect the pathogenesis and prognosis of lung disease by contributing to lung tissue repair, and consequently, to cure such diseases [2,3]. Accordingly, pre-clinical studies using stem-cell therapies, including hematopoietic stem cells (HSCs), mesenchymal stromal stem cells (MSCs), endothelial progenitor cells (EPCs), and embryonic stem cells (ESCs), have demonstrated the value of these therapeutic tools in Acute Respiratory Distress Syndrome (ARDS) [4], sepsis [5], acute lung injury [6], fibrotic lung diseases [7], and other lung-related diseases [8].
Subsequently, numerous clinical trials using mainly autologous or allogeneic MSCs in humans with these pathological conditions were performed rapidly after reports of efficacy in animal models by Brave and colleagues [9]. In COVID-19, MSCs and their secretome were very recently reported to mitigate the inflammatory process and consequently improve the lung function by exerting several cell contact-dependent and paracrine mechanisms, such as modulating the release of factors related to angiogenesis, fibrosis, and inflammation, altering the function and response of immune system cells, and communicating with the host niche cells via microvesicles as a source of cellular materials [10-14].
Notably, nebulized MSC-based therapies are at the forefront of treatment of lung diseases [9]. However, aerosol-based airway epithelial cell delivery was recently reported to improve airway regeneration and repair by Kardia and colleagues [15]. In addition, the same group showed that skin-derived fibroblasts following aerosolization maintain survivability and cell properties by activating the proliferation of airway epithelial cells leading to the reconstruction of a pseudostratified epithelium [16].
Still, cell loss and their low or failed engraftment in the host remain primary obstacles in cell therapy applications. The need for efficient delivery routes capable of maintaining cell viability and cell morphology to the targeted organ must be reassured for successful therapy. In this context, the appropriate choice of delivery devices appears to be as important as that of the therapeutics to be inhaled. Several studies have reported the intratracheal aerosol-based delivery technique as an effective way to deliver cells and related materials directly to the lungs, rather than by intravenous, intrapulmonary, and intraoral routes [17]. Various advantages make aerosol delivery a preferred route of administration over other types. Its benefits include immediately delivering biotherapeutics to the respiratory tract and reducing the dosage requirements (and any possible side effects). Subsequent studies have also demonstrated its capability to be faster acting than other various routes [18,19]. Aerosolized MSCs, extracellular vesicles derived-MSCs, and MSC-conditioned medium have shown high compatibility with different nebulizers [9,14]. Nevertheless, the administration route will still need extensive research before this strategy can be used in a clinical setting.
Increasing evidence suggests that peripheral blood (PB) is an alternative source of stem/progenitor cells for clinical application besides bone marrow (BM), including different subpopulations of HSCs and Non-Hematopoietic Stem Cells, Very Small Embryonic-like Stem Cells (VSELs), EPCs, and MSCs [20]. Studies have shown blood traffic of adult stem cells at a very low frequency at a steady state, which may be required to maintain homeostasis. In response to injury signals, the number of PB-derived stem cells in the bloodstream increases dramatically, indicating their possible role in tissue repair and regeneration [20]. Thus, in recent years, PB-derived stem cells, which can be collected with minimal invasion, have been identified as an important alternative to stem cells sourced from BM, cord blood, or leukapheresis for cell therapy. However, the nebulization of human peripheral blood-derived stem cells (hPBSCs) remains an innovative strategy despite their potential capabilities to improve lung inflammatory and fibrotic conditions.
This study aimed to assess the viability and morphology changes of hPBSCs using three types of nebulization methods: a compressor (jet), ultrasonic, and mesh nebulizers. These methods have the potential for use as an aerosol-based delivery system for stem cells, and provide a valuable path forward to future application in pre-clinical/clinical settings.
Materials and methods
This study is an analytic, translational, and in vitro research to assess the viability and morphological changes of hPBSCs after the three nebulization methods mentioned above.
hPBSCs collection
PB samples (300 mL) were drawn from patients during the SENTAD-COVID Study as per the following inclusion criteria: (1) RT-PCR Laboratory confirmation of COVID-19; (2) Male or female aged ≥18 years; (3) Interstitial lung change ≥3 judged by “Lungs lobar based scoring” according to computed tomography (CT) scans; (4) Hospitalized and symptomatic patients as described in the Protocol; (5) Ability to comply with test requirements and PB stem cells collection; and (6) Patient or legal representative signs the informed consent form. Furthermore, the exclusion criteria applied were: (1) Pediatric patients (aged <18 years); (2) Diagnosis of any kind of shock; (3) Organ transplants in the past 3 months; (4) Patients receiving immunosuppressive therapy; (5) Diagnostic of Hepatitis B Virus (HBV) infection; (6) Diagnostic of Human Immunodeficiency Virus (HIV) infection or Acquired Immunodeficiency Syndrome (AIDS); (7) Current diagnosis of cancer; (8) History of malignancies in the past five years; (9) Pregnant or lactating women; (10) Have participated in other clinical trials in the past three months; (11) Inability to comply with test requirements and PB stem cells collection; and (12) Inability to provide informed consent.
