Abstract
Cholesterol has been implicated in the pathophysiology and progression of several cancers now, although the mechanisms by which it influences cancer biology are just emerging. Two likely contributing mechanisms are the ability for cholesterol to directly regulate signaling molecules within the membrane, and certain metabolites acting as signaling molecules. One such metabolite is the oxysterol 27-hydroxycholesterol (27HC), which is a primary metabolite of cholesterol synthesized by the enzyme Cytochrome P450 27A1 (CYP27A1). Physiologically, 27HC is involved in the regulation of cholesterol homeostasis and contributes to cholesterol efflux through liver X receptor (LXR) and inhibition of de novo cholesterol synthesis through the insulin-induced proteins (INSIGs). 27HC is also a selective modulator of the estrogen receptors. An increasing number of studies have identified its importance in cancer progression of various origins, especially in breast cancer. In this review, we discuss the physiological roles of 27HC targeting these two nuclear receptors and the subsequent contribution to cancer progression. We describe how 27HC promotes tumor growth directly through cancer-intrinsic factors, and indirectly through its immunomodulatory roles which lead to decreased immune surveillance and increased tumor invasion. This review underscores the importance of the cholesterol metabolic pathway in cancer progression and the potential therapeutic utility of targeting this metabolic pathway.
1. Introduction
Obesity is a prevalent morbidity in the United States and worldwide, with over one third of US adults estimated to be obese [1]. Numerous clinical studies have reported obesity being associated with a variety of diseases, including diabetes, cardiovascular disease, metabolic syndrome, nonalcoholic fatty liver disease and even cancer [2]. However, many of the precise mechanistic links between obesity and the associated medical conditions remain to be elucidated. Elevated cholesterol is a common comorbidity of obesity, making it a possible mediator or exacerbating factor of obesity and its related diseases [3]. The role of cholesterol is well-known in cardiovascular disease, but less explored in other conditions. In breast cancer, meta-analysis and prospective studies have identified a positive correlation between elevated blood cholesterol and breast cancer risk, as well as recurrence. The use of cholesterol-lowering statin drugs is associated with a good prognosis [4-6]. In recent prospective trials or in vivo studies, co-treatment with either statins or antibodies against proprotein convertase subtilisin/kexin type 9 (PCSK9) improved the efficacy of immune checkpoint inhibitors for the treatment of non-small cell lung cancer (NSCLC) and colon cancer [7-9]. Thus, there is strong clinical data implicating cholesterol and cancer progression. In preclinical murine models, a diet high in cholesterol has been found to promote the growth and metastasis of several cancers, including breast [10-12]. The mechanisms by which cholesterol promotes cancer progression are likely multifactorial and include both direct effects of cholesterol on cancer cells, as well as effects of downstream cholesterol metabolites on both the cancer cells and the microenvironment. 27HC is one metabolite in particular that has emerged as being important for cancer progression [13]. In this review, we will provide a brief overview of cholesterol metabolism and some evidence for direct effects of cholesterol on cancer biology, and then focus on the described roles of 27HC in cancer progression (Figure 1). Many other cholesterol metabolites also contribute to cancer, and will be covered by other papers in this special issue.
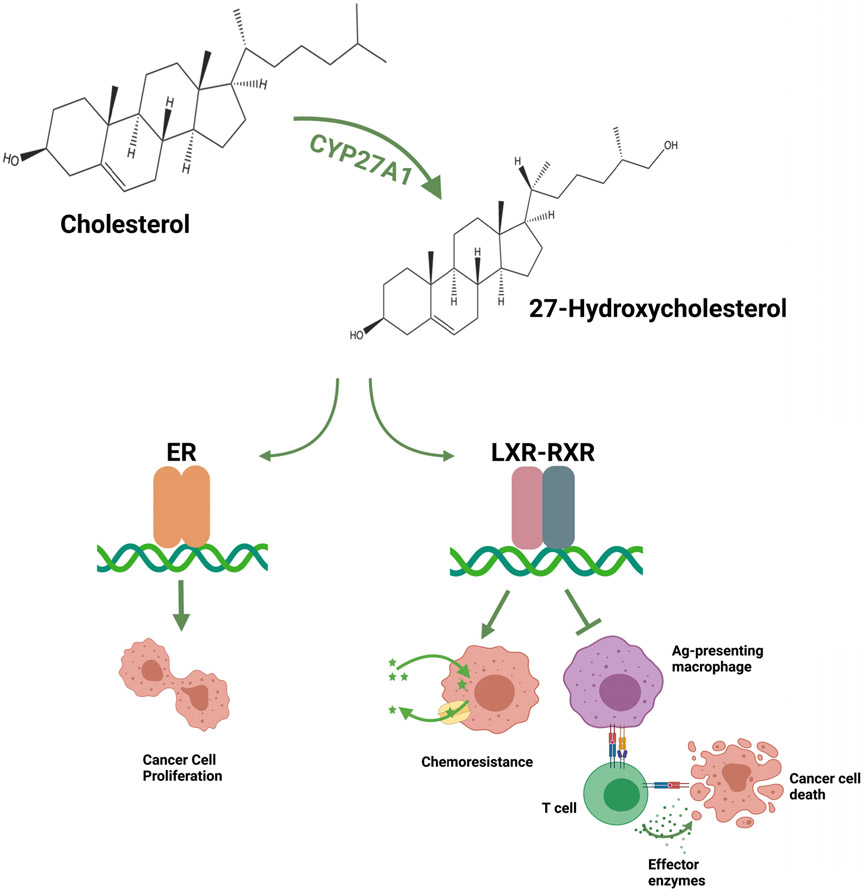
Figure 1. Cholesterol-27HC axis promotes tumor progression.
The enzyme CYP27A1 catalyzes the conversion of cholesterol to 27HC. 27HC is a ligand for both the ERs and LXRs. Through the ERs expressed in certain cancers (ERα for breast, ERβ or prostate; see text for details), 27HC promotes proliferation in a cancer cell-intrinsic manner. The activation of LXR in cancer cells leads to an upregulation of chemotherapy efflux pump Pgp and support chemoresistance of those cells, but has also been shown to induce cholesterol efflux and slow proliferation. In a cancer cell-extrinsic manner, 27HC works on LXRs in antigen (Ag)-presenting macrophages and other myeloid immune cells such as neutrophils to suppress T cell activity, which ultimately suppresses immune surveillance and drives cancer progression. Figure created with BioRender (BioRender.com).
2. Cholesterol metabolism, production and metabolism of 27HC
Intracellular cholesterol homeostasis is a tightly regulated cellular process, manifested by a controlled de novo synthesis and uptake/efflux of cholesterol molecules [14]. When cholesterol levels are low, biosynthesis of cholesterol is upregulated by sterol regulatory element-binding protein 2 (SREBP2) [13, 15]. SREBP2 interacts with SREBP-cleavage activating protein (SCAP), leading to the clustering of SREBP2 in coat protein complex II (COPII)-coated vesicles [13, 15]. This complex allows SREBP2 cleavage in the Golgi complex before translocating into the nucleus. Upon entering the nucleus, SREBP2 serves as a master transcription factor to promote cholesterol accumulation, in part by inducing the expression of HMG-CoA reductase (HMGCR), a rate-limiting enzyme of the cholesterol biosynthetic pathway, and low-density lipoprotein receptor (LDLR), thereby promoting cholesterol biosynthesis and uptake [14, 15]. On the contrary, when cholesterol levels are high, inhibition of cholesterol biosynthesis is mediated by the prevention of SREBP2 activation. This process is triggered by INSIGs binding to SCAP, which further precludes the binding of COPII to SCAP, the cleavage of SREBP2 and therefore its transport into the nucleus [13, 15].
To complement this system, activation of the LXR, which is found as a heterodimer with the retinoid X receptor (RXR), induces the transcription of the reverse cholesterol transporters such as ABCA1 and ABCG1. Activated LXR also upregulates the inducible degrader of the LDL receptor (IDOL), an E3 ligase that prompts ubiquitination of the LDLR [14, 16, 17]. Both recruitment of INSIG, which inhibits cholesterol biosynthesis, and the activation of LXR, which promotes cholesterol efflux, are dependent on the metabolic derivatives of cholesterol, especially the oxysterols inhibiting the SREBP2 system and activating the LXRs [13].
Oxysterols can be generated from cholesterol oxidation enzymatically and non-enzymatically. The enzymatic production is typically mediated by CYPs. Among them, CYP27A1, primarily expressed in liver and myeloid immune cells, converts cholesterol to 27HC. 27HC is the most abundant circulating oxysterol in humans, with concentrations ranging from 67 ng/mL (0.17 μM) to 199 ng/mL (0.5 μM) [18, 19]. Catabolizing 27HC requires another P450 enzyme, oxysterol 7α-hydroxylase (CYP7B1), which is abundantly expressed in the liver, but is also expressed in extra-hepatic tissues as well as tumors [20-23]. The deletion of CYP7B1 in mice has been shown to increase the plasma concentration of 27HC [24]. Furthermore, thyroid cancer tumors with elevated CYP7B1 mRNA expression had decreased 27HC concentrations compared to those with low CYP7B1 expression [23]. Ultimately, the metabolism of 27HC results in the production of bile acids, along the so-called acidic bile acid pathway.
3. Cholesterol itself has the ability to directly regulate cellular signaling pathways – implications for cancer progression.
Although cholesterol is synthesized in endoplasmic reticulum, a large majority of cellular unesterified cholesterol is found in the plasma membrane in mammalian cells [25, 26]. In addition to its structural roles at the plasma membrane, cholesterol has been implicated in cell signaling through the formation of membrane microdomains, including lipid rafts [27, 28], which may compartmentalize various cell signaling events, including cell proliferation [29-32]. However, this lipid raft hypothesis has remained controversial because lipid rafts have never been directly detected under physiological conditions [33]. Also, the level of exogenous cholesterol needed to stimulate cell proliferation is far lower than that required for lipid raft formation [34]. Recent studies have supported an alternative mechanism for the signaling function of cholesterol. First, multiple studies have reported that cholesterol directly regulates the structure and function of various integral membrane proteins involved in cell signaling [35], including G protein-coupled receptors [36]. Furthermore, accumulating evidence supports the notion that cholesterol in the inner leaflet of the plasma membrane regulates cytosolic signaling proteins through direct and specific interactions [37-40]. For example, it specifically binds and activates a scaffolding protein, dishevelled (Dvl), which plays a central role in growth-promoting canonical Wnt signaling [38-40]. Furthermore, simultaneous in situ quantitative imaging of cholesterol in each of the two leaflets of the plasma membrane showed that the concentration of cholesterol in the inner leaflet was increased in response to binding of a canonical Wnt ligand to its receptor, Frizzled. The increase was mediated by regulation of an ATP-binding cassette protein, ABCA1 that serves as a cholesterol translocase and led to plasma membrane recruitment and activation of Dvl, triggering the downstream canonical Wnt signaling pathway [41]. Similarly, binding of hedgehog to its receptor Patched induced an increase in the concentration of cholesterol in the inner leaflet of the cholesterol, leading to stimulation of hedgehog signaling [42]. Collectively, these recent results support the direct and specific signaling role of cholesterol, independent of its involvement in cholesterol-rich membrane domains and open up new horizons for cholesterol signaling research in cancer.
Whether cholesterol metabolites such as 27HC can behave at the membrane in a similar way to cholesterol and influence membrane stability or directly interact with membrane proteins is an area that requires further exploration. That 27HC has been found to be present in the membrane fractions of cells [43] and the high structural similarity between 27HC and cholesterol add support to this potential biology. 27HC has been found to disrupt signaling, purportedly through the disruption of lipid rafts [44]. Additionally, 27HC can shift cholesterol concentrations within the membrane, providing the possibility of indirect effects [45]. Further work is required to determine the relative distribution of 27HC within the inner versus outer leaflets of the membrane, whether this distribution can be regulated, and whether 27HC can directly interact with growth signaling proteins like G protein-coupled receptors (GPCRs or GPRs) and Dvl as cholesterol can.
