Introduction
Leprosy is a chronic, disfiguring infectious disease. It is traditionally classified into either tuberculoid (paucibacillary) leprosy or lepromatous (multibacillary) leprosy, which differ in the number of bacteria present in the skin and clinical manifestations. Patients in between the 2 types are classified as borderline. In the context of the COVID-19 pandemic, patients with leprosy will also receive the COVID-19 vaccination. Here we describe a case of multibacillary leprosy unmasked by COVID-19 vaccination.
Case report
A 24-year-old Indian man was admitted with swelling of the hands, feet, earlobes, and lips. He also had bilateral ankle pain and swelling. He developed symptoms 10 to 15 days after receiving the first dose of the Pfizer-BioNTech COVID-19 vaccination. His medical history was significant for COVID-19 infection 6 months previously (from which he recovered without complications) as well as urticaria. He was employed as a mooring worker.
On examination, he was noted to have diffuse and nodular edema of the ears (Fig 1), brows, and face. He had bilateral eyebrow hair loss. There was edema of the neck, and the greater auricular nerve was difficult to palpate. Peroneal and ulnar nerves were not thickened. He had conjunctival injection; however, his vision was unaffected. He had swelling of both hands (Fig 2).
Fig 1.
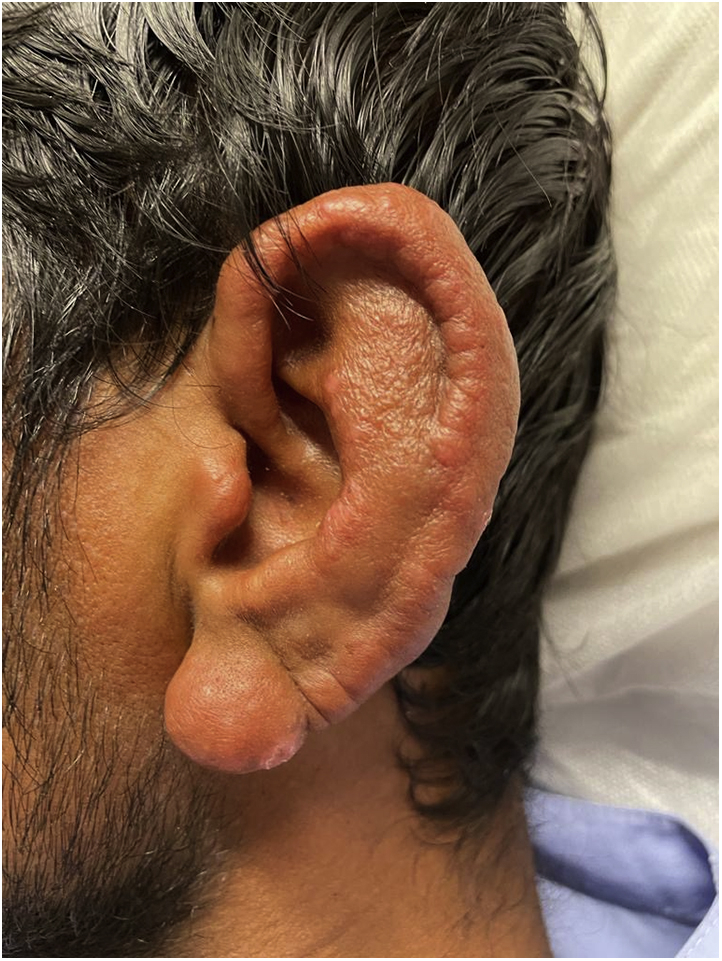
Ear edema.
Fig 2.
