Abstract
Background
Patients with cirrhosis and coronavirus disease-2019 (COVID-19) have high in-hospital mortality. The information on the outcome of cirrhosis patients in the posthospitalization period is limited.
Aims
We aimed to study the outcome of cirrhosis patients with COVID-19 after hospital discharge.
Methods
The records of the cirrhosis patients discharged after COVID-19 were reviewed. Their data were compared with a similar number of cirrhosis patients without COVID-19 after propensity score matching for age, sex, etiology of cirrhosis, and model for end-stage liver disease (MELD) score.
Results
Cirrhosis patients with (n = 92) or without (n = 92) COVID-19 were included in 1:1 ratio. The mortality among COVID-19 (22; 23.9%) and non-COVID-19 (19; 20.7%) were comparable (HR 1.224; 95% CI 0.663–2.263, P = 0.520), over a similar duration of follow-up [186 (86–271) vs. 183 (103–274)]. Among COVID-19 patients, 45; 48.9% developed a new acute decompensation-increased ascites (40; 43.5%), hepatic encephalopathy (20; 21.7%), or variceal bleeding (8; 8.7%) whereas 25 (27.2%) patients needed rehospitalization. A proportion of participants continued to have either fatigue/weakness (24/80; 30.0%), sleep disturbances (11/80; 13.7%), or joint pains (16/80; 20.0%). The most common causes of death in patients of both groups were end-stage liver disease: 16 (72.7%) vs. 9 (47.4%), followed by multiorgan dysfunction: 4 (18.2%) vs. 6 (31.6%), GI bleeding: 2 (9.1%) vs. 4 (21.0%), P = 0.484. A lower albumin level, higher international normalized ratio, bilirubin, Child-Turcotte-Pugh, and MELD scores at discharge predicted mortality in the COVID-19 group.
Conclusion
Short-term outcomes of patients with cirrhosis who survive the initial insult of COVID-19 are not different from patients without COVID-19, and survival is determined by the severity of liver disease at discharge.
Keywords: coronavirus, virus, pandemic, mortality, chronic liver disease
Abbreviations: AIH, autoimmune hepatitis; ACE2, Angiotensin-converting enzyme 2; AD, acute decompensation; ALT, alanine aminotransferase; Alk P, alkaline phosphatase; AST, aspartate aminotransferase; COVID-19, coronavirus disease-2019; CTP, Child-Turcotte-Pugh; GI, Gastrointestinal; HBV, hepatitis B virus; HCV, hepatitis C virus; INR, international normalized ratio; IQR, interquartile range; NAFLD, nonalcoholic fatty liver disease; TLC, Total leukocyte count; MELD, model for end-stage liver disease
The Coronavirus disease-2019 (COVID-19) has high mortality rates among those with risk factors such as old age, diabetes mellitus, renal failure, obesity, and chronic liver disease.1, 2, 3 Waning pandemic has surfaced the problem of its consequences such as multiorgan inflammatory syndrome and long COVID syndrome.4, 5, 6, 7 Now, COVID-19 is accepted as a multisystem disease with long-lasting consequences and not just an acute respiratory illness.8
Over 50% of those discharged after COVID-19 have troublesome symptoms related to various organ systems such as fatigue, loss of taste and/or smell, or a plethora of neuropsychiatric, pulmonary, and cardiovascular symptoms.5,9,10 The risk of developing post-COVID-19 symptoms is higher in old age.11 The exact pathogenesis of these symptoms is not clear. The post-COVID syndrome may result from ongoing organ damage secondary to prolonged subclinical inflammation, continued viral-mediated organ damage, immune complex-mediated injury, or induction of autoimmunity by antigen mimicry.7,11
Liver injury is common during active COVID-19 disease. Liver enzyme elevations are seen in up to 50–60% of patients.12 This high rate of liver injury can be explained by the high expression of ACE2 receptors on cholangiocytes, sinusoidal endothelial cells, and hepatocytes.13 There is a possibility of prolonged injury to hepatocytes or cholangiocytes, resulting in significant damage to the liver. Cirrhosis patients have limited residual hepatocytes, and any additional injury could lead to decompensation and death after discharge from the hospital. At present, the data are limited on the natural history of cirrhosis patients discharged after COVID-19 illness. We aimed to study the outcomes of cirrhosis patients with COVID-19 who were discharged from our COVID-19 care facility. In addition, we also assessed the development of new complications and predictors of mortality in the COVID-19 cirrhosis patients.
Patients and methods
Ethical clearance with a waiver of consent was obtained from the Institutional Ethics Committee of All India Institute of Medical Sciences, New Delhi (Ref No: IEC-253/17.04.2020).
Patient Population
We retrospectively reviewed the records of the cirrhosis patients admitted between January 2018 and April 2021 to select the cases and controls. Cirrhosis patients discharged after recovery from COVID-19 were selected as cases. Controls were selected from those who were admitted with cirrhosis-related complications without COVID-19. RT-PCR testing was available only for the controls admitted after the onset of the COVID-19 pandemic. The cases and controls were subjected to propensity score matching.
Definitions
Diagnosis of cirrhosis was made with a combination of clinical features, laboratory parameters, radiological and endoscopic evaluation. The diagnosis of COVID-19 was made with symptoms compatible with coronavirus infection supported with positive RT-PCR results in samples collected from nasal and/or throat swabs.
The clinical severity of COVID-19 was defined according to the Ministry of Health and Family Welfare (MOHFW) criteria: mild disease as patients with only upper respiratory tract symptoms without any signs of breathlessness and hypoxia. Moderate severity was defined as the presence of pneumonia with the respiratory rate (RR) between 24 and 30/min and SpO2 between 90% and 94% on room air, while the severe disease was defined by the presence of pneumonia with RR > 30/min or SpO2 < 90% on room air or severe respiratory distress.14
The relevant clinical, laboratory, radiological and endoscopic data information was retrieved to assess the liver disease severity. The liver disease severity was assessed with Child-Turcotte-Pugh (CTP)15 and MELD16 scores. All parameters available at hospital discharge were considered as baseline parameters for those with and without COVID-19 infection. The CTP and MELD score at discharge was recorded as the baseline value to assess the outcomes.
