Abstract
Introduction
Mycosis fungoides (MF) is the most frequent subtype of primary cutaneous T cell lymphomas (pCTCL). The diagnosis may be particularly difficult in the early stages as well as in atypical and rare clinical presentations. Furthermore, MF may simulate a large variety of common dermatologic disorders and patterns, both histopathologically and clinically.
Methods
A literature search was performed to provide a comprehensive update on the rare and atypical MF manifestations as well as the dermatoses and dermatological patterns that could be imitated by MF.
Results
A total of 114 publications were found describing a series of different dermatoses and dermatological patterns mimicked by MF, as well as some particular localizations of MF lesions and dermatoses that occur in preexisting MF lesions.
Conclusions
The number of dermatoses that can be imitated by MF is ever-increasing. Patients with common dermatologic conditions that prove to be treatment refractory should be biopsied without delay, and sequentially as necessary, to prevent delay in diagnosis and progression of disease. Clinicopathologic correlation is the best way of diagnosis.
Keywords: Mycosis fungoides, Primary cutaneous T cell lymphoma, pCTCL dermatology, Atypical manifestations, Diagnostic delay
Key Summary Points
| Why carry out this study? |
| Mycosis fungoides may present many atypical and rare forms, often imitating other dermatoses, delaying diagnosis. |
| What was learned from the study? |
| This study presents an update of the dermatological manifestations of mycosis fungoides and their corresponding histological presentations. |
| The number of dermatoses that can be imitated by mycosis fungoides is ever-increasing. |
| Patients with common dermatologic conditions that prove to be treatment refractory should be biopsied without delay, and sequentially as necessary, to prevent delay in diagnosis and progression of disease. Clinicopathologic correlation is the best way of diagnosis. |
Introduction
Primary cutaneous lymphomas are rare and, in terms of frequency, represent the third group behind digestive and hematologic lymphomas. Mycosis fungoides (MF) is the most frequent type of the primary cutaneous T cell lymphoma (pCTCL) group [1–8]. The annual incidence varies between 0.3 and 0.96 cases per 100,000 persons [9], typically affecting patients between 45 and 65 years of age, with a male to female sex ratio of 2:1. Childhood and adolescent MF cases are more exceptional [10–16].
Immunosuppression and underlying malignant hemopathies are recognized risk factors for developing MF [17]. It still remains unclear whether chronic inflammatory skin diseases, including atopic dermatitis, eczema, and psoriasis, or chronic exposure to chemical agents represent risk factors for MF [9, 18, 19]. Skin of color seems not to be a predisposing factor [20].
The early recognition and therapeutic management of MF is important as it has been demonstrated that a delayed diagnosis is associated with disease progression and a poorer long-term prognosis [15].
A thorough clinicopathologic correlation by a skilled dermatologist and pathologist determines the final diagnosis and the initial therapeutic approach. The long-term follow-up of a patient with MF requires a multidisciplinary tumor board with experience in patients with CTCL.
Recognition of MF is however a difficult task, particularly in the early stages of the disease. Furthermore, histology and immunohistochemical analyses may not be contributive in the early stages, and monoclonal T cell receptor (TCR) rearrangement is often not yet detectable. In fact, the clinical suspicion of MF is often present months to years before achieving the final evidence through a clinicopathological correlation involving histology, immunohistochemistry, and monoclonal TCR rearrangement [21, 22].
Hence, the initial and first suspicion of MF always remains a clinical doubt. Consequently, the recognition of the early and classic manifestations but also the rare and the atypical forms of MF as well as MF cases presenting as other dermatoses or presenting typical dermatological patterns or signs is of primordial importance for adequate management.
Since the last significant overviews dealing with the various clinical manifestations of MF are already some years of age [23–27], a review of existing data was performed as well as a literature update.
Methods
The following search terms were included in PubMed: mycosis fungoides, imitator, mimicking, masquerading, CTCL, pCNKTCL, atypical, cutaneous T cell lymphomas, between January 1986 until December 2020. The titles and the summaries of the retrieved articles were selected for eligibility, independently by two authors and then the entire publications were analyzed.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. All patients provided written consent for their pictures to be published.
Results
A total of 114 publications were retrieved where MF was initially misdiagnosed as an inflammatory, infectious, vascular, or neoplastic dermatological disorder, or imitating a particular dermatologic sign or pattern (Table 1). Some new clinical presentations that may be imitated by MF are also presented in Table 1, including keratosis punctata palmaris, seborrheic dermatitis, angular cheilitis, psoriasis inversa, rosacea, varicous eczema. Furthermore, some particular localizations of MF lesions and a series of dermatoses developing in preexisting MF lesions are presented. The histological subtype of the described MF cases is mentioned according to the ad hoc publications (Table 1).
Fig. 1.
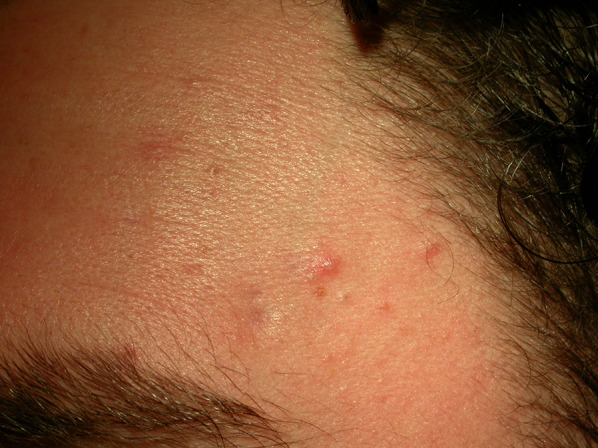
Folliculotropic MF mimicking acne vulgaris in a 19-year-old man
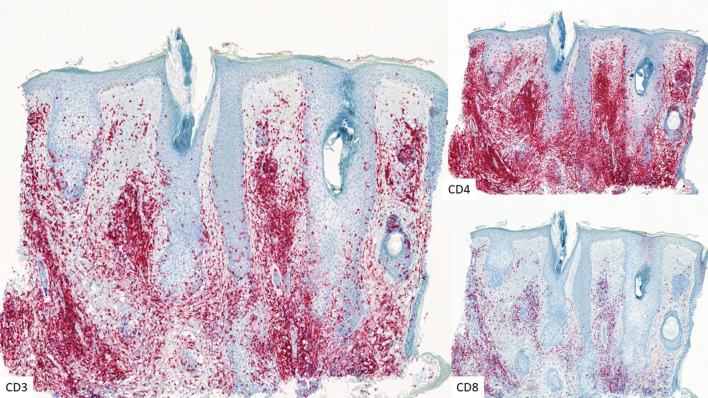
Fig. 2.
CD3, CD4, and CD8 immunostaining illustrating infiltrating lymphocytes in folliculotropic MF (× 5)
Fig. 3.
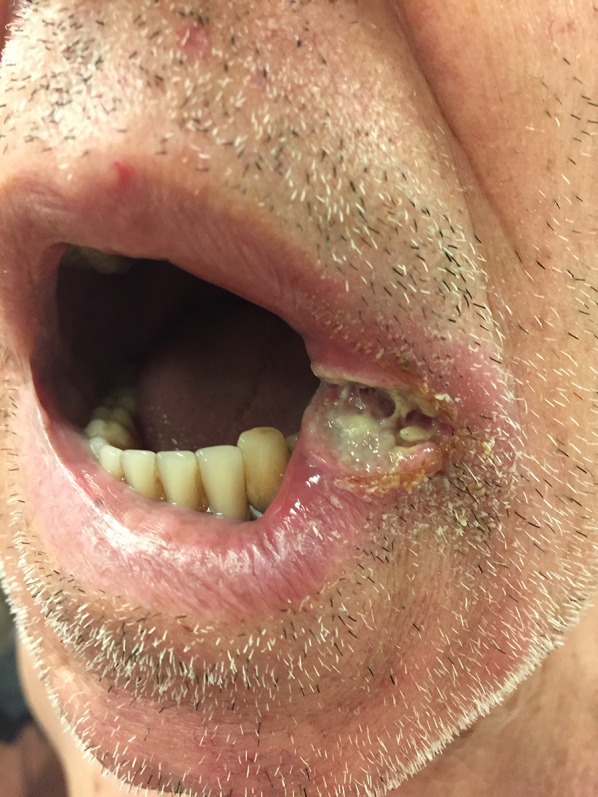
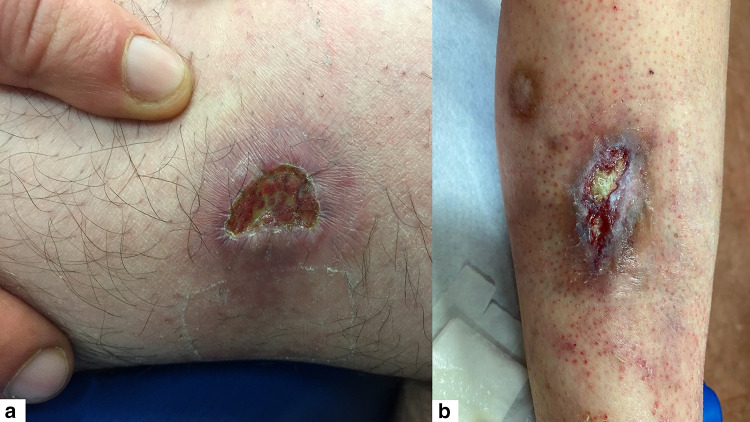
Tumoral MF mimicking angular cheilitis in a 73-year-old man
Fig. 4.
