Abstract
INTRODUCTION
Neoadjuvant chemotherapy is standard treatment for locally advanced (LABC) or inflammatory breast cancer (IBC). We hypothesized adding sunitinib, a tyrosine kinase inhibitor with anti-tumor and anti-angiogenic activity, to an anthracycline and taxane regimen would improve pathologic complete response (pCR) rates to a prespecified endpoint of 45% in patients with HER2 negative LABC or IBC.
PATIENTS AND METHODS
We conducted a multicenter, phase II trial of neoadjuvant sunitinib with paclitaxel (S+T) followed by doxorubicin and cyclophosphamide plus G-CSF for patients with HER2 negative LABC or IBC. Patients received sunitinib 25 mg PO daily with paclitaxel 80 mg/m2 IV weekly x12 followed by doxorubicin 24 mg/m2 IV weekly + cyclophosphamide 60 mg/m2 PO daily with G-CSF support. Response was evaluated using pCR in the breast and the Clinical-pathologic scoring + estrogen receptor (ER) and grade (CPS+EG) score.
RESULTS
Seventy patients enrolled and 66 were evaluable for efficacy. Eighteen patients (27%) had pCR in the breast (10 had ER+ disease and 8 had triple negative disease). When defining response as pCR and/or CPS+EG score ≤ 2, 47% were responders. In ER+ patients, 23 (64%) were responders. The most common toxicities were cytopenias and fatigue.
CONCLUSIONS
Neoadjuvant S+T followed by AC+G-CSF was safe and tolerable in LABC and IBC. The study did not meet the prespecified endpoint for pCR. However, 47% were responders using pCR and/or CPS+EG score ≤2. ER+ patients had the highest response rate (64%). The addition of sunitinib to neoadjuvant chemotherapy may provide promising incremental benefit for ER+ patients.
Keywords: Locally Advanced Breast Cancer (LABC), Inflammatory Breast Cancer, HER2 negative, Neoadjuvant, Sunitinib, Paclitaxel, Doxorubicin, Cyclophosphamide
MICROABSTRACT
Pathologic complete response (pCR) following neoadjuvant chemotherapy is associated with improved survival in locally advanced and inflammatory breast cancer. Neoadjuvant sunitinib + paclitaxel followed by doxorubicin + cyclophosphamide with G-CSF therapy resulted in a 27% pCR rate in this single-arm, phase II trial. ER+ patients had higher response rates (64%) using the CPS+EG score and pCR suggesting promising incremental benefit.
INTRODUCTION
Neoadjuvant chemotherapy remains the treatment of choice for locally advanced breast cancer (LABC) and inflammatory breast cancer (IBC). Unfortunately, pathologic complete response (pCR), which is associated with longer disease-free survival (DFS) and overall survival (OS), is attained only in a minority of patients treated with standard anthracycline and taxane regimens.1–5 This highlights the need for more effective treatment strategies.
Incorporating anti-angiogenic biologic agents into breast cancer therapy has demonstrated improvements in response rate and DFS when compared to chemotherapy alone.6–8 Sunitinib malate is an oral, multi-target tyrosine kinase inhibitor with anti-tumor and anti-angiogenic activity that is approved for treatment of metastatic renal cell carcinoma (RCC), advanced pediatric neuroendocrine tumors, and gastrointestinal stromal tumors (GIST) with progression or intolerance on imatinib.9 It functions as a potent broad specificity kinase inhibitor targeting VEGFR-1, 2, 3, FLT3, KIT, PDGFRα, and PDGFRβ.10 Activity against VEGFR-3 and dysregulation of pericyte homeostasis through interference of the PDGF/PDGFR pathway are potential anti-angiogenic activities which are unique to sunitinib, as compared to other angiogenesis inhibitors such as bevacizumab.11 A multi-targeted approach has several proposed benefits including improved targeting of orthogonal tumor growth pathways and decreased potential for resistance development.12 Moreover, preclinical models with dual inhibition of VEGF and PDGF signaling pathways were found to be synergistic in inhibiting tumor growth.13,14
Preliminary results from an earlier trial demonstrated that continuous daily cyclophosphamide and weekly doxorubicin (AC) plus scheduled G-CSF followed by paclitaxel as initial therapy in LABC and IBC was superior to standard, intermittent AC and with significant improvement in pCR (31% versus 19%, p=0.02).15 Though the final results ultimately showed no significant advantage in pCR in the investigational arm3, the initial results prompted the design for this trial and we hypothesized that adding sunitinib to a similar regimen may further improve pCR. Based upon data showing decreased tumor interstitial fluid pressure and improved oxygenation in patients treated with neoadjuvant weekly paclitaxel followed by dose dense doxorubicin and cyclophosphamide16 and the potential for dose-limiting neutropenia with sunitinib in combination with doxorubicin, we combined sunitinib (S) with weekly paclitaxel (S+T) prior to continuous AC. Phase I studies of combination S+T previously demonstrated this regimen was well tolerated in patients with locally advanced or metastatic breast cancer and there was preliminary evidence of antitumor activity.12,17
Herein we report outcomes regarding safety and efficacy of daily sunitinib in combination with weekly paclitaxel followed by weekly doxorubicin and daily cyclophosphamide plus G-CSF as neoadjuvant therapy for hormone receptor positive or negative, HER2 negative, LABC and IBC.
PATIENTS AND METHODS
Patient eligibility
Eligible patients had histologically confirmed, locally advanced or inflammatory HER2 negative breast cancer. Locally advanced was defined as clinical stages IIB, IIIA, IIIB, and IIIC or disease which was judged to be primarily unresectable by an experienced breast surgeon. Inflammatory breast cancer was defined by clinical criteria with erythema and peau d’orange of the breast with a histologic diagnosis of breast cancer. Additionally, patients were required to have an adequate performance status, adequate renal and kidney function, no significant neutropenia or thrombocytopenia, and normal ejection fraction measured within 3 months prior to enrollment.
Patients were excluded if they had any evidence of distant metastases, or, if they had received any prior chemotherapy/endocrine therapy, radiation therapy or definitive surgery for breast cancer. Additional exclusion criteria included significant medical comorbidities such as heart disease or a cerebrovascular event and contraindications to sunitinib including uncontrolled hypertension or thyroid abnormality.
This multicenter study was conducted at University of Washington/Seattle Cancer Care Alliance (SCCA), SCCA Network sites, and University of Arizona Cancer Center. Patients were enrolled between September 2007 and February 2012. All patients signed informed consent prior to any study-related procedures. This trial is registered with ClinicalTrials.gov, NCT00513695.
Drug administration
Patients were treated with paclitaxel 80 mg/m2 IV weekly and sunitinib 37.5 mg daily PO for 12 weeks. The dose of sunitinib was reduced to 25 mg from 37.5 mg in March 2008 by protocol amendment based on preliminary data from a phase I study of S+T which showed increased myelosuppression and neutropenia.17,18 Five patients received the 37.5 mg dose prior to the protocol amendment. After 12 weeks patients without significant toxicity that precluded further treatment then received doxorubicin 24 mg/m2 IV weekly and cyclophosphamide 60 mg/m2 PO daily for 15 weeks combined with growth factor support of G-CSF 5 ug/kg SC on days 2–7 for 15 weeks (Figure 1). Prior to starting this treatment, ANC had to be greater than 1,500/mm3 and platelets had to be greater than 100,000/mm3. Patients received pneumocystis carinii pneumonia prophylaxis with trimethoprim/sulfamethoxazole 1 single strength tablet daily for 15 weeks during doxorubicin and cyclophosphamide therapy per standard practice at the time.19–24 After completion of neoadjuvant treatment patients were offered definitive surgical resection.
