Abstract
Nanoparticles have revolutionized biomedicine especially in the field of drug delivery due to their intriguing properties such as systemic stability, level of solubility, and target site specificity. It can, however, be both beneficial and damaging depending on the properties in different environments, thus highlighting the importance of nanotoxicology studies before use in humans. Different types of nanoparticles have been used in drug delivery, and this review summarizes the recent toxicity studies of these nanoparticles. The toxicological evaluation of three widely used nanoparticles in drug delivery that are metal, lipid, and protein nanoparticles has been discussed in detail. Studies have recorded several toxic effects of various nanoparticles such as metal-based nanoparticles have been linked to increased oxidative stress and have the potential to infiltrate the cell nucleus and protein-based nanoparticles have been observed to have hepatotoxicity and nephrotoxicity as their adverse effects. Considering the increasing application of nanoparticles in drug delivery and the growing concerns of regulatory authorities regarding the toxicity of nanocarriers in living organisms, it requires urgent attention to identify the gap in toxicity studies. The review highlights the gap in toxicity studies and potential focus areas to overcome the existing challenges.
Keywords: Nanoparticles, Nanotoxicology, Toxicity assessment, Protein nanoparticles, Metal nanoparticles, Lipid nanoparticles
Introduction
The recent years have marked an unprecedented growth in nanotechnology due to its wide range of applications [1]. The rising popularity can be attributed to their astonishing properties which sets them apart from any other material. This has allowed their application to be expanded in the field of biomedicine, for the purpose of drug delivery, nutraceuticals, diagnostics/imaging, production of biocompatible materials, and much more [2, 3]. The technological advancements using nanoparticles have gained importance in such a short period due to impressive physical and chemical properties. A particle having size less than 100 nm (any one dimension) is characterized as a nanoparticle as per the new definition. These nanostructures possess great surface to area ratio, show quantum effects, and are highly mobile when present in free state [4]. The conventional drugs face problems like low specificity, less bioavailability, and high toxicity. These problems can be substantially overcome by use of nanoparticle-based drug delivery systems [5]. Metal, non-metal, polymers, and biopolymers have been used for preparation of nanoparticles/nanoformulations [6, 7]. For therapeutics, a nanoparticle can be used in two ways by acting itself (1) as a drug or (2) as a carrier for another substance [7, 8]. Since these nanoparticles are entering the biological system, they interact with various biological pathways and biomolecules found within living beings. Where on one side the unique properties of nanoparticles makes them ideal for drug delivery, the same properties also raise challenges, if not optimized efficiently [9]. As mentioned before, nanoparticles have a great surface to area ratio, which in one case provides benefit of efficient drug loading, but on the contrary, it can also increase chances of interaction with other biomolecules when present in a living organism. Some of the other properties that attract the concerns of scientists when using nanoparticles as drug delivery systems are their particle size (which allows easy access to various organs), charged surface (increasing chances of non-specific interaction), solubility, surface coating, and more. Various researches over the years support the claim that nanoparticles can be a bane or boon. We mostly know about the tremendous benefits that nanoparticles offer, but there is very limited information about toxicity, non-specific protein interaction, translocation to secondary target organs, etc. [10]. Some of the in vivo and in vitro studies have already highlighted the toxic effects of silica, gold, silver, and titanium nanoparticles. Since the size of the nanoparticles used in these studies is similar to the size used in biomedicine, it becomes imperative to assess the toxicological parameters. This review tries to highlight toxicity studies for some of the commonly used nanoparticles in drug delivery (Figs. 1 and 2).
Fig. 1.
Biomedical applications of nanotherapeutics
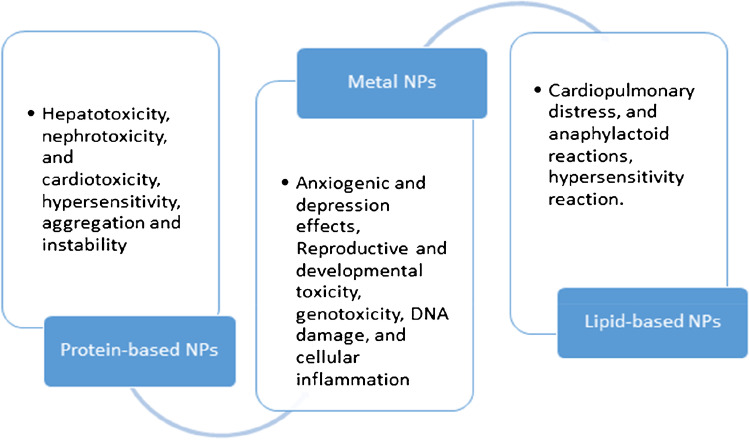
Fig. 2.
Toxicity recorded by protein-based nanoparticles, lipid-based nanoparticles, and metal nanoparticles
Carbon-Based Nanoparticles
Nanoparticle-based drug formulations have become prominent in recent years due to their properties of targeted and sustainable drug release, enhanced solubility of the drug, and minimum or no adverse drug reactions. Among numerous nanocarriers, carbon nanotubes have been one of the major focuses because of their wide application in cancer therapies [11]. However, the application of carbon nanotubes has been also expanded to non-anticancer drugs, either as a carrier or as an adjuvant [12]. Carbon nanotubes are made from graphene sheets. These sheets are rolled into different shapes (such as ellipsoid, hollow, sphere, and tubes) and sizes. The carbon nanotubes are majorly classified into two types that are SNWCT (single-walled carbon nanotubes) and MWCNT (multi-walled carbon nanotubes) [11, 13, 14]. The advanced physical, mechanical, and chemical properties of CNT raise some concern among researchers when used in drug delivery systems. This is because CNTs can move across the cell membranes while commuting the carrier biomolecule. Therefore, understanding the cytotoxicity of CNTs becomes very crucial [15].
Studies have shown that CNTs can cause neurotoxicity, pulmonary toxicity, immune toxicity, embryotoxicity, genotoxicity, hepatotoxicity, and cardiovascular toxicity [16–22]. Gholamine et al. studied the toxicological impact of SWNTs and MWCNT in mice. The mice were randomized into five groups and injected with MWCNT and SWNTs suspended in PBS (phosphate-buffered saline). After 2 weeks of observation, mice of each group were evaluated for a brain factor known as the brain-derived neurotrophic factor (BNDF). The factors support overall neuron health by promoting sprouting, improving survival, as well as controlling other aspects such as memory, learning, locomotion, and more. SWNTs and MWCNT showed anxiogenic and depression effects, while also impacting the gene expression of BNDF [16]. Fujita et al. conducted studies to understand the in vitro and in vivo pulmonary toxicity caused by SWNTS. It was found that SWNTS induced inflammation and slower recovery and enhanced the expression of genes governing cell proliferation, inflammation, and reactive oxygen species generation However, the level of toxic impact is dependent on the size of SWNTs used [23]. Reproductive and developmental toxicity of CNTs was observed in mice after intravenous administration. Fetal malformations, miscarriages, and teratogenic and high levels of ROS were some of the observations [24]. A study by Shang et al. highlights damage caused to the kidney and brain by SWCNTs by promoting ROS in mice [25]. Zeinabad et al. studied the CNTs interaction with tau protein and PC12 cells. Results showed that both MWCNT and SWCNT induced different modes of cell death pathway. Moreover, CNTs led to neurotoxicity and protein conformational changes [26••]. Cao et al. mention about the toxicological impact of CNT to the vascular system, wherein they induce formation of atherosclerotic plaque, poor heart rate, and vasomotor dysfunction among animal models [27]. CNTs have been used effectively to deliver anti-tumor drug paclitexal in animal models. The study showed significant tumor suppression and effective release of the drug with minimal side effects. However, the study lacks any toxicity assessment for short-term or long-term period [28].
Metal-Based Nanoparticles
Superparamagnetic iron oxide (SPIONS) is one of the FDA-approved nanoparticles, used widely in diagnostics as well as therapeutic application (hyperthermia, magnetic drug delivery, treatment of anemia) [29]. However, the recent years have observed expansion of SPIONs as potential drug delivery systems to treat cancer and other diseases [30]. But, the concerns related to toxicity of SPIONS have somehow limited their use until their property is enhanced. This is the reason that some of the previously FDA-approved SPIONS for use in medical imaging have been pulled off concerning their toxicity issues and anaphylactic reactions. Existing studies have demonstrated challenges like ROS, LDH leakage, inflammation, DNA damage, and alteration in mitochondrial functioning [31–33]. Currently, Feraheme™ or ferumoxytol of AMAG pharmaceuticals is the only approved drug used in anemic patients suffering from chronic kidney disease (CKD) [34–36]. Umirem, Gastromark, and Feridex are some of the other FDA-approved SPIONs used as imaging agents [34, 37].
