Abstract
Background: The anti-thrombotic strategy for patients with atrial fibrillation (AF) who have undergone percutaneous coronary intervention (PCI) for coronary artery disease (CAD) is a common and difficult challenge. This pilot study aimed to assess the feasibility and safety of “one-stop” left atrial appendage closure (LAAC) combined with PCI as an alternative stroke prophylaxis strategy. Methods: From March 2017 to October 2019, AF patients with elevated bleeding risk and significant stable CAD requiring PCI were recruited to undergo LAAC as alternative stroke prophylaxis in Fuwai Hospital, Beijing, China. LAAC was performed either in the same setting with PCI (i.e. “one-stop” LAAC/PCI), or as staged procedure after PCI. Dual antiplatelet therapy was given for all patients after LAAC. Peri-procedural and intermediate-term clinical outcomes were assessed through hospital clinical records review and standardized telephone interviews. Results: A total of 24 patients were recruited including 13 (54.2%) underwent stage procedure and 11 (45.8%) underwent “one-stop” procedure respectively. The mean CHA2DS2-VASc and HAS-BLED scores were 4.5±1.4 and 3 (IQR 3,4) respectively. Six patients (46.1%) in the staged procedure cohort were treated with triple anti-thrombotic following PCI, with 2 developed minor bleeding before LAAC. One patient (“one-stop” cohort) had gastrointestinal bleeding 1 day after procedure. Otherwise, there was no device related complication or peri-procedural stroke/myocardial infarction. After a mean 19±5.4 months follow-up, there was no death, myocardial infarction, stroke and systemic embolization detected. Conclusions: In this pilot study, “one-stop” LAAC with PCI was shown to be efficacious with no stroke, MI, VARC-2 major bleeding or CV death reported over a mean follow-up of 19 months, and safe with no major peri-procedural bleeding or device related complications.
Keywords: Atrial fibrillation (AF), left atrial appendage closure (LAAC), oral anticoagulants (OAC), percutaneous coronary Intervention (PCI), coronary artery disease (CAD)
Introduction
About 15% of patients with atrial fibrillation might be accompanying with coronary artery disease (CAD) [1]. Percutaneous coronary interventions (PCI) with stent placement to obstructive coronary artery is the basic treatment for CAD patients. However, the choice of anti-thrombotic regime for atrial fibrillation (AF) patients requiring percutaneous coronary intervention (PCI) for coronary artery disease (CAD) is challenging. It was known that dual antiplatelet therapy (DAPT) was superior to oral anticoagulants (OAC) in reducing cardiovascular events and stent thrombosis after PCI. On the other hand, OAC was superior to DAPT in the prevention of stroke in AF patients [2-4]. The 2018 ESC guidelines recommended triple therapy (OAC+DAPT) for at least one month followed by lifelong OAC in AF patients undergoing PCI, even with high bleeding risk [5]. This strategy intended to reduce thrombotic events at the cost of bleeding complications. However, in a number of recent clinical trials, double therapy (OAC with a single antiplatelet) was shown to significantly reduce the risk of bleeding (15.4-20.7% per year vs 25.6-44.4% per year) compared to triple therapy without a signal of harm with regard to stent thrombosis [6-10]. Therefore, the latest AHA/ACC/HRS Guideline recommended double therapy in AF patients undergoing PCI to reduce the bleeding risks. Yet, double therapy still carried a 15-20% annual bleeding risk. Left atrial appendage (LAA) closure (LAAC) is an effective stroke prophylaxis strategy in AF patients with contraindication to or at high risk of bleeding with OAC [11]. LAAC might be a good alternative in AF patients undergoing PCI, to reduce the risk of bleeding associated with double or triple therapy. Recent study reported favorable clinical outcomes in AF patients receiving both PCI and LAAC [12]. In this pilot study, we aimed to assess the feasibility and safety of LAAC combined with PCI and the use of short-term DAPT as an alternative stroke prophylaxis strategy.
Methods
Study population
From March 2017 to October 2019, we recruited AF patients with elevated bleeding risk and significant stable CAD requiring PCI to undergo LAAC as alternative stroke prophylaxis in Fuwai Hospital, Beijing, China. Inclusion criteria included, (1) Paroxysmal or persistent AF with elevated bleeding risk and thrombo-embolic risk as defined by a CHA2DS2-VASc score ≥2 and HAS-BLED score ≥3 AND; (2) Significant stable CAD requiring or had recent PCI; (3) Deemed physically fit to tolerate and mentally fit to consent for both PCI and LAAC AND; (4) Either contraindicated to OAC or poor compliance to OAC or experienced bleeding events with double/triple therapy post PCI. Patients with acute coronary syndrome or ST-segment elevation myocardial infarction were excluded. LAAC was performed either in the same procedure with PCI (“one-stop”) or as stage procedure (i.e. staged LAAC after PCI) (Figure 1). The study was approved by institutional ethics committee and complied with the Declaration of Helsinki.
