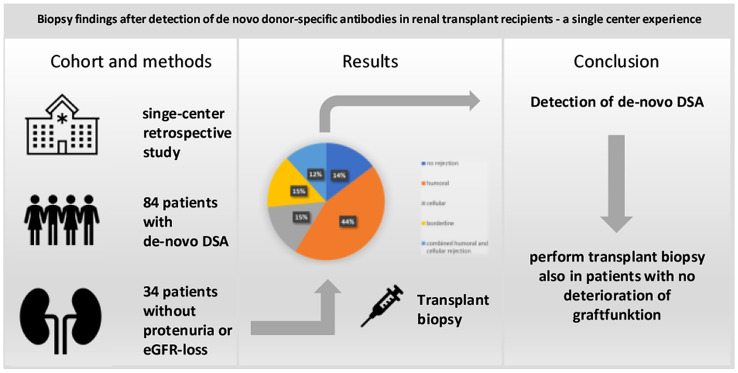
Abstract
Background
De novo donor-specific antibodies (DSA) are associated with an increased risk of antibody-mediated rejection and a substantial reduction of allograft survival. We hypothesized that detection of DSA should prompt a biopsy even in the absence of proteinuria and loss of estimated glomerular filtration rate (eGFR). However, data on a population without proteinuria or loss of kidney function is scant, and this is the main novelty of our study design.
Methods
Single center retrospective analysis on biopsy findings after detection of de novo DSA. One-hundred-thirty-two kidney and pancreas-kidney transplant recipients were included. Eighty-four of these patients (63.6%) underwent allograft biopsy. At the time of biopsy n = 50 (59.5%) had a protein/creatinine ratio (PCR) > 300 mg/g creatinine and/or a loss of eGFR ≥ 10 ml/min in the previous 12 months, whereas 40.5% did not. Diagnosis of rejection was performed according to Banff criteria.
Results
Seventy-seven (91.7%) of the biopsies had signs of rejection (47.6% antibody mediated rejection (ABMR), 13.1% cellular, 20.2% combined, 10.7% borderline). Among subjects without proteinuria or loss of eGFR ≥ 10 ml/min/a (n = 34), 29 patients (85.3%) showed signs of rejection (44.1% antibody mediated (ABMR), 14.7% cellular, 11.8% combined, 14.7% borderline).
Conclusion
The majority of subjects with de novo DSA have histological signs of rejection, even in the absence of proteinuria and deterioration of graft function. Thus, it appears reasonable to routinely perform an allograft biopsy after the detection of de novo DSA.
Graphic abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s40620-021-01040-y.
Keywords: Donor-specific antibodies, DSA, Kidney transplantation, Antibody-mediated rejection
Introduction
Occurrence of de novo donor-specific antibodies (DSA) is associated with an increased risk of antibody-mediated rejection (ABMR) and a substantial reduction of allograft survival [1]. Five years after detection of de novo DSA 50.0% of renal transplant recipients will have returned to dialysis [2]. Therefore, an increasing number of transplant centers screen for DSA on a regular basis—e. g. every three to twelve months. It remains elusive, however, what to do in case of a positive finding regarding both potential intensification of immunosuppression and carrying out a biopsy.
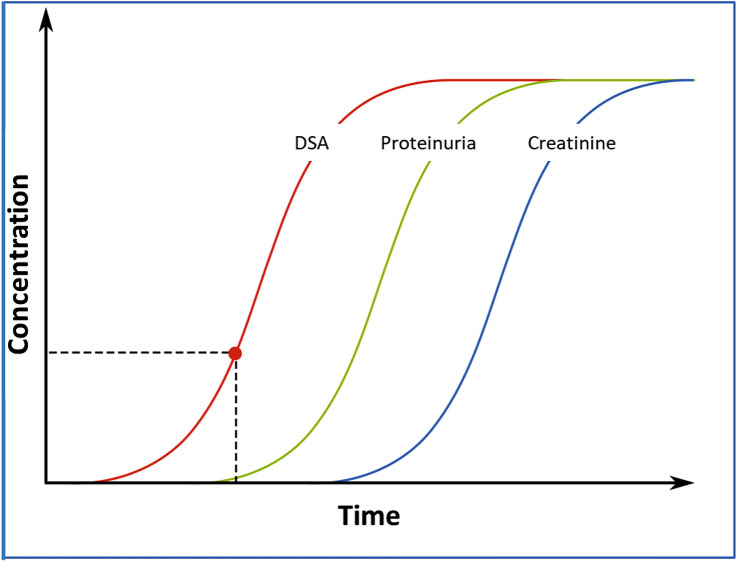
The development of DSA constitutes the first step in the evolution of ABMR. Second, the DSA initiate inflammation with consecutive glomerular damage resulting in impaired permselectivity and proteinuria [3]. Finally, there is a deterioration of glomerular filtration resulting in a clinically detectable rise in serum creatinine concentration (Fig. 1). Treatment of ABMR is one of the biggest challenges in current transplant medicine. The more advanced the glomerular pathology, the worse the efficacy of rejection therapy. We therefore hypothesized that detection of de novo DSA should be regarded as an indication for renal allograft biopsy even in the absence of proteinuria and impaired eGFR. In 2014 we started to screen for DSA on an annual basis and changed our standard operating procedure to recommend biopsy to every transplant recipient in case of a positive finding.
