Abstract
Introduction
Sonidegib is a Hedgehog pathway inhibitor approved to treat locally advanced basal cell carcinoma and, depending on regulatory approval, metastatic basal cell carcinoma. Results from the BOLT study demonstrated robust efficacy and continued tolerability through 42 months. This analysis evaluated the impact of sonidegib dose reductions and interruptions in patients with advanced basal cell carcinoma through 42 months.
Methods
BOLT was a randomized, double-blind, multicenter, phase 2 study. Adults with no previous Hedgehog pathway inhibitor therapy were randomized 1:2 to sonidegib 200 or 800 mg once daily. Primary endpoint was objective response rate. Dose modifications were permitted in patients unable to tolerate the dosing schedule or if a treatment-related adverse event was suspected.
Results
The incidence of dose interruptions was similar between the 200- and 800-mg groups (68.4% vs 65.3%, respectively). Dose reductions occurred more frequently in patients receiving sonidegib 800 mg (36.7%) than 200 mg (16.5%). Overall response rate for all patients receiving sonidegib 200 mg daily was 48.1% and was similar to those of patients without dose reduction or interruption (48.5%) and patients with at least one dose reduction or interruption (46.2%).
Conclusion
Dose reductions and interruptions were practical and did not impact the efficacy of sonidegib. In patients with advanced basal cell carcinoma who necessitate long-term treatment, dose interruptions may be beneficial for continued treatment and disease control.
Trial registration: ClinicalTrials.gov identifier, NCT01327053.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-021-00619-4.
Keywords: Basal cell carcinoma, Dose interruption, Dose reduction, Hedgehog pathway inhibitor, Sonidegib
Key Summary Points
| Why carry out this study? |
| Hedgehog pathway inhibitors (HHIs) inhibit aberrant Hedgehog signaling found in most cases of basal cell carcinoma (BCC) and are one of the few mechanistic-based pharmacologic treatment options available for patients with advanced BCC. |
| Adverse events are a substantial limiting factor for treatment duration with HHIs. |
| What was learned from the study? |
| Results from the 42-month BOLT study on sonidegib, an HHI, demonstrate that dose reductions and interruptions were well tolerated in patients and did not compromise the efficacy of sonidegib. |
| Dose interruptions may be a valuable approach for sustained treatment and disease control. |
Introduction
Basal cell carcinoma (BCC) is the most common malignancy and form of skin cancer worldwide [1, 2]. Advanced BCC (aBCC) can be characterized either as locally advanced BCC (laBCC) or metastatic BCC (mBCC); treatment options for patients with aBCC are limited [3].
Inhibition of the Hedgehog signaling pathway offers patients with aBCC a promising treatment option [1, 4]. Sonidegib, a Hedgehog pathway inhibitor (HHI), selectively targets Smoothened and is approved for the treatment of laBCC that has recurred following surgery or radiation therapy, or for patients who are not candidates for surgery or radiation therapy [5–8].
Results from the pivotal Basal Cell Carcinoma Outcomes with LDE225 (sonidegib) Treatment (BOLT) trial (NCT01327053) demonstrated durable efficacy of sonidegib to treat laBCC and mBCC [9–11]. At the final 42-month analysis, objective response rate (ORR) (95% confidence interval [CI]) was 48.1% (36.7–59.6%) vs 41.7% (33.8–50.0%) for the 200- and 800-mg groups, respectively [9]. In BOLT, adverse events (AEs) were the main cause of discontinuations [9, 10]. Accordingly, treatment interruption or dose modification is an important aspect of HHI treatment for BCC in order for clinicians to best manage their patients’ therapeutic courses. Here, we examine efficacy and safety outcomes associated with sonidegib treatment interruption or dose reduction in patients with aBCC enrolled in the BOLT trial.
Methods
Study Design
The study design for BOLT has been previously described (Fig. S1 in the supplementary material) [9–11]. The primary efficacy endpoint was ORR; secondary endpoints included duration of response (DOR) and progression-free survival (PFS).
