Abstract
Fetal cardiac intervention is an in-utero cardiac procedure done in fetuses with heart diseases like severe aortic stenosis with evolving hypoplastic left heart syndrome, hypoplastic left heart syndrome with an intact or restricted atrial septum, pulmonary atresia with an intact ventricular septum, fetal heart block obstructed total anomalous pulmonary venous return, pericardial collection. The successful biventricular repair can be done in postnatal life after aortic or pulmonary valvuloplasty. Fetal bypass is very challenging because of different physiology. Low prime volume with the high flow can be used to prevent an inflammatory response.
Keywords: Fetal cardiac intervention, fetal cardiac surgery, fetal bypass, fetal aortic valvuloplasty
Introduction
Fetal cardiac intervention and cardiac surgery is an emerging branch. Cardiac development is completed by eight weeks of gestation. With developed fetal imaging like fetal echocardiography and fetal MRI (Magnetic resonance imaging), most congenital heart disease can be detected by the 12th week. Primary morphological changes in the heart during the fetal period like valvular lesions can cause secondary changes in the heart like ventricular hypoplasia. Therefore early intervention during a fetal period can prevent secondary changes. Fetal cardiac intervention commonly used in fetal aortic stenosis, hypoplastic left heart syndrome, pulmonary atresia with an intact ventricular septum, fetal tachycardia, bradycardia, heart failure. For fetal cardiac surgery, appropriate extracorporeal support is needed as fetal physiology is different from neonatal physiology. Unfortunately, no study on human fetal bypass published to date. But there are few studies on the animal model which showed post bypass placental dysfunction is very much common due to fetal stress, prime volume and extracorporeal surface [1].
Fetal cardiac intervention
First-time fetal cardiac intervention was done in 1975 for fetal ventricular tachycardia [2]. In 1987, first fetal cardiac pacing was done due to complete heart block-induced fetal hydrops fetalis. The first fetal balloon aortic dilatation was done 25 years ago [3].
Fetal cardiac intervention is indicated in many cardiac lesions like restricted or intact atrial septum and severe aortic stenosis (AS) with evolving hypoplastic left heart syndrome, severe mitral stenosis, and pulmonary atresia with the intact ventricular septum (PA-IVS), pulmonary atresia with ventricular septal defect with small pulmonary artery, complete transposition of great vessel with restricted atrial communication or intact atrial septum. Contraindications are multiple gestations, major anomalies, contraindication to anesthetic drugs. Fetal cardiac intervention is mainly subdivided into pharmacological intervention and closed intervention [4].
Pharmacological intervention
Apart from transplacental injection other routes of medications are umbilical vein injection, fetal intramuscular injection, fetal intravascular injection and fetal intracardiac injection. Fetal medical therapy with digoxin is used in fetal heart failure due to poor placental transfer of digoxin due to hydrops [5].
Closed intervention
5% prenatally diagnosed congenital heart diseases (CHD) including Severe AS (1.6%), PA-IVS (1.9%), and severe pulmonary stenosis (1.5%) can be critical and therefore require intrauterine intervention. Early diagnosis and treatment of treatable CHD improves ventricular growth and angiogenesis [6].
Aortic valvuloplasty in evolving hypoplastic left heart syndrome
Fetuses with severe aortic stenosis evolve into hypoplastic left heart syndrome if not treated in the fetal period. Severe aortic stenosis causes systolic and diastolic dysfunction of the left ventricle and increased left atrial and left ventricle filling pressure which causes left ventricular underdevelopment due to redistribution of flow from the left atrium to the right atrium [7]. Following are the echocardiography criteria suggestive of progression to hypoplastic left heart syndrome-narrow aortic jet more than 2 m/s, dilated dysfunctional left ventricle, neo development of mitral regurgitation, monophasic mitral inflow Doppler, left to right shunt across foramen ovale, reversal of flow in the aortic arch, abnormal pulmonary vein flow, endocardial fibroelastosis [8]. Predicting criteria for biventricular repair was described like; left ventricle long axis z score >0, left ventricle short-axis z score >0, aortic annulus z score >-3.5, mitral valve annulus z score >-2, mitral regurgitation or aortic stenosis maximum gradient more than 20 mm of hg and left ventricular pressure more than 47 mm of hg and ascending aortic z score >0.57) [9,10]. Fetal aortic valvuloplasty is commonly performed between 21-32 weeks (Figure 1). 18 G/19 G Hawkins Atkins needle/Chiba needle/M3 coaxial needle, 0.014-inch coronary guidewire, Maverick/Hiyuru/Relysis coronary balloon are used. Apical left ventricular puncture is done well-aligned to left ventricular outflow tract and prepared guidewire balloon assembly is advanced through the needle. The balloon to annulus ratio of 1-1.2 provides the best result. Fetal aortic regurgitation is the most common complication which resolves within few weeks [11]. According to the study by Freud et al 43% of patients following aortic valvuloplasty underwent biventricular repair [12]. As per the International fetal cardiac intervention registry, in 2015 80% of patients in the fetal aortic valvuloplasty group lived and 40% underwent biventricular repair [13].
