Abstract
The increasing number of cancer survivors associated to a longer average life-span after diagnosis of an oncological disease facilitates the observation of deleterious long-term effects of both oncological disease and its treatment. Among these effects, chronic pain emerges as one of the most prevalent and, with its onset, there is a decrease in these patients’ functionality and quality of life. The main focus in oncological disease treatment has been tumour eradication and average life expectancy extension after diagnosis, neglecting these deleterious long-term effects. This study aims at assessing the prevalence and characteristics of chronic pain in cancer survivors as well as pain interference in their quality of life and functionality. The study selected cancer survivors (n = 85) after dismissal from oncology service to assess the presence and characteristics of chronic pain, their health-related quality of life (HRQoL) and pain-related disability through a combination of different questionnaires. Chronic pain prevalence was 23.5%. In total, 85% of patients reported neuropathic pain descriptors and 45% presented diagnostic criteria for neuropathic pain. Of these patients, 45% were followed-up for pain surveillance and 35% underwent analgesic medication. There was a median pain disability index of 20.50 (14.50–35.00) and an average HRQoL of 0.5338 in chronic pain patients and 0.8872 in patients without pain. We found that chronic pain was the main negative predictor of HRQoL and was associated with decreased functionality. This study also concluded that these patients often were not offered the appropriate long-term medical follow-up. These findings highlight a need to raise awareness among health professionals to the importance of timely diagnosis and treatment of pain and its impact on HRQoL and functionality of long-term cancer survivors as well as the need to change clinical practice in order to improve healthcare provided to these patients.
Keywords: Cancer survivor, chronic pain, disability, functionality, quality of life
Introduction
Medical technological breakthroughs in Oncology have provided tools for an earlier diagnosis of oncological disease and treatment options with a higher success rate.1–4 As a result, and in association with an increase in average life expectancy, the number of survivors and the number of life years after diagnosis of an oncological disease has increased over the years1–3,5,6 with an estimated 43.8 million survivors in 2018, up to 5 years after diagnosis. 7 In Portugal, the estimated number of survivors is 719.2 per 100,000 inhabitants. 7 Presently, it is estimated that globally more than 40% of patients diagnosed with cancer live more than 10 years after their diagnosis. 8
Consequently, an increase in the incidence of medium/long-term side effects has been seen, whose presentation depends on the location and extent of the disease, the presence of metastases, as well as the type of treatments such as chemotherapy, radiotherapy, hormone therapy, immunotherapy or surgery.6,9–12 Of the registered side effects, pain is one of the most prevalent4,10 and presents one of the biggest impacts on patients’ quality of life. 13 The estimated prevalence of chronic pain in cancer survivors can be as high as 40%,1,6,8,12,14 depending on the primary tumour location and performed treatment. Van den Beuken-van Everdingen et al. 15 establish the prevalence of chronic pain after curative treatment as 39.3% (CI 95% 33.3–45.3), 9 of which 27.6% reports moderate-to-severe pain.15,16 Other studies suggest that chronic pain can reach 50% in breast cancer survivors,6,11,15,17 40% in head and neck cancer survivors14,18 and 27% in colorectal cancer survivors.2,18,19
The aetiology of pain may be related to the tumour itself, the presence of metastases or as a secondary effect of treatment. However, the presence of pain may also be unrelated to cancer or its treatment.4,10 According to the World Health Organization’s International Classification of Diseases, 11th Edition (ICD-11), 20 chronic cancer–related pain can be caused by primary tumour or by metastases, referred as chronic cancer pain, or by its treatment, the latter referred as chronic post-cancer treatment pain. This pain persists or recurs for at least 3 months and presents no other better explanation from another mechanism.20,21
Chronic cancer pain results from tissue damage caused by tumour expansion or metastases’ development, activating inflammatory mechanisms and from neuropathic mechanisms such as compression and destruction of sensory nerve terminals and denervation of the area affected by the primary tumour and/or metastases. 21 Pain may persist even after tumour eradication, 16 through peripheral and central sensitization mechanisms.