Figure 1.
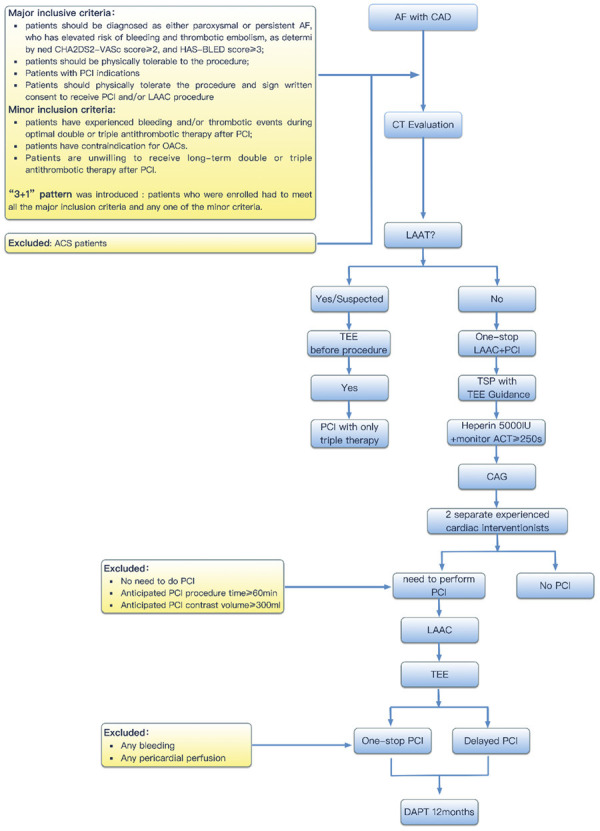
Protocol of “One-stop” Procedure of “LAAC+PCI”. AF: Atrial fibrillation; CAD: Coronary heart disease; CAG: Coronary angiography; PCI: Percutaneous coronary intervention; CT: computed tomography; LAAT: Left atrial appendage thrombus; TSP: Transseptal puncture; LAAC: Left atrial appendage.
Pre-procedural examination
All patients underwent myocardial injury markers, N-terminal-proBNP, routine 18 lead electrocardiogram (ECG) and transthoracic echocardiogram (TTE) to evaluate basic structure and function of heart. Pre-procedural cardiac computed tomography (CT) were performed to evaluate the morphology of left atrial appendage (LAA), to exclude left atrial and LAA thrombus and to assess for obstructive lesion of coronary artery. The procedure was performed under general anesthesia and transesophageal echocardiography (TEE) guidance.
Protocol for “one-stop” combined procedure
First, femoral venous and arterial access were obtained for LAAC and coronary angiography (CAG) + potential PCI respectively. Second, trans-septal puncture (TSP) was performed under TEE guidance. After that, heparin (5000 IU) was given. Third, CAG was performed and evaluated by 2 experienced interventional cardiologists for procedural planning. Patients with no significant CAD or significant CAD anticipated to require complex interventions (i.e. procedure time more than 60 minutes, and contrast volume ≥300 ml) were excluded (Figure 1). Forth, LAAC was performed prior to PCI. Watchman (Boston Scientific, Marlborough, MA, USA) and LAmbre (Lifetech Scientific, Shenzhen, China) devices were used, and devices were implanted according to individual instruction for use [13]. After confirming optimal device position and ruling out new pericardial effusion, PCI was proceeded. Active clotting time (ACT) was closely monitored and maintained above 250 s throughout the procedure. After procedure, femoral arterial access was closed with AngioSeal vascular closure devices and the femoral venous access was closed with hemostatic stitch.
Post procedure antithrombotic regime
All patients received DAPT (aspirin 100 mg daily with clopidogrel 75 mg daily or ticagrelor 90 mg twice a day) after LAAC. The duration of DAPT was at least 12 months from the latest PCI, followed by lifelong aspirin.
Follow up
Peri-procedural adverse events included hemodynamic significant pericardial effusion, device embolization, procedure-related stroke, VARC-2 major bleeding, cardiovascular or unexplained death occurred within 7 days from the procedure. Follow-up TEE or CT, for those could not tolerate or refused TEE, was performed at day 45 from the procedure to assess for device-related thrombus (DRT) and significant peri-device leak (PDL, >5 mm). Standardized patient phone interview was conducted to evaluate for clinical events before statistical analysis for the study. The clinical efficacy endpoints included ischemic or hemorrhagic stroke, systemic embolism or myocardial infarction (MI). The clinical safety endpoints included VARC-2 major or minor bleeding. Further review of hospital medical record, confirmation with primary care physicians and referring cardiologists were performed to verify clinical endpoints if any. All data were entered into a dedicated database. Patients were also divided into staged procedure cohort and “one-stop” procedure cohort, procedural and follow-up endpoints were compared between the two groups.