The follow-up data were retrieved for all the participants to assess the outcomes. Data were collected with either video call or physical visit to the hospital, with preference to the latter. The collected data included the appearance of new symptoms of decompensation, worsening of pre-existing decompensation. Patients were asked about any post-COVID complications like persistent fatigue/weakness, anosmia/dysgeusia, dyspnea, joint pains, difficulty in sleeping, cough, and headache. If the patient had died after discharge, verbal autopsy was done after discussion with the closest possible attendant who was with the patient during the last seven days before his/her death.
Statistical Analysis
Propensity score matching (PSM) was used for matching the groups. The propensity scores were based on the potential confounding variables, including age, sex, etiology of cirrhosis, and MELD score at the time of discharge. The patients were matched 1:1 from both the groups based on the propensity scores with a caliper width of 0.20 SD. PSM was also performed using Kernel weighting and logistic regression. PSM matching was done using STATA/SE version 14.2 (StataCorp LP, College Station, TX).
Qualitative data were expressed as number and percentage, and quantitative variables were expressed as median (interquartile range, IQR). Qualitative data were compared using the Chi-square test or Fisher’s Exact test as appropriate, while quantitative data were compared between two groups with the Mann–Whitney U test. Univariable and multivariable analysis was done for assessing independent predictors of the outcome using the Cox-regression method. Survival analysis was performed using the Kaplan–Meier (KM) method. A P-value of <0.05 was considered statistically significant. Statistical calculations were made using the statistical package for social sciences (SPSS) version 20.0 (IBM, Chicago, IL, USA). MedCalc Statistical Software version 18.2.1 (MedCalc Software bvba, Ostend, Belgium) was used to generate Kaplan–Meier curves for survival.
Results
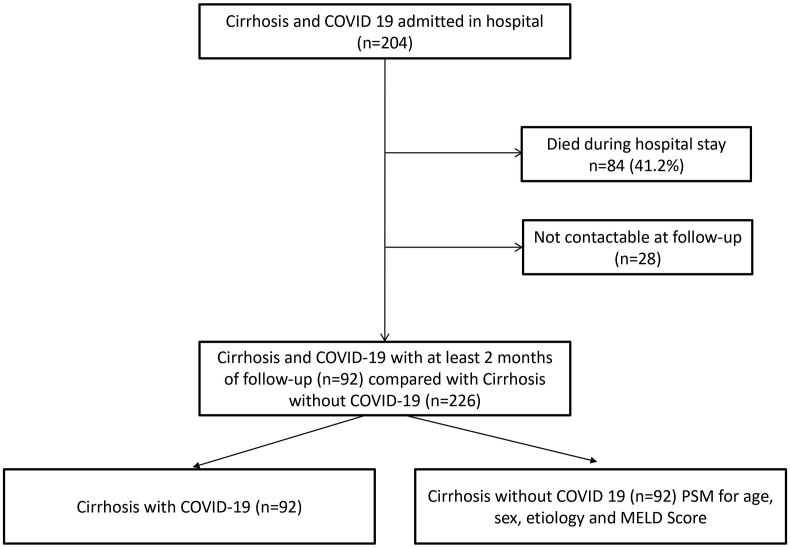
Of the 204 cirrhosis patients admitted with COVID-19 infection, 84 (41.1%) died, 28 (13.7%) could not be contacted after discharge, and 92 (45.1%) were included as cases. Propensity score-matched 92 controls were selected from 226 cirrhosis patients hospitalized with cirrhosis-related complications without COVID-19 and were discharged in hemodynamically stable conditions (Figure 1).
Figure 1.
The flow of patients in the study. PSM, Propensity-score matching
Comparison of Cirrhosis Patients with or without COVID-19
Overall, patients with cirrhosis and COVID-19 (n = 92) as compared to those without COVID-19 (n = 226) had higher age and hemoglobin. The platelet count, serum creatinine, alkaline phosphatase, and MELD score were lower in the COVID-19 patients (Table 1). The rest of the variables between the two groups were similar. The mortality in the two groups was similar, 23.9% vs. 25.7% (P = 0.778).
Table 1.
Comparison of Clinical Characteristics, Laboratory Parameters and Liver Disease Severity Scores among Cirrhosis Patients With or Without Covid-19 Infection.