SPIONs are also known to impact the complement system leading to innate immunity-related side effects. One such SPION-based drug known to alter the complement system is Feridex™, which is now discontinued by FDA [38–40]. Another concern with use of SPIONs is they show well-known Haber–Weiss and Fenton reactions as the free ions (Fe2 +) released after lysosomal ingestion react with oxygen or hydrogen peroxide in the powerhouse of cell (mitochondria), forming free radicals which are responsible for genotoxicity, DNA damage, and cellular inflammation [41, 42].
Some of the recent studies have highlighted that the toxicity of SPIONs can be reduced by use of coating agents. Polymers like polyethylene glycol (PEG), poly vinyl (PV), dextran, and chitosan can enhance the half-life and stability of SPIONs and prevent aggregation within cells [42, 43]. Recently, Ferreti et al. evaluated the biocompatibility of magnetic particles. The study finds that when magnetic iron nanoparticles are coated with zwitter-ionic ligands, they showed good stability and biodistribution and required levels of renal clearance [44]. Toxicity of SPIONs is a critical issue which needs to be considered before its extensive use as a drug delivery system. Though lots of research have been carried out to understand the toxicity relation, it is crucial to dig deeper to enhance their property further. This will lead to more safe use of SPION nanoparticles and FDA approval for drug-based purposes. Similar to SPIONs, other metal nanoparticles like titanium, gold, silver, and zinc have been used for drug delivery but showed significant toxicity.
Lipid-Based Nanoparticles
Alec D Bangham discovered liposome back in the 1960s at the University of Cambridge (Babraham Institute). Liposomes are small and spherical structures forming an enclosed compartment, composed of phospholipid layers. The cavity formed at the center due the interaction between hydrophilic head and hydrophobic tail allows them to act as amphipathic nanocarriers for a wide range of drugs in therapies [45, 46]. About more than 40 liposome-based drugs are either approved for use in the market or are undergoing clinical trials [47, 48]. This increases interest in understanding the interaction of liposomes within the living system and so does their toxic effects. Upon entry into the living system, liposomes interact with a wide range of biomolecules such as LDL, HDL, and opsonins. The encounter with opsonins leads to activation of RES recognition, which further tries to eliminate it from the system. Other lipoproteins in the blood are known to reduce the stability of liposomes within the biological system by leading to rearrangement of surface lipids. This is one of the major concerns when using liposomes as a drug delivery system [49]. To overcome this challenge, liposomes are PEGylated as they prevent opsonization and support evasion from phagocytic cells [47, 50]. Doxil® is an FDA-approved PEGylated liposome-based drug used for treatment of tumor [51]. However, Gabizon et al. highlighted that encapsulated doxorubicin alters particle clearance and phagocytic functioning of cells in the liver. Recently, FDA has approved emergency use of two lipid nanoparticle-based mRNA COVID vaccines of Pfizer-BioNTech, proven to be 95% effective [52, 53]. However, a major challenge of vaccine instability has been observed. Adjustments of lipid nanoparticle structure would be necessary to enhance and improvise the vaccine stability.
In 2018, FDA EC approved another lipid RNAi-based drug named ONPATTRO by Alnylam Pharmaceuticals for diseases caused due to altered TTR (Transthyretin) protein. Risk and safety studies associated with the drug use indicate infusion-related reactions, which can be reduced with vitamin A supplementation and premedication with certain drugs like antihistamines and corticosteroids. Some of the common adverse reactions observed with use of drugs include respiratory symptoms, headache, and change in blood pressure. However, there are limited studies to understand the use of this drug among pregnant women, patients with renal issues, etc. [48, 54, 55].
Some of the PEG-liposomal formulations are negatively charged; this often leads to complement activation, hypersensitivity reactions (HSRs), cardiopulmonary distress, and anaphylactoid reactions. Hoven et al. studied the impact of negatively charged PEGylated liposome nanocarriers. Liposomes with variations in PEG in terms of liposomal size, chain length, surface concentration, and anchor molecule were done. While other variations caused no to mild effects, PEG anchored with cholesterol showed maximum complement activation [56]. Another similar study is conducted by Szebeni et al., where they evaluated the doxil and hynic PEG liposomes for complement activation and hypersensitivity [57].
Protein-Based Nanoparticles
Protein-based nanoparticles have received much attention in recent times due to their biocompatibility, amphiphilicity, easy biodegradability, and reduced toxicity. Albumin, gelatin, ferritin, fibroin, and casein are some of the widely used protein-based nanoparticles for drug delivery [58].
Human serum albumin is a flexible protein that is used to make albumin-based nanocarriers (ANCs) for the administration of cancer treatments [59, 60]. Because of the various drug binding sites found in the albumin molecule, a large amount of drug can be integrated into the particle matrix with serum albumin nanoparticles [61]. HSA may be a valuable analytical indicator for people with autoimmune diseases. Thanks to breakthroughs in biotechnology and protein science, many albumin-associated pharmaceutical preparations, such as nab-paclitaxel (Abraxane®), albumin nanoparticles manufactured utilizing nab technology, are already on the market [62]. The FDA has also approved a paclitaxel formulation (Abraxane). Abraxane differs from previous paclitaxel formulations in that it is attached to albumin particles, which confer water solubility; previous formulations had to be administered in the solvent Cremophor®, which raised allergic reactions and limited dosing [63].
While looking at the preferred outcomes, we must not ignore the drawbacks. Albumin NPs have been claimed to be unstable and extremely damaging to healthy cells. To overcome this and to lengthen their half-life in an aqueous environment and/or limit the development of protein macro-aggregates, the freshly synthesized albumin NPs must be stabilized or cross-linked. Thermal treatment, high hydrodynamic pressure, and enzymatic cross-linking using genipin or transglutaminase are some of the ways that can be used to stabilize protein nanoparticles. Glutaraldehyde cross-linking is one of the most commonly utilized ways for stabilizing albumin nanoparticles in general [64]. Steinhauser et al. used heat denaturation of HSA to produce particles loaded with ASOs and found out that HSA nanoparticles cross-linked with glutaraldehyde exhibited no toxicity in cell culture at concentrations of up to 5 mg/ml [65].
Langer [66], used PH and buffer titration studies were used to assess the stability and electrical behavior of the HSA nanoparticles, with a typical titration profile of prepared nanoparticles throughout a pH range of 3 to 10. The HSA nanoparticles’ isoelectric point (pI) was calculated to be 5.05. The nanoparticles became unstable at pH values around pI, as evidenced by PCS: particle diameter increased from 250 nm to roughly 2.7 m (Langer, 2003). Even at higher pH values when the nanoparticles had a strong surface charge, the particle aggregation remained largely irreversible. They concluded that pH values that lead to neutral particle surface charges should be avoided when handling protein-based nanoparticles. In an HSA nanoparticle suspension, the pH value, as well as the buffer content, was found as critical criteria for particle stability [66].
Albumin nanocarriers are used in the treatment of a variety of disorders in addition to cancer. HSA-coupled TRAIL delivery, according to Byeon et al., could be a promising therapy for rheumatoid arthritis (RA), a chronic autoimmune disease marked by extreme synovial hyperplasia and joint destruction. They also found that rats given CLT-encapsulated HSA nanoparticles had milder hepatotoxicity, nephrotoxicity, and cardiotoxicity than rats given CLT alone, according to serum analysis and histopathological examination [67].
Mesken et al. recently released a study on plasmid transmission in HEK 293 T cells loaded onto HSA nanoparticles coated with cell-penetrating peptide (CPP). They were prepared using the desolvation method. There was little cytotoxicity and no significant change in performance when nanoparticle-mediated transfection was examined at low plasmid concentration levels [68]. Even though numerous studies and tests have been conducted to assess the efficiency of HSA nanocarriers and their potentially hazardous effects at various doses, sufficient evidence suggesting the detrimental toxicity of this nano-carrier is still lacking.
