Abstract
The p21-activated kinase PAK is targeted to focal complexes (FCs) through interactions with the SH3 domains of the PAK-interacting exchange factor PIX and Nck. PIX is a Rac GTP exchange factor that also binds the G-protein-coupled receptor kinase-interacting protein known as GIT1. Overexpression of GIT1 in fibroblasts or epithelial cells causes a loss of paxillin from FCs and stimulates cell motility. This is due to the direct interaction of a C-terminal 125-residue domain of GIT1 with paxillin, under the regulation of PIX. In its activated state, GIT1 can promote FC disassembly independent of actin-myosin contractile events. Additionally, GIT directly couples to a key component of FCs, focal adhesion kinase (FAK), via a conserved Spa2 homology domain. We propose that GIT1 and FAK cooperate to promote motility both by directly regulating focal complex dynamics and by the activation of Rac.
PAKs are a family of kinases activated on interacting with Cdc42 and Rac GTPases (3, 21). PAK associates in Drosophila with phosphotyrosine-rich cytoskeletal structures (10) and in cultured cells with focal complexes (FCs) which represent sites of attachment with the substratum (20). PAK association with FCs is independent of the Cdc42/Rac1-binding domain. Constitutively active PAK promotes FC disassembly (20) which can be blocked by the inhibitory PAK83-149 fragment (41). Cells expressing kinase-deficient PAK exhibit more stable FCs and increased formation of Rac-dependent lamellipodia (26). Rac1 activation can occur through the ubiquitous PAK-interacting exchange factor PIX, which specifically binds PAKs (1, 22). PAK plays an essential role in axonal guidance in Drosophila, achieved through its binding via the second SH3 domain of Nck (13). PAK binding to PIX through a unique SH3 interaction plays an important role in coordinating the activation of Cdc42 and Rac1 in a phosphatidylinositol 3-kinase (PI-3-(kinase)-dependent manner (40). It is an association with PIX that allows PAK to localize to FCs (22), although this interaction is negatively regulated by PAK activation and autophosphorylation (42).
Recently PIX has been shown to interact with a family of proteins first designated G-protein-coupled receptor kinase (GRK)-interacting targets (GIT [28]); these proteins contain at their N termini a domain that can function as an Arf GTPase-activating protein (GAP). This family of tyrosine-phosphorylated ∼90-kDa proteins can also bind paxillin (2, 37), indicating that PAK might be linked to FCs through this interaction. Interestingly, a close link between Arfs and FCs is indicated by the observation that the related Arf GAP ASAP (29) and PAG3 (16) both localize to FCs and can promote their turnover.
FCs are integrin-dependent sites linking the extracellular matrix (ECM) to the actin-rich cytoskeleton. Their formation is influenced particularly by RhoA, Cdc42, and Rac1 (24). RhoA induces the formation of steady-state FCs (or focal adhesions) which allow cultured cells to remain attached to the ECM. RhoA- and Cdc42-induced FCs require action of RhoA-binding ROK and Cdc42-binding MRCK (myotonin kinase-related Cdc42-binding kinase) action, respectively (17, 18). These kinases act through stimulating actin-myosin contractility, which promotes clustering of integrins (32).
Integrins form the transmembrane link to several FC proteins, thereby directly or indirectly recruiting vinculin, talin, α-actinin, focal adhesion kinase (FAK), p130Cas, and paxillin (reviewed by Hemler [11]). FAK was originally proposed to be essential for FC assembly; however, studies on FAK−/− cell lines show that FAK serves to facilitate FC turnover and promote motility (15). Most cultured cells exhibit migratory behavior which involves a balance of integrin-mediated adhesion at the leading edge and internally with integrin disengagement at the rear of the cell (23). Without FC breakdown, the adhesive contacts between the ECM and the cell are too strong to allow migration (33).
In this study we have investigated the structure and function of the PIX-associated GIT1. GIT1 has an activity which can promote FC disassembly through a mechanism that is PIX dependent and requires the C-terminal paxillin-binding domain. Interestingly, FAK contains a functionally similar C terminus which targets the same LD motif in paxillin. We find that GIT1 directly associates with FAK. Thus, GIT1 links FAK to PAK signaling, with both kinases apparently playing an important role in orchestrating FC dynamics which underlie cell motility.
MATERIALS AND METHODS
Antibodies.
Anti-glutathione S-transferase (GST) (polyclonal), antivinculin hVIN-1, and anti-Flag M2 monoclonal antibodies (MAbs) and M2-Sepharose were from Sigma. Anti-HA MAb was from Roche, antipaxillin was from Transduction Laboratories, rabbit anti-green fluorescent protein (GFP) from Clontech, and antiphosphotyrosine (MAb 4G10) was from Upstate Biotechnology. Rabbit polyclonal anti-GIT1 generated by injection of GST/GIT1646-770 and was purified using CNBr-coupled GST-GIT1. For immunofluorescence, we used MAbs at 5 to 10 μg/ml and polyclonal antibodies at 1:40.
Bacterial expression vectors.
The pGEX-Ras vector was derived from GEX4T-1 as follows. The Ras1-185 coding sequence was amplified (Vent, New England Biolabs) to include a BglII site adjacent to codon 1 and at the 3′ (adjacent to codon 185) a BamHI-EcoRI linker (GGATCCCCGAATTC). This BglII/EcoRI fragment was cloned into the pGEX-4T BamHI/EcoRI site, regenerating a BamHI site downstream of the Ras sequence (the new polylinker is identical to that in pGEX4T-3). pGEX-Ras/PIX496-554 was constructed by cloning the PCR-amplified GIT1-binding domain from βPIX (see Fig. 3A) into the pGEX-Ras vector at BamHI-XhoI sites. pGEX-Ras/GIT1646-770 was constructed by cloning the SmaI-NotI fragment from pGEX/GIT1 (see below) into the SmaI-NotI sites of pGEX-Ras.
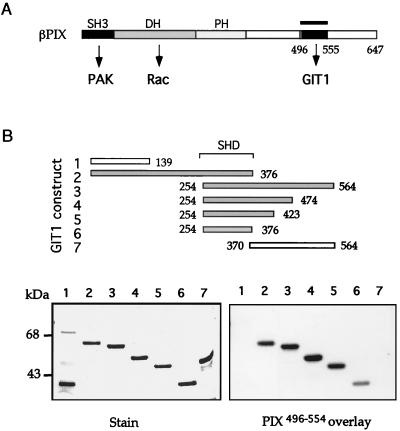
FIG. 3.
βPIX binds to GIT1 via the Spa2-related sequence. (A) The structural domains of βPIX include its N-terminal SH3 domain, which binds PAK, a catalytic Dbl homology (DH) region promoting nucleotide exchange on Rac1, and a C-terminal region (bar) which was used to bind GIT1 (B) by overlay. (B) GST fusion proteins corresponding to constructs 1 to 7 of GIT1 were separated on a 9% polyacrylamide gel and overlaid with PIX496-554 to localize the binding site. All positive binding constructs contain SHD-1 of GIT1254-376.
The GST-GIT1 full-length expression plasmid was constructed by amplifying a cDNA fragment encoding GIT1 residues 1 to 139 into the BamHI-EcoRI site of pGEX4T-1; the HindIII-EcoRI fragment corresponding to GIT1 residues 119 to 770 was derived from pBluescript SK-GIT1. GIT1 subclones were derived from restriction fragments as follows: GIT11-139 and GIT11-376 were derived from BamHI-XhoI cDNA fragments. GIT1254-564, GIT1254-474, GIT1254-423, GIT1254-376, and GIT1370-564 were derived from amplified cDNA using 5′ BamHI and 3′ EcoRI linkers. pFlag/FAK was constructed by moving the BamHI (filled site)-BglII fragment from pXJ-GST/FAK into the SmaI/BglII site of the pFlag.MAC vector (Kodak IBI). Full-length paxillin derived from human cDNA by PCR using 5′ HindIII and 3′ XhoI linkers for cloning. Paxillin250-281 (LD4) was derived by PCR using 5′ BamHI and 3′ XhoI linkers.