Fig. 1.
Scheme of natural course of clinical signs in antibody-mediated rejections. The red dot indicates the onset of clinically detectable proteinuria
There is a consensus guideline on testing and clinical management of HLA and non-HLA antibodies in transplantation, which recommends screening for DSA on a regular basis [4]. However, it describes that this decision was not unanimous and that there is a need for further research regarding “protocol biopsies at first appearance of de novo DSA to document pathologic correlation.” [4] The present study follows this research recommendation and aims to fill the gap of evidence regarding transplant recipients with DSA but without proteinuria. It describes 84 subjects after kidney or pancreas-kidney transplantation undergoing allograft biopsy after detection of de novo DSA irrespective of proteinuria and eGFR.
Methods
Study design and protocol
We performed a retrospective single center analysis including all renal transplant recipients with detection of de novo DSA at the transplant center of Ruhr University Bochum, Germany, between 2014 and 2018. Anti-HLA DSA are routinely screened once a year in our transplant center. Starting in 2016, patients were advised to undergo biopsy in case of de novo DSA irrespective of proteinuria or loss of eGFR. Analyses were performed using the LuminexR technology [5]. All anti-HLA antibodies were tested for donor-specificity and mean fluorescence intensity (MFI) levels. The lowest antibody concentration in this study was 500 MFI. Patients who tested positive for de novo DSA were encouraged to undergo biopsy of the renal allograft regardless of proteinuria and eGFR slope. The present work examines the histological findings of these biopsies including electron microscopy results, and describes the proportion of subjects with acute or chronic antibody-mediated rejection, cellular rejection, or a combination of both entities. In order to elucidate whether performing a biopsy is clinically conducive even in the absence of proteinuria, these subjects were analyzed in a predefined subgroup analysis.
Most of the biopsy specimens (89.3%) were analyzed by the same experienced histopathological institute. Diagnosis of cellular and ABMR was performed in accordance with 2013/2017 Banff criteria [6, 7]. Acute and chronic humoral rejections were summarized as “ABMR”. The center’s standard immunosuppressive regimen consisted of a calcineurin inhibitor (CNI; tacrolimus or cyclosporine), mycophenolic acid, prednisolone and induction therapy with either basiliximab or thymoglobulin.
Statistical analysis
Numeric data are presented as mean ± standard deviation or median and IQR. Data were tested for normal distribution by the Kolmogorov–Smirnov test. Numeric data of subjects with proteinuria and/or loss of eGFR were compared to subjects without proteinuria or loss of eGFR by Student’s t-test in case of normal distribution, otherwise by Mann–Whitney U test. Comparison of categorical parameters was performed by Pearson-Chi2-test. A logistic regression model was used to define the association of age, MFI of DSA, HLA class I/II antibodies, and time until detection of de novo DSA on the diagnosis of rejection in allograft biopsy analysis. p < 0.05 was regarded statistically significant. All statistical analyses were performed using SPSS Statistics 26 (SPSS Inc, Chicago, Illinois, USA) and Prism 5 (GraphPad Software, La Jolla, California, USA).
Results
In the period between 1997 and 2018, 1,878 patients received a kidney only or pancreas-kidney transplant in our center. Between 2014 and 2018 a total of 865 patients underwent screening for DSA. De novo DSA were detected in 132 (15.3%) renal or pancreas/kidney transplant recipients. One hundred twenty-eight (97.0%) of these subjects had an MFI level > 500. Eighty-four (63.6%) of the patients with de novo DSA agreed to undergo allograft biopsy irrespective of proteinuria and course of eGFR. Fifty-three (63.1%) were kidney only recipients, 36.9% (n = 31) were pancreas-kidney recipients. Among those with kidney only transplantation, the majority of subjects were transplanted after postmortal donation (66.0%), a minority (34.0%) after living donation. Among patients undergoing biopsy, 84.5% had their first renal allograft, 15.5% underwent two or more transplantations. At the time of de novo DSA detection, mean age of the transplant recipients undergoing biopsy was 52 years (IQR 44.8–57.0). The lowest antibody level of a patient having biopsy and proven ABMR was 999 MFI. Table 1 provides an overview on epidemiology, transplant data, and anti-HLA DSA findings. Table 2 provides data on the origin of end-stage renal disease (ESRD) and immunosuppression.
Table 1.