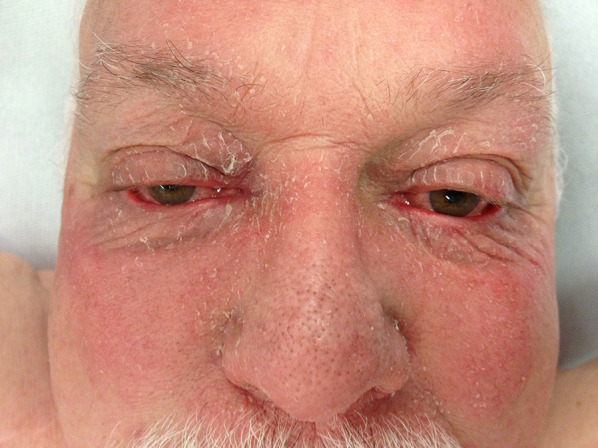
Classic MF imitating severe facial atopic dermatitis in a 69-year-old man
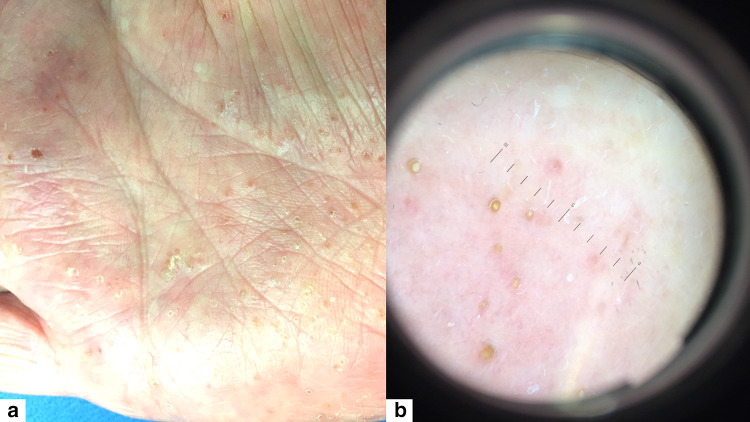
Fig. 5.
a Syringotropic MF presenting as keratosis punctata palmaris, b dermoscopic aspect
Fig. 6.
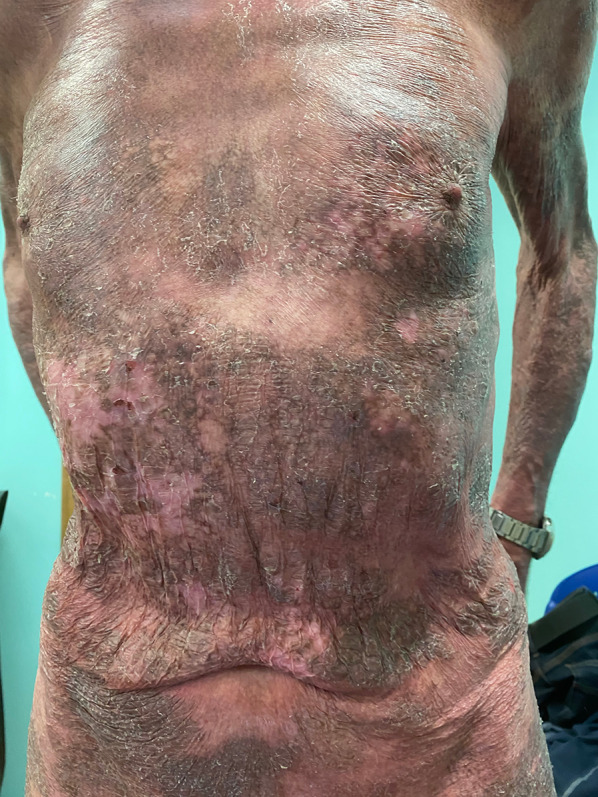
Poikilodermic MF mimicking pellagra
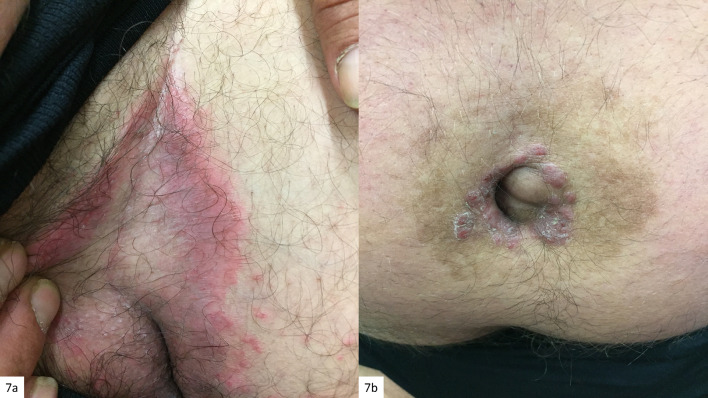
Fig. 7.
a Classic MF presenting as an intertriginous dermatosis. b Classic MF mimicking umbilical psoriasis inversa
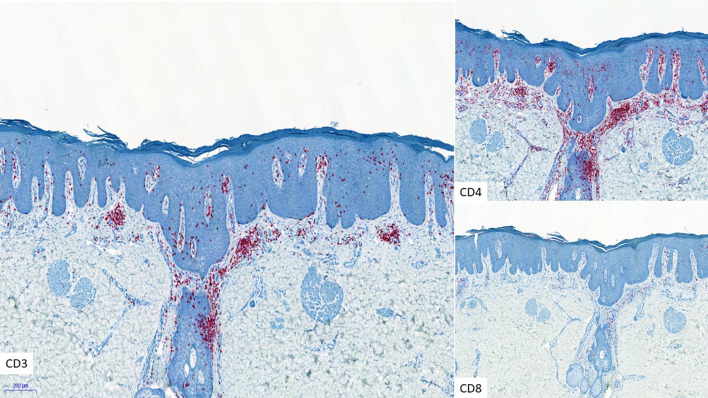
Fig. 8.
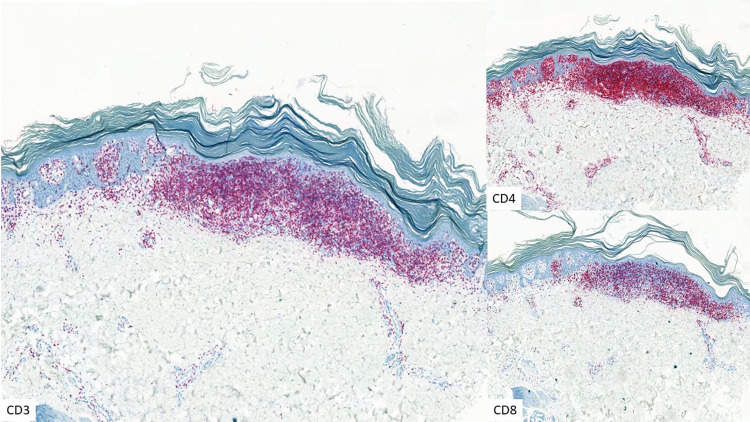
CD3, CD4, and CD8 immunostaining in psoriasiform MF (× 5)
Fig. 9.
a, b Tumoral MF imitating pyoderma gangrenosum
Fig. 10.
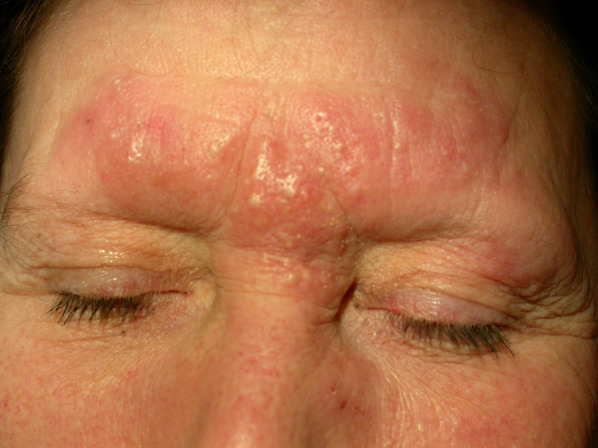
Folliculotropic MF presenting as granulomatous rosacea
Fig. 11.
Acanthosis nigricans-like MF
Fig. 12.
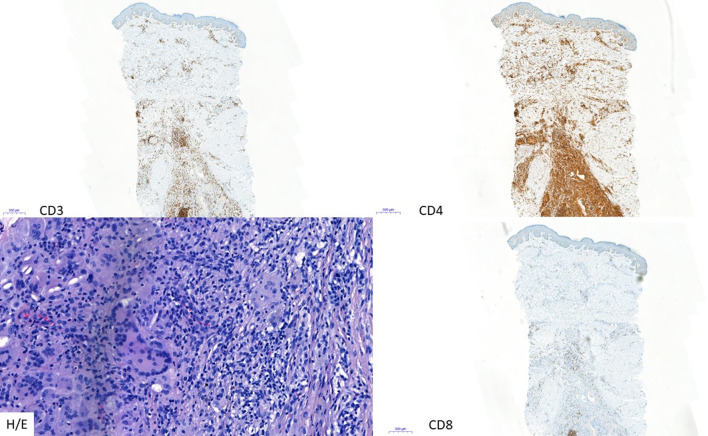
CD3, CD4, and CD8 immunostaining in granulomatous MF (× 5) and H/E (× 20) staining illustrating the granulomatous character
Fig. 13.
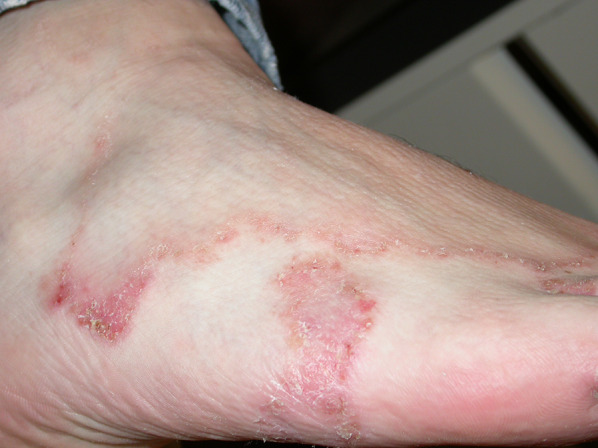
Tinea pedis-like MF
Fig. 14.
