Abstract
Background: Coronary no-reflow (NRF) following percutaneous coronary intervention (PCI) is infrequent but one of the most dreaded complication which results from impaired flow of microvascular bed. It is associated with adverse outcome if flow is not restored. Objective of this study was to find safety, effectiveness and outcome of intracoronary nikorandil (IC) administered using perforated balloon technique (PBT) to reverse NRF. Method: 2-4 mg of nicorandil was diluted with 5 ml of normal saline and administered using PBT over 5-minute. Its effectiveness was evaluated after 10 minute qualitatively using TIMI flow and quantitatively corrected TIMI frame count (cTFC) method. Result: Study comprised of 84 patients (out of 1789 patients undergoing PCI between January 2019 and February 2020). Their mean age was 57.8±17.9 years. Following PBT, TIMI III flow was successfully normalized in 71 subjects (84.5%), ten (12%) patients had TIMI II flow and it was not successful in three (3.5%) patients. TIMI flow grade got bettered from 1.03 to 2.58 and cTIMI frame count regressed from 52.9±11 to 16.5±5 (P < 0.001). PBT was well tolerated except short lived drop in blood pressure (n=10; 11.9%). Conclusion: This study, for the first time to the best our knowledge, demonstrated that PBT mediated intracoronary administration of nikorandil distally was rapid, safe, and efficacious method to deal with NRF.
Keywords: Perforated balloon technique, no-reflow, percutaneous coronary intervention, TIMI frame count, TIMI flow, nicorandil
Introduction
Restoration of flow mechanically by percutaneous coronary intervention (PCI) or pharmacologically using thrombolytics help to achieve only epicardial patency as in small subsets of patients, it does not translate into perfusion at distal microvascular bed which is defined as no-reflow or slow flow [1]. Incidence of no-reflow (NRF) ranges from 7% to 42% depending on the setting like primary PCI, plaque modification using rotablation, and intervention of degenerated venous graft [2,3]. Downstream embolization of thrombi and atheromatous debris causing clogging of microvasculature is primarily responsible for NRF. It is associated with poor outcome such as hypotension, shock, various arrhythmias, systolic dysfunction, and death [4,5]. Reduction of thrombus burden using thrombosuction or pharmacologically using GP IIb/IIIa antagonists, its distal embolization by minimum manipulation of hardwares, and minimizing inflammation by using adenosine, nicorandil, various calcium channel blockers (CCB) and sodium nitroprusside (SNP) have been shown to be effective. Therapeutic options for no-reflow is guided by amount of structural and functional damage of microvasculature as loss of structural integrity and clogging of distal vascular bed are responsible for structural and functional no-reflow respectively. Because of pathophysiology, former is usually irreversible while later, which is mostly seen in catheterization lab in setting of PCI, is dynamic and can be reversed. Functional NRF should be prevented by minimizing downstream embolization and treated by enhancing the targeted drug delivery at the potential site. In setting of no-reflow, downstream flow is already impaired; hence administration of pharmacological agents through guiding catheter may not be beneficial. Distal most delivery may be achieved using over-the-wire balloons (OTW) and micro-catheters which require removal of the workhorse wire which will mean loss of railroad. Moreover, prolonged and rapid infusion at times becomes difficult using this strategy. Therefore, ideal is maintaining the access through work horse wire and at the same time, rapid and targeted delivery using perforated balloon technique. Aim of the study was to access intracoronary delivery of nicorandil using perforated balloon technique (PBT) to facilitate its delivery in distal bed to mitigate no-reflow following PCI.
Methodology
Study subjects
This was a prospective study performed conducted among patients developing NRF following PCI between January 2019 and February 2020 at LPS Institute of Cardiology, Kanpur, India. Clinical indications for PCI were acute coronary syndrome (STEMI, NSTEMI, and UA) and chronic stable angina (CSA). The clinical, angiographic findings [6,7], and details of procedure of patients were recorded.
Written and informed consent from all patients were obtained before performing intervention and protocol was approved by institutional ethical committee (GSVM Medical College EC-81/December/2018). Exclusion criteria for PCI were allergy to acetyl salicylic acid (ASA), P2Y12 inhibitors (clopidogrel, ticagrelor, prasugrel), heparin, pregnant women, conditions warranting discontinuation of dual antiplatelets within 6-months following PTCA, and survival expectancy < 1 year. All patients in whom no-reflow was noted were treated using PBT. Patients who had impaired TIMI flow as a result of vascular spasm, edge dissection, thrombus, and no-reflow in vein grafts were excluded.
Details of PCTA
PCI was carried out in standard fashion using unfractionated heparin as anticoagulant on weight based regimen to achieve activated clotting time (ACT) >250 sec. Plaque modification was done before stent deployment except when they were soft and thrombotic, and post dilatation using noncompliant and OPN (open super high pressure) balloon were performed accordingly. All patients were loaded with acetyl salicylic acid (ASA)-325 mg and P2Y12 inhibitors and both were continued for at least 1-year followed by ASA alone thereafter.