Mammalian expression vectors.
The pXJ-HA (hemagglutinin epitope), pXJ-Flag, and pXJ-GST mammalian vectors used for transfection and microinjection are as described elsewhere (20, 41). The pXJ-GFP vector was derived from pXJ-Flag by replacing the N-terminal tag with the GFPS65T cDNA. The pXJ-GFP-Flag derivative was constructed using a restriction-compatible BglII-EDYKDDDDK-BamHI-XhoI sequence inserted into pXJ-GFP. The pXJ-Myr vector for expression of membrane-targeted proteins was constructed using sequence corresponding to the N-terminal myristoylation signal of Fyn fused to the Flag epitope. The corresponding 18-amino-acid sequence is MGCVQCKDKEDYKDDDDK, which was cloned using a 5′ EcoRI/Kozak linker (GAATTCACC) with a 3′ BamHI site.
The pXJ-GST/FAK expression plasmid was constructed by synthesizing residues 1 to 30 of chicken FAK as a BamHI-XhoI fragment in pXJ-GST then a SmaI-XhoI fragment (encoding FAK residues 30 to 1153) derived from a cDNA. Plasmids pXJ-GST/FAK-N′ (FAK1-427), pXJ-GST/FRNK (FAK693-1053), and pXJ-GST/FAT (FAK916-1053) were derived from the PCR-amplified cDNA fragments with BamHI/XhoI sites in the primers. pXJ-Flag/GIT1 and pXJ-myr/GIT1 were constructed by subcloning a BamHI/NotI fragment from pGEX/GIT1. pXJ-Flag/GIT11-253 was then derived by deleting the BglII-BglII fragment. GIT1253-424 represents the BglII-BglII fragment from pGEX-GIT1 cloned in the BamHI site of the vector. pXJ-Flag/GIT1424-770 (BglII-NotI) was constructed by PCR. PXJ-Flagmyr/GIT11-376 was derived from the BamHI-XhoI (partial digestion) fragment from pGEX-GIT1. PXJ-myr/GIT11-645 was derived from a BamHI-SmaI fragment. PXJ-myr/GIT1646-770 was derived from the SmaI-NotI fragment from pGEX/GIT1. PXJ-myr/GIT1424-770 was constructed by PCR. The GIT1 subclones in pXJ-GST and pXJ-myr vectors were constructed in the same way. pXJ-Flag/βPIX was described previously (22).
Overlays with [γ-32P]GTP-labeled proteins.
Proteins were separated on sodium dodecyl sulfate–10% polyacrylamide gels and transferred to polyvinylidene difluoride (NEN) membranes. The overlay protocol using radiolabeled GST/Ras-[γ-32P]GTP fusion proteins is essentially as described previously (20).
Cell migration assays.
COS-7 cells (35-mm-diameter dish, 70% confluence) were transiently transfected with various pXJ-Flag DNAs (2 μg of each), using the calcium phosphate protocol. Controls used pXJ-Flag/GFP (soluble) or pXJ-myr, which gave the same values (∼10% migration). Following overnight incubation in serum-free medium, haptotaxis was assayed using modified Boyden chambers (ChemTX; NeuroProbe Inc.) with a polycarbonate membrane (10-μm thickness, 8-μm pores). The membrane was precoated on the underside with fibronectin (20 μg/ml; Sigma) and collagen type I (10 μg/ml; Roche) in phosphate-buffered saline for 2 h at 37°C. The lower chamber contained serum-free Dulbecco modified Eagle medium with fibronectin (20 μg/ml) and collagen (10 μg/ml). Serum-starved transfected cells were suspended with 0.01% trypsin–5 mM EDTA–25 mM HEPES (pH 7.2). After two washes in serum-free culture medium, cells were resuspended at ∼1.25 × 105/ml. An equivalent of 2,500 cells was added on top of each well (6-mm diameter) and allowed to migrate to the underside of the membrane for 4 h at 37°C in 5% CO2. Each assay was performed in quadruplicate. Nonmigrating cells on the upper membrane surface were removed with a rubber scraper, and migratory cells attached to the bottom surface were fixed with 3% paraformaldehyde (20 min). After immunostaining for anti-Flag (50 μg/ml), transfected cells in each well were counted. Numbers of transfected cells (total) for each plasmid transfection were determined by dilution into medium plus 10% serum and plating on 15-well slides (in triplicate). Both flattened and rounded positive cells were counted. Migration was expressed as a percentage of the level for total expressing cells. Each determination represents the average of at least three individual wells, and error bars represent the standard deviation for three experiments.
Cell microinjection, transfection, and staining.
Subconfluent HeLa cells were microinjected into the nucleus with each expression plasmid DNA (50 ng/μl) using an Eppendorf microinjector (41). After 2 to 4 h, cells were fixed in 3% paraformaldehyde for 20 min and stained as described previously (20).
RESULTS
Identification of GIT1 in PAK complexes.
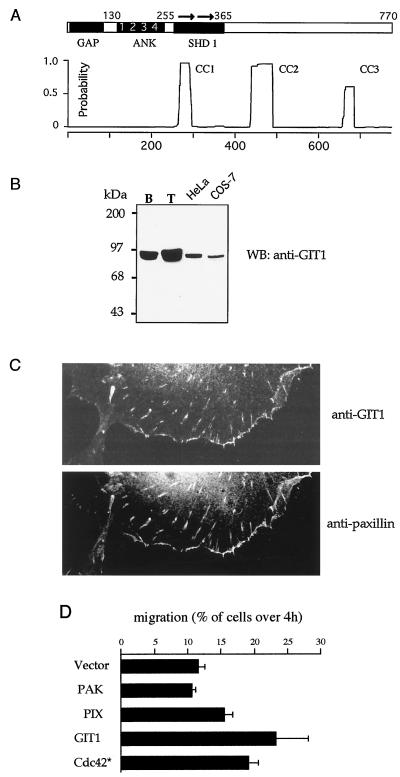
We previously identified PIX proteins binding the N-terminal regulatory region of αPAK and also a copurifying p90 species (22). This testis-enriched p90 becomes tyrosine phosphorylated when the complex is incubated with ATP (data not shown), indicating that protein tyrosine kinases are present. Peptides derived from p90 were used to isolate a rat cDNA which encoded a 770-residue protein identical with GIT1 (28) and highly related to PKL (37) and Cat (2). In addition to the primary structural features already described, namely, a cysteine-rich Arf GAP domain and four ankyrin repeats, we noted two novel features (Fig. 1A): (i) a region closely related to the yeast Spa2 homology domain 1 (SHD-1) and (ii) three regions predicted to form coiled-coil structures, potential sites of protein-protein interaction.
FIG. 1.
GIT1 expression stimulates cell motility. (A) Domain structure of GIT1. Regions with homology to recognized domains include the Arf GAP domain (GAP); a domain containing four ankyrin (ANK) repeats, and the direct repeat (arrows) comprising SHD-1. Three regions with coiled-coil potential include the conserved paxillin binding site at the C terminus. Residue numbers at the boundaries are marked. (B) GIT1 is present in cultured cells in FCs. Affinity-purified rabbit antibodies against GIT1646-770 identify a protein enriched in the testis, which is present as a single species in HeLa and COS-7 cells. B, brain; T, testis; WB, Western blot. (C) GIT1 localizes to FCs in COS-7 cells. Antipaxillin and anti-GIT1 identify punctate FCs in the center and at the periphery of the cells. (C) GIT1 is an efficient stimulator of cell motility. COS-7 cells were transfected with Flag-tagged expression constructs as shown and analyzed for migration through 8-μm membrane pores over 4 h under a soluble fibronectin-collagen gradient (see Materials and Methods).