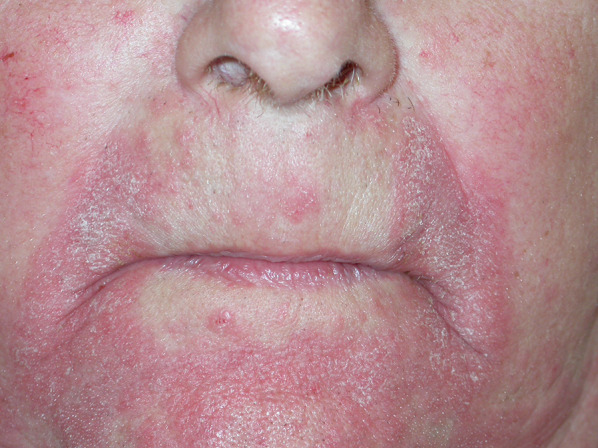
Classic MF presenting as seborrheic dermatosis of the face in a 74-year-old man
Fig. 15.
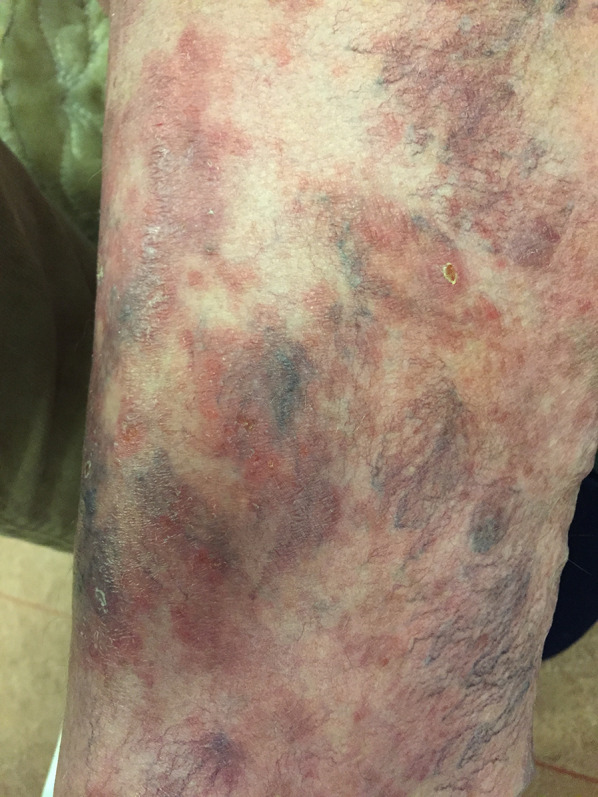
Classic MF mimicking as a varicous dermatosis of the legs in a 64-year-old woman
Fig. 16.
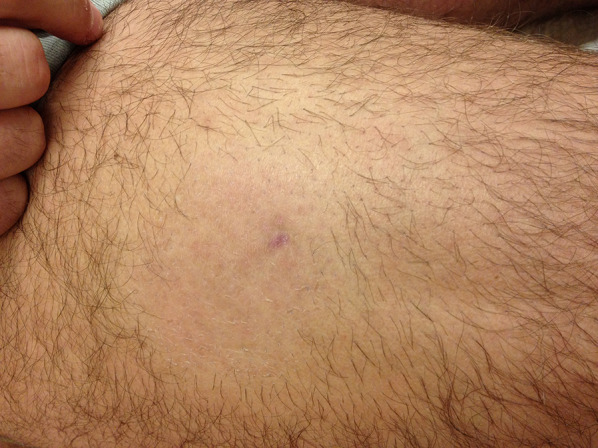
Folliculotropic MF presenting as an isolated alopecic patch on the thigh of a 34-year-old man
Fig. 17.
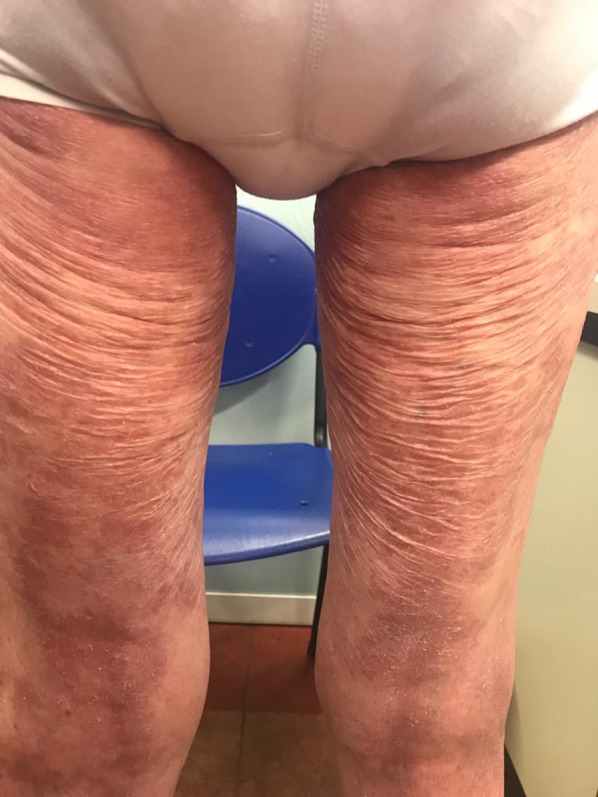
Elastophagic MF of the posterior aspect of the lower extremities in a 78-year-old man
Fig. 18.
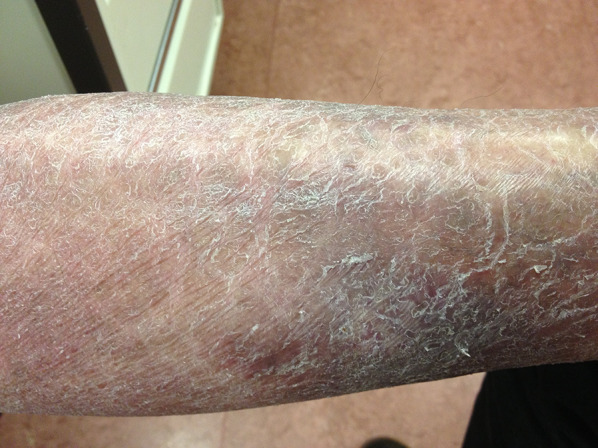
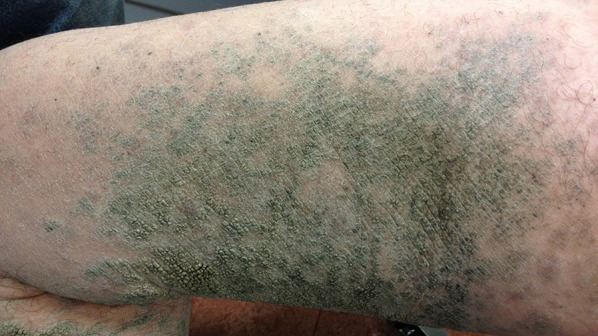
MF mimicking ichthyosis of the lower legs
Fig. 19.
CD3, CD4, and CD8 immunostaining in ichthyosiform MF (× 5)
Fig. 20.
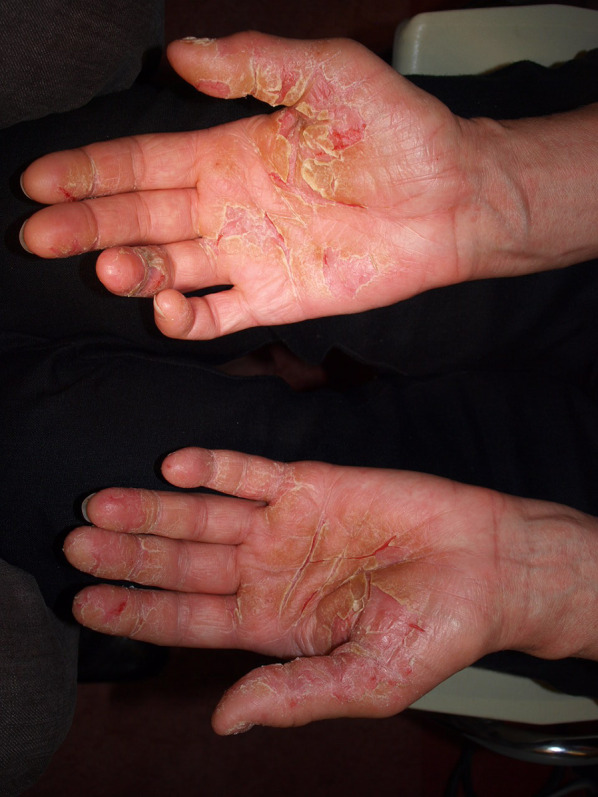
Palmar keratoderma-like MF
Table 1.