hPBSCs cocktails were provided by the Cells Processing Laboratory (CPL) of Abu Dhabi Stem Cells Center (ADSCC). Cell processing was performed according to the ADSCC Copyrighted Work of Science as per the INTEROCO Certificate EC-01-002811 [21]. No cell culture was performed before, during, or after nebulization, and cells were maintained at 4-6°C up to 24 h after collection. During this study, five different samples from COVID-19 patients were randomly selected and used for each nebulization method (n=15). The permuted block randomization was the way to randomly allocate five samples to any of the three nebulization groups. All the donors were males, met the inclusion/exclusion requirements, and were scored 5-6 as described in the SENTAD-COVID Study seven-category ordinal scale for clinical improvement.
hPBSCs nebulization
hPBSCs suspensions were nebulized by three different methods of nebulization, according to the approved experimental protocols, as follows: 1. Compressor (jet) nebulization: A MaxiNeb® nebulizer (Flexicare, UK) was used to aerosolize 4 mL of the hPBSCs suspension. The flow was set up on 5 L/min and was provided by an oxygen concentrator device (Drive DeVilbiss® Healthcare, USA) through the MaxiNeb® tubing system. 2. Mesh nebulization: Aerogen® Solo devices (Aerogen®, Ireland) were used to aerosolize 2 mL of the hPBSCs suspension. 3. Ultrasonic nebulization: An ultrasonic nebulizer UltraNeb (NOUVAG® AG, Switzerland) was used to aerosolize 8 mL of the hPBSCs suspension.
Differences in the required volume for nebulization were related to the medication chamber volume and the manufacturer recommendations. The minimum recovery volume was estimated at 0.5 mL for the post-nebulization analysis. All samples were collected in sterile, non-pyrogenic 50 mL conical centrifuge tubes (PP/HDPE, DNase/RNase-free, human DNA-free) from the nebulizer outlet (patient interface), not from the nebulization chamber, to guarantee that only aerosolized cells were considered. The nebulization process was performed under a Purifier Logic+ class II type A2 Biosafety Cabinet (Labconco Corporation, USA), providing both personnel and environmental protection. Appropriate Personal Protective Equipment (PPE) usage, disposal rules (applicable to sets intended for only a single-use), and other biosafety rules were followed by the involved staff.
Flow cytometry analyses
The determination of the hPBSCs phenotype was performed after 24 h of cell collection and after nebulization, by targeting CD45, CD34, CD133, and CD90 markers. Sample preparation was carried out according to the manufacturer’s specifications for cells immunophenotyping, using a protocol of red blood cells with OptiLyse® C buffer (Beckman Coulter Inc., USA). Briefly, 100 µl of a hPBSCs sample, containing approximately 1×106 nucleated cells were stained for 30 min at room temperature (RT) with the appropriate antibodies: Krome orange-(KRO-) conjugated anti-CD45 (Clone J33), phycoerythrin-(PE) conjugated anti-CD90 (Clone F15-42-1-5), Phycoerythrin-Texas Red-X-(ECD-) conjugated anti-CD34 (Clone 581), and Allophycocyanin-(APC-) conjugated anti-CD133 (Clone W6B3C1), all purchased from Beckman Coulter. Dead cells were stained with 7-aminoactinomycin D (BD Biosciences, USA), and nucleic DNA was stained with Hoechst 33342 (Sigma Aldrich, USA). Afterward, erythrocytes were lysed by incubating cells for 10 min at RT with 1 mL/tube of OptiLyse® C solution. Data acquisition was performed directly after labeling with a 10-colors flow cytometer Beckman Coulter Navios EX (Beckman Coulter Inc., USA). Cytometer quality control was performed daily with Flow-Check fluorospheres (Beckman Coulter, France) to align lasers and check the water system. Fluorescence intensity was controlled with Flow-Set fluorospheres from the same Company. Data analyses were performed with Kaluza Analysis Software v2.1 (Beckman Coulter Inc., USA), with a minimum of 50,000 acquired events. The gating strategy was manual, logical, and sequential (Figure 1), wherein each dot plot represents two parameters analyzed by the cytometer. Absolute values were determined through a single platform using Flow-Count Fluorospheres (Beckman Coulter Inc., USA).
Figure 1.