| Variables | Cohort before PSM |
P-value | Cohort after PSM |
P-value | ||
|---|---|---|---|---|---|---|
| Cirrhosis and COVID-19 (n = 92) | Cirrhosis without COVID-19 (n = 226) | Cirrhosis and COVID-19 (n = 92) | Cirrhosis without COVID-19 (n = 92) | |||
| Age (years) | 46 (38–54) | 43 (35–51) | 0.045 | 46 (38–54) | 46 (38–54) | 0.635 |
| Sex, male n (%) | 73 (79.3%) | 191 (84.5%) | 0.323 | 19 (20.7%) | 18 (19.6%) | 1.000 |
| Diabetes | 20 (21.7%) | 36 (15.9%) | 0.256 | 20 (21.7%) | 17 (18.5%) | 0.713 |
| Etiology of cirrhosis | 0.290 | 0.620 | ||||
| Alcohol | 38 (41.3%) | 114 (50.4%) | 38 (41.3%) | 38 (41.3%) | ||
| HBV | 7 (7.6%) | 24 (10.6%) | 7 (7.6%) | 6 (6.5%) | ||
| HCV | 9 (9.8%) | 12 (5.3%) | 9 (9.8%) | 7 (7.6%) | ||
| AIH | 8 (8.7%) | 10 (4.4%) | 8 (8.7%) | 3 (3.3%) | ||
| NAFLD | 10 (10.9%) | 15 (6.6%) | 10 (10.9%) | 9 (9.8%) | ||
| Cryptogenic | 16 (17.4%) | 41 (18.1%) | 16 (17.4%) | 22 (23.9%) | ||
| Others | 4 (4.3%) | 10 (4.4%) | 4 (4.3%) | 7 (7.6%) | ||
| Etiology | 0.173 | 1.000 | ||||
| Alcohol | 38 (41.3%) | 114 (50.4%) | 38 (41.3%) | 38 (41.3%) | ||
| Others | 54 (58.7%) | 112 (49.6%) | 54 (58.7%) | 54 (58.7%) | ||
| Hemoglobin (g/dL) | 8.5 (7.3–10.2) | 8.1 (7.0–9.6) | 0.017 | 8.5 (7.3–10.2) | 8.4 (6.7–9.6) | 0.116 |
| TLC (per mm3) | 5100 (3225–7975) | 5855 (3795–8656) | 0.301 | 5100 (3225–7975) | 6345 (4180–9300) | 0.178 |
| Platelet count ( × 103/mm3) | 69 (49–106) | 91 (60–144) | 0.002 | 69 (49–106) | 99 (65–150) | 0.001 |
| INR | 1.5 (1.2–1.8) | 1.6 (1.3–2.0) | 0.171 | 1.5 (1.2–1.8) | 1.4 (1.2–1.8) | 0.547 |
| Total bilirubin (mg/dL) | 1.8 (0.9–4.0) | 2.1 (1.2–3.2) | 0.640 | 1.8 (0.9–4.0) | 1.8 (0.9–2.9) | 0.435 |
| Creatinine (mg/dl) | 0.8 (0.6–1.1) | 0.9 (0.7–1.5) | 0.002 | 0.8 (0.6–1.1) | 0.9 (0.7–1.5) | 0.028 |
| AST (IU/L) | 51 (36–72) | 47 (34–84) | 0.693 | 51 (36–72) | 43 (34–70) | 0.227 |
| ALT (IU/L) | 31 (24–47) | 33 (22–55) | 0.903 | 31 (24–47) | 33 (22–42) | 0.749 |
| Alk P (IU/L) | 117 (78–142) | 166 (112–265) | <0.001 | 117 (78–142) | 171 (111–282) | <0.001 |
| Albumin (g/dL) | 2.8 (2.4–3.1) | 2.9 (2.5–3.2) | 0.301 | 2.8 (2.4–3.1) | 2.9 (2.5–3.2) | 0.168 |
| CTP score | 8 (7–10) | 8 (6–10) | 0.500 | 8 (7–10) | 7 (6–10) | 0.198 |
| CTP class | 0.648 | 0.419 | ||||
| A | 27 (29.3%) | 62 (27.4%) | 27 (29.3%) | 35 (38.0%) | ||
| B | 39 (42.4%) | 88 (38.9%) | 39 (42.4%) | 32 (34.8%) | ||
| C | 26 (28.3%) | 76 (33.6%) | 26 (28.3%) | 25 (27.2%) | ||
| MELD | 13.9 (10.6–20.2) | 17.0 (13–22) | 0.006 | 13.9 (10.6–20.2) | 15.0 (10.9–19.8) | 0.827 |
| Died at last FU | 22 (23.9%) | 58 (25.7%) | 0.778 | 22 (23.9%) | 19 (20.7%) | 0.723 |
Note: Qualitative and quantitative data are expressed as proportions and median (interquartile range), respectively.
Abbreviations: AIH, autoimmune hepatitis; HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease; TLC, total leukocyte count; INR, international normalized ratio; ALT, alanine aminotransferase; Alk P, alkaline phosphatase; AST, aspartate aminotransferase; CTP, Child-Turcotte-Pugh; MELD, model for end-stage liver disease.
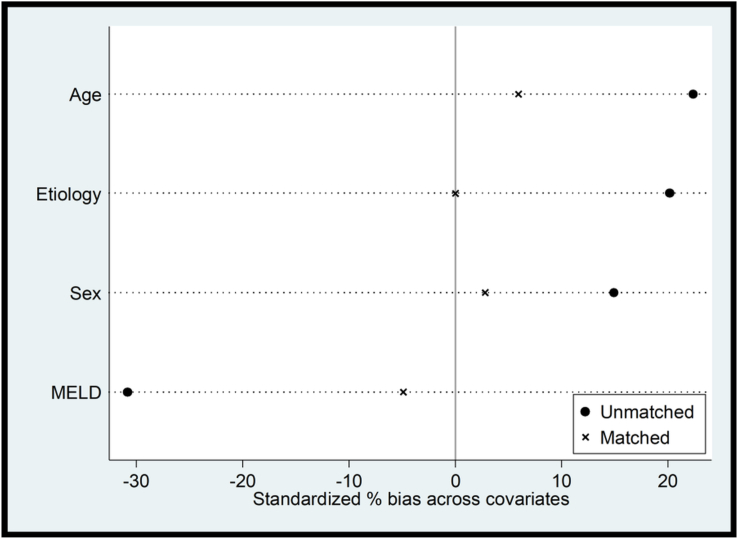
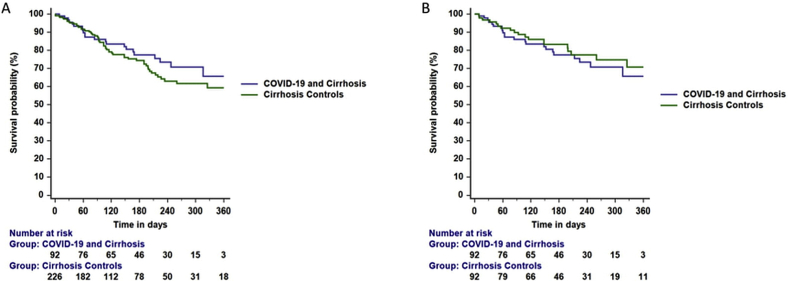
Postmatching balance assessment is represented using covariate balance plots (Figure 2). The standardized percentage differences in covariates were nearly eliminated by propensity matching. The differences in platelet count, serum creatinine, and alkaline phosphatase persisted after matching the groups (Table 1). Overall, among patients with COVID-19, the COVID severity was graded as mild in 76 (82.6%), moderate 9 (9.8%), and severe 7 (7.6%). The liver disease severity was as follows- CTP-A 27 (29.3%), B 39 (42.4%), and C 26 (28.3%); their median MELD score was 13.9 (10.6–20.2). The follow-up period (days) for the COVID-19 and controls was 186 (86–271) and 183 (102–274) days, respectively. The number of study participants who died during the follow-up included 22 (23.9%) in the COVID-19 group and 19 (20.7%) in the non-COVID-19 group. The KM survival curves of patients in the unmatched (log-rank P = 0.320) and matched (log-rank P = 0.519) groups are shown in Figure 3A and B.