Periprocedural estimation of coronary flow
Cineangiograms were analysed and labelled independently by two intervention cardiologists to qualify thrombolysis in myocardial infarction (TIMI) flow according to criteria laid down in TIMI trial at beginning of PCI following coronary angiogram, onset of NRF, and following completion of procedure [8]. NRF was labelled when contrast injection revealed TIMI flow < grade III after stent implantation. Mechanical causes (flap, tear, and thrombus) and vascular spasm of culprit artery were excluded before diagnosing NRF. Angiographic flow was quantified by corrected TIMI frame count (cTFC) method [9]. All angiographic run were recorded at speed of 30 frames per second in a field size that led to visualization of entrance of dye into culprit artery and its subsequent run-off from distal landmark branch (tele-tele branch). It was later reviewed by an independent investigator in arbitrary sequence that was blind to succession of injections. TFC was calculated as total number of angiographic run between initial and final frame where initial frame was considered as first frame in which dye fully entered the vessel while dye exiting the distal landmark branch was considered as final frame. An intra-observer variance was assessed using two different readings and kept as ±5 frames. cTFC was collated beforehand and following PBT.
Protocol of Perforated balloon technique
A semicompliant balloon (2.5×15 mm or longer) was chosen for PBT as it could easily cruise to distal most end of coronary artery and had sufficient volume to accommodate the dosage of nicorandil (Figure 1A). It was inflated using inflation device filled with 20 ml of normal saline (Figure 1B). Once inflated, 6 to 10 holes were made using micro puncture needle (26 gauze) in different parts of inflated balloon and sufficient amount of saline was allowed to ooze from the balloon to make it completely air free and then was disconnected from inflation device (Figure 2). The balloon was slowly pushed over the coronary wire into the guide catheter. The haemostatic valve of tuohy borst adapter was kept open till the balloon reached at the tip of guiding catheter to prevent any air suction and was subsequently advanced into distal most part of the culprit coronary artery. 2 mg of Nicorandil was diluted with 5 ml of 0.9% normal saline and was continuously administered using 5 ml syringe while pulling the balloon slowly from the distal segment to the proximal segment of the coronary artery. This manuover was repeated two or three times over 2-3 min while closely monitoring the electrocardiogram and vital parameters. TIMI flow and cTFC was finally re-evaluated at 10 minute after removing the perforated balloon. In refractory situation, other course of action like intracoronary SNP, adenosine, verapamil, diltiazem, and autologus blood transfusion were exercised after 15 minute.
Figure 1.

A semicompliant balloon (2.5×15 mm) was chosen for PBT (A); In-vitro inflation of the balloon catheter was performed at 10 atm pressure using the inflation device (B).
Figure 2.
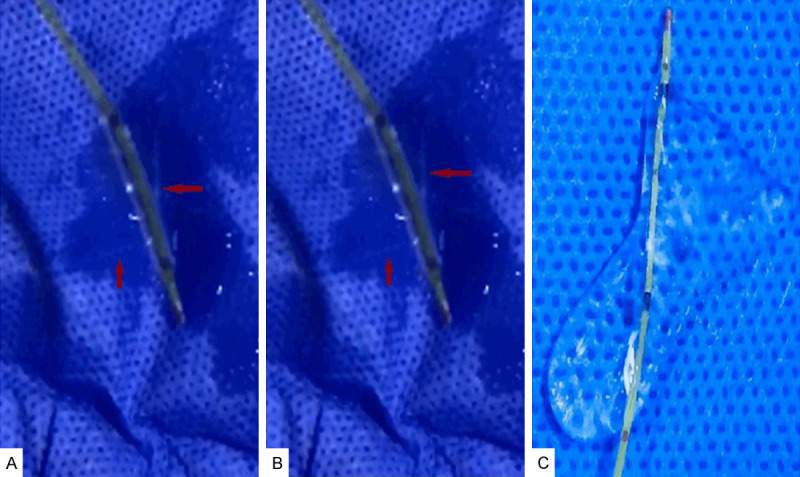
Six to ten punctures in different areas of inflated balloon segment were performed using a 26 gauze needle (A); The saline was allowed to ooze from the perforated segments to remove all possible micro-bubbles (B, C).
Statistical evaluation
Data were analysed using SPSS 20.0 using statistical package for the social sciences (IBM SPSS Statistics). All values were expressed as mean ± standard deviation, while categorical data were expressed as percentage. Unlikeness of TFC, pre and post PBT, were analyzed by Chi-square method. Statistical probability considered as P < 0.05 was assumed significant.