Mechanisms associated with peripheral and central sensitization reduce the threshold of nociceptor activation for noxious and non-noxious stimuli, being equally associated to central wind-up mechanisms and activation of N-methyl-D-aspartate (NMDA) receptors. 22
Exposure to inflammatory mediators and tissue damage of primary sensory neurons (peripheral nociceptors) result in peripheral sensitization, which translates into a threshold reduction and, consequently, an increase in these neurons’ responsiveness. This response is limited to the injury site. Thus, it represents a form of pain due to nociceptor activation requiring the presence of an active aggression (inflammatory mediators, tissue or neuronal injury).22–24
Prolonged exposure to an intense peripheral noxious stimulus, tissue injury or neuronal injury cause changes that include lowering the sensitive terminals’ threshold leading to an excitatory synaptic response increase (central hyperexcitability) associated with an inhibitory response and pain modulation decrease, as well as pain spreading to unaffected areas characterizing central sensitization. Hence, both noxious and non-noxious stimuli will cause a disproportional pain hypersensitivity as to the nature and extent of the lesion which translates into allodynia or secondary hyperalgesia.22–25
Post-surgical pain has a high prevalence in surgeries such as thoracotomy or mastectomy: 30–50% of patients who underwent thoracotomy 16 and in more than 50% of patients after breast surgery. 26 31% of rectal cancer patients undergoing surgery report chronic pain in the pelvic area or lower limbs and 41% of whom report daily pain. 27 The neuropathic pain component in chronic post-surgical pain is extremely prevalent, although not always identified by patients as pain. At least 6 months after chemotherapy treatment, 30% of patients report neuropathic pain, with the most reported descriptors being tingling, numbness and burning.8,9,28 Radiotherapy wise, 20% of breast cancer patients experience clinically significant pain (EN > 3/10 using the brief pain inventory (BPI) questionnaire) 29 and in patients who received curative radiotherapy for head or neck tumour at 5 years, 53% report chronic orofacial pain. 30
Chronic cancer–related pain presents a high negative impact, sometimes even superior to cancer itself, on patients’ quality of life, affecting their daily life activities, their relationships, mood, sleep and several other health aspects, in general.1,9,19,31–33 Survivors (10–20%) report experiencing severe chronic pain that affects their daily life and normal functioning, a number that reaches 40% in the initial period after treatment, and studies have shown that these limitations can persist up to 20 years after treatment.8,34,35
Most of cancer-related pain (chronic cancer–related pain) can be treated using multimodal pharmacological and non-pharmacological therapies.31,36,37 The impact of untreated or inadequately treated pain can be devastating to a patients’ quality of life, affecting one’s physical and psychological well-being as well as one’s social interactions. 36
Historically, the major treatment focus for cancer disease approach was tumour eradication and increasing average life expectancy after diagnosis, neglecting the medium- and long-term impact of the disease and prevention of chronic pain development.1,6,14,15,36 Currently, oncological diseases’ higher cure rate and the larger number of studies on the prevalence of chronic pain in this population have been an important vehicle to promote health professionals’ awareness, conducting studies in the context of the impact of chronic pain on patients’ health-related quality of life (HRQoL), as well as the development of therapeutic guidelines for the adequate treatment of these patients.
The objective of this study is to assess patient population discharged from the medical oncology appointment at Centro Hospitalar Universitário do Porto (CHUP) between March 2016 and June 2019, assessing the prevalence and characteristics of chronic pain in cancer survivors, as well as the interference of pain in their quality of life and functionality. Pain’s medical follow-up and analgesic medication intake were equally investigated.
Methods
We analysed retrospectively all patients discharged from the Medical Oncology consultation, at Centro Hospitalar Universitário do Porto (CHUP) between March 2016 and June 2019, for a total of 334 patients. Of these, 232 were excluded for the following reasons: 42 patients were excluded as they were discharged to another hospital for follow-up, 68 due to death, 39 for not presenting malignant disease, 36 for not having follow-up appointments, 34 for still being followed-up and 13 for incomplete clinical information. The selected 102 patients were contacted for a telephone interview and 17 of those did not respond/refused to respond to the study. Therefore, 85 patients (25.45%) were included in the study, having responded to a telephone interview.
The study was developed in two phases. The first phase involved consultation of clinical files (n = 334) and selection of participants (n = 102). The second phase involved contacting the selected patients (n = 102) for a telephone interview where a set of questionnaires was performed and answered verbally (n = 85).