GIT1 localizes to focal adhesions and stimulates motility.
Antibodies raised against the C-terminal 125 residues of GIT1 recognized p90 in all cell lines and tissues tested, with highest levels in testis (Fig. 1B). In cultured cells, the protein was present in FCs as previously observed with PIX (22). We noted that GFP/GIT1-expressing NIH 3T3 cells were more motile when viewed by time-lapse microscopy (data not shown). COS-7 cells contain endogenous GIT1 which colocalized to focal adhesions with paxillin (Fig. 1C). These cells were used to assay for haptotaxis using a modified Boyden chamber under a fibronectin-collagen gradient. The individual components in the Cdc42/PAK pathway were tested, with transfection normalized for each by counting total positively stained cells for the same total number (see Materials and Methods). GIT1 promoted cell motility as effectively as Cdc42G12V and was more potent than either PAK or PIX (Fig. 1D). Cdc42G12V both activates Rac and promotes loss of Rho-type FCs (20, 24).
GIT1 can stimulate FC disassembly.
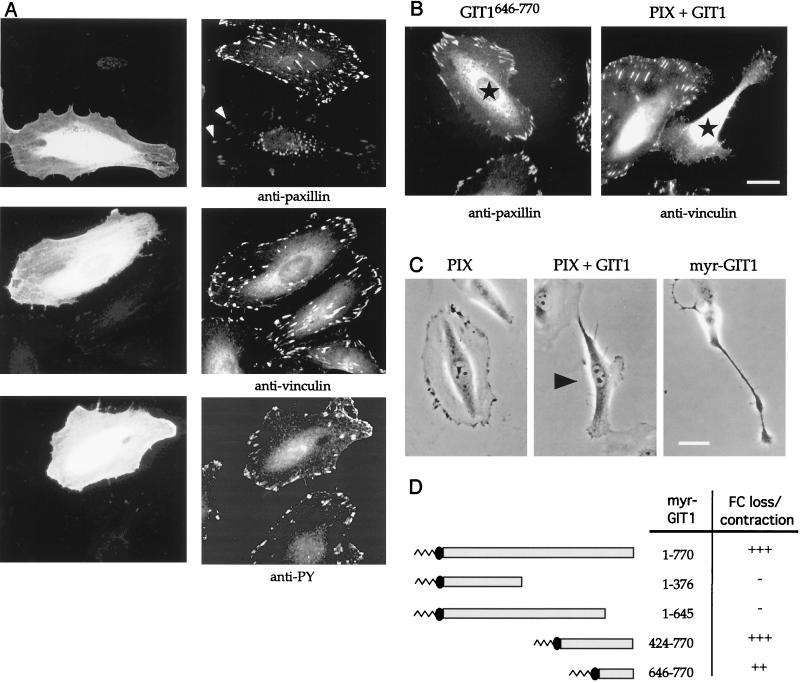
To investigate further the early responses to GIT1 expression, we microinjected HeLa cells, a protocol that has previously uncovered functions of Rho p21s and their targets such as PAK, ROK, and MRCK. After plasmid injection, GIT1-expressing cells exhibited no gross morphological alteration within 2 to 4 h; however, we noted a severe reduction in paxillin staining of FCs (Fig. 2A, top row) but not of other components including vinculin and phosphotyrosine (PY) or two other markers, talin and FAK (data not shown). To test that this was not due to epitope masking of paxillin by GIT, the paxillin-binding GIT C-terminal region was overexpressed (Fig. 2B, left); no loss of antipaxillin FC staining was observed under these conditions.
FIG. 2.
GIT1 promotes FC disassembly. (A) GIT1 expression causes the selective loss of paxillin from FCs which are faintly visible (arrows), while vinculin and phosphotyrosine (PY) levels are unaffected. The anti-Flag immunofluorescent photomicrographs of typical HeLa cells 4 h after injection are compared with images of uninjected controls. (B) The paxillin-binding domain GIT646-770 (left) does not affect antipaxillin staining. Coinjection of PIX and GIT1 (right) leads to focal adhesion loss, as assessed by antivinculin staining, and cell contraction. Injected cells are marked with stars. Bar, 10 μm. (C) Phase-contrast micrographs of cells showing induction of peripheral retraction. βPIX-expressing cells have small phase-dark ruffles, but coinjection of PIX plus GIT1 causes cell retraction (arrowhead). Membrane-tethered myrGIT1 similarly caused both cell body contraction and lamellipodia, driving neurite-like processes. Typical phenotypes were photographed 2 h after injection. (D) A C-terminal domain in GIT1 drives FC loss and cell contraction. The membrane-tethered constructs as shown were scored for cell contraction (B). The smallest myristoylated construct (646-770) was less efficient (++) in this regard. In each case, at least 15 microinjected cells were observed over a 3-h period.
In contrast to the effect of PIX or GIT1 alone, coinjection of expression plasmids led to loss of focal adhesions as assessed by antivinculin staining (Fig. 2B, right), causing a collapse of the cells (Fig. 2C). This phenotype superficially resembles that induced by active forms of PAK (20) or dominant inhibitory ROK (17), but we noted that PIX plus GIT1 did not cause any actin stress fiber loss. At early time points we also observed reduction in paxillin staining in FCs, suggesting that this is a primary event that then leads to FC loss. Targeting GIT1 to the membrane using an N-terminal myristoylation signal from Fyn (myrGIT1) led to similar cell contraction (independent of PIX expression). Time-lapse analysis also revealed a counteracting activity which often produced cells with neurite-like outgrowths (Fig. 2C, right). By comparison, myrβPIX-expressing cells were phenotypically normal (not shown). The myrGIT1 effects cannot be ascribed to the membrane anchor or indirectly to PIX recruitment to the plasma membrane.
Various regions of GIT1 were cloned into the pXJ-myr vector and tested for the ability to cause FC loss and cell body collapse, as summarized in Fig. 2D. The FC disassembly activity, typified by loss of vinculin staining with myrGIT1, was localized to the C-terminal 125 residues of GIT1. It seemed that PIX potentiated the activity of this C-terminal region when introduced with full-length GIT1, thereby promoting FC loss and cell contraction.
The GIT1 SHD-1 binds PIX.
To determine if these effects of GIT1 on FCs in vivo could be understood in terms of PIX regulation of the GIT C terminus, we localized the site of PIX binding. PIX496-554 (Fig. 3A) was used in overlay assays on various GIT1 fragments expressed as GST fusion proteins. Figure 3B shows that the fragments containing the SHD-1 bound PIX, but neither the ankyrin repeat nor central coiled-coil region (Fig. 1A) was essential. Sequences between positions 376 and 423 apparently enhanced this binding (Fig. 3B, compare lanes 5 and 6). Interestingly, only GIT1 and Spa2 family members contain SHD-1. The yeast SHD-1 covering ∼150 residues at the N terminus has recently been shown to bind to Mkk1p, Mkk2p, and Ste7p (34). Comparison of Mkk1p(1-197) with Mkk2p revealed a single region of similarity which is weakly related to the PIX GIT1-binding domain (not shown).
We concluded that the ability of GIT1 to cause FC disassembly (through residues 646 to 770) is not due to recruitment of PIX-PAK complexes. The coinjection experiments indicated, however, that PIX was able to modulate this activity, either through localization of GIT1 or by altering its conformation.
Regulated binding of GIT1 to paxillin.