Results
Baseline demographics and findings on coronary angiogram were recorded (Table 1). NRF was observed in 84 patients among 1789 interventions performed during index period. The male and female were 61 (73%) and 23 (27%) respectively. Mean age of subjects was 57.8±17.9 years. Tobacco consumption was most common (n=28; 33.3%) followed by hypertension (n=24; 28.6%). Clinical presentation were STEMI (n=60; 71%), NSTEMI (n=14; 17%), and UA (n=07; 8%). LV systolic function was critically depressed (EF < 35%) among 16 (18.5%) subjects while moderately impaired in 24 (28.1%) patients. Left anterior descending artery was the commonest artery involved (n=49; 58%).
Table 1.
Baseline and demographic characteristics of patients (N=84)
| Characterisyic | No (%) |
|---|---|
| Age (years) | 57.8±17.9 |
| Male | 61 (73%) |
| Female | 23 (27%) |
| CAD risk factors | |
| HTN | 24 (28.6%) |
| DM | 22 (26.2%) |
| Smoking | 28 (33.3%) |
| Family history of CAD | 9 (10%) |
| Dyslipidemia | 20 (21.5%) |
| Post CABG | 3 (3.5%) |
| Clinical Presentation | |
| STEMI | 60 (71%) |
| a. Primary | 22 (26%) |
| b. Pharmaco-invasive | 27 (32%) |
| c. Rescue PTCA | 11 (13%) |
| NSTEMI | 14 (17%) |
| UA | 07 (8%) |
| CCS | 03 (4%) |
| LVEF (%) | |
| a. >45% | 44 (52.4%) |
| b. 35-45% | 24 (28.1%) |
| c. 35% | 16 (18.5%) |
| SBP (mmHg) | 104±22 mmHg |
| DBP (mmHg) | 68±12 mmHg |
| High Polymorph Count (>8800 mm3) | 19 (22.6%) |
| Serum Creatinine (mg%) | 1.2 (0.9-1.9) |
| Medications | |
| Aspirin | 76 (90%) |
| Clopidogrel | 43 (51%) |
| Prasugrel | 19 (28%) |
| Ticagrelor | 22 (26%) |
| Statin | 72 (89%) |
| Carvidolol/Metoprolol/Bisoprolol | 57 (67%) |
| ACEI/ARB | 49 (58%) |
| Eplineron | 22 (33.7%) |
| Coronary artery involvement | |
| 1. SVD | 45 (54%) |
| 2. DVD | 23 (27%) |
| 3. TVD | 16 (19%) |
| Culprit lesion location | |
| a. LAD | 49 (58%) |
| b. RCA | 24 (28.5%) |
| c. LCx | 11 (13.5%) |
Data presented as mean ± standaid deviation oi niunbei (peicentage). CAD = Coionaiy artery cUsease; DM = Diabetes mellitiis; SBP = Systolic blood piessiue; DBP = Diastolic blood piessiue; PCI- = Peicutaneous Coionaiy Inteivention; STEMI = ST Segment Elevation Myocaiclial Infaiction; LVEF = ventricular ejection fraction; ACEI = Angiotensin-converting enzyme inhibitor; ARB = Angiotensin-receptor blocker; SVD = Single vessel disease; DVD = Double-vessel disease; TVD = Triple-vessel disease; LAD = Left anterior descending coronary artery; LCx = Left circumflex coronary artery; RCA = Right coronary artery.
Mean duration from onset of symptom to cannulation of culprit artery by guide catheter was 5.9±4.9 hrs in primary angioplasty group while 21.8±4.6 hrs and 28.5 hrs in rescue and pharmaco-invasive group respectively. Culprit vessel was supported either antegradely or retrogradely in 23 (24.7%) subjects and classified as grade 3 (n=9; 10.7%), grade 2 (n=10; 11.9%), and grade 1 (n=3; 3.5%). Tirofiban was administered upfront among 8 (9.5%) subjects and 12 (14.2%) received downstream following stenting when slow flow set in. NRF was encountered following stent deployment (n=61; 71%) and poststenting balloon dilatation (n=23; 29%) of whom perfusion was labelled as TIMI 1 in 51 (60.7%) subjects and while TIMI 2 in 31 (35.7%) subjects (Figure 3).
Figure 3.

Changes of TIMI flow grading at baseline, NRF after deployment of stent and reversal of no reflow following PBT which led improvement in TIMI flow.
Average dose of 2.9 mg (median-2.7 ml, range: 2-5.3 mg) of nicorandil was administered (Table 2). Changes in TIMI flow and cTFC following onset of NRF and changes after administration of nicorandil using PBT have been depicted in Figures 4, 5 respectively. A significant improvement in TIMI flow (progressed from 1.02 to 2.61) and successful attainment of TIMI 3 flow were observed among 71 subjects who had overall success rate of 84.5%. Subjects having the lowest TIMI flow demonstrated maximum improvement (Figure 3). Also, TIMI frame count came down to 19±5 following PBT from 64±13 following onset of NRF (P < 0.001) thus showing an average gain of 45±8 (Figure 5). A prototype scenario of NRF following multiple stent deployment in LAD and its successful reversal with nicorandil using PBT has been shown in Figures 6, 7, 8, 9 and 10.