Statistical analysis
Continuous variables were summarized as medians (interquartile range) or mean ± SD, and categorical variables were summarized as counts with percentage. Independent sample t-test was used to compare the means between two groups for normally distributed variables; Kruskal-Wallis test was used for non-normally distributed variables. Chi square test was used to compare categorical variables. Two-sided P-values less than 0.05 were considered as statistically significant. Statistical analyses were performed using SPSS software (PASW, v24; SPSS, Inc., Chicago, IL).
Results
Baseline characteristics
A total of 24 patients were enrolled, including 13 (54.2%) underwent staged procedures and 11 (45.8%) underwent “one-stop” combined procedure. The mean age was 70.1±7.6 (ranged 60-84), and 14 (58.3%) patients were male. Nineteen patients (79.2%) had persistent AF, while 5 patients (20.8%) had paroxysmal AF. Eleven patients (45.8%) had history of prior stroke or transient ischemic attack. The mean CHA2DS2-VASc score was 4.5±1.4, and the median HAS-BLED score 3 (IQR 3,4). The mean duration from previous PCI to LAAC in the staged group was 3.3±1.84 months. There were no statistically significant differences in the baseline characteristics between patients underwent stage procedure and “one-stop” combined procedure (Table 1).
Table 1.
Baseline characteristics of patients
| Total (n=24) | Staged (n=13) | One-stop (n=11) | P-value | |
|---|---|---|---|---|
| Male | 14 (58.3%) | 8 (61.5%) | 6 (54.5%) | 0.729 |
| Age (yrs) | 70.1±7.6 | 69.9±8.8 | 70.3±6.3 | 0.913 |
| CAD | 24 (100.0%) | 13 (100.0%) | 11 (100.0%) | NA |
| Diabetes mellitus | 12 (50.0%) | 7 (53.8%) | 5 (45.5%) | 0.682 |
| Hypertension | 21 (87.5%) | 11 (84.6%) | 10 (90.9%) | 1.000 |
| Hyperglycemia | 20 (83.3%) | 12 (92.3%) | 8 (72.7%) | 0.300 |
| Smoke | 11 (45.8%) | 7 (53.8%) | 4 (36.4%) | 0.392 |
| Drinking | 4 (16.7%) | 3 (23.1%) | 1 (9.1%) | 0.596 |
| Previous stroke/TIA | 11 (45.8%) | 5 (38.5%) | 6 (54.5%) | 0.431 |
| Pattern of AF | ||||
| persistent | 19 (79.2%) | 11 (84.6%) | 8 (72.7%) | 0.63 |
| paroxysmal | 5 (20.8%) | 2 (15.4%) | 3 (27.3%) | |
| Peripheral vascular disease | 11 (45.8%) | 7 (53.8%) | 4 (36.4%) | 0.392 |
| Heart failure (NYHA) | ||||
| I | 14 (58.3%) | 7 (53.8%) | 7 (63.6%) | 0.846 |
| II | 8 (33.3%) | 5 (38.5%) | 3 (27.3%) | |
| III | 2 (8.3%) | 1 (7.7%) | 1 (9.1%) | |
| CHA2DS2-VASc Score | 4.5±1.4 | 4.5±1.5 | 4.5±1.5 | 0.891 |
| HAS-BLED score | 3 (3,4) | 3 (3,3) | 3 (3,4) | 0.491 |
CAD: Coronary heart disease; AF: Atrial fibrillation; TIA: Transient ischemic attacks; NYHA: New York Heart Association.
Procedural data
LAAC device was successfully implanted in 23 (98.5%) patients with no significant intra-procedural PDL (>5 mm), and 1 aborted due to the small LAA ostium (maximal diameter <12 mm). Nine patients (37%) had chicken-wing, 2 (8.3%) had windsock (8.3%), 1 (4.1%) had cactus, and 12 (50%) had cauliflower LAA morphology respectively. Majority (n=20, 83.3%) of the patients received Watchman device, while the remaining (n=3) used LAmbre device for large LAA or multilobed LAA anatomy.
In staged procedure cohort, 26 coronary stents were implanted in 13 patients. Watchman device was used in 10 patients, LAmbre device in 2 patients and 1 failed LAAC. In one-stop cohort, PCI was performed with 13 coronary stents. Coronary lesions treated included left main coronary artery lesion (n=1, 9.1%), multi-vessel disease (n=1, 9.1%), calcified lesion (n=2, 18.2%), bifurcation lesion (n=2, 18.2%), and in-stent re-stenosis (n=1, 9.1%). Watchman device was used in 10 patients and LAmbre in 1 patient. Detailed procedure details were illustrated in Table 2.