Figure 2.
Covariate balance plot before and after propensity matching.
Figure 3.
A and B. Survival probability among patients with cirrhosis, with or without COVID-19 infection. The posthospital discharge mortality was similar in the A) unmatched (log-rank P = 0.320) and B) matched (log-rank P = 0.519) cohorts.
The KM survival curves of COVID-19 and non-COVID-19 patients in the unmatched groups (Child A, log-rank P = 0.114; Child B, log-rank P = 0.928 and Child C, log-rank P = 0.480) and matched groups (Child A, log-rank P = 0.370; Child B, log-rank P = 0.563 and Child C, log-rank P = 0.267) are shown in Supplementary Figures 1 and 2, respectively.
The most common causes of death in the COVID-19 and non-COVID-19 groups were end-stage liver disease: 16 (72.7%) vs. 9 (47.4%), followed by multiorgan dysfunction in 4 (18.2%) vs. 6 (31.6%), GI bleeding in 2 (9.1%) vs. 4 (21.0%), P = 0.484.
Predictors of Survival in the Prematch and PSM Groups
In the prematched group, independent predictors of mortality on unadjusted Cox-proportional hazard analysis included international normalized ratio, HR, 1.191 (95% CI, 1.062–1.337), creatinine, HR, 1.219 (95% CI, 1.065–1.397), alkaline phosphatase HR, 1.002 (95% CI, 1.001–1.003), albumin, HR, 0.598 (95% CI, 0.424–0.842), CTP score, HR, 1.224 (95% CI, 1.122–1.336), Child class B, HR, 2.684 (1.277–5.642), Child class C, HR, 4.680 (2.268–9.657), and MELD score, HR, 1.068 (1.034–1.103). The HR for mortality with COVID-19 infection compared to those without COVID-19 was 0.775 (0.474–1.268), P = 0.331 (Table 2).
Table 2.
Cox-Proportional Analysis of Variables Associated With Mortality in the Whole Cohort and After Propensity Score Matching (PSM).
| Whole cohort | PSM |
P-value | ||
|---|---|---|---|---|
| Characteristics | Univariate analysis HR (95% CI) | |||
| Age (years) | 1.014 (0.996–1.033) | 0.135 | 1.017 (0.991–1.044) | 0.201 |
| Sex | ||||
| Females | 1 | 1 | ||
| Male | 0.955 (0.544–1.675) | 0.872 | 1.065 (0.492–2.307) | 0.872 |
| Diabetes, yes | 0.943 (0.537–1.655) | 0.838 | 0.980 (0.467–2.059) | 0.958 |
| Etiology | ||||
| Others | 1 | 1 | ||
| Alcohol | 0.846 (0.544–1.316) | 0.458 | 0.878 (0.469–1.645) | 0.684 |
| Hemoglobin (g/dL) | 0.959 (0.871–1.057) | 0.402 | 0.912 (0.793–1.050) | 0.201 |
| TLC (per mm3) | 0.991 (0.947–1.036) | 0.692 | 1.002 (0.945–1.063) | 0.950 |
| Platelet count ( × 103/mm3) | 1.000 (0.997–1.002) | 0.760 | 0.999 (0.996–1.003) | 0.651 |
| INR | 1.191 (1.062–1.337) | 0.003 | 1.133 (0.945–1.359) | 0.178 |
| Total bilirubin (mg/dL) | 1.041 (0.989–1.097) | 0.123 | 1.095 (1.025–1.169) | 0.007 |
| Creatinine (mg/dl) | 1.219 (1.065–1.397) | 0.004 | 1.239 (0.931–1.648) | 0.142 |
| AST (IU/L) | 1.001 (0.998–1.003) | 0.631 | 0.998 (0.991–1.005) | 0.625 |
| ALT (IU/L) | 1.000 (0.998–1.003) | 0.811 | 0.996 (0.984–1.008) | 0.504 |
| Alk P (IU/L) | 1.002 (1.001–1.003) | 1.001 (0.999–1.003) | 0.287 | |
| Albumin (g/dL) | 0.598 (0.424–0.842) | 0.003 | 0.512 (0.325–0.807) | 0.004 |
| CTP | 1.224 (1.122–1.336) | <0.001 | 1.307 (1.154–1.480) | <0.001 |
| Child class | ||||
| A | 1 | 1 | ||
| B | 2.684 (1.277–5.642) | 0.009 | 4.415 (1.493–13.055) | 0.007 |
| C | 4.680 (2.268–9.657) | <0.001 | 8.098 (2.750–23.848) | <0.001 |
| MELD | 1.068 (1.034–1.103) | <0.001 | 1.074 (1.029–1.121) | 0.001 |
| COVID-19 | ||||
| No | 1 | 1 | ||
| Yes | 0.775 (0.474–1.268) | 0.331 | 1.224 (0.663–2.263) | 0.520 |
Abbreviations: TLC, total leukocyte count; INR, international normalized ratio; ALT, alanine aminotransferase; Alk P, alkaline phosphatase; AST, aspartate aminotransferase; CTP, Child-Turcotte-Pugh; MELD, model for end-stage liver disease.
