Abstract
Objective:
Associations among atrial fibrillation (AF) and heart failure (HF) have been established. We compared the extent to which AF is associated with each primary subtype of HF, with reduced (HFrEF) versus preserved ejection fraction (HFpEF).
Methods:
We included 25,787 participants free of baseline HF from the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort. Baseline AF was ascertained from electrocardiogram and self-reported history of physician diagnosis. Incident HF events were determined from physician-adjudicated review of hospitalization medical records and HF deaths. Based on ejection fraction (EF) at the time of HF event, HFrEF, HFpEF, and mid-range HF were defined as EF<40%, ≥50%, and 40–49%, respectively. Multivariable Cox proportional-hazards models examined the association between AF and HF. The Lunn-McNeil method was used to compare associations of AF with HFrEF versus HFpEF.
Results:
Over median 9 years follow-up, 1,109 HF events occurred (356 HFpEF, 388 HFrEF, 77 mid-range, and 288 unclassified). In a model adjusted for sociodemographics, cardiovascular risk factors, and incident coronary heart disease, AF was associated with increased risk of all HF events (hazard ratio [HR] 1.67, 95% confidence interval [CI] 1.38–2.01). The associations of AF with HFrEF versus HFpEF events did not differ significantly (HR [95% CI] 1.87 [1.38–2.54], and 1.65 [1.20–2.28], respectively; p-value for difference=0.581). These associations were consistent in sex and race subgroups.
Conclusions:
AF is associated with both HFrEF and HFpEF events, with no significant difference in the strength of association among these subtypes.
Keywords: atrial fibrillation, heart failure with preserved ejection fraction, heart failure with reduced ejection fraction
INTRODUCTION
Growth in the global burden of atrial fibrillation (AF)1 represents a troubling population health concern. While clinical management of AF is largely driven by symptom relief and abatement of stroke risk, new AF diagnosis appears to carry an approximately doubled risk of heart failure (HF) relative to that of stroke.2 As such, HF frequently develops in a population with AF, with which it has bidirectional associations3 driven through shared risk factors and pathophysiology.4 When concurrent, AF with HF carries a more than doubled mortality relative to either condition alone,5 so HF risk stratification in individuals with AF is an important research priority.6 Despite this, the extent to which AF is associated with each of the primary subtypes of HF, with reduced (HFrEF) and preserved (HFpEF) ejection fraction, remains uncertain.
Therefore, we aimed to compare the associations of AF with incident HF and HFrEF versus HFpEF events in the REasons for Geographic And Racial Differences in Stroke (REGARDS) Study, a racially and geographically diverse contemporary cohort. Furthermore, as appreciable sex and race-group differences are found in the epidemiology of AF7 and HF,8 we examined divergence in these differences across sex and race subgroups.
METHODS
Sample & Design
The REGARDS Study enrolled 30,239 Black or White participants aged ≥45 years living in the contiguous U.S. from 2003–2007.9 Potential participants were selected at random from publicly-available lists and enrolled by telephone and/or mail. Sampling design intentionally oversampled residents of the “Stroke Belt”, a region of excess stroke mortality in the southeastern United States,10 and Black Americans. Individuals were excluded from participation in the cohort for insufficient English proficiency, active treatment of a malignancy, residing in or waitlisting for a nursing home, any medical condition likely to preclude long-term follow-up, or interviewer suspicion of cognitive impairment.
An initial intake telephone interview was conducted to obtain verbal consent for participation and medical history. This was followed by an in-person assessment in the participant’s home, during which written consent, a resting electrocardiogram (ECG), biometric measurements, a medication inventory, and fasting blood and urine samples were obtained. ECGs were sent to the central electrocardiogram reading center (Epidemiological Cardiology Research Center, Wake Forest School of Medicine, Winston-Salem, North Carolina, USA) where they were interpreted and coded by clinicians blinded to other participant data. Glucose, total cholesterol, high-density lipoprotein, and triglyceride concentrations were measured in baseline blood samples via colorimetric reflectance spectrophotometry using the Orthos Vitros 950 IRC Clinical Analyzer (Johnson & Jonson Clinical Diagnostics, New Brunswick, New Jersey); the Friedewald equation11 was used to derive low-density lipoprotein (LDL) concentration. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation,12 with plasma creatinine measured by isotope dilution mass spectrometry-traceable methods.
Resting blood pressure was measured by a trained examiner following a protocol using an aneroid sphygmomanometer over the brachial artery.13 Height and weight were measured without shoes using a metal tape measure and 300-pound-calibrated scale, respectively.
A cohort of participants free of suspected heart failure at baseline was assembled using information on medication use and medical history, as recently described.14 Detailed exclusions are shown in Figure 1.
Figure 1.
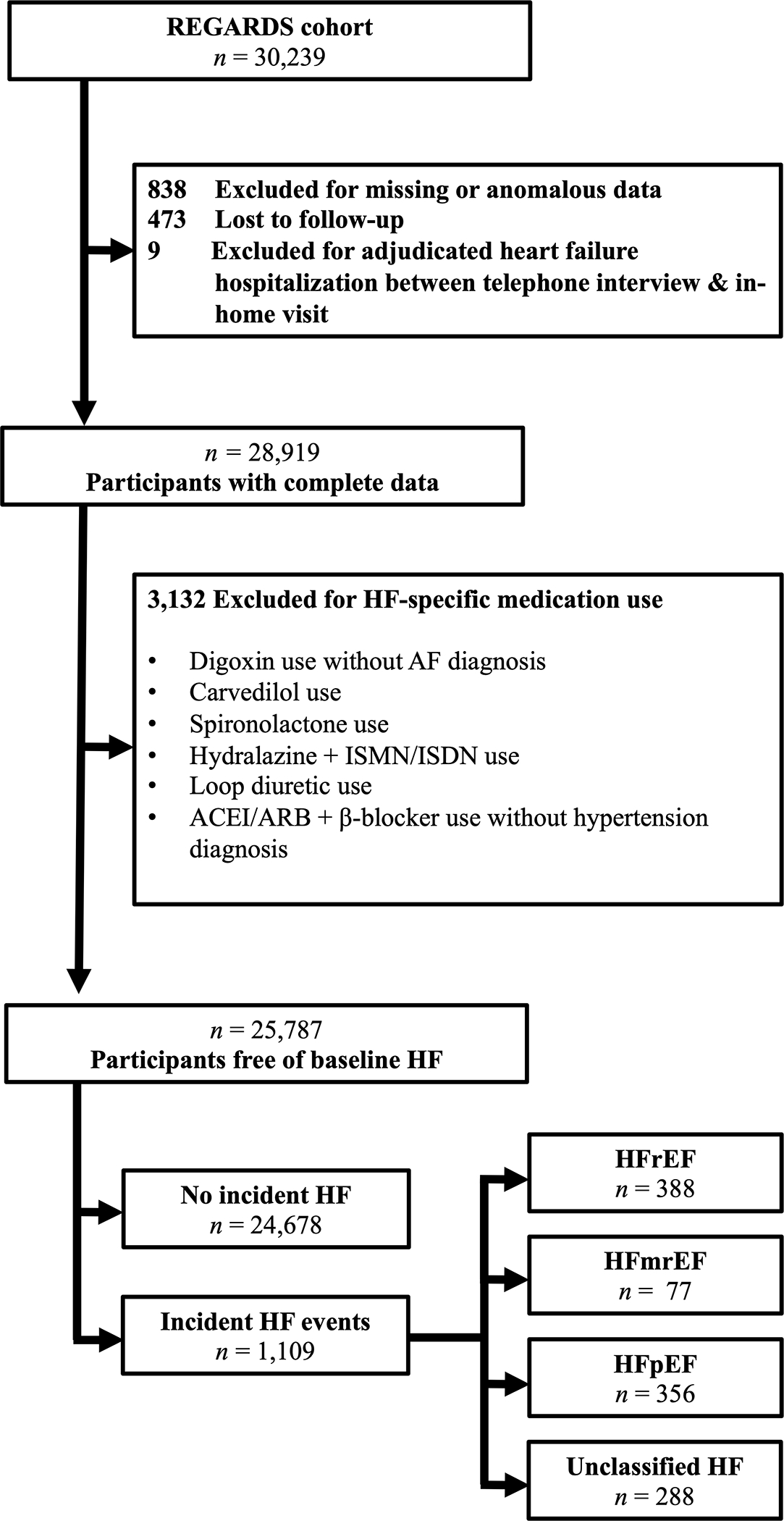
Exclusions. Abbreviations: ACEI, angiotensin converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; HFrEF, heart failure with reduced ejection fraction; HFpEF, heart failure with reduced ejection fraction; HFmrEF, heart failure with midrange ejection fraction; HF, heart failure; ISMN, isosorbide mononitrate; ISDN, isosorbide dinitrate; REGARDS, REasons for Geographic And Racial Differences in Stroke;
The institutional review boards of all institutions involved in data ascertainment and/or processing approved the methods of the REGARDS study. The public were not directly involved in the design or conduct of REGARDS. The REGARDS Publications Committee reviewed and approved the data analysis plan for this manuscript and reviewed and approved the final manuscript and adherence to this plan.