Epidemiological and transplant related data of the study population
| Overall study population | Subjects undergoing biopsy | Subjects not undergoing biopsy | P | Subjects undergoing biopsy with proteinuria > 300 mg/g creatinine and/or eGFR loss ≥ 10 ml/min in the past 12 months | Subjects undergoing biopsy with proteinuria ≤ 300 mg/g Creatinine and eGFR loss < 10 ml/min in the past 12 months | P | |
|---|---|---|---|---|---|---|---|
| General characteristics n, (%) | 132 | 84 (63.6%) | 48 (36.4%) | 50 (59.5%) | 34 (40.5%) | ||
|
Female gender n, (%) |
60 (45.5%) | 32 (38.1%) | 28 (58.3%) | 0.025 | 19 (38.0%) | 13 (38.2%) | 0.983 |
| Age at time of transplantation in years(median, IQR) |
47.5 38.0–55.25 |
46 38.0–53.25 |
50 38.0–60.0 |
0.289 |
44 37.0–52.0 |
50 43.25–55.75 |
0.043 |
| Time on dialysis in months (median, IQR) |
46 16.0–89.0 |
44 16.0–89.0 |
47 22.0–89.5 |
0.99 |
43 15.75–93.0 |
51 16.0–78.0 |
0.916 |
| Transplant characteristics | |||||||
|
Kidney only transplantation n, (%) |
84 (63.6%) | 53 (63.1%) | 31 (64.6%) | 0.895 | 30 (60.0%) | 23 (67.6%) | 0.318 |
|
Pancreas-Kidney Transplanation n, (%) |
48 (36.4%) | 31 (36.9%) | 17 (35.4%) | 0.895 | 20 (40.0%) | 11 (32.4%) | 0.318 |
|
Live donor transplantation n, (%) |
22 (26.2%) | 18 (33.9%) | 4 (12.9%) | 0.052 | 10 (20.0%) | 8 (23.5%) | 0.699 |
|
First renal transplant n, (%) |
112 (84.9%) | 71 (84.5%) | 41 (85.1%) | 0.889 | 43 (86.0%) | 28 (82.4%) | 0.642 |
|
HLA-mismatch – mean ± SD A B DR |
1.12 ± 0.65 1.31 ± 0.66 1.31 ± 0.72 |
1.17 ± 0.67 1.28 ± 0.68 1.35 ± 0.69 |
1.03 ± 0.61 1.38 ± 0.62 1.23 ± 0.76 |
0.384 0.674 0.586 |
1.22 ± 0.59 1.27 ± 0.71 1.38 ± 0.61 |
1.09 ± 0.75 1.30 ± 0.63 1.30 ± 0.80 |
0.131 0.602 0.061 |
|
DSA HLA Class I n, (%) |
45 (34.1%) |
23 (27.4%) |
22 (45.8%) |
0.717 |
11 (22.0%) |
12 (35.3%) |
0.285 |
|
DSA HLA Class II n, (%) |
66 (50.0%) |
43 (51.2%) |
23 (47.9%) |
0.031 |
28 (56.0%) |
15 (44.1%) |
0.185 |
|
DSA HLA Class I and II n, (%) |
21 (15.9%) |
18 (21.4%) |
3 (6.25%) |
0.022 |
11 (22.0%) |
7 (20.6%) |
0.877 |
| MFI (mean ± SD) | 8,478 ± 7,276 | 10,283 ± 7,339 | 5,394 ± 6,026 | < 0.001 |
10,941 ± 7,335 |
9,306 ± 7,235 |
0.258 |
| Time since transplant at DSA detection (months; median, IQR) |
55.5 12.0–100.25 |
44 3.0–94.25 |
61 36.75–123.0 |
0.016 |
63.5 3.75–119.25 |
27 3.25–71.0 |
0.073 |
| Time between detection of DSA and biopsy (months; median, IQR) |
1.0 0.0–12.5 |
1.0 0.0–12.5 |
– | – |
1.0 0.0–13.0 |
2.0 0.0–11.0 |
0.370 |
| eGFR (ml/min) at time of biopsy (mean ± SD) |
35.6 ± 18.5 |
31.7 ± 16.8 |
42 ± 19.3 |
0.001 |
25.9 ± 14.2 |
39.3 ± 16.9 |
< 0.001 |
| Proteinuria at time of biopsy (mg/g Creatinine, mean ± SD) | 510 ± 1,360 |
540 ± 850 |
440 ± 1,950 |
< 0.001 |
860 ± 1,040 |
160 ± 110 |
< 0.001 |
|
eGFR loss (ml/min) within 12 months before biopsy(mean ± SD) |
4.9 ± 9.3 |
7.0 ± 11.0 |
1.3 ± 2.9 |
0.002 |
11.8 ± 12.6 |
0.8 ± 2.1 |
< 0.001 |
Table 2.