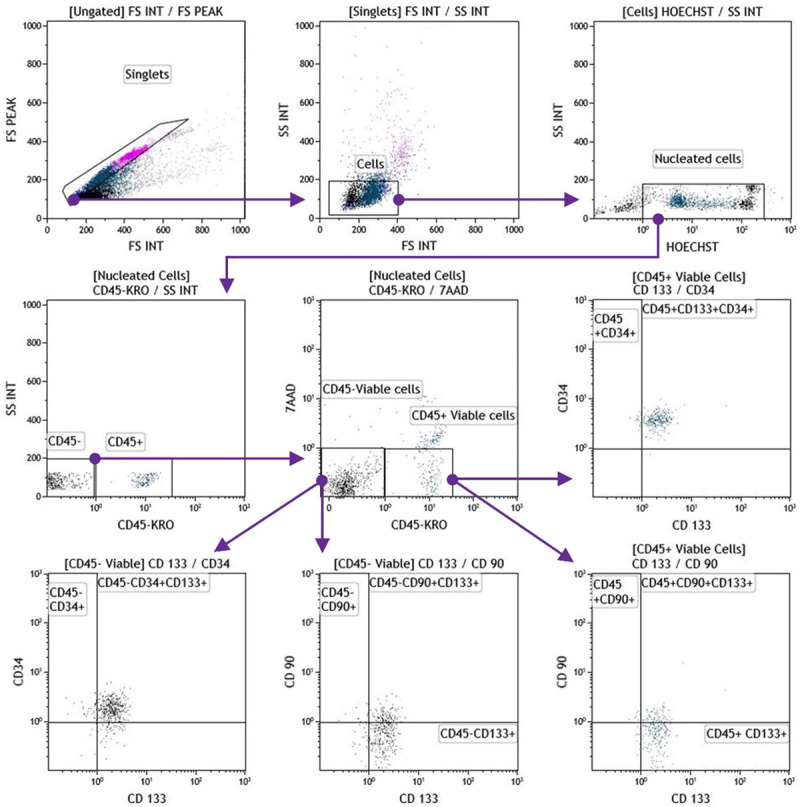
Flow cytometry sequential, logic and manual gating strategy for immunophenotypic characterization of hPBSCs. Visualized hPBSC on dot plots showed their FSC and SSC signals related to the cell’s size and granularity, respectively (upper panel gates). The cells were stained with Hoechst 33342, following 7ADD dye exclusion criteria, and immunofluorescence staining for CD45, CD34, CD133, and CD90. hPBSCs: human peripheral blood-derived stem cells; 7ADD: 7-aminoactinomycin D; APC: allophycocyanin; ECD: phycoerythrin-Texas Red conjugate; FITC: fluorescein isothiocyanate; FSC: forward scatter; PE: phycoerythrin; SSC: side scatter.
Fluorescence microscopy
For hPBSCs immunofluorescence staining, the expression of CD45 was examined before and after nebulization for three independent experiments: compressor, mesh, and ultrasound systems. hPBSCs samples were lysed in Beckman Coulter lysing buffer (Beckman Coulter, USA) for 15 min at 4°C and washed twice by centrifugation at 2500 rpm, 15 min, 4°C, with ice-cold phosphate-buffered saline (PBS). Cells were then fixed with 3.5% paraformaldehyde for 20 min, washed twice in ice-cold PBS, pre-blocked with 2% bovine serum albumin (BSA) for 10 min at RT, and subsequently stained with fluorescein isothiocyanate-(FITC-) conjugated CD45 (1:100, mouse monoclonal IgG; Beckman Coulter) for 30 min at room temperature in the dark. Hoechst 33342 nucleic acid stain (Sigma Aldrich, USA) was added at 10 µg/mL to the cell suspensions for 20 min at RT in the dark. After washing, hPBSCs were acquired using a laser scanning microscope (Leica SP8 confocal microscope, Germany) with the following settings: the emission bandwidth for FITC ranged from 496-598 nm using laser blue 488 and for Hoechst from 415-470 nm using laser blue 405 nm, using 63× objective water immersion with NA 1.2 and optical zoom 3-6.
Statistical analysis
The calculated variables include descriptive statistics, absolute frequencies, and medians. A normal distribution of the variables was evaluated using the Shapiro-Wilk test to define the range of values. Most of the variables did not follow a Gaussian distribution, and the results were expressed through medians. The Mann-Whitney U test was applied for independent samples to evaluate changes in the cell concentration before and after nebulization procedures. The significance thresholds were established at P<0.05, and statistical analysis was performed with GraphPad® Prism v8.4.3 (GraphPad® Software Inc., USA).
Ethical considerations
The clinical trial was approved by the Abu Dhabi Stem Cells Center (ADSCC) Research Ethics Committee (ADSCC.REC.001.1.1) and the Emirates Institutional Review Board (IRB) for COVID-19 Research (DOH/CVDC/2020/1172) with written informed consent obtained from each participant and/or their legal representative, as appropriate. The study was conducted following the provisions of the Declaration of Helsinki [22] and Good Clinical Practice guidelines and registered in ClinicalTrials.gov with Reference NCT04473170.
The information was protected under the principles of confidentiality without revealing patients’ identities.
Results
Immunophenotypic characterization
The full immunophenotypic characterization and cells count medians of the hPBSCs cocktail are shown in Table 1, before and after three methods of nebulization.
Table 1.