4. 27HC is an endogenous metabolite with signaling properties
In addition to the direct effects of cholesterol on signaling, emerging evidence supports the role of oxysterol metabolites as signaling molecules. In this regard, one of the best described metabolites is 27HC, which is also the most concentrated oxysterol in circulation. 27HC is a known ligand of two nuclear receptors, the estrogen receptor (ER) and LXR. We will elaborate on signaling through the ERs and LXRs in detail below. Initial screening found that 27HC had neither agonistic nor antagonistic activities on the androgen receptor (AR) [46]. However, in prostate epithelial RWPE-1 cells, 27HC increased the transcriptional activity of the AR and increased the abundance of prostate-specific antigen, a known AR target gene [47]. At this point, it is not known whether 27HC directly regulates the AR or it activates signaling pathways that indirectly result in increased AR activity. Besides binding to the nuclear receptors, there is a report that 27HC can also engage the GPCR, C-X-C chemokine receptor type 2 (CXCR2), leading to the recruitment of neutrophils to tumors, an activity that can be inhibited by a CXCR2 antagonist, SB225002 [48]. A related oxysterol, 25-hydroxychoelsterol has also been documented to bind to GPR183 [49]. Reportedly, oxysterols including 27HC and 22(R)-hydroxycholesterol (22RHC) can facilitate the recruitment of pro-tumor neutrophils through CXCR2. Additionally, 27HC binds to the INSIG proteins, resulting in the retention of SCAP:SREBP2 complex in the endoplasmic reticulum and consequent inhibition of cholesterol synthesis [50].
4.1. 27HC as an endogenous Selective Estrogen Receptor Modulator (SERM)
ERs are one of the members of the nuclear hormone receptor superfamily. As nuclear receptors share homology in their functional structures, the ERs also consist of five functional domains: an unstructured N-terminal region that contains the activation function 1 (AF1) surface, a zinc finger DNA-binding domain (DBD), a variable hinge region, and a ligand-binding domain (LBD) that not only binds to ligands but also interacts with co-regulators through the activation function 2 (AF-2) or the carboxyl-terminal domain [13, 51, 52]. Two major isoforms exist for the ERs, ERα (NR3A1) and ERβ (NR3A2), with ERβ having a shorter N-terminal region compared to ERα [13, 53]. ER signaling can be “genomic” or “non-genomic”. In classic or genomic signaling, upon ligand binding, a conformational change in ER occurs, and heat shock proteins dissociate from the ERs, allowing receptor dimerization and translocate into the nucleus. ER dimers then bind to the DNA estrogen response element (ERE) and/or enhancer regions, which then regulates the transcriptional activity of the target genes through the recruitment of co-regulators [53]. Besides directly binding DNA, indirect genomic signaling also takes place for the ER, where the ER interacts with other transcription factors, such as Sp1, already bound to DNA through protein-protein interactions [53, 54]. Non-genomic or rapid signaling of the ERs can be mediated through the membrane-bound G protein-coupled estrogen receptor 1 (GPER1), or direct interactions of the ER with other signaling proteins at the cytoplasmic membrane [54-56].
Estrogens including 17β-estradiol (E2) are known to be potent agonists of the ERs, inducing conformational changes of the receptor to promote transcriptional activities that play vital physiological roles ranging from reproduction to the regulation of cardiovascular, musculoskeletal, immune and brain functions [57-59]. Another class of molecules that binds the ERs and regulate transcriptional activities are known as SERMs [60-63]. The first described SERM was tamoxifen when it was being evaluated as a breast cancer treatment in the 1970s. Tamoxifen behaves like an ER antagonist in breast tissues, which blocks the proliferative actions of estrogens. However, and very unexpectedly at the time, tamoxifen exerted estrogenic activities in the bone, sparing bone loss [18]. We now know that the majority if not all ER ligands can be characterized as SERMS, having tissue- and context-specific activities. They can even have differential effects on the transcriptome within the same cell type [64]. This unique pharmacology has provided an opportunity for tailored drug development, and likely applies to all nuclear receptors [61], as it has now been described for the androgen receptor among others [65].
Mechanistically, it is generally thought that SERMs can manifest differential ER activity across different tissues through their ability to induce subtle yet distinct conformational changes in the three-dimensional structure of the ER [66-69]. Since the ability of the ER to regulate transcriptional activity essentially depends on the recruitment of and interaction with co-activators and corepressors, the distinct ligand-receptor structures ultimately affect the complex’s engagement of those co-regulators. Indeed, coactivators such as the SRC1/3, p300/CBP, p68 RNA helicase, and L7/SPA were found to promote the transcriptional activity of tamoxifen-bound ER, while corepressors, such as silencing mediator for retinoid and thyroid receptors (SMRT) and nuclear receptor co-repressor (NCoR), support the opposite [66, 70-74]. In addition to the distinct conformational changes of the ER upon SERM binding, other factors including the tissue-specific expression of co-regulators, tissue-specific expression of the ER isoforms and the binding of ER-SERM complex to distinct DNA motifs could also contribute to the selective modulatory nature of SERMs [18, 75]. Finally, certain ligands may activate receptors in addition to the ER, with the resulting crosstalk defining the biological outcome [62, 63].
The first endogenous ligand with SERM-like qualities to be described was 27HC, by Umetani et al., in an effort to understand the roles of 27HC in cardiovascular disease [76]. It was shown that 27HC competitively displaced E2 in EA.hy926 cells, suggesting that 27HC is a naturally occurring ER antagonist [76]. Furthermore, it was found that 27HC inhibited E2-induced, but not lipopolysaccharide (LPS)-induced, nitric oxide synthase (NOS) activity [76]. Outside vascular endothelial cells, 27HC also antagonized ER transactivation in BAECs and MCF-7 cells [76]. However, 27HC displayed properties similar to other ER agonists in HepG2 and Caco-2 cells [76]. 27HC nevertheless did not seem to impact the reproductive functions of mice [76]. In subsequent work, DuSell et al. used peptide display to demonstrate that upon binding, 27HC induced conformational changes in ER that were unique from those induced by E2, 4-hydroxy-tamoxifen (a SERM) or Fulvestrant (an ER antagonist) upon binding to ER [77]. Beyond inducing conformational changes, they further reported 27HC drives the recruitment of co-activators, including SRC1-NR, ASC2-NR, AIB1-NR and GRIP1-NR, to ERα, which then increased the expression of ER target genes and enhanced ER degradation (see abbreviation definitions in Table 1). Collectively, these data support the idea that 27HC is an endogenous SERM [77].
Table 1:
List of Abbreviations.
| Abbreviation | Definition |
|---|---|
| 22(R)HC | 22(R)-hydroxycholesterol |
| 24SHC | 24(S)-hydroxycholesterol |
| 25HC | 25-hydroxycholesterol |
| 27HC | 27-hydroxycholesterol |
| 4βHC | 4β-hydroxycholesterol |
| 7KC | 7-ketocholesterol |
| ABCA1 | ATP-binding cassette A1 |
| ABCG1 | ATP-binding cassette G1 |
| AF1 | activation function 1 |
| AIB1 | Amplified in breast cancer 1 |
| AR | Androgen Receptor |
| ATP | Adenosine triphosphate |
| BCG | Bacillus Calmette-Guerin |
| CCR7 | C-C chemokine receptor 7 |
| COPII | Coat protein complex II |
| CXCR2 | C-X-C chemokine receptor type 2 |
| CYP27A1 | Cytochrome P450 27A1 |
| CYP7B1 | Oxysterol 7α-hydroxylase |
| DBD | DNA-binding domain |
| DR | Direct repeats |
| Dvl | Dishevelled |
| E2 | 17β-estradiol |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial-mesenchymal transition |
| ER | Estrogen receptor |
| ERE | Estrogen response element |
| GH | Growth hormone |
| GPCR | G protein-coupled receptor |
| GPER1 | G protein-coupled estrogen receptor 1 |
| GPR183 | G-protein coupled receptor 183 |
| GRIP1 | Glutamate receptor-interacting protein 1 |
| HDAC3 | Histone deacetylase 3 |
| HDL | High-density lipoprotein |
| HER2 | Receptor tyrosine-protein kinase erbB-2, also known as human epidermal growth factor receptor 2, EGFR2 and CD340 |
| HIF | Hypoxia-inducible factor |
| HMGCR | HMG-CoA reductase |
| IDOL | Inducible degrader of the LDL receptor |
| IFN | Interferon |
| IGF-1 | Insulin-like growth factor-1 |
| INSIG | Insulin-induced protein |
| JAK | Janus kinase |
| LBD | Ligand-binding domain |
| LDL | Low-density lipoprotein |
| LDLR | Low-density lipoprotein receptor |
| LPS | Lipopolysaccharide |
| LXR | Liver X receptor |
| LXRE | Liver X response element |
| MAPK | Mitogen-activated protein kinase |
| MDM2 | Mouse double minute 2 |
| MDSC | Myeloid-derived suppressor cell |
| MMP9 | Matrix metallopeptidase 9 |
| NCoR | Nuclear receptor co-repressor |
| NOS | Nitric Oxide Synthase |
| NR | Nuclear receptor |
| NSCLC | Non-small cell lung cancer |
| PCSK9 | Proprotein convertase subtilisin/kexin type 9 |
| PD1 | Programmed cell death protein 1 |
| PDL1 | Programmed death-ligand 1 |
| PI3K | Phosphoinositide 3-kinase |
| PMN | Polymorphoneutrophils |
| PPAR | Peroxisome proliferator-activated receptor |
| ROS | Reactive oxygen species |
| RXR | Retinoid X receptor |
| SCAP | SREBP-cleavage activating protein |
| SERM | Selective estrogen receptor modulator |
| SIRT1 | NAD+-dependent deacetylases Sirtuin 1 |
| SMRT | Silencing mediator for retinoid and thyroid receptors |
| SRC1 | Steroid receptor coactivator-1 |
| SREBP2 | Sterol regulatory element-binding protein 2 |
| STAT | Signal transducer and activator of transcription |
| SUMO | Small ubiquitin-related modifier |
| TLR | Toll-like receptor |
| TNBC | Triple-negative breast cancer |
| VEGF | Vascular endothelial growth factor |
Besides 27HC, several other oxysterols also activate the ERs. Among them, 25HC was reported to inhibit the up-regulation of hypoxia-inducible factor (HIF)-1α and connective tissue growth factor in cardiomyocytes through ERα [78, 79]. It was described that the serum concentration of 25HC was in the range of 0.5 - 25 ng/mL for 25HC, compared to 5 - 250 ng/mL for 24(S)-hydroxycholesterol (24SHC) and 27HC [80]. A recent study also found 4β-hydroxycholesterol (4βHC), 7-ketocholesterol (7KC) and desmosterol being able to activate ER in T47D cells, measured by luciferase activity of the transfected ER reporter construct [22].