Dose modifications were allowed for patients who were unable to tolerate the protocol-specified dosing schedule or in the event of an adverse reaction suspected to be related to the study drug. In patients with study drug withheld because of suspected toxicity, scheduled visits and assessments continued with the exception of study drug dosing. In patients randomized to the 800-mg dose, a maximum of two dose reductions were permitted; if there was a need for further dose reduction, the patient was discontinued from study treatment (Fig. 1). A maximum of one dose reduction (to placebo treatment) was allowed for patients receiving the 200-mg dose, after which the patient was discontinued from study treatment if there was a need for further dose reduction. For dose interruptions, if the patient experienced the same toxicity following resumption of treatment, regardless of duration, the second reinitiation of study drug was resumed at a lower dose. Any dose interruption that exceeded 21 days from the previous dose resulted in discontinuation from study treatment. In patients who discontinued study treatment because of an AE or laboratory abnormality, assessments were continued until resolution of the event. All dosage interruptions and reductions were recorded in the Dosage Administration Record Case Report Form, as appropriate. Specific clinical strategies were established for managing AEs (Table 1).
Fig. 1.
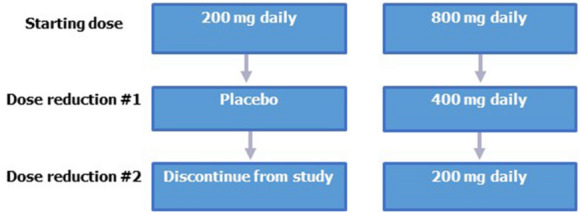
Dose modification steps for sonidegib
Table 1.
Recommended dose modifications and dose interruptions for suspected treatment-related muscle toxicity
| CK levels | Action |
|---|---|
| Normal CK with muscle-related symptoms (e.g., pain, spasms, or cramps) | Grade 1 or 2 symptoms: Continue sonidegib at same dose; consider symptomatic treatment for muscle-related toxicity |
| Grade 3: Hold sonidegib dose for up to 21 days; measure CK; resume sonidegib at a reduced dose if resolved or improved to Grade 1 | |
| Grade 1 or 2 CK elevationa | Asymptomatic (no new onset or worsening of muscle cramps, myalgia, or other muscle symptoms): Continue sonidegib at same dose |
|
Symptomatic: Continue sonidegib at same dose; monitor CK at least once weekly Hold sonidegib dose Check blood and/or urine myoglobin Monitor renal function Measure CK at least twice weekly | |
| Grade 3 or 4 CK elevationa | Consider electromyography and muscle biopsy |
| Consider resuming sonidegib at a reduced dose if renal function is not impaired and resolution to grade ≤ 1 occurs within 21 days | |
| Discontinue patient from study in the presence of renal impairment (serum creatine > 2 × ULN) |
CK creatine kinase, ULN upper limit of normal
aGraded according to National Cancer Institute Common Terminology Criteria for Adverse Events, v4.03 [15]
All patients provided written informed consent prior to the conduction of any study-specific procedures. The study protocol and all amendments were approved by the institutional review board/independent ethics committee for each center (Table S1 in the supplementary material). This study was carried out in accordance with the ethical principles of the Declaration of Helsinki.
Assessments
All patients received sonidegib 200 or 800 mg once daily until progressive disease (PD), intolerable toxicity, withdrawn consent, study discontinuation, or death. ORR was assessed applying Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 in patients with mBCC and modified RECIST in patients with laBCC. Safety assessments included monitoring and recording AEs and frequent monitoring of hematology, clinical chemistry, and electrocardiograms. The statistical methods were reported previously [9–11].
Results
Patient Disposition and Disease Characteristics
Of 230 patients enrolled and randomized, 79 received sonidegib 200 mg and 151 sonidegib 800 mg. At study completion, 11 (4.8%) patients remained on treatment (6 in the 200-mg group and 5 in the 800-mg group); median follow-up was 50.2 months. Most frequent study discontinuation causes included AEs (34.8%), PD (23.0%), withdrawal by patient (18.7%), and physician decision (10.4%), consistent with previous reports [10, 11]. Patient demographics and baseline disease characteristics were previously described (Table S2 in the supplementary material) [11].
Dose Reductions and Treatment Interruptions
Dose reductions and interruptions were predominantly attributed to AEs and consistent with the 30-month analysis results [10]. Through 42 months of sonidegib treatment, treatment interruptions were more common than dose reductions. The incidence of dose interruptions was comparable between the 200- and 800-mg groups (68.4% vs 65.3%, respectively; Table 2), while dose reductions were less common in the 200-mg group (16.5%) vs the 800-mg group (36.7%).
Table 2.