Immunophenotypic profiling of hPBSCs cocktail with cell count medians (cells/µL) analyzed by flow cytometry before and after nebulizations
| Immunophenotype | Compressor (n=5) | Mesh (n=5) | Ultrasound (n=5) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Median (cells/µL) | Pa | Median (cells/µL) | Pa | Median (cells/µL) | Pa | ||||
|
|
|
|
|||||||
| Before | After | Before | After | Before | After | ||||
| Nucleated CD45dim | 41 | 30 | .0238* | 16 | 3 | .0079** | 19 | 11 | .0079** |
| Nucleated CD45- | 19 | 15 | .5952 | 27 | 10 | .0397* | 31 | 30 | .4127 |
| Viable CD45dim | 38 | 26 | .2222 | 15 | 2 | .0159* | 10 | 7 | .2778 |
| Viable CD45- | 9 | 9 | >.9999 | 25 | 8 | .0317* | 25 | 26 | .8730 |
| CD45dim/CD34+ | 0 | 0 | >.9999 | 0 | 0 | >.9999 | 0 | 0 | >.9999 |
| CD45dim/CD90+ | 1 | 1 | .6825 | 1 | 1 | .4444 | 0 | 1 | 0.1667 |
| CD45dim/CD133+ | 15 | 8 | .0317 | 2 | 1 | .1270 | 5 | 2 | .0079** |
| CD45dim/CD34+/CD133+ | 15 | 12 | .6825 | 3 | 2 | .5079 | 1 | 2 | .6032 |
| CD45dim/CD90+/CD133+ | 30 | 19 | .4524 | 3 | 3 | >.9999 | 0 | 5 | .1667 |
| CD45-/CD34+ | 0 | 0 | .4444 | 1 | 1 | >.9999 | 10 | 1 | .0635 |
| CD45-/CD90+ | 1 | 2 | .5714 | 0 | 0 | >.9999 | 6 | 14 | .2222 |
| CD45-/CD133+ | 6 | 10 | .3889 | 5 | 2 | .2063 | 23 | 19 | .4206 |
| CD45-/CD34+/CD133+ | 1 | 0 | .1667 | 3 | 1 | .3019 | 12 | 2 | .0397* |
| CD45-/CD90+/CD133+ | 1 | 2 | .7778 | 0 | 1 | .4444 | 7 | 7 | .7143 |
hPBSCs: human peripheral blood-derived stem cells.
Mann Whitney U test:
P-value statistically significant differences (P<0.05);
P-value statistically highly significant differences (P<0.01).
Nucleated cell counts
The absolute cell count (cells/µL) of CD45- nucleated cells have shown significant differences (Mann-Whitney U test) only with the mesh nebulizer (*P=0.0397), while significant differences were found in CD45dim nucleated cells with the compressor method (*P=0.0238), and highly significant differences with mesh (**P=0.0079), and ultrasound (**P=0.0079) nebulizers (Figure 2).
Figure 2.
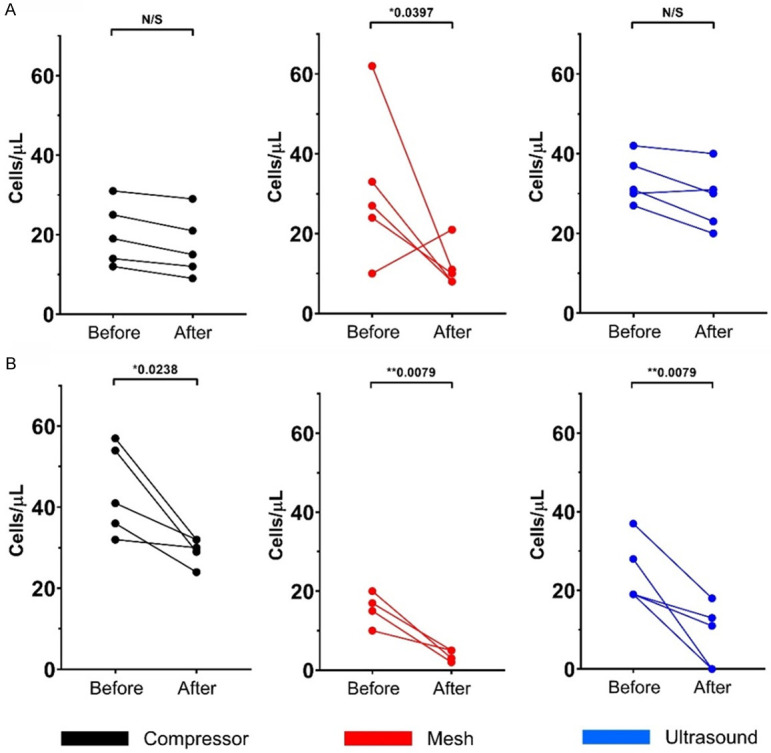
Changes in nucleated absolute cells counts (cells/µL) analyzed by flow cytometry. Cells were stained with Hoechst 33342 before and after three nebulization methods; each color represents one nebulization method [black: compressor (n=5); red: mesh (n=5); and blue: ultrasound nebulization (n=5)]. A. CD45- nucleated cells. B. CD45dim nucleated cells. *Statistically significant differences (P<0.05). **Statistically highly significant differences (P<0.01).
Viable cell counts
A significant loss of viability was found after mesh nebulization in both cell populations: CD45- and CD45dim (viable CD45- *P=0.0317, viable CD45dim *P=0.0159). In contrast, no significant differences were found in viability when comparing the compressor and ultrasound methods (Figure 3).
Figure 3.