Exposure & Outcomes
Prevalent AF was defined by detection of AF on baseline ECG and/or self-reported affirmative response to the telephone interview question “Has a physician or a health professional ever told you that you had atrial fibrillation?”15
Participants or their proxies were followed semiannually by telephone for monitoring of hospitalizations or deaths likely to involve HF and other outcomes such as stroke and coronary heart disease (CHD). Pertinent medical records for hospitalizations associated with suspected HF were obtained, and two clinicians independently adjudicated the diagnosis of heart failure using clinical documentation, left ventricular ejection fraction (LVEF) assessment from imaging studies (for example, echocardiography), and biomarkers such as natriuretic peptides. Discordance in adjudications was resolved by committee.
Incident HF events were defined as initial hospitalizations or deaths due to HF and were further subclassified into HF subtypes by LVEF. HFrEF was defined by documented LVEF <40% or qualitative report of reduced LVEF. HFpEF was defined by documented LVEF ≥50% or qualitatively normal LVEF. HF with mid-range ejection fraction (HFmrEF) was defined by documented LVEF ≥40% and <50%. Some HF events did not involve quantitative or qualitative evaluation of ventricular function and were therefore unclassified.
Variables
Baseline clinical and behavioral variables included age (years), sex, race, annual income (<$20,000, $20,000-$34,999, $35,000-$74,999, ≥$75,000, or declined to report), education level (< high school, high school graduate, some college, or ≥ college graduate), region (Stroke Belt, Stroke Buckle, or other10) and pack-year tobacco smoking history.
Prevalent medical conditions identified at baseline included diabetes mellitus (self-reported use of insulin or hypoglycemic medications, fasting glucose ≥6.99 mmol/L [126 mg/dL], or glucose ≥11.10 mmol/L [200 mg/dL] among those failing to fast), body mass index (BMI; calculated using height and weight obtained at baseline; continuous in kg/m2 or in categories: underweight [<18.5 kg/m2], normal weight [18.5–24.9 kg/m2], overweight [25.0–29.9 kg/m2], or obesity [≥30.0 kg/m2]), left ventricular hypertrophy (LVH; defined by Sokolow-Lyon ECG criteria16), and current use of warfarin, aspirin, statins, or antihypertensive medications of any dose or brand from home medications inventory. A history of coronary heart disease (CHD) was indicated by self-reported history of myocardial infarction (MI), evidence of prior infarct on baseline ECG, or history of coronary artery bypass graft surgery or percutaneous coronary intervention with use of stents or angioplasty.
Incident coronary heart disease (CHD) events, defined as definite or probable nonfatal MI or CHD death,17 were also identified by semiannual telephone call and independently adjudicated by two clinicians with discordance resolved by committee.
Statistical Analysis
Baseline characteristics were compared between participants with and without baseline AF using Fisher’s exact tests (categorical variables), one-way analysis of variance (ANOVA; continuous variables), or Wilcoxon rank-sum test (pack-year smoking history; skewed distribution).
Survival analysis was conducted using Cox proportional-hazards models. Survival time began on the in-home visit date and ended on HF event date (failure) or censoring at date of last follow-up or December 31, 2016 (creation of the heart failure analytic cohort). First, Cox proportional-hazards models were fitted to risk of all HF events (aggregately, including HFrEF, HFpEF, HFmrEF, and unclassified HF). Sex and race differences in the association of AF with HF were evaluated with AF*sex and AF*race multiplicative interaction terms in the all-events models. Next, the data set was augmented for competing risks analysis according to Lunn and McNeil.18 HFmrEF and unclassified HF events were censored in the main analysis. We also planned a sensitivity analysis in which HFmrEF events were incorporated into the HFpEF event group, thus considering LVEF ≥40% as a threshold for preserved EF. P-values for the difference in survival function between HFrEF and HFpEF events are reported from interaction terms for HF subtype*AF in the augmented data set. Estimates were also reported separately within each race and sex subgroup.
Four sequential sets of covariates were used in all multivariable modeling. Model 1 included demographics: age, sex, race, income, education, and region. Model 2 included Model 1 covariates and added heart failure risk factors: smoking history, systolic blood pressure, diabetes mellitus, BMI (continuous), LDL, LVH, eGFR, antihypertensive medication use, and baseline CHD. Model 3 included Model 2 covariates and added baseline use of medications that modify cardiovascular disease risk: aspirin, warfarin, and statins. Model 4 included Model 3 covariates and added incident CHD as a time-varying covariate.
Unadjusted Kaplan-Meier failure curves stratified by baseline AF status were plotted for all HF events in the overall sample and for HFrEF and HFpEF events in the augmented Lunn-McNeil dataset. All statistical tests were two-sided, with p-values considered statistically significant when below 0.10 for multiplicative interaction terms and below 0.05 for all other tests. Participants missing data were excluded from analyses in which relevant information was missing. This analysis was performed with Stata, version 16.1 (StataCorp, College Station, Texas).