4.2. 27HC and LXR signaling
The LXR is considered a major target receptor of 27HC, given its purported role in maintaining cholesterol homeostasis. Like the ER, LXR also has two isoforms, LXRα (NR1H3) and LXRβ (NR1H2), with LXRα being expressed mainly in the liver and tissues important for lipid metabolism and LXRβ expressed ubiquitously [81]. Originally thought to be an orphan nuclear receptor, LXRs were later identified as an endogenous target receptor of the oxysterols [82]. It is recognized that LXR forms a ‘permissive’ heterodimer with all isoforms of RXR and binds to LXRE of the target genes in an unliganded state. The consensus sequence is comprised of a direct repeat-4 (DR-4) DNA fragment with two AGGTCA half-sites separated by a 4-nucleotide spacer (5’-AGGTCA-NNNN-AGGTCA-3’) [13, 82]. Even though the dimers are already associated with the LXRE the in absence of ligand binding, LXR:RXR complexes suppress the transcriptional activity of the target genes by interacting with co-repressors, such as NCoR, SMRT and histone deacetylase 3 (HDAC3) [81]. Ligand binding triggers a conformational change in LXR:RXR dimer and leads to the release of co-repressors in exchange for co-activators, such as NAD+-dependent deacetylases Sirtuin 1 (SIRT1) [13]. Transrepression is also described for LXRs when LXRs become small ubiquitin-related modifier (SUMO)ylated upon agonist binding to LXR monomers. SUMOylated LXR monomer is often recruited to NFκB or AP1 promoters where it stabilizes the repressive nuclear complexes to inhibit the expression of inflammatory genes [81, 83]. For example, Lee et al. reported LXR-mediated repression of STAT1 signaling in interferon (IFN)-γ-stimulated brain astrocytes required SUMOylation [84]. However, transrepression is not the only way LXR participates in anti-inflammatory responses, as Ito et al. described that LXR activation could inhibit toll-like receptors (fs) 2, 4 and 9 signaling by inducing ABCA1 expression in macrophages [85].
The best described roles for LXRs are their ability to act as intracellular cholesterol sensors. It has been shown that elevated cholesterol levels induced by a high-cholesterol diet (HCD) led to a significant increase of 27HC, 4β-hydroxycholesterol (4βHC), 25-hydroxycholesterol (25HC) and 7KC in rabbit heart tissues [86]. A strong correlation between the concentration of 27HC in plasma and the concentration of cholesterol was also described in humans [87]. Additionally, high cholesterol levels induced the expression of LXRα [86]. These data indicate that, when cholesterol levels are raised, 27HC and other oxysterols are produced as a part of the feedback loop to engage and activate LXRs to elicit signaling pathways responsible for restoring the cholesterol homeostasis. The most prominent target genes involved in these pathways are the ATP binding cassette (ABC) transporters, promoting the efflux and reverse transport of cholesterol to the outside of cells. Studies have demonstrated that exogenous 27HC directly induces the expression of ABCA1 and ABCG1 [22, 88]. Mechanistically, ABCA1 promotes the transfer of free cholesterol and phospholipids to lipid-poor apolipoproteins (apoA-1), forming discoidal high-density lipoprotein (HDL) particles [89]. Unlike ABCA1, ABCG1 is capable of driving cholesterol efflux to mature HDL, LDL, and cyclodextrin, but not to lipid-poor apoA-1 [89]. Yvan-Charvet et al. described that combined loss of ABCA1 and ABCG1 in mice impaired cholesterol efflux to HDL or apoA-1, resulting in accelerated atherosclerotic lesion formation, suggesting complementary roles of ABCA1 and ABCG1 in cholesterol efflux [90].
5. 27HC and Cancer
27HC was first described as a biochemical mediator of the effects of a high cholesterol diet on breast tumor growth [91]. This oxysterol was also found to play a role in endocrine resistance of breast cancer, as 27HC administration stimulates the growth of tamoxifen-resistant MCF7 cells to levels at least comparable to tamoxifen or E2 [91]. Later, 27HC’s roles were further studied in a variety of cancer types, including prostate cancer, lung cancer, colorectal cancer and glioblastoma [92]. Interestingly, studies revealed that 27HC’s effects on tumor progression were both cancer cell-intrinsic and extrinsic.
5.1. The cancer cell intrinsic effects of 27HC on cancer progression
5.1.1. 27HC generally promotes the proliferation of cancers driven by the estrogen receptor
The ERs are a critical regulator of innumerable physiological functions and play recognized or suggestive roles in cancer progression of various cancer types, such as breast, lung and brain cancer [93-96]. Since the discovery of 27HC as a natural ligand of the ERs, it has raised a possibility that 27HC could also play a number of important physiological roles by signaling through this receptor. In 2008, soon after recognizing 27HC as an endogenous SERM, DuSell et al. first reported that 27HC could undertake similar roles as E2 in breast cancer cell lines, which promotes ER activation and subsequent cellular proliferation of ERα+ cell lines [77]. This suggests that 27HC signaling through ERα could facilitate cancer progression by cancer cell-intrinsic mechanisms. In support of this notion, Wu et al. found that 27HC induced ERα+ breast cancer cell proliferation in vitro, and a selective ERα antagonist, methyl-piperidino-pyrazole, ablates this effect, suggesting the requirement of ERα for 27HC-driven breast cancer cell proliferation [97]. Additionally, 27HC also stimulates the growth of ERα+ breast tumors in preclinical mouse models [91,97]. The growth of MCF7 xenografts was stimulated by exogenous 27HC, and this effect was attenuated by co-treatment with the ER antagonist Fulvestrant [91]. Germline deletion of CYP27A1 increased latency and decreased tumor growth in MMTV-PyMT mice, while loss of CYP7B1 (and subsequent increased circulating 27HC) increased growth [91].
To understand how 27HC promotes breast tumor growth through the ER, Raza et al. examined p53 activity downstream of ER in MCF-7 cells [98]. Interestingly, 27HC treatment resulted in reduced p53 activation and expression, which could be attributed to increased E3 ubiquitin-protein ligase (MDM2) expression and dimerization with p53, resulting in p53 degradation [98]. In support of a MDM2-dependent mechanism, inhibiting MDM2-p53 interaction by Nutlin-3 restored MCF-7 proliferation to basal level [98]. In addition, 27HC was also found to augment the expression of Vascular endothelial growth factor (VEGF) through ERα signaling in ER+ breast cancer cells [99].
Growth factor signaling such as EGFRvIII has been shown to increase the expression of the low-density lipoprotein receptor (LDLR) in glioblastoma cancer cells, allowing for increased cholesterol uptake. It was reported that these cells were in fact reliant on this cholesterol uptake mechanism, short-circuiting of which through LXR induced cholesterol efflux inhibited proliferation and tumor growth [100]. Interestingly, growth hormone and insulin like growth factor-1 (IGF-1) have been shown to increase CYP27A1 promotor use and subsequent CYP27A1 activity [101], suggesting that growth factor signaling not only can increase cholesterol uptake, but also its conversion to 27HC. It has also been suggested that 27HC itself may increase the expression of EGFR2 (HER2) in MCF7 cells, but the paper did not include quantified data with controls for readers to evaluate [102].
27HC’s promotion of tumor development through cancer-intrinsic mechanisms in breast cancer cells not involving the ERs were also reported. Among those mechanisms, STAT3 was a common regulator. As 27HC significantly increased the level of phosphorylated STAT3 (pSTAT3) in both MCF-7 and T47D cells, while E2 did not, an ER-independent mechanism driven by 27HC was proposed by Zhu et al. [99]. Further investigation revealed that reactive oxygen species (ROS) production induced by 27HC in breast cancer cells promoted STAT3/VEGF signaling, resulting in enhanced angiogenesis of both ER+ and ER− breast cancer cells [99]. On top of angiogenesis and VEGF, STAT3 activation by 27HC also increased breast cancer invasion and migration through epithelial-mesenchymal transition (EMT) [103]. Genetic knockdown of STAT3 attenuated the invasive and migratory capacity of MCF-7 and T47D cells by transwell assay and reduced the expression as well as the activation of MMP9 previously stimulated by 27HC [103]. Structurally similar to 27HC, 25HC was shown to support migration and invasion of lung adenocarcinoma cells (ADC) through the LXRs and the expression of Snail in ADC was reduced by the loss of LXR [104].
Extrapolating the relevance to humans, 27HC content was found to be elevated in normal breast tissue and further elevated in tumors of ER+ breast cancer patients, compared to that in cancer-free controls [97]. Roberg-Larsen et al. detected an increased level of 27HC in exosomes secreted by human ER+ breast cancer cell line compared to an ER− one and non-cancerous cell line, even though the ER− cell line had higher cytoplasmic 27HC concentration [105]. More importantly, the expression of CYP7B1 was predictive of the overall survival of breast cancer patients [97]. Additionally, increased expression of CYP27A1 in humans is correlated with a higher likelihood of having a higher tumor grade [91]. A recent cohort study on a Swedish-based population with invasive breast carcinoma showed that higher CYP27A1 expression not only correlated with higher tumor grade and larger tumor size, but also increased the risk of late lethal disease, especially in postmenopausal ERα+ breast cancer patients [106]. On the other hand, studies have shown that CYP27A1 expression was correlated with longer survival in prostate cancer and breast cancer among premenopausal women while this protective effect might be lost in post-menopausal women [107, 108]. These seemingly conflicting consequences of CYP27A overexpression in human patients indicate that well-controlled prospective clinical trials are needed in order to evaluate CYP27A1’s true influence on cancer progression.
Apart from breast cancer, 27HC was found to promote the growth of several other cancer types through the ERs, including endometrial cancer, thyroid cancer, prostate cancer, melanoma and lung cancer. Wu et al. first found that 27HC could induce Ishikawa cell growth in vitro, a model of endometrial cancer [97]. Hyperthyroidism is one of the most common co-morbidities of endometrial cancer and thyroid health is an important risk factor of thyroid carcinoma development [109]. Intriguingly, the 27HC was found to also promote thyroid cancer progression by Revilla et al. [23]. Thyroid tumor samples from advanced disease were found to have lower expression of CYP7B1 and a higher concentration of 27HC [23]. Moreover, overexpression of CYP7B1 in a thyroid anaplastic carcinoma model, but not in cells derived from normal thyroid epithelial cells, halted cell growth and migration after LDL treatment [23].
Tian et al. found that cholesterol promotes melanoma tumors in a 27HC-dependent fashion [110]. They demonstrated in vivo that CYP27A1 ablation attenuated the increased melanoma progression driven by a high cholesterol diet [110]. Further analysis suggested the involvement of the ERα and phosphorylation of Akt and MAPK pathways [110].
Given the high local concentration of 27HC in the lung [111], the oxysterol, 27HC expectedly could participate in paracrine and autocrine signaling of the local lung tissue. Previous work has reported that 27HC promoted cell proliferation in ERβ-positive lung cancer cells, and such effect by 27HC and estrogen could be obstructed by ERβ-specific inhibitors [79]. Akin to the case of melanoma, the proliferative outcome of lung cancer cells was driven by PI3K-Akt signaling, confirmed by kinase inhibitors [79].
Another endocrine-related cancer, prostate cancer, was found to be affected by 27HC [112]. Specifically, 27HC increased the proliferation of LNCaP and PC3 cells, which was inhibited by the ER antagonist, Fulvestrant [112]. To investigate the relative contribution of the two ER isoforms, Raza et al. found that 27HC’s effect on prostate cancer growth was dependent on ERβ, as 27HC-induced proliferation was mitigated by ERβ selective inhibitor, PHTPP [112]. However, two independent studies have indicated a protective (anti-proliferative) effect of 27HC in prostate cancer. As described below (section 5.1.2), this protective effect could emanate from the reduction of cellular cholesterol content (due to cholesterol efflux) and therefore reduced proliferative capacity [44, 108].
5.1.2. 27HC generally impairs or has limited effects on the proliferation of ER-negative cells.
In the absence of the ER, 27HC was found to have limited impact on or even inhibit cancer cell proliferation. In ER-negative lung cancer and cellular models of triple-negative breast cancer (TNBC), 27HC did not significantly impact cell proliferation [10, 79]. On the other hand, prostate cancer cells with little or no ER had reduced proliferation when CYP27A1 was overexpressed or were treated with 27HC [108]. Likewise, this was apparent in several ER-negative models of ovarian cancer, including ID8, MOE, HEYA8 and ES2: when exogenous 27HC was administered, there was a dose-dependent decrease in cellular proliferation of those cell lines [113]. It is generally thought that the anti-proliferative effects of 27HC and other LXR agonists are due to either inhibition of SREBP2 and/or activation of LXR and subsequent reduction in cellular cholesterol, as described for prostate cancer above [108]. Work in ovarian cancer cell lines suggested that activity through LXR and subsequent cholesterol efflux was most important for the anti-proliferative effects of 27HC [113].