FIGURE 1: Treatment Schema.
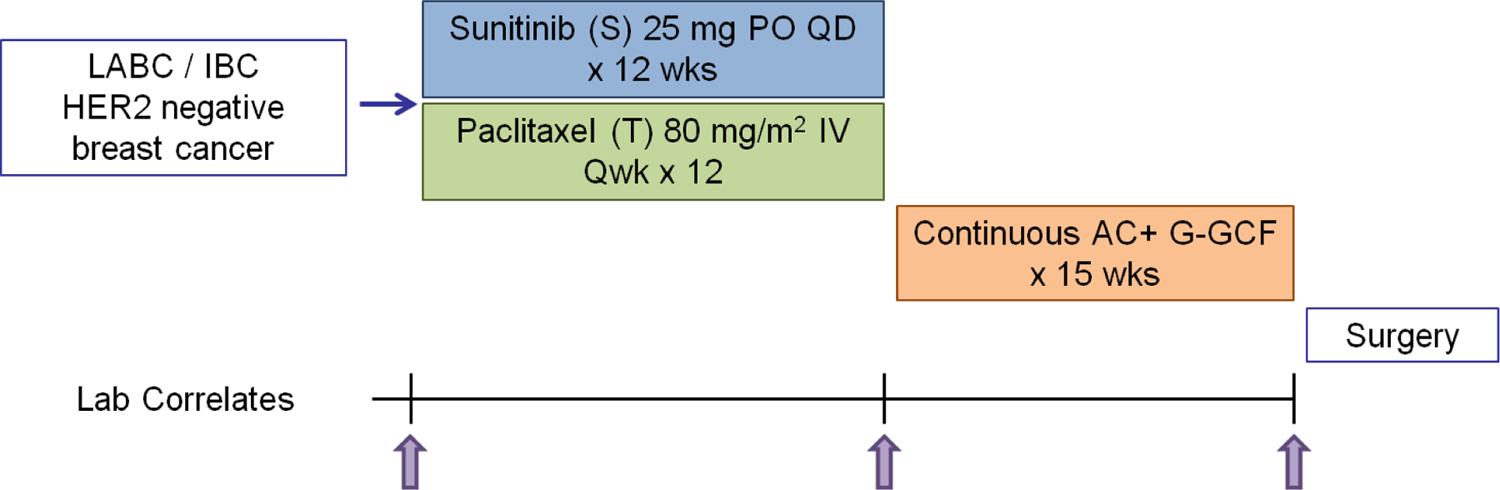
Schema for a Phase II trial of daily sunitinib in combination with weekly paclitaxel followed by weekly doxorubicin and daily cyclophosphamide plus G-CSF as neoadjuvant therapy, NCT00513695
Toxicity
Adverse events (AEs) were evaluated at each study visit for the duration of the trial. During investigational treatment laboratory toxicities were assessed at each infusion and clinical toxicity was assessed at each visit. Following completion of treatment, patients were clinically evaluated no less than quarterly for 2 years, then every 6 months for three years, and annually thereafter. For treatment related toxicity and AE reporting, the study used the NCI CTC (Common Terminology Criteria) Version 3.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf). Doses were adjusted or delayed per protocol according to the system showing the greatest degree of treatment related toxicity. AEs that meet severity grade 2 or greater were collected and reported.
Clinical Response:
The primary endpoint was pCR which was defined as no evidence of microscopic invasive tumor present in the surgical specimens including the primary tumor site (breast). Ellis et al. previously reported a pCR rate of 31% for patients treated with continuous doxorubicin and cyclophosphamide followed by paclitaxel in patients with inflammatory and locally advanced breast cancer.15 The pre-specified endpoint was selected based on this data and we hypothesized that incorporating sunitinib into this regimen would improve pCR to a rate of 45%. In addition to assessing pCR in the breast at the time of surgery, the MDACC Clinical-pathologic scoring +ER and grade (CPS+EG) was calculated. This scoring system incorporates pretreatment clinical stage, grade, and ER status with the final pathologic stage to more accurately stratify patients according to prognosis after administration of neoadjuvant chemotherapy.25,26 There is robust data to support the use of the CPS+EG score which has been validated multiple times both in internal and external patient cohorts.25–35
Secondary endpoints included clinical complete response, relapse rate, DFS, and OS. Prior to neoadjuvant chemotherapy, axillary nodes were assessed by sentinel node biopsy or image-guided biopsy. The optimal imaging method (mammogram, ultrasound or breast MRI) for assessing tumor volume was determined pre-study and then repeated every 4 – 8 weeks during investigational treatment. Radiologic test during the follow-up period was at the discretion of the treating physician.
Statistical Analysis
The study sample size of 70 patients (anticipating 64 evaluable) was determined to have 80% power to identify a 45% pCR rate as superior to a 30% null rate15, with a one-sided Type I error of 0.05. Baseline demographic and clinical characteristics were evaluated. For continuous variables, the median and range were calculated; for categorical variables, the number and percentage were calculated. For AE, the number of subjects under each AE category was summarized by different grades and by total, respectively.
For survival analysis, OS was defined as time from day 1 of protocol treatment to death and DFS was defined as time from day 1 of protocol treatment to disease progression or death whichever occurred first. DFS and OS were evaluated between different groups (e.g., CPS+EG score ≥ 3 vs CPS+EG score <3 vs indeterminate score) using Kaplan-Meier curves and Log-Rank test.
RESULTS
Patient and disease characteristics
From September 2007 to February 2012, a total of 70 patients provided informed consent and were enrolled from academic centers and community network oncology practices. Three patients were screen fails and 67 patients received protocol directed therapy. Patient and disease characteristics are shown in Table 1. Tumor characteristics were taken from diagnostic breast tumor biopsy prior to enrollment. Of the 67 patients treated, 59 (88%) had infiltrating ductal carcinoma and 5 (7%) had infiltrating lobular carcinoma. There were 37 patients with ER positive and/or PR positive disease and 30 patients with TNBC. Six patients had IBC of which 3 had ER positive and/or PR positive and 3 had TNBC.
TABLE 1:
Patient Characteristics
| Overall (N=67) | |
|---|---|
| Age | |
| Median (Range) | 50 (33, 79) |
| Menopausal Status | |
| Premenopausal | 34 (51%) |
| Perimenopausal | 3 (4%) |
| Menopausal | 26 (39%) |
| Missing | 4 (6%) |
| Study Site | |
| UWMC/SCCA | 31 (46%) |
| SCCA Network | 8 (12%) |
| UA/Arizona Cancer Center | 28 (42%) |
| Histology | |
| Ductal | 59 (88%) |
| Lobular | 5 (7%) |
| Other | 3 (4%) |
| Hormone Receptor Status | |
| ER and/or PR positive | 37 (55%) |
| TNBC | 30 (45%) |
| Ki67 | |
| Median (range) | 50 (5, 100) |
| Missing | 16 |
| Grade | |
| 1 | 2 (3%) |
| 2 | 13 (19%) |
| 3 | 43 (64%) |
| Missing | 9 (13%) |
| Clinical Stage | |
| IIB | 9 (13%) |
| IIIA | 40 (60%) |
| IIIB | 10 (15%) |
| IIIC | 8 (12%) |
| Inflammatory Breast Cancer | 6 (9%) |
| ER and/or PR positive | 3 (4%) |
| TNBC | 3 (4%) |
Selected patient and tumor characteristics from 67 patients
Safety analysis included all 67 patients who started therapy per protocol. The efficacy analysis included 66 patients as 1 patient withdrew consent after completing all therapy. Of the 66 patients, there were 61 patients who had surgery and were evaluable for pathologic response. The remaining 5 patients who did not complete surgery were considered non-responders for the primary endpoint of pCR. Of these 5 patients, 3 patients had progressive disease, 1 patient went off study due to toxicity, and 1 patient completed all therapy but elected not to have surgery.