Immunosuppression and cause of end-stage renal disease
| Immunosuppression at time of biopsy | Overall study population (n = 132) | Subjects undergoing biopsy (n = 84) | Subjects not undergoing biopsy (n = 48) | P | Subjects undergoing biopsy with proteinuria > 300 mg/g creatinine and/or eGFR loss ≥ 10 ml/min in the past 12 months (n = 50) | Subjects undergoing biopsy with proteinuria ≤ 300 mg/g creatinine and eGFR loss < 10 ml/min in the past 12 months (n = 34) | P |
|---|---|---|---|---|---|---|---|
| Triple immunosuppression | 113 (85.6%) | 74 (89.2%) | 39 (81.3%) | 0.142 | 42 (84.0%) | 32 (94.1%) | 0.320 |
| Mono/dual immunosuppression | 17 (12.9%) | 8 (9.5%) | 9 (18.4%) | 0.142 | 6 (12.0%) | 2 (5.9%) | 0.320 |
| Steroids | 124 (93.9%) | 81 (96.4%) | 43 (89.6%) | 0.042 | 47 (94.0%) | 34 (100%) | 0.397 |
| Azathioprin | 8 (6.1%) | 7 (8.5%) | 1 (2.1%) | 0.140 | 4 (8.0%) | 3 (8.8%) | 0.928 |
| Mycophenolic acid | 108 (81.8%) | 67 (79.8%) | 41 (85.4%) | 0.586 | 38 (76.0%) | 29 (85.3%) | 0.480 |
| Cyclosporine | 28 (21.2%) | 21 (25.0%) | 7 (14.6%) | 0.075 | 12 (24.0%) | 9 (26.5%) | 0.881 |
| Tacrolimus | 86 (65.2%) | 56 (66.6%) | 32 (66.7%) | 0.664 | 32 (64.0%) | 24 (70.6%) | 0.707 |
| mTOR inhibitors | 13 (9.9%) | 6 (7.1%) | 7 (14.6%) | 0.183 | 5 (10.0%) | 1 (2.9%) | 0.200 |
| Cause of end-stage renal disease | |||||||
| Nephrosclerosis | 11 (8.3%) | 9 (10.7%) | 2 (4.2%) | 0.513 | 4 (8.0%) | 5 (14.7%) | 0.564 |
| Glomerulonephritis | 33 (25.0%) | 21 (25.0%) | 12 (25.0%) | 0.792 | 13 (26.0%) | 8 (23.5%) | 0.797 |
| Polycystic kidney disease | 9 (6.8%) | 4 (4.8%) | 5 (5.9%) | 0.408 | 2 (4.0%) | 2 (5.9%) | 0.691 |
| Interstitial nephritis | 3 (2.3%) | 3 (3.6%) | 0 (0.0%) | 0.185 | 2 (4.0%) | 1 (2.9%) | 0.797 |
| Diabetic nephropathy | 48 (36.4%) | 30 (35.7%) | 18 (37.5%) | 0.864 | 19 (38.0%) | 11 (32.4%) | 0.476 |
| Alport’s Syndrome | 5 (3.8%) | 3 (3.6%) | 2 (4.2%) | 0.863 | 1 (2.0%) | 2 (5.9%) | 0.347 |
| Other | 15 (11.4%) | 9 (10.7%) | 6 (12.5%) | 0.756 | 6 (12.0%) | 3 (8.8%) | 0.797 |
| Unknown | 8 (6.1%) | 5 (5.9%) | 3 (6.3%) | 0.660 | 3 (6.0%) | 2 (5.9%) | 0.982 |
Thirty-four of 84 patients (40.5%) underwent biopsy without significant eGFR loss and/or proteinuria as defined above. Fifty (59.5%) of the patients undergoing biopsy had had an eGFR loss ≥ 10 ml/min 12 months prior to biopsy and/or proteinuria > 300 mg/g creatinine.
In subjects undergoing biopsy, de novo DSA were detected after a median of 44 months (IQR 3.0–94.25) post-transplant. The time between transplantation and detection of de novo DSA tended to be higher in subjects with proteinuria and/or deterioration of allograft function (67.5 vs. 27.5, p 0.073, Table 1), whereas the time between detection of DSA and biopsy was comparable (1.0 vs. 2.0, p 0.370, Table 1). Twenty-three (27.4%) of these antibodies corresponded to HLA class I, 51.2% (n = 43) to class II, 21.4% (n = 18) to a combination of class I and II. MFI levels ranged from 700 to 27,800 with a mean of 10,283 ± 7,339. MFI values were unavailable for 1 individual. At the time of biopsy, mean proteinuria was 540 ± 850 mg/g creatinine, mean eGFR was 31.7 ± 16.8 ml/min and mean decline of eGFR in the last 12 months was 7.0 ± 11.0 ml/min. Whereas data on quantified proteinuria at the time of biopsy were unavailable for 8 patients, and the 12-month course of eGFR was missing for 5 of the subjects undergoing indicated biopsy, the data set was complete for subjects undergoing biopsy without proteinuria or deterioration of eGFR.
The time until DSA detection differed significantly between patients, who agreed to undergo biopsy (44 months, IQR 3.0–94.25) vs. those who did not (61 months, IQR 36.75–123.0, p = 0.016). Moreover, these groups differed in mean MFI levels (10,283 ± 7,339 vs. 5,394 ± 6,026, p < 0.001), eGFR at time of biopsy (31.7 ± 16.8 vs. 42 ± 19.3 ml/min, p = 0.001), proteinuria at the time of biopsy (540 ± 850 vs. 440 ± 1950 mg/g creatinine) and the loss of eGFR in the year prior to the biopsy (7.0 ± 11.0 vs. 1.3 ± 2.9 ml/min, p = 0.002; Table 1).