Dose reduction and treatment interruptions in patients at 42 months
| Sonidegib 200 mg daily (n = 79) |
Sonidegib 800 mg daily (n = 151) |
All patients (N = 230) |
|
|---|---|---|---|
| Patients treated, n | 79 | 150 | 229 |
| Patients with any dose reduction, n (%) | 13 (16.5) | 55 (36.7) | 68 (29.7) |
| 1 reduction | 13 (16.5) | 44 (29.3) | 57 (24.8) |
| 2 reductions | 0 | 11 (7.3) | 11 (4.8) |
| Reasons for dose reduction, n | 13a | 55a | 68a |
| Adverse event | 12 | 57 | 69 |
| Dosing error | 1 | 3 | 4 |
| Lack of efficacy | 0 | 1 | 1 |
| Days full dose received, % | |||
| Median, range | 99.1 (8.1–100) | 94.2 (2.6–100) | 97.6 (2.6–100) |
| Patients with any interruption in treatment, n (%) | 54 (68.4) | 98 (65.3) | 152 (66.4) |
| 1 interruption | 19 (24.1) | 34 (22.7) | 53 (23.1) |
| ≥ 2 interruptions | 35 (44.3) | 64 (42.7) | 99 (43.2) |
| Days of sonidegib treatment, % | |||
| Median, range | 99.1 (76.7–100) | 97.8 (46.6–100) | 98.5 (46.6–100) |
| Reasons for treatment interruption, n (%) | 54a | 98a | 152a |
| Adverse event | 31 (39.2) | 77 (51.3) | 108 (47.2) |
| Dosing error | 28 (35.4) | 47 (31.3) | 75 (32.8) |
| Technical issue | 18 (22.8) | 24 (16.0) | 42 (18.3) |
| Dispensing error | 0 | 1 (0.7) | 1 (0.4) |
aPatient with multiple reasons for dose change or interruption is only counted once in the total row
Effect of Sonidegib Dose Reductions and Treatment Interruptions on Efficacy Endpoints
For patients receiving 200 mg, ORRs were comparable between all patients (48.1%) and subgroups of patients with at least one dose reduction or interruption (46.2%) and without dose reductions or interruptions (48.5%, Table 3). In patients with at least one dose reduction or interruption receiving sonidegib 800 mg, ORRs were higher compared with patients receiving the 200-mg dose who also had at least one dose reduction or interruption, although no statistical comparison was performed to determine significance. Overall, for all patients receiving sonidegib 800 mg, median DOR (95% CI) (23.3 [12.2–29.6] months) was comparable to that for patients with at least one dose reduction or interruption (24.8 [not estimable] months) (Table 3). However, in patients receiving sonidegib 200 mg, the effect of dose reduction or interruption on DOR was not able to be determined, given the small number of events.
Table 3.
Objective response rates, duration of response, and progression-free survival by central review at 42 months in patients with advanced basal cell carcinoma with and without dose reduction or interruption
| Sonidegib 200 mg daily (n = 79) |
Sonidegib 800 mg daily (n = 151) |
|
|---|---|---|
| Objective response rate | ||
| All patients (laBCC + mBCC) | ||
| Events/responders, n/N | 38/79 | 63/151 |
| ORR (95% CI) | 48.1% (36.7–59.6) | 41.7% (33.8–50.0) |
| No dose reduction or interruption | ||
| Events/responders, n/N | 32/66 | 31/96 |
| ORR (95% CI) | 48.5% (36.0–61.1) | 32.3% (23.1–42.6) |
| ≥ 1 dose reduction or interruption | ||
| Events/responders, n/N | 6/13 | 32/55 |
| ORR (95% CI) | 46.2% (19.2–74.9) | 58.2% (44.4–71.4) |
| Patients with laBCC | ||
| Events/responders, n/N | 37/66 | 59/128 |
| ORR (95% CI) | 56.1% (43.3–68.3) | 46.1% (37.3–55.1) |
| No dose reduction or interruption | ||
| Events/responders, n/N | 31/54 | 27/80 |
| ORR (95% CI) | 57.4% (43.2–70.8) | 33.8% (23.6–45.2) |
| ≥ 1 dose reduction or interruption | ||
| Events/responders, n/N | 6/12 | 32/48 |
| ORR (95% CI) | 50.0% (21.1–78.9) | 66.7% (51.6–79.6) |
| Duration of response | ||