Toxicity profile
Grade 2 or higher events as reported using CTCAE 3.0 were observed in 64 patients (96%) during S+T and 51 (88%) during AC+G-CSF (Tables 3 and 4). The most common toxicities of any grade during S+T included neutropenia (52 events), leukopenia (44), fatigue (22), and anemia (17). Seven patients developed grade 2 neuropathy. The most common toxicities of any grade during AC+G-CSF included leukopenia (22 events), neutropenia (21), anemia (19), mucositis (15), fatigue (14), and nail changes (14). No grade 5 toxicities were reported. These toxicities were similar in distribution and severity to other reports evaluating these agents and we report no new or unexpected toxicities.
TABLE 3:
Toxicity During Sunitinib+Paclitaxel
| Adverse Events during S+T | Grade 2 | Grade 3 | Grade 4 | Total |
|---|---|---|---|---|
| Neutropenia | 17 | 28 | 7 | 52 |
| Leukopenia | 26 | 17 | 1 | 44 |
| Fatigue | 19 | 3 | 0 | 22 |
| Anemia | 14 | 3 | 0 | 17 |
| Diarrhea | 6 | 4 | 0 | 10 |
| Mucositis | 6 | 3 | 0 | 9 |
| Hypertension | 7 | 0 | 0 | 7 |
| LFT abnormality | 6 | 1 | 0 | 7 |
| Neuropathy | 7 | 0 | 0 | 7 |
| Rash | 6 | 1 | 0 | 7 |
| Allergic reaction | 5 | 1 | 0 | 6 |
| Heartburn | 6 | 0 | 0 | 6 |
| Pain | 5 | 1 | 0 | 6 |
| Infection | 3 | 2 | 0 | 5 |
| Nausea/Vomiting | 5 | 0 | 0 | 5 |
| Alopecia | 4 | 0 | 0 | 4 |
| Mood alteration | 4 | 0 | 0 | 4 |
| Thrombocytopenia | 3 | 1 | 0 | 4 |
| Weakness | 4 | 0 | 0 | 4 |
| Hot flashes | 3 | 0 | 0 | 3 |
| Nail changes | 3 | 0 | 0 | 3 |
| Anorexia | 2 | 0 | 0 | 2 |
| Fever | 0 | 2 | 0 | 2 |
| Hand-foot | 1 | 1 | 0 | 2 |
| Lymphopenia | 1 | 1 | 0 | 2 |
| Taste alteration | 2 | 0 | 0 | 2 |
| Nose bleed | 0 | 1 | 0 | 1 |
| Total Events | 165 | 70 | 8 | 245 |
| Total Patients | 58 (87%) | 42 (63%) | 8 (12%) | 64 (96%) |
Most common adverse events during sunitinib plus paclitaxel phase. There were 245 total grade 2 or higher events that occurred in 64 (96% of patients). Neutropenia was most common grade 3/4 event and G-CSF 5 mcg/kg SC permitted. There were no grade 5 events.
TABLE 4:
Toxicity During AC+G-CSF
| Adverse Events during AC | Grade 2 | Grade 3 | Grade 4 | Total |
|---|---|---|---|---|
| Leukopenia | 13 | 6 | 3 | 22 |
| Neutropenia | 7 | 10 | 4 | 21 |
| Anemia | 15 | 4 | 0 | 19 |
| Mucositis | 6 | 9 | 0 | 15 |
| Fatigue | 13 | 1 | 0 | 14 |
| Nail changes | 12 | 2 | 0 | 14 |
| Nausea/Vomiting | 10 | 1 | 0 | 11 |
| Hand-foot | 3 | 3 | 0 | 6 |
| Alopecia | 5 | 0 | 0 | 5 |
| Infection | 5 | 0 | 0 | 5 |
| Mood alteration | 5 | 0 | 0 | 5 |
| Pain | 5 | 0 | 0 | 5 |
| Constipation | 4 | 0 | 0 | 4 |
| Lymphopenia | 3 | 0 | 1 | 4 |
| Neuropathy | 3 | 1 | 0 | 4 |
| Hot flashes | 3 | 0 | 0 | 3 |
| Diarrhea | 2 | 0 | 0 | 2 |
| Heartburn | 2 | 0 | 0 | 2 |
| Irregular menses | 2 | 0 | 0 | 2 |
| Nasal/paranasal reactions | 2 | 0 | 0 | 2 |
| Total Events | 120 | 37 | 8 | 165 |
| Total Patients | 50 (86%) | 27 (47%) | 5 (9%) | 51 (88%) |
Most common adverse events during doxorubicin and oral cyclophosphamide plus G-CSF phase. There were 165 total grade 2 or higher events which occurred in 51 (88%) of patients. Leukopenia was most common grade 3/4 event. There were no grade 5 events.
The median dose delivery by percent for sunitinib was 100% (range 54–115) based on the value after the dose reduction. The median dose for paclitaxel by percent was 100% (range 50–100). A total of 42 (63%) patients required dose modifications or a hold during the course of S+T, and 36 (62%) patients required dose modifications or a hold during the course of AC+G-CSF (Table 2). The most common reasons for dose modifications/holds during S+T included neutropenia (25 events), leukopenia (7), and diarrhea (3). Other reasons for dose modifications/holds during S+T included anorexia (2), fatigue (2), mucositis (2), rash (2), fever (1), hypertension (1), LFT abnormalities (1), epistaxis (1), hand-foot syndrome (1), and thrombocytopenia (1). The most common reasons for dose modifications/holds during AC+G-CSF included neutropenia (10 events), mucositis (6), leukopenia (6), and hand-foot syndrome (3). Other reasons for dose modifications/holds during AC+G-CSF included anemia (2), alopecia (1), constipation (1), infection (1), nail changes (1), neuropathy (1), and pain (1).
TABLE 2:
Dose Delivery
| S+T Phase (N=67) | Post S+T (N=64) | AC Phase (N=58) | Post AC (N=54) | |
|---|---|---|---|---|
| Treatment summary | ||||
| Completed without modification | 22 (33%) | 18 (31%) | ||
| Completed with modifications | 42 (63%) | 36 (62%) | ||
| Off study during of S+T | 3 (4%) | |||
| Off study after completing S+T | 6 (9%) | |||
| Off study during AC | 4 (7%) | |||
| Off study after completing AC | 4 (7%) | |||
| Reason for discontinuation | ||||
| Progressive disease | 1 (33%) | 3 (50%) | 0 (0%) | 2 (50%) |
| Toxicity (by protocol specifications) | 2 (67%) | 0 (0%) | 1 (25%) | 0 (0%) |
| Patient choice | 0 (0%) | 1 (17%) | 3 (75%) | 1 (25%) |
| MD decision | 0 (0%) | 1 (17%) | 0 (0%) | 0 (0%) |
| Other | 0 (0%) | 1 (17%) | 0 (0%) | 1 (25%) |
Dose delivery of neoadjuvant therapy. G-CSF 5 mcg/kg days 2–7 each wk administered per protocol during AC+G-CSF period. Sunitinib (S) dose was reduced from 37.5 to 25 mg PO daily by protocol amendment March 2008.