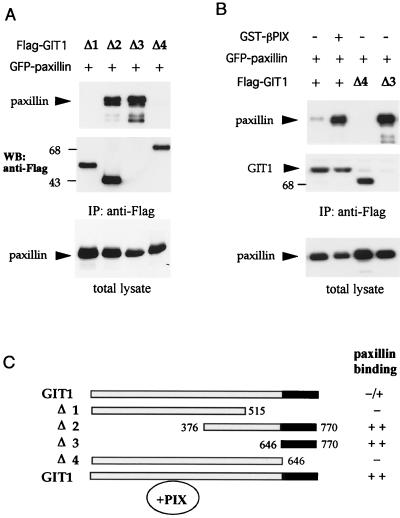
It has recently been reported that a region of paxillin (LD4 motif) binds to GIT1-like proteins, through a C-terminal region of PKL (37). By cotransfection of fragments of GIT1 (Fig. 4C) with paxillin, we localized the C-terminal paxillin-binding domain of GIT1 further (Fig. 4A). The C-terminal 125-residue GIT1646-770 bound paxillin as efficiently as the larger GIT1376-770 fragment; functional constructs smaller than this could not be derived (data not shown). Significantly, full-length GIT1 is a poor paxillin binder, but upon cotransfection with βPIX there was a dramatic increase in paxillin binding (Fig. 4B, compare lanes 1 and 2). Thus, the paxillin-binding domain of GIT1 appears masked and is regulated by PIX. When GIT1 and PIX were cotransfected into cells, the potentiation of FC loss appeared to result from an increased affinity for paxillin.
FIG. 4.
GIT1 association with paxillin LD4 is regulated by PIX binding. (A) Paxillin is immunoprecipitated with C-terminal constructs of GIT1. COS-7 cells were transfected with expression vectors containing Flag-tagged ΔGIT1 constructs depicted in panel C and GFP-paxillin. Western blots (WB) of anti-Flag immunoprecipitates or total lysates (50 μg per lane) were probed by antipaxillin. (The smallest Δ3 GIT1 [residues 646 to 770] is detected in separate gels but not shown here.) (B) Coexpression of PIX potentiates the ability of GIT1 to associate with paxillin. GIT1 or Δ4 and Δ3 represent cotransfection. A significant increase of paxillin (top) associated with Flag-GIT1 immunoprecipitates (IP) (middle) was observed when PIX was also present. Total GFP-paxillin expression is shown at the bottom. (C) Summary of constructs used and their ability to bind paxillin via the conserved C-terminal region (black).
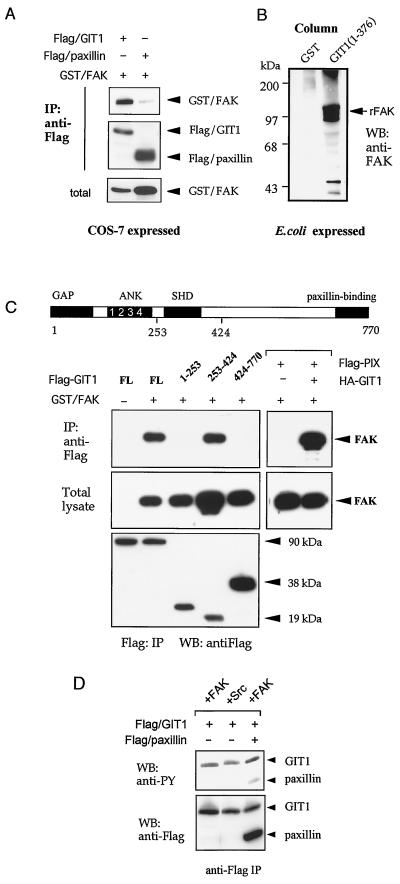
GIT1 can interact directly with FAK.
The localization of GIT1 to focal adhesions suggested that FAK might be the tyrosine kinase that copurifies with PIX-GIT1 complexes (22). It has been reported that GIT1 is a FAK substrate whose phosphorylation is up-regulated upon integrin ligation (2). To test whether FAK might be indirectly recruited to PIX-GIT1 complexes by paxillin, we examined GIT1 or paxillin immunoprecipitates for the presence FAK (Fig. 5A). Surprisingly, GIT1 could bind a far greater proportion of FAK than paxillin (compare bottom and top panels), suggesting that FAK directly interacts with GIT1. This was confirmed using GST-GIT11-376 and recombinant (Escherichia coli-expressed) FAK (Fig. 5B). The FAK-binding region was further determined by cotransfecting FAK separately with three fragments of GIT1 in COS-7 cells (Fig. 5C). Only GIT1253-424 (containing SHD-1) was capable of binding FAK (in the absence of the paxillin-binding domain). To test whether a trimeric complex of PIX, GIT1, and FAK could be formed, Flag-PIX was used to immunoprecipitate FAK in the presence or absence of HA-GIT1 (Fig. 5C, right panel). Since GIT1 homodimers were not detected when we cotransfected different tagged forms of full-length GIT1 (data not shown), we conclude that the SHD-1 region can simultaneously bind FAK and PIX.
FIG. 5.
GIT1 directly binds FAK through SHD-1. (A) Flag-GIT1 or Flag-paxillin was coexpressed with GST-FAK, and the anti-Flag immunoprecipitates (IP) were analyzed for the presence of FAK. Little FAK was detected in paxillin immunoprecipitates compared to the amount in the total lysate. (B) Full-length FAK was expressed from pFlag-FAK vector. E. coli cell lysates (300 μl, 8 mg/ml) were passed through a 30-μl column of glutathione-Sepharose containing 2 mg of GST or GST/GIT11-376 per ml. rFak, recombinant FAK. (C) FAK and PIX can both associate with the GIT1 SHD-1. Flag-GIT1 constructs which were coexpressed with GST-FAK in COS-7 cells cover the entire GIT1 protein as shown schematically (domains as in Fig. 1). The Flag-tagged immunoprecipitates (lower panel) were used to detect the presence of FAK. The right panels show that Flag-PIX was able to immunoprecipitate FAK only in the presence of HA-GIT1. (D) FAK or Src was cotransfected with GIT1 and/or paxillin and analyzed for phosphotyrosine (PY) content. The amount of phosphotyrosine relative to anti-Flag signal indicates the efficiency with which GIT1 and paxillin are substrates for FAK (right lane).
GIT1, which is phosphorylated on three sites (data not shown), also served as a more efficient FAK substrate than paxillin, as judged from the relative levels of tyrosine phosphorylation after appropriate cotransfection (Fig. 5D). GIT1 was also found to be a substrate for the Src kinase.
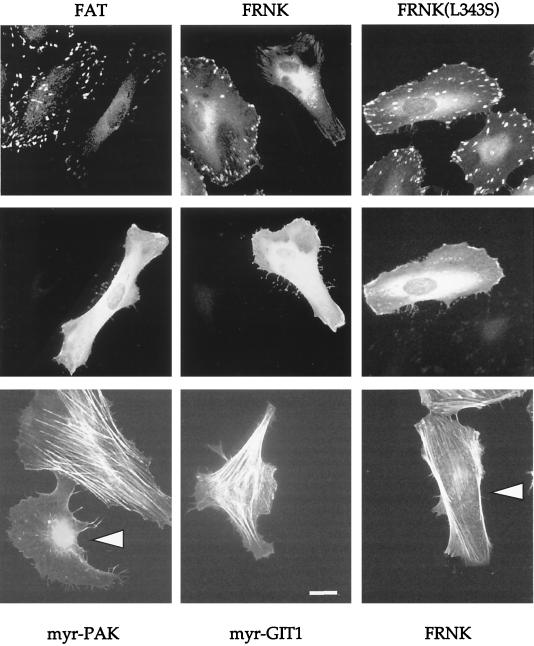
GIT and FAK cause FC loss without affecting actin stress fibers.