However, the anti-proliferative effects in ovarian cancers and TNBC were at first counterintuitive, given that high expression of CYP27A1 was associated with decreased progression-free survival (PFS) of ovarian cancer patients, while high expression of CYP7B1 was associated with increased PFS [113], and CYP27A1 protein expression was associated with increased tumor grade in breast cancer [10]. These data and the previous work showing the ablation of 27HC’s pro-proliferative effect by blocking the ERs suggest that 27HC promotes cancer cell proliferation primarily through this receptor. However, when the ERs are not expressed or blocked by antagonists, 27HC could potentially persistently drive cancer progression through alternate mechanisms including therapy resistance or cancer cell-extrinsic mechanisms.
Interestingly, recent work has implicated LXR as a mediator of chemoresistance in ER-negative breast cancer [114]. Both the synthetic ligand of LXR, GW3965, and oxysterols, including 27HC, were able to diminish the effect of epirubicin in TNBC cell lines, which could be attributed to the upregulation of chemotherapy efflux pump Pgp in an LXRα-dependent manner [114]. An LXRα target gene signature was correlated with Pgp in human breast tumor samples. On the other hand, in vitro work indicated that 27HC did not alter sensitivity to doxorubicin in MCF7 cells [115]. In ovarian cancer however, 27HC was also shown to confer chemoprotective property in mice grafted with MOE cells [113].
5.2. 27HC’s tumor extrinsic effect on cancer progression
The conflicting effects of 27HC on different cancers could potentially be explained by the presence or absence of ER. However, in certain cases such as in ovarian cancer, the tumoral expression of CYP27A1 was associated with a poor prognosis in human patients while 27HC treatment of ovarian cancer cells was antiproliferative [113]. Furthermore, 27HC treatment resulted in increased breast cancer metastasis even in ER-negative models [12]. These apparently paradoxical observations suggested that alternative targets may mediate the effects of 27HC.
There is rising awareness of the tumor microenvironment’s roles in cancer development and progression, and growing number of studies have revealed cancer-extrinsic factors involved in dictating disease progression and response to therapy. Of those, tumor-infiltrating immune cell populations have been shown to have high predictive value in disease prognosis, even compared to traditional indicators in breast cancer, colorectal cancer, melanoma and pancreatic cancer [116-119]. Anti-PD1 (Pembrolizumab) and anti-PDL1 (Atezolizumab) are now approved for the treatment of several solid tumors, including a subset of patients with advanced TNBC [120-122]. However, these two immune checkpoint inhibitions have limited efficacy in the treatment of many solid tumors, including breast and ovarian. The efficacy of immune checkpoint inhibitors relies on the functionalities of the host immune system and studies have shown that immune cell infiltration is associated with the response to immunotherapy [123-126]. Therefore, it was of interest as to whether 27HC could modulate immune cell recruitment or function within the tumor microenvironments.
Previous work showed that human and mouse tumor-derived LXR ligands could inhibit C-C chemokine receptor 7 (CCR7) expression on mature dendritic cells, thereby hindering their migration to the lymphoid organs [127]. These findings back the possibility of 27HC, as an LXR ligand, acting on the cells of myeloid origin at the tumor site during tumor progression. In support of this, Baek et al. showed that 27HC treatment of metastasis-bearing mice led to increased myeloid cell infiltration, both inflammatory monocytes and polymorphoneutrophils (PMNs), and a decrease of intratumoral CD8+ cytotoxic T cells [12]. Follow-up experiments found that the prometastatic effect of 27HC required myeloid immune cells, and specifically PMNs. γδ-T cells were also found to be critical, likely playing a role in conditioning the PMNs to be pro-tumorigenic [12].
Interestingly, in models of ovarian cancer, it was found that inflammatory monocytes (monocytic myeloid-derived suppressor cells, M-MDSCs, defined as CD11b+Ly6C+Ly6G−) and immature macrophages (CD11b+Ly6C+IA/IE+) were enriched post administration of 27HC [113]. Tumors grown in CYP27A1−/− mice failed to thrive and regressed to non-detectable levels in many of the CYP27A1−/− mice. Bone marrow-derived cells were implicated as a marrow transplant from wildtype mice allowed tumors to establish and grow in CYP27A1−/− mice. These results strongly implicate the immune system in mediating the effects of 27HC on cancer progression. They also support the notion that 27HC can work through different myeloid immune cell types to suppress adaptive immune function.
In this regard, we have recently determined that 27HC can act through myeloid immune cells to inhibit T cell expansion and function [22]. Since tumor-infiltrating macrophages are a prototypical myeloid immune cell, and one of the most abundant cell types residing in and constantly being recruited to the tumor site, we focused on how 27HC regulates the function of this cell type. In this study, 27HC was shown to modulate the expression of antigen presentation genes in macrophages, again supporting the notion that 27HC modulates myeloid – T cell interactions. Using co-culture systems, it was found that 27HC-treated macrophages impaired T cell activation and expansion [22]. This phenomenon was regardless of whether CD8+, CD4+ or naive CD4+ T cells were co-cultured with the macrophages. Interestingly, the conditioned media from 27HC-treated macrophages also inhibited T cell expansion, indicating that the effects were being mediated through the secretion of a soluble factor. The inhibitory effects were also observed when PMNs were treated with 27HC, again indicating that 27HC can work through different myeloid immune cell types to suppress adaptive immune cells.
It was found that T cells also underwent a greater level of advanced apoptosis after being exposed to 27HC-treated macrophages [22]. Importantly, those CD8+ T cells that did expand were less functionally active, as they expressed decreased levels of the effector enzymes perforin and granzyme B and had decreased cytolytic capacity when co-cultured with tumor cells expressing the antigen specific for their T cell receptor [22]. In an effort to demonstrate the clinical relevance of these findings, the study surveyed the Human Cancer Genome Atlas (TCGA) and found positive correlations between tumoral CYP7B1 mRNA in invasive breast cancer carcinoma and the expression of several genes encoding T cell effector proteins including CD3G, CD8A, perforin 1, granzyme B, and IFN-γ [22].
Because 27HC is a ligand of the ER and LXR, roles of these two nuclear receptors in mediating 27HC’s suppressive effect were assessed. Using both pharmacologic and RNA-interference approaches, it was found that the ER within macrophages was dispensable for the inhibitory actions of 27HC-treated macrophages [22]. However, the LXR within macrophages was required for the subsequent inhibitory effects on T cells. Expanding on these findings, it was found that several oxysterols and other LXR ligands also worked on macrophages to reduce T cell expansion. Further, there was a positive correlation of the ability of a ligand to induce the LXR target gene ABCA1, and T cell expansion [22]. Previous work described that LXR activation within T cells impairs their proliferation [128]. This work on the other hand demonstrates that the LXR-mediated suppression on T cells could also be indirect and mediated through macrophages.
In preclinical models, mice lacking CYP27A1 in the myeloid cell population developed smaller breast tumors and exhibited less metastasis, indicating that targeting the production of 27HC or the 27HC-LXR axis may attenuate the suppressive effects of 27HC in the tumor microenvironment [22]. Such attenuation may eventually help alleviate T cell restraint, improve checkpoint inhibition efficacy and reduce tumor progression. Indeed, one study found that administration of a CYP27A1 inhibitor along with checkpoint inhibitor, anti-PDL1, helped overcome immunotherapy resistance in the 4T1 model of metastatic breast cancer [22]. Additionally, this combination therapy was also shown to be effective in reducing ovarian tumor burden in mice [113]. Recent findings by Yu et al. also support the possible influence of cholesterol and/or cholesterol metabolites on macrophage-driven T cell death, as the study showed that F4/80+ cells isolated from liver metastasis, a high cholesterol microenvironment, induced apoptosis of OT-1 T cells [129]. Another group of researchers showed that an inverse agonist of LXR promotes CD8+ T cell activity and enhances tumor destruction, although whether these effects were myeloid cell-driven was not investigated [130]. Furthermore, inhibiting cholesterol synthesis by zaragozic acid A, which decreased LXR target gene expression in the tumor microenvironment, potentiates the tumoricidal ability of antitumor T cells in vivo [131]. Similarly, blocking PCSK9 to reduce cholesterol metabolism synergized with immune checkpoint blockade, anti-PD1, to reduce the tumor burden of B16F10, MC38, 4T1 or CT26 models, some of which are known to be resistant to immune checkpoint monotherapy [7]. Subsequent analysis showed that depleting PCSK9 could promote intratumoral infiltration of T cells [7]. These data provide robust support for developing therapeutic targets along the cholesterol metabolic axis (cholesterol-CYP27A1-27HC-LXR) for bolstering the immune response to various cancers.
However, LXR signaling and effects on immune function may be ligand- and context-specific. Several studies also suggested the immune-protective roles of LXR. In 2018, Tavazoie et al. demonstrated that agonism of LXR led to decreased MDSC abundance in murine melanoma models as well as in human samples. This same ligand improved checkpoint blockade efficacy in the B16F10 mouse model [132]. Recent work also showed that pharmacological activation of LXR using T0901317 downregulates the expression of Ccl7 in macrophages, a Treg-attracting chemokine [133]. Another study suggested that activation of LXR through synthetic ligand, GW3965, could promote natural killer (NK) cell-mediated cytotoxicity against cancer cells [134]. Outside cancer, an LXR agonist was found to augment the pro-inflammatory signal in human monocytes trained by Bacillus Calmette-Guerin (BCG) vaccine [135]. However, an earlier study also suggested that activation of LXR through tumor-derived factors (oxysterols) dampened the maturation and therefore the anti-tumor response of dendritic cells [127].
The conflicting roles of LXR in immunoregulation and anti-tumor functions could be due to several reasons: 1. the consequence of LXR activation is likely context-dependent, being tissue-type and cell-type specific. 2. Cholesterol metabolite signaling (e.g., 27HC) may result in the crosstalk between multiple receptors, so the observed effects may not be solely accredited to the activation of LXR. 3. LXR activation by synthetic agonists could yield a protective effect, yet natural ligands (oxysterols and cholesterol metabolites) may lead to pro-tumor consequences due to their selective modulatory activities [136]. 4. There are possible off-target effects of synthetic LXR agonist that render enhanced immune surveillance and anti-tumor outcome [137]. Nevertheless, further investigations are needed to better understand the immunoregulatory roles of LXR and its relationship to anti-tumor functions of immune cells. It is also important to delineate the differences in LXR activation and downstream signaling cascade driven by 27HC and other cholesterol metabolites versus the synthetic ligands in various tissue types and cancer types to better understand the pro-tumor and anti-tumor paradox of LXR.
6. Conclusion
27HC has been historically understood as being important in the regulation of cholesterol metabolism and homeostasis. When cholesterol is in excess, 27HC can bind to INSIGs to inhibit cholesterol synthesis through SREBPs, and LXRs to promote cholesterol efflux through ABCA1 and ABCG1 and decrease uptake by downregulating LDLR. The ERs are an additional nuclear receptor whose activity 27HC and several other oxysterols can modulate. As a SERM, 27HC has been demonstrated to activate or antagonize the ERs under a tissue-specific context. Signaling through the ER, 27HC plays important roles in the cardiovascular system, where it is the most common oxysterol present in atherosclerotic lesions [138]. Specifically, it was observed that 27HC competes with estradiol to regulate iNOS and eNOS and impairs the E2-stimulated reendothelialization upon artificial injury [76]. On the other hand, 27HC does not seem to influence all estradiol-ER-driven functions, as 27HC did not disrupt the reproductive functions of mice lacking CYP7B1 [76, 139]. Therefore, the precise physiological roles of 27HC signaling through the ERs remain to be elucidated. Nevertheless, behaving as an ER agonist, 27HC stimulates tumor growth of various origins, including endocrine-related breast and prostate tumors. However, in the case of prostate and ovarian cancer, the contributions of 27HC to its progression appear to be a balanced outcome between 27HC serving as an ER agonist and its role as a cholesterol regulator; on one hand, 27HC was shown to promote prostate cancer cell proliferation through ERβ, while on the other, the reduction of cellular cholesterol content signaled by 27HC was unfavorable to tumor growth [108, 112].