In the matched group, independent predictors of mortality on unadjusted Cox-proportional hazard analysis included bilirubin, HR, 1.095 (95% CI, 1.025–1.169), albumin, HR, 0.512 (95% CI, 0.325–0.807), CTP score, HR, 1.307 (95% CI, 1.154–1.480), Child class B, HR, 4.415 (1.493–13.055), Child class C, HR, 8.098 (2.750–23.848), and MELD score, HR, 1.074 (1.029–1.121). The HR for mortality with COVID-19 infection compared to those without COVID-19 was 1.224 (0.663–2.263), P = 0.520 (Table 3).
Table 3.
Comparison of Cirrhosis With COVID-19 Survivors and Non-survivors, After Hospital Discharge.
| Characteristics | Survived N = 70 | Died N = 22 | Univariate HR | P-value |
|---|---|---|---|---|
| Age (years) | 47 (38–54) | 45 (40–54) | 0.991 (0.954–1.029) | 0.636 |
| Sex, male n (%) | 57 (81.4%) | 16 (72.7%) | 0.779 (0.304–1.995) | 0.779 |
| Diabetes | 15 (21.4%) | 5 (22.7%) | 0.890 (0.326–2.428) | 0.820 |
| Etiology of cirrhosis | ||||
| Alcohol | 31 (44.3%) | 7 (31.8%) | ||
| HBV | 4 (5.7%) | 3 (13.6%) | ||
| HCV | 8 (11.4%) | 1 (4.5%) | ||
| AIH | 7 (10.0%) | 1 (4.5%) | ||
| NAFLD | 6 (8.6%) | 4 (18.2%) | ||
| Cryptogenic | 11 (15.7%) | 5 (22.7%) | ||
| Others | 3 (4.3%) | 1 (4.5%) | ||
| Etiology | ||||
| Alcohol | 31 (44.3%) | 7 (31.8%) | 0.595 (0.242–1.460) | 0.257 |
| Others | 39 (55.7%) | 15 (68.2%) | ||
| Hemoglobin (g/dL) | 8.8 (7.4–10.5) | 7.9 (7.1–8.8) | 0.880 (0.731–1.059) | 0.176 |
| TLC (per mm3) | 5100 (3375–7825) | 5640 (2800–8675) | 0.985 (0.875–1.109) | 0.801 |
| Platelet count ( × 103/mm3) | 71 (50–109) | 66 (40–98) | 0.998 (0.992–1.004) | 0.560 |
| INR | 1.4 (1.2–1.7) | 1.7 (1.3–2.2) | 1.652 (1.133–2.408) | 0.009 |
| Total bilirubin (mg/dL) | 1.5 (0.8–3.4) | 3.2 (1.5–4.9) | 1.087 (1.011–1.169) | 0.024 |
| Creatinine (mg/dl) | 0.8 (0.6–0.9) | 1.1 (0.7–1.4) | 1.269 (0.842–1.914) | 0.255 |
| AST (IU/L) | 51 (36–72) | 54 (41–73) | 1.003 (0.996–1.010) | 0.421 |
| ALT (IU/L) | 31 (24–44) | 31 (23–51) | 1.000 (0.986–1.015) | 0.991 |
| Alk P (IU/L) | 106 (70–142) | 129 (98–145) | 1.003 (0.998–1.009) | 0.190 |
| Albumin (g/dL) | 2.9 (2.5–3.3) | 2.6 (2.1–2.8) | 0.338 (0.178–0.642) | 0.001 |
| CTP | 7 (6–9) | 9 (8–11) | 1.598 (1.273–2.005) | <0.001 |
| Child class | ||||
| A | 26 (37.1%) | 1 (4.5%) | 1 | |
| B | 28 (40.0%) | 11 (50.0%) | 5.418 (0.699–41.985) | 0.106 |
| C | 16 (22.9%) | 10 (45.5%) | 14.799 (1.877–116.687) | 0.011 |
| MELD | 12.7 (10.0–19.3) | 19.2 (12.3–22.7) | 1.092 (1.031–1.157) | 0.003 |
| COVID severity | ||||
| Mild | 58 (82.9%) | 18 (81.8%) | 1 | |
| Moderate | 7 (10.0%) | 2 (9.1%) | 0.901 (0.208–3.898) | 0.890 |
| Severity | 5 (7.1%) | 2 (9.1%) | 2.014 (0.461–8.794) | 0.352 |
Note: Qualitative and quantitative data are expressed as proportions and median (interquartile range), respectively.
Abbreviations: AIH, autoimmune hepatitis; HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease; TLC, total leukocyte count; INR, international normalized ratio; ALT, alanine aminotransferase; Alk P, alkaline phosphatase; AST, aspartate aminotransferase; CTP, Child-Turcotte-Pugh; MELD, model for end-stage liver disease.
On multivariate analysis adjusting for age, sex, etiology, and MELD score/CTP score, the presence of COVID-19 infection was not associated with mortality in both the whole cohort and PSM cohort.
Predictors of Survival in Patients with Cirrhosis and COVID-19 after Hospital Discharge
In the COVID-19 group, 22 died during follow-up. On univariate analysis, the only significant predictors of mortality included CTP score/CTP class and individual components of Child score, namely INR, bilirubin, and albumin. MELD score also independently predicted outcome (Table 3). Multivariate analysis was not done as the significant factors included MELD/CTP and their components.
For predicting mortality, the area under the receiver operating characteristic curve (AUROC) of MELD and CTP score was 0.708 (0.597–0.820) and 0.714 (0.594–0.834), respectively.