Dermatoses and patterns mimicked by MF and their histological presentation
| Mimicking disease | Histological subtype of MF | References |
|---|---|---|
|
Skin diseases Inflammatory | ||
| Acne | Folliculotropic/syringotropic MF | |
| Angular cheilitis | Tumoral MF | Figure 3 |
| Atopic dermatitis | Classic MF with epidermotropic atypical Lc |
[8] Figure 4 |
| Drug eruption | Classic MF with epidermotropic atypical Lc | [8] |
| Dyshidrosis | Classic MF with severe spongiosis | [30] |
| Erythema annulare centrifugum | Superficial perivascular and lichenoid lymphocytic infiltrate with exocytosis of predominantly small atypical Lc | [31–35] |
| Erythema multiforme | Classic MF | [36] |
| Folliculitis decalvans/dissecting cellulitis | Folliculotropic MF | [37] |
| ILVEN (inflammatory linear verrucous epidermal nevus) | Classic MF with Pautrier's microabscesses, follicular epitheliotropism, wiry bundles of collagen | [38] |
| Keratosis lichenoides chronica | Epidermotropic infiltrate of small irregular CD4+ Lc | [39] |
| Keratosis punctata palmaris | Syringotropic MF | Figure 5 |
| Lichen sclerosus | Interstitial MF: atrophic epidermis, band-like infiltration, composed of small- to medium-sized lymphocytes, with hyperchromatic and slightly convoluted nuclei. At the lower parts of the epidermis: atypical Lc, arranged in either solitary units with perinuclear haloes or in small collections. Wiry collagen in the papillary dermis entrapped within the infiltration. In the reticular dermis: interstitial infiltration of atypical Lc between the collagen bundles, reminiscent of interstitial granuloma annulare or inflammatory stage of morphea | [40] |
| Morphea | Lymphocytic epidermotropism arranged in small Pautrier-like collections as well as linear arrangements in dermal-epidermal junction. Replacement of subcutaneous fat with closely packed thick collagen bundles under eccrine glands | [41] |
| Necrobiosis | Granulomatous MF | [42] |
| Ofuji's papuloerythroderma | Classic MF with Pautrier’s microabscess, haloed Lc, disproportionate epidermotropism, and wiry collagen bundles | [43] |
| Pellagra | Poikiloderma MF |
[12] Figure 6 |
| Perioral dermatitis | Folliculotropic MF | [44] |
| Pigmented purpuric dermatosis | Pigmented purpuric dermatitis with classic MF | [31, 45–52] |
| Pityriasis alba | Classic MF with epidermotropic atypical Lc | [8, 53] |
| Pityriasis lichenoides | Classic MF, lymphocytic epidermotropism, and Lc tagging the dermo-epidermal junction. Hyperchromatic and irregular nuclei of atypical Lc, the infiltrating lymphocytes: CD2, CD3, CD5, CD7, and CD8: +. CD4, CD20, CD30, CD68, and CD163: −. TCR: rearrangement of the gamma chain | [54, 55] |
| Pseudolymphomatous angiokeratoma | Granulomatous MF | [14] |
| Psoriasis inversa | Classic MF; marked psoriasiform epidermal hyperplasia with epidermotropic atypical Lc | Figures 7, 8 |
| Psoriasis vulgaris | Classic MF; marked psoriasiform epidermal hyperplasia with epidermotropic atypical Lc | [31, 56, 57] |
| Pyoderma gangrenosum | Neutrophil-rich MF |
[58] Figure 9 |
| Reticular erythematous mucinosis | Classic MF | [59] |
| Rosacea | Folliculotropic MF | Figure 10 |
| Sarcoidosis | Granulomatous MF. Granulomatous infiltrate rich in giant cells, emperipolesis, histiocytic cells, and scattered eosinophils, sometimes reaching the fascia and muscle; the absence of elastic fibers or their phagocytosis by giant cells; and Lc with atypia and epidermotropism | [60, 61] |
| Seborrheic dermatitis | Classic MF | Figure 11 |
| Urticaria | Classic MF | [62] |
| Varicous eczema | Classic MF | Figure 12 |
|
Skin diseases Infectious | ||
| Facial erysipelas | Classic MF with cellulitis, with only focal epidermo- and folliculotropism of atypical Lc | [63] |
| Tinea pedis | Folliculotropic MF |
Figure 13 |
| Gangrene | Classic MF with epidermal vesiculation | [66] |
|
Skin diseases Vascular | ||
| Ischemic toe | Ulcerated plaque stage MF | [67] |
| Telangiectasia | Atypical band-like epidermotropic Lc infiltration along the basal layer and upper dermis, surrounding prominent dilated vessels | [68] |
|
Skin diseases Tumoral | ||
| Pagetoid reticulosis | Intraepidermal infiltrate of atypical Lc | [45, 69] |
| Sarcoma | Tumoral stage MF | [70] |
|
Skin diseases Other | ||
| Cysts | Tumoral stage MF | [71–75] |
| Skin signs/patterns | ||
| Acanthosis nigricans | Granulomatous MF (Slack skin-like) | |
| Alopecia | Folliculotropic MF |
Figure 16 |
| Elastolysis | Classic MF with elastophagy |
[76] Figure 17 |
| Erythroderma | Classic tumoral MF | [8] |
| Hypopigmented/vitiligo | Classic MF predominance of CD8+ T cells, intense epidermotropism | [53, 80–86] |
| Hyperpigmented | Classic MF | [60] |
| Intertriginous lesions | Classic MF | Figure 7 |
| Ichthyosis | Classic MF | |
| Invisible dermatosis | Classic MF | [93] |
| Leonine facies | Folliculotropic MF | [92] |
| Pachyderma | Classic MF with significant dermal infiltrate | [94] |
| Palmoplantar keratoderma | Classic MF with atypical Lc in the upper dermis. Immunostaining of the atypical lymphocytes with strong expression of CD3, CD4 and CD5; reduced expression of CD7 and CD8; and no expression of CD20, with invasion into the deeper layers of skin |
Figure 20 |
| Palmoplantar pustulosis | Classic MF with neutrophilic epidermal infiltrate | [97–99] |
| Poikiloderma | Classic MF, epidermal atrophy |
Figure 4 |
| Porokeratosis | Classic MF, epidermal atrophy | [99, 103] |
| Pseudocarcinomatous hyperplasia | Verrucous MF | [104] |
| Vesicular/bullous lesions | Classic MF with significant spongiosis | [31, 52, 105–107] |
| Serpiginous | Classic MF | [29] |
| Zosteriform | Classic MF | [107] |
| Particular localizations | ||
| Mucosal tongue, palate, and gingiva | Tumoral MF with large cell transformation | [108–110] |
| Vocal cord or laryngeal involvement, hoarseness | Tumoral MF with large atypical Lc | [111] |
| Herpes zoster scar | Classic MF | [107, 112] |
| Palpebral | Classic MF | [113] |
| MF restricted to traumatized skin | Classic MF | [114] |
| MF lesions harboring other dermatoses | ||
| Dermatofibroma | Mixed fibro-histiocytic proliferation as well as atypical intraepidermal and dermal Lc. No large-cell transformation. dermatofibroma-like process arising within a lesion of MF | [115] |
| Keratoma | Classic MF with beta-HPV infection of keratinocytes | [116] |
| HSV infection | Classic MF with epidermal HSV infection | [117] |
| Malassezia | Classic MF with Malassezia in the upper epidermal layers | [117] |
| Staphylococcus aureus | Classic MF with bacterial presence in the upper epidermal layers | [117] |
Lc lymphocytes, MF mycosis fungoides, HSV herpes simplex virus, HPV human papillomavirus
Discussion
About 70–75% of patients with MF present the classic form of the disease, characterized by patches and plaques [1, 9, 21]. MF patches are defined as clinically non-infiltrated lesions whereas plaques feature palpable, infiltrated skin lesions. The classic MF skin lesions are more or less pruritic, erythematous, and slightly squamous. They are often ill defined, particularly in the beginning of the disease [25, 118, 119]. The fixed character of the lesions, the waxing and waning of the skin lesions over months or even years, the localization of the lesions on photo-protected sites, particularly on the hips, are other indicators of a potential diagnosis of MF. A serpiginous distribution of the lesions, without a dermatomal or blaschkoid pattern, is also evocative of MF. The clinical manifestations of childhood MF are as heterogenous as those observed in the adult population [10].
The classical appearance of full-blown MF often takes years to develop and unfortunately the early manifestations of MF are usually very mild, spontaneously regressing and recurring, and hence very complicated to diagnose on clinical grounds. The absence of clear histological signs during early MF disease renders the diagnosis even more difficult.
In order to improve the early diagnosis of MF, a series of criteria were developed, including clinical signs, histological and immunohistological data as well as and TCR rearrangement clues [120, 121]. A score of 4 points or more is highly suggestive of “early MF”. Other complementary diagnostic techniques including dermoscopy [122, 123] and in vivo reflectance confocal microscopy [124] may also be helpful in the case of a suspicion of MF. The PROCLIPI (PROspective Cutaneous Lymphoma International Prognostic Index) study is another ongoing attempt to develop a prognostic index for MF, using a web-based data collection system for early-stage MF [21].
The presence of accompanying signs may sometimes be helpful in the diagnostic puzzle, although the majority are not pathognomonic. The most important accompanying sign of MF and also of early MF is pruritus, often the main reason for the impairment of the quality of life (QoL) index [1, 125]. The management of pruritus in patients with MF merits an important place in the treatment strategy [125]. Other accompanying skin signs may be encountered, usually in the later (T4) stages, such as palmoplantar dishidrosis, alopecia of the vertex, onychodystrophy, and palmoplantar keratoderma. The most striking ophthalmologic signs are blepharitis (50.0%), thickened eyelids (37.5%), and flaking (25.0%) [112]. Another particularity of MF cutaneous lesions is their propensity for bacterial (staphylococcal) or viral (herpes simplex virus (HSV), varicella zoster virus (VZV)) infections of the patch/plaque lesions, again, particularly in long-standing MF [117].
The clinical diagnosis of MF may also be complicated by the rare and/or atypical presentations. Atypical and rare variants [45] include the folliculotropic MF (FMF)/syringotropic forms [24, 78, 126, 127] accounting for about 10–15% of the total MF cases, the chalazoderma-type MF, also termed granulomatous slack skin [76], pagetoid reticulosis, ichthyosiform MF [87], blastic MF, granulomatous MF [60, 128], hypopigmented MF, useful considered as a surrogate marker of cytotoxic immunity targeting the malignant T cells and associated with a good prognosis [80–82], hyperpigmented MF, palmoplantar MF [95, 96], bullous MF [83, 105, 106, 129–131], papillomatous MF, verrucous MF [104], poikilodermic MF [100–102, 132], and invisible MF where pruritus is the only clinical sign [24, 93, 118]. All these aforementioned clinical subtypes of MF may imitate a large array of other dermatological manifestations [6, 26, 27, 31, 133–135], again hindering prompt diagnosis. This fact has already been described [133], relating that MF can mimic more than 50 different clinical entities. The reason behind these very heterogenous presentations remains unclear.