Table 2.
Procedural characteristics and outcome of patients (N=84)
| Variables | No (%) |
|---|---|
| AHA/ACC lesion class | |
| a. A | 06 (7.1%) |
| b. B1 | 11 (13.1%) |
| c. B2 | 23 (27.4%) |
| d. C | 44 (52.4%) |
| Lesion characteristics | |
| a. Ulcerated | 11 (13.1%) |
| b. Thrombus | 59 (70.2%) |
| c. Acute total occlusion | 34 (40.5%) |
| d. Dissection | 04 (4.7%) |
| Collaterals present (grade 1-3) | 19 (22.7%) |
| Size of vessels | |
| a. 2.5-3 mm | 32 (38%) |
| b. 3-4 mm | 45 (54%) |
| c. ≥4 mm | 07 (8%) |
| TTMI flow pre procedure | |
| a. Grade 0 | 38 (45%) |
| b. Grade 1 | 20 (24%) |
| c. Grade 2 | 15 (18%) |
| d. Grade 3 | 11 (13%) |
| Median length of stent per patient (mm) | 32±11 |
| Procedural Details | |
| Lesion Preparation | |
| a. Dnect Stentnig | 09 (11%) |
| b. Tlrombosuction | 13 (15%) |
| c. Predilatation | 65 (77%) |
| d. GP IIb/IIIa antagonist (Tirofiban) | 20 (23%) |
| Post-dilation | 67 (79%) |
| Nicorandil (mg) | 2.9 (2-4) |
| Transient hypotension | 10 (11.9%) |
| Corrected TIMI Frame count (Pre PBT) | 52.9±11 |
| Corrected TIMI Frame count (Post PBT) | 16.5±5 |
| TIMI flow post PBT | |
| a. Grade 0 | 00 (00%) |
| b. Grade 1 | 03 (3.5%) |
| c. Grade 2 | 10 (12%) |
| d. Grade 3 | 71 (84.5%) |
CV end points-Cardiovascular end points; MACCE-Major acute cardio cerebrovascular events-Composite of CV events and all deaths; TLR-Target lesion revascularization; PBT-Perforated balloon technique.
Figure 4.

Changes of TIMI flow grading at baseline, after deployment of stent and after PBT. There was significant improvement in TIMI flow following PBT (P < 0.001) as TIMI flow was impaired in all patients prior to treatment.
Figure 5.

cTFC at the time of no-reflow and after PBT showing significant reduction in TIMI frame count (P < 0.001).
Figure 6.

Angiogram of left system showing diffuse disease of left anterior descending artery (A); After predilatation, it was stented using 3×33 mm Xience Prime everolimus eluting stent (Abott Vascular; USA) at 12 atm pressure proximally (B) and 2.75×33 mm Xience Prime distally (C).
Figure 7.
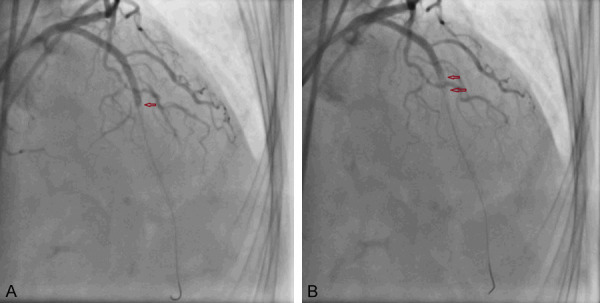
No reflow of LAD with no filling of contrast from mid segment of stent (A, B).
Figure 8.

Nicorandil was administered using PBT from distal most segment to proximal segment of LAD (A, B).
Figure 9.

Nicorandil was administered using PBT from distal most segment to proximal segment of LAD (A-C).
Figure 10.

Reversal of no reflow after Nicorandil administration with TIMI 3 flow in LAD.
Sequel of hospitalization
In 3 patients with no-reflow who were non responsive to PBT, 2 subjects experienced limited improvement (TIMI 2) after receiving SNP. Both got relieved from hospital after 9.7±2.4 days. Third patient got detoriated as a result of progressive pump dysfunction and died on seventh day. Re-infarction and stent thrombosis were not observed among any patients during period of hospital stay.
Discussion
Out study revealed the safety and effectiveness of IC nicorandil using PBT to rescind no-reflow phenomenon following PCI with overall success rate of 84.5% and was safe and efficacious.
NRF can mitigate the advantages gained from opening the occluded artery and portends adverse cardiovascular prognosis. Underlying microvascular dysfunction, ischaemia reperfusion cascade, thrombus load, collateral perfusion, diabetes mellitus and dyslipidemia are few of the contributing factors. Nicorandil is nitrate like congener which selectively opens ATP-sensitive potassium channel (K+ATP) in vascular endothelium of arterioles. It decreases sarcolemmal calcium release which reduces intracellular calcium burden thereby causes vasodilatation of arteriolar bed and increases blood flow. It also mediates cardio protection by ischemic preconditioning, anti-arrhythmic effects, and reduction of ischemia mediated reperfusion injury [10,11]. Contrary to nitrates which causes vasodilatation of large conductive vessels (epicardial vessels), nicorandil acts at arteriolar level which is the seed of disease in NRF and thus helps to restore the flow [12].