The complexity of 27HC’s tumor-promoting roles was further revealed through its influence of the tumor microenvironment. To date, this facet of 27HC biology seems to lie in its target receptor, LXR. LXR activation itself was found to suppress various inflammatory gene expression in macrophages and other immune cells [85, 140, 141]. Expectedly, under tumor development, abundant infiltrating macrophages and cancer cells themselves could induce LXR activity through an increased activity of cholesterol metabolism brought by cells, especially in the context of hypercholesterolemia. This therefore could lead to increased anti-inflammatory phenotype, decreased immune surveillance and increased tumor growth. Indeed, studies have found that oxysterols such as 27HC led to increased metastasis, likely due to increased tumor-associated myeloid immune cells and decreased T cell cytotoxicity. LXR was shown to play an indispensable role in some of these processes. Blockade of targets along the cholesterol-metabolic axis boosted the immune functions against tumor cells in various tumor models. However, it was also shown that LXR activation could be beneficial to anti-tumor activities, as it depletes MDSC infiltration into the tumor microenvironment. Studies also showed direct pro-inflammatory actions of LXR activation by synthetic agonists. One example was increasing Bacillus Calmette-Guerin (BCG) vaccine-trained immunity in monocytes [135]. We discussed several possibilities that might have caused this paradox, especially in the tumor context: 1. the tissue-type and cell-type specific consequence of LXR activation. 2. Effects mediated by cholesterol metabolite (e.g., 27HC) signaling could involve crosstalk between multiple nuclear receptors. 3. Natural ligands (oxysterols and cholesterol metabolites) of cholesterol could have selective modulatory effects. 4. The off-target effects of LXR agonists may play a role in the anti-tumor effect. Further investigation will be needed to validate the participation of LXR and the difference between LXR agonists and cholesterol metabolites in their influence on cancer development.
Despite the confounding contributions of LXR, cholesterol metabolism and 27HC, the most prevalent oxysterol present in circulation, has been shown to directly and indirectly promote breast cancer progression. Targeting the synthesis of 27HC presents a logical option to circumvent the to-be-scrutinized roles of LXR. Various independent studies have shown that inhibiting the enzyme CYP27A1 that generates 27HC could reduce tumor development and improve checkpoint inhibition efficacy [12, 22, 113]. Additionally, a marketed aromatase inhibitor, anastrozole, had off-target effects as an inhibitor of CYP27A1 without altering plasma and hepatic cholesterol content, implying the minimal impact this approach may put on cholesterol homeostasis [142], and that the efficacy of aromatase inhibitors may be in part due to their influence on this axis. Upstream of CYP27A1, a recent study targeting PCSK9 to reduce cholesterol levels also poses a resolution to improve checkpoint inhibition efficacy through targeting this axis [7]. The use of statin to directly inhibit cholesterol synthesis may also be effective. Indeed, observational studies have shown statin use is associated better response rate or clinical outcome of checkpoint inhibitors in NSCLC [8, 9]. Yet, it will be important to determine the long-term efficacy of this approach, as cholesterol homeostasis might be restored, and the beneficial effect could be counteracted by increased cholesterol synthesis in response to the lowered cellular cholesterol level.
In order to realize the therapeutic translation of this axis, we call for future research with the following directions: (1) Prospective clinical trials to evaluate the influence of and interactions between circulating cholesterol/27HC, CYP27A1 and CYP7B1 and statin therapy on cancer progression. (2) Characterization of 27HC within the inner and outer leaflets of the plasma membrane. (3) Determine if and how 27HC interacts with membrane associated proteins, and how this may influence subsequent signaling or the effects of cholesterol itself. (4) Mechanistic studies to determine the selective modulator activity of different ligands on the LXR. (5) Development of more pharmacologically suitable small molecule inhibitors of CYP27A1.
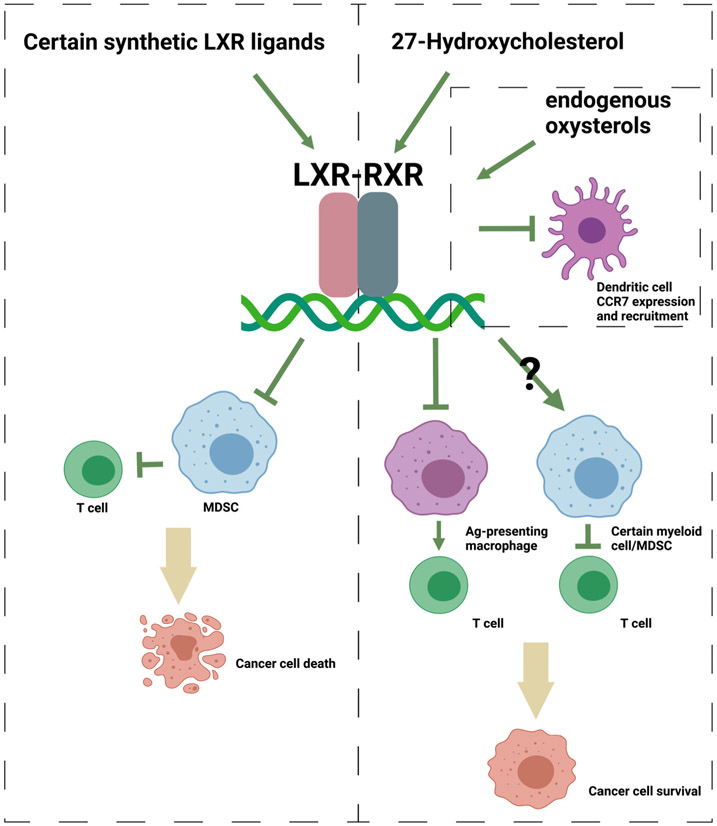
Figure 2. The LXRs have multifaceted contributions to cancer progression.
When the LXRs were activated by certain synthetic agonists, MDSC population was depleted, leading to augmented T cell activity and cancer death in various cancer models. On the other hand, 27HC has been shown to increase the recruitment and differentiation of other types of myeloid cells including MDSCs to the tumor site, resulting in impaired T cell function. The precise role of LXR in this process has not been determined. 27HC could activate LXR in Ag-presenting macrophages and other myeloid cells such as neutrophils, resulting in a phenotype that confers suppressive effects on T cells. Additionally, endogenously produced oxysterols have been shown to decrease the expression of CCR7 within dendritic cells, impairing their ability to migrate towards tumor sites. Figure created with BioRender.
Acknowledgments:
This work was supported by the National Cancer Institute of the National Institutes of Health (R01CA234025; ERN), the National Institute of General Medical Sciences of the National Institutes of Health (R35GM122530; WC) and the Department of Defense Breast Cancer Research Program Era of Hope Scholar Award (W81XWH-20-BCRP-EOHS / BC200206; ERN). LM was supported by a Julie and David Mead Endowed Graduate Student Fellowship.
Footnotes
Conflict of interest: The authors have nothing to declare
References
- [1].Inoue Y, Qin B, Poti J, Sokol R, Gordon-Larsen P, Epidemiology of Obesity in Adults: Latest Trends, Curr Obes Rep, 7 (2018) 276–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pi-Sunyer X, The medical risks of obesity, Postgrad Med, 121 (2009) 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gostynski M, Gutzwiller F, Kuulasmaa K, Doring A, Ferrario M, Grafnetter D, Pajak A, Project WM, Analysis of the relationship between total cholesterol, age, body mass index among males and females in the WHO MONICA Project, Int J Obes Relat Metab Disord, 28 (2004)1082–1090. [DOI] [PubMed] [Google Scholar]
- [4].Garcia-Estevez L, Moreno-Bueno G, Updating the role of obesity and cholesterol in breast cancer, Breast Cancer Res, 21 (2019) 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kwan ML, Habel LA, Flick ED, Quesenberry CP, Caan B, Post-diagnosis statin use and breast cancer recurrence in a prospective cohort study of early stage breast cancer survivors, Breast Cancer Res Treat, 109 (2008) 573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nielsen SF, Nordestgaard BG, Bojesen SE, Statin use and reduced cancer-related mortality, N Engl J Med, 367 (2012) 1792–1802. [DOI] [PubMed] [Google Scholar]
- [7].Liu X, Bao X, Hu M, Chang H, Jiao M, Cheng J, Xie L, Huang Q, Li F, Li CY, Inhibition of PCSK9 potentiates immune checkpoint therapy for cancer, Nature, 588 (2020) 693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cantini L, Pecci F, Hurkmans DP, Belderbos RA, Lanese A, Copparoni C, Aerts S, Cornelissen R, Dumoulin DW, Fiordoliva I, Rinaldi S, Aerts J, Berardi R, High-intensity statins are associated with improved clinical activity of PD-1 inhibitors in malignant pleural mesothelioma and advanced non-small cell lung cancer patients, Eur J Cancer, 144 (2021) 41–48. [DOI] [PubMed] [Google Scholar]
- [9].Omori M, Okuma Y, Hakozaki T, Hosomi Y, Statins improve survival in patients previously treated with nivolumab for advanced non-small cell lung cancer: An observational study, Mol Clin Oncol, 10 (2019) 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nelson ER, Wardell SE, Jasper JS, Park S, Suchindran S, Howe MK, Carver NJ, Pillai RV, Sullivan PM, Sondhi V, Umetani M, Geradts J, McDonnell DP, 27-Hydroxycholesterol Links Hypercholesterolemia and Breast Cancer Pathophysiology, Science (New York, N.Y.), 342 (2013) 1094–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Baek AE, Nelson ER, The Contribution of Cholesterol and Its Metabolites to the Pathophysiology of Breast Cancer, Horm Cancer, 7 (2016) 219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Baek AE, Yu YA, He S, Wardell SE, Chang CY, Kwon S, Pillai RV, McDowell HB, Thompson JW, Dubois LG, Sullivan PM, Kemper JK, Gunn MD, McDonnell DP, Nelson ER, The cholesterol metabolite 27 hydroxycholesterol facilitates breast cancer metastasis through its actions on immune cells, Nat Commun, 8 (2017) 864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ma L, Nelson ER, Oxysterols and nuclear receptors, Mol Cell Endocrinol, 484 (2019) 42–51. [DOI] [PubMed] [Google Scholar]
- [14].McDonnell DP, Park S, Goulet MT, Jasper J, Wardell SE, Chang CY, Norris JD, Guyton JR, Nelson ER, Obesity, cholesterol metabolism, and breast cancer pathogenesis, Cancer Res, 74 (2014) 4976–4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Luo J, Yang H, Song BL, Mechanisms and regulation of cholesterol homeostasis, Nat Rev Mol Cell Biol, 21 (2020) 225–245. [DOI] [PubMed] [Google Scholar]
- [16].Zelcer N, Hong C, Boyadjian R, Tontonoz P, LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor, Science, 325 (2009) 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ouvrier A, Cadet R, Vernet P, Laillet B, Chardigny JM, Lobaccaro JM, Drevet JR, Saez F, LXR and ABCA1 control cholesterol homeostasis in the proximal mouse epididymis in a cell-specific manner, J Lipid Res, 50 (2009) 1766–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].He S, Nelson ER, 27-Hydroxycholesterol, an endogenous selective estrogen receptor modulator, Maturitas, 104 (2017) 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Burkard I, Rentsch KM, von Eckardstein A, Determination of 24S- and 27-hydroxycholesterol in plasma by high-performance liquid chromatography-mass spectrometry, J Lipid Res, 45 (2004) 776–781. [DOI] [PubMed] [Google Scholar]