Table 2.
Details of LAAC and PCI Procedure
| Sex | Age | Pre-stroke/TIA | CHA2DS2-VASC | HAS-BLED | Heart failure (NYHA) | Previous PCI | Procedure time (min) | LAAC procedure | Present PCI | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||||||||||||
| Y/N | Target lesion | Stent | LAA shape | Depth (mm) | OD (mm) | Success | Leak (mm) | Release | Compression (%) | Divice | Size (mm) | Y/N | Target lesion | Stent | DEB | ||||||||
| staged | |||||||||||||||||||||||
| 1 | M | 84 | N | 6 | 3 | III | Y | RCA/LAD | 1/1 | 70 | cauliflower | 23 | 20 | Y | N | 1 | 16 | Watchman | 24 | N | |||
| 2 | M | 60 | N | 2 | 3 | I | Y | RCA | 3 | 80 | cauliflower | 35 | 19 | Y | N | 2 | 26 | Watchman | 27 | N | |||
| 3 | M | 63 | Y | 4 | 2 | II | Y | LAD | 1 | 60 | chicken-wing | 27 | 20 | Y | 1 | 2 | 29 | Watchman | 27 | N | |||
| 4 | M | 62 | Y | 4 | 3 | I | Y | LAD | 1 | 50 | chicken-wing | 28 | 22 | Y | N | 2 | NA | Lambre | 26-32 | N | |||
| 5 | M | 72 | N | 6 | 4 | II | Y | LAD | 1 | 60 | chicken-wing | 30 | 21 | Y | 2 | 2 | 20 | Watchman | 30 | N | |||
| 6 | F | 71 | Y | 6 | 3 | I | Y | LAD/LCX/RCA | 2/1/1 | 60 | chicken-wing | 17 | 12 | N | N | ||||||||
| 7 | F | 74 | Y | 7 | 5 | II | Y | LAD | 1 | 60 | cauliflower | 27 | 27 | Y | N | 3 | 16 | Watchman | 30 | N | |||
| 8 | F | 61 | N | 3 | 2 | I | Y | LAD | 1 | 40 | windsock | 27 | 20 | Y | N | 1 | 18 | Watchman | 27 | N | |||
| 9 | M | 63 | N | 4 | 3 | II | Y | LAD | 1 | 40 | cauliflower | 27 | 21 | Y | N | 2 | 12.5 | Watchman | 24 | N | |||
| 10 | M | 83 | N | 4 | 3 | I | Y | RCA | 2 | 75 | cauliflower | 24 | 30 | Y | 2 | 3 | 23 | Watchman | 30 | N | |||
| 11 | F | 68 | Y | 5 | 4 | I | Y | RCA | 3 | 60 | chicken-wing | 27 | 19 | Y | 3 | 2 | 20 | Watchman | 24 | N | |||
| 12 | M | 65 | N | 3 | 3 | I | Y | RCA | 1 | 60 | cauliflower | 22 | 20 | Y | N | 1 | 16.7 | Watchman | 24 | N | |||
| 13 | F | 83 | N | 5 | 3 | II | Y | RCA/LCX/LAD | 2/1/2 | 50 | chicken-wing | 30 | 28 | Y | N | 1 | NA | Lambre | 28-38 | N | |||
| One-stop | |||||||||||||||||||||||
| 1 | M | 74 | N | 3 | 2 | III | Y | RCA | 1 | 65 | cauliflower | 19 | 24 | Y | N | 2 | 25 | Watchman | 30 | Y | LAD | 1 | N |
| 2 | M | 71 | Y | 4 | 3 | I | N | 90 | cactus | 27 | 21 | Y | 4 | 2 | 28 | Watchman | 27 | Y | LAD | 1 | N | ||
| 3 | F | 73 | N | 5 | 3 | II | Y | LCX | 2 | 70 | cauliflower | 27 | 21 | Y | N | 1 | 15 | Watchman | 27 | Y | LAD | 1 | N |
| 4 | F | 61 | Y | 6 | 4 | II | N | 60 | cauliflower | 27 | 20 | Y | N | 2 | 12 | Watchman | 24 | Y | LCX | 1 | N | ||
| 5 | F | 73 | N | 3 | 4 | I | N | 60 | windsock | 27 | 21 | Y | N | 1 | 12.5 | Watchman | 24 | Y | LAD | 1 | N | ||
| 6 | M | 80 | Y | 7 | 4 | II | Y | RCA | 2 | 60 | cauliflower | 28 | 22 | Y | N | 1 | 14.8 | Watchman | 27 | Y | LCX | 1 | N |
| 7 | M | 60 | Y | 5 | 3 | I | Y | RCA/LAD | 2/2+1DEB | 50 | chicken-wing | 18 | 19 | Y | N | 1 | 22 | Watchman | 24 | Y | LM-LAD | 2 | N |
| 8 | M | 63 | N | 2 | 2 | I | Y | RCA/LAD | 2/1 | 50 | chicken-wing | 22 | 26 | Y | N | 1 | 17 | Watchman | 30 | Y | RCA | N | 1 |
| 9 | M | 71 | Y | 4 | 3 | I | N | 50 | chicken-wing | 20 | 30 | Y | N | 1 | 22 | Watchman | 27 | Y | LAD | 1 | N | ||
| 10 | F | 73 | Y | 6 | 4 | I | N | 50 | cauliflower | 26 | 28 | Y | N | 2 | NA | Lambre | 20-32 | Y | LAD/LCX | 2/1 | N | ||
| 11 | F | 74 | N | 4 | 5 | I | N | 60 | cauliflower | 16 | 20 | Y | N | 1 | 19 | Watchaman | 21 | Y | LAD | 1 | N | ||
LAD: Left anterior descending branch; RCA: Right coronary artery; LCX: Left circumflex artery; DEB: Drug eluting balloon; LAAC: Left atrial appendage closure; LAA: Left atrial appendage; PCI: percutaneous coronary intervention; TIA: Transient ischemic attacks; OD: Ostium diameter.