Although its efficacy has already been demonstrated by Kostic and co-workers for treating NRF, [12] it was evaluated in setting of primary PCI and number of patients (n=32) were limited in contrast to our study which included all substrate of PCI and included fairly large number of patients (n=84) showing its effectiveness among patients with acute as well as chronic coronary syndrome.
While TIMI flow grade correlates with epicardial perfusion, TIMI myocardial perfusion grade (TMPG) corresponds to myocardial perfusion [13]. PBT mediated rapid delivery of nicorandil at microvasculature and achieved its therapeutic blood concentrations. Our findings were concordant with report by Pang who demonstrated its beneficial effect in NRF following elective PCI [12]. In their study, patients already received nikorandil both upstream and downstream following onset of NRF. Therefore, it might have acted as confounder and all beneficial effects are difficult to be attributed to downstream administration of nicorandil alone. In our study, nicorandil was administered only downstream with excellent result.
Its safety was also proven in our study as hypotension was noted among 11.9% patients in our study and consistent with findings reported by Qi [14]. It was significantly lower compared to sodium nitroprusside (25%). Our technique ensured focussed delivery of nicorandil at microvascular bed only in the culprit artery, thereby curtailed its systemic release. Moreover, all episodes were transient with its spontaneous recovery. This was also significant considering the fact that 18.5% of study population had already severely impaired systolic function (< 35%). It also indicated that it may score over sodium nitroprusside (SNP) to reverse NRF in background of STEMI as hypotension is poorly tolerated among them and SNP has tendency to reduce blood pressure further. Moreover, SNP was found to be effective in only 82% when used as 100 µg bolus while results were negative when used as 60 µg bolus [15]. When it was combined with adenosine, results were little better and concordant with our findings but side effects were more [15].
The success rate of PBT using nikorandil (84.5%) in our study was comparable to other pharmacological measures like adenosine and nitroprusside either in isolation or combination, various calcium channel antagonists (nicardipine, diltiazem, verapamil) and epinephrine which had success rate of 65%-95% [16]. These were associated with various side effects like atrio-ventricular (AV) block, need of temporary pacemaker, chronotropic drugs (atropine) and ST segment changes (elevation/depression).
Adenosine has also been used and is quite effective for no-reflow but require quick and multiple administration because of ultra short half life (6 seconds) and therefore makes it time consuming and costlier. It also causes bronchospasm, chest tightness, momentary lowering of blood pressure, and short lived AV block [17,18]. In REOPEN TRIAL, IC adenosine was demonstrated to be superior over nitroprusside and placebo among patients with STEMI where efficacy was shown to be 71%, 54% and 50% respectively. Our study demonstrated better efficacy than either of three (84.5%) despite the fact that only 79% of patients received upfront infusion of GpIIb/IIIa compared to 100% in their study [19]. In ADAPT trial, adenosine failed to show superiority over placebo among patients with no-reflow which was evaluated using myocardial blush and TIMI flow. However, thrombus aspiration and abciximab infusion might have dampened the results [20]. Adenosine was shown to be effective for no-reflow in AMISTAD II trial but required upstream administration prior to PCI and continous infusion over 3 hours [21]. Contrary to adenosine, nicorandil through PBT mostly requires single administration and is quite safe because of minimal side effects. In contrast to adenosine, it has got limited role in combating no-reflow following vein graft [22-24].
CCBs (nicardipine, diltiazem, nifedipine) have been reported to be productive but biggest apprehension remains different degree of atrio-ventricular block and momentary lowering of blood pressure. Their efficacy has been demonstrated to be 87% to 96% among various studies with average reduction of TFC of 32±5 frames [15,25,26]. Our findings were concordant with these findings. In some studies CCB were delivered distally beyond the stent using microcatheter but they had potential chance of loss of wire access further whereas wire access was always maintained using our technique. In comparison to different CCBs, PBT is better and safer as arrhythmias were not observed among any patients in our study [27-29]. In our study, tirofiban was used in 23% of subjects who might have been benefitted by this therapy as well.