Gelatin nanoparticles have recently been offered as a feasible option for parenteral formulations due to their low cost, biocompatibility, biodegradability, minimal antigenicity, and application in a variety of formulations [69]. The US Food and Drug Administration considers gelatin to be a natural, biodegradable, and biocompatible substance that can be utilized to deliver resveratrol, cycloheximide, doxorubicin, and other medications for tumor therapy [70].
In terms of nanoparticle formation, gelatin has low mechanical strength and a fast breakdown rate. To boost mechanical strength and minimize the rate of breakdown and solubility in aqueous solutions, gelatin nanoparticles must be physically, physiologically, or chemically cross-linked with various cross-linking agents, such as GA [71]. In comparison to uncross-linked particles, cross-linking of GNPs is necessary to provide stability, spherical shape, and increased in vivo circulation time. GNPs that have not been cross-linked have been discovered to be unstable and to agglomerate during storage. Furthermore, substantial applications of GNPs as a drug/vaccine delivery vehicle have been explored in a variety of sectors. However, there is still a serious issue with the usage of animal-derived gelatin, which poses a danger of infection with transmissible spongiform encephalopathy [72].
Experiments on the toxicity of GNPs after administration have been carried out. In a study, paclitaxel-loaded gelatin nanoparticles were used to treat dogs with intravesical bladder cancer, and they achieved a 2.6 higher drug concentration in tumors than control dogs given Cremophor EL with commercial paclitaxel injection. Intraperitoneal injections of doxorubicin-loaded glutaraldehyde cross-linked gelatin nanoparticles into rats were used in the experiment. The electrocardiograms and body weights of the animals were monitored for side effects, and the results revealed that control nanoparticles (no medication) had no toxicity. Although the anticancer drug’s potency was increased, repeated administration of the formulation (doxorubicin-loaded gelatin nanoparticles) revealed substantial cardiotoxicity [73].
In another study, healthy rats were exposed to doxorubicin-loaded gelatin nanoparticles that had been cross-linked by glutaraldehyde. The researchers discovered that linking DXR to gelatin nanoparticles boosted the drug’s cardiotoxicity in the lab. The significant toxicity of DXR-loaded nanoparticles might be due to the drug’s covalent binding to the carrier since DXR was attached to the protein matrix of nanoparticles through glutaraldehyde [74].
Gelatin nanofibers have also been proposed for wound healing processes in tissue engineering. Although electrospun pure gelatin nanofibers have been successfully developed, their poor mechanical characteristics and quick disintegration profile have limited their use in wound healing. Many people have tried to combine gelatin with other natural and synthetic polymers to take advantage of gelatin’s excellent biocompatibility while increasing the mechanical and physical qualities of nanofibers [75].
Ferritin has unique qualities in various disciplines, such as restricted synthesis, nanodevices, disease detection and therapy, drug delivery, vaccine development, and bioassay. These qualities make ferritin a suitable and powerful nanoplatform [76]. Given its presence not just within every cell of the human body but also in the extracellular space and circulating plasma, the iron storage protein ferritin is likely the greatest prospect for therapeutic use among NPs. The production of ferritin nanoparticles in microorganisms necessitates time-consuming purifying procedures. As a result, producing ferritin nanoparticles in large quantities is challenging. Furthermore, ferritin nanoparticles’ high drug loading capacity is limited by their tiny size [77].
The most widely studied technique for anticancer drug delivery is doxorubicin (DXR) encapsulation in ferritin nanocages. DXR is a commonly used cytotoxic medication that has good anticancer activity and is used to treat a variety of solid cancers. However, because it is linked with numerous significant toxicities when supplied at large dosages, its usage is dose-limited. This tailored nanoformulation enhances the drug tumor uptake and accumulation, tumor growth suppression, and circulation half-life and decreased DXR cardiotoxicity [78].
In the experiment conducted by Todd et al., an exceptional tumor inhibition rate of 83.64 percent was re-counted when a genetically improved ferritin derivative apoferritin (RFRTs) was photo-irradiated by a 671-nm laser. Toxicity of the skin is one of the most prevalent side effects of photodynamic treatment; with this RFRT formulation, they found low toxicity to the skin and other tissues [79].
Although ferritin-based drug delivery has been employed for cancer therapy, ferritin was nearly always supplemented with recognition ligands to accomplish tumor-specific targeting in practically all published studies. It causes problems because these additional surface changes undermine the inherent tumor-specific binding of natural ferritin and disrupt its in vivo performance and biocompatibility due to the changed surface physicochemical characteristics of ferritin nanoparticles. Furthermore, many existing approaches for drug loading into ferritin include dismantling ferritin nanocages under a harsh acidic pH, which irreversibly destroys ferritin protein cages and causes harm to the spherical protein surface. Ferritin’s irreparable destruction will significantly impact their in vivo stability and medication delivery efficiency [80].
Despite the fact that numerous researches, in their clinical trials, have revealed that this NP can decrease the harmful effects of cytotoxic medications, there is little information on its harmful effects. Before the probable application of this substance in humans, the safety profile, including pharmacology and toxicology, should be thoroughly examined. The development of gliadin NPs has benefitted medication delivery and controlled release applications [81]. Although the desolvation approach can be used to make gliadin particles, it has significant drawbacks, such as limited drug loading efficiency and the difficulty to separate particles from the aqueous phase [82]. Antisolvent precipitated gliadin NPs are vulnerable to pH, heating, and salt effects, resulting in aggregation and instability. They were particularly susceptible to aggregation at pH 6.0–7.0, with a rapid rise in particle size and even the formation of a precipitate [83].
Furthermore, when it comes to achieving the desired outcome with the usage of gliadin nanoparticles while avoiding serious side effects, it is critical to pay attention to the dosage. TIMP-GLIA tolerance was investigated in mice after immunization by Freitag et al. TIMP-GLIA was given several hours after priming (day 0) and again after immunization on day 7. While 0.025 mg/mouse (1.25 mg/kg) therapy failed to show effect, dosages of 0.25–2.5 mg/mouse (12.5–125 mg/kg) were linked to significant reductions in ear edema when compared to controls. Experiments using gliadin recall also revealed a dose-dependent impact. T cell proliferation was unaffected in spleen cells from animals given 0.025 mg/mouse TIMP-GLIA, but two infusions of 0.25–2.5 mg/mouse/dose resulted in a considerable reduction of T cell growth. TIMP-GLIA had no observed adverse impact level of 75 mg/kg, according to the study [84].
Multiple studies have been conducted to determine the stability of GNPs [85]. In line with the research, the gliadin nanoparticle suspensions showed fair stability to aggregation and sedimentation over a very slim pH range that is from pH 4.5 to 6.0, when exposed to a selection of food processing conditions. Short-term thermal handlings at temperatures above 40 °C disrupted particle suspensions, increasing the size of the particle and sedimentation. The increased hydrophobic attraction between the protein particles and/or the increased particle collision frequency may have caused the instability seen at higher temperatures. The protein nanoparticles’ poor aggregation stability in pH, salt, and temperature conditions often encountered in food products would be a serious issue for their use in many real-world food systems. As a result, more tactics are needed to improve aggregation stability. Coating gliadin nanoparticles with pectin is a viable method of creating stable functional ingredients, particularly for application in the food sector [86].
The creation of fibroin nanoparticles (FNPs) for a variety of biomedical applications has recently received a lot of attention. FNPs can encapsulate a variety of medicinal chemicals, including small and large molecules, proteins, enzymes, vaccines, and genetic elements, due to their versatility and chemical modifiability [87]. Pandey et al. used the MTT assay to assess the cytotoxicity of the medication containing silk fibroin nanoparticles on the rat and human glioblastoma cell lines C-6 and LN-229, respectively. They also calculated the capacity of free and drug-bearing nanoparticles to impede cell growth. SFN-DXR had an IC50 value of 3.68 M and 1.44 M for 24 and 48 h against C-6 rat glioma cell lines, respectively, which was lower than DXR. Similarly, when TSFN (Tween 80–coated silk fibroin nanoparticles) with DXR is compared to free DXR, the TSFN with DXR has a considerably larger cytotoxicity impact [88].