Through the consultation of electronic clinical files, the following information was collected
sociodemographic variables such as age, gender, marital status, education and employment status;
oncological disease characteristics such as location of the primary tumour, months since diagnosis and presence of metastasis;
cancer treatment performed such as chemotherapy, radiotherapy, hormone therapy and immunotherapy and/or surgery.
Through the telephone interview, patients were verbally inquired about the
4. presence or absence of chronic pain;
5. HRQoL, through the application of the Portuguese version of ‘EuroQoL five dimensions questionnaire (EQ-5D-3L)’;
Patients, who reported pain, were also verbally inquired regarding
6. factors related to chronic pain such as anatomical location, type of medical follow-up and analgesic medication intake;
7. the characterization of pain, through the application of the following questionnaires ‘BPI’ (adapted), ‘pain disability index (PDI)’ and ‘specific questionnaire for screening of pain neuropathic pain – Douleur Neuropathique en 4 Questions (DN4)’ (adapted), validated for the Portuguese language.
Pain
Pain was assessed using a set of questionnaires: BPI, PDI and DN4.
From the BPI questionnaire, the first four items were applied, namely, the dichotomous item for verification of the existence of pain; an item for the location of pain, indicating pain areas in a human body representative diagram (converted into coding for anatomical pain localization); and a two-item pain intensity scale (maximum and minimum), featuring numerical rating scales (from 0 to 10). The other items on BPI questionnaire were not applied because they would not add any valuable information, since there is some overlapping with other questions included in the telephone survey and some were not relevant to the objectives of this study. As such, we decided to skip the other sections in order to decrease the time of survey and therefore increase the response rate and patient cooperation. 38
The PDI questionnaire aims to evaluate important dimensions of disability and functional pain interference, regardless of the location and the diagnosis of chronic pain. It consists of seven dimensions, with a numerical scale of 11 points, where 0 represents the total absence of disability and 10 represents total disability (⩾2 – mild, ⩾5 – moderate and ⩾8 – severe), assessing the pain-related disability in family and home responsibilities, recreational activities, social activities, at work/occupation, sexual behaviour, self-care and life-support activities. The results obtained for each dimension can be added up to obtain a value of the pain-related disability index, measured on an increasing disability scale from 0 to 70 points, reflecting the interference associated with pain in daily life activities. 38
The DN4 questionnaire was developed by the French Group of Neuropathic Pain to differentiate neuropathic pain from nociceptive pain. It consists of ten items grouped into four sections. The first seven, applied in this study, are related to the quality of pain (burning sensation, painful cold sensation and electric shock sensation) and its association with abnormal sensations (tingling, pricking, numbness and itching). The other three items are related to neurological examination in the painful area (touch hypoesthesia, prick hypoesthesia and touch allodynia) and once our study was based in telephonic survey and data collection, physical examination of patients was not possible. A positive item receives a value of 1 and a negative item receives a value of 0. 38
Quality of life
HRQoL was assessed based on the application of the EQ-5D-3L, which comprises the following five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each dimension has three levels (1 – no problems, 2 – some problems and 3 – extreme problems). The HRQoL value can be calculated based on results obtained and a predetermined value set, with values expressed on a scale of 1 corresponding to total health to 0 corresponding to death. Negative values are also considered for quality of life states considered worse than death. Greiner et al. established the questionnaire’s value set for the European population.32,39–41
Quality of life predictors
In order to understand which variables best predict HRQoL, we performed a linear regression analysis, having predictors organized in three blocks as follows
(1) variables related to the patient, including gender (female), age and marital status (married);
(2) variables related to the disease, such as the number of months since diagnosis and the number of treatments (three or more treatments);
(3 variables related to chronic pain.
The studied variables were obtained through electronic clinical processes and telephone interviews, with the authorization and approval by the Ethics Committee for Health, the Department of Education, Training and Research and the Responsible for Clinical Information Access of Centro Hospitalar Universitário do Porto.
Statistical analysis was performed using the IBM SPSS Statistics® (version 26.0).
Results
Individuals had an average age of 65.33 years, 50.6% (n = 43) being female and 49.4% (n = 42) being male. Of these, 72.9% (n = 62) were aged 60 years or older, 70.6% (n = 60) were married, 50.6% (n = 43) attended between the first and fourth grade of education and 68.2% (n = 58) were retired (Table 1).