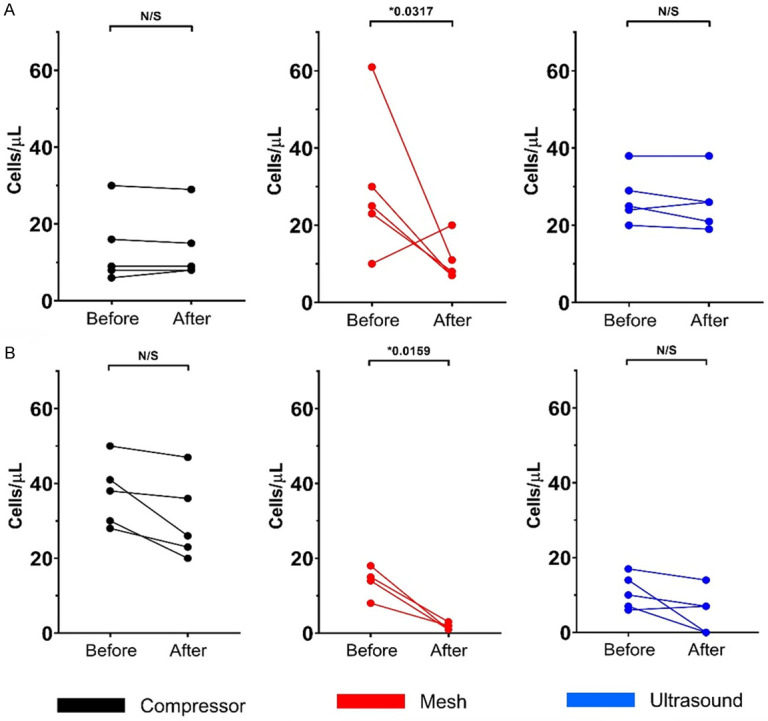
Changes in viable absolute cells counts (cells/µL) analyzed by flow cytometry. Cells were stained with 7ADD before and after three nebulization methods; each color represents one nebulization method [black: compressor (n=5); red: mesh (n=5); and blue: ultrasound nebulization (n=5)]. A. CD45- nucleated cells. B. CD45dim nucleated cells. *Statistically significant differences (P<0.05). 7ADD: 7-aminoactinomycin D.
Cell morphology assessment
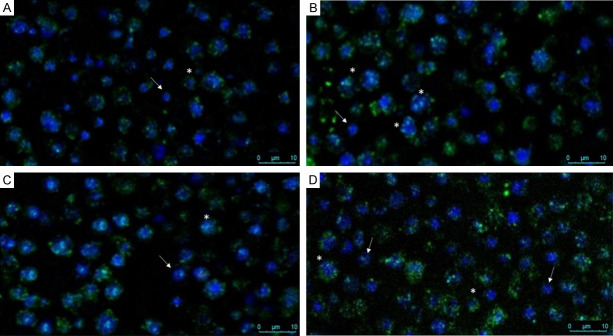
Based on the above results, we were motivated to evaluate the effect on cell morphology after delivering hPBSCs product by the three methods of nebulization. To do this, we next performed immunofluorescence staining for Hoechst 33342 as a nuclear indicator of cell death and CD45 as a cell surface marker antigen, to differentiate between two main subpopulations in hPBSCs: hematopoietic and non-hematopoietic stem cells. A representative image of hPBSCs before nebulization shows very small cells, negative for CD45, with a diameter of approximately 5-7 µm, and a fraction of cells dimly stained with CD45 and slightly higher in number compared with the negatively stained cells (Figure 4A). Figure 4B and 4C show a cytotoxic effect on the CD45dim fraction represented by morphological changes, such as condensation and fragmentation of nuclei, and exhibited brilliant blue fluorescence, indicating cell death. On the other hand, CD45- cells maintain their shape and viability after ultrasound nebulization (Figure 4B), but a cytotoxic effect was shown in Figure 4C. Interestingly, the compressor nebulization method shows no adverse effects on hPBSCs viability and morphology, and CD45dim and CD45- fractions maintain their phenotype after compressor nebulization. These cells maintained a normal appearance, and the fluorescent dye stained morphologically normal nuclei, with a dimly blue fluorescence (Figure 4D).
Figure 4.
Effect of nebulization on cell morphology and viability. Representative immunofluorescence images of hPBSCs cocktail before (A) and after ultrasound (B), mesh (C) and compressor (D) nebulization. hPBSCs were stained with FITC-conjugated monoclonal surface antibody CD45 (1:100) and Hoechst nucleic acid dye 33342 (10 µg/mL). Images were acquired using Leica SP8 confocal microscope using 63× objective. Two main populations were found: hematopoietic (*) and non-hematopoietic cells (arrow). Compressor nebulization shows no adverse effects on hPBSCs morphology. Please note three, one, and two hematopoietic cells along with one, one, and two non-hematopoietic cells were pointed out after ultrasound, mesh, and compressor nebulization, respectively. hPBSCs: Human peripheral blood-derived stem cells; FITC: fluorescein isothiocyanate.
Discussion
hPBSCs are present in a small proportion in the bloodstream as a heterogeneous group composed of distinct subsets [23]. Using a manual gating strategy, we first sorted the small-sized nucleated cells positively stained with Hoechst 33342 as a nucleic DNA probe combined with scattering characteristics, while the inclusion of Hoechst 33342 improves the precision in the measurement of nucleated cells by eliminating the effects of debris by flow cytometry analyses [24].
For identifying the presence of subpopulations in hPBSCs, we chose the CD34 and CD133 stem cell markers in combination with CD45, a well-known hematopoietic cell-surface antigen [23]. Notably, we found that hPBSCs were characterized by two main subpopulations, CD45dim and CD45-, with both fractions expressing CD34 and CD133. The CD45dim/CD34+/CD133+ fraction of cells represents the circulating hematopoietic stem/progenitors cells [25,26].