Of the 67 patients who received protocol therapy, a total of 3 patients discontinued therapy during treatment with S+T (2 due to toxicity and 1 due to progressive disease). Another 6 patients went off therapy after completion of all S+T, but prior to receiving AC (3 due to progressive disease, 1 due to patient choice, 1 due to physician choice, and 1 due to a femur fracture unrelated to treatment). A total of 4 patients went off study during AC (3 due to patient wishes and 1 due to toxicity). There were 4 patients who went off study after completion of all therapy, but prior to surgery (2 due to progressive disease, 1 due to patient wishes, and 1 patient withdrew consent). In the 3 patients who discontinued treatment due to toxicity, the AE resulting in termination of study treatment included hyponatremia, infusion reaction to paclitaxel during S+T, and neutropenia during AC+G-CSF.
Efficacy data
Of the 66 patients in the efficacy cohort, 18 (27%) had pCR in the breast and 15 (23%) had pCR in the breast and axilla, with similar pCR rates for patients with ER/PR+ disease and TNBC (Table 5, Chi-square test of independence p=0.99 for both). None of the 6 patients with IBC had a pCR. Patients were also evaluated with CPS+EG scores (Table 6). Twenty patients (30%) had a CPS+EG scores ≤ 2 and 38 (58%) had a CPS+EG score ≥ 3. There were 8 patients for whom CPS+EG was not available (3 left the study before surgery due to progressive disease, 2 left due to patient choice, 1 left due to toxicity, and 2 had insufficient information to calculate scores). Of the patients with indeterminant scores, 7 had TNBC disease and 1 had ER/PR+ disease. Of ER/PR+ patients, 18 (50%) had CPS+EG scores ≤ 2. Two (7%) patients with TNBC had a score ≤ 2. Responders were defined as patients with pCR in the breast and/or CPS+EG score ≤ 2. Overall, 31 (47%) patients were responders. Within the ER/PR+ cohort 23 patients (64%) were responders and within the TNBC cohort 8 (27%) were responders (p=0.006).
TABLE 5:
Pathologic Complete Response
| Evaluable Patients (N=66) | ER and/or PR positive (N=36) | TNBC (N=30) | |
|---|---|---|---|
| pCR in breast | |||
| Yes | 18 | 10 | 8 |
| No | 48 | 26 | 22 |
| pCR rate, n (%) | 18 (27%) | 10 (28%) | 8 (27%) |
| pCR in breast and axilla | |||
| Yes | 15 | 8 | 7 |
| No | 51 | 28 | 23 |
| pCR in breast and axilla rate, n (%) | 15 (23%) | 8 (22%) | 7 (23%) |
Efficacy assessment of pathologic complete response in breast, breast and axilla for evaluable patients.
TABLE 6:
CPS+EG Scores
| Evaluable patients (N=66) | ER and/or PR positive (N=36) | TNBC (N=30) | |
|---|---|---|---|
| CPS+EG score | |||
| ≤ 2 | 20 (30%) | 18 (50%) | 2 (7%) |
| ≥ 3 | 38 (58%) | 17 (47%) | 21 (70%) |
| Indeterminate | 8 (12%) | 1 (3%) | 7 (23%) |
| Responder = pCR and/or CPS+EG ≤ 2 | 31 (47%) | 23 (64%) | 8 (27%) |
| Non-responder = non-pCR or CPS+EG score ≥ 3 | 35 (53%) | 13 (36%) | 22 (73%) |
Efficacy assessment by CPS+EG score. Responders included patients with pCR in the breast and/or CPS+EG score ≤ 2. Non-responders were patients with no pCR or CPS+EG score ≥ 3.
Secondary endpoints included DFS and OS (Figure 2). At 5 years, 45 patients (67%) were disease-free. DFS was not reached in both the ER+ and TNBC subsets and was statistically similar between the two groups (p=0.068). Median DFS was significantly longer in patients with CPS+EG scores ≤ 2 (DFS not reached for CPS+EG ≤ 2 vs 8.23 years for scores ≥ 3 vs 1.02 years for indeterminate scores, p=0.0035). Patients who were responders had significantly better DFS compared to those who were non-responders. The median DFS was not reached for responders vs 3.03 years for non-responders (p=0.00013).
Figure 2: Disease Free Survival and Overall Survival.
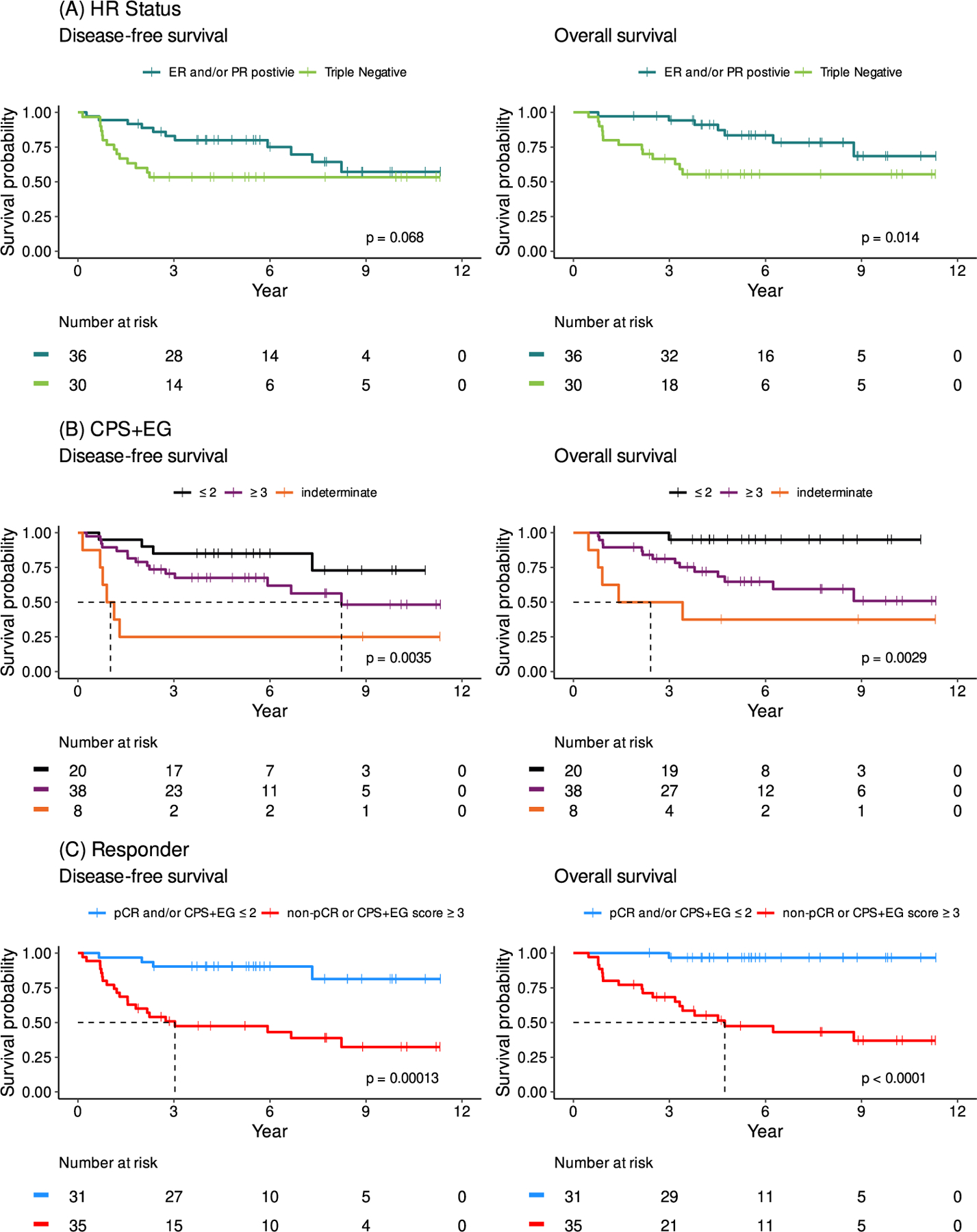
(A) DFS and OS based on hormone receptor status. Median OS and median DFS were not reached for ER/PR+ or TNBC disease. (B) DFS and OS based on CPS+EG score. Median DFS was 1.02 years for those with indeterminate CPS+EG, 8.23 years for patients with CPS+EG ≥ 3, and was not reached for patients with CPS+EG ≤ 2 (p=0.0035). Median OS for patients with indeterminate CPS+EG score 2.41 years and was not reached for patients with CPS+EG ≤ 2 and CPS+EG ≥ 3 (p=0.0029). (C) DFS and OS based on response to treatment. Patients were deemed responders if they had a pathologic complete response and/or had CPS+EG ≤ 2. Median DFS for non-responders was 3.03 years and was not reached for responders (p=0.00013). Median OS for non-responders was 4.73 years and was not reached for responders (p=<0.0001). N=66.