Table 1.
Sociodemographic characterization.
| Variable | Total N (%) | Pain N (%) | No pain N (%) | p value |
|---|---|---|---|---|
| Gender | ||||
| Feminine | 43 (50.6) | 16 (80.0) | 27 (41.5) | p = 0.003 |
| Masculine | 42 (49.4) | 4 (20.0) | 38 (58.5) | |
| Age (years) | ||||
| <30 | 0 (0) | 0 (0.0) | 0 (0.0) | p = 0.066 |
| 30–39 | 3 (3.5) | 0 (0.0) | 3 (4.6) | |
| 40–49 | 6 (7.1) | 2 (10.0) | 4 (6.2) | |
| 50–59 | 14 (16.5) | 4 (20.0) | 10 (15.4) | |
| 60–69 | 25 (29.4) | 4 (20.0) | 21 (32.3) | |
| 70–79 | 30 (35.3) | 9 (45.0) | 21 (32.3) | |
| ⩾80 | 7 (8.2) | 1 (20.0) | 6 (9.2) | |
| Marital status | ||||
| Single | 3 (3.5) | 0 (0.0) | 3 (4.6) | p = 0.558 |
| Married | 60 (70.6) | 17 (85.0) | 43 (66.2) | |
| Widower | 7 (8.2) | 1 (5.0) | 6 (9.29) | |
| Partnership | 1 (1.2) | 0 (0.0) | 1 (1.5) | |
| Divorced | 14 (16.5) | 2 (10.0) | 12 (18.5) | |
| Schooling | ||||
| No formal education | 2 (2.4) | 1 (5.0) | 1 (1.5) | p = 0.759 |
| First to fourth grade | 43 (50.6) | 11 (55.0) | 32 (49.2) | |
| Fifth to ninth grade | 20 (23.5) | 4 (20.0) | 16 (24.6) | |
| 10th to 12th grade | 11 (12.9) | 3 (15.0) | 8 (12.3) | |
| University education | 9 (10.6) | 1 (5.0) | 8 (12.3) | |
| Employment status | ||||
| Employed | 19 (22.4) | 3 (15.0) | 16 (24.6) | p = 0.427 |
| Unemployed | 8 (9.4) | 1 (5.0) | 7 (10.8) | |
| Retired | 58 (68.2) | 16 (80.0) | 42 (64.6) | |
χ2 test, p < 0.05.
Primary tumour’s most prevalent locations were digestive (50.6% n = 43), head and neck (15.3%, n = 13) and breast (14.1%, n = 12). Of these patients, 69.4% (n = 59) were in the period between 5 and 10 years after diagnosis and 87.1% (n = 74) did not present metastasis (Table 2).
Table 2.
Oncological characterization.
| Variable | Total N (%) | Pain N (%) | No pain N (%) | p value |
|---|---|---|---|---|
| Primary tumour location | ||||
| Head and neck | 13 (15.3) | 1 (5.0) | 12 (18.5) | p = 0.070 |
| Digestive | 43 (50.6) | 11 (55.0) | 32 (49.2) | |
| Genitourinary | 5 (5.9) | 0 (0.0) | 5 (7.7) | |
| Breast | 12 (14.1) | 6 (30.0) | 6 (9.2) | |
| Bone | 2 (2.4) | 0 (0.0) | 2 (3.1) | |
| Hidden primary | 1 (1.2) | 1 (5.0) | 0 (0.0) | |
| CNS | 3 (3.5) | 0 (0.0) | 3 (4.6) | |
| Synchronous | 6 (7.1) | 1 (5.0) | 5 (7.7) | |
| Months since diagnosis (months) | ||||
| ⩾60 | 3 (3.5) | 2 (10.0) | 1 (1.5) | p = 0.079 |
| 61–72 | 1 (1.2) | 0 (0.0) | 1 (1.5) | |
| 73–84 | 12 (14.1) | 3 (15.0) | 9 (13.8) | |
| 85–96 | 12 (14.1) | 1 (5.0) | 11 (16.9) | |
| 97–108 | 16 (18.8) | 4 (20.0) | 12 (18.6) | |
| 109–120 | 18 (21.2) | 1 (5.0) | 17 (26.5) | |
| ⩽121 | 23 (27.1) | 9 (45.0) | 14 (21.5) | |
| Metastization | ||||
| No | 74 (87.1) | 17 (85.0) | 57 (87.7) | p = 0.754 |
| Yes | 11 (12.9) | 3 (15.0) | 8 (12.3) | |
χ2 test, p < 0.05. CNS: Central Nervous System.