RESULTS
Sample Characteristics
This analysis included 25,787 participants who were HF-free at baseline. Prevalent AF at baseline was detected in 7.4% (n=1,896; 105 by ECG only, 1,637 by self-reported medical history only, and 154 by ECG and self-reported medical history). Table 1 compares baseline characteristics of included participants with and without AF at baseline. Participants with baseline AF were more likely to be older, less educated, with lower LDL, eGFR, and annual income, with more White participants, CHD history, diabetes mellitus, incident CHD events, use of warfarin, statins, or aspirin, and higher pack-year smoking history.
Table 1.
Baseline Characteristics of Included Participants by Baseline Atrial Fibrillation Status
| No Atrial Fibrillation (n=23,889) | Atrial Fibrillation (n=1,896) | p | |
|---|---|---|---|
| Age (years; mean [95% CI]) | 64.2 (64.1, 64.4) | 66.8 (66.3, 67.2) | <0.001 |
| Male Sex (%) | 45.0 | 44.8 | 0.829 |
| Black Race (%) | 40.6 | 35.8 | <0.001 |
| Annual Income (%) | |||
| <$20,000 | 16.6 | 20.1 | |
| $20,000–$34,999 | 23.8 | 25.3 | |
| $35,000–$74,999 | 30.6 | 28.0 | <0.001 |
| ≥$75,000 | 17.2 | 13.1 | |
| Refused | 11.9 | 13.5 | |
| Education (%) | |||
| < High School | 11.4 | 12.0 | |
| High School Graduate | 25.3 | 27.6 | 0.030 |
| Some College | 26.9 | 27.0 | |
| ≥ College | 36.4 | 33.3 | |
| Region (%) | |||
| Stroke Belt | 34.4 | 33.8 | 0.019 |
| Stroke Buckle | 20.6 | 23.3 | |
| Other | 45.0 | 42.9 | |
| Estimated glomerular filtration rate (mL/min/1.73m2; mean [95% CI]) | 86.6 (86.4, 86.8) | 82.5 (81.8, 83.2) | <0.001 |
| Smoking History (pack-years; median [IQR]) | 0.3 [0, 19] | 1.5 [0, 22] | <0.001 |
| Systolic Blood Pressure (mmHg, mean [95% CI]) | 127.2 (127.0, 127.4) | 127.9 (127.2, 128.7) | 0.07 |
| Diabetes Mellitus (%) | 18.7 | 21.0 | 0.014 |
| Body Mass Index (%) | |||
| Underweight (<18.5 kg/m2) | 1.1 | 1.2 | |
| Normal Weight (18.5–24.9 kg/m2) | 24.8 | 26.3 | 0.401 |
| Overweight (25–29.9 kg/m2) | 38.0 | 36.5 | |
| Obesity (≥30 kg/m2) | 36.1 | 36.0 | |
| Warfarin Use (%) | 1.4 | 17.9 | <0.001 |
| Statin Use (%) | 28.8 | 35.6 | <0.001 |
| Aspirin Use (%) | 41.2 | 47.5 | <0.001 |
| Low-Density Lipoprotein (mmol/L; mean [95% CI]) | 3.00 (2.98, 3.01) | 2.84 (2.80, 2.88) | <0.001 |
| Left Ventricular Hypertrophy (%) | 9.2 | 9.0 | 0.867 |
| Antihypertensive Drug Use (%) | 51.8 | 64.1 | <0.001 |
| Baseline Coronary Heart Disease (%) | 13.4 | 30.9 | <0.001 |
| Coronary Heart Disease Events in Follow-Up (%) | 5.1 | 8.8 | <0.001 |
Abbreviations: CI, confidence interval; mmHg, millimeters of mercury; mmol/L, millimoles per liter; IQR, interquartile range
A total of 1,109 incident heart failure events were identified over mean follow-up of 9.0 years (standard deviation 3.6 years), including 356 HFpEF, 388 HFrEF, 77 HFmrEF, and 288 unclassified HF events.
Association of Atrial Fibrillation with All Heart Failure Events
Table 2 presents the association of AF with all incident HF events. Figure 1 depicts Kaplan-Meier curves for all HF events, stratified by baseline AF status. Baseline AF increased risk of all incident HF events in all models; this association was moderately attenuated across subsequent models. Associations of AF with all HF events were consistent across race and sex.
Table 2.
Distributions of Heart Failure Event Subtypes by Baseline Atrial Fibrillation Status
| HF Event Subtype | No Atrial Fibrillation | Atrial Fibrillation | Total |
|---|---|---|---|
| All HF | 915 | 194 | 1,109 |
| HFpEF | 295 | 61 | 356 |
| HFmrEF | 69 | 8 | 77 |
| HFrEF | 316 | 72 | 388 |
| Unclassified HF | 235 | 53 | 288 |
| No HF | 22,976 | 1,702 | 24,678 |
| Total | 23,891 | 1,896 | 25,787 |
Abbreviations: HF, heart failure; HFmrEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with reduced ejection fraction; HFrEF, heart failure with reduced ejection fraction
Association of Atrial Fibrillation with HFrEF & HFpEF Events
Figure 2 depicts separate Kaplan-Meier curves for incident HFpEF and HFrEF events in the augmented dataset, stratified by baseline AF status. The separate associations of AF with incident HFrEF and HFpEF events in the augmented dataset are presented in Table 2. The cause-specific association of AF with HFpEF differed by sex (Model 4 sex difference interaction p = 0.024). In men, AF was associated with HFpEF in the demographic model, but this was attenuated and was no longer statistically significant when considering other risk factors.
Figure 2.