Recent studies by Ratajzcak and colleagues report the presence of human adult stem cells (VSELs), known by their pluripotency phenotype, their very primitive morphology (5-7 µm as diameter), and considered to be at the top of the stem cell hierarchy in normal BM, giving rise to HSCs, MSCs, and EPCs [27]. VSELs are classified by the absence of CD45 due to their non-hematopoietic origin. In this study, we identified the presence of small-sized cells CD45-/CD34+/CD133+ that probably represent VSELs, but more analysis will be needed. Furthermore, a small fraction of hPBSCs was identified to express CD90, known as immature progenitor cells. Indeed, CD34+/CD90+ cells have been demonstrated to reconstitute human hematopoiesis in vitro and in vivo [28].
An appropriate understanding of the working principle and the factors influencing the nebulizers’ performance is mandatory for interpreting our results. Nebulization produces fine aqueous droplets, and aerosolized cells can give unanticipatedly dynamic aerosol behavior [29,30]. Interestingly, there are no reports in the medical literature related to the nebulization of hPBSCs. Aver’yanov and colleagues have performed nebulization of MSCs in suspension, demonstrating that the compressor showed higher viability and cell count compared to ultrasonic and mesh nebulization [31].
In our study, a statistically significant difference in the CD45dim nucleated cell count was found after compressor nebulization, without statistically significant differences among the other identified subsets. In our opinion, the nebulization method by compressor represents physical stress likely to induce changes in the hPBSCs suspensions [32], even though it was the gentlest method as per our results.
Besides, there was a significant decrease in the CD45dim nucleated cell count in the ultrasonic-nebulized samples. We attribute the viscosity changes, combined with ultrasonic device-induced temperature increases, might be responsible for this decrease [32,33].
Still, statistically significant differences in both CD45dim and CD45- nucleated cell counts and viability were found after mesh nebulization. Vibrating-mesh nebulizers force samples through multiple apertures in a plate to generate aerosols [33], while not all available apertures produce droplets all of the time and orifices can get clogged over time [34], resulting in a loss of viability and cell count after nebulization.
In that regard, the average particle size for Aerogen® Solo devices is 3.1-3.9 μm, as per manufacturer specifications [35], in such a way the hPBSCs size heterogeneity are impacting this nebulizer performance.
The evaporation of the solvent is another influencing factor: the sample concentration and viscosity increased as nebulization progressed, with a higher magnitude of increase on ultrasound devices than compressor nebulizers, while concentration changes are negligible with mesh nebulizers [36,37]. With our ultrasound-nebulized samples, we detected an increased concentration of viable CD45- cells, but this did not reach statistical significance.
Regarding the temperature changes in compressor nebulizers, Dennis and colleagues found that the solution temperature started decreasing as soon as nebulization began [38]. Conversely, ultrasonic and mesh nebulizers increase the sample temperatures [32], and we attributed the temperature changes as one of the contributing factors for explaining the best conservation of the analyzed subsets after the compressor nebulization.
All this evidence suggests CD45dim cells are less resistant to mechanical or physical stresses than CD45- cells. We hypothesize that cell size and nuclear-cytoplasmic ratio are determinant factors to resistance during nebulization. Smaller cells with less cytoplasm volume seem to better resist the stresses produced by nebulization compared to CD45dim cells.
Thus, the changes in temperature and viscosity of the hPBSCs samples in the context of the physical principles that govern the aerosolization processes seem to be most noticeable (in decreasing order) after mesh, ultrasound, and compression nebulization. In addition, the morphological changes described in Figure 4 support these findings.
Consequently, the compressor platform has demonstrated proper feasibility to nebulize hPBSCs cocktails isolated from COVID-19 patients. However, considering that the safety and effectiveness of the hPBSCs nebulization are based on different mechanisms, we cannot exclude the possibility that different types of nebulizers will produce distinct clinical effects. Our main limitations overlap with the unavoidable limitations of in vitro research, so it will be necessary to conduct additional pre-clinical and clinical studies. Complex interactions occurring on validated in vivo models, airway deposition assays, and emerging technologies for aerosol generation require further studies.
Conclusions
The nebulized cell delivery technique has been described as an effective delivery route for cell-based therapy to treat patients with lung-related diseases. This study provides the first evidence that stem cells derived from PB of COVID-19 patients can be nebulized without substantial loss of cell viability, cell count, and morphological changes using the compressor device. Our group currently provides hPBSCs therapies delivered through compressor nebulization (under Expanded Access Programs) to seriously sick COVID-19 patients who have exhausted all viable treatment options within the UAE. We hope this will lead to a novel treatment with high cell survival, cell homing, and engraftment into the lungs.
Acknowledgements
Authors acknowledge ADSCC Management and New York University-Abu Dhabi’s Core Technology Platforms for supporting the research reported in this manuscript.
Disclosure of conflict of interest
None.