Our technique was less time consuming, easy to use and reproducible. Multiple holes on the balloon helped in delivering nicorandil in controlled, uniform and targeted manner, and prohibited any high velocity traumatic jet to the arterial wall. In a similar study which used adenosine through PBT, only four holes were punctured contrary to six to ten holes in our study [30]. More number of holes facilitates delivery of drug quickly. Using PBT, desired drug could be delivered multiple times through several passes without losing the wire access. Distal drug delivery can also be performed using over-the-wire balloon or micro-catheter (Finecross, Crusade, Caravel, Tornus) but they require exchange of wire and drug is released only through distal lumen. This potentially reduces time area curve of drug thus reduces its effectiveness and is time consuming. It can also be performed using dual lumen catheters (e.g. Twinpass) or thrombosuction catheter (e.g. Export) but they are bulkier in size, costly and may cause thromboembolism [31,32]. Similarly, tenecteplase was used to treat NRF by Kelly and co-workers using dedicated delivery catheters (Export catheter, Pronto Catheter) but had the similar limitations [33]. Despite aggressive administration of different vasodilators (adenosine, nitroglycerin, SNP, verapamil, diltiazem, and nikorandil) directly through the guide catheter, NRF sometimes may be non-responsive because of drug may fail to reach its target (microvasculature) [34].
Guide catheter mediated injection of drugs may lead to overflow to the aortic sinus, delivery to non-affected artery, and systemic release into circulation which not only account for suboptimal concentration at coronary microvasculature but also side effects [35]. It also demonstrated rapid recovery of blood flow with minimal hemodynamic compromise indicating that this was safe and effective method of drug administration. One advantage which can be extrapolated from our study using PBT is that it can be used for prolonged infusion of thrombolytic agents (urokinase, streptokinase) in patients with pulmonary embolism, or critical limb ischaemia [36].
Study limitations
Although this was a prospective study, it lacked randomization, had smaller sample size, and no long term follow up was made.
Conclusions
Phenomenon of no-reflow is not infrequent following percutaneous coronary intervention. This study, for the first time to the best our knowledge, demonstrated the safety as well as efficacy of intracoronary nicorandil using perforated balloon technique and may be one of the alternative to treat no-reflow following PCI.
Disclosure of conflict of interest
None.
References
- 1.Rezkalla SH, Kloner RA. No-reflow phenomenon. Circulation. 2002;105:656–662. doi: 10.1161/hc0502.102867. [DOI] [PubMed] [Google Scholar]
- 2.Ndrepepa G, Tiroch K, Keta D, Fusaro M, Seyfarth M, Pache J, Mehilli J, Schömig A, Kastrati A. Predictive factors and impact of no-reflow after primary percutaneous coronary intervention in patients with acute myocardial infarction. Circ Cardiovasc Interv. 2010;3:27–33. doi: 10.1161/CIRCINTERVENTIONS.109.896225. [DOI] [PubMed] [Google Scholar]
- 3.Resnic FS, Wainstein M, Lee MK, Behrendt D, Wainstein RV, Ohno-Machado L, Kirshenbaum JM, Rogers CD, Popma JJ, Piana R. No-reflow is an independent predictor of death and myocardial infarction after percutaneous coronary intervention. Am Heart J. 2003;145:42–46. doi: 10.1067/mhj.2003.36. [DOI] [PubMed] [Google Scholar]
- 4.Morishima I, Sone T, Okumura K, Tsuboi H, Kondo J, Mukawa H, Matsui H, Toki Y, Ito T, Hayakawa T. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first myocardial infarction. J Am Coll Cardiol. 2000;36:1202–1209. doi: 10.1016/s0735-1097(00)00865-2. [DOI] [PubMed] [Google Scholar]
- 5.Bolognese L, Carrabba N, Parodi G, Santoro GM, Buonamici P, Cerisano G, Antoniucci D. Impact of microvascular dysfunction on left ventricular remodelling and long-term clinical outcome after primary coronary angioplasty for acute myocardial infarction. Circulation. 2004;109:1121–1126. doi: 10.1161/01.CIR.0000118496.44135.A7. [DOI] [PubMed] [Google Scholar]
- 6.Jaffe R, Charron T, Puley G, Dick A, Strauss BH. Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation. 2008;117:3152–6. doi: 10.1161/CIRCULATIONAHA.107.742312. [DOI] [PubMed] [Google Scholar]
- 7.Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol. 1985;5:587–592. doi: 10.1016/s0735-1097(85)80380-6. [DOI] [PubMed] [Google Scholar]