Similarly, Mishra et al. used solvent precipitation procedures to make curcumin-loaded SF nanoparticles and tested their efficiency in triggering apoptosis in a metastatic breast cancer cell line in vitro. Curcumin release from nanoparticles indicated an initial burst release of more than 50% in the first 24 h, with curcumin release continuing for up to 7 days. Although lower concentrations of curcumin-loaded SF nanoparticles seemed to be less hazardous after 96 h of treatment compared to 24 h of therapy, prolonged exposure to greater concentrations of curcumin-loaded SF nanoparticles resulted in noteworthy cell viability reductions [89].
Several previous studies have shown that fibroin nanoparticles can be used as a nanocarrier to minimize toxicity and improve the bioavailability of many medications. Although the silk fibroin protein nanoparticles have many advantages, they also have certain drawbacks. Dong et al. employed HPLC to measure the amount of free and encapsulated ibuprofen in liposomes after separation and learned that silk fibroin-coated liposomes had a lower drug encapsulation efficiency than liposomes. It was found out that the presence of SF reduced drug encapsulation, which could be the result of SF hydrophobic chains being inserted into lipid bilayers, causing breaking in the liposomes’ outer layers, resulting in medication leakage following methanol treatment. The drug release rate was positively associated with SF dissolving time, but negatively with SF concentration, according to the results of an in vitro release investigation [90].
The delayed degradation of the fibroin crystalline antiparallel sheet domain has also been reported to be a drawback in some applications that need the removal of nanoparticle carriers quickly and completely. Furthermore, because fibrin is a protein, it can be attacked by immune systems such as macrophages and giant cells, resulting in encapsulation and granuloma development inside these cells, which can lead to drug release outside the target. Finally, while fibroin can be extracted from a variety of sources, comprising those that are similar to other natural products, the nature of each batch may differ slightly due to post-conversion process alterations in both species and entities [91].
Casein is a fascinating protein because it has excellent structural and physicochemical features that make it ideal for drug delivery. Gandhi et al. made casein nanoparticles with doxorubicin, an anticancer medicine. The team used fluorescence studies with varied quantities of DXR combined with a 2% casein solution to maximize DXR binding to casein. Casein NP was found to be an efficient drug delivery carrier at both physiological and acidic pH levels [92].
Microspheres made of hydrophobic proteins, casein, may easily encapsulate hydrophobic pharmaceuticals like progesterone, and the release rate and amount of drug molecules may be regulated by adjusting the glutaraldehyde-to-protein ratio and particle size [89, 93].
Pealva et al. explored the use of casein NPs as resveratrol transporters. Once the nanoparticles were created by coacervation, they were then refined and spray-dried. The mean size of NPs was calculated to be roughly 200 nm with a resveratrol payload of roughly 30 g/mg. The experiments that took place in vitro indicated that the release of resveratrol from casein nanoparticles did not show any effect by the pH and followed a kinetic of zero order. When resveratrol was loaded with casein nanoparticles, its oral bioavailability resulted in 10 times greater than the polyphenol delivery as an oral solution, thus proving the hypothesis of improved efficacy of casein nanoparticles as oral carriers [94].
Toxicity and therapeutic efficacy such as the IC50, LD50, and EC50 of the casein can be evaluated by standard pharmaceutical techniques. A 3-month dose-repeated toxicity test was conducted to examine the safety of casein nanoparticles when administered orally to animals. It was reported that the males and females treated with the maximum dose of 500 mg/Kg bw developed comprehensive hyperchloremia after almost a month. A few cases of hypernatremia in the females were also reported. It was concluded that the formation of drug aggregates with casein nanoparticles results in highly localized concentrations at the sites of deposition that are associated with local toxicity [95]. This study indicates the side effects of protein-based nanoparticles, casein, although further studies are required for the detailed analysis of casein nanoparticle’s toxicity and adverse effects.
Polymeric Nanoparticles
Polymeric nanoparticles are made from natural or synthetic polymers and are especially evident in smart drug delivery. They are being studied extensively in the pharmaceutical industry as carriers for controlled/sustained release in medication delivery systems. Safety difficulties, toxicity threats, inadequate biocompatibility, and physiological challenges all contribute to the activity of these nanopharmaceuticals being limited [96]. The downside of these nanoparticles is toxic monomer aggregation, residual material linked with them, and toxic degradation process [97].
Polymeric NPs’ toxicity is affected by quantum size effects, which are linked to oxidative stress, cytotoxicity, and genotoxicity [98]. Upon investigation of the toxicity of a variety of poly(lactide-co-glycolic acid) (PLGA) nanoparticles on human-like THP-1 macrophages, Nadège et al. found that the slightly cytotoxic chitosan polymer conferred significant cytotoxicity to PLGA nanoparticles when used as a nanoparticle stabilizer. Poly vinyl alcohol and poloxamer 188 polymers also conferred significant cytotoxicity to PLGA nano. These findings revealed that stabilizers employed in the formulation of PLGA nanoparticles have a significant toxicological role when used at high concentrations, which could have consequences for local toxicity of PLGA-based nanomedicine [99].
The differential lung toxicity of positively and negatively charged PEG-polylactic acid (PLA) nanoparticles was explored following daily endotracheal instillation of BALB/c mice while examining the impacts of nanoparticle surface charge for polymeric particles. PEG-PLA nanoparticles based on cationic stearylamine induced higher local and systemic harmful effects [100]. Synthetic nanoparticles and organic solvents that have been demonstrated to be harmful are employed to manufacture these nanoparticles. Natural polymers and synthesis methods using less harmful solvents have been investigated as potential alternatives to synthetic polymers [101]. Because of their poor circulation stability and targeting inefficiency, using polymeric NPs as chemotherapeutic drug delivery vehicles is generally difficult [102]. Multiple experiments have been going on in order to address these issues such as Palanikumar et al. developed biocompatible and biodegradable pH-responsive hybrid NPs. A cross-linked bovine serum albumin shell was added to these nanosystems based on a drug-loaded PLGA core to decrease interactions with serum proteins and macrophages. As a result, the drug-loaded NPs demonstrated strong anticancer action both in vitro and in vivo while causing no harm to healthy tissue [103].
Silica Nanoparticles
Properties like ease of surface modification, synthesis, tunable pore size, efficient thermal stability, and biocompatibility make silica nanoparticles a great choice for drug delivery [104, 105]. Recently, silica nanoparticles have been used to deliver cargos like anti-cancer drugs (camptothecin and doxorubicin in breast cancer treatment), nucleic acid, and proteins (e.g., RGD peptide and aptamers) via controlled released method [106–108]. The growing application often demands assessment of potential risks of toxicity associated with the nanoparticles. Some studies have highlighted the toxicity of silica nanoparticles which leads to lung diseases and neurotoxicity and impacts the immune system. The physicochemical properties of silica nanoparticles are known to induce toxicity at cellular level. In a study by Bancos et al., the impact of silica nanoparticles on macrophages was evaluated using the RAW 264.7 cell line. Exposure to 10-nm silica nanoparticles led to reduced phagocytosis and impacted cytokine production [109]. Gonzalez et al. identified that silica nanoparticles induce cytotoxic effects on lung carcinoma cells [110]. Similar studies by Yang et al. highlight cytotoxicity in human vascular endothelial cells due to silica nanoparticles [111]. Micronucleus assay and in vitro comet-based analysis have been carried out in some studies to understand the damage caused by silica nanoparticles at DNA level. It was observed that silica nanoparticles show dose-dependent genotoxic effects in these studies on lung cells [112]. In another study, Lucarelli et al. used U-937 cells and ELISA-based assay to evaluate the change in response to silica nanoparticles; they observed M1 polarization and altered IL-1 beta and TNF-alpha production [113]. Xifei et al.’s study highlights that silica nanoparticles lead to increased levels of reactive oxygen species and depletion of glutathione at intracellular levels [114]. Thus, it is very important to evaluate and regulate the toxicity assessment of nanoparticles before use in humans.
Challenges and Future Prospects
The field of nanotechnology has seen remarkable improvement post-1980 as many nano-based drug delivery products have been approved in the market. However, a range of challenges are faced before these nano-based agents enter into the market which include efficient development of nano-carriers, toxicity assessment, and ethical and regulatory requirements. The safety and risk ratio both to the patient and to the manufacturer is not yet clearly defined. The risk/benefit ratio itself faces challenges in laying the details as a strong framework for evaluation has not been demarcated. For nano-based products to be used, it is very crucial that the pre-clinical and clinical testing guidelines be set in detail. Nanoscience has recently stepped in drug delivery and understanding of toxicity, pharmacology, and immune responses of these nano-products still need a lot of deep research.
