Abstract
Inflammation is one of the primary factors associated with the causation and/or progression of several lifestyle disorders, including obesity, type 2 diabetes and non-alcoholic fatty liver disease (NAFLD). NAFLD is a spectrum of disorders, and starts with simple steatosis, progresses to non-alcoholic steatohepatitis, and then advances to fibrosis, cirrhosis and finally, hepatocellular carcinoma, due to perpetual cycles of insults caused by inflammation and other cellular stress. Emerging evidence has documented that patients with NAFLD have severe coronavirus disease 2019 (COVID-19), and patients with COVID-19 have a higher liver injury and mortality. Although the exact cause or mechanism is not known, inflammatory cytokine storm is a characteristic feature of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and is known to be associated with higher mortality among COVID-19 patients. Therefore, the COVID-19 pandemic seems to be a major concern in NAFLD patients, who have contracted SARS-CoV-2 infection and develop COVID-19. This is evident in patients at any stage of the NAFLD spectrum, as the inflammatory cytokine storm may cause and/or aggravate the progression or severity of NAFLD. Thus, there is a need for resolution of the inflammatory cytokine storm in these patients. A large body of evidence has demonstrated the efficacy of omega-3 long-chain polyunsaturated fatty acids (ω-3 LCPUFA) in NAFLD conditions, due to their anti-inflammatory, immunomodulatory and anti-viral properties. Therefore, intervention with ω-3 LCPUFA, an effective pharmaconutrient along with the standard treatment for COVID-19 may be useful in the management of the NAFLD spectrum in COVID-19 patients with pre-existing NAFLD conditions by resolving the inflammatory cytokine storm and thereby attenuating its progression. Although there are challenges in implementation, optimistically they can be circumvented and the pharmaconutrition strategy may be potentially helpful in tackling both the pandemics; NAFLD and COVID-19 at least in this subset of patients.
Keywords: Lipids, Virus, Inflammation, Infection, Fish oil, Supplementation
Core Tip: Inflammatory cytokine storm seems to pose a risk for patients with non-alcoholic fatty liver disease (NAFLD), as these patients have shown severe coronavirus disease 2019 (COVID-19) compared to those without NAFLD, while, liver injury has been reported to be high among COVID-19 patients. Thus, this bi-directional relationship between NAFLD and COVID-19 may worsen both conditions at least in this subset of patients. Therefore, resolving the inflammatory cytokine storm is an important target, not only in the management of NAFLD but also for COVID-19. In this context, we highlight the pharmacological potential of omega-3 long-chain polyunsaturated fatty acids in the clinical nutrition therapy strategy to resolve inflammatory cytokine storm and its associated tissue injury.
INTRODUCTION
The global epidemic, non-alcoholic fatty liver disease (NAFLD) is one of the most prevalent public health problems, affecting nearly 25% of the population worldwide and one of the leading causes of liver-associated mortality[1]. During this era of the non-communicable disease epidemic, the current pandemic of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has claimed over 2.2 million lives (regardless of race and ethnicity) and continues to spread and infect more people (> 102 million as on 2nd February 2021) globally[2]. During metabolic insult, injury, allergy, or infection, inflammation is initiated by coordinated regulation of biological networks involving different cell types (including immune cells), receptors, signaling molecules, proteins and transcriptional regulators to eliminate the causative factor and maintain tissue/organ homeostasis. Inflammation is a normal physiological process and often self-limiting. However, the uncontrolled or overt/hyper-inflammation leads to severe cellular/ tissue damage and causes other pathological complications both in communicable and non-communicable disease conditions[3]. Inflammation plays a detrimental role in the development and progression of NAFLD, from simple steatosis to hepatocellular carcinoma and the associated complications, such as obesity, insulin resistance, type 2 diabetes and metabolic syndrome[4]. Similarly, clinical studies on novel coronavirus disease (COVID-19) across the continents have invariably reported the inflammatory cytokine storm or overt-inflammation among COVID-19 patients[5-11]. Furthermore, clinical studies have reported that the patients with pre-existing NAFLD conditions show severe COVID-19, on the other hand, COVID-19 patients are at higher risk for developing liver injury during SARS-CoV-2 infection[12,13]. From these perspectives, it is apparent that inflammation is a double-edged sword for NAFLD patients, who have contracted SARS-CoV-2 infection and develop COVID-19. Therefore, controlling the inflammatory cytokine storm in these patients is crucial not only for the management of NAFLD or its progression but also for the COVID-associated complications. Keeping this in mind, even the current medical treatment for COVID-19 is aimed at controlling and resolving the inflammatory cytokine storm. In this context, nutritional intervention has been considered one of the safest strategies in the management of various human diseases and for patient care for several decades. Among various nutrients, the long-chain polyunsaturated fatty acids (LCPUFA) are known for their potent anti-inflammatory properties, besides their role in normal physiological functions and human health[14]. Previously, the health benefits of omega-3 long-chain polyunsaturated fatty acids (ω-3 LCPUFA) and their therapeutic efficacy have been reported in critically ill patients and human diseases (including NAFLD), associated with infection and/or inflammation[15]. In this context, here we reviewed the anti-inflammatory and immunomodulatory potential of ω-3 LCPUFA and its plausible therapeutic usefulness as a pharmaconutrition intervention strategy in the resolution and management of the inflammatory cytokine storm associated with COVID-19, with special reference to NAFLD, in addition to its feasibility and the potential challenges in implementation and the way forward.
NAFLD
NAFLD is one of the public health epidemic diseases of both developed and developing nations. The recent meta-analysis data from various countries have shown a higher prevalence of NAFLD among the general population of the Middle East (32%) and South America (31%) followed by the United States (24%), while the lowest was found in Africa (14%)[1]. Recently, Younossi et al[16], have reported a prevalence of NAFLD ranging from < 10% to ≥ 30%, across the globe. NAFLD is a spectrum of progressive diseases with multiple etiologies and the leading cause of mortality associated with liver dysfunction. In this spectrum, fatty liver/hepatic steatosis is the first event to occur and defined clinically, when triglyceride accumulation of hepatocytes exceeds 5% of hepatic tissue weight[17]. Although fatty liver/hepatic steatosis is often self-limiting; the condition can escalate to non-alcoholic steatohepatitis (NASH), wherein infiltration of immune cells, ballooning of hepatocytes, activation of stellate cells and cell death occur. NASH is a reversible form of liver injury, however, a continuous and perpetual cycle of inflammatory insult and stellate cell activation aggravate NASH to the fibrotic stage, characterized by excessive accumulation of extracellular matrix proteins (including collagen type 1) in the hepatocytes. Overt inflammation and other cellular stress-mediated insults result in fibrosis changing to cirrhosis, in which scar tissue replaces hepatocytes primarily with type 1 collagen. Liver cirrhosis eventually progresses to hepatocellular carcinoma (HCC) and hepatocellular death[18]. NAFLD progression from simple hepatic steatosis to HCC involves the interplay of several cascades of events mediated by various cell types of both liver and systemic origin, and this was explained by the parallel and multiple hit hypothesis[19]. The role of inflammation, various pathways and factors associated with the development and progression of NAFLD has been extensively reviewed by several investigators, and unambiguously concluded that the inflammation-mediated effects are the central players in hepatic injury to malignancy[20-25].
INFLAMMATORY CYTOKINE STORM OF SARS-COV-2 INFECTION
Ever since the outbreak occurred in Wuhan, China, in December 2019, the spread of SARS-CoV-2 and thus COVID-19 has imposed a tremendous burden not only on the health and economic status of nations but also on the psychological and social aspects of individuals. As the epidemiology, various symptoms and current medical practices for treating COVID-19 have been extensively reviewed previously[26-28], the focus of our review is on the inflammatory cytokine storm in COVID-19. An observational study conducted in eight severely/critically ill COVID-19 patients, aged between 2 mo and 15 years has reported that cytokine storm was observed by an elevation of circulatory interleukin 6 (IL-6), IL-10 and interferon γ (IFNγ) levels[5]. Studies on the clinical, immunological and pathophysiological features associated with infection caused by various coronaviruses; SARS-CoV-2, SARS and Middle East respiratory syndrome (MERS) have reported pronounced lung injury associated with cytokine storm in critically ill patients, as observed from plasma cytokine profiling, i.e., elevation of pro-inflammatory cytokines such as IL-6, IL-12, IL-1β, tumor necrosis factor α (TNFα), IFNγ, along with anti-inflammatory cytokines, including IL-4, IL-10, IL-13 and transforming growth factor 1β (TGF-1β) (although the levels of IL-10 are reported to be very low with SARS infection)[6-8]. Zhang et al[29] who have examined the clinical characteristics of 140 COVID-19 patients from Wuhan, China reported a significant elevation in serum C-reactive protein (CRP) levels, an acute inflammatory molecule, in 96.4% of severe cases, compared to non-severe patients, although various other co-morbidities, particularly hypertension, diabetes mellitus, fatty liver and coronary heart disease (CHD) were comparable. Similarly, Chen et al[30] have also reported significantly increased serum CRP and IL-6 levels among 86% and 52% of patients diagnosed with COVID-19, respectively.
Another study on COVID-19 patients in Wuhan, China has reported significant increases in various adipocytokines associated with inflammation, such as interleukins (IL-1B, IL-7, IL-8, IL-9 and IL-10), macrophage chemoattractant protein 1, macrophage inflammatory proteins 1α (MIP-1α) and 1β, TNFα and INFγ-inducible protein 10 (IP-10), compared to healthy adults. Furthermore, the levels of these inflammatory adipocytokines were higher in patients admitted to the intensive care unit (ICU), than those not in the ICU, despite a comparable prevalence of other co-morbidities, such as diabetes, cardiovascular diseases and chronic liver disease[9].