Post procedure antithrombotic regime
Six patients (46.1%) in staged cohort were treated with triple therapy after PCI, with VARC-2 minor bleeding occurred in 2 patients (33.3%). After LAAC, patients received DAPT for a mean of 8.2±1.7 months (range: 6-12 months). In the one-stop cohort, 10 patients received aspirin and clopidogrel for 12 months, while 1 patient received aspirin and ticagrelor. During follow up, all patients adhered to long-term aspirin 100 mg/day after DAPT.
Periprocedural adverse events (≤7 days)
Two patients had pseudoaneurysm at formal artery access site confirmed by ultrasound, one in staged cohort and the other in one-stop cohort. One patient in the one-stop cohort had gastrointestinal bleeding 1 day after LAAC/PCI. This patient had prior gastrointestinal bleeding history. His bleeding was stopped with proton pump inhibitor infusion successfully without endoscopic therapy and did not report any recurrent bleeding event after discharge. Otherwise, there were no major bleeding events. Besides, there were no pericardial effusion, device embolization, or stroke during the LAAC and/or PCI procedure. The details were summarized in Table 3.
Table 3.
Safety events of periprocedural follow-up
| Events (≤7 days) | staged | one-stop |
| Death | 0 | 0 |
| Stroke/TIA | 0 | 0 |
| Femoral bleeding | 1 | 1 |
| Cardiac tamponade | 0 | 0 |
| Device embolization | 0 | 0 |
| Major bleeding | 0 | 1 |
| Myocardial infarction | 0 | 0 |
| Systemic embolism | 0 | 0 |
| Need for surgery | 0 | 0 |
| Minor bleeding | 0 | 0 |
| Events (>7 days) | ||
| Death | 0 | 0 |
| All stroke | 0 | 0 |
| Ischemic stroke | 0 | 0 |
| Hemorrhagic stroke | 0 | 0 |
| Cardiac imaging (CT or TEE) 45 days | ||
| Cardiac CT | 3 | 0 |
| TEE | 9 | 11 |
| Peri-device leak | 2 | 1 |
| Device-related thrombus | 0 | |
| Device embolization | 0 | |
| Antithrombotic therapy post LAAC | ||
| Lifelong (ASP/clopidogrel) | 13 | 11 |
| DAPT | ||
| 12 months | 11 | |
| <12 months | 13 |
TIA: Transient ischemic attacks; TEE: Transesophageal ultrasound; DAPT: Dual antiplatelet therapy; CT: computed tomography.
45 days follow-up imaging
Twenty patients (83.3%) undergone TEE and 3 patients undergone cardiac CT. There was no significant PDL (>5 mm) nor DRT detected. Minor PDL were detected in 3 patients (13%), which measured 2 mm, 2 mm, and 4 mm respectively.
Telephone follow-up
After a mean follow-up of 19±5.4 months, no ischemic/hemorrhagic stroke, systemic embolism or myocardial infarction (MI), VARC-2 major bleeding or CV death occurred. Follow-up events were summarized in Table 3.
Safety and efficacy outcomes compared to previous studies
Compared with PROTACT-AF, PREVAIL and EWOLUTIN-1Y studies, the stroke, DRT and death rate were zero in our study. One patient suffered gastrointestinal bleeding (4.0%), which was lower than PROTECT-AF (7.4%) but higher than PREVAIL (2.3%) and EWOLUTION-1Y (2.6%) study. The details are summarized in Figure 2.