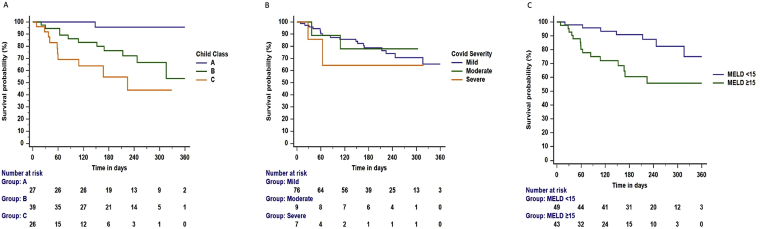
On follow-up, the mortality was higher in patients with Child C: 38.5% (10/26) than those with Child A: 3.7% (1/27) and B: 28.2% (11/39) (log-rank P < 0.001)- Figure 4A. There was no difference in the survival after discharge as per the admission COVID-19 severity (log-rank P = 0.619)- Figure 4B. The mortality was higher in patients with MELD score ≥15: 34.9% (15/43) than those with MELD <15: 14.3% (7/49) (log-rank P = 0.005)- Figure 4C.
Figure 4.
A–C. Survival probability posthospital discharge among patients with cirrhosis and COVID-19 infection. A. The mortality was higher in Child C than in Child A and B (log-rank test, P < 0.001). B. The mortality among mild, moderate, and severe COVID-19 at admission was similar (log-rank test, P = 0.619). C. The mortality was higher in patients with MELD scores ≥15 than <15 (log-rank test, P = 0.005).
Development of Complications after Hospital Discharge in the COVID-19 Patients
Of the 92 patients discharged alive from the hospital, 22 died during follow-up. Of the 92 patients, 45 (48.9%) developed a new acute decompensation during follow-up: 40 (43.5%) had increased ascites, 20 (21.7%) developed hepatic encephalopathy, and 8 (8.7%) suffered from GI bleeding. A total of 25 (27.2%) patients required readmission to the hospital.
We could assess other symptoms in 80 out of the 92 patients; the remaining 12 had died before the first contact. The other symptoms included persistent fatigue/weakness in 24 (30.0%), difficulty sleeping in 11 (13.7%), joint pains in 16 (20.0%), dyspnea in 4 (5.0%), anosmia/dysgeusia in 1 (1.3%). Six out of 92 (6.5%) were actively consuming alcohol.
Comparison of COVID-19 Patients whom we Could Not or Could Contact for Follow-up
The patients we could not contact for follow-up had a higher MELD score (statistically not significant), and a significantly larger proportion of them had alcohol as the etiology of cirrhosis compared to patients we could contact. Other clinical and laboratory variables were comparable between the two groups (Table 4). The CTP score scores and COVID-19 disease severity were comparable between the two groups.
Table 4.
Comparison of Clinical Characteristics, Laboratory Parameters, and Liver Disease Severity Scores Among Cirrhosis Patients With Covid-19 Infection Whom We Could or Failed to Contact for Follow-Up.
| Clinical characteristics | Failed to contact on follow-up (n = 28) | Contacted on follow-up (n = 92) | P-value |
|---|---|---|---|
| Age (years) | 45 (33–52) | 46 (38–54) | 0.260 |
| Sex, male n (%) | 24 (85.7%) | 73 (79.3%) | 0.588 |
| Diabetes | 20 (21.7%) | ||
| Etiology of cirrhosis | 0.077 | ||
| Alcohol | 18 (64.3%) | 38 (41.3%) | |
| HBV | 0 | 7 (7.6%) | |
| HCV | 2 (7.1%) | 9 (9.8%) | |
| AIH | 1 (3.6%) | 8 (8.7%) | |
| NAFLD | 1 (3.6%) | 10 (10.9%) | |
| cryptogenic | 2 (7.1%) | 16 (17.4%) | |
| Others | 4 (14.3%) | 4 (4.3%) | |
| Etiology | 0.050 | ||
| Alcohol | 18 (64.3%) | 38 (41.3%) | |
| Others | 10 (35.7%) | 54 (58.7%) | |
| Hemoglobin (g/dL) | 8.7 (7.4–8.9) | 8.5 (7.3–10.2) | 0.876 |
| TLC (per mm3) | 5600 (2500–9000) | 5100 (3225–7975) | 0.805 |
| Platelet count ( × 103/mm3) | 72 (47–131) | 69 (49–106) | 0.871 |
| INR | 1.4 (1.2–2.2) | 1.5 (1.2–1.8) | 0.747 |
| Total bilirubin (mg/dL) | 2.9 (1.1–6.5) | 1.8 (0.9–4.0) | 0.131 |
| Creatinine (mg/dl) | 0.9 (0.6–1.3) | 0.8 (0.6–1.1) | 0.356 |
| AST (IU/L) | 49 (39–80) | 51 (36–72) | 0.695 |
| ALT (IU/L) | 30 (22–42) | 31 (24–47) | 0.644 |
| Alk P (IU/L) | 101 (69–134) | 117 (78–142) | 0.487 |
| Albumin (g/dL) | 2.8 (2.3–3.2) | 2.8 (2.4–3.1) | 0.462 |
| CTP score | 9 (7–10) | 8 (6–10) | 0.272 |
| CTP class | 0.463 | ||
| A | 5 (17.9%) | 27 (29.3%) | |
| B | 13 (46.4%) | 39 (42.4%) | |
| C | 10 (35.7%) | 26 (28.3%) | |
| MELD | 18.0 (11.6–27.9) | 13.9 (10.6–20.2) | 0.087 |
| COVID severity | 0.798 | ||
| Mild | 22 (78.6%) | 76 (82.6%) | |
| Moderate | 4 (14.3%) | 9 (9.8%) | |
| Severity | 2 (7.1%) | 7 (7.6%) | |
Qualitative and quantitative data are expressed as proportions and median (interquartile range), respectively.
Abbreviations: AIH, autoimmune hepatitis; HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease; TLC, Total leukocyte count; INR, international normalized ratio; ALT, alanine aminotransferase; Alk P, alkaline phosphatase; AST, aspartate aminotransferase; CTP, Child-Turcotte-Pugh; MELD, model for end-stage liver disease.