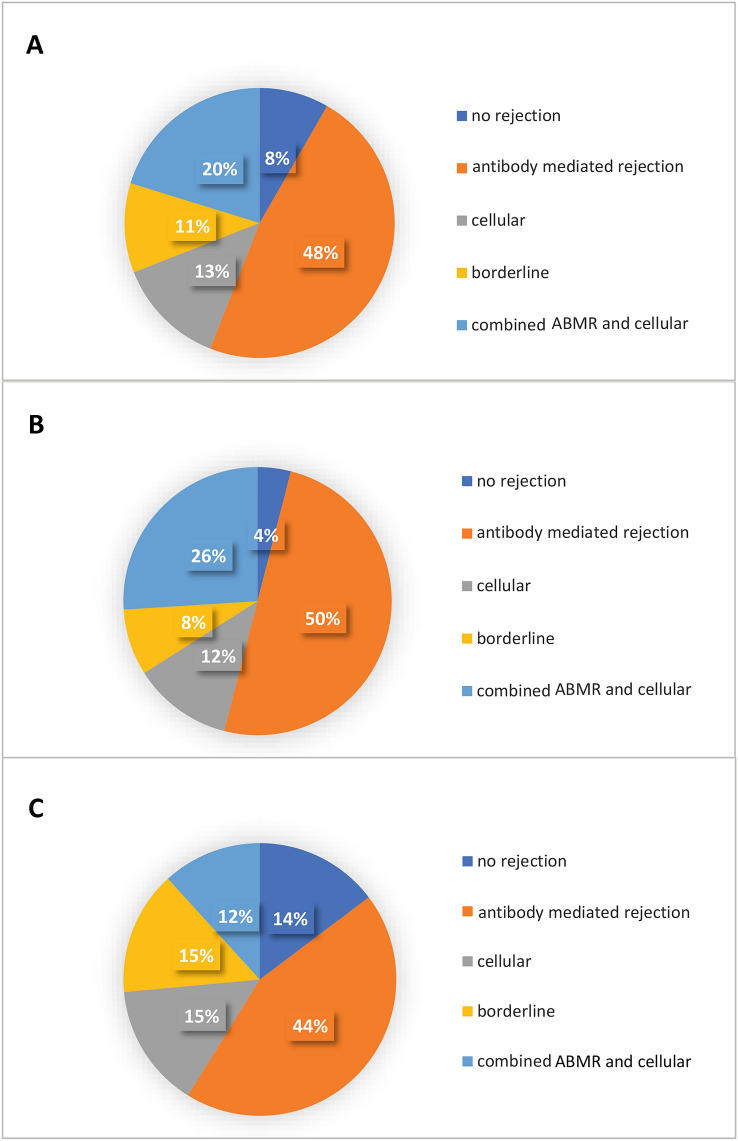
Seventy-seven of the biopsies resulted in a diagnosis of rejection, thereby, corresponding to 91.7% of the study population. There was no significant difference in rejections between the population of patients that had combined pancreas/kidney transplantation and kidney only transplantation (p = 0.971). Among patients in whom rejection occurred, ABMR criteria were met in n = 40 (47.6%) of them. Cellular rejection was found in n = 11 (13.1%), and n = 17 patients (20.2%) showed signs of both ABMR and cellular rejection. Borderline rejection was found in n = 9 patients (10.7%), while 7 patients (8.3%) had no rejection. Among subjects undergoing biopsy without proteinuria > 300 mg/g creatinine or loss of eGFR ≥ 10 ml/a (n = 34), 85.3% (n = 29) had biopsy-proven rejection (44.1% ABMR, 14.7% cellular rejection, 11.8% combined, 14.7% borderline, 14.7% no rejection). Among subjects with proteinuria and/or deterioration of eGFR, n = 48 (96.0%) had biopsy-proven rejection (50.0% ABMR, 17.7% cellular, 38.2% combined, 8.0% borderline, 4.0% no rejection). Electron microscopy was performed in 26 cases. Thirty point seven% (n = 8) showed signs of chronic transplant glomerulopathy. There was no difference in frequency of peritubular capillary basement membrane multilayering between the group with proteinuria and/or eGFR loss and the group without proteinuria and eGFR-loss (p = 0.393). The findings are summarized in Fig. 2 and Table 3. Banff lesion scores are presented in Supplemental Table 1 [8]. The prevalence of biopsy-proven rejection tended to be higher in those with proteinuria/deterioration of graft function (p = 0.08).
Fig. 2.
Biopsy findings in subjects undergoing biopsy after detection of de novo donor-specific antibodies (DSA). The figure describes the proportion of subjects with antibody-mediated rejections (ABMR), cellular rejections, borderline rejections, a combination of ABMR and cellular rejections, or exclusion of rejection in a the overall population with de novo DSA (n = 84), b those with proteinuria > 300 mg/g creatinine and/or loss of eGFR ≥ 10 ml/min in the previous 12 months (n = 50), and c those with proteinuria < 300 mg/g creatinine and loss of eGFR < 10 ml/min (n = 34)
Table 3.