At 5 years, 31 (86%) percent of patients with ER/PR+ disease were still alive and 16 (63%) of patients with TNBC were alive. Overall, 47 (71%) of patients were alive. Median OS was not reached but was significantly better in the ER+ group compared to TNBC (p=0.014). Median OS was also significantly longer in patients with CPS+EG scores ≤ 2 (OS not reached for both CPS+EG scores ≤ 2 and ≥ 3 vs 2.41 years for indeterminant scores, p=0.0029). Responders had significantly better OS compared to non-responders: the median OS was not reached for responders vs 4.73 years for non-responders (p=<0.0001)
DISCUSSION
We found that the addition of S+T followed by AC+G-CSF was safe and tolerable as neoadjuvant therapy for LABC and IBC. The majority of patients (81%) completed protocol therapy. The primary endpoint for the study was pCR: overall pCR rate was 27%, 27% for TNBC, and 28% for hormone receptor-positive disease. Though the observed pCR rate did not meet the pre-specified criteria to reject the null hypothesis, the pCR rate observed in patients with ER+ LABC exceeded rates previously reported in randomized clinical trials.15 Additionally, when response was defined by pCR and/or CPS+EG ≤ 2, 31 patients (47%) were responders. Interestingly in the SWOG S0800 trial, which examined bevacizumab in combination with nab-paclitaxel followed by dose-dense AC compared to nab-paclitaxel followed or preceded by AC as neoadjuvant treatment for HER2-negative LABC or IBC, they found the addition of the anti-angiogenic agent bevacizumab significantly increased the pCR rate overall (36 vs 21 %; p = 0.019) and in TNBC (59 vs 29 %; p = 0.014), but not in hormone receptor-positive disease (24 vs 18%; p = 0.41).36
However, in our study the addition of sunitinib to neoadjuvant chemotherapy was not associated with improvement in pCR for TNBC patients compared to historical controls alone while patients with ER or PR+ disease had higher response rates than previously reported (64% when response was defined by pCR and/or CPS+EG ≤ 2). This suggests that the addition of sunitinib to neoadjuvant chemotherapy may result in promising incremental benefits for patients with ER+ LABC. The biological rationale for why sunitinib might be more effective in ER+ patients is not fully understood. VEGF and the estrogen receptor are co-expressed in many breast tumors and several studies show estrogen can modulate VEGF expression in multiple tissues.37,38 Heer et al. reported elevated VEGF levels immediately prior to surgery for patients with ER+ tumors while those with lobular carcinoma and ER− tumors had serum VEGF levels comparable to healthy controls.39 Therefore it is possible that the tumorigenesis of some ER+ positive breast cancers may also be dependent on VEGF signaling. However, other studies have shown that VEGF levels are higher in TNBC.40 As sunitinib is a broad specificity kinase inhibitor, it is also possible the effect we observed in the ER+ subtype is due to sunitinib’s inhibition of a pathway other than VEGF. For example, sunitinib is also known to be an inhibitor of RET. RET and ER expression have been shown to be significantly correlated in prior studies.41–44 In a preclinical in vivo model Spanheimer et al. showed inhibition of RET via sunitinib reduced growth of luminal breast cancer cells primarily via increased apoptosis.45 Overall, additional studies are needed to further elucidate why the ER+ subtype may be more sensitive to sunitinib.
The most common toxicities during treatment were neutropenia, leukopenia, fatigue, and anemia with no new safety signals observed. A total of 42 (63%) patients required dose modifications or a hold during the course of S+T treatment and 36 (62%) required dose modifications or a hold during the course of AC+G-CSF treatment. No grade 5 toxicities occurred. The number of AEs reported was relatively high, perhaps in part due to frequent assessment at weekly administration of dose-dense chemotherapy. Overall, the treatment combination was safe and tolerable.
One of the limitations of this work is that the continuous AC regimen that forms the backbone of the study is not the current standard of care. Selection of this regimen was based on data from SWOG 9625 and SWOG 0012 as well as institutional experience.15,46 In SWOG 0012 Ellis et al. compared standard AC followed by paclitaxel to a continuous dosing schedule with daily oral cyclophosphamide and weekly doxorubicin followed by weekly paclitaxel in the neoadjuvant setting for patients with LABC or IBC. Initial results showed improved pCR rates in the continuous arm (31% in the continuous arm versus 19% pCR in the standard arm, p=0.02). At the time, this was highest reported pCR for this patient population. This regimen was consequently chosen as our backbone and the pre-specified endpoint for pCR was based on these results. Our study was powered to detect improvement in pCR when compared to this regimen. Additionally, the final results of the Ellis et al. study showed no difference in pCR including in the hormone receptor positive subgroup which had a pCR rate of 15.3% (n=98) in the continuous arm and 10.7% (n=84) in the standard arm. This supports that the difference observed in our trial was primarily driven by addition of sunitinib. Our study provides a provocative observation particularly in the setting of ER+ disease which has traditionally had unimpressive pCR rates following neoadjuvant chemotherapy. This highlights the continued need to better understand which patients may derive benefit from treatment with anti-angiogenic agents.