A retrospective study conducted in deceased patients with COVID-19 has found higher serum IL-6, IL-8, IL-10 and TNFα levels, while 91% had undetectable IL-1β, compared with patients who recovered from the disease[10]. The study by Yang et al[11] in which analysis of 48 cytokines in the plasma from COVID-19 patients has revealed elevated levels of nearly 14 cytokines of both pro- and anti-inflammatory nature, such as IP-10, macrophage chemoattractant protein 3 (MCP3), IL-1ra, IL-2ra, IL-6, IL-10 and IL-18, IFNγ, hepatocyte growth factor, macrophage colony-stimulating factor, granulocyte colony-stimulating factor, MIP-1α and cutaneous T-cell attracting chemokine in patients infected with SARS-CoV-2 compared to healthy individuals, while higher levels of IP-10, MCP3 and IL-1ra were associated with a more severe form of the disease. Recently, Mehta et al[31] who have collated various findings related to severe COVID-19 cases and compared with them with secondary hemophagocytic lymphohistiocytosis (sHLH), described that the cytokine profile of the former resembles that of sHLH and thus COVID-19 is considered a “cytokine storm syndrome” at least in subgroups of infected patients. Similarly, Ye et al[32] have reported that SARS-CoV-2 infection-induced inflammatory cytokine storm is a key detrimental cause of pathogenesis, including severity, multi-organ failure and mortality among COVID-19 patients. Importantly, Tang et al[33] have opined that controlling the cytokine storm during COVID-19 is critical for better treatment outcome. Undoubtedly, our understanding of COVID-19 and its complications (during disease and post-recovery period) is very limited to date.
EICOSANOID PATHWAY AND INFLAMMATORY CYTOKINE STORM
Unlike inflammation caused by metabolic insult, which is detrimental, the infection-induced inflammatory response is one of the defense mechanisms, primarily to remove or eliminate the pathogen from the body. However, the uncontrolled/high inflammatory response also leads to several serious consequences, including tissue damage and cell death. In this context, LCPUFA-derived lipid mediators are the key modulators of the inflammatory process, as they can induce and resolve inflammation[34]. Following viral infection, besides inflammatory cytokines (namely IL-6, IL-1β, TNFα) several pro-inflammatory lipid mediators, such as prostaglandins (PG), thromboxanes (TX) and leukotrienes (LT) (collectively known as eicosanoids) are also formed through enzymatic [cyclooxygenase (COX) and lipoxygenase (LOX), respectively] conversion of free arachidonic acid (ω-6 LCPUFA) released by the action of phospholipase A2 (PLA2) from the cell membrane (of macrophages, monocytes and immune cells; T cells, B cells, to name a few)[35,36]. These released pro-inflammatory eicosanoids [such as prostaglandin E2 (PGE2), PGD2, PGF2α, TXB2 and LT4] initiate inflammation and perpetuate the pro-inflammatory condition, by interacting with several other cells and elevate the release of several inflammatory mediators and cytokines including IL-6[35-37]. A previous experimental study has shown TXA2-mediated regulation of TNFα and IL-1β synthesis in non-adherent human monocytes[38]. Furthermore, the activity of PLA2 plays a central role in the inflammatory process[39,40] and the activity is elevated by inflammatory cytokines, such as IL-1β, and TNF[41,42]. Furthermore, the role of eicosanoids in airway inflammation and other inflammatory respiratory diseases is well recognized[43,44]. Overall, it is apparent that eicosanoid and inflammatory cytokine pathways interact and regulate each other during infection. However, the molecular link between these two phenomena and/or the association between eicosanoids and cytokine storms are poorly studied and understood in SARS-CoV-2 infection to date. Therefore, several researchers have speculated on the importance of the eicosanoids pathway and pro-inflammatory lipid mediators in the inflammatory cytokine storm syndrome of COVID-19 and urged further investigations for a better understanding to evolve strategies for resolution of eicosanoids storm and thereby the inflammatory cytokine storm[45-47].
NAFLD AND COVID-19
A retrospective study conducted on COVID-19 patients (202 consecutive patients) admitted to hospital has reported liver injury in 50% of patients (mostly hepatocellular injury) during admission, while 75.2% of patients showed liver injury during hospitalization. Furthermore, the authors have found frequent mild liver injury in patients with COVID-19, while patients with NAFLD had a higher chance of developing severe COVID-19 (possibly due to polarization of pro-inflammatory M1 to anti-inflammatory M2 macrophages, thus suppressing the inflammatory process) and longer viral shedding time[48]. Huang et al[49] who have studied 280 consecutive patients with confirmed COVID-19 reported the presence of NAFLD in 30.7% patients with COVID-19, whereas 35.7% had abnormal liver function on admission. However, the authors have observed comparable complications and other clinical outcomes in COVID-19 patients with or without NAFLD and no cases of liver failure during hospitalization[49]. A retrospective case-control study has reported NAFLD among 31% of COVID-19 patients[50] Furthermore, the authors have observed more severe COVID-19 disease among patients with NAFLD (39.7%) than those without NAFLD (11.7%) and significantly higher levels of CRP in the circulation. In addition, the unadjusted logistic regression model analysis also showed a significant association between NAFLD and the severity of COVID-19, thus indicating that patients with NAFLD were likely to have severe COVID-19[50]. A large population-based study that analyzed various comorbid conditions (such as hypertension, obesity, diabetes, hyperlipidemia and NASH) associated with COVID-19 has found the strongest association between NASH and COVID-19. From the multivariable binary logistic regression model, the authors have found higher COVID-19, in patients with NASH with an adjusted odds ratio (OR) of 4.93 and a 95%CI of 4.06-6.00[51]. A single-center cohort study by Chen et al[52], has reported that the presence of hepatic steatosis in 52% of patients was associated with admission to the ICU with an OR of 1.6 and a 95%CI of 1.00-2.57 (according to multivariable analysis) among patients admitted to hospital for COVID-19. Furthermore, severe COVID-19 was observed among the patients with hepatic steatosis. Another study has reported higher in-hospital mortality in males with NAFLD among COVID-19 patients admitted to hospital, although liver cirrhosis did not show this association. Furthermore, the study has reported higher plasma CRP levels in patients with COVID-19 and NAFLD than those without NAFLD. However, neither ICU admission nor in-hospital mortality among COVID-19 patients had an association with NAFLD[53]. A pooled analysis on NAFLD and COVID-19 study data by Sachdeva et al[54], has reported that NAFLD was a predictor of severe COVID-19, even after adjusting for a common risk factor; obesity. Furthermore, the association was significant with an OR of 2.358 and a 95%CI of 1.902-2.923. Hence, the authors have concluded that NAFLD is associated with severe COVID-19, although the relationship between these two is poorly understood. In line with this, nascent pre-print article has also reproted that NAFLD/NASH was one of the significant risk factors associated with hospitalization among COVID-19 patients[55]. Portincasa et al[13], who have collated information from the literature on NAFLD and COVID-19 observed that fibrosis was one of the additional and independent risk factors for severe COVID-19 in a sub-group of patients with NAFLD; thus affecting the outcome of COVID-19. Furthermore the authors have stated that the inflammatory interplay of chronic and acute conditions of NAFLD and COVID-19, respectively, can be deleterious to liver health and may aggravate liver injury, at least in metabolically compromised patients and therefore, suggested that monitoring of liver health is imperative in patients with NAFLD, who have recovered from COVID-19[13]. In a recent review, Xu et al[12], have summarized liver injury during infection caused by various human coronaviruses and found that SARS-CoV-2 infection-induced liver injury ranged from 14.8% to 53% among COVID-19 patients, while in deceased patients, it could be as high as 58.06%. The authors have concluded that the underlying cause was unclear, but speculated that the detrimental role of SARS-CoV-2 infection-induced cytopathic effects and/or hyper-inflammatory response mediated by immunopathology was responsible for the occurrence of liver injury among COVID-19 patients. The authors also have emphasized monitoring of liver health among COVID-19 patients, (during and after recovery) and usage/choice of drugs (which could protect liver health, while arresting the hyper-inflammatory response), so that the disease is primarily treated, eventually protecting the liver from damage, which would improve overall disease recovery and treatment outcome[12]. Collectively, it has been shown that SARS-CoV-2 infection causes liver damage and NAFLD patients are at risk of developing a severe form of COVID-19, at least in this sub-group of the population and the underlying cause is yet to be identified. The data on the impact of COVID-19 (both short- and long-term) on NAFLD (development and/or progression) are lacking, at least for now. However, based on the available clinical and immunological data (including inflammatory cytokine storm), COVID-19 may pose a greater risk for progression in patients with pre-existing NAFLD conditions, including simple hepatic steatosis. NAFLD is an inflammatory disease, and it is necessary to resolve the inflammatory cytokine storm induced during SARS-CoV-2 infection, either through pharmacological or nutritional interventions. However, it is known that drugs can often induce liver toxicity or injury, and the current treatment protocol (against symptoms) for COVID-19 involves several drugs (although vaccines have recently become available against SARS-CoV-2);, thus, there is a possibility of drug-induced liver injury in these patients. Therefore, in this context, nutritional intervention seems to be safe and may be an effective approach in resolving the inflammatory cytokine storm at least in COVID-19 patients with NAFLD.
PHARMACONUTRITION - Ω-3 LCPUFA
Pharmaconutrition intervention is one of the therapeutic strategies where nutrients are used along with standard treatment, particularly for critically ill patients. Recently, Pierre et al[56], have reviewed the literature on several potent pharmaconutrients of several classes, such as micro- and macro-nutrients, prebiotics, probiotics, synbiotics and nucleotides, to name a few for clinical therapy. More than two decades ago, the macronutrient; ω-3 LCPUFA was identified as a pharmaconutrient hence its use in immunomodulation[57].