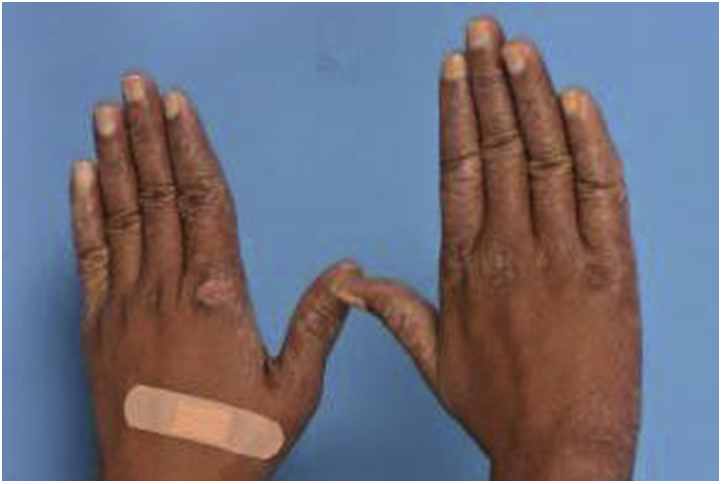
Swelling of the hands.
A skin punch biopsy from the ear and the dorsum of the left hand showed a diffuse infiltrate of macrophages. Fite stain was positive (Fig 3).
Fig 3.
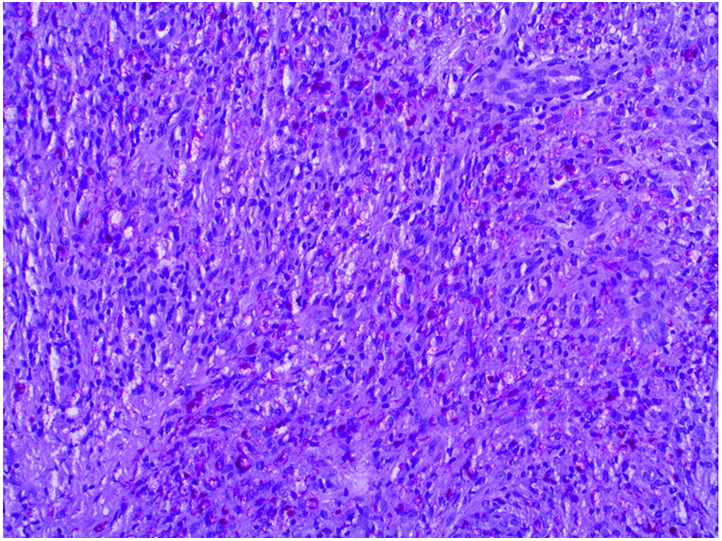
Fite stain showing diffuse infiltration with acid-fast bacilli. (Original magnification: ×40.)
Slit skin smear results were positive from the ear (6+ <1%), hands (5+ <1%), and feet (5+ <1%). A full blood count revealed bicytopenia (anemia and leukopenia).
The patient's symptoms and clinical and histologic findings were consistent with multibacillary leprosy. Given the temporal relationship with COVID-19 vaccination 10 to 15 days previously, his reaction was likely unmasked by the COVID-19 vaccine.
Treatment was commenced. Conventional multi-drug therapy was not chosen in view of his bicytopenia, which was being evaluated. Instead, he was treated with clarithromycin, minocycline, and ofloxacin, as alternative multi-drug therapy, with a slow taper of prednisolone.
Within 2 weeks of treatment, he had marked improvement of facial and ear swelling, as well as improvement of hand and feet swelling and pain.