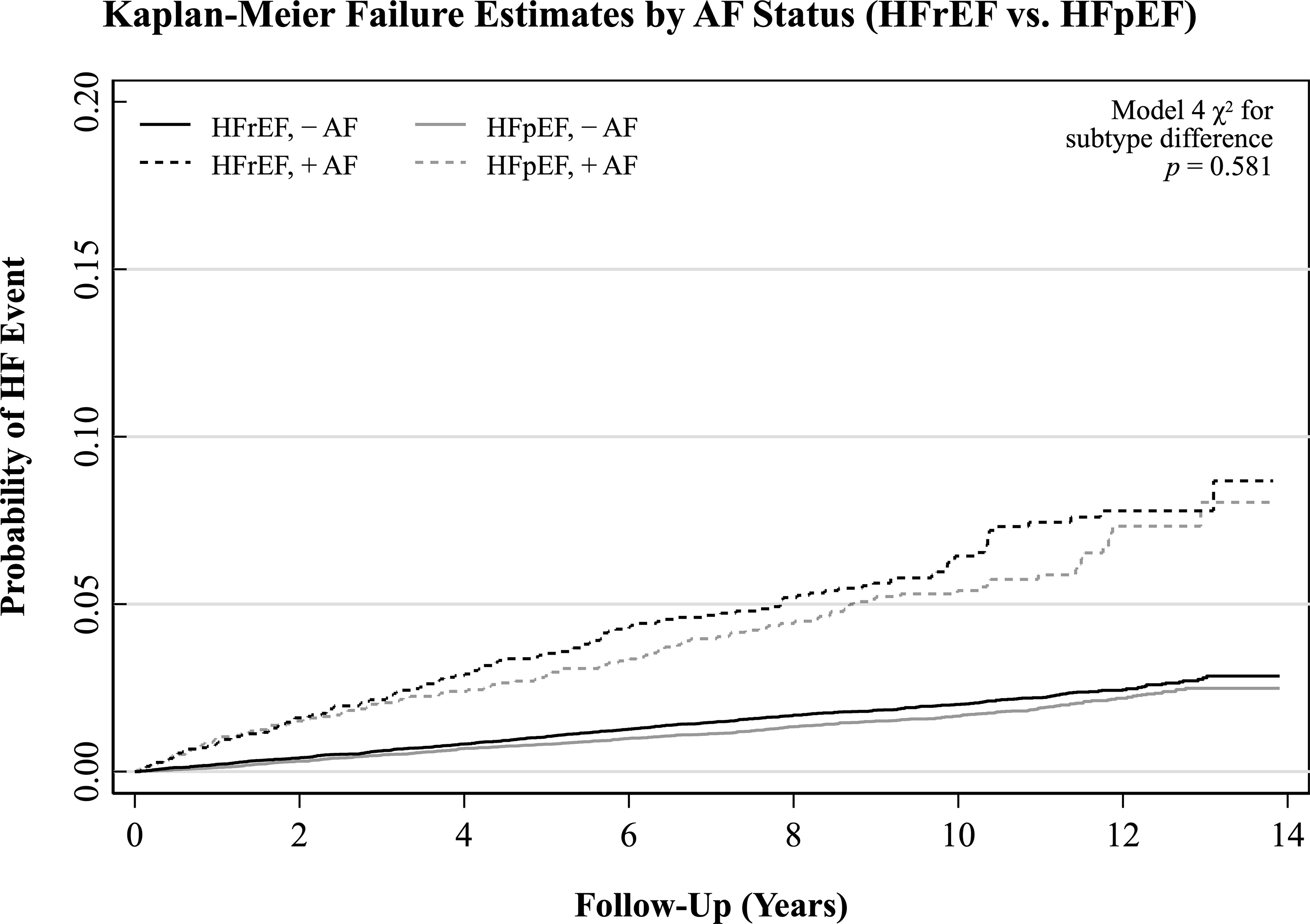
Unadjusted Kaplan-Meier failure curves for all incident heart failure events stratified by baseline atrial fibrillation status. Failure includes heart failure with reduced, preserved, midrange, and unclassified ejection fraction. Abbreviations: AF, atrial fibrillation; HF, heart failure.
Difference in Associations of AF with HFrEF vs. HFpEF
No significant differences in the associations of AF with HFrEF vs. HFpEF events were observed in the overall group, although a subjectively larger-magnitude association of AF with HFrEF was consistently observed across models.
No significant differences in the associations of AF with HFrEF vs. HFpEF events were observed in the Black or White subgroups. Despite the lack of a significant association of AF with HFpEF in men in models, 2–4, the associations of AF with HFrEF vs. HFpEF events did not statistically differ in either sex subgroup.
Sensitivity Analysis
Results of a planned sensitivity analysis redefining HFpEF as LVEF ≥40% are reported in Table 3 and Figure 3. Results did not differ substantially from those of the primary analysis, except for a subjectively lower magnitude of associations of AF with HFpEF events when defining HFpEF as LVEF ≥40% vs. LVEF ≥50%.
Table 3.
Association of Atrial Fibrillation With Incident Heart Failure Events
| n events / n at risk | Model 1 | Model 2 | Model 3 | Model 4 | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| HF Group* | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | χ2 p For Subtype Difference | ||
| Overall | All HF | 1,109/25,787 | 2.61 (2.24, 3.05) | 2.11 (1.79, 2.50) | 1.75 (1.46, 2.11) | 1.67 (1.38, 2.01) | |
| HFrEF | 388/25,787 | 2.88 (2.22, 3.72) | 2.36 (1.79, 3.12) | 2.02 (1.48, 2.74) | 1.87 (1.38, 2.54) | 0.581 | |
| HFpEF | 356/25,787 | 2.54 (1.92, 3.34) | 1.99 (1.48, 2.68) | 1.74 (1.26, 2.39) | 1.65 (1.20, 2.28) | ||
| Women † | All HF | 493/14,176 | 2.40 (1.89, 3.04) | 1.92 (1.47, 2.50) | 1.75 (1.33, 2.30) | 1.76 (1.34, 2.32) | |
| HFrEF | 141/14,176 | 2.89 (1.90, 4.40) | 2.23 (1.39, 3.57) | 2.16 (1.33, 3.52) | 2.21 (1.36, 3.59) | 0.145 | |
| HFpEF | 198/14,176 | 2.82 (1.98, 4.02) | 2.49 (1.70, 3.65) | 2.28 (1.54, 3.40) | 2.26 (1.52, 3.36) | ||
| Men † | All HF | 616/11,611 | 2.73 (2.22, 3.36) | 2.27 (1.82, 2.83) | 1.74 (1.34, 2.24) | 1.59 (1.23, 2.06) | |
| HFrEF | 245/11,611 | 2.84 (2.05, 3.94) | 2.51 (1.77, 3.55) | 1.94 (1.30, 2.90) | 1.75 (1.17, 2.60) | 0.943 | |
| HFpEF | 158/11,611 | 2.07 (1.33, 3.24) | 1.46 (0.90, 2.36) | 1.15 (0.67, 2.00) | 1.05 (0.61, 1.83) | ||
| Black ‡ | All HF | 445/10,310 | 2.46 (1.88, 3.23) | 1.81 (1.34, 2.44) | 1.64 (1.20, 2.23) | 1.60 (1.17, 2.18) | |
| HFrEF | 146/10,310 | 2.92 (1.87, 4.57) | 1.91 (1.16, 3.14) | 1.77 (1.06, 2.96) | 1.80 (1.08, 3.02) | 0.434 | |
| HFpEF | 144/10,310 | 2.15 (1.31, 3.53) | 1.89 (1.12, 3.20) | 1.79 (1.05, 3.05) | 1.74 (1.02, 2.96) | ||
| White ‡ | All HF | 664/15,477 | 2.60 (2.15, 3.15) | 2.24 (1.82, 2.75) | 1.84 (1.45, 2.32) | 1.75 (1.38, 2.22) | |
| HFrEF | 242/15,477 | 2.74 (2.00, 3.77) | 2.54 (1.81, 3.55) | 2.14 (1.46, 3.16) | 2.01 (1.37, 2.97) | 0.918 | |
| HFpEF | 212/15,477 | 2.66 (1.89, 3.72) | 2.00 (1.39, 2.87) | 1.70 (1.13, 2.54) | 1.61 (1.07, 2.42) | ||
Abbreviations: AF, atrial fibrillation; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HR, hazard ratio; CI, confidence interval
Covariates in Multivariable Models:
Model 1: age, sex, race, income, education, & region
Model 2: Model 1 covariates + smoking (pack-years), systolic blood pressure, diabetes mellitus, body mass index (continuous), low-density lipoprotein, left ventricular hypertrophy, estimated glomerular filtration rate, antihypertensive drug use, and coronary heart disease history.