Figure 2.
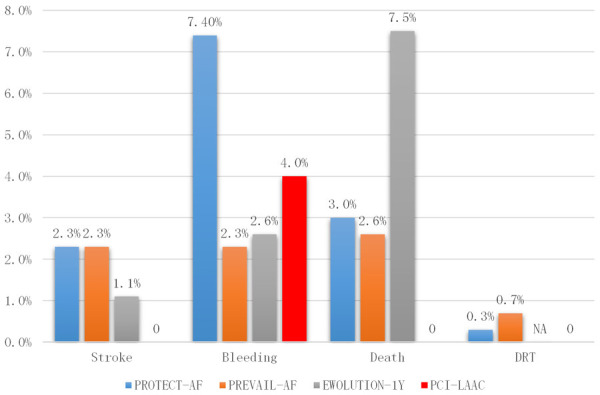
Safety and Efficacy of LAAC/PCI compared to Previous Studies. Stroke include ischemic or hemorrhagic stroke; Bleeding: any bleeding annual; Death including all cause death; DRT: device related thrombus.
Discussion
We described an alternative approach for stroke prophylaxis for patients with AF requiring PCI and high bleeding risk, by combining LAAC and PCI either as stage or as “one-stop” procedure. In this pilot study, this approach was shown to be efficacious with no stroke, MI, VARC-2 major bleeding or CV death over a mean follow-up of 19 months, and safe with no major peri-procedural bleeding or device related complications. When performed in stage procedures compared to “one-stop” combined procedure, exposure to triple therapy after PCI before LAAC might be associated with increased bleeding.
The role of LAAC in patients with PCI
The management of AF patients underwent PCI is challenging, as we need to balance the risks of AF-related thromboembolism, anti-thrombotic-related bleeding, and post PCI stent thrombosis. Current guidelines recommended triple therapy (OAC+DAPT) for at least one month and OAC for lifelong in AF patients undergoing PCI, even with high bleeding risk. This strategy reduces thrombotic events at the cost of increasing bleeding risk [14,15]. In this study, 6 patients (46.1%) in the staged cohort were exposed to short term triple therapy after PCI before LAAC, which resulted in minor bleeding events in 2 patients (2/6, 33.3%). LAAC is a rapidly emerging option for AF patients with high risk of bleeding or contraindicated for long-term OAC [16]. The long-term outcomes of LAAC randomized controlled trials (PROTECT-AF and PREVAIL) and their accompanying registries (CAP and CAP2) demonstrated non-inferiority of the LAAC devices to warfarin for preventing stroke and superiority in reducing hemorrhagic stroke, cardiovascular mortality, and non-procedure related bleeding [17-20]. In patients with high bleeding risk and significant CAD, combining LAAC and PCI in the same procedure followed by a short term DAPT might be the ideal therapy due to its potential advantages of preventing AF-associated cardio-embolism and reducing the risk of multiple anti-thrombotic related bleeding. In our study, there was only one major bleeding events because of stress ulcer in patients accepted one-stop LAAC and PCI procedural. No bleeding events happened during long term follow, which was comparable with previous studies.
The safety concerns of “one-stop” LAAC and PCI
Concomitant PCI and LAAC procedure had been described in one previous study [11], which showed bleeding events were higher in the LAAC/PCI group than the double therapy and triple therapy group at 30-day follow-up (1.92% vs 1.31%), and raised a safety concern of combining the two procedures [12]. To minimize the risk significant pericardial effusion during “one-stop” LAAC and PCI, we performed TSP before CAG and full heparinization. On the other hand, after successful LAAC, TEE was performed meticulously to rule out new pericardial effusion before proceeding to coronary stenting. Besides, CAG was reviewed by two experienced interventional cardiologists to determine the complexity of PCI and decide whether to proceed as “one-stop” procedure or stage procedure. This further reduced the risk incurred with prolonged procedure and excessive contrast used. With these, no device related complication or stroke occurred in the “one-stop” procedure cohort. At 45-day follow-up, TEE/cardiac CT confirmed no major PDL or DRT in all patients. These supported the safety of “one-stop” LAAC with PCI.
DAPT post PCI and LAAC procedure
The optimal antithrombotic regime after LAAC is still controversial and varied between different LAAC devices. According to Watchman device instruction for use, patients should be treated with warfarin for 45 days, followed by a 4.5-month of DAPT and lifelong aspirin. However, DAPT had been shown to be an effective alternative in patients with OAC contraindication in the ASAP study [21]. In the EWOLUTION real-life registry that enrolled >1000 patients, DAPT was frequently used (60%) with no increase in DRT risk [22]. In our study, all patients were given DAPT after LAAC with no DRT detected in follow-up. In summary, our study found that the “one-stop” LAAC and PCI followed by short term DAPT therapy was a feasible therapeutic strategy for high bleeding risk AF patients requiring coronary intervention.