Among the various nutrients, LCPUFA are one of the important nutrients required for maintaining optimal health and wellbeing of humans. In particular, ω-3 LCPUFA otherwise known as n-3 LCPUFA, namely, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are potent biologically active lipids, essential for fetal development in a wide range of biological functions, and are thus known to play a pivotal role in human health and disease[58]. The consumption of marine fish is the main source of these ω-3 fatty acids, although, the human body can synthesize them from the precursor, α-linolenic acid (ALA) an essential fatty acid (from dietary fat sources, such as flaxseed, soybean and canola oil, walnuts, etc.) through a series of enzymatic elongation and desaturation processes. However, the conversion efficiency of ALA to LCPUFA is low, particularly for the formation of DHA, thus the pre-formed sources of marine origin are recommended for consumption to meet the required EPA and DHA levels[15].
These LCPUFA are the key structural components of the cell membrane lipid bilayer; however, during normal and various pathophysiological conditions, the activation of phospholipases (e.g., phospholipase A2) releases these membrane-bound LCPUFA into the cytoplasm, which serve as substrates for the production of various bioactive lipid mediators, namely resolvins, protectins (PD) and maresins (collectively called “autocoids”) by the action of COX and LOX. Importantly, these enzymes are also involved in the production of ω-6 LCPUFA; i.e., arachidonic acid (AA)-derived lipid mediators, and except lipoxin, PG, LT and TX are known as eicosanoids and are pro-inflammatory. Notably, the enzymes involved in the production of these lipid mediators are common for both ω-6 and ω-3 fatty acids and hence the cellular inflammatory status is dramatically influenced by their availability/concentration. Therefore, optimum levels of both ω-6 and ω-3 fatty acids are required to balance most of the cellular physiological and biochemical processes[59]. Based on the proven biological functions and the data from basic, clinical and epidemiological studies, various national and international agencies have recommended adequate intake of ω-3 PUFA to maintain optimum health. WHO/FAO has recommended the daily allowance of ω-3 LCPUFA for the general population of different age, sex and physiological states; e.g. in adult men or women, the acceptable dietary intake of ω-3 LCPUFA ranges from 250 to 2000 mg/d including fish/algal oil supplementation for secondary prevention of CHD, while 200-250 mg/d is recommended for girls and boys aged between 10-18 years[60]. Among the various sources, fish-derived fat is considered one of the nutritional supplements of ω-3 PUFA, as it has been shown to reduce the inflammation, cellular stress and the incidence of acute respiratory distress syndrome and sepsis in critically ill patients, due to the presence of pre-formed ω-3 LCPUFA; EPA and DHA[61-63]. In this context, here we highlight the therapeutic potential of ω-3 LCPUFA, based on the evidence of their anti-inflammatory, immunomodulatory, and anti-viral properties in clinical and/or experimental settings and discuss the scope for intervention to resolve the inflammatory cytokine storm of COVID-19, which in turn helps in the management of NAFLD, at least for COVID-19 patients with pre-existing NAFLD conditions.
ω-3 LCPUFA– perspective on NAFLD, and anti-inflammatory, immunomodulatory and anti-viral potentials
In a randomized placebo-controlled trial, ω-3 PUFA supplementation in the form of seal oil (2 g, thrice a day) to NAFLD patients has shown to improve hypertriglyceridemia and liver function at the end of 24 wk. Furthermore, it resulted in a reduction of hepatic fat accumulation and reversed the condition in 50% of patients receiving ω-3 PUFA, compared to the placebo group[64]. Another randomized controlled trial carried out in histologically proven NAFLD children has reported that the group receiving DHA (250 mg/d and 500 mg/d) for 6 mo displayed improvement in liver histology and hypertriglyceridemia, compared to the control group[65]. A double-blind randomized placebo-controlled trial has reported that daily intake of 4 g ω-3 PUFA, containing 1840 mg EPA and 1520 mg DHA as ethyl esters for 15-18 mo, resulted in a reduction of liver fat% in NAFLD patients without improving the fibrosis scores. In addition, the authors have found enrichment of erythrocytes with ω-3 LCPUFA and 6% of DHA enrichment associated with 20% hepatic fat reduction[66]. Argo et al[67], who have conducted a double-blind randomized controlled trial in non-cirrhotic NASH patients reported that the consumption of ω-3 PUFA-rich fish oil of 3 g/d for approximately 1 year reduced hepatic fat accumulation and had no impact on the NASH-activity score. A double-blind, randomized, placebo-controlled trial that tested the efficacy of DHA supplementation (250 mg/d) in the form of oil from algae for 6 mo has found a 53.4% reduction of fat in the liver of overweight children with NAFLD, in addition to other clinical outcomes[68]. In a double-blind, randomized, placebo-controlled trial, the investigators have reported ω-3 PUFA supplementation (315 mg as oil from flaxseed and fish, to provide 64% ALA from flaxseed oil, 16% EPA and 21% DHA from fish oil) to NASH patients for 6 mo resulted in improved liver histology and dyslipidemia, compared to the placebo group[69]. A double-blind randomized clinical trial has shown that NAFLD patients with hyperlipidemia, who received ω-3 PUFA in the form of fish oil (4 g/d) for 3 mo displayed significant improvement in several clinical and inflammatory parameters, including triglyceride, ALT, γ-glutamyl transpeptidase, TNFα, fibroblast growth factor 21, cytokeratin 18 fragment M30, leukotriene B4 and PGE2, compared to the control group receiving corn oil, after adjusting for age, gender and BMI, and the author have concluded that ω-3 PUFA is beneficial in reducing metabolic abnormalities in NAFLD patients[70]. Although a few randomized trials have found no improvement associated with NAFLD conditions[71-73], several meta-analyses have observed the health benefits of ω-3 PUFA supplementation in NAFLD patients, as it reduced hepatic fat accumulation and NAFLD progression, possibly by improving associated metabolic abnormalities including hypertriglyceridemia, hepatic enzymes and dyslipidemia in patients with NAFLD spectrum[74-77].
For the first time, González-Périz et al[78], have shown DHA-mediated reduction in necro-inflammatory liver injury and oxidative damage in an experimental liver-injury model, possibly through the formation of DHA-derived lipid mediators, namely protectin D1 (PD1) and 17S-hydroxy docosahexaenoic acid (HDHA), while arresting the synthesis of the pro-inflammatory mediator of n-6 PUFA; PGE2. Furthermore, the authors have reported 17-hydroxy docosahexaenoic acid (17-HDHA)-mediated suppression of TNFα release and 5-lipoxygenase (5-LOX) activity in the in vitro hepatocyte cell line and macrophage culture, respectively. González-Périz and colleague[79] also have shown attenuation of hepatic steatosis and insulin resistance upon DHA supplementation in an ob/ob mouse model, which corroborated increased DHA-derived lipid mediators, such as resolvin D1 (RvD1), PD1 and 17-HDHA in adipose tissue with a concomitant reduction in AA-derived eicosanoids, namely PGE2 and 2-hydroxy eicosatetraenoic acid (2-HETE). Waylandt et al[80], who have studied liver tumorigenesis in a mouse model of transgenic over-expressing Fat-1 gene (converts endogenous ω-6 PUFA to ω-3 PUFA), reported decreased formation of liver tumor (chemically induced) and suppression of hepatic fibrotic activity and COX2 expression. In addition, the transgenic mice displayed significantly higher levels of ω-3 LCPUFA-derived lipid mediators; namely 18-hydroxy eicosapentaenoic acid (18-HEPH) and 17-HDHA in the liver and lower levels of TNFα in the circulation. Furthermore, even in a murine macrophage cell line, these ω-3 LCPUFA-derived lipid mediators were shown to suppress lipopolysaccharide (LPS)-induced TNFα formation. Previously, Rius et al[81] have reported macrophage polarization; pro-inflammatory M1 to anti-inflammatory M2 by RvD1, besides a reduction in hepatic steatosis and macrophage infiltration in an obese mouse model of NASH. Furthermore, RvD1 has been shown to inhibit tunicamycin-induced triglyceride accumulation in the in vitro hepatocyte cell line model; HepG2[82]. In line with this, a study from our laboratory also showed a negative association of RvD1 levels with triglyceride levels of both liver and plasma in a high fructose-induced NAFLD rat model. Furthermore, we concluded that RvD1 may be a key player in hepatic triglyceride metabolism and therefore it has a regulatory effect in the development of NAFLD[83]. Another study has demonstrated that the administration of RvD1 offers protection against chemically-induced liver injury in mice and shows improvement in liver pathology by suppressing inflammation and oxidative stress[84]. Furthermore, Jung et al[85] have shown suppression of triglyceride accumulation and hepatic steatosis by another DHA-derived lipid mediator; protectin DX (PDX), in hepatocytes (in vitro) and a high fat diet-fed mouse (in vivo) model, respectively. The novel DHA-derived lipid mediator; maresin 1 (MaR1) has been shown to arrest the inflammatory insult of hepatocytes and increased phagocytic activity of Kupffer cells. Notably, the administration of MaR1 resulted in significant reductions in hepatic triglyceride accumulation and circulatory liver enzymes in both genetically (ob/ob) diet-induced obese mouse models[86]. The study by Jung and colleagues have also shown that maresin 1 (MaR1) treated mice displayed suppression of hepatic lipogenesis, thus triglyceride accumulation and steatosis due to high fat-diet ingestion[87].