Model 3: Model 2 covariates + aspirin, warfarin, and statin use.
Model 4: Model 3 covariates + incident coronary heart disease events (time-varying)
The entire sample, including non-cases and HFrEF, HFpEF, HFmrEF, and unclassified events, was included in analysis for “all HF”. Unclassified and HFmrEF cases were censored in the Lunn-McNeil analysis for “HFpEF” and “HFrEF”
Model 4 AF*sex interaction p-values (cause-specific): All HF: 0.697; HFrEF: 0.692; HFpEF: 0.024; HFmrEF: 0.908
Model 4 AF*race interaction p-values (cause-specific): All HF: 0.945; HFrEF: 0.995; HFpEF: 0.917; HFmrEF: 0.744
Figure 3.
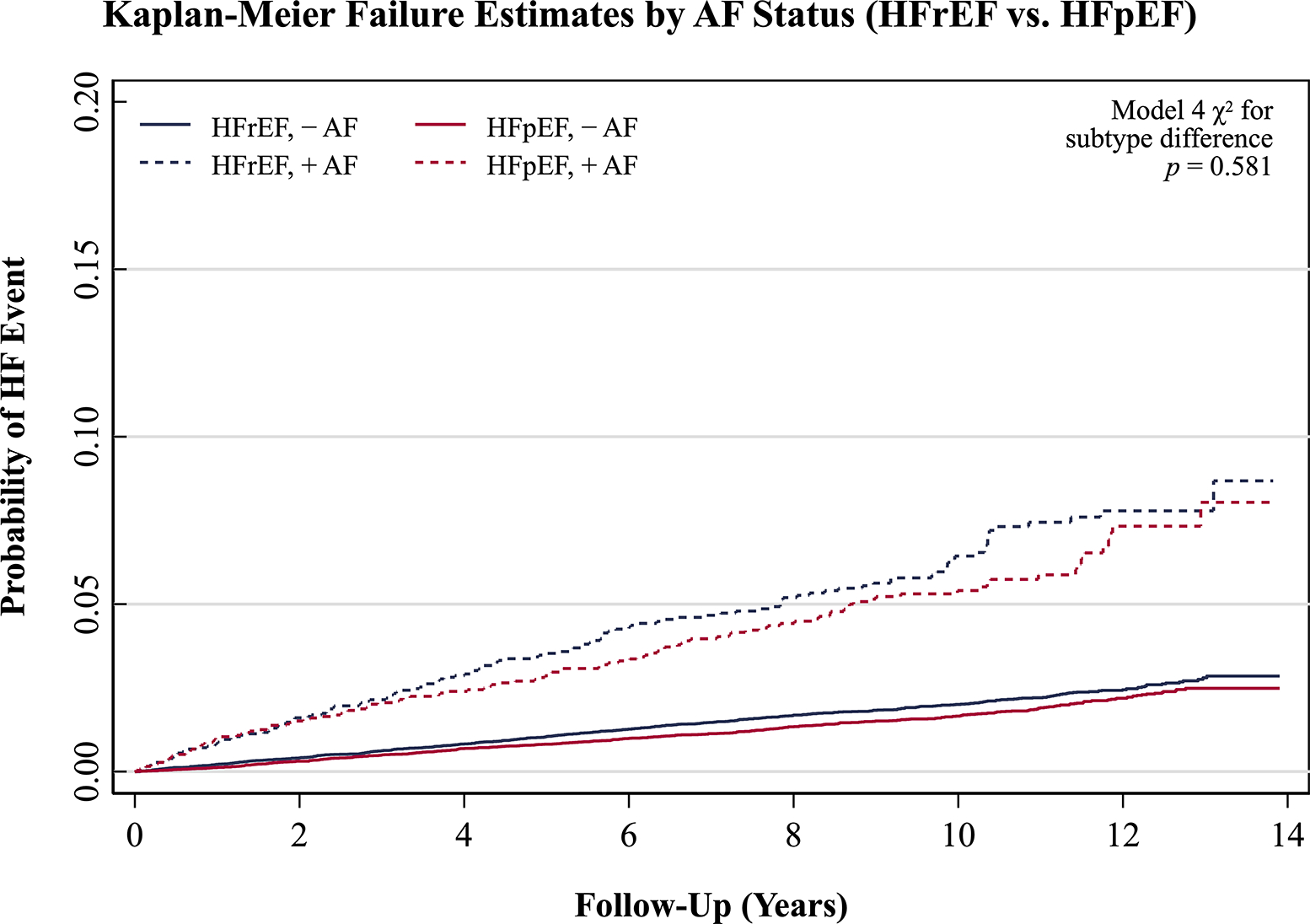
Unadjusted Kaplan-Meier failure curves for HFrEF and HFpEF events in the augmented dataset for Lunn-McNeil analysis, with each HF subtype stratified by baseline atrial fibrillation status. Abbreviations: AF, atrial fibrillation; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction.
DISCUSSION
In this prospective analysis of the contemporary and biracial REGARDS cohort, we showed that the associations of baseline AF with incident HFrEF vs. HFpEF events did not significantly differ over 9 years of follow-up, independent of risk factors, medication use, and incident CHD events. This suggests that AF is a similarly important risk factor for both primary subtypes of HF. Specifically, participants with vs. without prevalent AF had a 65% increased risk of all heart failure events, 86% increased risk of HFrEF events, and 64% increased risk of HFpEF events in the maximally adjusted model.
Our finding of an overall association of AF with incident HF events is consistent with multiple prior studies of various populations.3 19–21 However, our finding of no significant difference in the associations of AF with HFrEF vs. HFpEF events contrasts with one study of Framingham Heart Study participants reporting an association of AF with HFpEF, but not with HFrEF, and a resulting difference in the association between subtypes.22 This discordance is likely due to the smaller sample size, enrollment in an earlier era, and limited inclusion of non-White participants in that cohort.
The reasons underlying these associations remain uncertain. While individuals with AF may represent a population at higher risk for HF, little research has focused on how the pathophysiology of AF could also contribute to separate associations between AF and HFrEF and HFpEF. A causal relationship of AF with HF has been purported to occur through compromised diastolic filling and cardiac output as a result of elevated ventricular rate, irregular cycle length, and loss of atrial systole, as well as concurrent neurohormonal changes and molecular alterations.5 It is possible that different pathophysiologic characteristics of AF are associated with each subtype in similarly important ways. For example, loss of the physiologic increase in inotropy with increased contraction rate could play a more important role in the association of AF with HFrEF,23 while decoupling of myocardial relaxation and contraction functions as a result of irregular cycle length5 could contribute more to the association of AF with HFpEF. However, the observed associations of AF with HF and its subtypes may be better attributed to a unifying disease process or overlapping or synergistic pathophysiology, although our findings and modeling approach suggest these separate associations are not entirely due to shared traditional risk factors between these phenotypes. Thus, further basic and translational investigation into the pathophysiology underlying associations of AF with HFrEF and HFpEF is necessary.