The same is true for the histological subtypes. For example, even if the major histopathological alteration is classified as FMF, various clinical presentations can occur. In a series of 27 patients with FMF, the following atypical clinical presentations were found; lichen spinulosus-like lesions in association with hypopigmentation (n = 3), alopecia (n = 2), infiltrated/elevated erythematous facial plaques initially considered to be lupus tumidus (n = 2), pseudotumoral lesions clinically mimicking tumor-stage MF (n = 1), persistent excoriations (n = 1) and erythematous facial papules mimicking rosacea (n = 1), as well as white dome-shaped asymptomatic papules/nodules filled with mucin (n = 2) [78].
Despite the help of histology, immunohistology, and molecular biology tools, the majority of the publications report important diagnostic delays. In these dermatological mimickers this diagnostic delay seems even more important than the usual diagnostic delays in classic MF.
A suspicion of MF should always be considered if a given dermatosis does not respond to the recognized standard treatments, and/or worsens using immunosuppressive treatments. A case series of patients with psoriasiform MF that had all been treated as psoriasis vulgaris for many years were finally identified as MF particularly after deterioration induced by immunosuppressive therapies including ciclosporin [56]. In addition, repetitive discordant clinicopathological results seem also to be an indicator of a possible MF.
It is furthermore important to recognize these entities in terms of prognosis. In fact, the ichthyosiform and poikiloderma patterns are associated with a rather favorable prognosis, whereas when MF is mimicking necrobiosis [136] or presenting lesions on the eyelids [113] or with involvement of the ENT region [108–110], the prognosis is generally unfavorable because of a risk of blastic transformation of a tumoral MF stage.
Conclusions
The recognition of the different presentations of MF, both the classic, the atypical and rare types as well as the cutaneous disorders and dermatological patterns imitated by MF is important for the clinician, in order to detect MF as soon as possible, particularly as the prognosis is better if the disease is adequately managed in the early stages. One should not refrain from multiple and repetitive skin biopsies searching for signs of MF using histology, immunohistochemistry, and a search for TCR monoclonal rearrangement, combined with a thorough clinicopathological correlation.
Acknowledgements
Funding
No funding or sponsorship was received for the study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
EL, PC, JS, and AFN all participated equally in the concept and design, as well as in the final drafting of the manuscript.
Disclosures
Arjen F. Nikkels, Joan Somja, Patrick Collins, and Eve Lebas have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. All patients provided written consent for their pictures to be published.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.Jawed SI, Myskowski PL, Horwitz S, Moskowitz A, Querfeld C. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part I. Diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205. doi: 10.1016/j.jaad.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 2.Oluwole OO, Zic JA, Douds JJ, Thompson MA, Greer JP. Cutaneous manifestations and management of hematologic neoplasms. Semin Oncol. 2016;43:370–383. doi: 10.1053/j.seminoncol.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 3.Wilcox RA. Cutaneous T-cell lymphoma: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol. 2017;92:1085–1102. doi: 10.1002/ajh.24876. [DOI] [PubMed] [Google Scholar]
- 4.Cerroni L. Mycosis fungoides-clinical and histopathologic features, differential diagnosis, and treatment. Semin Cutan Med Surg. 2018;37:2–10. doi: 10.12788/j.sder.2018.002. [DOI] [PubMed] [Google Scholar]
- 5.Larocca CA, LeBoeuf NR. Overview of cutaneous T-cell lymphomas. Hematol Oncol Clin North Am. 2019;33:669–686. doi: 10.1016/j.hoc.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Peterson E, Weed J, Lo Sicco K, Latkowski J-A. Cutaneous T cell lymphoma: a difficult diagnosis demystified. Dermatol Clin. 2019;37:455–469. doi: 10.1016/j.det.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Specht L, Skov L. Cutaneous lymphomas. Clin Oncol (R Coll Radiol) 2019;31:797–807. doi: 10.1016/j.clon.2019.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Vaidya T, Badri T. Mycosis fungoides. In: StatPearls. Treasure Island: StatPearls Publishing; 2020. (PMID: 30137856). [PubMed]
- 9.Dobos G, Pohrt A, Ram-Wolff C, et al. Epidemiology of cutaneous T-cell lymphomas: a systematic review and meta-analysis of 16,953 patients. Cancers. 2020;12:E2921. doi: 10.3390/cancers12102921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heng YK, Aan Koh MJ, Giam YC, Yang Tang MB, Chong WS, Tan SH. Pediatric mycosis fungoides in Singapore: a series of 46 children. Pediatr Dermatol. 2014;31:477–482. doi: 10.1111/pde.12352. [DOI] [PubMed] [Google Scholar]
- 11.Kempf W, Kazakov DV, Belousova IE, Mitteldorf C, Kerl K. Paediatric cutaneous lymphomas: a review and comparison with adult counterparts. J Eur Acad Dermatol Venereol. 2015;29:1696–1709. doi: 10.1111/jdv.13044. [DOI] [PubMed] [Google Scholar]
- 12.Poppe H, Kerstan A, Beckers M, et al. Childhood mycosis fungoides with a CD8+ CD56+ cytotoxic immunophenotype. J Cutan Pathol. 2015;42:258–264. doi: 10.1111/cup.12452. [DOI] [PubMed] [Google Scholar]
- 13.Ceppi F, Pope E, Ngan B, Abla O. Primary cutaneous lymphomas in children and adolescents. Pediatr Blood Cancer. 2016;63:1886–1894. doi: 10.1002/pbc.26076. [DOI] [PubMed] [Google Scholar]
- 14.Evans MS, Burkhart CN, Bowers EV, et al. Solitary plaque on the leg of a child: a report of two cases and a brief review of acral pseudolymphomatous angiokeratoma of children and unilesional mycosis fungoides. Pediatr Dermatol. 2019;36:e1–e5. doi: 10.1111/pde.13686. [DOI] [PubMed] [Google Scholar]
- 15.Wu JH, Cohen BA, Sweren RJ. Mycosis fungoides in pediatric patients: clinical features, diagnostic challenges, and advances in therapeutic management. Pediatr Dermatol. 2020;37:18–28. doi: 10.1111/pde.14026. [DOI] [PubMed] [Google Scholar]
- 16.Biondo G, Cerroni L, Brunasso AMG, et al. Risk of mycosis fungoides in psoriatic patients: a critical review. J Eur Acad Dermatol Venereol. 2020;34:1186–1195. doi: 10.1111/jdv.16160. [DOI] [PubMed] [Google Scholar]
- 17.Seßkin D. Cutaneous lymphoproliferative disorders in organ transplant recipients: update 2014. Ital Dermatol Venereol. 2014;149:401–408. [PubMed] [Google Scholar]
- 18.Nikkels AF, Quatresooz P, Delvenne P, Balsat A, Piérard GE. Mycosis fungoides progression and chronic solvent exposure. Dermatology. 2004;208:171–173. doi: 10.1159/000076496. [DOI] [PubMed] [Google Scholar]
- 19.Morales-Suárez-Varela MM, Olsen J, Villeneuve S, et al. Occupational exposure to chlorinated and petroleum solvents and mycosis fungoides. J Occup Environ Med. 2013;55:924–931. doi: 10.1097/JOM.0b013e3182941a1c. [DOI] [PubMed] [Google Scholar]
- 20.Huang AH, Kwatra SG, Khanna R, Semenov YR, Okoye GA, Sweren RJ. Racial disparities in the clinical presentation and prognosis of patients with mycosis fungoides. J Natl Med Assoc. 2019;111:633–639. doi: 10.1016/j.jnma.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Scarisbrick JJ, Quaglino P, Prince HM, et al. The PROCLIPI international registry of early-stage mycosis fungoides identifies substantial diagnostic delay in most patients. Br J Dermatol. 2019;181:350–357. doi: 10.1111/bjd.17258. [DOI] [PubMed] [Google Scholar]
- 22.Oschlies I, King RL, Dotlic S, et al. The clinico-pathological spectrum of primary cutaneous lymphoma other than mycosis fungoides/Sezary syndrome. Virchows Arch. 2020;476:683–699. doi: 10.1007/s00428-019-02713-7. [DOI] [PubMed] [Google Scholar]
- 23.Howard MS, Smoller BR. Mycosis fungoides: classic disease and variant presentations. Semin Cutan Med Surg. 2000;19:91–99. doi: 10.1016/s1085-5629(00)80005-x. [DOI] [PubMed] [Google Scholar]