Discussion
Our propensity score-matched case–control study showed that cirrhosis patients’ posthospitalization survival was comparable among those admitted with or without COVID-19. Their survival was primarily determined by the liver disease severity scores at discharge. On follow-up of about six months, over half of the patients had acute decompensation, and one-fourth required rehospitalization. A significant proportion of these patients continued to have post-COVID symptoms such as fatigue, difficulty sleeping, and joint pains.
This study aimed to assess the outcomes of cirrhosis patients with COVID-19 after discharge from the hospital. In order to reduce the effect of various confounding variables, which could influence the outcome in cirrhosis patients, we used propensity score matching. Study groups were matched for age, gender, etiology, and MELD score. These factors are known to influence outcomes in patients with cirrhosis and COVID-19.3,17 Our results suggest that posthospital discharge, survival of cirrhosis patients with COVID-19 is similar to those without COVID-19. There were no differences in the survival across Child classes among patients with and without COVID-19.
Post-COVID-19 recovery was associated with significant morbidity. Almost half of the patients developed new acute decompensation, and one-fourth required hospital admission. The most common new acute decompensation was ascites, followed by HE and GI bleeding. The risk of acute decompensation development on follow-up in patients with cirrhosis and SARS-CoV2 infection was similar to that reported for patients with cirrhosis without SARS-CoV2 infection.18,19 In contrast, an episode of bacterial infection independent of the MELD score alters patients’ natural history and reduces survival.20 The outcomes of cirrhosis patients are also affected by the underlying etiology of liver disease.21 Projections suggest that the COVID-19 pandemic will be responsible for increased mortality in cirrhosis patients, both directly and indirectly, due to care delivery issues.22,23 A study from China reported persistence of abnormal liver enzymes up to 2 months after infection and association with the worse recovery of COVID-19 patients.24
Our results suggest that persistent fatigue/weakness, difficulty sleeping, joint pains, and dyspnea are prevalent postrecovery in patients with cirrhosis and COVID-19 infection. These findings are consistent with those reported in the literature.25, 26, 27 We did not compare these symptoms in patients and controls, as the recall for such symptoms may be fallacious. We may have overestimated the prevalence of these symptoms as these were leading questions to patients. Nevertheless, it is important to note that postrecovery cirrhosis patients have sequelae that need to be addressed. In addition, other symptoms/signs reported in COVID-19 patients on follow-up include cardiac arrhythmias, myocarditis, pulmonary fibrosis, seizures, encephalitis, mood swings, brain fog, chronic fatigue syndrome, depression, anxiety, post-traumatic stress disorder, and substance abuse.9,10,28,29
We could not contact almost 20% of our patients who were discharged from the hospital. Unfortunately, we could not trace these patients despite multiple attempts. These patients had higher MELD scores, which is an important determinant of outcomes in patients with cirrhosis.30, 31, 32 It is, therefore, possible that the overall mortality in this subgroup would have been higher than 25%. Nonetheless, our analysis suggests a similar short-term outcome in COVID-19 and those without COVID-19 patients with similar MELD scores. Our results indicate that the MELD and Child scores and their components independently predict the outcome after discharge from the hospital.
We are aware of the following limitations of our study. First, many patients with high MELD scores were not contactable at the last follow-up, possibly because a large population migration occurred at the time of lockdown. We did not assess for the quality of life and symptoms such as depression and anxiety. We could not correlate the outcomes with markers of disease severity such as IL-6, ferritin, CRP due to the nonavailability of these investigations. Postdischarge from the hospital, MELD and CTP scores were unavailable as many patients could not get a repeat blood investigation. The indications of hospital admission for the controls may have been different from the COVID-19 patients, where respiratory causes may have been more common (20% of patients had moderate or severe COVID-19). The causes of death were ascertained by verbal autopsy, which has its limitations.
In conclusion, the short-term outcome of patients with cirrhosis who survive the initial insult of COVID-19 is not different from patients without COVID-19, and survival is determined by the severity of liver disease at discharge.
Credit authorship contribution statement
Manas Vaishnav, Department of Gastroenterology and Human Nutrition Unit, All India Institute of Medical Sciences, New Delhi: acquisition of data, analysis and interpretation of data, drafting of the manuscript.
Anshuman Elhence, Department of Gastroenterology and Human Nutrition Unit, All India Institute of Medical Sciences, New Delhi: acquisition of data, analysis and interpretation of data, drafting of the manuscript.
Sagnik Biswas, Department of Gastroenterology and Human Nutrition Unit, All India Institute of Medical Sciences, New Delhi: acquisition of data; analysis and interpretation of data; drafting of the manuscript.
Piyush Pathak, Department of Gastroenterology and Human Nutrition Unit, All India Institute of Medical Sciences, New Delhi: acquisition of data; analysis and interpretation of data; drafting of the manuscript.
Abhinav Anand, Department of Gastroenterology and Human Nutrition Unit, All India Institute of Medical Sciences, New Delhi: acquisition of data; analysis and interpretation of data; drafting of the manuscript.
Sabreena Sheikh, Department of Gastroenterology and Human Nutrition Unit, All India Institute of Medical Sciences, New Delhi: acquisition of data; drafting of the manuscript.
Vishwajeet Singh, Biostatistician, Department of Geriatric Medicine, All India Institute of Medical Sciences, New Delhi, India: data interpretation; analysis; critical revision.
Souvik Maitra, Department of Anaesthesiology, Pain Medicine & Critical Care, All India Institute of Medical Sciences, New Delhi: data interpretation; analysis; critical revision.
Amit Goel, Department of Gastroenterology, Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGI), Lucknow: manuscript writing; data interpretation; critical revision.
Shalimar, Department of Gastroenterology and Human Nutrition Unit, All India Institute of Medical Sciences, New Delhi: concept; acquisition of data; analysis and interpretation of data; critical revision.