Limitations
As a pilot study, we acknowledged that the number of patients enrolled was small. Besides, there is lack of comparative data to the current standard double or triple therapy after PCI. Randomized clinical trial with lager sample size is warranted to verify the safety and efficacy of this “one-stop” combined LAAC/PCI to prevent stroke and reduce risk of bleeding associated with multiple anti-thrombotic.
Conclusions
In this pilot study, “one-stop” LAAC with PCI was efficacious with no stroke, MI, VARC-2 major bleeding or CV death reported over a mean follow-up of 19 months, and safe with no major peri-procedural bleeding or device related complications.
Acknowledgements
This work was supported by grants from the China Postdoctoral Science Foundation Grants (Postdoctoral Science Foundation, Beijing, China) and National Key R&D Program of China (2020YFC2008100).
Disclosure of conflict of interest
None.
Abbreviations
- ACT
active clotting time
- AF
Atrial fibrillation
- CAD
coronary artery disease
- CAG
coronary angiography
- CT
computed tomography
- DAPT
dual anti-platelet therapy
- DRT
device related thrombus
- LAA
left atrial appendage
- LAAC
left atrial appendage closure
- MI
myocardial infarction
- OAC
oral anticoagulants
- PCI
percutaneous coronary intervention
- PE
pericardial effusion
- TEE
transesophageal echocardiography
- TSP
trans-septal puncture
References
- 1.Capodanno D, Huber K, Mehran R, Lip GYH, Faxon DP, Granger CB, Vranckx P, Lopes RD, Montalescot G, Cannon CP, Ten Berg J, Gersh BJ, Bhatt DL, Angiolillo DJ. Management of antithrombotic therapy in atrial fibrillation patients undergoing PCI: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74:83–99. doi: 10.1016/j.jacc.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 2.ACTIVE Writing Group of the ACTIVE Investigators. Connolly S, Pogue J, Hart R, Pfeffer M, Hohnloser S, Chrolavicius S, Pfeffer M, Hohnloser S, Yusuf S. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the atrial fibrillation clopidogrel trial with irbesartan for prevention of vascular events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367:1903–1912. doi: 10.1016/S0140-6736(06)68845-4. [DOI] [PubMed] [Google Scholar]
- 3.Writing Group Members. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM, Yancy CW. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2019;16:e66–e93. doi: 10.1016/j.hrthm.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 4.Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139–e228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 5.Lip GYH, Collet JP, Haude M, Byrne R, Chung EH, Fauchier L, Halvorsen S, Lau D, Lopez-Cabanillas N, Lettino M, Marin F, Obel I, Rubboli A, Storey RF, Valgimigli M, Huber K ESC Scientific Document Group. 2018 Joint European consensus document on the management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous cardiovascular interventions: a joint consensus document of the European Heart Rhythm Association (EHRA), European Society of Cardiology Working Group on Thrombosis, European Association of Percutaneous Cardiovascular Interventions (EAPCI), and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), Latin America Heart Rhythm Society (LAHRS), and Cardiac Arrhythmia Society of Southern Africa (CASSA) Europace. 2019;21:192–193. doi: 10.1093/europace/euy174. [DOI] [PubMed] [Google Scholar]
- 6.Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, De Smet BJ, Herrman JP, Adriaenssens T, Vrolix M, Heestermans AA, Vis MM, Tijsen JG, van’t Hof AW, ten Berg JM WOEST study investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381:1107–1115. doi: 10.1016/S0140-6736(12)62177-1. [DOI] [PubMed] [Google Scholar]
- 7.Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, Birmingham M, Ianus J, Burton P, van Eickels M, Korjian S, Daaboul Y, Lip GY, Cohen M, Husted S, Peterson ED, Fox KA. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375:2423–2434. doi: 10.1056/NEJMoa1611594. [DOI] [PubMed] [Google Scholar]
- 8.Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura T, Maeng M, Merkely B, Zeymer U, Gropper S, Nordaby M, Kleine E, Harper R, Manassie J, Januzzi JL, Ten Berg JM, Steg PG, Hohnloser SH RE-DUAL PCI Steering Committee and Investigators. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513–1524. doi: 10.1056/NEJMoa1708454. [DOI] [PubMed] [Google Scholar]