- [20].Lee WR, Ishikawa T, Umetani M, The interaction between metabolism, cancer and cardiovascular disease, connected by 27-hydroxycholesterol, Clinical lipidology, 9 (2014) 617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Umetani M, Shaul PW, 27-Hydroxycholesterol: the first identified endogenous SERM, Trends Endocrinol Metab, 22 (2011) 130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ma L, Wang L, Nelson AT, Han C, He S, Henn MA, Menon K, Chen JJ, Baek AE, Vardanyan A, Shahoei SH, Park S, Shapiro DJ, Nanjappa SG, Nelson ER, 27-Hydroxycholesterol acts on myeloid immune cells to induce T cell dysfunction, promoting breast cancer progression, Cancer letters, 493 (2020) 266–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Revilla G, Pons MP, Baila-Rueda L, Garcia-Leon A, Santos D, Cenarro A, Magalhaes M, Blanco RM, Moral A, Ignacio Perez J, Sabe G, Gonzalez C, Fuste V, Lerma E, Faria MDS, de Leiva A, Corcoy R, Carles Escola-Gil J, Mato E, Cholesterol and 27-hydroxycholesterol promote thyroid carcinoma aggressiveness, Sci Rep, 9 (2019) 10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Umetani M, Ghosh P, Ishikawa T, Umetani J, Ahmed M, Mineo C, Shaul PW, The cholesterol metabolite 27-hydroxycholesterol promotes atherosclerosis via proinflammatory processes mediated by estrogen receptor alpha, Cell Metab, 20 (2014) 172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lange Y, Swaisgood MH, Ramos BV, Steck TL, Plasma membranes contain half the phospholipid and 90% of the cholesterol and sphingomyelin in cultured human fibroblasts, J Biol Chem, 264 (1989) 3786–3793. [PubMed] [Google Scholar]
- [26].Yeagle PL, Cholesterol and the cell membrane, Biochim Biophys Acta, 822 (1985) 267–287. [DOI] [PubMed] [Google Scholar]
- [27].Lingwood D, Simons K, Lipid rafts as a membrane-organizing principle, Science, 327 (2010) 46–50. [DOI] [PubMed] [Google Scholar]
- [28].Sezgin E, Levental I, Mayor S, Eggeling C, The mystery of membrane organization: composition, regulation and roles of lipid rafts, Nat Rev Mol Cell Biol, 18 (2017) 361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen Q, Pan Z, Zhao M, Wang Q, Qiao C, Miao L, Ding X, High cholesterol in lipid rafts reduces the sensitivity to EGFR-TKI therapy in non-small cell lung cancer, Journal of cellular physiology, 233 (2018) 6722–6732. [DOI] [PubMed] [Google Scholar]
- [30].Irwin ME, Bohin N, Boerner JL, Src family kinases mediate epidermal growth factor receptor signaling from lipid rafts in breast cancer cells, Cancer Biol Ther, 12 (2011) 718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li YC, Park MJ, Ye SK, Kim CW, Kim YN, Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents, Am J Pathol, 168 (2006) 1107–1118; quiz 1404-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhuang L, Lin J, Lu ML, Solomon KR, Freeman MR, Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells, Cancer Res, 62 (2002) 2227–2231. [PubMed] [Google Scholar]
- [33].Klymchenko AS, Kreder R, Fluorescent probes for lipid rafts: from model membranes to living cells, Chem. Biol, 21 (2014) 97–113. [DOI] [PubMed] [Google Scholar]
- [34].Nelson ER, Chang CY, McDonnell DP, Cholesterol and breast cancer pathophysiology, Trends Endocrinol Metab, 25 (2014) 649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fantini J, Barrantes FJ, How cholesterol interacts with membrane proteins: an exploration of cholesterol-binding sites including CRAC, CARC, and tilted domains, Front Physiol, 4 (2013) 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kiriakidi S, Kolocouris A, Liapakis G, Ikram S, Durdagi S, Mavromoustakos T, Effects of Cholesterol on GPCR Function: Insights from Computational and Experimental Studies, Adv. Exp. Med. Biol, 1135 (2019) 89–103. [DOI] [PubMed] [Google Scholar]
- [37].Wang PY, Weng J, Anderson RG, OSBP is a cholesterol-regulated scaffolding protein in control of ERK 1/2 activation, Science, 307 (2005) 1472–1476. [DOI] [PubMed] [Google Scholar]
- [38].Sheng R, Chen Y, Yung Gee H, Stec E, Melowic HR, Blatner NR, Tun MP, Kim Y, Kallberg M, Fujiwara TK, Hye Hong J, Pyo Kim K, Lu H, Kusumi A, Goo Lee M, Cho W, Cholesterol modulates cell signaling and protein networking by specifically interacting with PDZ domain-containing scaffold proteins, Nat Commun, 3 (2012) 1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sheng R, Kim H, Lee H, Xin Y, Chen Y, Tian W, Cui Y, Choi JC, Doh J, Han JK, Cho W, Cholesterol selectively activates canonical Wnt signalling over non-canonical Wnt signalling, Nat Commun, 5 (2014) 4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Francis KR, Ton AN, Xin Y, O'Halloran PE, Wassif CA, Malik N, Williams IM, Cluzeau CV, Trivedi NS, Pavan WJ, Cho W, Westphal H, Porter FD, Modeling Smith-Lemli-Opitz syndrome with induced pluripotent stem cells reveals a causal role for Wnt/beta-catenin defects in neuronal cholesterol synthesis phenotypes, Nat. Med, 22 (2016) 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Liu SL, Sheng R, Jung JH, Wang L, Stec E, O'Connor MJ, Song S, Bikkavilli RK, Winn RA, Lee D, Baek K, Ueda K, Levitan I, Kim KP, Cho W, Orthogonal lipid sensors identify transbilayer asymmetry of plasma membrane cholesterol, Nature chemical biology, 13 (2017) 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhang Y, Bulkley DP, Xin Y, Roberts KJ, Asarnow DE, Sharma A, Myers BR, Cho W, Cheng Y, Beachy PA, Structural Basis for Cholesterol Transport-like Activity of the Hedgehog Receptor Patched, Cell, 175 (2018) 1352–1364 e1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ragot K, Mackrill JJ, Zarrouk A, Nury T, Aires V, Jacquin A, Athias A, Pais de Barros JP, Véjux A, Riedinger JM, Delmas D, Lizard G, Absence of correlation between oxysterol accumulation in lipid raft microdomains, calcium increase, and apoptosis induction on 158N murine oligodendrocytes, Biochem Pharmacol, 86 (2013) 67–79. [DOI] [PubMed] [Google Scholar]
- [44].Dambal S, Alfaqih M, Sanders S, Maravilla E, Ramirez-Torres A, Galvan GC, Reis-Sobreiro M, Rotinen M, Driver LM, Behrove MS, Talisman TJ, Yoon J, You S, Turkson J, Chi JT, Freeman MR, Macias E, Freedland SJ, 27-Hydroxycholesterol Impairs Plasma Membrane Lipid Raft Signaling as Evidenced by Inhibition of IL6-JAK-STAT3 Signaling in Prostate Cancer Cells, Mol Cancer Res, 18 (2020) 671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wang Y, Zhang X, Wang T, Liu W, Wang L, Hao L, Ju M, Xiao R, 27-Hydroxycholesterol Promotes the Transfer of Astrocyte-Derived Cholesterol to Neurons in Co-cultured SH-SY5Y Cells and C6 Cells, Front Cell Dev Biol, 8 (2020) 580599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Umetani M, Domoto H, Gormley A, Yuhanna IS, Cummins CL, Javitt NB, Korach KS, Shaul PW, Mangelsdorf DJ, 27-hydroxycholesterol is an endogenous selective estrogen receptor modulator that inhibits the cardiovascular effects of estrogen, Nature Med., 13 (2007) 1185–1192. [DOI] [PubMed] [Google Scholar]
- [47].Raza S, Meyer M, Schommer J, Hammer KD, Guo B, Ghribi O, 27-Hydroxycholesterol stimulates cell proliferation and resistance to docetaxel-induced apoptosis in prostate epithelial cells, Medical oncology (Northwood, London, England), 33 (2016) 12. [DOI] [PubMed] [Google Scholar]
- [48].Raccosta L, Fontana R, Maggioni D, Lanterna C, Villablanca EJ, Paniccia A, Musumeci A, Chiricozzi E, Trincavelli ML, Daniele S, Martini C, Gustafsson JA, Doglioni C, Feo SG, Leiva A, Ciampa MG, Mauri L, Sensi C, Prinetti A, Eberini I, Mora JR, Bordignon C, Steffensen KR, Sonnino S, Sozzani S, Traversari C, Russo V, The oxysterol-CXCR2 axis plays a key role in the recruitment of tumor-promoting neutrophils, J Exp Med, 210 (2013) 1711–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hannedouche S, Zhang J, Yi T, Shen W, Nguyen D, Pereira JP, Guerini D, Baumgarten BU, Roggo S, Wen B, Knochenmuss R, Noël S, Gessier F, Kelly LM, Vanek M, Laurent S, Preuss I, Miault C, Christen I, Karuna R, Li W, Koo D.l., Suply T, Schmedt C, Peters EC, Falchetto R, Katopodis A, Spanka C, Roy MO, Detheux M, Chen YA, Schultz PG, Cho CY, Seuwen K, Cyster JG, Sailer AW, Oxysterols direct immune cell migration via EBI2, Nature, 475 (2011) 524–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Radhakrishnan A, Ikeda Y, Kwon HJ, Brown MS, Goldstein JL, Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: oxysterols block transport by binding to Insig, Proc Natl Acad Sci U S A, 104 (2007) 6511–6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Weikum ER, Liu X, Ortlund EA, The nuclear receptor superfamily: A structural perspective, Protein Sci, 27 (2018) 1876–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Umetani M, Re-adopting classical nuclear receptors by cholesterol metabolites, The Journal of steroid biochemistry and molecular biology, 157 (2016) 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Fuentes N, Silveyra P, Estrogen receptor signaling mechanisms, Adv Protein Chem Struct Biol, 116 (2019) 135–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Vrtačnik P, Ostanek B, Mencej-Bedrač S, Marc J, The many faces of estrogen signaling, Biochem Med (Zagreb), 24 (2014) 329–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Barton M, Filardo EJ, Lolait SJ, Thomas P, Maggiolini M, Prossnitz ER, Twenty years of the G protein-coupled estrogen receptor GPER: Historical and personal perspectives, J Steroid Biochem Mol Biol, 176 (2018) 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Levin ER, Extranuclear estrogen receptor's roles in physiology: lessons from mouse models, Am J Physiol Endocrinol Metab, 307 (2014) E133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Gruber CJ, Tschugguel W, Schneeberger C, Huber JC, Production and actions of estrogens, N Engl J Med, 346 (2002) 340–352. [DOI] [PubMed] [Google Scholar]
- [58].Nelson LR, Bulun SE, Estrogen production and action, J Am Acad Dermatol, 45 (2001) S116–124. [DOI] [PubMed] [Google Scholar]
- [59].Yaşar P, Ayaz G, User SD, Güpür G, Muyan M, Molecular mechanism of estrogenestrogen receptor signaling, Reprod Med Biol, 16 (2017) 4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].An KC, Selective Estrogen Receptor Modulators, Asian Spine J, 10 (2016) 787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wardell SE, Nelson ER, McDonnell DP, From empirical to mechanism-based discovery of clinically useful Selective Estrogen Receptor Modulators (SERMs), Steroids, 90 (2014) 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Nelson ER, Wardell SE, McDonnell DP, The molecular mechanisms underlying the pharmacological actions of estrogens, SERMs and oxysterols: implications for the treatment and prevention of osteoporosis, Bone, 53 (2013) 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Nelson ER, Habibi HR, Estrogen receptor function and regulation in fish and other vertebrates, Gen Comp Endocrinol, 192 (2013) 15–24. [DOI] [PubMed] [Google Scholar]