There have since been several trials evaluating the use of sunitinib in advanced breast cancer. A phase II study by Burstein et al showed that sunitinib was active and safe in patients with heavily pretreated metastatic breast cancer with an objective response rate (ORR) of 11%.47 However, multiple studies have shown minimal benefit in terms of survival or response, but have raised concern for increased toxicity. A phase II study by Bergh et al showed sunitinib plus docetaxel improved ORR but did not prolong either DFS or OS compared with docetaxel alone in HER2 negative advanced breast cancer.48 Similarly, there have been two phase III trials evaluating sunitinib in combination with capecitabine in advanced breast cancer both of which showed no improvement in progression free survival, OS, or ORR though the frequency and severity of toxicity was increased in patients receiving sunitinib.49,50
In the neoadjuvant setting, a phase I/II trial of sunitinib administered with weekly paclitaxel/carboplatin in patients with locally advanced TNBC showed a pCR rate of 35%. While these results were comparable to other trials, substantial toxicity was observed with resulting dose reductions/omissions including discontinuation of sunitinib in 27% of patients.51
Lack of a predictive biomarker has hindered the use of sunitinib in the treatment of breast cancer and other solid tumors.48 Biomarker data collected during this trial was reported previously by Brown-Glaberman et al.52 These results validated circulating carbonic anhydrase IX (CAIX) levels as a biomarker of hypoxia and HIF-1α upregulation suggesting this as a potential identifier for tumors unlikely to respond to anti-angiogenic therapies. Pre-clinical studies have suggested that anti-angiogenic agents may overprune the tumor vasculature, causing tumor hypoxia and genetic drift which leads to more aggressive phenotypes.53–55 Areas of hypoxia may promote resistance by upregulation of pro-angiogenic factors that allow tumors to circumvent anti-angiogenic agents such as bevacizumab and sunitinib.56 The biomarker data collected during this trial is consistent with this observation and suggests that tumors adapted to a hypoxic environment, as determined by baseline VCAM and CAIX levels, may be less sensitive to anti-angiogenic therapy.52 Additionally, in vivo models have shown that intra-tumoral hypoxia generated by treatment with sunitinib or bevacizumab increases cancer stem cell populations via HIF-1α upregulation and Akt/β-catenin activation,57 thus limiting effectiveness of anti-angiogenic agents. Therefore a combination therapy that additionally targets resistance pathways and/or cancer stem proliferation may be necessary.57
Another theory proposed by Jain et al is the concept of vascular normalization, suggesting that judicious use of anti-angiogenic therapy may temporarily stabilize the vasculature and therefore enhance drug delivery.58 Conversely prolonged therapy or high dose therapy would lead to blood vessel destruction which in turn would decrease drug delivery.58,59 Wong et al implemented this strategy in a phase Ib/II of neoadjuvant doxorubicin and cyclophosphamide with or without short course sunitinib (12.5 mg given daily for 7 days prior to chemotherapy.60 While the addition of sunitinib did not improve pCR, they observed evidence of vascular normalization on IHC which was accompanied by improved vascular perfusion seen on imaging. However, there is no study that addresses the optimal timing or duration of anti-angiogenic therapy in relation to chemotherapy which would be critical in order to implement a vascular normalization strategy.60
CONCLUSIONS
Neoadjuvant treatment with S+T followed by AC+G-CSF was found to be safe and tolerable in patients with locally advanced breast cancer. Using a combined definition of response of pCR and/or CPS+EG score ≤2, 47% of patients had a response to treatment. Patients with ER positive disease had the highest response rate (64%). Our data provide evidence that the addition of sunitinib to neoadjuvant chemotherapy may result in promising incremental benefit for patients with ER positive breast cancer. While other trials in breast cancer have not shown significant benefit with the addition of sunitinib, the present study suggests that carefully selected patients may derive benefit. This work highlights the continued challenges of the use sunitinib and other anti-angiogenic agents which have shown varying degrees of benefit in patients with breast cancer.
CLINICAL PRACTICE POINTS:
Neoadjuvant chemotherapy is the treatment of choice for locally advanced breast cancer and inflammatory breast cancer. Achieving a pathologic complete response (pCR) following treatment is associated with improved survival, but unfortunately pCR is achieved in a minority of patients with standard anthracycline and taxane regimens.1–5
Sunitinib is a tyrosine kinase inhibitor with anti-tumor and anti-angiogenic activity.
This multicenter, phase II clinical trial showed neoadjuvant treatment with sunitinib plus weekly paclitaxel (S+T) followed by doxorubicin and daily oral cyclophosphamide plus G-CSF (AC+G-CSF) was safe and tolerable patients with HER2 negative locally advanced breast cancer and inflammatory breast cancer. The most common toxicities were uncomplicated neutropenia, leukopenia, fatigue, and anemia.
While pathologic complete response (pCR) did not meet the pre-specified criteria of interest, it was similar to comparable trials.
The MDACC Clinical-pathologic scoring +ER and (CPS+EG) system incorporates pretreatment clinical stage, grade, and ER status with the final pathologic stage to more accurately stratify patients according to prognosis after administration of neoadjuvant chemotherapy.25,26
When response to S+T followed by AC+G-CSF was evaluated using a combined definition of response of pCR and/or CPS+EG score ≤2, there were a significant number of responders among ER+ patients suggesting there may be in promising incremental benefits in the subset of patients with ER+ tumors.
ACKNOWLEDGEMENTS
We thank the patients who participated in this trial and their families. We also thank the research staff, clinical staff, and providers involved in this trial. We also wish to thank the late Dr. Robert B. Livingston for mentorship in trial design, clinical research and patient care.
FUNDING
This study was approved and funded by the National Comprehensive Cancer Network (NCCN) Oncology Research Program from general research support provided by Pfizer, Inc (SI11, CC-IRB #6488) and unrestricted research funds from Safeway. Additional support was provided by the Biostatistics Shared Resource Facility of the Fred Hutchinson Cancer Research Center (P30 CA015704) UL1 RR025014 (University of Washington ITHS REDCap installation).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The following authors report they have conflicts of interest to disclose:
JRG: Roche/Genentech (DSMC, Steering committee), Astra Zeneca (DSMC, consultant), Novartis (DSMC), Puma (advisory board), Immunomedics (DSMC), Radia (DSMC), Pfizer (advisory board), InBiomotion (advisory board), Sandoz/Hexal (Consultant), Genomic Health (advisory board)
VG: SEngine Precision Medicine (ownership), AmunBio (Ownership), New Equilibrium Biosciences (Ownership), Seagen (Honoria/Consulting), Puma (Honoraria, Consulting), Sanofu (Honoraria/Consulting), Roche (research funding)
BK: eResearch Technologies (Employer)
AS: Amgen (grant funding/honoraria), Astazeneca (consulting), Athenex (consulting)
The remaining authors have no conflicts of interest to disclose.