Figure 1.

Fetal aortic valvuloplasty is performed using 18 G/19 G Hawkins Atkins needle, 0.014 inch coronary guide wire and Maverick coronary balloon.
Pulmonary valve perforation in pulmonary atresia with intact ventricular septum
Fetuses with PA-IVS developed hypertrophy and hyperplasia of the right ventricular muscle with low right ventricular volume. Pulmonary valvuloplasty is done in the fetus of 21-32 week with membranous pulmonary atresia with tricuspid valve z score <-2. The right ventricular outflow tract is approached through via subcostal approach or intercostals space next to the sternum. Cannula tip is directed towards pulmonary valve and inserted to right ventricular outflow tract with 10-15 degree angulation (Figure 2). Balloon- annulus ratio is 1.2-1.3 and balloon length should be 8-10 mm to prevent right ventricular free wall dilatation [14]. Some of the predictors of single ventricular outcome are tricuspid valve: mitral valve ratio ≤0.83, right ventricle: left ventricle length ratio ≤0.64, pulmonary valve: aortic valve ratio ≤0.75, tricuspid inflow duration/cardiac cycle length ≤36.5% [15]. Biventricular repair has been achieved in some center yielding good results where fetal pulmonary valvuloplasty has been performed in utero in pulmonary atresia with an intact ventricular septum [16].
Figure 2.

Pulmonary valve perforation with catheter inserted to right ventricular outflow tract with 10-15 degree angulations.
Atrial septoplasty or atrial stenting in hypoplastic left heart syndrome with intact atrial septum or restricted atrial septum
Hypoplastic left heart syndrome with intact or restricted atrial septum causes an increase in left atrial pressure followed by increase pulmonary vascular pressure which increases mortality and morbidity after stage 1 palliation. Indications of intervention are pulmonary venous doppler forward: retrograde velocity-time integral ratio less than 3, maternal hyper-oxygenation (post 28 weeks) less than 10% reactivity [17]. Right atrium is punctured perpendicular to inter atrial septum with an 18 G/17 G needle which can be advanced to perforate the septum. Then either balloon is inserted (Figure 3) or short length stent (3.5×13 mm) (Figure 4) is inserted. As per international fetal cardiac intervention registry data 65% of patients underwent successful intervention but there was no difference in discharge survival [13]. According to the largest single-center experience creation of atrial communication of more than 3 mm was associated with higher postnatal oxygen saturation and better outcome after stage 1 palliation [18].
Figure 3.

Perforation of the inter-atrial septum after piercing the right atrium with 18 G/17 G needle.
Figure 4.

Stenting of the inter-atrial septum defect with short length stent (3.5×13 mm).
Fetal cardiac surgery
Fetal cardiac surgery is the most challenging part of cardiac surgery as fetal physiology and fetal response to extracorporeal circulation is different from the neonate. To date no original article, a case report was published but few articles were published regarding the effect of extracorporeal circulation on the animal fetus. Non-physiological flow causes placental vasoconstriction followed by placental dysfunction due to reduced placental blood flow. Hypothermia increases sympathetic response which escalates blood viscosity and finally reduces placental blood flow. From a maternal point of view hypothermia also increases uterine contraction especially during the rewarming phase. So, hypothermia is not favorable like neonate [19]. Pulsatile blood flow with high blood flow is needed for adequate fetoplacental perfusion and to reduce placental dysfunction. It reduces vascular resistance through preserving feto-maternal nitric oxide biosynthesis and prevents fetal renin-angiotensin-aldosterone pathway [20]. No or ultralow prime volume is needed to reduce the endothelial reaction. More amount of primed maternal blood dilutes fetal hemoglobin [21]. There are two types of fetal bypass circuits with or without placenta. In case of prolonging bypass without placenta is preferred because of chances of hypoxemia [22]. Continuous hemodiafiltration combined with steroid can reduce inflammatory response [23]. Experimental animal research works without the use of prime volume with the inline axial flow, the pump has also yielded promising outcomes (Figure 5) [24].
Figure 5.