Biopsy findings on rejection in transplant recipients after detection of de novo donor- specific antibodies (DSA)
| Kind of rejection | All biopsies n = 84 |
Patients with eGFR loss ≥ 10 ml/a and/or proteinuria > 300 mg/g creatinine n = 50 |
Patients with eGFR loss < 10 ml/a and proteinuria < 300 mg/g creatinine n = 34 |
p |
|---|---|---|---|---|
|
Borderline n, (%) |
9 (10.7%) |
4 (8.0%) |
5 (14.7%) |
0.329 |
|
ABMR n, (%) - acute - chronic - combined |
40 (47.6%) 26 (65.0%) 6 (15.0%) 8 (20.0%) |
25 (50.0%) 15 (60.0%) 3 (12.0%) 7 (28.0%) |
15 (44.1%) 11 (73.3%) 3 (20.0%) 1 (6.7%) |
0.596 0.237 0.602 0.066 |
|
Cellular n, (%) - Banff 1a - Banff 1b - Banff 2 |
11 (13.1%) 6 (54.55%) 3 (27.27%) 2 (18.18%) |
6 (17.7%) 5 (83.3%) 1 (16.7%) 0 (0.0%) |
5 (14.7%) 1 (20.0%) 2 (40.0%) 2 (40.0%) |
0.718 0.066 0.201 0.039 |
|
Combined cellular and ABMR |
17 (20.2%) |
13 (38.2%) |
4 (11.8%) |
0.111 |
|
No rejection n, (%) |
7 (8.3%) |
2 (4.0%) |
5 (14.7%) |
0.175 |
ABMR antibody-mediated rejection
In the logistic regression model, neither age, MFI of DSA, HLA class I/II antibodies, nor time until detection of de novo DSA predicted the overall rejections or ABMR (p > 0.05 each; Table 4).
Table 4.
Logistic regression model on the association of age, MFI of donor-specific antibodies (DSA), HLA class I/II antibodies, and time until detection of de novo DSA with the diagnosis of rejection (overall) and antibody-mediated rejection (ABMR) in allograft biopsy analysis
| N = 84 | HR (95%CI) for detection of any kind of rejection | P | HR (95%CI) for detection of ABMR | p |
|---|---|---|---|---|
| Age at time of transplantation in years | 0.996 (0.919–1.080) | 0.926 | 1.045 (0.996–1.096) | 0.070 |
| Time since transplant at DSA | 1.006 (0.989–1.023) | 0.480 | 1.001 (0.993–1.010) | 0.779 |
| MFI | 1.000 (1.000–1.000) | 0.297 | 1.000 (1.000–1.000) | 0.068 |
| HLA I | 0.965 (0.077–12.113) | 0.978 | 0.676 (0.202–2.261) | 0.525 |
| HLA II | 2.556 (0.223–29.267) | 0.450 | 1.812 (0.436–7.530) | 0.413 |
Discussion
Nowadays, screening for DSA is performed on a regular basis by many but not all transplant centers. The present data strongly support this approach. Moreover, they provide implications for the diagnostic approach after detection of de novo DSA. The majority of patients in this cohort showed histological signs of rejection irrespective of proteinuria or loss of allograft function. Interestingly, the histological findings did not only present signs of ABMR but also of rejections mediated by cellular immunity.
Reports from the last three decades consistently describe the crucial role of allograft biopsies to guide immunosuppressive medication after renal transplantation. Historical reports demonstrate that indication biopsies (biopsies due to proteinuria or deterioration of eGFR) are associated with change in immunosuppression in approximately 40.0% of the patients [9]. In contrast to indication biopsies, (surveillance) intend to detect subclinical renal pathologies including rejections, viral nephritides, recurrence of glomerular diseases, and calcineurin inhibitor-induced tissue damage. A potential prognostic benefit of performing biopsies in these cases is less well established than by indication biopsies. However, biopsies in subclinical patients doubtlessly provide prognostic information, e. g. by quantification of interstitial fibrosis and tubular injury (IF/TA) [10, 11]. The proportion of patients with a subsequent change of immunosuppressive medication is necessarily lower than in indication biopsies. The present findings show that de novo DSA constitute a valuable biomarker that selects renal transplant recipients who might benefit from a biopsy despite not having proteinuria or impaired allograft function. The presence of de novo DSA indicates a highly increased probability of pathological allograft histology with therapeutic implications.
In a retrospective French study, subclinical ABMR was detected in 41.0% of biopsies following the detection of de novo DSA [12]. These data necessitate confirmation by other transplant centers. Our data are very much in line with the findings from France: 44.0% had a histological diagnosis of ABMR without proteinuria or impairment of allograft dysfunction. In the French study, stable allograft function was defined by eGFR and covered a period of three months. Proteinuria, however, was not defined as an exclusion criterion. Our data expand the implications of the French results to renal transplant recipients with stable renal allograft function in the previous 12 months and to those without proteinuria. Thus, the present findings show that biopsy is decisive even in the absence of proteinuria, which usually precedes deterioration of GFR. A study from Wisconsin reports the histological results of 29 renal transplant recipients with DSA and stable allograft function with comparable results [13]. However, only new-onset proteinuria was defined as an exclusion criterion, whereas preexisting proteinuria was not. Interestingly, the proportion of patients with rejections was only slightly higher among subjects with proteinuria and/or loss of eGFR. ABMR occurred in 50.0% of patients with allograft dysfunction and in 44.0% without proteinuria and/or loss of eGFR.
A study from the United States reports 54 transplant patients with de novo DSA and demonstrates that the dnDSA class and sum MFI at baseline appear to be prognostic [14]. Moreover, it shows that patients without ABMR at time of detection may benefit from a follow-up biopsy within one year. It does not describe, however, a population without proteinuria.