REFERENCES
- 1.Kuerer HM, Newman LA, Smith TL, et al. Clinical Course of Breast Cancer Patients With Complete Pathologic Primary Tumor and Axillary Lymph Node Response to Doxorubicin-Based Neoadjuvant Chemotherapy. Journal of Clinical Oncology 1999;17(2):460–460. [DOI] [PubMed] [Google Scholar]
- 2.Colleoni M, Viale G, Zahrieh D, et al. Chemotherapy Is More Effective in Patients with Breast Cancer Not Expressing Steroid Hormone Receptors. A Study of Preoperative Treatment 2004;10(19):6622–6628. [DOI] [PubMed] [Google Scholar]
- 3.Ellis GK, Barlow WE, Gralow JR, et al. Phase III Comparison of Standard Doxorubicin and Cyclophosphamide Versus Weekly Doxorubicin and Daily Oral Cyclophosphamide Plus Granulocyte Colony-Stimulating Factor As Neoadjuvant Therapy for Inflammatory and Locally Advanced Breast Cancer: SWOG 0012. Journal of Clinical Oncology 2011;29(8):1014–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. Journal of Clinical Oncology 1998;16(8):2672–2685. [DOI] [PubMed] [Google Scholar]
- 5.Aapro MS. Neoadjuvant Therapy in Breast Cancer: Can We Define Its Role? The Oncologist 2001;6(S3):36–39. [DOI] [PubMed] [Google Scholar]
- 6.Miller KD, Chap LI, Holmes FA, et al. Randomized Phase III Trial of Capecitabine Compared With Bevacizumab Plus Capecitabine in Patients With Previously Treated Metastatic Breast Cancer. Journal of Clinical Oncology 2005;23(4):792–799. [DOI] [PubMed] [Google Scholar]
- 7.Miller K, Wang M, Gralow J, et al. Paclitaxel plus Bevacizumab versus Paclitaxel Alone for Metastatic Breast Cancer. New England Journal of Medicine 2007;357(26):2666–2676. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez RH, Guarneri V, Icli F, et al. Bevacizumab Treatment for Advanced Breast Cancer. The Oncologist 2011;16(12):1684–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robert NJ, Saleh MN, Paul D, et al. Sunitinib Plus Paclitaxel Versus Bevacizumab Plus Paclitaxel for First-Line Treatment of Patients With Advanced Breast Cancer: A Phase III, Randomized, Open-Label Trial. Clinical Breast Cancer 2011;11(2):82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abrams TJ, Murray LJ, Pesenti E, et al. Preclinical evaluation of the tyrosine kinase inhibitor SU11248 as a single agent and in combination with “standard of care” therapeutic agents for the treatment of breast cancer. Molecular Cancer Therapeutics 2003;2(10):1011–1021. [PubMed] [Google Scholar]
- 11.Pietras K, Hanahan D. A Multitargeted, Metronomic, and Maximum-Tolerated Dose “Chemo-Switch” Regimen is Antiangiogenic, Producing Objective Responses and Survival Benefit in a Mouse Model of Cancer. Journal of Clinical Oncology 2005;23(5):939–952. [DOI] [PubMed] [Google Scholar]
- 12.Faivre S, Demetri G, Sargent W, Raymond E. Molecular basis for sunitinib efficacy and future clinical development. Nature Reviews Drug Discovery 2007;6:734. [DOI] [PubMed] [Google Scholar]
- 13.Potapova O, Laird AD, Nannini MA, et al. Contribution of individual targets to the antitumor efficacy of the multitargeted receptor tyrosine kinase inhibitor SU11248. Molecular Cancer Therapeutics 2006;5(5):1280–1289. [DOI] [PubMed] [Google Scholar]
- 14.Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. The Journal of Clinical Investigation 2003;111(9):1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellis GK, Barlow WE, Russell CA, Royce ME, Perez EA, Livingston RB. SWOG 0012, a randomized phase III comparison of standard doxorubicin (A) and cyclophosphamide (C) followed by weekly paclitaxel (T) versus weekly doxorubicin and daily oral cyclophosphamide plus G-CSF (G) followed by weekly paclitaxel as neoadjuvant therapy for inflammatory and locally advanced breast cancer. Journal of Clinical Oncology 2006;24(18_suppl):LBA537–LBA537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taghian AG, Abi-Raad R, Assaad SI, et al. Paclitaxel Decreases the Interstitial Fluid Pressure and Improves Oxygenation in Breast Cancers in Patients Treated With Neoadjuvant Chemotherapy: Clinical Implications. Journal of Clinical Oncology 2005;23(9):1951–1961. [DOI] [PubMed] [Google Scholar]
- 17.Kozloff M, Chuang E, Starr A, et al. An exploratory study of sunitinib plus paclitaxel as first-line treatment for patients with advanced breast cancer. Annals of Oncology 2010;21(7):1436–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MF Kozloff EC. SABCS Abstract 60782007
- 19.Ellis GK, Livingston RB, Gralow JR, Green SJ, Thompson T. Dose-Dense Anthracycline-Based Chemotherapy for Node-Positive Breast Cancer. Journal of Clinical Oncology 2002;20(17):3637–3643. [DOI] [PubMed] [Google Scholar]
- 20.Waks AG, Tolaney SM, Galar A, et al. Pneumocystis jiroveci pneumonia (PCP) in patients receiving neoadjuvant and adjuvant anthracycline-based chemotherapy for breast cancer: incidence and risk factors. Breast Cancer Res Treat 2015;154(2):359–367. [DOI] [PubMed] [Google Scholar]
- 21.Brunvand MW, Collins C, Livingston RB, Raghu G. Pneumocystis carinii pneumonia associated with profound lymphopenia and abnormal T-lymphocyte subset ratios during treatment for early-stage breast carcinoma. Cancer 1991;67(9):2407–2409. [DOI] [PubMed] [Google Scholar]
- 22.Siminski J, Kidd P, Phillips GD, Collins C, Raghu G. Reversed helper/suppressor T-lymphocyte ratio in bronchoalveolar lavage fluid from patients with breast cancer and Pneumocystis carinii pneumonia. Am Rev Respir Dis 1991;143(2):437–440. [DOI] [PubMed] [Google Scholar]
- 23.Tolaney SM, Partridge AH, Sheib RG, Burstein HJ, Winer EP. Pneumocystis Carinii Pneumonia During Dose-Dense Chemotherapy for Breast Cancer. Journal of Clinical Oncology 2006;24(33):5330–5331. [DOI] [PubMed] [Google Scholar]
- 24.Kulke MH, Vance EA. Pneumocystis carinii pneumonia in patients receiving chemotherapy for breast cancer. Clin Infect Dis 1997;25(2):215–218. [DOI] [PubMed] [Google Scholar]
- 25.Jeruss JS, Mittendorf EA, Tucker SL, et al. Combined use of clinical and pathologic staging variables to define outcomes for breast cancer patients treated with neoadjuvant therapy. J Clin Oncol 2008;26(2):246–252. [DOI] [PubMed] [Google Scholar]
- 26.Mittendorf EA, Jeruss JS, Tucker SL, et al. Validation of a Novel Staging System for Disease-Specific Survival in Patients With Breast Cancer Treated With Neoadjuvant Chemotherapy. Journal of Clinical Oncology 2011;29(15):1956–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marmé F, Lederer B, Blohmer JU, et al. Utility of the CPS+EG staging system in hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer treated with neoadjuvant chemotherapy. Eur J Cancer 2016;53:65–74. [DOI] [PubMed] [Google Scholar]
- 28.Bergquist JR, Murphy BL, Storlie CB, Habermann EB, Boughey JC. Incorporation of Treatment Response, Tumor Grade and Receptor Status Improves Staging Quality in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy. Ann Surg Oncol 2017;24(12):3510–3517. [DOI] [PubMed] [Google Scholar]
- 29.Marmé F, Aigner J, Lorenzo Bermejo J, et al. Neoadjuvant epirubicin, gemcitabine and docetaxel for primary breast cancer: Long-term survival data and major prognostic factors based on two consecutive neoadjuvant phase I/II trials. International Journal of Cancer 2013;133(4):1006–1015. [DOI] [PubMed] [Google Scholar]
- 30.Michel LL, Sommer L, González Silos R, et al. Locoregional risk assessment after neoadjuvant chemotherapy in patients with primary breast cancer: clinical utility of the CPS + EG score. Breast Cancer Res Treat 2019;177(2):437–446. [DOI] [PubMed] [Google Scholar]