| All patients (laBCC + mBCC) | ||
| Events/responders, n/N | 13/38 | 24/63 |
| Median, months (95% CI) | 26.1 (NE) | 23.3 (12.2–29.6) |
| No dose reduction or interruption | ||
| Events/responders, n/N | 12/32 | 13/31 |
| Median, months (95% CI) | 24.0 (NE) | 14.7 (8.3–26.4) |
| ≥ 1 dose reduction or interruption | ||
| Events/responders, n/N | 1/6 | 11/32 |
| Median, months (95% CI) | NE (NE) | 24.8 (NE) |
| Patients with laBCC | ||
| Events/responders, n/N | 12/37 | 23/59 |
| Median, months (95% CI) | 26.1 (NE) | 23.3 (12.2–29.6) |
| No dose reduction or interruption | ||
| Events/responders, n/N | 11/31 | 12/27 |
| Median, months (95% CI) | 26.1 (NE) | 14.7 (8.8–26.4) |
| ≥ 1 dose reduction or interruption | ||
| Events/responders, n/N | 1/6 | 11/32 |
| Median, months (95% CI) | NE (NE) | 24.8 (NE) |
| Progression-free survival | ||
| All patients (laBCC + mBCC) | ||
| Events/responders, n/N | 25/79 | 46/151 |
| Median, months (95% CI) | 22.1 (14.4–33.1) | 21.5 (16.1–28.4) |
| No dose reduction or interruption | ||
| Events/responders, n/N | 22/66 | 28/96 |
| Median, months (95% CI) | 22.1 (14.4–30.7) | 16.7 (12.1–28.4) |
| ≥ 1 dose reduction or interruption | ||
| Events/responders, n/N | 3/13 | 18/55 |
| Median, months (95% CI) | NE (NE) | 24.9 (16.6–42.8) |
| Patients with laBCC | ||
| Events/responders, n/N | 17/66 | 33/128 |
| Median, months (95% CI) | 22.1 (NE) | 24.9 (19.2–33.4) |
| No dose reduction or interruption | ||
| Events/responders, n/N | 15/54 | 20/80 |
| Median, months (95% CI) | 22.1 (14.4–39.6) | 21.5 (13.2–33.4) |
| ≥ 1 dose reduction or interruption | ||
| Events/responders, n/N | 2/12 | 13/48 |
| Median, months (95% CI) | NE (NE) | 29.3 (19.3–43.3) |
Median DOR and PFS were calculated using the Kaplan–Meier method
BCC basal cell carcinoma, CI confidence interval, laBCC locally advanced BCC, mBCC metastatic BCC, NE not estimable, ORR objective response rate
Similar to DOR efficacy outcomes, median PFS (95% CI) for all patients receiving sonidegib 800 mg (21.5 [16.1–28.4] months) was similar to patients with at least one dose reduction or interruption receiving the 800-mg dose (24.9 [16.6–42.8] months, Table 3). Moreover, in patients receiving sonidegib 800 mg, median PFS (95% CI) was higher in patients with at least one dose reduction or interruption (29.3 [19.3–43.3] months) compared with patients without a dose reduction or interruption (21.5 [13.2–33.4] months). As with DOR outcomes, median PFS in patients receiving sonidegib 200 mg with at least one dose reduction or interruption was unable to be calculated because of the low number of events.
Safety
As described previously, most AEs were manageable and consistent with previous analyses [9–11]. Overall, AEs were predominantly Grade 1 or 2 in patients receiving sonidegib 200 mg. Grade 3–4 AEs were reported in 43.0% and 64.0% of patients receiving sonidegib 200 and 800 mg, respectively. In addition, Grade 3–4 AEs resulted in discontinuation in 13.9% and 14.7% of patients in the 200- and 800-mg dosing groups, respectively. In patients with dose reductions or interruptions, AEs were primarily reversible.
Most frequent AEs (> 5%) leading to dose reductions or interruptions in the 200-mg group were elevated serum creatine kinase (CK; 6.3%), nausea (6.3%), vomiting (6.3%), diarrhea (5.1%), and increased lipase (5.1%). In the 800-mg group, AEs (> 5%) leading to dose reductions or interruptions consisted of muscle spasms (18.7%), nausea (12.7%), elevated serum CK (12.0%), dysgeusia (8.0%), and vomiting (8.0%).