Discussion
Leprosy is a chronic infectious disease caused by Mycobacterium leprae, primarily affecting the skin and peripheral nerves. The diagnosis of leprosy is often challenging, as there is a wide array of clinicopathological manifestations based on the host immune response.1 Clinically, leprosy in reaction can resemble urticaria, and leprosy can cause a secondary asteatosis or xerosis, which may mimic eczema. The clinical features of leprosy are often determined by the host response to leprosy bacilli, where patients with tuberculous leprosy demonstrate T-cell immunity and delayed-type hypersensitivity to M. leprae, while patients with lepromatous leprosy exhibit abundant antibody-mediated inflammatory responses but minimal T-cell responses.1
The Pfizer-BioNTech BNT162b2 vaccine contains lipid nanoparticle-formulated, nucleoside-modified RNA, which encodes for the SARS-CoV-2 spike protein.2 This triggers an immunologic response, leading to the formation of SARS-CoV-2 neutralizing antibodies.3 The vaccine can generate significant titres of neutralizing antibodies and upregulate T-cell responses within 2 to 4 weeks of inoculation. To trigger adaptive immunity, a vaccine requires both a pathogen-specific immunogen and an adjuvant to stimulate the innate immunity that provides the cellular signals required for T-cell activation. The Pfizer-BioNTech messenger RNA (mRNA) serves as both the immunogen and adjuvant upon entry into dendritic cells, leading to cellular activation and production of type 1 interferon and other inflammatory mediators, which prime differentiation of CD4 and CD8 effector T cells with production of CD4+ follicular helper T cells that promote differentiation of B cells into antibody-secreting plasma cells.3 Studies have shown that vaccination can induce production of neutralizing antibodies for several months.4
Delayed large local reactions to the Moderna mRNA vaccine were reported in a case series of 12 patients, proposed to be delayed-type or T cell-mediated hypersensitivity reaction.5 This process is similar to the immune reconstitution inflammatory syndrome seen in HIV patients following immune reconstitution mediated by upregulated CD4+ and CD8+ T-cell responses with anti-retroviral therapy. Similar paradoxical response with worsening of symptoms related to infections may be observed in non-HIV infected patients following cessation of immunosuppressive medications.6,7
Clinically, our patient did not have fever, myalgias, or malaise. He had no lesions of erythema nodosum or systemic symptoms. He was therefore clinically diagnosed as having a type 1 leprosy reaction. In type 1 leprosy reaction, there is a hypersensitivity reaction to M. leprae that is clinically characterized by urticarial swelling of leprosy skin lesions. Hand edema, observed in our patient, may occur in both type 1 reactions8 and type 2 leprosy reactions. Type 1 reactions represent the most common form of reaction and are usually seen in borderline leprosy and triggered by therapies, comorbidities, and pregnancy.9 The underlying pathophysiological basis of type 1 leprosy reaction is related to T cell-mediated inflammatory responses toward M. leprae.1
mRNA vaccination has been shown to elicit T-cell responses early on, and early T-cell and binding antibody responses are associated with COVID-19 RNA vaccine efficacy onset of vaccine effect.10 Therefore, it is plausible that T-cell mediated immune upregulation elicited by the COVID-19 vaccine may result in a type 1 leprosy reaction. However, the exact pathophysiological basis of this heightened immunologic response leading to unmasking of leprosy remains to be elucidated.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Nath I., Saini C., Valluri V.L. Immunology of leprosy and diagnostic challenges. Clin Dermatol. 2015;33(1):90–98. doi: 10.1016/j.clindermatol.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teijaro J.R., Farber D.L. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21(4):195–197. doi: 10.1038/s41577-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sahin U., Muik A., Derhovanessian E., et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 5.Blumenthal K.G., Freeman E.E., Saff R.R., et al. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N Engl J Med. 2021;384(13):1273–1277. doi: 10.1056/NEJMc2102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cadena J., Thompson G.R., III, Ho T.T., Medina E., Hughes D.W., Patterson T.F. Immune reconstitution inflammatory syndrome after cessation of the tumor necrosis factor alpha blocker adalimumab in cryptococcal pneumonia. Diagn Microbiol Infect Dis. 2009;64(3):327–330. doi: 10.1016/j.diagmicrobio.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Garcia Vidal C., Rodríguez Fernández S., Martínez Lacasa J., et al. Paradoxical response to antituberculous therapy in infliximab-treated patients with disseminated tuberculosis. Clin Infect Dis. 2005;40(5):756–759. doi: 10.1086/427941. [DOI] [PubMed] [Google Scholar]
- 8.Kamath S., Vaccaro S.A., Rea T.H., Ochoa M.T. Recognizing and managing the immunologic reactions in leprosy. J Am Acad Dermatol. 2014;71(4):795–803. doi: 10.1016/j.jaad.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 9.Fischer M. Leprosy–an overview of clinical features, diagnosis, and treatment. J Dtsch Dermatol Ges. 2017;15(8):801–827. doi: 10.1111/ddg.13301. [DOI] [PubMed] [Google Scholar]
- 10.Kalimuddin S., Tham C.Y.L., Qui M., et al. Early T cell and binding antibody responses are associated with COVID-19 RNA vaccine efficacy onset. Med (N Y) 2021;2(6):682–688.e4. doi: 10.1016/j.medj.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