That no association of AF with HFpEF events was observed in men in Models 2–4 is consistent with established sex differences in the epidemiology of HFpEF.24 The racial epidemiology of AF appears paradoxical,25 whereby Black Americans have a higher prevalence of established AF risk factors, but White Americans appear to have higher risk for AF. Conversely, risk for HF appears to be greater in Black vs. White Americans.26 Despite these racial differences in the separate epidemiology of AF and HF, no significant differences between Black and White participants were observed in the association of AF with all HF, HFrEF, or HFpEF. This importantly suggests that AF holds similar relevance to HF, regardless of race or HF subtype.
Strengths & Limitations
Several limitations of this study must be considered. Firstly, findings from studies of the REGARDS cohort may have limited generalizability to race groups other than Black or White. As AF is more realistically considered as a continuum of frequency rather than a dichotomy (present or absent),27 we are unable to account for AF burden or frequency-based categories (paroxysmal, persistent, or long-standing persistent AF). AF burden likely has an impact on HF outcomes, as participants with permanent vs. paroxysmal AF had higher risk of incident HF in a recent study.28 Nevertheless, studies evaluating the association of AF burden with risk of HF are sparse; further research on the association of AF burden with subtypes of HF is needed. Baseline assessment of cardiac function parameters (i.e. LVEF) was not available in REGARDS, so the possibility exists that some participants had subclinical HF at baseline. Participants developing HF not resulting in hospitalization or death, such as that managed in the outpatient care setting, did not meet the criteria for incident HF because of difficulty in detecting this across in a large cohort. Lastly, although we enhanced AF detection at baseline with electrocardiography, 15 the proportion of participants with AF observed on study-scheduled ECG was relatively low. The prevalence of subclinical or undetected AF is likely 2.5 to 4%29 30 and we cannot exclude that some participants developed AF after the initial visit. However, misclassification of participants with AF as not having AF would be expected to bias findings towards the null.
This study has several noteworthy strengths. We evaluated the difference in the associations of AF with HFpEF vs. HFrEF events in a contemporary and diverse cohort with ongoing follow-up. Events were rigorously and conservatively adjudicated, and we used a conservative threshold in defining preserved LVEF in the primary analysis. REGARDS has a similar number of HFpEF and HFrEF events. Importantly, in comparing the survival functions for HFrEF and HFpEF across baseline AF status, we used a Lunn & McNeil augmented dataset approach rather than cause-specific hazard functions (in which other relevant failure types are censored). This was critical to direct comparison of competing risks and the integrity of resulting Kaplan-Meier plots, given that the risks of HFrEF and HFpEF are unlikely to be independent of one another.
In conclusion, over median 9 years’ follow-up of the REGARDS study, a cohort of contemporary Black and White Americans, AF was associated with all HF, HFrEF, and HFpEF events, and there was no significant difference in the associations of AF with incident HFrEF vs. HFpEF events. This suggests that AF increases risk for each of these primary subtypes to a similar magnitude. No differences in the associations of AF with HFrEF vs. HFpEF events were observed in any sex or race group, and findings were corroborated in sensitivity analysis using a less conservative definition of HFpEF. Further basic and translational research is needed to differentiate the mechanisms underlying the separate associations of AF with HFrEF and HFpEF.
Figure 4.
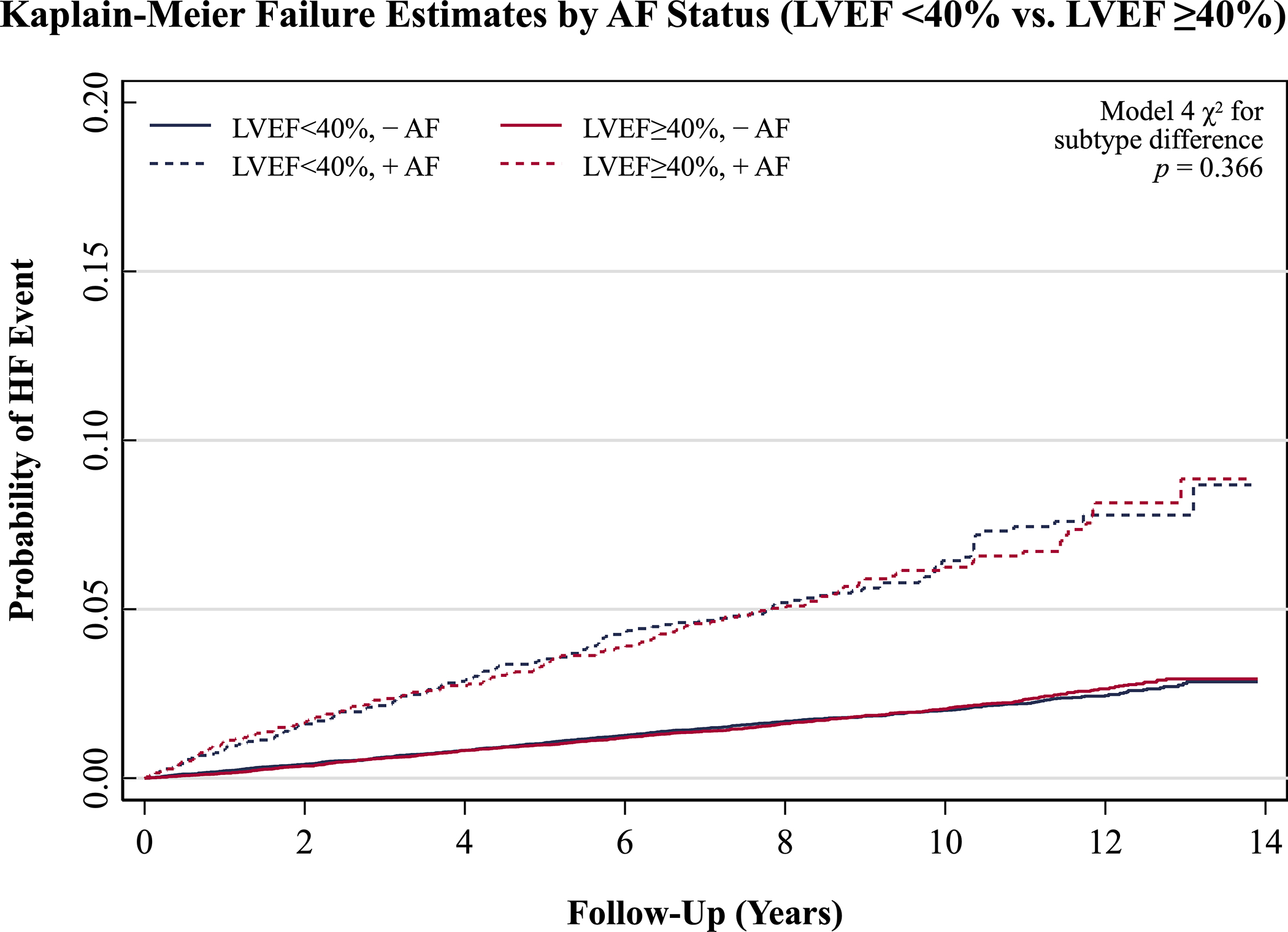
Unadjusted Kaplan-Meier failure curves in sensitivity analysis for heart failure events with left ventricular ejection fraction <40% and ≥40% in the augmented dataset for Lunn-McNeil analysis, each stratified by baseline atrial fibrillation status. Abbreviations: AF, atrial fibrillation; HF, heart failure; LVEF, left ventricular ejection fraction.