- [64].Wardell SE, Kazmin D, McDonnell DP, Research resource: Transcriptional profiling in a cellular model of breast cancer reveals functional and mechanistic differences between clinically relevant SERM and between SERM/estrogen complexes, Mol Endocrinol, 26 (2012) 1235–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Christiansen AR, Lipshultz L.l., Hotaling JM, Pastuszak AW, Selective androgen receptor modulators: the future of androgen therapy?, Transl Androl Urol, 9 (2020) S135–S148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Dutertre M, Smith CL, Molecular mechanisms of selective estrogen receptor modulator (SERM) action, J Pharmacol Exp Ther, 295 (2000) 431–437. [PubMed] [Google Scholar]
- [67].Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, Greene GL, The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen, Cell, 95 (1998) 927–937. [DOI] [PubMed] [Google Scholar]
- [68].Paige LA, Christensen DJ, Gron H, Norris JD, Gottlin EB, Padilla KM, Chang CY, Balias LM, Hamilton PT, McDonnell DP, Fowlkes DM, Estrogen receptor (ER) modulators each induce distinct conformational changes in ER alpha and ER beta, Proc Natl Acad Sci U S A, 96 (1999) 3999–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Norris JD, Paige LA, Christensen DJ, Chang CY, Huacani MR, Fan D, Hamilton PT, Fowlkes DM, McDonnell DP, Peptide antagonists of the human estrogen receptor, Science, 285 (1999) 744–746. [DOI] [PubMed] [Google Scholar]
- [70].Lonard DM, Lanz RB, O'Malley BW, Nuclear receptor coregulators and human disease, Endocr Rev, 28 (2007) 575–587. [DOI] [PubMed] [Google Scholar]
- [71].Varlakhanova N, Snyder C, Jose S, Hahm JB, Privalsky ML, Estrogen receptors recruit SMRT and N-CoR corepressors through newly recognized contacts between the corepressor N terminus and the receptor DNA binding domain, Mol Cell Biol, 30 (2010) 1434–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].McKenna NJ, O'Malley BW, Combinatorial control of gene expression by nuclear receptors and coregulators, Cell, 108 (2002) 465–474. [DOI] [PubMed] [Google Scholar]
- [73].Auboeuf D, Hönig A, Berget SM, O'Malley BW, Coordinate regulation of transcription and splicing by steroid receptor coregulators, Science, 298 (2002) 416–419. [DOI] [PubMed] [Google Scholar]
- [74].Smith CL, Nawaz Z, O'Malley BW, Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen, Mol Endocrinol, 11 (1997) 657–666. [DOI] [PubMed] [Google Scholar]
- [75].Martinkovich S, Shah D, Planey SL, Arnott JA, Selective estrogen receptor modulators: tissue specificity and clinical utility, Clin Interv Aging, 9 (2014) 1437–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Umetani M, Domoto H, Gormley AK, Yuhanna IS, Cummins CL, Javitt NB, Korach KS, Shaul PW, Mangelsdorf DJ, 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen, Nat Med, 13 (2007) 1185–1192. [DOI] [PubMed] [Google Scholar]
- [77].DuSell CD, Umetani M, Shaul PW, Mangelsdorf DJ, McDonnell DP, 27-hydroxycholesterol is an endogenous selective estrogen receptor modulator, Mol Endocrinol, 22 (2008) 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Lappano R, Recchia AG, De Francesco EM, Angelone T, Cerra MC, Picard D, Maggiolini M, The cholesterol metabolite 25-hydroxycholesterol activates estrogen receptor alpha-mediated signaling in cancer cells and in cardiomyocytes, PLoS One, 6 (2011) e16631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Hiramitsu S, Ishikawa T, Lee WR, Khan T, Crumbley C, Khwaja N, Zamanian F, Asghari A, Sen M, Zhang Y, Hawse JR, Minna JD, Umetani M, Estrogen Receptor Beta-Mediated Modulation of Lung Cancer Cell Proliferation by 27-Hydroxycholesterol, Front Endocrinol (Lausanne), 9 (2018) 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Cha E, Lee KM, Park KD, Park KS, Lee KW, Kim SM, Lee J, Hydroxycholesterol Levels in the Serum and Cerebrospinal Fluid of Patients with Neuromyelitis Optica Revealed by LC-Ag+CIS/MS/MS and LC-ESI/MS/MS with Picolinic Derivatization: Increased Levels and Association with Disability during Acute Attack, PLoS One, 11 (2016) e0167819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Courtney R, Landreth GE, LXR Regulation of Brain Cholesterol: From Development to Disease, Trends in endocrinology and metabolism: TEM, 27 (2016) 404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Wójcicka G, Jamroz-Wiśniewska A, Horoszewicz K, Bełtowski J, Liver X receptors (LXRs). Part I: structure, function, regulation of activity, and role in lipid metabolism, Postepy Hig Med Dosw (Online), 61 (2007) 736–759. [PubMed] [Google Scholar]
- [83].Ghisletti S, Huang W, Ogawa S, Pascual G, Lin ME, Willson TM, Rosenfeld MG, Glass CK, Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARgamma, Mol Cell, 25 (2007) 57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Lee JH, Park SM, Kim OS, Lee CS, Woo JH, Park SJ, Joe EH, Jou I, Differential SUMOylation of LXRalpha and LXRbeta mediates transrepression of STAT1 inflammatory signaling in IFN-gamma-stimulated brain astrocytes, Mol Cell, 35 (2009) 806–817. [DOI] [PubMed] [Google Scholar]
- [85].Ito A, Hong C, Rong X, Zhu X, Tarling EJ, Hedde PN, Gratton E, Parks J, Tontonoz P, LXRs link metabolism to inflammation through Abca1-dependent regulation of membrane composition and TLR signaling, Elife, 4 (2015) e08009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Sozen E, Yazgan B, Sahin A, Ince U, Ozer NK, High Cholesterol Diet-Induced Changes in Oxysterol and Scavenger Receptor Levels in Heart Tissue, Oxid Med Cell Longev, 2018 (2018) 8520746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Burkard I, von Eckardstein A, Waeber G, Vollenweider P, Rentsch KM, Lipoprotein distribution and biological variation of 24S- and 27-hydroxycholesterol in healthy volunteers, Atherosclerosis, 194 (2007) 71–78. [DOI] [PubMed] [Google Scholar]
- [88].Fu X, Menke JG, Chen Y, Zhou G, MacNaul KL, Wright SD, Sparrow CP, Lund EG, 27-hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells, J Biol Chem, 276 (2001) 38378–38387. [DOI] [PubMed] [Google Scholar]
- [89].Oram JF, Lawn RM, ABCA1. The gatekeeper for eliminating excess tissue cholesterol, J Lipid Res, 42 (2001) 1173–1179. [PubMed] [Google Scholar]
- [90].Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR, Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice, J Clin Invest, 117 (2007) 3900–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Nelson ER, Wardell SE, Jasper JS, Park S, Suchindran S, Howe MK, Carver NJ, Pillai RV, Sullivan PM, Sondhi V, Umetani M, Geradts J, McDonnell DP, 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology, Science, 342 (2013) 1094–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Asghari A, Umetani M, Obesity and Cancer: 27-Hydroxycholesterol, the Missing Link, Int J Mol Sci, 21 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME, Etiology of hormone receptor-defined breast cancer: a systematic review of the literature, Cancer Epidemiol Biomarkers Prev, 13 (2004) 1558–1568. [PubMed] [Google Scholar]
- [94].Siegfried JM, Hershberger PA, Stabile LP, Estrogen receptor signaling in lung cancer, Semin Oncol, 36 (2009) 524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Liu J, Sareddy GR, Zhou M, Viswanadhapalli S, Li X, Lai Z, Tekmal RR, Brenner A, Vadlamudi RK, Differential Effects of Estrogen Receptor β Isoforms on Glioblastoma Progression, Cancer Res, 78 (2018) 3176–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Sareddy GR, Li X, Liu J, Viswanadhapalli S, Garcia L, Gruslova A, Cavazos D, Garcia M, Strom AM, Gustafsson JA, Tekmal RR, Brenner A, Vadlamudi RK, Selective Estrogen Receptor β Agonist LY500307 as a Novel Therapeutic Agent for Glioblastoma, Sci Rep, 6 (2016) 24185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Wu Q, Ishikawa T, Sirianni R, Tang H, McDonald JG, Yuhanna IS, Thompson B, Girard L, Mineo C, Brekken RA, Umetani M, Euhus DM, Xie Y, Shaul PW, 27-Hydroxycholesterol promotes cell-autonomous, ER-positive breast cancer growth, Cell reports, 5 (2013) 637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Raza S, Ohm JE, Dhasarathy A, Schommer J, Roche C, Hammer KD, Ghribi O, The cholesterol metabolite 27-hydroxycholesterol regulates p53 activity and increases cell proliferation via MDM2 in breast cancer cells, Mol Cell Biochem, 410 (2015) 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Zhu D, Shen Z, Liu J, Chen J, Liu Y, Hu C, Li Z, Li Y, The ROS-mediated activation of STAT-3/VEGF signaling is involved in the 27-hydroxycholesterol-induced angiogenesis in human breast cancer cells, Toxicol Lett, 264 (2016) 79–86. [DOI] [PubMed] [Google Scholar]
- [100].Guo D, Reinitz F, Youssef M, Hong C, Nathanson D, Akhavan D, Kuga D, Amzajerdi AN, Soto H, Zhu S, Babic I, Tanaka K, Dang J, Iwanami A, Gini B, Dejesus J, Lisiero DD, Huang TT, Prins RM, Wen PY, Robins H.l., Prados MD, Deangelis LM, Mellinghoff IK, Mehta MP, James CD, Chakravarti A, Cloughesy TF, Tontonoz P, Mischel PS, An LXR agonist promotes glioblastoma cell death through inhibition of an EGFR/AKT/SREBP-1/LDLR-dependent pathway, Cancer Discov, 1 (2011) 442–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Araya Z, Tang W, Wikvall K, Hormonal regulation of the human sterol 27-hydroxylase gene CYP27A1, Biochem J, 372 (2003) 529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Torres CG, Ramirez ME, Cruz P, Epunan MJ, Valladares LE, Sierralta WD, 27-hydroxycholesterol induces the transition of MCF7 cells into a mesenchymal phenotype, Oncol Rep, 26 (2011) 389–397. [DOI] [PubMed] [Google Scholar]
- [103].Shen Z, Zhu D, Liu J, Chen J, Liu Y, Hu C, Li Z, Li Y, 27-Hydroxycholesterol induces invasion and migration of breast cancer cells by increasing MMP9 and generating EMT through activation of STAT-3, Environmental toxicology and pharmacology, 51 (2017) 1–8. [DOI] [PubMed] [Google Scholar]
- [104].Chen L, Zhang L, Xian G, Lv Y, Lin Y, Wang Y, 25-Hydroxycholesterol promotes migration and invasion of lung adenocarcinoma cells, Biochem Biophys Res Commun, 484 (2017) 857–863. [DOI] [PubMed] [Google Scholar]
- [105].Roberg-Larsen H, Lund K, Seterdal KE, Solheim S, Vehus T, Solberg N, Krauss S, Lundanes E, Wilson SR, Mass spectrometric detection of 27-hydroxycholesterol in breast cancer exosomes, The Journal of steroid biochemistry and molecular biology, 169 (2017) 22–28. [DOI] [PubMed] [Google Scholar]