- 24.Munoz-Gonzalez H, Molina-Ruiz AM, Requena L. Clinicopathologic variants of mycosis fungoides. Actas Dermosifiliogr. 2017;108:192–208. doi: 10.1016/j.ad.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Willemze R. Mycosis fungoides variants-clinicopathologic features, differential diagnosis, and treatment. Semin Cutan Med Surg. 2018;37:11–17. doi: 10.12788/j.sder.2018.004. [DOI] [PubMed] [Google Scholar]
- 26.Hodak E, Amitay-Laish I. Mycosis fungoides: a great imitator. Clin Dermatol. 2019;37:255–267. doi: 10.1016/j.clindermatol.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Sidiropoulou P, Nikolaou V, Marinos L, et al. The different faces of mycosis fungoides: results of a single-center study. Int J Dermatol. 2020;59:314–320. doi: 10.1111/ijd.14735. [DOI] [PubMed] [Google Scholar]
- 28.Venturini A, Zane C, Rodella R, Leali C, Calzavara Pinton P, Zorzi F. Syringotropic cutaneous T cell lymphoma treated with PUVA therapy. Eur J Dermatol. 2005;15:262–264. [PubMed] [Google Scholar]
- 29.Yost JM, Do TT, Kovalszki K, Su L, Anderson TF, Gudjonsson JE. Two cases of syringotropic cutaneous T-cell lymphoma and review of the literature. J Am Acad Dermatol. 2009;61:133–138. doi: 10.1016/j.jaad.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Soyer HP, Smolle J, Kerl H. Dyshidrotic mycosis fungoides. J Cutan Pathol. 1987;14:372. [Google Scholar]
- 31.Zackheim HS, McCalmont TH. Mycosis fungoides: the great imitator. J Am Acad Dermatol. 2002;47:914–918. doi: 10.1067/mjd.2002.124696. [DOI] [PubMed] [Google Scholar]
- 32.Notay M, Petukhova TA, Kiuru M, Kunder CA, Hwang ST. Mycosis fungoides presenting as symmetric concentric patches mimicking figurate erythema. JAAD Case Rep. 2017;3:288–290. doi: 10.1016/j.jdcr.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moura FN, Thomas L, Balme B, Dalle S. Mycosis fungoides mimicking an annular erythema. Clin Exp Dermatol. 2009;34:e581–e583. doi: 10.1111/j.1365-2230.2009.03249.x. [DOI] [PubMed] [Google Scholar]
- 34.Ceyhan AM, Akkaya VB, Chen W, Bircan S. Erythema annulare centrifugum-like mycosis fungoides. Australas J Dermatol. 2011;52:e11–e13. doi: 10.1111/j.1440-0960.2010.00683.x. [DOI] [PubMed] [Google Scholar]
- 35.Qiang JK, Marinas JE, Sajic D, Yeung J. Serpiginous mycosis fungoides in a 21-year-old man. JAAD Case Rep. 2015;1:82–84. doi: 10.1016/j.jdcr.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krebs A, Zala A, Graber W, Meyer A, Jenni C. Mycosis fungoides. Dermatologica. 1978;157:312–315. [Google Scholar]
- 37.Gilliam AC, Lessin SR, Wilson DM, Salhany KE. Folliculotropic mycosis fungoides with large-cell transformation presenting as dissecting cellulitis of the scalp. J Cutan Pathol. 1997;24:169–175. doi: 10.1111/j.1600-0560.1997.tb01572.x. [DOI] [PubMed] [Google Scholar]
- 38.Jang JG, Sim HJ, Kim SH, et al. Mycosis fungoides mimicking inflammatory linear verrucous epidermal nevus. J Eur Acad Dermatol Venereol. 2004;18:218–220. doi: 10.1111/j.1468-3083.2004.00891.x. [DOI] [PubMed] [Google Scholar]
- 39.Bahadoran P, Wechsler J, Delfau-Larue MH, Gabison G, Revuz J, Bagot M. Mycosis fungoides presenting as keratosis lichenoides chronica. Br J Dermatol. 1998;138:1067–1069. doi: 10.1046/j.1365-2133.1998.02282.x. [DOI] [PubMed] [Google Scholar]
- 40.Tekin B, Kempf W, Seckin D, Ergun T, Yucelten D, Demirkesen C. Interstitial mycosis fungoides with lichen sclerosus-like clinical and histopathological features. Am J Dermatopathol. 2016;38:138–143. doi: 10.1097/DAD.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 41.Basir HRG, Alirezaei P, Rezanejad A, Daneshyar S. Early morphea simulating patch-stage mycosis fungoides in two cases. Dermatol Rep. 2018;10:7471. doi: 10.4081/dr.2018.7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hur J, Seong JY, Choi TS, et al. Mycosis fungoides presenting as Ofuji's papuloerythroderma. J Eur Acad Dermatol Venereol. 2002;16:393–396. doi: 10.1046/j.1468-3083.2002.00486.x. [DOI] [PubMed] [Google Scholar]
- 43.Wolf P, Cerroni L, Kerl H. Mycosis fungoides mimicking perioral dermatitis. Clin Exp Dermatol. 1992;17:132–134. doi: 10.1111/j.1365-2230.1992.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 44.Riyaz N, Sasidharanpillai S, Abdul Latheef EN, Davul H, Ashraf F. Pigmented purpuric dermatosis or mycosis fungoides: a diagnostic dilemma. Indian Dermatol Online J. 2016;7:183–185. doi: 10.4103/2229-5178.182361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martinez-Escala ME, Rubio Gonzalez B, Guitart J. Mycosis fungoides variants. Surg Pathol Clin. 2014;7:169–189. doi: 10.1016/j.path.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Barnhill RL, Braverman IM. Progression of pigmented purpura-like eruptions to mycosis fungoides: report of three cases. J Am Acad Dermatol. 1988;19:25–31. doi: 10.1016/s0190-9622(88)70147-4. [DOI] [PubMed] [Google Scholar]
- 47.Cather JC, Farmer A, Jackow C, Manning JT, Shin DM, Duvic M. Unusual presentation of mycosis fungoides as pigmented purpura with malignant thymoma. J Am Acad Dermatol. 1998;39:858–863. doi: 10.1016/s0190-9622(98)70366-4. [DOI] [PubMed] [Google Scholar]
- 48.Lipsker D. The pigmented and purpuric dermatitis and the many faces of mycosis fungoides. Dermatology. 2003;207:246–247. doi: 10.1159/000073083. [DOI] [PubMed] [Google Scholar]
- 49.Georgala S, Katoulis AC, Symeonidou S, Georgala C, Vayopoulos G. Persistent pigmented purpuric eruption associated with mycosis fungoides: a case report and review of the literature. J Eur Acad Dermatol Venereol. 2001;15:62–64. doi: 10.1046/j.1468-3083.2001.00198.x. [DOI] [PubMed] [Google Scholar]
- 50.Toro JR, Sander CA, LeBoit PE. Persistent pigmented purpuric dermatitis and mycosis fungoides: simulant, precursor, or both? A study by light microscopy and molecular methods. Am J Dermatopathol. 1997;19:108–118. doi: 10.1097/00000372. [DOI] [PubMed] [Google Scholar]
- 51.Dyduch G, Zuber Z, Turowska-Heydel D, Sobczyk M, Wielowieyska-Szybińska D, Białas M. Granulomatous pigmented purpura in an adolescent girl: a precursor of mycosis fungoides? Pol J Pathol. 2013;64:157–159. doi: 10.5114/pjp.2013.36008. [DOI] [PubMed] [Google Scholar]
- 52.Whitmore SE, Simmons-O'Brien E, Rotter FS. Hypopigmented mycosis fungoides. Dermatology. 1994;130:476–480. [PubMed] [Google Scholar]
- 53.Lin TL, Chen YJ, Wen YC, Yang CS, Juan CK. Pityriasis lichenoid-like mycosis fungoides in a 9-year-old boy: a case report. Acta Dermatovenerol Croat. 2019;27:37–39. [PubMed] [Google Scholar]
- 54.Ko JW, Seong JY, Suh KS, Kim ST. Pityriasis lichenoides-like mycosis fungoides in children. Br J Dermatol. 2000;142:347–352. doi: 10.1046/j.1365-2133.2000.03307.x. [DOI] [PubMed] [Google Scholar]
- 55.Vaudreuil AM, Stroud CM, Hsu S. Psoriasis mimicking mycosis fungoides clinically. Dermatol Online J. 2017;23:13030/qt3r4942kh. [PubMed] [Google Scholar]
- 56.Azizpour A, Ghanadan A, Nasimi M, Etesami I. Psoriasiform mycosis fungoides: a rare form of the disease with review of the literature. Dermatol Online J. 2017;23:13030/qt9mv8r0j7. [PubMed] [Google Scholar]
- 57.Ho KK, Browne A, Fitzgibbons J, Carney D, Powell FC. Mycosis fungoides bullosa simulating pyoderma gangrenosum. Br J Dermatol. 2000;142:124–127. doi: 10.1046/j.1365-2133.2000.03253.x. [DOI] [PubMed] [Google Scholar]
- 58.Twersky JM, Mutasim DF. Mycosis fungoides presenting as reticular erythematous mucinosis. Int J Dermatol. 2006;45:230–233. doi: 10.1111/j.1365-4632.2004.02434.x. [DOI] [PubMed] [Google Scholar]
- 59.Bessis D, Sotto A, Farcet JP, Barnéon G, Guilhou JJ. Granulomatous mycosis fungoides presenting as sarcoidosis. Dermatology. 1996;193:330–332. doi: 10.1159/000246283. [DOI] [PubMed] [Google Scholar]
- 60.Rodriguez G, Tullez A. Perineural and intraneural cutaneous granulomas in granulomatous mycosis fungoides mimicking tuberculoid leprosy. Int J Dermatol. 2016;55:1336–1340. doi: 10.1111/ijd.13398. [DOI] [PubMed] [Google Scholar]
- 61.Niesert AC, Gürtler A, Schlaak M, Flaig MJ. Urticarial mycosis fungoides. Hautarzt. 2020;71(Suppl 1):21–23. doi: 10.1007/s00105-020-04642-y. [DOI] [PubMed] [Google Scholar]
- 62.Weyers W, Diaz-Cascajo C, Preinfalk P, Lofler H. Mycosis fungoides mimicking erysipelas. J Dtsch Dermatol Ges. 2008;6:298–301. doi: 10.1111/j.1610-0387.2007.06576.x. [DOI] [PubMed] [Google Scholar]