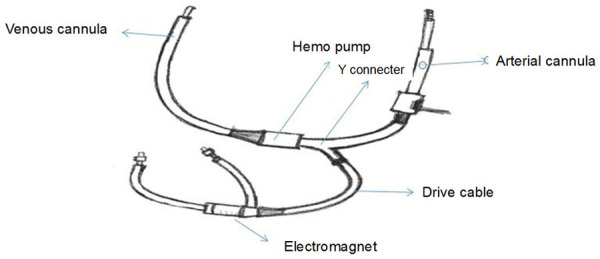
Inline axial flow pump.
Conclusion
Successful planning and implementation of fetal cardiac intervention can increase biventricular repair in postnatal life in patients with severe aortic stenosis with evolving hypoplastic left heart syndrome and pulmonary atresia with an intact ventricular septum. Minimal prime volume with high flow normothermic bypass can be used with good outcomes.
Disclosure of conflict of interest
None.
References
- 1.Bradley SM, Hanley FL, Duncan BW, Jennings RW, Jester JA, Harrison MR, Verrier ED. Fetal cardiac bypass alters regional blood flows, arterial blood gases, and hemodynamics in sheep. Am J Physiol. 1992;263:H919–H928. doi: 10.1152/ajpheart.1992.263.3.H919. [DOI] [PubMed] [Google Scholar]
- 2.Eibschitz I, Abinader EG, Klein A, Sharf M. Intrauterine diagnosis and control of fetal ventricular arrhythmia during labor. Am J Obstet Gynecol. 1975;122:597–600. doi: 10.1016/0002-9378(75)90056-3. [DOI] [PubMed] [Google Scholar]
- 3.Gardiner H. Progression of fetal heart disease and rationale for fetal intracardiac interventions. Semin Fetal Neonatal Med. 2005;10:578–585. doi: 10.1016/j.siny.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Yuan SM. Fetal cardiac interventions. Pediatr Neonatol. 2015;56:81–7. doi: 10.1016/j.pedneo.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Zhou K, Hua Y, Zhu Q, Liu H, Yang S, Zhou R, Guo N. Transplacental digoxin therapy for fetal tachyarrhythmia with multiple evaluation systems. J Matern Fetal Neonatal Med. 2011;24:1378–83. doi: 10.3109/14767058.2011.554924. [DOI] [PubMed] [Google Scholar]
- 6.Wohlmuth C, Tulzer G, Artz W, Gitter R, Wertaschnigg D. Maternal aspects of fetal cardiac intervention. Ultrasound Obstet Gynecol. 2014;44:532–537. doi: 10.1002/uog.13438. [DOI] [PubMed] [Google Scholar]
- 7.Allan LD, Sharland G, Tynan MJ. The natural history of the hypoplastic left heart syndrome. Int J Cardiol. 1989;25:341–343. doi: 10.1016/0167-5273(89)90226-x. [DOI] [PubMed] [Google Scholar]
- 8.Mäkikallio K, McElhinney DB, Levine JC, Marx GR, Colan SD, Marshall AC, Lock JE, Marcus EN, Tworetzky W. Fetal aortic valve stenosis and the evolution of hypoplastic left heart syndrome: patient selection for fetal intervention. Circulation. 2006;113:1401–1405. doi: 10.1161/CIRCULATIONAHA.105.588194. [DOI] [PubMed] [Google Scholar]
- 9.McElhinney DB, Marshall AC, Wilkins-Haug LE, Brown DW, Benson CB, Silva V, Marx GR, Mizrahi-Arnaud A, Lock JE, Tworetzky W. Predictors of technical success and postnatal biventricular outcome after in utero aortic valvuloplasty for aortic stenosis with evolving hypoplastic left heart syndrome. Circulation. 2009;120:1482–1490. doi: 10.1161/CIRCULATIONAHA.109.848994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman KG, Freud L, Escobar-Diaz M, Banka P, Emani S, Tworetzky W. Left ventricular remodeling and function in children with biventricular circulation after fetal aortic valvuloplasty. Pediatr Cardiol. 2015;36:1502–1509. doi: 10.1007/s00246-015-1193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atiyah M, Kurdi A, Al Tuwaijry O, Al Sahari A, Al Rakaf M, Babic I, Al Habshan F, Alhalees Z, Al Najashi K. Fetal aortic valvuloplasty: first report of two cases from Saudi Arabia. J Cardiothorac Surg. 2020;15:150. doi: 10.1186/s13019-020-01195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freud LR, McElhinney DB, Marshall AC, Marx GR, Friedman KG, del Nido PJ, Emani SM, Lafranchi T, Silva V, Wilkins-Haug LE, Benson CB, Lock JE, Tworetzky W. Fetal aortic valvuloplasty for evolving hypoplastic left heart syndrome: postnatal outcomes of the first 100 patients. Circulation. 