- 9.Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R, Goodman SG, Windecker S, Darius H, Li J, Averkov O, Bahit MC, Berwanger O, Budaj A, Hijazi Z, Parkhomenko A, Sinnaeve P, Storey RF, Thiele H, Vinereanu D, Granger CB, Alexander JH AUGUSTUS Investigators. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019;380:1509–1524. doi: 10.1056/NEJMoa1817083. [DOI] [PubMed] [Google Scholar]
- 10.Vranckx P, Valgimigli M, Eckardt L, Tijssen J, Lewalter T, Gargiulo G, Batushkin V, Campo G, Lysak Z, Vakaliuk I, Milewski K, Laeis P, Reimitz PE, Smolnik R, Zierhut W, Goette A. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet. 2019;394:1335–1343. doi: 10.1016/S0140-6736(19)31872-0. [DOI] [PubMed] [Google Scholar]
- 11.Bergmann MW, Betts TR, Sievert H, Schmidt B, Pokushalov E, Kische S, Schmitz T, Meincke F, Stein KM, Boersma LVA, Ince H. Safety and efficacy of early anticoagulation drug regimens after WATCHMAN left atrial appendage closure: three-month data from the EWOLUTION prospective, multicentre, monitored international WATCHMAN LAA closure registry. EuroIntervention. 2017;13:877–884. doi: 10.4244/EIJ-D-17-00042. [DOI] [PubMed] [Google Scholar]
- 12.Körmendy D, Pilgrim T, Pulver C, Shakir S, Zanchin T, Gloekler S, Nietlispach F, Rat-Wirtzler J, Moschovitis A, Khattab AA. Outcome after simultaneous PCI and left atrial appendage occlusion. The Journal of Cardiovascular Medicine. 2015;18:96–102. [Google Scholar]
- 13.Glikson M, Wolff R, Hindricks G, Mandrola J, Camm AJ, Lip GYH, Fauchier L, Betts TR, Lewalter T, Saw J, Tzikas A, Sternik L, Nietlispach F, Berti S, Sievert H, Bertog S, Meier B. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion-an update. EuroIntervention. 2020;22:184–184. doi: 10.4244/EIJY19M08_01. [DOI] [PubMed] [Google Scholar]
- 14.Rubboli A, Colletta M, Valencia J, Capecchi A, Franco N, Zanolla L, La Vecchia L, Piovaccari G, Di Pasquale G WARfarin and Coronary STENTing (WAR-STENT) Study Group. Periprocedural management and in-hospital outcome of patients with indication for oral anticoagulation undergoing coronary artery stenting. J Interv Cardiol. 2009;22:390–397. doi: 10.1111/j.1540-8183.2009.00468.x. [DOI] [PubMed] [Google Scholar]
- 15.Leon MB, Baim DS, Popma JJ, Gordon PC, Cutlip DE, Ho KK, Giambartolomei A, Diver DJ, Lasorda DM, Williams DO, Pocock SJ, Kuntz RE. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent anticoagulation restenosis study investigators. N Engl J Med. 1998;339:1665–1671. doi: 10.1056/NEJM199812033392303. [DOI] [PubMed] [Google Scholar]
- 16.Peritz DC, Chung EH. Left atrial appendage closure: an emerging option in atrial fibrillation when oral anticoagulants are not tolerated. Cleve Clin J Med. 2015;82:167–176. doi: 10.3949/ccjm.82a.14117. [DOI] [PubMed] [Google Scholar]
- 17.Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, Mullin CM, Sick P PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534–542. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 18.Reddy VY, Doshi SK, Sievert H, Buchbinder M, Neuzil P, Huber K, Halperin JL, Holmes D PROTECT AF Investigators. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3-year follow-up of the PROTECT AF (watchman left atrial appendage system for embolic protection in patients with atrial fibrillation) trial. Circulation. 2013;127:720–729. doi: 10.1161/CIRCULATIONAHA.112.114389. [DOI] [PubMed] [Google Scholar]
- 19.Holmes DR Jr, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, Huber K, Reddy VY. Prospective randomized evaluation of the watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1–12. doi: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 20.Reddy VY, Holmes D, Doshi SK, Neuzil P, Kar S. Safety of percutaneous left atrial appendage closure: results from the watchman left atrial appendage system for embolic protection in patients with AF (PROTECT AF) clinical trial and the continued access registry. Circulation. 2011;123:417–424. doi: 10.1161/CIRCULATIONAHA.110.976449. [DOI] [PubMed] [Google Scholar]
- 21.Reddy VY, Mobius-Winkler S, Miller MA, Neuzil P, Schuler G, Wiebe J, Sick P, Sievert H. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA plavix feasibility study with watchman left atrial appendage closure technology) J Am Coll Cardiol. 2013;61:2551–2556. doi: 10.1016/j.jacc.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 22.Ciccarelli G, D’Amico C, Izzo M, Cimmino G, Morello A. Safety of percutaneous left atrial appendage occlusion in patients with atrial fibrillation after acute coronary syndrome: a single center experience. International Journal of Cardiovascular Research. 2017;8602:2. [Google Scholar]