Figure 1 schematically illustrates the natural course of ABMR. The occurrence of de novo DSA usually precedes the onset of proteinuria, which in turn precedes a deterioration of eGFR. The prognosis of ABMR, however, crucially depends on the time of diagnosis. The later the diagnosis, the more irreversible the damage in renal tissue. The present approach of performing a biopsy prior to the onset of proteinuria and allograft dysfunction may therefore improve the outcome of these patients. To this end, the interval of DSA screening may be critically discussed. As mentioned above, the interval was 12 months in the present cohort. De novo DSA frequently develop after the first month post-transplant with an average time of onset of 4–5 years [15–17]. With regard to the high percentage of patients with rejections, it may be wise to shorten the DSA screening interval in the early period after transplantation. Six patients had MFI levels of 500–999, of whom two had had a transplant biopsy. None of these patients developed histological signs of rejection indicating a potentially lower immunological risk with very low DSA concentrations. This finding is in line with current recommendations to regard an MFI of 1000 as the cut-off for further diagnostic investigations [18].
The time between transplantation and detection of DSA tended to be higher in subjects with proteinuria and/or deterioration of GFR, whereas there was no difference in the time between detection of DSA and biopsy. Thus, a delay in biopsy cannot explain the finding that histological signs of rejections occur in a comparable frequency in subjects with and without an impairment of allograft function.
Interestingly, several patients did not show signs of ABMR but of acute cellular rejections. This finding is somewhat surprising with regard to the median time of 57 months after transplantation. Acute cellular rejections usually occur in the first year post-transplant. However, a high coincidence of both entities was reported in heart transplant recipients as well [19]. De novo DSA may be regarded as a biomarker of “underimmunosuppression”, which not only increases the risk of ABMR but also of cellular rejections. In our study population the coincidence of signs of cellular and antibody-mediated rejections occurred in 8.0% of the overall study population and in 14.0% of subjects without proteinuria and allograft dysfunction.
Fifteen% of ABMR met the histological signs of chronicity, whereas 65.0% were regarded as acute (20.0% combined). Concerning cellular rejections, the most prevalent Banff stage after borderline-rejection was Ia. Neither acute or chronic antibody-mediated rejections, nor the individual categories of cellular rejection occurred significantly more often in subjects with impaired allograft function than in those without it. Thus, the findings on individual rejection categories widely correspond to the overall findings in this population. In patients with subclinical ABMR, the timespan between transplantation and DSA detection was far shorter than in those with graft dysfunction. This finding is likely to be explained by the fact that screening for DSA was introduced in the routine post-transplant management in this center in 2014. Thus, patients who underwent transplant before 2014 had a higher probability of developing graft dysfunction prior to detection of DSA.
The logistic regression model did not detect a predictive value of age, MFI, HLA class I/II antibodies, and time until detection of de novo DSA on the diagnosis of rejection in allograft biopsy. Thus, obtaining a biopsy may be wise in all patients with de novo DSA and not only in a subgroup of them. HLA class II mismatch and younger age (e.g. due to noncompliance) have been described as risk factors for ABMR [20]. These are actually risk factors for the genesis of de novo DSA. Once DSA have been established—like in the present study population—the risk of developing ABMR is obviously independent of these parameters.
Our study is limited by its single-center character and the study size. To the best of our knowledge, however, it constitutes the first analysis of biopsy findings in renal transplant recipients with de novo DSA who have neither deterioration of allograft function, nor proteinuria. Moreover, the definition of < 10 ml eGFR loss in the previous 12 months as “stable renal allografts” encompasses patients with milder allograft dysfunctions. We decided for this value, however, due to the high frequency of unspecific prerenal changes in serum creatinine concentrations after kidney transplantation.
In conclusion, the present study shows that the majority of patients with de novo DSA present signs of rejection in allograft biopsies, even in the absence of proteinuria or eGFR loss. Since the prognosis of rejection therapy crucially depends on early diagnosis, it appears reasonable to perform an allograft biopsy after the detection of de novo DSA, irrespective of proteinuria or eGFR loss.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Mrs. Hajt for keeping the databases up to date and providing us with the data this study is based on.
Abbreviations
- ABMR
Antibody-mediated rejection
- DSA
Donor-specific antibodies
- eGFR
Estimated glomerular filtration rate
- HLA
Human leukocyte antigen
- IQR
Interquartile range
- MFI
Median fluorescence intensity
- PCR
Proteinuria creatinine ratio
Author contributions
CBW participated in research design, writing of the paper, data analysis, performance of the research, data collection, PZ participated in data collection, FSS participated in data analysis, SG participated in data collection, PS participated in data collection, Frederic Bauer participated in data analysis, BR participated in data analysis, RV participated in design of the study, NB participated in revising the manuscript, TW participated in research design, writing of the paper, data analysis, performance of the research.
Funding
Open Access funding enabled and organized by Projekt DEAL. No funding was received for conducting this study.
Code availability
Software application (SPSS, Excel).
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical statement
There was no ethical conflict, either.