2014;130:638–645. doi: 10.1161/CIRCULATIONAHA.114.009032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moon-Grady AJ, Morris SA, Belfort M, Chmait R, Dangel J, Devlieger R, Emery S, Frommelt M, Galindo A, Gelehrter S, Gembruch U, Grinenco S, Habli M, Herberg U, Jaeggi E, Kilby M, Kontopoulos E, Marantz P, Miller O, Otaño L, Pedra C, Pedra S, Pruetz J, Quintero R, Ryan G, Sharland G, Simpson J, Vlastos E, Tworetzky W, Wilkins-Haug L, Oepkes D International Fetal Cardiac Intervention Registry. International fetal cardiac intervention registry: a worldwide collaborative description and preliminary outcomes. J Am Coll Cardiol. 2015;66:388–399. doi: 10.1016/j.jacc.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 14.Tworetzky W, McElhinney DB, Marx GR, Benson CB, Brusseau R, Morash D, Wilkins-Haug LE, Lock JE, Marshall AC. In utero valvuloplasty for pulmonary atresia with hypoplastic right ventricle: techniques and outcomes. Pediatrics. 2009;124:e510–e518. doi: 10.1542/peds.2008-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez-Montes E, Herraiz I, Medoza A, Galindo A. Fetal intervention in right outflow tract obstructive disease: selection of candidates and results. Cardiol Res Pract. 2012;2012:592403. doi: 10.1155/2012/592403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tulzer G, Gardiner H. Cardiac interventions in the fetus: potential for right-sided lesions. Fetal interventions in right heart disease. Prog Pediatr Cardiol. 2006;22:79–83. [Google Scholar]
- 17.Schidlow DN, Freud L, Friedman K, Tworetzky W. Fetal interventions for structural heart disease. Echocardiography. 2017;34:1834–1841. doi: 10.1111/echo.13667. [DOI] [PubMed] [Google Scholar]
- 18.Marshall AC, Levine J, Morash D, Silva V, Lock JE, Benson CB, Wilkins-Haug LE, McElhinney DB, Tworetzky W. Results of in utero atrial septoplasty in fetuses with hypoplastic left heart syndrome. Prenat Diagn. 2008;28:1023–1028. doi: 10.1002/pd.2114. [DOI] [PubMed] [Google Scholar]
- 19.Kohl T, Szabo Z, Suda K, Petrossian E, Ko E, Kececioglu D, Moore P, Silverman NH, Harrison MR, Chou TM, Hanley FL. Fetoscopic and open transumbilical fetal cardiac catheterization in sheep: potential approaches for human fetal cardiac intervention. Circulation. 1997;95:1048–1053. doi: 10.1161/01.cir.95.4.1048. [DOI] [PubMed] [Google Scholar]
- 20.Pardi G, Ferrari MM, Iorio F, Acocella F, Boero V, Berlanda N, Monaco A, Reato C, Santoro F, Cetin I. The effect of maternal hypothermic cardiopulmonary bypass on fetal lamb temperature, hemodynamics, oxygenation, and acid-base balance. J Thorac Cardiovasc Surg. 2004;127:1728–1735. doi: 10.1016/j.jtcvs.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 21.Champaur G, Vedrinne C, Martinot S, Tronc F, Robin J, Ninet J, Franck M. Flow induced release of endothelium derived relaxing factor during pulsatile bypass: experimental study in the fetal lamb. J Thorac Cardiovasc Surg. 1997;114:738–745. doi: 10.1016/S0022-5223(97)70077-1. [DOI] [PubMed] [Google Scholar]
- 22.Sakata M, Hisano K, Okada M, Yasufuku M. A new artificial placenta with a central pump: long-term total extrauterine support of goat fetuses. J Thorac Cardiovasc Surg. 1998;115:1023–1031. doi: 10.1016/S0022-5223(98)70401-5. [DOI] [PubMed] [Google Scholar]
- 23.Carotti A, Emma F, Picca S, Iannace E, Albanese SB, Grigioni M, Meo F, Sciarra M, Di Donato RM. Inflammatory response to cardiac bypass in ewe fetuses: effects of steroid administration or continuous hemodiafiltration. J Thorac Cardiovasc Surg. 2003;126:1839–1850. doi: 10.1016/s0022-5223(03)01293-5. [DOI] [PubMed] [Google Scholar]
- 24.Reddy VM, Liddicoat JR, Klein JR, Wampler RK, Hanley FL. Long-term fetal outcome after fetal cardiac bypass: fetal survival to full term and organ abnormalities. J Thorac Cardiovasc Surg. 1996;111:536–544. doi: 10.1016/s0022-5223(96)70305-7. [DOI] [PubMed] [Google Scholar]