Table 4.
Sensitivity Analysis Defining HFpEF as LVEF≥40%: Association of Atrial Fibrillation With Incident Heart Failure Events
|
|
|||||||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | ||||
|
| |||||||
| HF Group* | n events / n at risk | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | χ2 p For Subtype Difference | |
|
| |||||||
| Overall | LVEF <40% | 388/ 25,787 | 2.87 (2.22, 3.72) | 2.35 (1.78, 3.11) | 2.01 (1.48, 2.72) | 1.85 (1.36, 2.51) | 0.366 |
| LVEF ≥40% | 433/ 25,787 | 2.33 (1.80, 3.02) | 1.84 (1.39, 2.42) | 1.61 (1.20, 2.17) | 1.52 (1.13, 2.05) | ||
| Women † | LVEF <40% | 141/14,176 | 2.89 (1.90, 4.40) | 2.22 (1.39, 3.55) | 2.16 (1.33, 3.51) | 2.20 (1.35, 3.57) | 0.114 |
| LVEF ≥40% | 224/14,176 | 2.57 (1.83, 3.63) | 2.23 (1.54, 3.23) | 2.05 (1.40, 3.01) | 2.03 (1.39, 2.98) | ||
| Men † | LVEF <40% | 247/11,611 | 2.84 (2.05, 3.94) | 2.50 (1.77, 3.54) | 1.94 (1.30, 2.89) | 1.72 (1.16, 2.56) | 0.803 |
| LVEF ≥40% | 209/11,611 | 1.96 (1.32, 2.92) | 1.43 (0.93, 2.17) | 1.15 (0.72, 1.85) | 1.04 (0.65, 1.68) | ||
| Black ‡ | LVEF <40% | 146/10,310 | 2.89 (1.85, 4.52) | 1.88 (1.14, 3.10) | 1.75 (1.05, 2.92) | 1.78 (1.07, 2.98) | 0.334 |
| LVEF ≥40% | 173/10,310 | 1.99 (1.25, 3.18) | 1.73 (1.06, 2.83) | 1.64 (1.00, 2.70) | 1.58 (0.96, 2.61) | ||
| White ‡ | LVEF <40% | 242/15,477 | 2.74 (2.00, 3.76) | 2.51 (1.79, 3.52) | 2.12 (1.44, 3.13) | 1.97 (1.34, 2.91) | 0.745 |
| LVEF ≥40% | 260/15,477 | 2.44 (1.79, 3.34) | 1.86 (1.33, 2.61) | 1.61 (1.11, 2.33) | 1.51 (1.04, 2.20) | ||
Abbreviations: AF, atrial fibrillation; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; EF, ejection fraction; HR, hazard ratio; CI, confidence interval
Covariates in Multivariable Models:
Model 1: age, sex, race, income, education, & region
Model 2: Model 1 covariates + smoking (pack-years), systolic blood pressure, diabetes mellitus, body mass index (continuous), low-density lipoprotein, left ventricular hypertrophy, estimated glomerular filtration rate, antihypertensive drug use, and coronary heart disease history.
Model 3: Model 2 covariates + aspirin, warfarin, and statin use.
Model 4: Model 3 covariates + incident coronary heart disease events (time-varying)
Unclassified cases were censored in the Lunn-McNeil analysis.
Model 4 AF*sex interaction p-values (cause-specific): LVEF <40%: 0.692; LVEF ≥40%: 0.022
Model 4 AF*race interaction p-values (cause-specific): LVEF <40%: 0.991; LVEF ≥40%: 0.971
KEY QUESTIONS.
What is already known about this subject?
Atrial fibrillation and heart failure have bidirectional associations that appear driven through shared risk factors and pathophysiology.
What does this study add?
We confirmed an association of atrial fibrillation with all incident heart failure events exists in a contemporary and biracial cohort.
We found that the associations of atrial fibrillation with heart failure with reduced versus preserved ejection fraction events do not differ significantly.
We showed that our finding of no significant difference in the associations of atrial fibrillation with heart failure subtypes is consistent across sex and race subgroups.
How might this impact on clinical practice?
Clinicians should be aware that their patients with atrial fibrillation appear to be at similar risk for both primary subtypes of heart failure.
Further clinical and translational research examining the pathophysiology through which atrial fibrillation and each subtype of heart failure are associated may allow for strategies to prevent these often-concurrent diseases.
ACKNOWLEDGEMENTS:
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at: https://www.uab.edu/soph/regardsstudy/.
DISCLOSURES:
Nicoli: No disclosures.
O’Neal: No disclosures
Levitan: Research funding from Amgen, scientific consulting for a research project funded by Novartis.
Singleton: No disclosures
Judd: No disclosures
Howard: No disclosures
Safford: No disclosures
Soliman: No disclosures
FUNDING:
This research project is supported by cooperative agreement U01 NS041588 co-funded by the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute on Aging (NIA), National Institutes of Health, Department of Health and Human Service. Additional funding was provided by R01 HL80477 from the National Heart Lung and Blood Institute (NHLBI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIA. Representatives of the NINDS were involved in the review of the manuscript but were not directly involved in the collection, management, analysis or interpretation of the data.
Footnotes
FEDERAL EMPLOYEE DISCLAIMER (Dr. Nicoli, must be included in publication): The identification of specific products or scientific instrumentation is considered an integral part of the scientific endeavor and does not constitute endorsement or implied endorsement on the part of the author, Department of Defense (DoD), or any component agency. The views expressed in this manuscript are those of the author and do not reflect the official policy of the Department of Army/Navy/Air Force, DoD, or U.S. Government.
DATA AVAILABILITY STATEMENT
The data used in these analyses include potentially identifying participant information and therefore are not publicly available due to legal and ethical restrictions. Qualified investigators may request access from the University of Alabama at Birmingham to obtain de-identified data (regardsadmin@uab.edu).