Abbreviations
- 7ADD
7-aminoactinomycin D
- ADSCC
Abu Dhabi Stem Cells Center
- AIDS
Acquired Immunodeficiency Syndrome
- APC
Allophycocyanin
- ARDS
Acute Respiratory Distress Syndrome
- BM
bone marrow
- BSA
Bovine serum albumin
- COVID-19
2019 novel coronavirus disease
- CPL
Cells Processing Laboratory
- ECD
Phycoerythrin-Texas Red conjugate
- EPCs
Endothelial progenitor cells
- ESCs
Embryonic stem cells
- FITC
Fluorescein isothiocyanate
- FSC
Forward scatter
- HBV
Hepatitis B Virus
- HIV
Human Immunodeficiency Virus
- hPBSCs
Human peripheral blood-derived stem cells
- HSCs
Hematopoietic stem cells
- KRO
Krome orange
- MMAD
Median mass aerodynamic diameter
- MSCs
Mesenchymal stromal stem cells
- PB
Peripheral blood
- PBNHE-SCC
Peripheral Blood Non-Hematopoietic Enriched Stem Cell Cocktail
- PE
Phycoerythrin
- PPE
Personal Protective Equipment
- RT
room temperature
- RT-PCR
Real-time Polymerase Chain Reaction
- SENTAD-COVID
Adaptive open-label study evaluating the Safety and Efficacy of Autologous Non-Hematopoietic Peripheral Blood Stem Cells Therapy in COVID-19 outbreak in Abu Dhabi, 2020
- SSC
Side scatter
- UAE
United Arab Emirates
- VSELs
Very small embryonic like-stem cells
References
- 1.GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen F, Fine A. Stem cells in lung injury and repair. Am J Pathol. 2016;186:2544–2550. doi: 10.1016/j.ajpath.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parekh KR, Nawroth J, Pai A, Busch SM, Senger CN, Ryan AL. Stem cells and lung regeneration. Am J Physiol Cell Physiol. 2020;319:C675–C693. doi: 10.1152/ajpcell.00036.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horie S, Gonzalez HE, Laffey JG, Masterson CH. Cell therapy in acute respiratory distress syndrome. J Thorac Dis. 2018;10:5607–5620. doi: 10.21037/jtd.2018.08.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cribbs SK, Martin GS. Stem cells in sepsis and acute lung injury. Am J Med Sci. 2011;341:325–332. doi: 10.1097/MAJ.0b013e3181f30dee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lian J, Lin J, Zakaria N, Yahaya BH. Acute lung injury: disease modelling and the therapeutic potential of stem cells. Adv Exp Med Biol. 2020;1298:149–166. doi: 10.1007/5584_2020_538. [DOI] [PubMed] [Google Scholar]
- 7.Hostettler KE, Gazdhar A, Khan P, Savic S, Tamo L, Lardinois D, Roth M, Tamm M, Geiser T. Multipotent mesenchymal stem cells in lung fibrosis. PLoS One. 2017;12:e0181946. doi: 10.1371/journal.pone.0181946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sueblinvong V, Weiss DJ. Stem cells and cell therapy approaches in lung biology and diseases. Transl Res. 2010;156:188–205. doi: 10.1016/j.trsl.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brave H, MacLoughlin R. State of the art review of cell therapy in the treatment of lung disease, and the potential for aerosol delivery. Int J Mol Sci. 2020;21:6435. doi: 10.3390/ijms21176435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsuchiya A, Takeuchi S, Iwasawa T, Kumagai M, Sato T, Motegi S, Ishii Y, Koseki Y, Tomiyoshi K, Natsui K, Takeda N, Yoshida Y, Yamazaki F, Kojima Y, Watanabe Y, Kimura N, Tominaga K, Kamimura H, Takamura M, Terai S. Therapeutic potential of mesenchymal stem cells and their exosomes in severe novel coronavirus disease 2019 (COVID-19) cases. Inflamm Regen. 2020;40:14. doi: 10.1186/s41232-020-00121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta A, Kashte S, Gupta M, Rodriguez HC, Gautam SS, Kadam S. Mesenchymal stem cells and exosome therapy for COVID-19: current status and future perspective. Hum Cell. 2020;33:907–918. doi: 10.1007/s13577-020-00407-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020;29:747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu S, Peng D, Qiu H, Yang K, Fu Z, Zou L. Mesenchymal stem cells as a potential therapy for COVID-19. Stem Cell Res Ther. 2020;11:169. doi: 10.1186/s13287-020-01678-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu A, Zhang X, He H, Zhou L, Naito Y, Sugita S, Lee JW. Therapeutic potential of mesenchymal stem/stromal cell-derived secretome and vesicles for lung injury and disease. Expert Opin Biol Ther. 2020;20:125–140. doi: 10.1080/14712598.2020.1689954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kardia E, Ch’ng ES, Yahaya BH. Aerosol-based airway epithelial cell delivery improves airway regeneration and repair. J Tissue Eng Regen Med. 2018;12:e995–e1007. doi: 10.1002/term.2421. [DOI] [PubMed] [Google Scholar]
- 16.Kardia E, Yusoff NM, Zakaria Z, Yahaya B. Aerosol-based delivery of fibroblast cells for treatment of lung diseases. J Aerosol Med Pulm Drug Deliv. 2014;27:30–34. doi: 10.1089/jamp.2012.1020. [DOI] [PubMed] [Google Scholar]