One common challenge in defining the safety and risk of nanoparticles is that they have different properties depending upon their size, surface area, and surface charge, making them difficult to categorize the risks. Moreover, nanoparticles can move within the biological system easily due to their effective biocompatibility. They can cross cell membranes, placenta, blood–brain barrier, and more, thus increasing the chances of non-specific interaction, which can eventually lead to unwanted accumulation and toxicity at cellular level. Clinical trials to assess these risks can be of great advantage, but the current scenario faces challenges of efficient risk assessment, risk management, and risk communication. Sometimes, extrapolation of animal-based studies into humans can be detrimental. For example, a study involving monoclonal antibodies showed severe illness among the human subjects, even though the results were promising in animal subjects. Learning from these kinds of cases needs to be considered when working with nanomedicines. Post-clinical trials, the side effects need to be informed to the respective safety agency to avoid potential risks.
Despite the above challenges, it can be anticipated that the efficiency of nanomedicines will make their demand grow further in coming years. The application of nano-carriers in cancer treatment has attracted the eye of many researchers, which becomes more evident from the fact that most of the FDA-approved nano-based drugs were cancer related. All in all, the coming years will see a rise in the application of nano-based drug delivery agents for a variety of diseases, and there will be significant growth in the market size of nanomedicines.
Conclusion
Over decades, nanoparticles have been considered to be a wonder of modern science due to their wide applications. The use of nanoparticles in biomedicine has very well marked that standard, as superior therapeutic systems have emerged, helping us conquer various diseases and enhance the quality of human life. However, there still exist many challenges to effectively utilize the full potential of nanoparticles. Toxicity of nanoparticles is one the major barriers that need urgent attention. The safety assessment is vital for use in living beings to recognize potential risks and create preventive measures. As we have seen, there is very limited data available for certain types of nanoparticles used in drug delivery. Therefore, before pushing nanoparticles into the therapeutic market, it is crucial to assess the risk to benefit ratio. Another reason is that the property of nanoformulations within a biological system is yet to be studied in great detail. This has made the regulatory authorities take necessary steps which emphasizes understanding the safety and toxicity concerns associated with nanoparticles used in drug delivery systems. Thus, toxicological studies are needed to provide a more detailed analysis of nanoparticles used in drug delivery. It will help researchers to enhance the properties of nanoparticles further to reduce the toxicity and create nanoformulations which are effective but also non-toxic for therapeutic use.
Abbreviations
- DXR
Doxorubicin
- HSA
Human serum albumin
- RA
Rheumatoid arthritis
- CLT
Celastrol
- CPP
Cell-penetrating peptide
- GNPs
Gelatin nanoparticles
- RFRTs
Ferritin derivative apoferritin
- TIMP-GLIA
Tolerogenic immune-modifying nanoparticles containing gliadin
- FNPs
Fibroin nanoparticles
- SFN-DXR
Silk fibroin nanoparticle bearing doxorubicin
- TSFN
Tween 80–coated silk fibroin nanoparticles
- SF
Silk fibroin
- HPLC
High-performance liquid chromatography
- IC50
Half maximal inhibitory concentration
- LD50
Median lethal dose
Declarations
Conflict of Interest
Swati Sharma, Roza Parveen, and Dr. Biswa Prasun Chatterji declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Nanoparticle-based Drug Delivery.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Swati Sharma, Email: vatsyaswati@gmail.com.
Roza Parveen, Email: rozaparveen786@gmail.com.
Biswa Prasun Chatterji, Email: biswaprasun.chatterji@adypu.edu.in.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
- 1.Khana I, Saeed K, IdreesKhan. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2019; 908–93. 10.1016/j.arabjc.2017.05.011.
- 2.Mehta RV. Synthesis of magnetic nanoparticles and their dispersions with special reference to applications in biomedicine and biotechnology. Mater Sci Eng C. 2017;79:901–916. doi: 10.1016/j.msec.2017.05.135. [DOI] [PubMed] [Google Scholar]
- 3.Zahin Nuzhat, Anwar Raihanatul, Tewari Devesh, Kabir MT, et al. Nanoparticles and its biomedical applications in health and diseases: special focus on drug delivery. Environ Sci Pollut Res. 2019;27:19151–19168. doi: 10.1007/s11356-019-05211-0. [DOI] [PubMed] [Google Scholar]
- 4.Hasan A, Morshed M, Memic A, Hassan S, et al. Nanoparticles in tissue engineering: applications, challenges and prospects. 2018:13: 5637–5655. 10.2147/IJN.S153758. [DOI] [PMC free article] [PubMed]
- 5.Prasad M, Lambe UP, Brar B, lShah I, et al. Nanotherapeutics: an insight into healthcare and multi-dimensional applications in the medical sector of the modern world 2018:97: 1521–1537. 10.1016/j.biopha.2017.11.026. [DOI] [PubMed]
- 6.Nikolova M, Chavali M. Metal oxide nanoparticles as biomedical materials. Biomimetics. 2020;5(2):27. doi: 10.3390/biomimetics5020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patra J, Das G, Fraceto L, Campos E, Rodriguez-Torres M, Acosta-Torres L, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 2018;16(1). 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed]
- 8.De Jong WH, Borm PJA. Drug delivery and nanoparticles: applications and hazards. Int J Nanomed. 2008;3(2):133–149. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasut G. Grand challenges in nano-based drug delivery. Front Med Technol. 2019;1(1). 10.3389/fmedt.2019.00001. [DOI] [PMC free article] [PubMed]
- 10.Faria M, Björnmalm M, Thurecht KJ, Kent SJ, Parton RG, et al. Minimum information reporting in bio–nano experimental literature. Nat Nanotechnol. 2018;13:777–785. doi: 10.1038/s41565-018-0246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan H, Xue Z, Xie J, Dong Y, Ma Z, Sun X, et al. Toxicity of carbon nanotubes as anti-tumor drug carriers. Int J Nanomed. 2019;14:10179–10194. doi: 10.2147/IJN.S220087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong B, Yoong S, Jagusiak A, Panczyk T, Ho H, Ang W, et al. Carbon nanotubes for delivery of small molecule drugs. Adv Drug Deliv Rev. 2013;65(15):1964–2015. doi: 10.1016/j.addr.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Singh B, Lohan S, Sandhu P, Jain A, Mehta S. Functionalized carbon nanotubes and their promising applications in therapeutics and diagnostics. Nanobiomater Med Imaging. 2016;455–478. 10.1016/B978-0-323-41736-5.00015-7.
- 14.Saifuddin N, Raziah A, Junizah A. Carbon nanotubes: a review on structure and their interaction with proteins. J Chem. 2013:1–18. 10.1155/2013/676815.