The paxillin LD4 motif that is targeted by GIT1 also interacts with FAK but not with other paxillin binders such as vinculin or talin which associate with different regions (4, 5). Since our experiments suggested that the function of GIT1 interaction with the paxillin LD4 motif is to promote paxillin loss from FCs, we reckoned that the paxillin-binding domain of FAK would exhibit a similar effect. Indeed, microinjection of an expression plasmid encoding FAK916-1053 (the so-called FAT [focal adhesion targeting] region) caused FC disassembly as assessed by vinculin staining, leaving smaller residual structures (Fig. 6). The larger FAK-related nonkinase (FRNK) construct could also promote FC disassembly and eventual cell contraction as efficiently as the FAT construct. Mutant FRNK (L343S), which cannot bind paxillin (reference 36 and our unpublished data), caused no alteration in either vinculin or paxillin distribution in FCs. As with myrGIT1 expression, neither FRNK nor FAT domains induced significant actin stress fiber disassembly (Fig. 6, bottom row); in contrast, myrPAK, like other activated versions of PAK, caused complete stress fiber loss.
FIG. 6.
The FAK C-terminal domain can cause FC disassembly. (Top) FAK916-1053 and FRNK result in marked loss of FCs from HeLa cells as visualized by vinculin staining, but the mutant FRNK(L343S) is ineffective. Expression of each construct was by anti-Flag (middle). Typical cells are shown 2 h after injection with expression plasmid (100 μg/ml). FAK916-1053-expressing cells begin to narrow and undergo cell body retraction. Similar effects were noted with full-length FAK. (Bottom) Distribution of polymerized actin as visualized by phalloidin-fluorescein isothiocyanate in typical cells expressing myrαPAK, myrGIT1, or FRNK. Only in the first case is there loss of actin stress fibers. Arrowheads indicate injected cells. Bar, 10 μm.
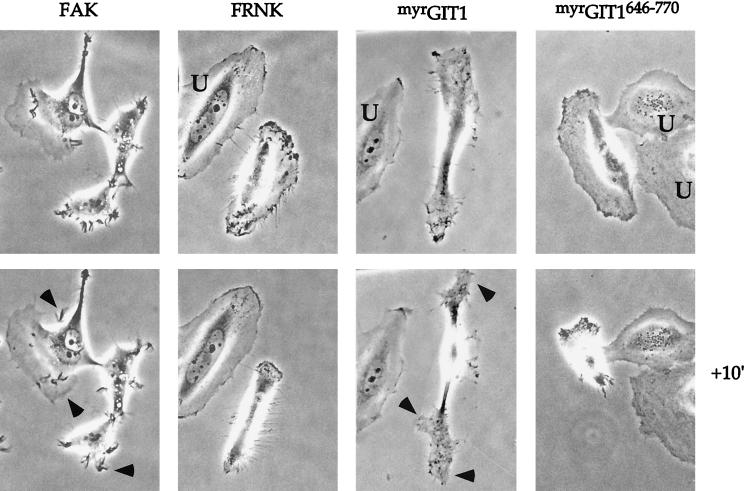
Both GIT1 and FAK can promote Rac activities.
Full-length FAK showed similar abilities to cause FC loss in NIH 3T3 and HeLa cells stained for vinculin. However, while the FRNK construct showed only peripheral collapse, FAK (full length) produced a counteracting membrane protrusive activity characterized by phase-dark dynamic lamellipodia (Fig. 7). For time-lapse experiments, HeLa cells were chosen because they are relatively static; FAK-expressing HeLa cells became very motile. Similarly, myrGIT1-injected cells are characterized by cell body rounding but concomitantly exhibit peripheral extension by active lamellipodial production. In both cases, the protrusive activity was blocked by coexpression of Rac1N17 (not shown). The paxillin-binding myrGIT1646-770 domain did not induce a similar lamellipodial formation, and once cells began to show signs of collapse, we noted that the process is extremely rapid. This might be attributable to internal tension generated by the intact actin-myosin system. Thus, full-length FAK and GIT1 promote two distinct activities: a decrease in cell adhesion (FC loss) and a Rac1-induced increase in peripheral membrane extensions, both facilitating migration.
FIG. 7.
FAK and GIT1 can drive lamellipodial formation. Phase-contrast micrographs of HeLa cells microinjected with expression plasmids (0.1 μg/μl) as shown. FAK-expressing cells typically extend many phase-dark membrane ruffles (arrowheads), while myrGIT1 cells become bipolar with lamellipodia forming at the ends (arrows). Uninjected cells (U) do not show movement over this time frame. These new structures were completely blocked by coexpression of RacN17. The FRNK and myrGIT646-770 cells were captured at an intermediate stage of collapse: photomicrographs were taken with a 10-min interval approximately 1 h after injection.
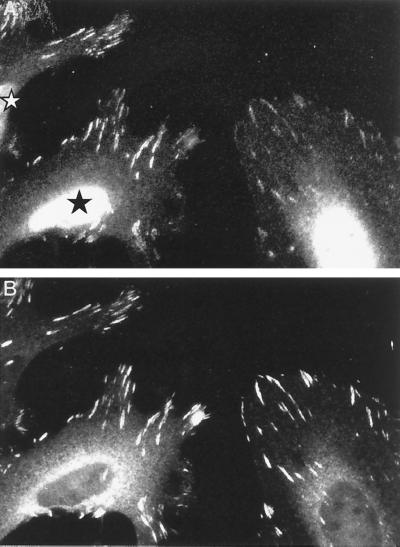
Events downstream of Cdc42 and PAK involve GIT1 and paxillin.
We have recently shown that PAK (which is not usually detected in the RhoA-type FCs of cultured cells) becomes concentrated in such FCs when we introduce its autoinhibitory region PAK83-149 (41). Thus, the kinase does play a role in these complexes but interacts only transiently, due to a negative regulation of its association with PIX (42). We tested whether increased levels of FC-localized PAK would lead to additional recruitment of GIT1, therefore indicating that PAK does recruit GIT1. Indeed, endogenous GIT1 was significantly concentrated in the FCs of HeLa cells expressing the PAK inhibitor (Fig. 8A) relative to vinculin levels, which were unaffected. Thus, PAK is probably a limiting factor for GIT1 localization to FCs, which explains the rather weak FC staining pattern obtained with GIT1 antibodies.
FIG. 8.
PAK can recruit GIT1 to FCs. (A) Increased FC-localized GIT1 in cells expressing the kinase inhibitor PAK83-149. The photomicrograph shows that in HeLa cells expressing the inhibitor (star), endogenous GIT1 staining is more concentrated in FCs relative to vinculin (B).
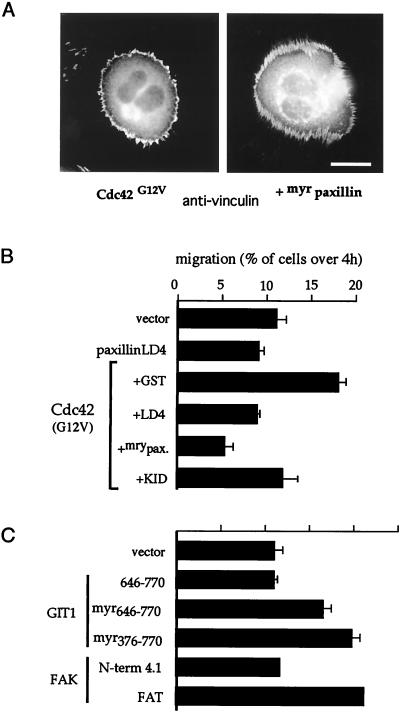
In the microinjection experiments, myrGIT1646-770 or the FAK916-1053 caused FC disassembly (via the LD4 region of paxillin), though these constructs might be predicted to merely drive the association of paxillin with the plasma membrane or FCs, respectively. Could these effects be attributable simply to such targeting of paxillin to juxtamembrane regions? Rather than causing FC loss, myrpaxillin expression increased FC numbers, particularly at peripheral locations (not shown). This suggested that myrpaxillin might stabilize newly emergent peripheral FCs (under the influence of Cdc42/Rac1). We then examined the effects of coexpressing myrpaxillin with Cdc42G12V (Fig. 9A). Typical vinculin staining patterns are shown: the coexpressing cells consistently exhibited much denser, brush-like peripheral FCs than cells expressing Cdc42G12V alone (Fig. 9A). We interpret this as myrpaxillin stabilizing these FCs by countering the effects of GIT1 (and FAK) which normally drive paxillin loss from FCs. In any case, paxillin recruitment to the membrane certainly does not result in FC disassembly.