For patients who experienced Grade ≥ 2 elevations in serum CK, 14.3% (n = 2) of patients in the 200-mg group and 36.2% (n = 17) of patients in the 800-mg group required dose interruptions within 2 weeks of onset of the AE. Following dose interruption, patients in the 200-mg group received placebo, while for the 800-mg group, 11 of 17 patients resumed at a reduced dose level and 6 patients resumed at 800 mg. Overall, the median times to a dose interruption due to Grade ≥ 2 serum CK elevation were 12.5 days and 18.0 days for patients receiving 200 and 800 mg, respectively.
Discussion
This analysis of the BOLT trial demonstrated robust and continued efficacy of sonidegib in patients with aBCC who required dose reductions and/or interruptions through 42 months of treatment. More patients necessitated treatment interruptions than dose reductions. Importantly, patients with dose reductions or interruptions still showed clinically meaningful ORRs. Notably, patients receiving sonidegib 800 mg that had at least one treatment reduction or interruption had greater ORRs compared with the total patient population as well as patients without dose reductions or interruptions.
While HHIs provide a promising treatment option for patients with aBCC, AEs can be difficult for patients to endure. Therefore, treatment with HHIs needs to maintain a balance between disease control and potential adverse reactions. In this analysis, AEs were mostly Grade 1–2; however, AEs were the primary reason for discontinuation [10, 11]. Therefore, since AEs are commonly the source of treatment interruptions and discontinuations and may potentially impact disease outcome, treatment interruptions are a frequent approach in patient management, especially with more severe AEs [12].
In a phase 2 study assessing the efficacy and safety of intermittent doses of the HHI vismodegib (Erivedge®, Genentech, San Francisco, CA) in patients with multiple BCCs, treatment interruption did not substantially affect the efficacy of vismodegib [13]. However, 23% of patients discontinued treatment because of AEs [13]. Notably, an increased number of treatment interruptions were associated with longer median duration of vismodegib treatment [14]. Although there is no direct comparable study evaluating dose interruptions of a prespecified length with sonidegib, the results reported here support the long-term efficacy of sonidegib in patients that experienced treatment interruptions.
It is important to note that for patients receiving the approved dose of sonidegib 200 mg who required a dose reduction, the decreased dose was placebo treatment [5–8]. Consequently, in clinical practice, dose reductions of sonidegib are not a practical option for patients who require dose adjustments due to treatment-related AEs. However, in these patients, treatment interruptions offer a viable option to manage a patient’s care while safeguarding continued course of treatment.
Study limitations include small sample size, especially for patients with mBCC, and the effect of sonidegib dose reduction or interruption on DOR and PFS could not be determined because of the low number of responders for the 200-mg dose. Additionally, since patients with recurrent disease following previous therapy with an HHI were excluded from this study, efficacy of sonidegib in these patients is unknown [10].
Conclusions
Overall, dose reductions and interruptions were feasible in patients and did not compromise the efficacy of sonidegib. Furthermore, preemptive management of AEs through treatment interruptions may improve tolerability and optimize sonidegib treatment duration. In patients requiring long-term treatment for aBCC, dose interruptions may be a valuable approach for sustained treatment and disease control.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study was sponsored and funded by Novartis. The journal’s rapid service fee was funded by Sun Pharmaceutical Industries, Inc. (Princeton, NJ, USA). Writing and editorial support for manuscript preparation were provided by Zehra Gundogan, VMD, of AlphaBioCom, LLC, and funded by Sun Pharmaceutical Industries, Inc., Princeton, NJ, USA.
Authorship
All listed authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors and have significantly contributed to, seen, and approved the final submitted version of the manuscript.
Authorship Contributions
KL, RD, ASF, AG, NS, and MM drafted and critically reviewed the manuscript and approved the final version.
Medical Writing, Editorial, and Other Assistance
Medical writing and editorial assistance were provided by Zehra Gundogan, VMD, of AlphaBioCom, LLC, under the direction of the authors and was funded by Sun Pharmaceutical Industries, Inc.