Conflicts of interest
The authors have none to declare.
Acknowledgment
Mr Amar Negi, Hemant, and Dilshad for data maintenance.
Funding
The study was supported by a grant from the All India Institute of Medical Sciences, New Delhi. Grant number A-COVID-28; 02.06.2020.
Disclosures
None of the authors has any financial, professional, or personal conflicts that are relevant to the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2021.11.009.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Figure 1.
Supplementary Figure 2.
References
- 1.Williamson E.J., Walker A.J., Bhaskaran K., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shalimar, Elhence A., Vaishnav M., et al. Poor outcomes in patients with cirrhosis and corona virus disease-19. Indian J Gastroenterol. 2020;39:285–291. doi: 10.1007/s12664-020-01074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marjot T., Moon A.M., Cook J.A., et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.09.024. 0(0). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubens J.H., Akindele N.P., Tschudy M.M., Sick-Samuels A.C. Acute covid-19 and multisystem inflammatory syndrome in children. BMJ. 2021;372:n385. doi: 10.1136/bmj.n385. [DOI] [PubMed] [Google Scholar]
- 5.Xiong Q., Xu M., Li J., et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect. 2021;27:89–95. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carfì A., Bernabei R., Landi F. Gemelli against COVID-19 post-acute care study group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garg P., Arora U., Kumar A., Wig N. The “post-COVID” syndrome: how deep is the damage? J Med Virol. 2021;93:673–674. doi: 10.1002/jmv.26465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elhence A., Shalimar COVID-19: beyond respiratory tract. J Dig Endosc. 2020;11:24–26. doi: 10.1055/s-0040-1712550. [DOI] [Google Scholar]
- 9.Puntmann V.O., Carerj M.L., Wieters I., et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zubair A.S., McAlpine L.S., Gardin T., Farhadian S., Kuruvilla D.E., Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77:1018–1027. doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nalbandian A., Sehgal K., Gupta A., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kulkarni A.V., Kumar P., Tevethia H.V., et al. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52:584–599. doi: 10.1111/apt.15916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elhence A., Vaishnav M., Biswas S., Chauhan A., Anand A., Shalimar Coronavirus disease-2019 (COVID-19) and the liver. J Clin Transl Hepatol. 2021;9:247–255. doi: 10.14218/JCTH.2021.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.UpdatedDetailedClinicalManagementProtocolforCOVID19adultsdated24052021.pdf. Accessed September 1, 2021. https://www.mohfw.gov.in/pdf/UpdatedDetailedClinicalManagementProtocolforCOVID19adultsdated24052021.pdf.
- 15.Pugh R.N., Murray-Lyon I.M., Dawson J.L., Pietroni M.C., Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 16.Malinchoc M., Kamath P.S., Gordon F.D., Peine C.J., Rank J., ter Borg P.C. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 17.Kim D., Adeniji N., Latt N., et al. Predictors of outcomes of COVID-19 in patients with chronic liver disease: US multi-center study. Clin Gastroenterol Hepatol. Published online September. 2020;17 doi: 10.1016/j.cgh.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Amico G., Garcia-Tsao G., Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Shah A.S., Amarapurkar D.N. Natural history of cirrhosis of liver after first decompensation: a prospective study in India. J Clin Exp Hepatol. 2018;8:50–57. doi: 10.1016/j.jceh.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dionigi E., Garcovich M., Borzio M., et al. Bacterial infections change natural history of cirrhosis irrespective of liver disease severity. Am J Gastroenterol. 2017;112:588–596. doi: 10.1038/ajg.2017.19. [DOI] [PubMed] [Google Scholar]
- 21.Shalimar Kedia S., Mahapatra S.J., et al. Severity and outcome of acute-on-chronic liver failure is dependent on the etiology of acute hepatic insults: analysis of 368 patients. J Clin Gastroenterol. 2017;51:734–741. doi: 10.1097/MCG.0000000000000823. [DOI] [PubMed] [Google Scholar]
- 22.Tapper E.B., Asrani S.K. The COVID-19 pandemic will have a long-lasting impact on the quality of cirrhosis care. J Hepatol. 2020;73:441–445. doi: 10.1016/j.jhep.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pawlotsky J.-M. COVID-19 and the liver-related deaths to come. Nat Rev Gastroenterol Hepatol. June 11, 2020:1–3. doi: 10.1038/s41575-020-0328-2. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.An Y.-W., Song S., Li W.-X., et al. Liver function recovery of COVID-19 patients after discharge, a follow-up study. Int J Med Sci. 2021;18:176–186. doi: 10.7150/ijms.50691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Logue J.K., Franko N.M., McCulloch D.J., et al. Sequelae in adults at 6 Months after COVID-19 infection. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garrigues E., Janvier P., Kherabi Y., et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81:e4–e6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang C., Huang L., Wang Y., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.del Rio C., Collins L.F., Malani P. Long-term health consequences of COVID-19. JAMA. 2020;324:1723–1724. doi: 10.1001/jama.2020.19719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Y.-M., Shang Y.-M., Song W.-B., et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shalimar, Kumar D., Vadiraja P.K., et al. Acute on chronic liver failure because of acute hepatic insults: etiologies, course, extrahepatic organ failure and predictors of mortality. J Gastroenterol Hepatol. 2016;31:856–864. doi: 10.1111/jgh.13213. [DOI] [PubMed] [Google Scholar]
- 31.Shalimar, Rout G., Kumar R., et al. Persistent or incident hyperammonemia is associated with poor outcomes in acute decompensation and acute-on-chronic liver failure. JGH Open. 2020;4:843–850. doi: 10.1002/jgh3.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sonika U., Jadaun S., Ranjan G., et al. Alcohol-related acute-on-chronic liver failure-Comparison of various prognostic scores in predicting outcome. Indian J Gastroenterol. 2018;37:50–57. doi: 10.1007/s12664-018-0827-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.