FIG. 9.
PAK can recruit GIT1 to FCs. (A) Cdc42-induced peripheral FCs are stabilized by coexpression of membrane-targeted myrpaxillin. The typical rounded Cdc42G12V phenotype is seen 4 h after injection with or without coexpressed myrpaxillin. Note that with myrpaxillin the peripheral FCs are much denser. (B) COS-7 cells were transfected with Flag expression plasmids as shown. Motility of positively stained cells was assessed as for Fig. 1 (4 h after plating onto transwells). The top panel shows that myrpaxillin and the paxillin LD4 constructs effectively reduce Cdc42G12V-driven cell motility. KID, the PAK inhibitor αPAK83-149. (C) Smaller GIT1 and FAK constructs also promote motility. FAK1-427 corresponds to the band 4.1-like domain, and FAK916-1053 corresponds to the FAT domain.
Since our experiments indicated a Cdc42/PAK/PIX/GIT1/FAK pathway acting on FC-bound paxillin to promote FC turnover (thereby facilitating motility), we tested the effects of myrpaxillin and paxillin250-281 (an LD4 peptide derived independently of Turner et al. [37] that binds to GIT and FAK) on Cdc42G12V-induced cell motility. Both constructs attenuated the motility driven by Cdc42G12V (Fig. 9B), though LD4 had little effect on basal migration. This was also partially blocked by the kinase-inhibitory PAK83-149 construct, consistent with PAK activity contributing to FC disassembly. By masking the GIT1 and FAK C-terminal regions, the LD4 peptide can block FC turnover.
Can FC turnover increase motility?
We also tested directly whether smaller constructs that promoted FC loss via paxillin (without other effects) could increase the number of transfected COS-7 cells traversing a Boyden chamber membrane. Indeed, myrGIT1 constructs enhanced migration (Fig. 9C) as did FAK916-1053 protein (FAT domain), but the FAK N-terminal domain (band 4.1 homology domain) had no effect in this assay. Thus, under these conditions a loosening of cell attachment aided migration, as has been discussed previously (33). However, because FAT and FRNK will compete for endogenous FAK, these might also interfere with cell migration under different circumstances.
DISCUSSION
Functional implications of the regulated binding of GIT1 (and FAK) to paxillin.
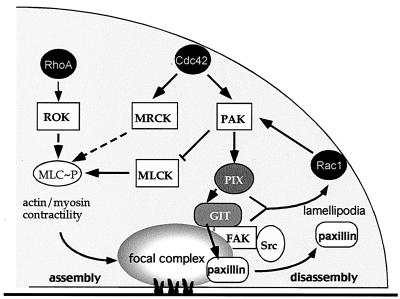
In investigating the mechanisms underlying PAK targeting to, and disassembling, FCs (20), we have isolated the PIX-interacting protein GIT1 as part of a multicomponent complex with GIT1 involved in stimulating motility. Our results lead us to propose a model (Fig. 10) in which Cdc42 recruitment of PAK and PIX drives the association of GIT1 with FCs; this favors dissociation of paxillin from FCs, which then become destabilized. FC turnover can also occur via PAK inhibition of myosin light-chain (MLC) phosphorylation, leading to decreased myosin contractility. The resultant decreased adherence facilitates motility, together with Rac1-induced peripheral activities. FAK, which similarly promotes motility, also binds to the GIT1 complex, and indeed more strongly than to paxillin. This raises the possibility that the paxillin binding region of FAK might be under some form of intramolecular control, as for GIT1. GIT1 and FAK could act synergistically in promoting motility.
FIG. 10.
PAK and its putative roles in FC regulation. Events downstream of Cdc42 affect FCs. A cascade via PAK leads to paxillin dissociation from FCs, promoting their turnover. The PIX-GIT1 complex also activates Rac1, leading to formation of lamellipodia. PAK can down-regulate MLCK, which normally induces actin-myosin contractility and formation of FCs. ROK drives the process by inhibiting MLC dephosphorylation; MRCK also promotes FCs. FC turnover and resultant decrease in cell adherence, together with Rac1-driven peripheral changes, promote cell motility. FAK, which also act binds to GIT1, may act synergistically with the PAK/PIX/GIT1 pathway to facilitate motility. For details, see Discussion.
The paxillin-binding C-terminal residues 646 to 770 are 78% identical within GIT1 and GIT2/Cat whereas adjacent N-terminal regions are dissimilar, compatible with their forming a functionally discrete domain. An intramolecular interaction probably maintains GIT1 in an inactive conformation, until disruption or activation by PIX binding unmasks the C-terminal domain. Such regulation seems common among FC components; for example, phosphatidylinositol-4,5-bisphosphate regulates the activity of vinculin's binding sites for talin and actin (9). Thus, PIX is permissive for GIT function and acts upstream in the pathway. The selective loss of paxillin from FCs, but not pronounced loss of FCs themselves, on GIT1 microinjection can be ascribed to a weak phenotype (i.e., sufficient paxillin remains to stabilize the FCs). However, when PIX is coexpressed and a greater fraction of the GIT1 has the exposed C terminus, FC disassembly results. Selective paxillin loss is observed at early times, prior to the FC loss per se, as assessed by vinculin staining (Fig. 2B).
The N terminus LD1, LD2, and LD4 motifs of paxillin which can potentially form parallel two-stranded coiled coils (>0.5 probability, using the coils algorithm of Lupas et al. [19]) exhibit protein-binding activity (37). The LD4-interacting domains of GIT1 and FAK each contain complementary regions with coiled-coil potential (cf. GIT1656-679 in Fig. 1A). We suggest that binding to LD4 triggers an alteration in paxillin to promote its dissociation from FCs, driven through conformational and/or subsequent phosphorylation events. The selective FC paxillin loss resulting from elevated GIT1 levels (in stable lines or by transient expression) via such a mechanism must alter the properties of the C-terminal LIM2 and LIM3 domains that act as FC localization modules (5).
Our motility assay results are consistent with paxillin263-282 LD4 effects on wound healing in NIH 3T3 cells (37), although the mechanism of action proposed differs in that PKL (highly related to GIT1) binding to paxillin drives FC recruitment of the PIX-PAK complex (i.e., in the opposite direction). If this were the case, PAK should be observed in all FCs that contain paxillin; however, experimentally PAK concentrates only in Cdc42- or Rac-induced structures (20). Our model of PIX activating GIT1 to act on paxillin (facilitating its dissociation) should be further tested once we understand how paxillin associates with FCs. There are multiple paxillin-related proteins (paxillinβ, Hic5, and leupaxin) containing the LD4 motif, and these are probably regulated in the same manner.
Cdc42 and Rac1 antagonism of RhoA function which results in dissolution of RhoA-dependent FCs (41) and facilitation of motility probably occurs through two mechanisms utilizing PAK (Fig. 10). PAK can directly inhibit MLC kinase (MLCK) (31), adversely affecting actin-myosin contractility; second, PAK-PIX recruitment activates GIT1. PIX itself, being Dbl related, can activate Rac1 and Cdc42 (22), an activity that has recently reported to be both PI-3-kinase and PAK dependent (40). Catalytic activity of PAK alone is insufficient for dissolution of RhoV14-driven FCs (41). With Cdc42G12V-induced peripheral FCs (Fig. 9A), the disassembling effect of Cdc42 involving PAK is balanced by Cdc42's contrary and known action of driving formation of these FCs (24) via MRCK (18). These peripheral FCs (and those of Rac) are considerably smaller than Rho-driven structures and may serve to provide transient and necessary contacts with the ECM to support cell migration and motility.