Unlike eicosanoids (except lipoxins), the lipid mediators derived from AA (ω-6 LCPUFA), the ω-3 LCPUFA (EPA and DHA)-derived lipid mediators possess anti-inflammatory and pro-resolving properties on tissue inflammation and wound healing, thus, are termed specialized pro-resolving mediators (SPM) and exhaustive literature has demonstrated the anti-inflammatory functions and health impact of ω-3 LCPUFA and their lipid mediators[88-92]. A previous study has reported the formation of various pro-resolving lipid mediators, including RvD1, RvD2 and PD1 at biologically active levels in healthy human subjects, who received fish oil supplementation for 3 wk[93]. Similarly, another study has reported plasma RvE1 levels 4 h after receiving fish oil orally in healthy human subjects[94]. Although the formation of these lipid mediators is known to vary with different physiological and pathological conditions, ω-3 LCPUFA is being used for the secondary prevention and/or management of inflammatory diseases in humans, including cardiovascular disease, atherosclerosis, and cancer (non-communicable diseases), Alzheimer’s disease (neurodegenerative diseases), arthritis, asthma and skin diseases (dermatitis and psoriasis)[15,92,95-97]. Overall, the aforementioned literature has indicated multiple, interlinked pathways and mechanisms in exerting the anti-inflammatory and pro-resolving activities of ω-3 LCPUFA and their lipid mediators, such as inhibition of the cellular AA metabolic pathway (including immune cells such as neutrophils, monocytes, leukocytes, etc.,), its production and conversion to various pro-inflammatory lipid mediators [PG2 series, leukotriene 4 (LT4) series and thromboxane 2 (TX2) series eicosanoids] and platelet activation and its aggregation (anti-thrombosis), cessation of pro-inflammatory cytokine (TNFα, IL-6 and IL-1) release and leukocyte infiltration, elevation of anti-inflammatory cytokines/factors (IL-4, IL-10 and TGFβ) by mononuclear cells, induction of pro-resolving mediator (RvD/RvE, PD, MaR) synthesis, macrophage polarization and activation (classically activated/pro-inflammatory-M1 to alternatively activated/reparative-M2), induction of apoptosis of polymorphonuclear monocytes (PMN), their removal by phagocytosis, resolution of inflammation, initiation of wound healing/tissue repair and tissue regeneration, besides, regulating genes, proteins, receptor activation and cell signaling.
A large body of evidence on the immunomodulatory effects mediated by ω-3 LCPUFA (EPA and DHA) and their lipid mediators, during infections and the underlying mechanisms have emerged from various experimental studies, involving animal and cell line models (both in vitro and ex vivo) and exhaustively reviewed earlier by several researchers[98-106]. In general, ω-3 LCPUFA administration results in EPA and DHA incorporation into membrane phospholipids, which increase the fluidity of the membrane, due to their long unsaturated acyl chain and thus alter the overall physicochemical properties of cells, including, membrane microdomains; lipid rafts, transporters, receptors, signaling proteins, etc. Furthermore, ω-3 LCPUFA have been shown to alter the functions of immune cells, such as monocytes/macrophages, natural killer (NK) cells, T cells, B cells, dendritic cells and other immune cells, and affect the cell interaction, antigen-presenting, major histocompatibility complex (MHC) class II molecule and intracellular adhesion molecule-1 (IVCAM-1)], secretory cytokines (namely IL-1β, TNFα and INFγ) and factors of immune cells involved in the innate and adaptive response, thus decreasing the host’s defense against microorganisms and increasing susceptibility to infection and mostly associated with excessive ω-3 PUFA concentration. However, extensive pre-clinical studies have shown that the ω-3 PUFA increases B cell activation by modulating antigen presentation, cytokine release and antibody production through multiple pathways, including the regulation of cytokine production by T helper cell type 2 (Th2 cell type)[103,104]. A study by Gorjã et al[105] has demonstrated increased neutrophil phagocytic capacity in human volunteers upon fish oil intake for 2 mo. Importantly, it appears that the ω-3 LCPUFA bring about these changes possibly through their bio-active lipid mediators and by altering the membrane composition and properties, in addition to their direct involvement. However, the lack of direct/supportive evidence from clinical studies with infectious diseases suggests that the immunomodulatory effects of ω-3 LCPUFA are still debatable and inconclusive, as they are derived mainly from experimental and in vitro and ex vivo studies. Nevertheless, the experimental evidence has unequivocally established the profound effect of ω-3 LCPUFA on almost all immune cells that are being investigated[98-105].
Several studies have shown the role of ω-3 LCPUFA/their lipid mediators as anti-infectious agents against various pathogenic organisms, including viruses in experimental models. However, in the present context, we report a couple of studies that have reported the effect of ω-3 PUFA-lipid mediators on viral infection, particularly RNA viruses. Morita et al[106] have reported that influenza A virus; HINI/PR8 strain-infected human lung epithelial cells treated with various LCPUFA-derived lipid mediators have displayed a reduction in viral nucleoprotein transcript levels and marked inhibition of viral replication. This is evident from the observed decrease in mRNA expression of viral M protein and viral titer observed in the infected cells treated with the ω-3 LCPUFA-lipid mediator; the PD1 isomer, PDX. Furthermore, the treatment inhibited viral mRNA and vRNA translocation into the cytoplasm (otherwise called nuclear export of virus RNAs). In addition, evaluation of the therapeutic efficacy of PDX in influenza virus-infected mice suggests that even late administration of PDX along with the anti-viral drug; peramivir, prevents the fatality associated with influenza viral infection, whereas the anti-viral drug alone fails to show a therapeutic effect. Overall, the study has highlighted the protective role of ω-3 PUFA-derived lipid mediators against influenza viral infection[106]. Bryan et al[107] who have studied respiratory syncytial virus (RSV) infection using human alveolar epithelial cells, reported that the cells treated with various LCPUFA increased the incorporation of these fatty acids i.e., AA, EPA and DHA into phospholipids of the cell membrane, which are associated with PGE2 production, i.e., higher levels in AA-treated cells, whereas this decreased with EPA or DHA treatment. Hence, this study suggests that the ratio of ω-6 to ω-3 LCPUFA plays a crucial role in bronchiolar and vascular changes (dilation and constriction) in lungs. During viral infection, through cytokine production and their incorporation into epithelial cells, ω-3 LCPUFA protects the lungs against long-term detrimental effects of RSV infection[107].
Although some of the ω-3 LCPUFA-derived lipid mediators were identified several years ago, data on their levels in human NAFLD conditions are still lacking, while none exist for COVID-19. Therefore, studies are required to understand the basal level and/or changes in various classes of fatty acids (in biological fluids and membranes) and the lipid mediators of both ω-6 and ω-3 LCPUFA in NAFLD patients and those who developed COVID-19. Clinical studies in these directions are needed, and are perhaps vital to understand the role of this pharmaconutrient and hence its application in clinical nutrition therapy. Nevertheless, ω-3 LCPUFA and its derived lipid mediators are potent nutrients in regulating hepatic fat accumulation, liver injury, inflammation, immunomodulation and viral infection (Schematic summary Figure 1). Hence, there is ample scope/opportunity for their use in pharmaconutrition during the COVID-19 pandemic, at least for the subset of patients with pre-existing NAFLD spectrum.
Figure 1.
Schematic summary of the therapeutic potential of omega-3 long-chain polyunsaturated fatty acids and their derived lipid mediators. ω-3 LCPUFA: Omega-3 long-chain polyunsaturated fatty acids; COX: Cyclooxygenase; DHA: Docosahexaenoic acid; EPD: Eicosapentaenoic acid; IL: Interleukin; LOX: Lipoxygenase; MHC: Major histocompatibility complex; PG: Prostaglandin; TX: Thromboxane; TGFβ: Transforming growth factor β; TNFα: Tumor necrosis factor α; ⊗: Inhibitory effect, ↓: Decrease; ↑: Increase.
PHARMACONUTRITION STRATEGY: Ω-3 LCPUFA INTERVENTION FOR COVID-19 PATIENTS WITH NAFLD - FAR FROM REALITY?