- 15.Jin H, Heller D, Strano M. Single-particle tracking of endocytosis and exocytosis of single-walled carbon nanotubes in NIH-3T3 cells. Nano Lett. 2008;8(6):1577–1585. doi: 10.1021/nl072969s. [DOI] [PubMed] [Google Scholar]
- 16.Gholamine B, Karimi I, Salimi A, et al. Neurobehavioral toxicity of carbon nanotubes in mice. Toxicol Ind Health. 2017;33:340–350. doi: 10.1177/0748233716644381. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Zheng X, Nicholas J, et al. Single-walled carbon nanotubes modulate pulmonary immune responses and increase pandemic influenza virus titers in mice. Virol J. 2017;14:242. doi: 10.1186/s12985-017-0909-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park EJ, Choi J, Kim JH, et al. Subchronic immunotoxicity and screening of reproductive toxicity and developmental immunotoxicity following single instillation of HIPCO-single-walled carbon nanotubes: purity-based comparison. Nanotoxicology. 2016;10:1188–1202. doi: 10.1080/17435390.2016.1202348. [DOI] [PubMed] [Google Scholar]
- 19.Lee S, Khang D, Kim S-H. High dispersity of carbon nanotubes diminishes immunotoxicity in the spleen. Int J Nanomed. 2015;10:2697–2710. doi: 10.2217/nnm.15.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bottini M, Bruckner S, Nika K, et al. Multi-walled carbon nanotubes induce T lymphocyte apoptosis. Toxicol Lett. 2006;160:121–126. doi: 10.1016/j.toxlet.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Larner SF, Wang J, Goodman J, et al. In vitro neurotoxicity resulting from exposure of cultured neural cells to several types of nanoparticles. J Cell Death. 2017;10:1179670717694523. doi: 10.1177/1179670717694523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aragon MJ, Topper L, Tyler CR, et al. Serum-borne bioactivity caused by pulmonary multiwalled carbon nanotubes induces neuroinflammation via blood-brain barrier impairment. Proc Natl Acad Sci U S A. 2017;114:E1968–E1976. doi: 10.1073/pnas.1616070114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujita K, Fukuda M, Endoh S, Maru J, Kato H, Nakamura A, et al. Size effects of single-walled carbon nanotubes on in-vivo and in-vitro pulmonary toxicity. Inhalation Toxicol. 2015;27(4):207–223. doi: 10.3109/08958378.2015.1026620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi N, Izumi H, Morimoto Y. Review of toxicity studies of carbon nanotubes. J Occup Health. 2017;59(5):394–407. doi: 10.1539/joh.17-0089-RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shang S, Yang S, Liu Z, Yang X. Oxidative damage in the kidney and brain of mice induced by different nano-materials. Frontiers in Biology. 2015;10(1):91–96. doi: 10.1007/s11515-015-1345-3. [DOI] [Google Scholar]
- 26.•• Kavosi A, Hosseini Ghale Noei S, Madani S, Khalighfard S, Khodayari S, Khodayari H et al. The toxicity and therapeutic effects of single-and multi-wall carbon nanotubes on mice breast cancer. Sci Reports. 2018;8:8375. 10.1038/s41598-018-26790-x. In this study, Researchers evaluated the toxicology of SWCNTs and MWCNTs and a typical animal model of breast cancer in order to gain insights into the effects of CNTs on MC4L2 cells and mice. As a result, this study has high scientific value since the authors address the toxicity of carbon nanotubes to humans at high doses as CNTs at high doses cause inflammation in the liver and spleen. In addition, they also address the cardiovascular and neurotoxicity of carbon nanotubes. Using SWCNTs as a model for carbon nanoparticles, the paper briefly. [DOI] [PMC free article] [PubMed] [Retracted]
- 27.Cao Y, Luo Y. Pharmacological and toxicological aspects of carbon nanotubes (CNTs) to the vascular system: a review. Toxicol Appl Pharmacol. 2019;385:114801. doi: 10.1016/j.taap.2019.114801. [DOI] [PubMed] [Google Scholar]
- 28.Liu Z, Chen K, Davis C, Sherlock S, Cao Q, Chen X, et al. Drug delivery with carbon nanotubes for in vivo cancer treatment. Can Res. 2008;68(16):6652–6660. doi: 10.1158/0008-5472.CAN-08-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madkour LH, et al. Toxicological considerations of clinically applicable nanoparticles. Nucleic Acids Gene Anticancer Drug Deliv Ther. 2019:425–483. 10.1016/b978-0-12-819777-6.00019-6.
- 30.Bakhtiary Z, Saei A, Hajipour M, Raoufi M, Vermesh O, Mahmoudi M. Targeted superparamagnetic iron oxide nanoparticles for early detection of cancer: possibilities and challenges. Nanomed Nanotechnol Biol Med. 2016;12(2):287–307. doi: 10.1016/j.nano.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 31.Veranth J, Kaser E, Veranth M, Koch M, Yost G. Cytokine responses of human lung cells (BEAS-2B) treated with micron-sized and nanoparticles of metal oxides compared to soil dusts. Part Fibre Toxicol. 2007;4(1):2. doi: 10.1186/1743-8977-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Häfeli U, Riffle J, Harris-Shekhawat L, Carmichael-Baranauskas A, Mark F, Dailey J, et al. Cell uptake and in vitro toxicity of magnetic nanoparticles suitable for drug delivery. Mol Pharm. 2009;6(5):1417–1428. doi: 10.1021/mp900083m. [DOI] [PubMed] [Google Scholar]
- 33.Jeng H, Swanson J. Toxicity of metal oxide nanoparticles in mammalian cells. J Environ Sci Health A. 2006;41(12):2699–2711. doi: 10.1080/10934520600966177. [DOI] [PubMed] [Google Scholar]
- 34.Bobo D, Robinson K, Islam J, Thurecht K, Corrie S. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. 2016;33(10):2373–2387. doi: 10.1007/s11095-016-1958-5. [DOI] [PubMed] [Google Scholar]
- 35.Thakor A, Jokerst J, Ghanouni P, Campbell J, Mittra E, Gambhir S. Clinically approved nanoparticle imaging agents. J Nucl Med. 2016;57(12):1833–1837. doi: 10.2967/jnumed.116.181362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y. Current status of superparamagnetic iron oxide contrast agents for liver magnetic resonance imaging. World J Gastroenterol. 2015;21(47):13400. doi: 10.3748/wjg.v21.i47.13400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vakili-Ghartavol R, Momtazi-Borojeni A, Vakili-Ghartavol Z, Aiyelabegan H, Jaafari M, Rezayat S, et al. Toxicity assessment of superparamagnetic iron oxide nanoparticles in different tissues. Artif Cells Nanomed Biotechnol. 2020;48(1):443–451. doi: 10.1080/21691401.2019.1709855. [DOI] [PubMed] [Google Scholar]
- 38.Banda N, Mehta G, Chao Y, Wang G, Inturi S, Fossati-Jimack L, et al. Mechanisms of complement activation by dextran-coated superparamagnetic iron oxide (SPIO) nanoworms in mouse versus human serum. Part Fibre Toxicol. 2014;11(1):64. doi: 10.1186/s12989-014-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drugs@FDA: FDA-Approved Drugs [Internet]. Accessdata.fda.gov. 2021 [cited 6 June 2021]. Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.processand ApplNo=020416
- 40.Benasutti H, Wang G, Vu V, Scheinman R, Groman E, Saba L, et al. Variability of complement response toward preclinical and clinical nanocarriers in the general population. Bioconjug Chem. 2017;28(11):2747–2755. doi: 10.1021/acs.bioconjchem.7b00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ameta S. Advanced oxidation processes for wastewater treatment (2018)
- 42.Dulińska-Litewka J, Łazarczyk A, Hałubiec P, Szafrański O, Karnas K, Karewicz A. Superparamagnetic iron oxide nanoparticles—current and prospective medical applications. Materials. 2019;12(4):617. doi: 10.3390/ma12040617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Unterweger H, Dézsi L, Matuszak J, Janko C, Poettler M, Jordan J, et al. Dextran-coated superparamagnetic iron oxide nanoparticles for magnetic resonance imaging: evaluation of size-dependent imaging properties, storage stability and safety. Int J Nanomed. 2018;13:1899–1915. doi: 10.2147/IJN.S156528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferretti A, Usseglio S, Mondini S, Drago C, La Mattina R, Chini B, et al. Towards bio-compatible magnetic nanoparticles: Immune-related effects, in-vitro internalization, and in-vivo bio-distribution of zwitterionic ferrite nanoparticles with unexpected renal clearance. J Colloid Interface Sci. 2021;582:678–700. doi: 10.1016/j.jcis.2020.08.026. [DOI] [PubMed] [Google Scholar]
- 45.Daraee H, Etemadi A, Kouhi M, Alimirzalu S, Akbarzadeh A. Application of liposomes in medicine and drug delivery. Artif Cells Nanomed Biotechnol. 2014;44(1):381–391. doi: 10.3109/21691401.2014.953633. [DOI] [PubMed] [Google Scholar]
- 46.Alavi M, Karimi N, Safaei M. Application of various types of liposomes in drug delivery systems. Adv Pharm Bull. 2017;7(1):3–9. doi: 10.15171/apb.2017.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomed. 2015;10:975–999. doi: 10.2147/IJN.S68861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anselmo A, Mitragotri S. Nanoparticles in the clinic: an update. Bioeng Transl Med. 2019;4(3):e10143. doi: 10.1002/btm2.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fanciullino R, Ciccolini J. Liposome-encapsulated anticancer drugs: still waiting for the magic bullet? Curr Med Chem. 2009;16(33):4361–4373. doi: 10.2174/092986709789712916. [DOI] [PubMed] [Google Scholar]
- 50.Anilkumar Parambath. Engineering of biomaterials for drug delivery systems: beyond polyethylene glycol. Woodhead Publishing; 2018.