- 63.Hanna SA, Kirchhof MG. Mycosis fungoides mimicking tinea pedis. CMAJ. 2016;188:E539. doi: 10.1503/cmaj.160038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang RF, Sokumbi O, Chiu YE. Folliculotropic mycosis fungoides in a pediatric patient mimicking black dot tinea capitis. Pediatr Dermatol. 2019;36:386–387. doi: 10.1111/pde.13768. [DOI] [PubMed] [Google Scholar]
- 65.Lund KA, Parker CM, Norins AL, Tejada E. Vesicular cutaneous T cell lymphoma presenting with gangrene. J Am Acad Dermatol. 1990;23:1169–1171. doi: 10.1016/s0190-9622(08)80921-8. [DOI] [PubMed] [Google Scholar]
- 66.Goldstein LJ, Williams JD, Zackheim HS, Helfend LK. Mycosis fungoides masquerading as an ischemic foot. Ann Vasc Surg. 1999;13:305–307. doi: 10.1007/s100169900262. [DOI] [PubMed] [Google Scholar]
- 67.García-Colmenero L, Curto-Barredo L, Gómez-Martin I, Gallardo F, Pujol RM. Telangiectatic mycosis fungoides: a new clinicopathological presentation mimicking acquired naevoid telangiectasia. Acta Derm Venereol. 2017;97:651–652. doi: 10.2340/00015555-2613. [DOI] [PubMed] [Google Scholar]
- 68.Kaufmann F, Kettelhack N, Hilty N, Kempf W. Unilesional plantar mycosis fungoides treated with topical photodynamic therapy—case report and review of the literature. J Eur Acad Dermatol Venereol. 2017;31:1633–1637. doi: 10.1111/jdv.14160. [DOI] [PubMed] [Google Scholar]
- 69.Machler BC, Elgart GW, Kerdel FA. Extracutaneous mycosis fungoides of the gastrocnemius muscle mimicking sarcoma. J Am Acad Dermatol. 1994;31:673–675. doi: 10.1016/s0190-9622(08)81739-2. [DOI] [PubMed] [Google Scholar]
- 70.Radeff B, Mérot Y, Saurat JH. Acquired epidermal cysts and mycosis fungoids. A possible pitfall in clinical staging. Am J Dermatopathol. 1988;10:424–429. [PubMed] [Google Scholar]
- 71.Oliwiecki S, Ashworth J. Mycosis fungoides with a widespread follicular eruption, comedones and cysts. Br J Dermatol. 1992;127:54–56. doi: 10.1111/j.1365-2133.1992.tb14828.x. [DOI] [PubMed] [Google Scholar]
- 72.Aloi F, Tomasini C, Pippione M. Mycosis fungoides and eruptive epidermoid cysts: a unique response of follicular and eccrine structures. Dermatology. 1993;187:273–277. doi: 10.1159/000247263. [DOI] [PubMed] [Google Scholar]
- 73.Lacour JP, Castanet J, Perrin C, Ortonne JP. Follicular mycosis fungoids. A clinical and histologic variant of cutaneous T-cell lymphoma: report of two cases. J Am Acad Dermatol. 1993;29:330–334. doi: 10.1016/0190-9622(93)70188-Y. [DOI] [PubMed] [Google Scholar]
- 74.Peris K, Chimenti S, Sacerdoti G, Muscardin L, Fazio M. Pilotropic mycosis fungoides. Dermatology. 1999;199:192–194. doi: 10.1159/000018242. [DOI] [PubMed] [Google Scholar]
- 75.Willemze R, Scheffer E, Van Vloten WA. Mycosis fungoides simulating acanthosis nigricans. Am J Dermatopathol. 1985;7:367–371. doi: 10.1097/00000372-198508000-00010. [DOI] [PubMed] [Google Scholar]
- 76.El-Khoury J, Kurban M, Abbas O. Elastophagocytosis: underlying mechanisms and associated cutaneous entities. J Am Acad Dermatol. 2014;70:934–944. doi: 10.1016/j.jaad.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 77.Kossard S, White A, Killingsworth M. Basaloid folliculolymphoid hyperplasia with alopecia as an expression of mycosis fungoides (CTCL) J Cutan Pathol. 1995;22:466–471. doi: 10.1111/j.1600-0560.1995.tb00764.x. [DOI] [PubMed] [Google Scholar]
- 78.Baykal C, Atci T, Ozturk Sari S, Polat Ekinci A, Buyukbabani N. Underrecognized clinical features of folliculotropic mycosis fungoides: a large clinical series. J Dtsch Dermatol Ges. 2017;15:289–299. doi: 10.1111/ddg.12976. [DOI] [PubMed] [Google Scholar]
- 79.Kim JC, Kim YC. Hypopigmented mycosis fungoides mimicking vitiligo. Am J Dermatopathol. 2020 doi: 10.1097/DAD.0000000000001750. [DOI] [PubMed] [Google Scholar]
- 80.Cecanho Furlan F, Sanches JA. Hypopigmented mycosis fungoides: a review of its clinical features and pathophysiology. An Bras Dermatol. 2013;88:954–960. doi: 10.1590/abd1806-4841.20132336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rodney IJ, Kindred C, Angra K, Qutub ON, Villanueva AR, Halder RM. Hypopigmented mycosis fungoides: a retrospective clinicohistopathologic study. J Eur Acad Dermatol Venereol. 2017;31:808–814. doi: 10.1111/jdv.13843. [DOI] [PubMed] [Google Scholar]
- 82.Martinez Villarreal A, Gantchev J, Lagace F, et al. Hypopigmented mycosis fungoides: loss of pigmentation reflects antitumor immune response in young patients. Cancers. 2020;12:2007. doi: 10.3390/cancers12082007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zackheim HS, Epstein EH, Jr, Grekin DA, McNutt NS. Mycosis fungoides presenting as areas of hypopigmentation: a report of three cases. J Am Acad Dermatol. 1982;6:340–345. doi: 10.1016/s0190-9622(82)70026-x. [DOI] [PubMed] [Google Scholar]
- 84.Cooper D, Jacobson M, Bart RS. Hypopigmented macules. Hypopigmented mycosis fungoides (MF). Arch Dermatol. 1992;128:1266–7, 1269–70. 10.1001/archderm.128.9.1266. [DOI] [PubMed]
- 85.Lambroza E, Cohen SR, Phelps R, Lebwohl M, Braverman IM, DiCostanzo D. Hypopigmented variant of mycosis fungoides: demography, histopathology, and treatment of seven cases. J Am Acad Dermatol. 1995;32:987–993. doi: 10.1016/0190-9622(95)91337-8. [DOI] [PubMed] [Google Scholar]
- 86.Badawy E, D'Incan M, El Majjaoui S, et al. Ichthyosiform mycosis fungoides. Eur J Dermatol. 2002;12:594–596. [PubMed] [Google Scholar]
- 87.Kütting B, Metze D, Luger TA, Bonsmann G. Mycosis fungoides presenting as an acquired ichthyosis. J Am Acad Dermatol. 1996;34:887–889. doi: 10.1016/s0190-9622(96)90072-9. [DOI] [PubMed] [Google Scholar]
- 88.Ryan C, Whittaker S, D'Arcy C, O'Regan GM, Rogers S. Juvenile folliculotropic and ichthyosiform mycosis fungoides. Clin Exp Dermatol. 2009;34:e160–e162. doi: 10.1111/j.1365-2230.2008.03051.x. [DOI] [PubMed] [Google Scholar]
- 89.Hodak E, Amitay I, Feinmesser M, Aviram A, David M. Ichthyosiform mycosis fungoides: an atypical variant of cutaneous T-cell lymphoma. J Am Acad Dermatol. 2004;50:368–374. doi: 10.1016/j.jaad.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 90.Sato M, Sohara M, Kitamura Y, Hatamochi A, Yamazaki S. Ichthyosiform mycosis fungoides: report of a case associated with IgA nephropathy. Dermatology. 2005;210:324–328. doi: 10.1159/000084759. [DOI] [PubMed] [Google Scholar]
- 91.Brown DN, Wieser I, Wang C, Dabaja BS, Duvic M. Leonine facies (LF) and mycosis fungoides (MF): a single-center study and systematic review of the literature. J Am Acad Dermatol. 2015;73:976–986. doi: 10.1016/j.jaad.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 92.Lebas E, Chian C, Nikkels-Tassoudji N, Arrese JE, Nikkels AF. Pachyderma in primary cutaneous NK and T-cell lymphoma and leukemia cutis. Case Rep Dermatol. 2017;9:151–157. doi: 10.1159/000480068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pujol RM, Gallardo F, Llistosella E, et al. Invisible mycosis fungoides: a diagnostic challenge. J Am Acad Dermatol. 2002;47:S168–S171. doi: 10.1067/mjd.2002.107231. [DOI] [PubMed] [Google Scholar]
- 94.Moreno JC, Ortega M, Conejo-Mir JS, Sanchez-Pedreño P. Palmoplantar pustulosis as a manifestation of cutaneous T cell lymphoma (mycosis fungoides) J Am Acad Dermatol. 1990;23:758–759. doi: 10.1016/s0190-9622(08)81083-3. [DOI] [PubMed] [Google Scholar]
- 95.Nakai N, Hagura A, Yamazato S, Katoh N. Mycosis fungoides palmaris et plantaris successfully treated with radiotherapy: case report and mini-review of the published work. J Dermatol. 2014;41:63–67. doi: 10.1111/1346-8138.12308. [DOI] [PubMed] [Google Scholar]
- 96.Beiser I, Yim J, Robles-Sherman E, Mirkin GS, Hao X. Mycosis fungoides palmaris et plantaris on the plantar aspect of the foot: a case report. J Case Rep. 2020;21:e923361. doi: 10.12659/AJCR.923361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tagami H, Aiba S, Ohkouchi K. Palmoplantar pustular lesions in mycosis fungoides. J Am Acad Dermatol. 1991;25:733–734. doi: 10.1016/s0190-9622(08)80688-3. [DOI] [PubMed] [Google Scholar]
- 98.Camisa C, Aulisio A. Pustular mycosis fungoides. Cutis. 1994;54:202–204. [PubMed] [Google Scholar]
- 99.Hsu WT, Toporcer MB, Kantor GR, Vonderheid EC, Kadin ME. Cutaneous T-cell lymphoma with porokeratosis-like lesions. J Am Acad Dermatol. 1992;27:327–330. doi: 10.1016/0190-9622(92)70192-i. [DOI] [PubMed] [Google Scholar]