ω-3 LCPUFA intervention for infectious disease is skeptical to date; however, during this COVID-19 pandemic, several researchers have reviewed the existing literature and opined the possibilities of using ω-3 LCPUFA for COVID-19 as adjuvant therapy[108-112]. Furthermore, very recently, Santos et al[113], who have reviewed the evidence of various macro and micronutrients in clinical nutrition therapy have outlined the functional potency of ω-3 LCPUFA in the clinical management of COVID-19 as a pharmaconutrition strategy. On the other hand, the literature on ω-3 PUFA intervention for NAFLD is flooded with several highly influential studies, which have convincingly concluded their therapeutic potential against NAFLD both in humans and experimental models as discussed in the earlier section. Although, the anti-inflammatory, immunomodulatory and anti-viral potentials of ω-3 LCPUFA have been demonstrated in various experimental studies, besides improving NAFLD, in the absence of well-powered/-designed clinical trials/studies, speculating on the efficacy of ω-3 LCPUFA in resolving inflammatory cytokine storm during SARS-CoV-2 infection in NAFLD patients may be a matter of concern. Besides, applying the experimental knowledge to the therapeutic practice for COVID-19 patients with NAFLD is far beyond reality for various reasons. One of the key shortcomings in the area of NAFLD research is that the existing reports have used different doses of ω-3 LCPUFA, particularly EPA and DHA, during their evaluation/intervention studies, whereas studies assessing a specific dose of ω-3 LCPUFA for its efficacy evaluation, across race and/or ethnicity are lacking. However, in a recent review, Calder et al[114] have strongly urged public health officials for the inclusion of nutrition intervention strategies to limit the emerging viral infection, including SARS-CoV-2, in addition to standard treatment, as they are known to improve the immune system and are considered safe and cost-effective[114]. Based on the data from various clinical trials/studies evaluating the efficacy of ω-3 LCPUFA on inflammatory diseases in humans, the author has reported that the daily intake of at least 2000 mg of ω-3 LCPUFA is required to elicit the anti-inflammatory potency[92]. Furthermore, Husson et al[98] have highlighted that a daily dose of 500 mg of EPA and DHA improved the outcome of experimental infection caused by extracellular pathogens in healthy human volunteers, partly resulting from anti-inflammatory functions. Notably, several meta-analyses of various clinical trials, evaluated the clinical outcomes of fish oil supplementation in critically ill patients and reported a reduction in length of stay in the ICU, duration of mechanical ventilation and mortality[115-118]. However, there are a few challenges in using ω-3 LCPUFA in COVID-19 patients with NAFLD, such as (1) Dose (what is the optimal dose required to exert the pharmacological effect to resolve the “cytokine storm” and improve inflammation?); (2) Time of intervention (at what stage of the viral infection/immune response, should the nutrition intervention be introduced along with bio-medical treatment, to balance the immunomodulatory and anti-inflammatory actions for a better outcome?); and finally (3) Route of administration; oral, enteral or parenteral (which one would offer maximal benefits during the disease condition?). Nonetheless, in the absence of standard/specific treatment (although vaccination has been rolled out very recently in several countries), optimistically, these challenges can be circumvented. Therefore, considering the therapeutic potential (to name a few, anti-inflammation, immunomodulation, anti-thrombosis, pro-resolution, tissue repair and regeneration), there is scope for ω-3 LCPUFA intervention, at least for COVID-19 patients with NAFLD and may be for critically ill patients or patients admitted to ICU, as one of the strategies along with current biomedical treatment, which may yield a better clinical outcome, in terms of resolution and management of inflammatory cytokine storm/hyper-inflammation, associated-cellular and tissue damage. This can disconnect the bi-directional relationship between COVID-19 and NAFLD and thus would certainly help COVID-19 patients, particularly those with pre-existing NAFLD conditions, by attenuating the disease progression and/or liver injury, not only caused by SARS-CoV-2 infection, but possibly due to drugs as well (Schematic summary Figure 2).
Figure 2.
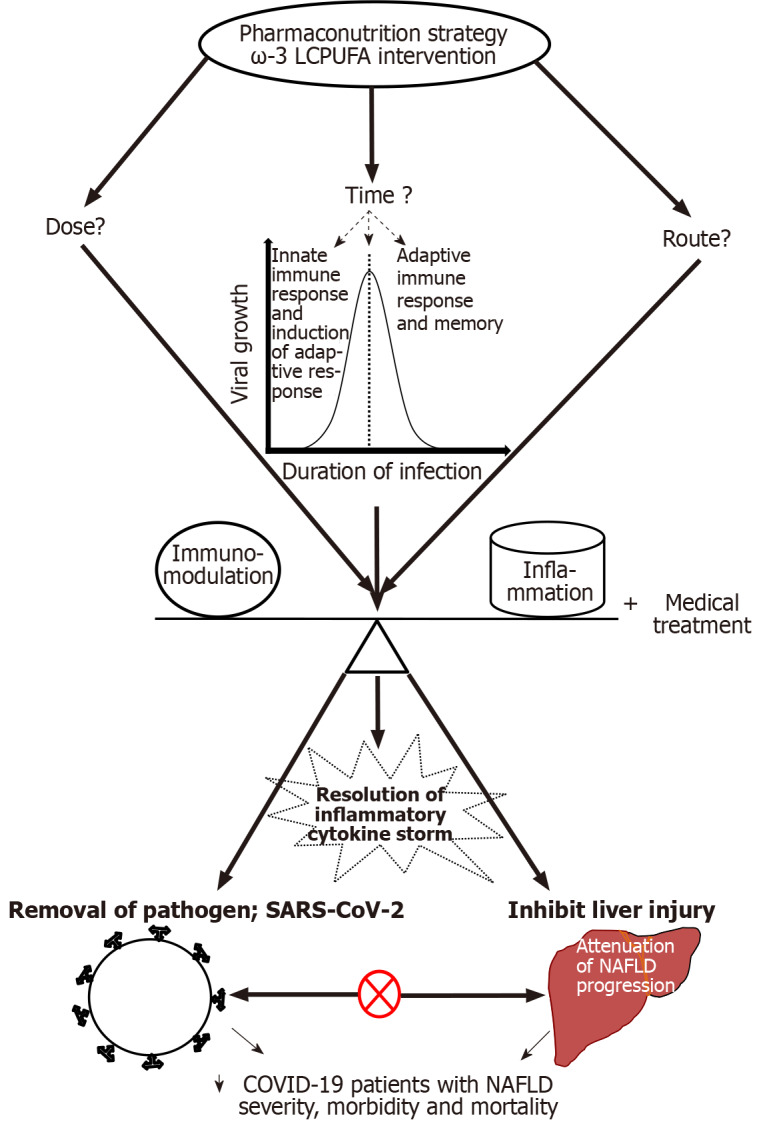
Schematic summary of the pharmaconutrition strategy and possible outcomes: ⊗ - Disconnecting bi-directional relationship between severe acute respiratory syndrome coronavirus 2 and non-alcoholic fatty liver disease and ↓ - decrease. ω-3 LCPUFA: Omega-3 long-chain polyunsaturated fatty acids; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; NAFLD: Non-alcoholic fatty liver disease; COVID-19: Coronavirus disease 2019.
CONCLUSION
Several randomized clinical trials and the meta-analysis data from randomized controlled trials have shown the beneficial effects of ω-3 LCPUFA in the management of NAFLD. The anti-inflammatory potential of ω-3 LCPUFA has been unequivocally shown in some of the overt/hyper-inflammatory (both acute and chronic) conditions associated with human diseases, including in critically ill patients, in addition to their immunomodulatory and anti-viral properties in various experimental models. However, whether these effects are mediated either directly by ω-3 LCPUFA or their derived bio-active lipid mediators or by both are not fully understood. Nevertheless, the emerging evidence has documented the involvement of multiple pathways and mechanisms that include modulation of immune cells, their functions and membrane fatty acid composition, augmented production of anti-inflammatory cytokines/ mediators and suppression of pro-inflammatory cytokines/mediators and, accelerated resolution of tissue inflammation, injury, faster wound healing and tissue regeneration, in addition to regulation of genes and cell signaling proteins, receptors/ molecules of various interlinked inflammatory pathways, to name a few. Therefore, the administration of ω-3 LCPUFA as a pharmaconutrient in the intervention strategy for COVID-19 patients with NAFLD may be a viable and promising option to mitigate and manage the inflammatory cytokine storm and associated-tissue injury in SARS-CoV-2 infection. Besides, it may complement the current treatment protocol and deliver a better clinical outcome in the management of NAFLD and COVID-19 pandemics.
Footnotes
Conflict-of-interest statement: The authors declare no conflicts of interest associated with this article.
Manuscript source: Invited manuscript
Peer-review started: February 25, 2021
First decision: May 13, 2021
Article in press: August 17, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cordeiro A S-Editor: Ma YJ L-Editor: Webster JR P-Editor: Wang LYT
Contributor Information
Shanmugam M Jeyakumar, Department of Clinical Pharmacology, ICMR-National Institute for Research in Tuberculosis, Chennai 600031, Tamil Nadu, India. smjkumar@gmail.com.
Ayyalasomayajula Vajreswari, Department of Biochemistry, National Institute of Nutrition, Hyderabad 500007, Telangana, India.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Weekly Epidemiological Update- 2 February 2021. Available from: https://www.who.int/publications/m/item/weekly-epidemiological-update---2-february-2021 .