GIT1 a multifunctional protein linking PAK and FAK pathways.
The direct repeat SHD-1 mediates GIT1 association with both PIX and FAK. This domain is found uniquely in GIT1 family and yeast Spa2-like proteins. Both are indirectly linked to Cdc42: Spa2p colocalizes with Cdc42 to the emerging bud site and is thought to bind directly to Ste11p and Ste7p (34), which lie downstream of the PAK-like Ste20p; GIT proteins bind PIX and thereby PAK. The direct association of FAK with GIT1 as well as its integrin-dependent tyrosine phosphorylation in vivo (2) suggest that GIT1 is a bona fide FAK substrate. Although the C-terminal domains of both FAK and GIT1 (138 and 125 residues, respectively) form a paxillin-binding domain (targeting LD4), the sequences are essentially unrelated. However, both contain a region with a high potential to form a coiled-coil structure (Fig. 1A).
Interestingly, GIT1 was first identified as being involved in heterotrimeric G-protein signaling (28), but how GIT1 binds to GRKs and whether this binding affects the various activities of GIT1 with respect to the cytoskeleton remains to be investigated. Two related Arf GAPs have recently been shown to associate with FCs: ASAP/centaurin β4 binds Src and phosphatidylinositol-4,5-bisphosphate and is localized to FCs (29); PAG3 contains a paxillin-binding domain and is suggested to regulate transport of paxillin to FCs (16). It has also been suggested that Arf1 mediates paxillin recruitment to FCs and potentiates RhoA function (25).
FAK and GIT1 affect the cytoskeleton through common mechanisms.
Our findings that the FAK C terminus acts to disassemble FCs tally with studies on FAK−/− cells revealing a role for FAK in FC turnover (15). That the noncatalytic FAK C terminus fosters FC turnover correlates with the report that inducible expression of FAK668-1052 (FRNK) causes irreversible loss of adhesion and subsequent anoikis (38). However, the above findings disagree with those obtained by microinjection of GST/FAK765-1052 protein, which apparently decreased cell migration (8). We suggest that these differences arise because FAK, unlike FRNK, stimulates Rac1-dependent formation of lamellipodia. Although not unexpected, this behavior of FAK has not been previously reported. An ability to promote motility appears to rely on interactions with Src family kinases and p130Cas (7, 14). Similar conclusions were recently reported for FAK mutants introduced in FAK−/− cells (35). In addition, FAK, by recruiting GIT1 and the associated partners PIX and PAK, could promote FC turnover by antagonizing RhoA (Fig. 10).
The introduction of myrGIT1 into cells would be expected to activate Rac1 via PIX, and possibly the associated p85 PI-3-kinase subunit (40). FAK-mediated Rac1 activation, on the other hand, may be predominantly via Crk interactions with phosphorylated p130Cas (7). Thus, it is interesting that the phenotypes of HeLa cells expressing FAK or myrGIT1 are somewhat different (Fig. 7). In relation to cell motility, Rac activation, which is dependent on FAK, will be adversely affected by its competition with FRNK. On the other hand, where Rac is activated by other mechanisms, FRNK expression, which decreases adhesion (via paxillin, as seen here), can also enhance motility. We have shown that the paxillin-binding-defective mutant FRNK(L343S) fails to cause FC loss. Focal adhesion targeting and paxillin binding are separate functions in FAK (12).
Our results support the notion that interaction of the paxillin LD4 with FAK or GIT1 provides a mechanism for disengagement of paxillin from, and consequent disassembly of, FCs (perhaps requiring phosphorylation events). The corollary that cell adhesion is enhanced by the stabilization of paxillin-FC interactions was shown with certain paxillin LIM3 mutants (6). Thus, paxillin is identified as a linchpin to stabilize FCs, cross-linking components via its C-terminal and N-terminal domains. Providing evidence for this conjecture through targeted disruption of combinations of the four known paxillin-related genes (4) will take some time.
What evidence is there that paxillin plays such a key role in regulating FC dynamics? Mitotic cells, which lack well-formed focal adhesions, appear to use two mechanisms to regulate paxillin. First, paxillin levels are reduced whereas other FC proteins including talin, vinculin, and FAK are unaffected (39); second, Yamaguchi et al. (39) note that altered phosphorylation of paxillin (primarily on serine) regulates its interaction with FC partners. Small cell lung cancer cells, which are aggressive and metastasize quickly, are characterized by a loss of paxillin expression (30). Conversely, FAK, which we suggest opposes paxillin in function, is elevated in invasive human tumors (27). Interestingly, overexpression of GIT1 in NIH 3T3 cells (which require attachment for propagation) is facilitated by a compensatory up-regulation of paxillin (data not shown).
In conclusion, our observations suggest that in addition to altering FC behavior through interplay between Rho proteins, a PAK-driven mechanism exists to directly control FC turnover through GIT1 (Fig. 9). GIT1 also binds to and is phosphorylated by FAK (and its partner Src). This model addresses some of the questions raised by previous studies of FAK and provides a basis for future investigations into FC regulation and its relationship to cell motility.
ACKNOWLEDGMENT
This work is supported by the Glaxo Singapore Research Fund.
REFERENCES
- 1.Bagrodia S, Taylor S J, Jordon K A, Van Aelst L, Cerione R A. A novel regulator of p21-activated kinases. J Biol Chem. 1998;273:23633–23636. doi: 10.1074/jbc.273.37.23633. [DOI] [PubMed] [Google Scholar]
- 2.Bagrodia S, Bailey D, Lenard Z, Hart M, Guan J L, Premont R T, Taylor S J, Cerione R A. A tyrosine-phosphorylated protein that binds to an important regulatory region on the cool family of p21-activated kinase-binding proteins. J Biol Chem. 1999;274:22393–22400. doi: 10.1074/jbc.274.32.22393. [DOI] [PubMed] [Google Scholar]
- 3.Bagrodia S, Cerione R A. Pak to the future. Trends Cell Biol. 1999;9:350–355. doi: 10.1016/s0962-8924(99)01618-9. [DOI] [PubMed] [Google Scholar]
- 4.Brown M C, Curtis M S, Turner C E. Paxillin LD motifs may define a new family of protein recognition domains. Nat Struct Biol. 1998;5:677–678. doi: 10.1038/1370. [DOI] [PubMed] [Google Scholar]
- 5.Brown M C, Perrotta J A, Turner C E. Identification of LIM3 as the principal determinant of paxillin focal adhesion localization and characterization of a novel motif on paxillin directing vinculin and focal adhesion kinase binding. J Cell Biol. 1996;135:1109–1123. doi: 10.1083/jcb.135.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown M C, Perrotta J A, Turner C E. Serine and threonine phosphorylation of the paxillin LIM domains regulates paxillin focal adhesion localization and cell adhesion to fibronectin. Mol Biol Cell. 1998;9:1803–1816. doi: 10.1091/mbc.9.7.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cary L A, Han D C, Polte T R, Hanks S K, Guan J L. Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration. J Cell Biol. 1998;140:211–221. doi: 10.1083/jcb.140.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilmore A P, Romer L H. Inhibition of focal adhesion kinase (FAK) signaling in focal adhesions decreases cell motility and proliferation. Nature. 1996;381:531–535. doi: 10.1091/mbc.7.8.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilmore A P, Burridge K. Regulation of vinculin binding to talin and actin by phosphatidyl-inositol-4,5-bisphosphate. Mol Biol Cell. 1996;7:1209–1224. doi: 10.1038/381531a0. [DOI] [PubMed] [Google Scholar]