- 51.Gabizon A. Pegylated liposomal doxorubicin: metamorphosis of an old drug into a new form of chemotherapy. Cancer Invest. 2001;19(4):424–436. doi: 10.1081/cnv-100103136. [DOI] [PubMed] [Google Scholar]
- 52.Baden L, El Sahly H, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Polack F, Thomas S, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Drug Approval Package: Onpattro (patisiran) [Internet]. Accessdata.fda.gov. 2021 [cited 26 June 2021]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210922Orig1s000TOC.cfm
- 55.Urits I, Swanson D, Swett M, Patel A, Berardino K, Amgalan A, et al. Correction to: a review of patisiran (ONPATTRO®) for the treatment of polyneuropathy in people with hereditary transthyretin amyloidosis. Neurol Ther. 2021;10(1):407–407. doi: 10.1007/s40120-020-00208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van den Hoven J, Nemes R, Metselaar J, Nuijen B, Beijnen J, Storm G, et al. Complement activation by PEGylated liposomes containing prednisolone. Eur J Pharm Sci. 2013;49(2):265–271. doi: 10.1016/j.ejps.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 57.Szebeni J, Baranyi L, Savay S, Milosevits J, Bunger R, Laverman P, et al. Role of complement activation in hypersensitivity reactions to doxil and hynic PEG liposomes: experimental and clinical studies. J Liposome Res. 2002;12(1–2):165–172. doi: 10.1081/LPR-120004790. [DOI] [PubMed] [Google Scholar]
- 58.Tarhini M, Greige-Gerges H, Elaissari A. Protein-based nanoparticles: from preparation to encapsulation of active molecules. Int J Pharm. 2017;522:172–197. doi: 10.1016/j.ijpharm.2017.01.067. [DOI] [PubMed] [Google Scholar]
- 59.Mariam J, Sivakami S, Dongre PM. Albumin corona on nanoparticles – a strategic approach in drug delivery. Drug Delivery. 2015;23:2668–2676. doi: 10.3109/10717544.2015.1048488. [DOI] [PubMed] [Google Scholar]
- 60.Zeeshan F, Madheswaran T, Panneerselvam J, Taliyan R, Kesharwani P. Human serum albumin as multifunctional nanocarrier for cancer therapy. J Pharm Sci. 2021 doi: 10.1016/j.xphs.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 61.Wartlick H, Spänkuch-Schmitt B, Strebhardt K, Kreuter J, Langer K. Tumour cell delivery of antisense oligonucleotides by human serum albumin nanoparticles. J Control Release. 2004;96:483–495. doi: 10.1016/j.jconrel.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 62.Taguchi K, Okamoto Y, Matsumoto K, Otagiri M, Chuang V. When albumin meets liposomes: a feasible drug carrier for biomedical applications. Pharmaceuticals. 2021;14:296. doi: 10.3390/ph14040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee Villano J, Mehta D, Radhakrishnan L. Abraxane® induced life-threatening toxicities with metastatic breast cancer and hepatic insufficiency. Invest New Drugs. 2006;24:455–456. doi: 10.1007/s10637-006-6214-0. [DOI] [PubMed] [Google Scholar]
- 64.Luis de Redín I, Boiero C, Martínez-Ohárriz M, Agüeros M, Ramos R, Peñuelas I, Allemandi D, Llabot J, Irache J. Human serum albumin nanoparticles for ocular delivery of bevacizumab. Int J Pharm. 2018;541:214–223. doi: 10.1016/j.ijpharm.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 65.Steinhauser I, Langer K, Strebhardt K, Spänkuch B. Effect of trastuzumab-modified antisense oligonucleotide-loaded human serum albumin nanoparticles prepared by heat denaturation. Biomaterials. 2008;29:4022–4028. doi: 10.1016/j.biomaterials.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 66.Langer K, Balthasar S, Vogel V, Dinauer N, von Briesen H, Schubert D. Optimization of the preparation process for human serum albumin (HSA) nanoparticles. Int J Pharm. 2003;257:169–180. doi: 10.1016/s0378-5173(03)00134-0. [DOI] [PubMed] [Google Scholar]
- 67.Rahimizadeh P, Yang S, Lim S. Albumin: an emerging opportunity in drug delivery. Biotechnol Bioprocess Eng. 2020;25:985–995. doi: 10.1007/s12257-019-0512-9. [DOI] [Google Scholar]
- 68.Langiu M, Dadparvar M, Kreuter J, Ruonala M. Human serum albumin-based nanoparticle-mediated in vitro gene delivery. PLoS ONE. 2014;9:e107603. doi: 10.1371/journal.pone.0107603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nahar M, Mishra D, Dubey V, Jain N. Development, characterization, and toxicity evaluation of amphotericin B–loaded gelatin nanoparticles. Nanomed Nanotechnol Biol Med. 2008;4:252–261. doi: 10.1016/j.nano.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 70.Chou M, Yu H, Hsia J, Chen Y, Hung T, Chao H, Chern E, Huang Y. Highly efficient intracellular protein delivery by cationic polyethyleneimine-modified gelatin nanoparticles. Materials. 2018;11:301. doi: 10.3390/ma11020301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hong S, Choi DW, Kim HN, Park CG, Lee W, Park HH. Protein-based nanoparticles as drug delivery systems. Pharmaceutics. 2020;12(7):604. doi: 10.3390/pharmaceutics12070604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sahoo N, Sahoo RK, Biswas N, Guha A, Kuotsu K. Recent advancement of gelatin nanoparticles in drug and vaccine delivery. Int J Biol Macromol. 2015;81:317–331. doi: 10.1016/j.ijbiomac.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 73.Kommareddy S, Shenoy DB, Amiji MM. Gelatin nanoparticles and their biofunctionalization. Nanotechnol Life Sci. 2007 doi: 10.1002/9783527610419.ntls0011. [DOI] [Google Scholar]
- 74.Leo E, Arletti R, Forni F, Cameroni R. General and cardiac toxicity of doxorubicin-loaded gelatin nanoparticles. Farmaco. 1997;52(6–7):385–8. [PubMed] [Google Scholar]
- 75.Mohiti-Asli M, Loboa EG. Nanofibrous smart bandages for wound care. Wound Heal Biomater. 2016;483–499. 10.1016/b978-1-78242-456-7.00023-4.
- 76.Wang Z, Gao H, Zhang Y, Liu G, Niu G, Chen X. Functional ferritin nanoparticles for biomedical applications. Front Chem Sci Eng. 2017;11:633–646. doi: 10.1007/s11705-017-1620-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Singh B, Yang S, Krishna A, Sridhar S. Nanoparticle formulations of poly (ADP-ribose) polymerase inhibitors for cancer therapy. Front Chem. 2020 doi: 10.3389/fchem.2020.594619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Truffi M, Fiandra L, Sorrentino L, Monieri M, Corsi F, Mazzucchelli S. Ferritin nanocages: a biological platform for drug delivery, imaging and theranostics in cancer. Pharmacol Res. 2016;107:57–65. doi: 10.1016/j.phrs.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 79.Todd TJ, Zhen Z, Xie J. Ferritin nanocages: great potential as clinically translatable drug delivery vehicles? Nanomedicine. 2013;8:1555–1557. doi: 10.2217/nnm.13.141. [DOI] [PubMed] [Google Scholar]
- 80.Liang M, Fan K, Zhou M, Duan D, Zheng J, Yang D, Feng J, Yan X. H-ferritin-nanocaged doxorubicin nanoparticles specifically target and kill tumors with a single-dose injection. Proc Natl Acad Sci. 2014;111:14900–14905. doi: 10.1073/pnas.1407808111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gulfam M, J-eun K, Lee JM, Ku B, Chung BH, Chung BG. Anticancer drug-loaded gliadin nanoparticles induce apoptosis in breast cancer cells. Langmuir. 2012;28:8216–8223. doi: 10.1021/la300691n. [DOI] [PubMed] [Google Scholar]
- 82.Elzoghby AO, Elgohary MM, Kamel NM. Implications of protein- and peptide-based nanoparticles as potential vehicles for anticancer drugs. Adv Protein Chem Struct Biol. 2015;169–221. 10.1016/bs.apcsb.2014.12.002. [DOI] [PubMed]
- 83.Wu W, Kong X, Zhang C, Hua Y, Chen Y. Improving the stability of wheat gliadin nanoparticles – effect of gum arabic addition. Food Hydrocolloids. 2018;80:78–87. doi: 10.1016/j.foodhyd.2018.01.042. [DOI] [Google Scholar]
- 84.Freitag TL, Podojil JR, Pearson RM, et al. Gliadin nanoparticles induce immune tolerance to gliadin in mouse models of celiac disease. Gastroenterology. 2020. 10.1053/j.gastro.2020.01.045. [DOI] [PMC free article] [PubMed]
- 85.Peng D, Jin W, Li J, Xiong W, Pei Y, Wang Y, Li Y, Li B. Adsorption and distribution of edible gliadin nanoparticles at the air/water interface. J Agric Food Chem. 2017;65:2454–2460. doi: 10.1021/acs.jafc.6b05757. [DOI] [PubMed] [Google Scholar]
- 86.Joye IJ, Nelis VA, McClements DJ. Gliadin-based nanoparticles: fabrication and stability of food-grade colloidal delivery systems. Food Hydrocolloids. 2015;44:86–93. doi: 10.1016/j.foodhyd.2014.09.008. [DOI] [Google Scholar]
- 87.Pham DT, Tiyaboonchai W. Fibroin nanoparticles: a promising drug delivery system. Drug Deliv. 2020;27:431–448. doi: 10.1080/10717544.2020.1736208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pandey V, Haider T, Chandak AR, Chakraborty A, Banerjee S, Soni V. Surface modified silk fibroin nanoparticles for improved delivery of doxorubicin: development, characterization, in-vitro studies. In: International Journal of Biological Macromolecules. 2020. https://www.sciencedirect.com/science/article/pii/S014181302034085X. Accessed 27 Jun 2021. 10.1016/j.ijbiomac.2020.07.326. [DOI] [PubMed]
- 89.Mishra D, Iyyanki TS, Hubenak JR, Zhang Q, Mathur AB. Silk fibroin nanoparticles and cancer therapy. Nanotechnology in Cancer. 2017;19–44. 10.1016/b978-0-323-39080-4.00002-1.