- 3.Feehan KT, Gilroy DW. Is Resolution the End of Inflammation? Trends Mol Med. 2019;25:198–214. doi: 10.1016/j.molmed.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Cariou B, Byrne CD, Loomba R, Sanyal AJ. Nonalcoholic fatty liver disease as a metabolic disease in humans: A literature review. Diabetes Obes Metab. 2021;23:1069–1083. doi: 10.1111/dom.14322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun D, Li H, Lu XX, Xiao H, Ren J, Zhang FR, Liu ZS. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J Pediatr. 2020;16:251–259. doi: 10.1007/s12519-020-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li G, Fan Y, Lai Y, Han T, Li Z, Zhou P, Pan P, Wang W, Hu D, Liu X, Zhang Q, Wu J. Coronavirus infections and immune responses. J Med Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azhar EI, Hui DSC, Memish ZA, Drosten C, Zumla A. The Middle East Respiratory Syndrome (MERS) Infect Dis Clin North Am. 2019;33:891–905. doi: 10.1016/j.idc.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hui DSC, Zumla A. Severe Acute Respiratory Syndrome: Historical, Epidemiologic, and Clinical Features. Infect Dis Clin North Am. 2019;33:869–889. doi: 10.1016/j.idc.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y, Shen C, Li J, Yuan J, Yang M, Wang F, Li G, Li Y, Xing L, Peng L, Wei J. Cao M, Zheng H, Wu W, Zou R, Li D, Xu Z, Wang H, Zhang M, Zhang Z, Liu L, Liu Y. Exuberant elevation of IP-10, MCP-3 and IL-1 1ra during SARS-CoV-2. medRxiv. :2020.03.02.20029975. [Google Scholar]
- 12.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Portincasa P, Krawczyk M, Smyk W, Lammert F, Di Ciaula A. COVID-19 and non-alcoholic fatty liver disease: Two intersecting pandemics. Eur J Clin Invest. 2020;50:e13338. doi: 10.1111/eci.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margină D, Ungurianu A, Purdel C, Nițulescu GM, Tsoukalas D, Sarandi E, Thanasoula M, Burykina TI, Tekos F, Buha A, Nikitovic D, Kouretas D, Tsatsakis AM. Analysis of the intricate effects of polyunsaturated fatty acids and polyphenols on inflammatory pathways in health and disease. Food Chem Toxicol. 2020;143:111558. doi: 10.1016/j.fct.2020.111558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shahidi F, Ambigaipalan P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu Rev Food Sci Technol. 2018;9:345–381. doi: 10.1146/annurev-food-111317-095850. [DOI] [PubMed] [Google Scholar]
- 16.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 17.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 18.Agosti P, Sabbà C, Mazzocca A. Emerging metabolic risk factors in hepatocellular carcinoma and their influence on the liver microenvironment. Biochim Biophys Acta Mol Basis Dis. 2018;1864:607–617. doi: 10.1016/j.bbadis.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 19.Tilg H, Adolph TE, Moschen AR. Multiple Parallel Hits Hypothesis in Nonalcoholic Fatty Liver Disease: Revisited After a Decade. Hepatology. 2021;73:833–842. doi: 10.1002/hep.31518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49:608–612. doi: 10.1016/j.jhep.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Farrell GC, van Rooyen D, Gan L, Chitturi S. NASH is an Inflammatory Disorder: Pathogenic, Prognostic and Therapeutic Implications. Gut Liver. 2012;6:149–171. doi: 10.5009/gnl.2012.6.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo Y, Lin H. Inflammation initiates a vicious cycle between obesity and nonalcoholic fatty liver disease. Immun Inflamm Dis. 2021;9:59–73. doi: 10.1002/iid3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luci C, Bourinet M, Leclère PS, Anty R, Gual P. Chronic Inflammation in Non-Alcoholic Steatohepatitis: Molecular Mechanisms and Therapeutic Strategies. Front Endocrinol (Lausanne) 2020;11:597648. doi: 10.3389/fendo.2020.597648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bessone F, Razori MV, Roma MG. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell Mol Life Sci. 2019;76:99–128. doi: 10.1007/s00018-018-2947-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alisi A, Carpino G, Oliveira FL, Panera N, Nobili V, Gaudio E. The Role of Tissue Macrophage-Mediated Inflammation on NAFLD Pathogenesis and Its Clinical Implications. Mediators Inflamm. 2017;2017:8162421. doi: 10.1155/2017/8162421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ge H, Wang X, Yuan X, Xiao G, Wang C, Deng T, Yuan Q, Xiao X. The epidemiology and clinical information about COVID-19. Eur J Clin Microbiol Infect Dis. 2020;39:1011–1019. doi: 10.1007/s10096-020-03874-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qu L, Li J, Ren H. COVID-19: the epidemiology and treatment. Br J Hosp Med (Lond) 2020;81:1–9. doi: 10.12968/hmed.2020.0580. [DOI] [PubMed] [Google Scholar]
- 28.Krishnan A, Hamilton JP, Alqahtani SA, Woreta TA. COVID-19: An overview and a clinical update. World J Clin Cases. 2021;9:8–23. doi: 10.12998/wjcc.v9.i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Akdis CA, Gao YD. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 30.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang L, Yin Z, Hu Y, Mei H. Controlling Cytokine Storm Is Vital in COVID-19. Front Immunol. 2020;11:570993. doi: 10.3389/fimmu.2020.570993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nat Rev Immunol. 2015;15:511–523. doi: 10.1038/nri3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCarthy MK, Weinberg JB. Eicosanoids and respiratory viral infection: coordinators of inflammation and potential therapeutic targets. Mediators Inflamm. 2012;2012:236345. doi: 10.1155/2012/236345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sander WJ, O'Neill HG, Pohl CH. Prostaglandin E2 As a Modulator of Viral Infections. Front Physiol. 2017;8:89. doi: 10.3389/fphys.2017.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uozumi N, Kita Y, Shimizu T. Modulation of lipid and protein mediators of inflammation by cytosolic phospholipase A2alpha during experimental sepsis. J Immunol. 2008;181:3558–3566. doi: 10.4049/jimmunol.181.5.3558. [DOI] [PubMed] [Google Scholar]
- 38.Caughey GE, Pouliot M, Cleland LG, James MJ. Regulation of tumor necrosis factor-alpha and IL-1 beta synthesis by thromboxane A2 in nonadherent human monocytes. J Immunol. 1997;158:351–358. [PubMed] [Google Scholar]
- 39.Cirino G. Multiple controls in inflammation. Extracellular and intracellular phospholipase A2, inducible and constitutive cyclooxygenase, and inducible nitric oxide synthase. Biochem Pharmacol. 1998;55:105–111. doi: 10.1016/s0006-2952(97)00215-3. [DOI] [PubMed] [Google Scholar]
- 40.Gilroy DW, Newson J, Sawmynaden P, Willoughby DA, Croxtall JD. A novel role for phospholipase A2 isoforms in the checkpoint control of acute inflammation. FASEB J. 2004;18:489–498. doi: 10.1096/fj.03-0837com. [DOI] [PubMed] [Google Scholar]
- 41.Schalkwijk C, Pfeilschifter J, Märki F, van den Bosch H. Interleukin-1 beta, tumor necrosis factor and forskolin stimulate the synthesis and secretion of group II phospholipase A2 in rat mesangial cells. Biochem Biophys Res Commun. 1991;174:268–275. doi: 10.1016/0006-291x(91)90515-9. [DOI] [PubMed] [Google Scholar]
- 42.Schalkwijk CG, de Vet E, Pfeilschifter J, van den Bosch H. Interleukin-1 beta and transforming growth factor-beta 2 enhance cytosolic high-molecular-mass phospholipase A2 activity and induce prostaglandin E2 formation in rat mesangial cells. Eur J Biochem. 1992;210:169–176. doi: 10.1111/j.1432-1033.1992.tb17405.x. [DOI] [PubMed] [Google Scholar]
- 43.Sanak M. Eicosanoid Mediators in the Airway Inflammation of Asthmatic Patients: What is New? Allergy Asthma Immunol Res. 2016;8:481–490. doi: 10.4168/aair.2016.8.6.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Claar D, Hartert TV, Peebles RS Jr. The role of prostaglandins in allergic lung inflammation and asthma. Expert Rev Respir Med. 2015;9:55–72. doi: 10.1586/17476348.2015.992783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoxha M. What about COVID-19 and arachidonic acid pathway? Eur J Clin Pharmacol. 2020;76:1501–1504. doi: 10.1007/s00228-020-02941-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hammock BD, Wang W, Gilligan MM, Panigrahy D. Eicosanoids: The Overlooked Storm in Coronavirus Disease 2019 (COVID-19)? Am J Pathol. 2020;190:1782–1788. doi: 10.1016/j.ajpath.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ripon MAR, Bhowmik DR, Amin MT, Hossain MS. Role of arachidonic cascade in COVID-19 infection: A review. Prostaglandins Other Lipid Mediat. 2021;154:106539. doi: 10.1016/j.prostaglandins.2021.106539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang R, Zhu L, Wang J, Xue L, Liu L, Yan X, Huang S, Li Y, Zhang B, Xu T, Li C, Ji F, Ming F, Zhao Y, Cheng J, Wang Y, Zhao H, Hong S, Chen K, Zhao XA, Zou L, Sang D, Shao H, Guan X, Chen X, Chen Y, Wei J, Zhu C, Wu C. Clinical features of COVID-19 patients with non-alcoholic fatty liver disease. Hepatol Commun. 2020 doi: 10.1002/hep4.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahamid M, Nseir W, Khoury T, Mahamid B, Nubania A, Sub-Laban K, Schifter J, Mari A, Sbeit W, Goldin E. Nonalcoholic fatty liver disease is associated with COVID-19 severity independently of metabolic syndrome: a retrospective case-control study. Eur J Gastroenterol Hepatol. 2020 doi: 10.1097/MEG.0000000000001902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghoneim S, Butt MU, Hamid O, Shah A, Asaad I. The incidence of COVID-19 in patients with metabolic syndrome and non-alcoholic steatohepatitis: A population-based study. Metabol Open. 2020;8:100057. doi: 10.1016/j.metop.2020.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen VL, Hawa F, Berinstein JA, Reddy CA, Kassab I, Platt KD, Hsu CY, Steiner CA, Louissaint J, Gunaratnam NT, Sharma P. Hepatic Steatosis Is Associated with Increased Disease Severity and Liver Injury in Coronavirus Disease-19. Dig Dis Sci . 2021;66:3192–3198. doi: 10.1007/s10620-020-06618-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Forlano R, Mullish BH, Mukherjee SK, Nathwani R, Harlow C, Crook P, Judge R, Soubieres A, Middleton P, Daunt A, Perez-Guzman P, Selvapatt N, Lemoine M, Dhar A, Thursz MR, Nayagam S, Manousou P. In-hospital mortality is associated with inflammatory response in NAFLD patients admitted for COVID-19. PLoS One. 2020;15:e0240400. doi: 10.1371/journal.pone.0240400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sachdeva S, Khandait H, Kopel J, Aloysius MM, Desai R, Goyal H. NAFLD and COVID-19: a Pooled Analysis. SN Compr Clin Med. 2020:1–4. doi: 10.1007/s42399-020-00631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bramante C, Tignanelli CJ, Dutta N, Jones E, Tamariz L, Clark JM, Usher M, Metlon-Meaux G, Ikramuddin S. Non-alcoholic fatty liver disease (NAFLD) and risk of hospitalization for Covid-19. medRxiv. 2020 [Google Scholar]