Availability for data and material
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Garg N, Parajuli S, Mandelbrot DA, et al. Donor-specific antibodies in kidney transplantation: the University of Wisconsin experience. Curr Opin Organ Transpl. 2020;25:543–548. doi: 10.1097/MOT.0000000000000814. [DOI] [PubMed] [Google Scholar]
- 2.Lachmann N, Schönemann C. Tracing clinically relevant HLA antibodies prior to kidney transplantation: commentary on "Pre-transplant HLA antibodies detected by single antigen bead assay are a risk factor for long-term kidney graft loss even in the absence of donor specific antibodies". Transpl Int. 2016;29:985–987. doi: 10.1111/tri.12796. [DOI] [PubMed] [Google Scholar]
- 3.(1997) Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet 349:1857–1863 [PubMed]
- 4.Tait BD, Süsal C, Gebel HM, et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation. 2013;95:19–47. doi: 10.1097/TP.0b013e31827a19cc. [DOI] [PubMed] [Google Scholar]
- 5.Tait BD, Hudson F, Cantwell L, et al. Review article: Luminex technology for HLA antibody detection in organ transplantation. Nephrology (Carlton) 2009;14:247–254. doi: 10.1111/j.1440-1797.2008.01074.x. [DOI] [PubMed] [Google Scholar]
- 6.Haas M, Loupy A, Lefaucheur C, et al. The Banff 2017 Kidney Meeting Report: revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transpl. 2018;18:293–307. doi: 10.1111/ajt.14625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transpl. 2014;14:272–283. doi: 10.1111/ajt.12590. [DOI] [PubMed] [Google Scholar]
- 8.Roufosse C, Simmonds N, Clahsen-van Groningen M, et al. A 2018 reference guide to the Banff classification of renal allograft pathology. Transplantation. 2018;102:1795–1814. doi: 10.1097/TP.0000000000002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pascual M, Vallhonrat H, Cosimi AB, et al. The clinical usefulness of the renal allograft biopsy in the cyclosporine era: a prospective study. Transplantation. 1999;67:737–741. doi: 10.1097/00007890-199903150-00016. [DOI] [PubMed] [Google Scholar]
- 10.Serón D, Moreso F. Protocol biopsies in renal transplantation: prognostic value of structural monitoring. Kidney Int. 2007;72:690–697. doi: 10.1038/sj.ki.5002396. [DOI] [PubMed] [Google Scholar]
- 11.Nankivell BJ, Shingde M, Keung KL, et al. The causes, significance and consequences of inflammatory fibrosis in kidney transplantation: the Banff i-IFTA lesion. Am J Transpl. 2018;18:364–376. doi: 10.1111/ajt.14609. [DOI] [PubMed] [Google Scholar]
- 12.Bertrand D, Gatault P, Jauréguy M, et al. Protocol biopsies in patients with subclinical de novo donor-specific antibodies after kidney transplantation: a multicentric study. Transplantation. 2020;104:1726–1737. doi: 10.1097/TP.0000000000003055. [DOI] [PubMed] [Google Scholar]
- 13.Parajuli S, Reville PK, Ellis TM, et al. Utility of protocol kidney biopsies for de novo donor-specific antibodies. Am J Transpl. 2017;17:3210–3218. doi: 10.1111/ajt.14466. [DOI] [PubMed] [Google Scholar]
- 14.Schinstock CA, Cosio F, Cheungpasitporn W, et al. The value of protocol biopsies to identify patients with de novo donor-specific antibody at high risk for allograft loss. Am J Transpl. 2017;17:1574–1584. doi: 10.1111/ajt.14161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everly MJ, Rebellato LM, Haisch CE, et al. Incidence and impact of de novo donor-specific alloantibody in primary renal allografts. Transplantation. 2013;95:410–417. doi: 10.1097/TP.0b013e31827d62e3. [DOI] [PubMed] [Google Scholar]
- 16.Liefeldt L, Brakemeier S, Glander P, et al. Donor-specific HLA antibodies in a cohort comparing everolimus with cyclosporine after kidney transplantation. Am J Transpl. 2012;12:1192–1198. doi: 10.1111/j.1600-6143.2011.03961.x. [DOI] [PubMed] [Google Scholar]
- 17.Wiebe C, Gibson IW, Blydt-Hansen TD, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transpl. 2012;12:1157–1167. doi: 10.1111/j.1600-6143.2012.04013.x. [DOI] [PubMed] [Google Scholar]
- 18.Schinstock CA, Mannon RB, Budde K, et al. Recommended treatment for antibody-mediated rejection after kidney transplantation: the 2019 expert consensus from the transplantion society working group. Transplantation. 2020;104:911–922. doi: 10.1097/TP.0000000000003095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zakliczyński M, Nożyński J, Konecka-Mrówka D, et al. Coincidence of cellular and antibody mediated rejection in heart transplant recipients—preliminary report. Kardiochir Torakochirurgia Pol. 2014;11:52–55. doi: 10.5114/kitp.2014.41932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nickerson PW. What have we learned about how to prevent and treat antibody-mediated rejection in kidney transplantation? Am J Transpl. 2020;20(Suppl 4):12–22. doi: 10.1111/ajt.15859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Software application (SPSS, Excel).