- [106].Kimbung S, Inasu M, Stalhammar T, Nodin B, Elebro K, Tryggvadottir H, Ygland Rodstrom M, Jirstrom K, Isaksson K, Jernstrom H, Borgquist S, CYP27A1 expression is associated with risk of late lethal estrogen receptor-positive breast cancer in postmenopausal patients, Breast cancer research : BCR, 22 (2020) 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Kimbung S, Chang CY, Bendahl PO, Dubois L, Thompson JW, McDonnell DP, Borgquist S, Impact of 27-hydroxylase (CYP27A1) and 27-hydroxycholesterol in breast cancer, Endocr Relat Cancer, 24 (2017) 339–349. [DOI] [PubMed] [Google Scholar]
- [108].Alfaqih MA, Nelson ER, Liu W, Safi R, Jasper JS, Macias E, Geradts J, Thompson JW, Dubois LG, Freeman MR, Chang CY, Chi JT, McDonnell DP, Freedland SJ, CYP27A1 Loss Dysregulates Cholesterol Homeostasis in Prostate Cancer, Cancer Res, 77 (2017) 1662–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Wang Y, Zhou R, Wang J, Relationship between Hypothyroidism and Endometrial Cancer, Aging Dis, 10 (2019) 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Tian W, Pang W, Ge Y, He X, Wang D, Li X, Hou H, Zhou D, Feng S, Chen Z, Yang Y, Hepatocyte-generated 27-hydroxycholesterol promotes the growth of melanoma by activation of estrogen receptor alpha, J Cell Biochem, 119 (2018) 2929–2938. [DOI] [PubMed] [Google Scholar]
- [111].Babiker A, Andersson O, Lindblom D, van der Linden J, Wiklund B, Lütjohann D, Diczfalusy U, Björkhem I, Elimination of cholesterol as cholestenoic acid in human lung by sterol 27-hydroxylase: evidence that most of this steroid in the circulation is of pulmonary origin, J Lipid Res, 40 (1999) 1417–1425. [PubMed] [Google Scholar]
- [112].Raza S, Meyer M, Goodyear C, Hammer KDP, Guo B, Ghribi O, The cholesterol metabolite 27-hydroxycholesterol stimulates cell proliferation via ERβ in prostate cancer cells, Cancer Cell Int, 17 (2017) 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].He S, Ma L, Baek AE, Vardanyan A, Vembar V, Chen JJ, Nelson AT, Burdette JE, Nelson ER, Host CYP27A1 expression is essential for ovarian cancer progression, Endocr Relat Cancer, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Hutchinson SA, Websdale A, Cioccoloni G, Røberg-Larsen H, Lianto P, Kim B, Rose A, Soteriou C, Pramanik A, Wastall LM, Williams BJ, Henn MA, Chen JJ, Ma L, Moore JB, Nelson E, Hughes TA, Thorne JL, Liver x receptor alpha drives chemoresistance in response to side-chain hydroxycholesterols in triple negative breast cancer, Oncogene, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Wang CW, Huang CC, Chou PH, Chang YP, Wei S, Guengerich FP, Chou YC, Wang SF, Lai PS, Soucek P, Ueng YF, 7-ketocholesterol and 27-hydroxycholesterol decreased doxorubicin sensitivity in breast cancer cells: estrogenic activity and mTOR pathway, Oncotarget, 8 (2017) 66033–66050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Ye L, Zhang T, Kang Z, Guo G, Sun Y, Lin K, Huang Q, Shi X, Ni Z, Ding N, Zhao KN, Chang W, Wang J, Lin F, Xue X, Tumor-Infiltrating Immune Cells Act as a Marker for Prognosis in Colorectal Cancer, Front Immunol, 10 (2019) 2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Zhang SC, Hu ZQ, Long JH, Zhu GM, Wang Y, Jia Y, Zhou J, Ouyang Y, Zeng Z, Clinical Implications of Tumor-Infiltrating Immune Cells in Breast Cancer, J Cancer, 10 (2019) 6175–6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Fu Q, Chen N, Ge C, Li R, Li Z, Zeng B, Li C, Wang Y, Xue Y, Song X, Li H, Li G, Prognostic value of tumor-infiltrating lymphocytes in melanoma: a systematic review and meta-analysis, Oncoimmunology, 8 (2019) 1593806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Miksch RC, Schoenberg MB, Weniger M, Bösch F, Ormanns S, Mayer B, Werner J, Bazhin AV, D'Haese JG, Prognostic Impact of Tumor-Infiltrating Lymphocytes and Neutrophils on Survival of Patients with Upfront Resection of Pancreatic Cancer, Cancers (Basel), 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Polk A, Svane IM, Andersson M, Nielsen D, Checkpoint inhibitors in breast cancer - Current status, Cancer Treat Rev, 63 (2018) 122–134. [DOI] [PubMed] [Google Scholar]
- [121].du Rusquec P, de Calbiac O, Robert M, Campone M, Frenel JS, Clinical utility of pembrolizumab in the management of advanced solid tumors: an evidence-based review on the emerging new data, Cancer Manag Res, 11 (2019) 4297–4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Tie Y, Yang H, Zhao R, Zheng H, Yang D, Zhao J, Liu M, Safety and efficacy of atezolizumab in the treatment of cancers: a systematic review and pooled-analysis, Drug Des Devel Ther, 13 (2019) 523–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Kümpers C, Jokic M, Haase O, Offermann A, Vogel W, Grätz V, Langan EA, Perner S, Terheyden P, Immune Cell Infiltration of the Primary Tumor, Not PD-L1 Status, Is Associated With Improved Response to Checkpoint Inhibition in Metastatic Melanoma, Front Med (Lausanne), 6 (2019) 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Jochems C, Schlom J, Tumor-infiltrating immune cells and prognosis: the potential link between conventional cancer therapy and immunity, Exp Biol Med (Maywood), 236 (2011) 567–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Plesca I, Tunger A, Müller L, Wehner R, Lai X, Grimm MO, Rutella S, Bachmann M, Schmitz M, Characteristics of Tumor-Infiltrating Lymphocytes Prior to and During Immune Checkpoint Inhibitor Therapy, Front Immunol, 11 (2020) 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Zhang C, Zheng JH, Lin ZH, Lv HY, Ye ZM, Chen YP, Zhang XY, Profiles of immune cell infiltration and immune-related genes in the tumor microenvironment of osteosarcoma, Aging (Albany NY), 12 (2020) 3486–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Villablanca EJ, Raccosta L, Zhou D, Fontana R, Maggioni D, Negro A, Sanvito F, Ponzoni M, Valentinis B, Bregni M, Prinetti A, Steffensen KR, Sonnino S, Gustafsson JA, Doglioni C, Bordignon C, Traversari C, Russo V, Tumor-mediated liver X receptor-alpha activation inhibits CC chemokine receptor-7 expression on dendritic cells and dampens antitumor responses, Nat Med, 16 (2010) 98–105. [DOI] [PubMed] [Google Scholar]
- [128].Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, Hausner MA, Shih R, Parks JS, Edwards PA, Jamieson BD, Tontonoz P, LXR signaling couples sterol metabolism to proliferation in the acquired immune response, Cell, 134 (2008) 97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, Rizvi SM, Qin A, Waninger JJ, Lang X, Chopra Z, El Naqa I, Zhou J, Bian Y, Jiang L, Tezel A, Skvarce J, Achar RK, Sitto M, Rosen BS, Su F, Narayanan SP, Cao X, Wei S, Szeliga W, Vatan L, Mayo C, Morgan MA, Schonewolf CA, Cuneo K, Kryczek I, Ma VT, Lao CD, Lawrence TS, Ramnath N, Wen F, Chinnaiyan AM, Cieslik M, Alva A, Zou W, Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination, Nat Med, 27 (2021) 152–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Carpenter KJ, Valfort AC, Steinauer N, Chatterjee A, Abuirqeba S, Majidi S, Sengupta M, Di Paolo RJ, Shornick LP, Zhang J, Flaveny CA, LXR-inverse agonism stimulates immune-mediated tumor destruction by enhancing CD8 T-cell activity in triple negative breast cancer, Sci Rep, 9 (2019) 19530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Lanterna C, Musumeci A, Raccosta L, Corna G, Moresco M, Maggioni D, Fontana R, Doglioni C, Bordignon C, Traversari C, Russo V, The administration of drugs inhibiting cholesterol/oxysterol synthesis is safe and increases the efficacy of immunotherapeutic regimens in tumor-bearing mice, Cancer Immunol Immunother, 65 (2016) 1303–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Tavazoie MF, Pollack I, Tanqueco R, Ostendorf BN, Reis BS, Gonsalves FC, Kurth I, Andreu-Agullo C, Derbyshire ML, Posada J, Takeda S, Tafreshian KN, Rowinsky E, Szarek M, Waltzman RJ, McMillan EA, Zhao C, Mita M, Mita A, Chmielowski B, Postow MA, Ribas A, Mucida D, Tavazoie SF, LXR/ApoE Activation Restricts Innate Immune Suppression in Cancer, Cell, 172 (2018) 825–840 e818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Carbó JM, León TE, Font-Díaz J, De la Rosa JV, Castrillo A, Picard FR, Staudenraus D, Huber M, Cedó L, Escolà-Gil JC, Campos L, Bakiri L, Wagner EF, Caelles C, Stratmann T, Van Ginderachter JA, Valledor AF, Pharmacological activation of LXR alters the expression profile of tumor-associated macrophages and the abundance of regulatory T cells in the tumor microenvironment, Cancer Res, (2020). [DOI] [PubMed] [Google Scholar]
- [134].Bilotta MT, Abruzzese MP, Molfetta R, Scarno G, Fionda C, Zingoni A, Soriani A, Garofalo T, Petrucci MT, Ricciardi MR, Paolini R, Santoni A, Cippitelli M, Activation of liver X receptor up-regulates the expression of the NKG2D ligands MICA and MICB in multiple myeloma through different molecular mechanisms, Faseb j, 33 (2019) 9489–9504. [DOI] [PubMed] [Google Scholar]
- [135].Sohrabi Y, Sonntag GVH, Braun LC, Lagache SMM, Liebmann M, Klotz L, Godfrey R, Kahles F, Waltenberger J, Findeisen HM, LXR Activation Induces a Proinflammatory Trained Innate Immunity-Phenotype in Human Monocytes, Front Immunol, 11 (2020) 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Muse ED, Yu S, Edillor CR, Tao J, Spann NJ, Troutman TD, Seidman JS, Henke A, Roland JT, Ozeki KA, Thompson BM, McDonald JG, Bahadorani J, Tsimikas S, Grossman TR, Tremblay MS, Glass CK, Cell-specific discrimination of desmosterol and desmosterol mimetics confers selective regulation of LXR and SREBP in macrophages, Proc Natl Acad Sci U S A, 115 (2018) E4680–e4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Fessler MB, The challenges and promise of targeting the Liver X Receptors for treatment of inflammatory disease, Pharmacol Ther, 181 (2018) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Brown AJ, Jessup W, Oxysterols and atherosclerosis, Atherosclerosis, 142 (1999) 1–28. [DOI] [PubMed] [Google Scholar]
- [139].DuSell CD, McDonnell DP, 27-Hydroxycholesterol: a potential endogenous regulator of estrogen receptor signaling, Trends Pharmacol Sci, 29 (2008) 510–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P, Reciprocal regulation of inflammation and lipid metabolism by liver X receptors, Nat Med, 9 (2003) 213–219. [DOI] [PubMed] [Google Scholar]
- [141].Castrillo A, Joseph SB, Marathe C, Mangelsdorf DJ, Tontonoz P, Liver X receptor-dependent repression of matrix metalloproteinase-9 expression in macrophages, J Biol Chem, 278 (2003) 10443–10449. [DOI] [PubMed] [Google Scholar]
- [142].Mast N, Lin JB, Pikuleva IA, Marketed Drugs Can Inhibit Cytochrome P450 27A1, a Potential New Target for Breast Cancer Adjuvant Therapy, Molecular pharmacology, 88 (2015) 428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]