- 56.Pierre JF, Heneghan AF, Lawson CM, Wischmeyer PE, Kozar RA, Kudsk KA. Pharmaconutrition review: physiological mechanisms. JPEN J Parenter Enteral Nutr. 2013;37:51S–65S. doi: 10.1177/0148607113493326. [DOI] [PubMed] [Google Scholar]
- 57.Tashiro T. N-3 polyunsaturated fatty acids in pharmaconutrition and immunonutrition. J Gastroenterol. 2000;35 Suppl 12:24. [PubMed] [Google Scholar]
- 58.Zárate R, El Jaber-Vazdekis N, Tejera N, Pérez JA, Rodríguez C. Significance of long chain polyunsaturated fatty acids in human health. Clin Transl Med. 2017;6:25. doi: 10.1186/s40169-017-0153-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeyakumar SM, Vajreswari A. Dietary management of non-alcoholic fatty liver disease (NAFLD) by n-3 polyunsaturated fatty acid (PUFA) supplementation: A perspective on the role of n-3 PUFA-derived lipid mediators. In: Watson RR, Preedy VR, editors, Dietary Interventions in Liver Disease: Foods, Nutrients and Dietary Supplements. London: Elsevier, 2019: 373-389. [Google Scholar]
- 60.WHO/FAO . Fats and fatty acids in human nutrition, Reports of an expert consultation 2008 November 10-14. Rome, Italy. Geneva: FAO. 2008: 1-161. [Google Scholar]
- 61.Parolini C. Marine n-3 polyunsaturated fatty acids: Efficacy on inflammatory-based disorders. Life Sci. 2020;263:118591. doi: 10.1016/j.lfs.2020.118591. [DOI] [PubMed] [Google Scholar]
- 62.Langlois PL, D'Aragon F, Hardy G, Manzanares W. Omega-3 polyunsaturated fatty acids in critically ill patients with acute respiratory distress syndrome: A systematic review and meta-analysis. Nutrition. 2019;61:84–92. doi: 10.1016/j.nut.2018.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu C, Sharma S, McIntyre L, Rhodes A, Evans L, Almenawer S, Leduc L, Angus DC, Alhazzani W. Omega-3 supplementation in patients with sepsis: a systematic review and meta-analysis of randomized trials. Ann Intensive Care. 2017;7:58. doi: 10.1186/s13613-017-0282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu FS, Liu S, Chen XM, Huang ZG, Zhang DW. Effects of n-3 polyunsaturated fatty acids from seal oils on nonalcoholic fatty liver disease associated with hyperlipidemia. World J Gastroenterol. 2008;14:6395–6400. doi: 10.3748/wjg.14.6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nobili V, Bedogni G, Alisi A, Pietrobattista A, Risé P, Galli C, Agostoni C. Docosahexaenoic acid supplementation decreases liver fat content in children with non-alcoholic fatty liver disease: double-blind randomised controlled clinical trial. Arch Dis Child. 2011;96:350–353. doi: 10.1136/adc.2010.192401. [DOI] [PubMed] [Google Scholar]
- 66.Scorletti E, Bhatia L, McCormick KG, Clough GF, Nash K, Hodson L, Moyses HE, Calder PC, Byrne CD WELCOME Study. Effects of purified eicosapentaenoic and docosahexaenoic acids in nonalcoholic fatty liver disease: results from the Welcome* study. Hepatology. 2014;60:1211–1221. doi: 10.1002/hep.27289. [DOI] [PubMed] [Google Scholar]
- 67.Argo CK, Patrie JT, Lackner C, Henry TD, de Lange EE, Weltman AL, Shah NL, Al-Osaimi AM, Pramoonjago P, Jayakumar S, Binder LP, Simmons-Egolf WD, Burks SG, Bao Y, Taylor AG, Rodriguez J, Caldwell SH. Effects of n-3 fish oil on metabolic and histological parameters in NASH: a double-blind, randomized, placebo-controlled trial. J Hepatol. 2015;62:190–197. doi: 10.1016/j.jhep.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pacifico L, Bonci E, Di Martino M, Versacci P, Andreoli G, Silvestri LM, Chiesa C. A double-blind, placebo-controlled randomized trial to evaluate the efficacy of docosahexaenoic acid supplementation on hepatic fat and associated cardiovascular risk factors in overweight children with nonalcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2015;25:734–741. doi: 10.1016/j.numecd.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 69.Nogueira MA, Oliveira CP, Ferreira Alves VA, Stefano JT, Rodrigues LS, Torrinhas RS, Cogliati B, Barbeiro H, Carrilho FJ, Waitzberg DL. Omega-3 polyunsaturated fatty acids in treating non-alcoholic steatohepatitis: A randomized, double-blind, placebo-controlled trial. Clin Nutr. 2016;35:578–586. doi: 10.1016/j.clnu.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 70.Qin Y, Zhou Y, Chen SH, Zhao XL, Ran L, Zeng XL, Wu Y, Chen JL, Kang C, Shu FR, Zhang QY, Mi MT. Fish Oil Supplements Lower Serum Lipids and Glucose in Correlation with a Reduction in Plasma Fibroblast Growth Factor 21 and Prostaglandin E2 in Nonalcoholic Fatty Liver Disease Associated with Hyperlipidemia: A Randomized Clinical Trial. PLoS One. 2015;10:e0133496. doi: 10.1371/journal.pone.0133496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sanyal AJ, Abdelmalek MF, Suzuki A, Cummings OW, Chojkier M EPE-A Study Group. No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology. 2014;147:377–84.e1. doi: 10.1053/j.gastro.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 72.Janczyk W, Lebensztejn D, Wierzbicka-Rucińska A, Mazur A, Neuhoff-Murawska J, Matusik P, Socha P. Omega-3 Fatty acids therapy in children with nonalcoholic Fatty liver disease: a randomized controlled trial. J Pediatr. 2015;166:1358–63.e1. doi: 10.1016/j.jpeds.2015.01.056. [DOI] [PubMed] [Google Scholar]
- 73.Dasarathy S, Dasarathy J, Khiyami A, Yerian L, Hawkins C, Sargent R, McCullough AJ. Double-blind randomized placebo-controlled clinical trial of omega 3 fatty acids for the treatment of diabetic patients with nonalcoholic steatohepatitis. J Clin Gastroenterol. 2015;49:137–144. doi: 10.1097/MCG.0000000000000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu W, Li S, Li J, Wang J, Zhang R, Zhou Y, Yin Q, Zheng Y, Wang F, Xia Y, Chen K, Liu T, Lu J, Guo C. Effects of Omega-3 Fatty Acid in Nonalcoholic Fatty Liver Disease: A Meta-Analysis. Gastroenterol Res Pract. 2016;2016:1459790. doi: 10.1155/2016/1459790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.He XX, Wu XL, Chen RP, Chen C, Liu XG, Wu BJ, Huang ZM. Effectiveness of Omega-3 Polyunsaturated Fatty Acids in Non-Alcoholic Fatty Liver Disease: A Meta-Analysis of Randomized Controlled Trials. PLoS One. 2016;11:e0162368. doi: 10.1371/journal.pone.0162368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guo XF, Yang B, Tang J, Li D. Fatty acid and non-alcoholic fatty liver disease: Meta-analyses of case-control and randomized controlled trials. Clin Nutr. 2018;37:113–122. doi: 10.1016/j.clnu.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 77.Chen LH, Wang YF, Xu QH, Chen SS. Omega-3 fatty acids as a treatment for non-alcoholic fatty liver disease in children: A systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2018;37:516–521. doi: 10.1016/j.clnu.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 78.González-Périz A, Planagumà A, Gronert K, Miquel R, López-Parra M, Titos E, Horrillo R, Ferré N, Deulofeu R, Arroyo V, Rodés J, Clària J. Docosahexaenoic acid (DHA) blunts liver injury by conversion to protective lipid mediators: protectin D1 and 17S-hydroxy-DHA. FASEB J. 2006;20:2537–2539. doi: 10.1096/fj.06-6250fje. [DOI] [PubMed] [Google Scholar]
- 79.González-Périz A, Horrillo R, Ferré N, Gronert K, Dong B, Morán-Salvador E, Titos E, Martínez-Clemente M, López-Parra M, Arroyo V, Clària J. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. 2009;23:1946–1957. doi: 10.1096/fj.08-125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weylandt KH, Krause LF, Gomolka B, Chiu CY, Bilal S, Nadolny A, Waechter SF, Fischer A, Rothe M, Kang JX. Suppressed liver tumorigenesis in fat-1 mice with elevated omega-3 fatty acids is associated with increased omega-3 derived lipid mediators and reduced TNF-α. Carcinogenesis. 2011;32:897–903. doi: 10.1093/carcin/bgr049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rius B, Titos E, Morán-Salvador E, López-Vicario C, García-Alonso V, González-Périz A, Arroyo V, Clària J. Resolvin D1 primes the resolution process initiated by calorie restriction in obesity-induced steatohepatitis. FASEB J. 2014;28:836–848. doi: 10.1096/fj.13-235614. [DOI] [PubMed] [Google Scholar]