- 31.Abdelsattar JM, Al-Hilli Z, Hoskin TL, Heins CN, Boughey JC. Validation of the CPS + EG Staging System for Disease-Specific Survival in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy. Ann Surg Oncol 2016;23(10):3206–3211. [DOI] [PubMed] [Google Scholar]
- 32.Vila J, Teshome M, Tucker SL, et al. Combining Clinical and Pathologic Staging Variables Has Prognostic Value in Predicting Local-regional Recurrence Following Neoadjuvant Chemotherapy for Breast Cancer. Ann Surg 2017;265(3):574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loibl S, Weber K, Huober J, et al. Risk Assessment after Neoadjuvant Chemotherapy in Luminal Breast Cancer Using a Clinicomolecular Predictor. Clin Cancer Res 2018;24(14):3358–3365. [DOI] [PubMed] [Google Scholar]
- 34.LeVasseur N, Willemsma KA, Li H, et al. Efficacy of Neoadjuvant Endocrine Therapy Versus Neoadjuvant Chemotherapy in ER-positive Breast Cancer: Results From a Prospective Institutional Database. Clin Breast Cancer 2019;19(6):e683–e689. [DOI] [PubMed] [Google Scholar]
- 35.Loibl S, Marmé F, Martin M, et al. Palbociclib for Residual High-Risk Invasive HR-Positive and HER2-Negative Early Breast Cancer—The Penelope-B Trial. Journal of Clinical Oncology 0(0):JCO.20.03639. [DOI] [PubMed] [Google Scholar]
- 36.Nahleh ZA, Barlow WE, Hayes DF, et al. SWOG S0800 (NCI CDR0000636131): addition of bevacizumab to neoadjuvant nab-paclitaxel with dose-dense doxorubicin and cyclophosphamide improves pathologic complete response (pCR) rates in inflammatory or locally advanced breast cancer. Breast Cancer Res Treat 2016;158(3):485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stoner M, Wormke M, Saville B, et al. Estrogen regulation of vascular endothelial growth factor gene expression in ZR-75 breast cancer cells through interaction of estrogen receptor α and SP proteins. Oncogene 2004;23(5):1052–1063. [DOI] [PubMed] [Google Scholar]
- 38.Cullinan-Bove K, Koos RD. Vascular endothelial growth factor/vascular permeability factor expression in the rat uterus: rapid stimulation by estrogen correlates with estrogen-induced increases in uterine capillary permeability and growth. Endocrinology 1993;133(2):829–837. [DOI] [PubMed] [Google Scholar]
- 39.Heer K, Kumar H, Read JR, Fox JN, Monson JR, Kerin MJ. Serum vascular endothelial growth factor in breast cancer: its relation with cancer type and estrogen receptor status. Clin Cancer Res 2001;7(11):3491–3494. [PubMed] [Google Scholar]
- 40.Linderholm BK, Hellborg H, Johansson U, et al. Significantly higher levels of vascular endothelial growth factor (VEGF) and shorter survival times for patients with primary operable triple-negative breast cancer. Ann Oncol 2009;20(10):1639–1646. [DOI] [PubMed] [Google Scholar]
- 41.Mechera R, Soysal SD, Piscuoglio S, et al. Expression of RET is associated with Oestrogen receptor expression but lacks prognostic significance in breast cancer. BMC Cancer 2019;19(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tozlu S, Girault I, Vacher S, et al. Identification of novel genes that co-cluster with estrogen receptor alpha in breast tumor biopsy specimens, using a large-scale real-time reverse transcription-PCR approach. Endocr Relat Cancer 2006;13(4):1109–1120. [DOI] [PubMed] [Google Scholar]
- 43.Kao J, Salari K, Bocanegra M, et al. Molecular Profiling of Breast Cancer Cell Lines Defines Relevant Tumor Models and Provides a Resource for Cancer Gene Discovery. PLOS ONE 2009;4(7):e6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Plaza-Menacho I, Morandi A, Robertson D, et al. Targeting the receptor tyrosine kinase RET sensitizes breast cancer cells to tamoxifen treatment and reveals a role for RET in endocrine resistance. Oncogene 2010;29(33):4648–4657. [DOI] [PubMed] [Google Scholar]
- 45.Spanheimer PM, Cyr AR, Gillum MP, Woodfield GW, Askeland RW, Weigel RJ. Distinct pathways regulated by RET and estrogen receptor in luminal breast cancer demonstrate the biological basis for combination therapy. Ann Surg 2014;259(4):793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ellis G GS, Livingston R, et al. Neoadjuvant doxorubicin, cyclophosphamide and G-CSF (AC+G) for locally advanced breast cancer (LABC), a Southwest Oncology Group phase II study. Proc Am Soc Clin Oncol 2000(19(suppl):85a. abstr 326). [Google Scholar]
- 47.Burstein HJ, Elias AD, Rugo HS, et al. Phase II Study of Sunitinib Malate, an Oral Multitargeted Tyrosine Kinase Inhibitor, in Patients With Metastatic Breast Cancer Previously Treated With an Anthracycline and a Taxane. Journal of Clinical Oncology 2008;26(11):1810–1816. [DOI] [PubMed] [Google Scholar]
- 48.Bergh J, Bondarenko IM, Lichinitser MR, et al. First-Line Treatment of Advanced Breast Cancer With Sunitinib in Combination With Docetaxel Versus Docetaxel Alone: Results of a Prospective, Randomized Phase III Study. Journal of Clinical Oncology 2012;30(9):921–929. [DOI] [PubMed] [Google Scholar]
- 49.Barrios CH, Liu M-C, Lee SC, et al. Phase III randomized trial of sunitinib versus capecitabine in patients with previously treated HER2-negative advanced breast cancer. Breast Cancer Research and Treatment 2010;121(1):121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crown J, Dieras V, Staroslawska E, et al. Phase III trial of sunitinib (SU) in combination with capecitabine (C) versus C in previously treated advanced breast cancer (ABC). Journal of Clinical Oncology 2010;28(18_suppl):LBA1011–LBA1011. [Google Scholar]
- 51.Yardley DA, Shipley DL, Peacock NW et al. Phase I/II trial of neoadjuvant sunitinib administered with weekly paclitaxel/carboplatin in patients with locally advanced triple-negative breast cancer. Breast Cancer Res Treat 2015; 152(557). [DOI] [PubMed] [Google Scholar]
- 52.Brown-Glaberman U, Marron M, Chalasani P, et al. Circulating Carbonic Anhydrase IX and Antiangiogenic Therapy in Breast Cancer. Disease Markers 2016;2016:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ebos JML, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated Metastasis after Short-Term Treatment with a Potent Inhibitor of Tumor Angiogenesis. Cancer Cell 2009;15(3):232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pàez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic Therapy Elicits Malignant Progression of Tumors to Increased Local Invasion and Distant Metastasis. Cancer Cell 2009;15(3):220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loges S, Mazzone M, Hohensinner P, Carmeliet P. Silencing or Fueling Metastasis with VEGF Inhibitors: Antiangiogenesis Revisited. Cancer Cell 2009;15(3):167–170. [DOI] [PubMed] [Google Scholar]
- 56.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 2005;8(4):299–309. [DOI] [PubMed] [Google Scholar]
- 57.Conley SJ, Gheordunescu E, Kakarala P, et al. Antiangiogenic agents increase breast cancer stem cells via the generation of tumor hypoxia. Proceedings of the National Academy of Sciences 2012;109(8):2784–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nature Medicine 2001;7:987. [DOI] [PubMed] [Google Scholar]
- 59.Goel S, Wong AH-K, Jain RK. Vascular Normalization as a Therapeutic Strategy for Malignant and Nonmalignant Disease. Cold Spring Harbor Perspectives in Medicine 2012;2(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong ALA, Sundar R, Wang T-T, et al. Phase Ib/II randomized, open-label study of doxorubicin and cyclophosphamide with or without low-dose, short-course sunitinib in the pre-operative treatment of breast cancer. Oncotarget 2016;7(39):64089–64099. [DOI] [PMC free article] [PubMed] [Google Scholar]