- 100.Abbott RA, Sahni D, Robson A, Agar N, Whittaker S, Scarisbrick JJ. Poikilodermatous mycosis fungoides: a study of its clinicopathological, immunophenotypic, and prognostic features. J Am Acad Dermatol. 2011;65:313–319. doi: 10.1016/j.jaad.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 101.Shiomi T, Monobe Y, Kuwabara C, Hayashi H, Yamamoto T, Sadahira Y. Poikilodermatous mycosis fungoides with a CD8+ CD56+ immunophenotype: a case report and literature review. J Cutan Pathol. 2013;40:317–320. doi: 10.1111/cup.12067. [DOI] [PubMed] [Google Scholar]
- 102.Pankratov O, Gradova S, Tarasevich S, Pankratov V. Poikilodermatous mycosis fungoides: clinical and histopathological analysis of a case and literature review. Acta Dermatovenerol Alp Pannonica Adriat. 2015;24:37–41. doi: 10.15570/actaapa.2015.10. [DOI] [PubMed] [Google Scholar]
- 103.Breneman DL, Breneman JC. Cutaneous T-cell lymphoma mimicking porokeratosis of Mibelli. J Am Acad Dermatol. 1993;29:1046–1048. doi: 10.1016/s0190-9622(08)82045-2. [DOI] [PubMed] [Google Scholar]
- 104.Jeunon T, Assoni A, Verdolin A. Pseudocarcinomatous hyperplasia, squamous cell carcinoma, and keratoacanthoma associated to lymphomas of the skin and external mucous membranes: a case report and literature review. Am J Dermatopathol. 2020;42:662–672. doi: 10.1097/DAD.0000000000001587. [DOI] [PubMed] [Google Scholar]
- 105.Juzot C, Josselin N, Dréno B, Quéreux G. Mycosis fungoides bullosa: a rare clinical presentation. Ann Dermatol Venereol. 2020;147:760–763. doi: 10.1016/j.annder.2020.04.017. [DOI] [PubMed] [Google Scholar]
- 106.Korekawa A, Kaneko T, Nakajima K, et al. Mycosis fungoides bullosa associated with bullous pemphigoid. Int J Dermatol. 2015;54:e366–e368. doi: 10.1111/ijd.12821. [DOI] [PubMed] [Google Scholar]
- 107.Córdoba S, Fernández-Herrera J, Sánchez-Pérez J, Fraga J, García-Díez A. Vesiculobullous mycosis fungoides. Br J Dermatol. 1999;141:164–166. doi: 10.1046/j.1365-2133.1999.02946.x. [DOI] [PubMed] [Google Scholar]
- 108.Huang S, Kim EJ, Lewis DJ, Chan WH, Miranda RN, Duvic M. Mycosis fungoides occurring at the site of previous herpes zoster eruption. Australas J Dermatol. 2018;59:217–219. doi: 10.1111/ajd.12756. [DOI] [PubMed] [Google Scholar]
- 109.Sultan AS, Mostoufi B, Papadimitriou JC, Koka R, Basile J, Younis RH. Large cell transformation of oral mycosis fungoides. Head Neck Pathol. 2018;12:247–251. doi: 10.1007/s12105-017-0840-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rosebush MS, Allen CA, Accurso BT, Baiocchi RA, Cordell KG. Oral mycosis fungoides: a report of three cases and review of the literature. Head Neck Pathol. 2019;13:492–499. doi: 10.1007/s12105-018-0923-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bauman TM, Wichterman CM, Musiek AC, Nemer KM. Hoarseness as a presentation of mycosis fungoides infiltrating the larynx. BMJ Case Rep. 2017 doi: 10.1136/bcr-2017-221531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Colossi CG, Mondadori J, Barreto PKM, Valeneßa FM, Duquia R, Vilela MAP. Mycosis fungoides: analysis of ophthalmologic findings in a series of cases. Case Rep Dermatol Med. 2019;2019:2380598. doi: 10.1155/2019/2380598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Holm Svendsen F, Heegaard S. Lymphoma of the eyelid. Surv Ophthalmol. 2017;6:312–331. doi: 10.1016/j.survophthal.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 114.Lebas E, Libon F, Nikkels AF. Koebner phenomenon and mycosis fungoides. Case Rep Dermatol. 2015;7:287–291. doi: 10.1159/000440856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Morcos SM, Girardi M, Subtil A, Wilson LD, Cowper SE. Mycosis fungoides exhibiting features of a dermatofibroma: a case report and review of the literature. J Cutan Pathol. 2012;39:40–46. doi: 10.1111/j.1600-0560.2011.01804.x. [DOI] [PubMed] [Google Scholar]
- 116.Lebas E, Quatresooz P, Arrese JE, Nikkels AF. Eruptive seborrheic keratoses restricted to plaque/patch-stage mycosis fungoides. Case Rep Dermatol. 2017;9:35–39. doi: 10.1159/000471787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lebas E, Arrese JE, Nikkels AF. Risk factors for skin infections in mycosis fungoides. Dermatology. 2016;232:731–737. doi: 10.1159/000455944. [DOI] [PubMed] [Google Scholar]
- 118.Cho-Vega JH, Tschen JA, Duvic M, Vega F. Early-stage mycosis fungoides variants: case-based review. Ann Diagn Pathol. 2010;14:369–385. doi: 10.1016/j.anndiagpath.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 119.Maguire A, Puelles J, Raboisson P, Chavda R, Gabriel S, Thornton S. Early-stage mycosis fungoides: epidemiology and prognosis. Acta Derm Venereol. 2020;100:adv00013. doi: 10.2340/00015555-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoids. International Society for Cutaneous Lymphoma. J Am Acad Dermatol. 2005;53:1053–1063. doi: 10.1016/j.jaad.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 121.Kaufman AE, Patel K, Goyal K, et al. Mycosis fungoides: developments in incidence, treatment and survival. J Eur Acad Dermatol Venereol. 2020;34:2288–2294. doi: 10.1111/jdv.16325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Errichetti E, Stinco G. Dermatoscopy in life-threatening and severe acute rashes. Clin Dermatol. 2020;38:113–121. doi: 10.1016/j.clindermatol.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 123.Piccolo V, Russo T, Agozzino M, et al. Dermoscopy of cutaneous lymphoproliferative disorders: where are we now? Dermatology. 2018;234:131–136. doi: 10.1159/000490412. [DOI] [PubMed] [Google Scholar]
- 124.Melhoranse Gouveia B, Wells J, Kim J, Consuegra G, Longo C, Fernandez-Penas P. Systematic review and proposal of an in vivo reflectance confocal microscopy assessment tool for cutaneous lymphoma. J Cutan Pathol. 2020;47:295–304. doi: 10.1111/cup.13598. [DOI] [PubMed] [Google Scholar]
- 125.Ahern K, Gilmore ES, Poligone B. Pruritus in cutaneous T-cell lymphoma: a review. J Am Acad Dermatol. 2012;67:760–768. doi: 10.1016/j.jaad.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Geller S, Pulitzer M, Myskowski PL. Acneiform follicular mucinosis: an indolent follicular mucinosis variant unrelated to mycosis fungoides? Clin Exp Dermatol. 2018;43:921–924. doi: 10.1111/ced.13671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Magro CM, Telang GH, Momtahen S. Unilesional follicular mycosis fungoides: report of 6 cases and review of the literature. Am J Dermatopathol. 2018;40:329–336. doi: 10.1097/DAD.0000000000000997. [DOI] [PubMed] [Google Scholar]
- 128.Wieser I, Wohlmuth C, Duvic M. Granulomatous mycosis fungoides in an adolescent-a rare encounter and review of the literature. Pediatr Dermatol. 2016;33:e296–e298. doi: 10.1111/pde.12959. [DOI] [PubMed] [Google Scholar]
- 129.Roenigk HH, Jr, Castrovinci AJ. Mycosis fungoides bullosa. Arch Dermatol. 1971;104:402–406. doi: 10.1001/archderm.1971.04000220060011. [DOI] [PubMed] [Google Scholar]
- 130.McBride SR, Dahl G, Slater DN, Sviland L. Vesicular mycosis fungoides. Br J Dermatol. 1998;138:141–144. doi: 10.1046/j.1365-2133.1998.02041.x. [DOI] [PubMed] [Google Scholar]
- 131.Maeda K, Jimbow K, Takahashi M. Association of vesiculobullous eruptions with mycosis fungoides. Dermatologica. 1987;174:34–38. doi: 10.1159/000248978. [DOI] [PubMed] [Google Scholar]
- 132.Nofal A, Salah E. Acquired poikiloderma: proposed classification and diagnostic approach. J Am Acad Dermatol. 2013;69:e129–e140. doi: 10.1016/j.jaad.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 133.Nashan D, Faulhaber D, Ständer S, Luger TA, Stadler R. Mycosis fungoides: a dermatological masquerader. Br J Dermatol. 2007;156:1–10. doi: 10.1111/j.1365-2133.2006.07526.x. [DOI] [PubMed] [Google Scholar]
- 134.Ahmad A, Semkova K, Stefanato CM, Calonje EJ, Choczaj-Kukula A, Palamaras I. Tiger-like mycosis fungoides: an unusual clinical presentation of a rare variant of mycosis fungoides. Dermatol Online J. 2019;25:13030/qt0w1035f7. [PubMed] [Google Scholar]
- 135.Alsayyah A. Is it mycosis fungoides? A comprehensive guide to reaching the diagnosis and avoiding common pitfalls. Ann Diagn Pathol. 2020;47:151546. doi: 10.1016/j.anndiagpath.2020.151546. [DOI] [PubMed] [Google Scholar]
- 136.Woollons A, Darvay A, Khorshid SM, Whittaker S, Jones RR. Necrobiotic cutaneous T-cell lymphoma. J Am Acad Dermatol. 1999;41:815–819. doi: 10.1016/s0190-9622(99)70332-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.