- 8.Sheehan FH, Braunwald E, Canner P, Dodge HT, Gore J, Van Natta P, Passamani ER, Williams DO, Zaret B. The effect of intravenous thrombolytic therapy on left ventricular function: a report on tissue-type plasminogen activator and streptokinase from the Thrombolysis in Myocardial Infarction (TIMI Phase I) trial. Circulation. 1987;72:817–829. doi: 10.1161/01.cir.75.4.817. [DOI] [PubMed] [Google Scholar]
- 9.Gibson CM, Cannon CP, Daley WL, Dodge JT Jr, Alexander B Jr, Marble SJ, McCabe CH, Raymond L, Fortin T, Poole WK, Braunwald E. TIMI frame count. A quantitative method for assessing coronary artery flow. Circulation. 1996;93:879–888. doi: 10.1161/01.cir.93.5.879. [DOI] [PubMed] [Google Scholar]
- 10.Neda P, Homa F. Nicorandil in patients with acute coronary syndrome and stable angina undergoing percutaneous coronary intervention: literature review. Rev Clin Med. 2015;2:42–44. [Google Scholar]
- 11.Pang Z, Zhao W, Yao Z. Cardioprotective effects of nicorandil on coronary heart disease patients undergoing elective percutaneous coronary intervention. Med Sci Monit. 2017;23:2924–2930. doi: 10.12659/MSM.902324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kostic J, Djordjevic-Dikic A, Dobric M, Milasinovic D, Nedeljkovic M, Stojkovic S, Stepanovic J, Tesic M, Trifunovic Z, Zamaklar-Tifunovic D, Radosavljevic-Radovanovic M, Ostojic M, Beleslin B. The effects of nicorandil on microvascular function in patients with ST segment elevation myocardial infarction undergoing primary PCI. Cardiovasc Ultrasound. 2015;13:26. doi: 10.1186/s12947-015-0020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen CH, Fu XH, Li W. Intracoronary administration of anisodamine and nicorandil in individuals undergoing primary percutaneous coronary intervention for acute inferior myocardial infarction: a randomized factorial trial. Exp Ther Med. 2015;10:1059–1065. doi: 10.3892/etm.2015.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi Q, Niu J, Chen T, Yin H, Wang T, Jiang Z. Intracoronary nicorandil and the prevention of the no-reflow phenomenon during primary percutaneous coronary intervention in patients with acute ST-segment elevation myocardial infarction. Med Sci Monit. 2018;24:2767–2776. doi: 10.12659/MSM.906815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vijayalakshmi K, Whittaker VJ, Sutton A, Campbell P, Wright RA, Hall JA, Harcombe AA, Linker NJ, Stewart MJ, Davies A, de Belder MA. Prospective, randomized con-trolled trial to study the effect of intracoronary injection of verapamil and adenosine on coronary blood flow during percutaneous coronary intervention in patients with acute coronary syndromes. Heart. 2006;92:1278–1284. doi: 10.1136/hrt.2005.075077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barcin C, Denktas AE, Lennon RJ, Hammes L, Higano ST, Holmes DR Jr, Garratt KN, Lerman A. Comparison of combination therapy of adenosine and nitroprusside with adenosine alone in the treatment of angiographic no-refl ow phenomenon. Catheter Cardiovasc Interv. 2004;61:484–91. doi: 10.1002/ccd.20010. [DOI] [PubMed] [Google Scholar]
- 17.Assali AR, Sdringola S, Ghani M, Denkats AE, Yepes A, Hanna GP, Schroth G, Fujise K, Anderson HV, Smalling RW, Rosales OR. Intracoronary adenosine administered during percutaneous intervention in acute myocardial infarction and reduction in the incidence of ‘no-reflow’ phenomenon. Catheter Cardiovasc Interv. 2000;51:27–31. doi: 10.1002/1522-726x(200009)51:1<27::aid-ccd7>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 18.Fischell TA, Carter AJ, Foster MT, Hempsall K, DeVries J, Kim DH, Kloostra A. Reversal of ‘no-reflow’ during vein graft stenting using high velocity boluses of intracoronary adenosine. Cathet Cardiovasc Diagn. 1998;45:366–367. doi: 10.1002/(sici)1097-0304(199812)45:4<360::aid-ccd1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 19.Niccoli G, Rigattieri S, De Vita MR, Valgimigli M, Corvo P, Fabbiocchi F, Romagnoli E, De Caterina AR, La Torre G, Lo Schiavo P, Tarantino F, Ferrari R, Tomai F, Olivares P, Cosentino N, D’Amario D, Leone AM, Porto I, Burzotta F, Trani C, Crea F. Open-label, randomized, placebo-controlled evaluation of intracoronary adenosine or nitroprusside after thrombus aspiration during primary percutaneous coronary intervention for the prevention of microvascular obstruction in acute myocardial infarction: the REOPEN-AMI study (intracoronary nitroprusside versus adenosine in acute myocardial infarction) JACC Cardiovasc Interv. 2013;6:580–9. doi: 10.1016/j.jcin.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Fokkema ML, Vlaar PJ, Vogelzang M, Gu YL, Kampinga MA, de Smet BJ, Jessurun GA, Anthonio RL, van den Heuvel AF, Tan ES, Zijlstra F. Effect of high-dose intracoronary adenosine administration during primary percutaneous coronary intervention in acute myocardial infarction: a randomized controlled trial. Circ Cardiovasc Interv. 2009;2:323–9. doi: 10.1161/CIRCINTERVENTIONS.109.858977.109.858977. [DOI] [PubMed] [Google Scholar]