Disclosures
Karl Lewis has received grants and personal fees from Amgen, Bristol-Myers Squibb, Genentech, GlaxoSmithKline, Merck Sharp & Dohme, Novartis, and Roche. Reinhard Dummer has participated on advisory boards and consulted for Amgen; Bristol-Myers Squibb; Catalym; Merck Sharpe & Dohme; Novartis Pharmaceutical Corporation; Pierre Fabre; Roche; Sanofi; Second Genome; Sun Pharmaceutical Industries, Inc.; and Takeda. Aaron S. Farberg has participated on advisory boards and received honoraria from Ortho Dermatologics and Sun Pharmaceutical Industries, Inc. Alexander Guminski has participated on advisory boards for Bristol-Myers Squibb, Pfizer, and Sanofi; received honoraria from Novartis; and received travel support from Astellas; Bristol-Myers Squibb; and Sun Pharmaceutical Industries, Inc. Nicholas Squittieri is an employee of Sun Pharmaceutical Industries, Inc. Michael Migden has participated on advisory boards and received honoraria from Genentech; Novartis; Sun Pharmaceutical Industries, Inc.; and Regeneron.
Compliance with Ethics Guidelines
This study was conducted according to the ethical principles of the Declaration of Helsinki. All patients provided informed consent for publication of this report. The study protocol and all amendments were approved by the institutional review board/independent ethics committee for each center (Table S1 in the supplementary material).
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
References
- 1.Bakshi A, Chaudhary SC, Rana M, Elmets CA, Athar M. Basal cell carcinoma pathogenesis and therapy involving hedgehog signaling and beyond. Mol Carcinog. 2017;56(12):2543–2557. doi: 10.1002/mc.22690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88(2):167–179. [PMC free article] [PubMed] [Google Scholar]
- 3.Lear JT, Corner C, Dziewulski P, et al. Challenges and new horizons in the management of advanced basal cell carcinoma: a UK perspective. Br J Cancer. 2014;111(8):1476–1481. doi: 10.1038/bjc.2014.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epstein EH. Basal cell carcinomas: attack of the hedgehog. Nat Rev Cancer. 2008;8(10):743–754. doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Odomzo (sonidegib capsules). Full Prescribing Information. Sun Pharmaceutical Industries, Inc., Cranbury, NJ, USA.
- 6.European Medicines Agency. Summary of Product Characteristics, WC500188762. https://www.ema.europa.eu/en/documents/product-information/odomzo-epar-product-information_en.pdf
- 7.Swissmedic, Authorization Number 65065. 2015. https://www.swissmedic.ch/swissmedic/de/home/humanarzneimittel/authorisations/new-medicines/odomzo--200mg--kapseln--sonidegibum-.html
- 8.Australian Government Department of Health, ARTG 292262. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2017-PI-02511-1&d=2018030216114622483&d=20210929172310101
- 9.Dummer R, Guminksi A, Gutzmer R, et al. Long-term efficacy and safety of sonidegib in patients with advanced basal cell carcinoma: 42-month analysis of the phase II randomized, double-blind BOLT study. Br J Dermatol. 2020;182(6):1369–1378. doi: 10.1111/bjd.18552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lear JT, Migden MR, Lewis KD, et al. Long-term efficacy and safety of sonidegib in patients with locally advanced and metastatic basal cell carcinoma: 30-month analysis of the randomized phase 2 BOLT study. J Eur Acad Dermatol Venereol. 2018;32(3):372–381. doi: 10.1111/jdv.14542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Migden MR, Guminski A, Gutzmer R, et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2 trial. Lancet Oncol. 2015;16(6):716–728. doi: 10.1016/S1470-2045(15)70100-2. [DOI] [PubMed] [Google Scholar]
- 12.Lacouture ME, Dréno B, Ascierto PA, et al. Characterization and management of hedgehog pathway inhibitor-related adverse events in patients with advanced basal cell carcinoma. Oncologist. 2016;21(10):1218–1229. doi: 10.1634/theoncologist.2016-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dréno B, Kunstfeld R, Hauschild A, et al. Two intermittent vismodegib dosing regimens in patients with multiple basal-cell carcinomas (MIKIE): a randomised, regimen-controlled, double-blind, phase 2 trial. Lancet Oncol. 2017;18(3):404–412. doi: 10.1016/S1470-2045(17)30072-4. [DOI] [PubMed] [Google Scholar]
- 14.Dummer R, Basset-Seguin N, Hansson J, et al. Impact of treatment breaks on vismodegib patient outcomes: exploratory analysis of the STEVIE study. J Clin Oncol. 2015;33(15_suppl):9024.
- 15.US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0 Published: May 28, 2009 (v4. 03: June 14, 2010). 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article/as supplementary information files.