REFERENCES
- 1.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129(8):837–47. doi: 10.1161/circulationaha.113.005119 [published Online First: 2013/12/19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piccini JP, Hammill BG, Sinner MF, et al. Clinical course of atrial fibrillation in older adults: the importance of cardiovascular events beyond stroke. European heart journal 2014;35(4):250–6. doi: 10.1093/eurheartj/eht483 [published Online First: 2013/11/28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Neal WT, Qureshi W, Zhang ZM, et al. Bidirectional association between atrial fibrillation and congestive heart failure in the elderly. J Cardiovasc Med (Hagerstown) 2016; 17(3):181–6. doi: 10.2459/jcm.0000000000000289 [published Online First: 2016/01/31] [DOI] [PubMed] [Google Scholar]
- 4.Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. The American journal of cardiology 2003;91(6a):2d–8d. doi: 10.1016/s0002-9149(02)03373-8 [published Online First: 2003/04/03] [DOI] [PubMed] [Google Scholar]
- 5.Ling LH, Kistler PM, Kalman JM, et al. Comorbidity of atrial fibrillation and heart failure. Nature reviews Cardiology 2016;13(3): 131–47. doi: 10.1038/nrcardio.2015.191 [published Online First: 2015/12/15] [DOI] [PubMed] [Google Scholar]
- 6.Al-Khatib SM, Benjamin EJ, Albert CM, et al. Advancing Research on the Complex Interrelations Between Atrial Fibrillation and Heart Failure: A Report From a US National Heart, Lung, and Blood Institute Virtual Workshop. Circulation 2020;141(23): 1915–26. doi: 10.1161/circulationaha.119.045204 [published Online First: 2020/06/09] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. Jama 2001;285(18):2370–5. doi: 10.1001/jama.285.18.2370 [published Online First: 2001/05/10] [DOI] [PubMed] [Google Scholar]
- 8.Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nature reviews Cardiology 2016;13(6):368–78. doi: 10.1038/nrcardio.2016.25 [published Online First: 2016/03/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology 2005;25(3): 135–43. [DOI] [PubMed] [Google Scholar]
- 10.Howard G, Anderson R, Johnson NJ, et al. Evaluation of social status as a contributing factor to the stroke belt region of the United States. Stroke 1997;28(5):936–40. [DOI] [PubMed] [Google Scholar]
- 11.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clinical Chemistry 1972;18(6):499–502. doi: 10.1093/clinchem/18.6.499 [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006 [published Online First: 2009/05/06] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howard VJ, Woolson RF, Egan BM, et al. Prevalence of hypertension by duration and age at exposure to the stroke belt. J Am Soc Hypertens 2010;4(1):32–41. doi: 10.1016/j.jash.2010.02.001 [published Online First: 2010/04/09] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goyal P, Mefford MT, Chen L, et al. Assembling and validating a heart failure-free cohort from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. BMC Med Res Methodol 2020;20(1):53. doi: 10.1186/s12874-019-0890-x [published Online First: 2020/03/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prineas RJ, Soliman EZ, Howard G, et al. The sensitivity of the method used to detect atrial fibrillation in population studies affects group-specific prevalence estimates: ethnic and regional distribution of atrial fibrillation in the REGARDS study. J Epidemiol 2009;19(4):177–81. doi: 10.2188/jea.je20081032 [published Online First: 2009/06/30] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. American heart journal 1949;37(2): 161–86. doi: 10.1016/0002-8703(49)90562-1 [published Online First: 1949/02/01] [DOI] [PubMed] [Google Scholar]
- 17.Safford MM, Brown TM, Muntner PM, et al. Association of race and sex with risk of incident acute coronary heart disease events. Jama 2012;308(17): 1768–74. doi: 10.1001/jama.2012.14306 [published Online First: 2012/11/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics 1995;51(2):524–32. [published Online First: 1995/06/01] [PubMed] [Google Scholar]
- 19.Goto S, Bhatt DL, Röther J, et al. Prevalence, clinical profile, and cardiovascular outcomes of atrial fibrillation patients with atherothrombosis. American heart journal 2008;156(5):855–63, 63.e2. doi: 10.1016/j.ahj.2008.06.029 [published Online First: 2008/12/09] [DOI] [PubMed] [Google Scholar]
- 20.Vermond RA, Geelhoed B, Verweij N, et al. Incidence of Atrial Fibrillation and Relationship With Cardiovascular Events, Heart Failure, and Mortality: A Community-Based Study From the Netherlands. J Am Coll Cardiol 2015;66(9):1000–7. doi: 10.1016/j.jacc.2015.06.1314 [published Online First: 2015/09/01] [DOI] [PubMed] [Google Scholar]
- 21.Andersson T, Magnuson A, Bryngelsson IL, et al. Gender-related differences in risk of cardiovascular morbidity and all-cause mortality in patients hospitalized with incident atrial fibrillation without concomitant diseases: a nationwide cohort study of 9519 patients. International journal of cardiology 2014;177(1):91–9. doi: 10.1016/j.ijcard.2014.09.092 [published Online First: 2014/12/17] [DOI] [PubMed] [Google Scholar]
- 22.Santhanakrishnan R, Wang N, Larson MG, et al. Atrial Fibrillation Begets Heart Failure and Vice Versa: Temporal Associations and Differences in Preserved Versus Reduced Ejection Fraction. Circulation 2016;133(5):484–92. doi: 10.1161/circulationaha.115.018614 [published Online First: 2016/01/10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gwathmey JK, Slawsky MT, Hajjar RJ, et al. Role of intracellular calcium handling in force-interval relationships of human ventricular myocardium. J Clin Invest 1990;85(5): 1599–613. doi: 10.1172/jci114611 [published Online First: 1990/05/01] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhingra A, Garg A, Kaur S, et al. Epidemiology of heart failure with preserved ejection fraction. Curr Heart Fail Rep 2014;11(4):354–65. doi: 10.1007/s11897-014-0223-7 [published Online First: 2014/09/17] [DOI] [PubMed] [Google Scholar]
- 25.Soliman EZ, Alonso A, Goff DC Jr. Atrial fibrillation and ethnicity: the known, the unknown and the paradox. Future Cardiol 2009;5(6):547–56. doi: 10.2217/fca.09.49 [published Online First: 2009/11/06] [DOI] [PubMed] [Google Scholar]
- 26.Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Archives of internal medicine 2008;168(19):2138–45. doi: 10.1001/archinte.168.19.2138 [published Online First: 2008/10/29] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen LY, Chung MK, Allen LA, et al. Atrial Fibrillation Burden: Moving Beyond Atrial Fibrillation as a Binary Entity: A Scientific Statement From the American Heart Association. Circulation 2018;137(20):e623–e44. doi: 10.1161/cir.0000000000000568 [published Online First:2018/04/18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pandey A, Kim S, Moore C, et al. Predictors and Prognostic Implications of Incident Heart Failure in Patients With Prevalent Atrial Fibrillation. JACC Heart Fail 2017;5(1):44–52. doi: 10.1016/j.jchf.2016.09.016 [published Online First: 2016/12/31] [DOI] [PubMed] [Google Scholar]
- 29.Rooney MR, Soliman EZ, Lutsey PL, et al. Prevalence and Characteristics of Subclinical Atrial Fibrillation in a Community-Dwelling Elderly Population: The ARIC Study. Circ Arrhythm Electrophysiol 2019;12(10):e007390. doi: 10.1161/circep.119.007390 [published Online First: 2019/10/15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Syennberg Engdahl J, Al-Khalili F, et al. Mass Screening for Untreated Atrial Fibrillation: The STROKESTOP Study. Circulation 2015;131(25):2176–84. doi: 10.1161/circulationaha.114.014343 [published Online First: 2015/04/26] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in these analyses include potentially identifying participant information and therefore are not publicly available due to legal and ethical restrictions. Qualified investigators may request access from the University of Alabama at Birmingham to obtain de-identified data (regardsadmin@uab.edu).