- 17.Jin JF, Zhu LL, Chen M, Xu HM, Wang HF, Feng XQ, Zhu XP, Zhou Q. The optimal choice of medication administration route regarding intravenous, intramuscular, and subcutaneous injection. Patient Prefer Adherence. 2015;9:923–942. doi: 10.2147/PPA.S87271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Contreras L, Yadav KS. Inhaled formulation design for the treatment of lung infections. Curr Pharm Des. 2015;21:3875–3901. doi: 10.2174/1381612821666150820110305. [DOI] [PubMed] [Google Scholar]
- 19.Ibrahim M, Verma R, Garcia-Contreras L. Inhalation drug delivery devices: technology update. Med Devices (Auckl) 2015;8:131–139. doi: 10.2147/MDER.S48888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Huang B. Peripheral blood stem cells: phenotypic diversity and potential clinical applications. Stem Cell Rev Rep. 2012;8:917–925. doi: 10.1007/s12015-012-9361-z. [DOI] [PubMed] [Google Scholar]
- 21.Abu Dhabi Stem Cells Center (ADSCC) Work of science: cell obtaining procedure for a method for harvesting autologous peripheral blood non-hematopoietic enriched stem cell cocktail (PBNHE-SCC) from adult human peripheral blood. International Online Copyright Office (INTEROCO) 2020. Available at https://interoco.com/all-materials/work-of-science/3129-work-of-science-cell-obtaining-procedure.html (Accessed on October 17th, 2020)
- 22.World Medical Association. World Medical Association Declaration of Helsinki ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 23.Roland J. Hematopoietic stem cell heterogeneity. Adv Exp Med Biol. 2019;1169:195–211. doi: 10.1007/978-3-030-24108-7_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.An X, Chen L. Flow cytometric analysis of erythroblast enucleation. Methods Mol Biol. 2018;1698:193–203. doi: 10.1007/978-1-4939-7428-3_11. [DOI] [PubMed] [Google Scholar]
- 25.King KY, Goodell MA. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat Rev Immunol. 2011;11:685–692. doi: 10.1038/nri3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bujko K, Kucia M, Ratajczak J, Ratajczak MZ. Hematopoietic stem and progenitor cells (HSPCs) Adv Exp Med Biol. 2019;1201:49–77. doi: 10.1007/978-3-030-31206-0_3. [DOI] [PubMed] [Google Scholar]
- 27.Ratajczak MZ, Ratajczak J, Kucia M. Very small embryonic-like stem cells (VSELs) Circ Res. 2019;124:208–210. doi: 10.1161/CIRCRESAHA.118.314287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimazaki C, Sumikuma T, Inaba T. CD34+ CD90+ cells and late hematopoietic reconstitution after autologous peripheral blood stem cell transplantation. Leuk Lymphoma. 2004;45:661–668. doi: 10.1080/1042819031000140997. [DOI] [PubMed] [Google Scholar]
- 29.Martin AR, Finlay WH. Nebulizers for drug delivery to the lungs. Expert Opin Drug Deliv. 2015;12:889–900. doi: 10.1517/17425247.2015.995087. [DOI] [PubMed] [Google Scholar]
- 30.Saeed H, Ali AMA, Elberry AA, Eldin AS, Rabea H, Abdelrahim MEA. Modeling and optimization of nebulizers’ performance in non-invasive ventilation using different fill volumes: comparative study between vibrating mesh and jet nebulizers. Pulm Pharmacol Ther. 2018;50:62–71. doi: 10.1016/j.pupt.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Aver’yanov AV, Konoplyannikov AG, Antonov NS, Osipova GL, Vasil’eva OS, Sakharova MG, Tatarskii AR, Kobylyansky VI. Survival of mesenchymal stem cells in different methods of nebulization. Bull Exp Biol Med. 2018;164:576–578. doi: 10.1007/s10517-018-4034-9. [DOI] [PubMed] [Google Scholar]
- 32.Carvalho TC, McConville JT. The function and performance of aqueous aerosol devices for inhalation therapy. J Pharm Pharmacol. 2016;68:556–578. doi: 10.1111/jphp.12541. [DOI] [PubMed] [Google Scholar]
- 33.Ari A. Jet, ultrasonic, and mesh nebulizers: an evaluation of nebulizers for better clinical outcomes. Eurasian J Pulmonol. 2014;16:1–7. [Google Scholar]
- 34.Lin HL, Chen CS, Fink JB, Lee GH, Huang CW, Chen JC, Chiang ZY. In vitro evaluation of a vibrating-mesh nebulizer repeatedly use over 28 days. Pharmaceutics. 2020;12:971. doi: 10.3390/pharmaceutics12100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aerogen® Solo System Instruction Manual, 2016: 27. Available at https://www.aerogen.com/wp-content/uploads/2017/03/30-354-Rev-P-Aerogen-Solo-System-IM-English-WEB.pdf (Accessed on February 27th, 2021)
- 36.Le Brun PP, de Boer AH, Heijerman HG, Frijlink HW. A review of the technical aspects of drug nebulization. Pharm World Sci. 2000;22:75–81. doi: 10.1023/a:1008786600530. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Y, Ahuja A, Irvin CM, Kracko DA, McDonald JD, Cheng YS. Medical nebulizer performance: effects of cascade impactor temperature. Respir Care. 2005;50:1077–1082. [PubMed] [Google Scholar]
- 38.Dennis JH, Stenton SC, Beach JR, Avery AJ, Walters EH, Hendrick DJ. Jet and ultrasonic nebuliser output: use of a new method for direct measurement of aerosol output. Thorax. 1990;45:728–732. doi: 10.1136/thx.45.10.728. [DOI] [PMC free article] [PubMed] [Google Scholar]