- 10.Harden N, Lee J, Loh H Y, Ong Y M, Tan I, Leung T, Manser E, Lim L. A Drosophila homolog of the Rac- and Cdc42-activated serine/threonine kinase PAK is a potential focal adhesion and focal complex protein that colocalizes with dynamic actin structures. Mol Cell Biol. 1996;16:1896–1908. doi: 10.1128/mcb.16.5.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemler M E. Integrin associated proteins. Curr Opin Cell Biol. 1998;10:578–585. doi: 10.1016/s0955-0674(98)80032-x. [DOI] [PubMed] [Google Scholar]
- 12.Hildebrand J D, Schaller M D, Parsons J T. Paxillin, a tyrosine phosphorylated focal adhesion-associated protein binds to the carboxyl terminal domain of focal adhesion kinase. Mol Biol Cell. 1995;6:637–647. doi: 10.1091/mbc.6.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hing H, Xiao J, Harden N, Lim L, Zipursky S L. Pak functions downstream of Dock to regulate photoreceptor axon guidance in Drosophila. Cell. 1999;97:853–863. doi: 10.1016/s0092-8674(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 14.Ilic D, Damsky C H, Yamamoto T. Focal adhesion kinase: at the crossroads of signal transduction. J Cell Sci. 1997;110:401–407. doi: 10.1242/jcs.110.4.401. [DOI] [PubMed] [Google Scholar]
- 15.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 16.Kondo A, Hashimoto S, Yano H, Nagayama K, Mazaki Y, Sabe H. A new paxillin-binding protein, PAG3/Papα/KIAA0400, bearing an ADP-ribosylation factor GTPase-activating protein activity, is involved in paxillin recruitment to focal adhesions and cell migration. Mol Cell Biol. 2000;20:1315–1327. doi: 10.1091/mbc.11.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leung T, Chen X Q, Manser E, Lim L. The p160 RhoA-binding kinase ROKαis a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16:5313–5327. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung T, Chen X Q, Tan I, Manser E, Lim L. Myotonic dystrophy kinase-related Cdc42-binding kinase acts as a Cdc42 effector in promoting cytoskeletal reorganization. Mol Cell Biol. 1998;18:130–140. doi: 10.1128/mcb.18.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 20.Manser E, Huang H Y, Loo T-H, Chen X-Q, Dong J-M, Leung T, Lim L. Expression of constitutively active αPAK reveals effects of the kinase on actin and focal complexes. Mol Cell Biol. 1997;17:1129–1143. doi: 10.1128/mcb.17.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manser E, Leung T, Salihuddin H, Zhao Z-S, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 22.Manser E, Loo T-H, Koh C-G, Zhao Z-S, Chen X-Q, Tan L, Tan I, Leung T, Lim L. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell. 1998;1:183–192. doi: 10.1016/s1097-2765(00)80019-2. [DOI] [PubMed] [Google Scholar]
- 23.Mitchison T J, Cramer L P. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- 24.Nobes C D, Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 25.Norman J C, Jones D, Barry S T, Holt M R, Cockcroft S, Critchley D R. ARF1 mediates paxillin recruitment to focal adhesions and potentiates Rho-stimulated stress fiber formation in intact and permeabilized Swiss 3T3 fibroblasts. J Cell Biol. 1998;143:1981–1995. doi: 10.1083/jcb.143.7.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obermeier A, Ahmed S, Manser E, Yen S-C, Hall C, Lim L. PAK promotes morphological changes by acting upstream of Rac. EMBO J. 1998;17:4328–4339. doi: 10.1093/emboj/17.15.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owens L V, Xu L, Craven R J, Dent G A, Weiner T M, Kornberg L, Liu E T, Cance W G. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 1995;55:2752–2755. [PubMed] [Google Scholar]
- 28.Premont R T, Claing A, Vitale N, Freeman J L, Pitcher J A, Patton W A, Moss J, Vaughan M, Lefkowitz R J. β2-Adrenergic receptor regulation by GIT1, a G protein-coupled receptor kinase-associated ADP ribosylation factor GTPase-activating protein. Proc Natl Acad Sci USA. 1998;95:14082–14087. doi: 10.1073/pnas.95.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Randazzo P A, Andrade J, Miura K, Brown M T, Long Y-Q, Stauffer S, Roller P, Cooper J. The Arf GTPase-activating protein ASAP1 regulates the actin cytoskeleton. Proc Natl Acad Sci USA. 2000;97:4011–4016. doi: 10.1073/pnas.070552297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salgia R, Li J L, Ewaniuk D S, Wang Y B, Sattler M, Chen W C, Richards W, Pisick E, Shapiro G L, Rollins B J, Chen L B, Griffin J D, Sugarbaker D J. Expression of the focal adhesion protein paxillin in lung cancer and its relation to cell motility. Oncogene. 1999;18:67–77. doi: 10.1038/sj.onc.1202273. [DOI] [PubMed] [Google Scholar]
- 31.Sanders L C, Matsumura F, Bokoch G M, de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science. 1999;283:2083–2085. doi: 10.1126/science.283.5410.2083. [DOI] [PubMed] [Google Scholar]
- 32.Schoenwaelder S M, Burridge K. Bidirectional signaling between the cytoskeleton and integrins. Curr Opin Cell Biol. 1999;11:274–286. doi: 10.1016/s0955-0674(99)80037-4. [DOI] [PubMed] [Google Scholar]
- 33.Schwarzbauer J E. Cell migration: may the force be with you. Curr Biol. 1997;7:R292–R294. doi: 10.1016/s0960-9822(06)00140-0. [DOI] [PubMed] [Google Scholar]
- 34.Sheu Y J, Santos B, Fortin N, Costigan C, Snyder M. Spa2p interacts with cell polarity proteins and signaling components involved in yeast cell morphogenesis. Mol Cell Biol. 1998;18:4053–4069. doi: 10.1128/mcb.18.7.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sieg D J, Hauck C R, Schlaepfer D D. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J Cell Sci. 1999;112:2677–91. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- 36.Tachibana K, Sato T, D'Avirro N, Morimoto C. Direct association of pp125FAK with paxillin, the focal adhesion-targeting mechanism of pp125FAK. J Exp Med. 1995;182:1089–1100. doi: 10.1084/jem.182.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turner C E, Brown M C, Perrotta J A, Riedy M C, Nikolopoulos S N, McDonald A R, Bagrodia S, Thomas S, Leventhal P S. Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: a role in cytoskeletal remodeling. J Cell Biol. 1999;145:851–863. doi: 10.1083/jcb.145.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu L H, Yang X, Craven R J, Cance W G. The COOH-terminal domain of the focal adhesion kinase induces loss of adhesion and cell death in human tumor cells. Cell Growth Differ. 1998;9:999–1005. [PubMed] [Google Scholar]
- 39.Yamaguchi R, Mazaki Y, Hirota K, Hashimoto S, Sabe H. Mitosis specific serine phosphorylation and downregulation of one of the focal adhesion protein, paxillin. Oncogene. 1997;15:1753–1761. doi: 10.1038/sj.onc.1201345. [DOI] [PubMed] [Google Scholar]
- 40.Yoshii S, Tanaka M, Otsuki Y, Wang D-Y, Guo R-J, Zhu Y, Takeda R, Hanai H, Kaneko E, Sugimura H. αPIX nucleotide exchange factor is activated by interaction with phosphatidylinositol 3-kinase. Oncogene. 1999;18:5680–5690. doi: 10.1038/sj.onc.1202936. [DOI] [PubMed] [Google Scholar]
- 41.Zhao Z S, Manser E, Chen X Q, Chong C, Leung T, Lim L. A conserved negative regulatory region in αPAK: inhibition of PAK kinases reveals their morphological roles downstream of Cdc42 and Rac1. Mol Cell Biol. 1998;18:2153–2163. doi: 10.1128/mcb.18.4.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao Z S, Manser E, Lim L. Interaction between PAK and Nck: a template for Nck targets and role of PAK autophosphorylation. Mol Cell Biol. 2000;20:3906–3917. doi: 10.1128/mcb.20.11.3906-3917.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]