- 21.Ross AM, Gibbons RJ, Stone GW, Kloner RA, Alexander RW AMISTAD-II Investigators. A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II) J Am Coll Cardiol. 2005;45:1775–80. doi: 10.1016/j.jacc.2005.02.061. [DOI] [PubMed] [Google Scholar]
- 22.Ito H, Taniyama Y, Iwakura K, Nishikawa N, Masuyama T, Kuzuya T, Hori M, Higashino Y, Fujii K, Minamino T. Intravenous nicorandil can preserve microvascular integrity and myocardial viability in patients with reperfused anterior wall myocardial infarction. J Am Coll Cardiol. 1999;33:654–660. doi: 10.1016/s0735-1097(98)00604-4. [DOI] [PubMed] [Google Scholar]
- 23.Tsubokawa A, Ueda K, Sakamoto H, Iwase T, Tamaki S. Effect of intracoronary nicorandil administration on preventing no-reflow/slow flow phenomenon during rotational atherectomy. Circ J. 2002;66:1119–1123. doi: 10.1253/circj.66.1119. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan BM, Benzuly KH, Kinn JW, Bowers TR, Tilli FV, Grines CL, O’Neill WW, Safian RD. Treatment of no-reflow in degenerated saphenous vein graft intervention: comparison of intracoronary verapamil and nitroglycerin. Cathet Cardiovasc Diagn. 1996;39:113–118. doi: 10.1002/(SICI)1097-0304(199610)39:2<113::AID-CCD1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 25.Werner GS, Lang K, Kuehnert H, Figulla HR. Intracoronary verapamil for reversal of no-reflow during coronary angioplasty for acute myocardial infarction. Catheter Cardiovasc Interv. 2002;57:444–451. doi: 10.1002/ccd.10375. [DOI] [PubMed] [Google Scholar]
- 26.Gupta S, Gupta MM. No-reflow phenomenon in percutaneous coronary interventions in ST-segment elevation myocardial infarction. Indian Heart J. 2016;68:539–51. doi: 10.1016/j.ihj.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Werner GS, Lang K, Kuehnert H, Figulla HR. Intracoronary verapamil for reversal of no-reflow during coronary angioplasty for acute myocardial infarction. Catheter Cardiovasc Interv. 2002;57:444–451. doi: 10.1002/ccd.10375. [DOI] [PubMed] [Google Scholar]
- 28.Weyrens FJ, Mooney J, Lesser J, Mooney MR. Intracoronary diltiazem for microvascular spasm after interventional therapy. Am J Cardiol. 1995;75:849–850. doi: 10.1016/s0002-9149(99)80430-5. [DOI] [PubMed] [Google Scholar]
- 29.Jalinours F, Mooney JA, Mooney MR. Pretreatment with intracoronary diltiazem reduces non-Q wave myocardial infarction following directional atherectomy. J Invasive Cardiol. 1997;9:270–273. [PubMed] [Google Scholar]
- 30.Patel T, Shah S, Gulati R, Kwan T, Cohen MG, Pancholy S. Perforated balloon technique: a simple and handy technique to combat no-reflow phenomenon in coronary system. Cath Cardiovasc Interv. 2018;92:890–894. doi: 10.1002/ccd.27477. [DOI] [PubMed] [Google Scholar]
- 31.Meerkin D, Balkin J, Shaheen J, Tzivoni D. The twin-pass dual access catheter for assessment of the no-reflow phenomenon. J Invasive Cardiol. 2010;22:125–129. [PubMed] [Google Scholar]
- 32.Movahed M, Baweja G. Distal administration of very high doses of intracoronary adenosine for the treatment of resistant no-reflow. Exp Clin Cardiol. 2008;13:141–143. [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly RV, Crouch E, Krumnacher H, Cohen MG, Stouffer GA. Safety of adjunctive intracoronary thrombolytic therapy during complex percutaneous coronary intervention: Initial experience with intracoronary tenecteplase. Catheter Cardiovasc Interv. 2005;66:327–332. doi: 10.1002/ccd.20521. [DOI] [PubMed] [Google Scholar]
- 34.Forman M, Hou D, Jackson E. Treating acute “no-reflow” with intracoronary adenosine. Tex Heart Inst J. 2008;35:439–446. [PMC free article] [PubMed] [Google Scholar]
- 35.Maluenda G, Ben-Dor I, Delhaye C. Clinical experience with a novel intraeoronary perfusion catheter to treat no-reflow phenomenon in acute coronary syndromes. J Interv Cardiol. 2010;23:109–13. doi: 10.1111/j.1540-8183.2010.00539.x. [DOI] [PubMed] [Google Scholar]
- 36.Barsness GW, Buller C, Ohman EM, Schechter E, Pucillo A, Taylor MA, Miller MJ, Reiner JS, Churchill D, Chandler AB, Gonzalez M, Smith J, Tommaso C, Berdan LG, Wildermann NM, Hasdai D, Holmes DR. Reduced thrombus burden with abciximab delivered locally before percutaneous intervention in saphenous vein grafts. Am Heart J. 2000;139:824–829. doi: 10.1016/s0002-8703(00)90014-0. [DOI] [PubMed] [Google Scholar]