- 82.Jung TW, Hwang HJ, Hong HC, Choi HY, Yoo HJ, Baik SH, Choi KM. Resolvin D1 reduces ER stress-induced apoptosis and triglyceride accumulation through JNK pathway in HepG2 cells. Mol Cell Endocrinol. 2014;391:30–40. doi: 10.1016/j.mce.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 83.Raja Gopal Reddy M, Pavan Kumar C, Mahesh M, Sravan Kumar M, Mullapudi Venkata S, Putcha UK, Vajreswari A, Jeyakumar SM. Vitamin A deficiency suppresses high fructose-induced triglyceride synthesis and elevates resolvin D1 Levels. Biochim Biophys Acta. 2016;1861:156–165. doi: 10.1016/j.bbalip.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 84.Chen X, Gong X, Jiang R, Wang B, Kuang G, Li K, Wan J. Resolvin D1 attenuates CCl4-induced acute liver injury involving up-regulation of HO-1 in mice. Immunopharmacol Immunotoxicol. 2016;38:61–67. doi: 10.3109/08923973.2015.1115517. [DOI] [PubMed] [Google Scholar]
- 85.Jung TW, Kyung EJ, Kim HC, Shin YK, Lee SH, Park ES, Hacımüftüoğlu A, Abd El-Aty AM, Jeong JH. Protectin DX Ameliorates Hepatic Steatosis by Suppression of Endoplasmic Reticulum Stress via AMPK-Induced ORP150 Expression. J Pharmacol Exp Ther. 2018;365:485–493. doi: 10.1124/jpet.117.246686. [DOI] [PubMed] [Google Scholar]
- 86.Laiglesia LM, Lorente-Cebrián S, Martínez-Fernández L, Sáinz N, Prieto-Hontoria PL, Burrell MA, Rodríguez-Ortigosa CM, Martínez JA, Moreno-Aliaga MJ. Maresin 1 mitigates liver steatosis in ob/ob and diet-induced obese mice. Int J Obes (Lond) 2018;42:572–579. doi: 10.1038/ijo.2017.226. [DOI] [PubMed] [Google Scholar]
- 87.Jung TW, Kim HC, Abd El-Aty AM, Jeong JH. Maresin 1 attenuates NAFLD by suppression of endoplasmic reticulum stress via AMPK-SERCA2b pathway. J Biol Chem. 2018;293:3981–3988. doi: 10.1074/jbc.RA117.000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ishihara T, Yoshida M, Arita M. Omega-3 fatty acid-derived mediators that control inflammation and tissue homeostasis. Int Immunol. 2019;31:559–567. doi: 10.1093/intimm/dxz001. [DOI] [PubMed] [Google Scholar]
- 89.Serhan CN, Chiang N, Dalli J. New pro-resolving n-3 mediators bridge resolution of infectious inflammation to tissue regeneration. Mol Aspects Med. 2018;64:1–17. doi: 10.1016/j.mam.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Molfino A, Amabile MI, Monti M, Muscaritoli M. Omega-3 Polyunsaturated Fatty Acids in Critical Illness: Anti-Inflammatory, Proresolving, or Both? Oxid Med Cell Longev. 2017;2017:5987082. doi: 10.1155/2017/5987082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Basil MC, Levy BD. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat Rev Immunol. 2016;16:51–67. doi: 10.1038/nri.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Calder PC. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015;1851:469–484. doi: 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 93.Mas E, Croft KD, Zahra P, Barden A, Mori TA. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin Chem. 2012;58:1476–1484. doi: 10.1373/clinchem.2012.190199. [DOI] [PubMed] [Google Scholar]
- 94.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 Lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rangel-Huerta OD, Aguilera CM, Mesa MD, Gil A. Omega-3 Long-chain polyunsaturated fatty acids supplementation on inflammatory biomakers: a systematic review of randomised clinical trials. Br J Nutr. 2012;107 Suppl 2:S159–S170. doi: 10.1017/S0007114512001559. [DOI] [PubMed] [Google Scholar]
- 96.Nindrea RD, Aryandono T, Lazuardi L, Dwiprahasto I. Protective Effect of Omega-3 Fatty Acids in Fish Consumption Against Breast Cancer in Asian Patients: A Meta-Analysis. Asian Pac J Cancer Prev. 2019;20:327–332. doi: 10.31557/APJCP.2019.20.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Spite M. Deciphering the role of n-3 polyunsaturated fatty acid-derived lipid mediators in health and disease. Proc Nutr Soc. 2013;72:441–450. doi: 10.1017/S0029665113003030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Husson MO, Ley D, Portal C, Gottrand M, Hueso T, Desseyn JL, Gottrand F. Modulation of host defence against bacterial and viral infections by omega-3 polyunsaturated fatty acids. J Infect. 2016;73:523–535. doi: 10.1016/j.jinf.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 99.Imai Y. Role of omega-3 PUFA-derived mediators, the protectins, in influenza virus infection. Biochim Biophys Acta. 2015;1851:496–502. doi: 10.1016/j.bbalip.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 100.Fenton JI, Hord NG, Ghosh S, Gurzell EA. Immunomodulation by dietary long chain omega-3 fatty acids and the potential for adverse health outcomes. Prostaglandins Leukot Essent Fatty Acids. 2013;89:379–390. doi: 10.1016/j.plefa.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pae M, Meydani SN, Wu D. The role of nutrition in enhancing immunity in aging. Aging Dis. 2012;3:91–129. [PMC free article] [PubMed] [Google Scholar]
- 102.Sijben JW, Calder PC. Differential immunomodulation with long-chain n-3 PUFA in health and chronic disease. Proc Nutr Soc. 2007;66:237–259. doi: 10.1017/S0029665107005472. [DOI] [PubMed] [Google Scholar]
- 103.Gutiérrez S, Svahn SL, Johansson ME. Effects of Omega-3 Fatty Acids on Immune Cells. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20205028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Whelan J, Gowdy KM, Shaikh SR. N-3 polyunsaturated fatty acids modulate B cell activity in pre-clinical models: Implications for the immune response to infections. Eur J Pharmacol. 2016;785:10–17. doi: 10.1016/j.ejphar.2015.03.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gorjão R, Verlengia R, Lima TM, Soriano FG, Boaventura MF, Kanunfre CC, Peres CM, Sampaio SC, Otton R, Folador A, Martins EF, Curi TC, Portiolli EP, Newsholme P, Curi R. Effect of docosahexaenoic acid-rich fish oil supplementation on human leukocyte function. Clin Nutr. 2006;25:923–938. doi: 10.1016/j.clnu.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 106.Morita M, Kuba K, Ichikawa A, Nakayama M, Katahira J, Iwamoto R, Watanebe T, Sakabe S, Daidoji T, Nakamura S, Kadowaki A, Ohto T, Nakanishi H, Taguchi R, Nakaya T, Murakami M, Yoneda Y, Arai H, Kawaoka Y, Penninger JM, Arita M, Imai Y. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell. 2013;153:112–125. doi: 10.1016/j.cell.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 107.Bryan DL, Hart P, Forsyth K, Gibson R. Modulation of respiratory syncytial virus-induced prostaglandin E2 production by n-3 Long-chain polyunsaturated fatty acids in human respiratory epithelium. Lipids. 2005;40:1007–1011. doi: 10.1007/s11745-005-1463-4. [DOI] [PubMed] [Google Scholar]
- 108.Weill P, Plissonneau C, Legrand P, Rioux V, Thibault R. May omega-3 fatty acid dietary supplementation help reduce severe complications in Covid-19 patients? Biochimie. 2020;179:275–280. doi: 10.1016/j.biochi.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Darwesh AM, Bassiouni W, Sosnowski DK, Seubert JM. Can N-3 polyunsaturated fatty acids be considered a potential adjuvant therapy for COVID-19-associated cardiovascular complications? Pharmacol Ther. 2021;219:107703. doi: 10.1016/j.pharmthera.2020.107703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Arnardottir H, Pawelzik SC, Öhlund Wistbacka U, Artiach G, Hofmann R, Reinholdsson I, Braunschweig F, Tornvall P, Religa D, Bäck M. Stimulating the Resolution of Inflammation Through Omega-3 Polyunsaturated Fatty Acids in COVID-19: Rationale for the COVID-Omega-F Trial. Front Physiol. 2020;11:624657. doi: 10.3389/fphys.2020.624657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hathaway D, Pandav K, Patel M, Riva-Moscoso A, Singh BM, Patel A, Min ZC, Singh-Makkar S, Sana MK, Sanchez-Dopazo R, Desir R, Fahem MMM, Manella S, Rodriguez I, Alvarez A, Abreu R. Omega 3 Fatty Acids and COVID-19: A Comprehensive Review. Infect Chemother. 2020;52:478–495. doi: 10.3947/ic.2020.52.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rogero MM, Leão MC, Santana TM, Pimentel MVMB, Carlini GCG, da Silveira TFF, Gonçalves RC, Castro IA. Potential benefits and risks of omega-3 fatty acids supplementation to patients with COVID-19. Free Radic Biol Med. 2020;156:190–199. doi: 10.1016/j.freeradbiomed.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Santos HO, Tinsley GM, da Silva GAR, Bueno AA. Pharmaconutrition in the Clinical Management of COVID-19: A Lack of Evidence-Based Research But Clues to Personalized Prescription. J Pers Med. 2020;10 doi: 10.3390/jpm10040145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Calder PC, Carr AC, Gombart AF, Eggersdorfer M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients. 2020;12 doi: 10.3390/nu12041181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kristine Koekkoek W, Panteleon V, van Zanten AR. Current evidence on ω-3 fatty acids in enteral nutrition in the critically ill: A systematic review and meta-analysis. Nutrition. 2019;59:56–68. doi: 10.1016/j.nut.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 116.Wan X, Gao X, Bi J, Tian F, Wang X. Use of n-3 PUFAs can decrease the mortality in patients with systemic inflammatory response syndrome: a systematic review and meta-analysis. Lipids Health Dis. 2015;14:23. doi: 10.1186/s12944-015-0022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Manzanares W, Langlois PL, Dhaliwal R, Lemieux M, Heyland DK. Intravenous fish oil lipid emulsions in critically ill patients: an updated systematic review and meta-analysis. Crit Care. 2015;19:167. doi: 10.1186/s13054-015-0888-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Manzanares W, Dhaliwal R, Jurewitsch B, Stapleton RD, Jeejeebhoy KN, Heyland DK. Parenteral fish oil lipid emulsions in the critically ill: a systematic review and meta-analysis. JPEN J Parenter Enteral Nutr. 2014;38:20–28. doi: 10.1177/0148607113486006. [DOI] [PMC free article] [PubMed] [Google Scholar]