- 90.Dong Y, Dong P, Huang D, Mei L, Xia Y, Wang Z, Pan X, Li G, Wu C. Fabrication and characterization of silk fibroin-coated liposomes for ocular drug delivery. Eur J Pharm Biopharm. 2015;91:82–90. doi: 10.1016/j.ejpb.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 91.Hong S, Choi DW, Kim HN, Park CG, Lee W, Park HH. Protein-based nanoparticles as drug delivery systems. Pharmaceutics. 2020;12:604. doi: 10.3390/pharmaceutics12070604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gandhi S, Roy I. Doxorubicin-loaded casein nanoparticles for drug delivery: preparation, characterization and in vitro evaluation. Int J Biol Macromol. 2019;121:6–12. doi: 10.1016/j.ijbiomac.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 93.Sinha VR, Trehan A. Biodegradable microspheres for protein delivery. J Control Release. 2003;90:261–280. doi: 10.1016/S0168-3659(03)00194-9. [DOI] [PubMed] [Google Scholar]
- 94.Peñalva R, Morales J, González-Navarro C, Larrañeta E, Quincoces G, Peñuelas I, Irache J. Increased oral bioavailability of resveratrol by its encapsulation in casein nanoparticles. Int J Mol Sci. 2018;19:2816. doi: 10.3390/ijms19092816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gil AG, Irache JM, Peñuelas I, González Navarro CJ, López de Cerain A. Toxicity and biodistribution of orally administered casein nanoparticles. Food Chem Toxicol. 2017;106:477–486. doi: 10.1016/j.fct.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 96.Anagnostou K, Stylianakis M, Michaleas S, Skouras A (2020) Biodegradable nanomaterials. Nanomaterials for Clinical Applications 123–157
- 97.Singh N, Joshi A, Toor A, Verma G. Drug delivery: advancements and challenges. Nanostruct Drug Deliv. 2017;865–886. 10.1016/B978-0-323-46143-6.00027-0.
- 98.Suriya Prabha A, Dorothy R, Jancirani S, Rajendran S, Singh G, Senthil Kumaran S. Recent advances in the study of toxicity of polymer-based nanomaterials. Nanotoxicity. 2020;143–165. 10.1016/B978-0-12-819943-5.00007-5.
- 99.Grabowski N, Hillaireau H, Vergnaud J, Tsapis N, Pallardy M, Kerdine-Römer S, Fattal E. Surface coating mediates the toxicity of polymeric nanoparticles towards human-like macrophages. Int J Pharm. 2015;482:75–83. doi: 10.1016/j.ijpharm.2014.11.042. [DOI] [PubMed] [Google Scholar]
- 100.Das S, Khadka P, Shah R, McGill S, Smyth H. Nanomedicine in pulmonary delivery. Theory Appl Nonparent Nanomed. 2021;319–354. 10.1016/B978-0-12-820466-5.00014-4.
- 101.Krishnaswamy K, Orsat V. Sustainable delivery systems through green nanotechnology. Nano- Microscale Drug Deliv Syst. 2017;17–32. 10.1016/B978-0-323-52727-9.00002-9.
- 102.Zielińska A, Carreiró F, Oliveira A, et al. Polymeric nanoparticles: production, characterization, toxicology and ecotoxicology. Molecules. 2020;25:3731. doi: 10.3390/molecules25163731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Palanikumar L, Al-Hosani S, Kalmouni M, Nguyen V, Ali L, Pasricha R, Barrera F, Magzoub M. pH-responsive high stability polymeric nanoparticles for targeted delivery of anticancer therapeutics. Commun Biol. 2020. 10.1038/s42003-020-0817-4. [DOI] [PMC free article] [PubMed]
- 104.Biju V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem Soc Rev. 2014;43(3):744–764. doi: 10.1039/c3cs60273g. [DOI] [PubMed] [Google Scholar]
- 105.Slowing I, Viveroescoto J, Wu C, Lin V. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv Drug Deliv Rev. 2008;60(11):1278–1288. doi: 10.1016/j.addr.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 106.Yang P, Gaib S, Lin J. Chem Soc Rev. 2012;41:3679–3698. doi: 10.1039/C2CS15308D. [DOI] [PubMed] [Google Scholar]
- 107.Li Z, Barnes JC, Bosoy A, Stoddar JF, Zink JI. Chem Soc Rev. 2012;41:2590–2605. doi: 10.1039/C1CS15246G. [DOI] [PubMed] [Google Scholar]
- 108.Lu J, Liong M, Li Z, Zink JI, Tamanoi F. Biocompatibility, biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small. 2010;6(16):1794–1805. doi: 10.1002/smll.201000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tyner K, Bancos S, Stevens D. Effect of silica and gold nanoparticles on macrophage proliferation, activation markers, cytokine production, and phagocytosis in vitro. Int J Nanomed. 2014;183. 10.2147/ijn.s72580. [DOI] [PMC free article] [PubMed]
- 110.Gonzalez L, et al. Co-assessment of cell cycle and micronucleus frequencies demonstrates the influence of serum on the in vitro genotoxic response to amorphous monodisperse silica nanoparticles of varying sizes. Nanotoxicology. 2014;8(8):876–884. doi: 10.3109/17435390.2013.842266. [DOI] [PubMed] [Google Scholar]
- 111.Yang L, et al. The role of potassium channel in silica nanoparticle-induced inflammatory effect in human vascular endothelial cells in vitro. Toxicol Lett. 2013;223:16–24. doi: 10.1016/j.toxlet.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 112.Maser E, Schulz M, Sauer UG, et al. In vitro and in vivo genotoxicity investigations of differently sized amorphous SiO2 nanomaterials. Mutat Res Genet Toxicol Environ Mutagen. 2015;794:57–74. doi: 10.1016/j.mrgentox.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 113.Lucarelli M, Gatti AM, Savarino G, et al. Innate defence functions of macrophages can be biased by nano-sized ceramic and metallic particles. Eur Cytokine Netw. 2004;15(4):339–346. [PubMed] [Google Scholar]
- 114.Xifei Y, et al. Uptake of silica nanoparticles: neurotoxicity and Alzheimer-like pathology in human SK-N-SH and mouse neuro2a neuroblastoma cells. Toxicol Lett. 2014;229(1):240–249. doi: 10.1016/j.toxlet.2014.05.009. [DOI] [PubMed] [Google Scholar]