Of these patients, 95.3% (n = 81) underwent chemotherapy, 49.4% (n = 42) underwent radiotherapy, 10.6% (n = 9) underwent hormone therapy, 2.4% (n = 2) underwent immunotherapy and 88.2% (n = 75) underwent surgery. Of these patients, 62.4% (n = 53) underwent up to two types of treatment (Table 3).
Table 3.
Treatment characterization.
| Variable | Total N (%) | Pain N (%) | No pain N (%) | p value |
|---|---|---|---|---|
| Chemotherapy | ||||
| No | 4 (4.7) | 2 (10.0) | 2 (3.1) | p = 0.201 |
| Yes | 81 (95.3) | 18 (90.0) | 63 (96.9) | |
| Radiotherapy | ||||
| No | 43 (50.6) | 8 (40.0) | 35 (53.8) | p = 0.279 |
| Yes | 42 (49.4) | 12 (60.0) | 30 (46.2) | |
| Hormone therapy | ||||
| No | 76 (89.4) | 16 (80.0) | 60 (92.3) | p = 0.118 |
| Yes | 9 (10.6) | 4 (20.0) | 5 (7.7) | |
| Immunotherapy | ||||
| No | 83 (97.6) | 19 (95.0) | 64 (98.5) | p = 0.372 |
| Yes | 2 (2.4) | 1 (5.0) | 1 (1.5) | |
| Surgery | ||||
| No | 10 (11.8) | 2 (10.0) | 8 (12.3) | p = 0.779 |
| Yes | 75 (88.2) | 18 (90.0) | 57 (87.7) | |
| Number of treatments | ||||
| 1 | 2 (2.4) | 0 (0.0) | 2 (3.1) | p = 0.238 |
| 2 | 51 (60.0) | 11 (55.0) | 40 (61.5) | |
| 3 | 24 (28.2) | 5 (25.0) | 19 (29.2) | |
| 4 | 7 (8.2) | 4 (20.0) | 3 (4.6) | |
| 5 | 1 (1.2) | 0 (0.0) | 1 (1.5) | |
χ2 test, p < 0.05.
Of the patients surveyed, 23.5% (n = 20) present chronic pain. The most referred sources of pain locations were lumbar area (25%, n = 5), abdomen (20%, n = 4) and left upper limb (15%, n = 3), followed by thorax, upper right limb and lower limbs (10%, n = 2, each) and lower right limb and lower left limb (5%, n = 1, each). Of these patients, 45% (n = 9) were followed by their Assistant Physician/Family Physician and 55% (n = 11) had no medical follow-up for their pain. Of the patients reporting chronic pain, 35% (n = 7) took analgesic medication.
In the week prior to the telephone survey, 85% (n = 17) of patients with chronic pain experienced pain. The median of this pain minimum intensity was 4 (2.5–4.5) and the median of this pain maximum intensity was 7 (5.5–8.5), on a 0–10 scale. The most referred as the source of pain were lumbar area (29.4%, n = 5) and abdomen (17.6%, n = 3), as shown in Table 4.
Table 4.
Brief pain inventory (last week).
| Variable | N (%) | |
|---|---|---|
| Pain | ||
| No | 3 (15.0) | |
| Yes | 17 (85.0) | |
| Location | ||
| Thorax | 2 (11.8) | |
| Abdomen | 3 (17.6) | |
| Lumbar area | 5 (29.4) | |
| Upper left limb | 2 (11.8) | |
| Upper right limb | 2 (11.8) | |
| Lower right limb | 1 (5.9) | |
| Lower limbs | 2 (11.8) | |
| Pain intensity | Maximum | Minimum |
| Median (P25–P75) | 7 (5.5–8.5) | 4 (2.5–4.5) |
| Average ± standard deviation | 7.18 ± 1.976 | 3.53 ± 1.125 |
Patients with chronic pain demonstrated a total median pain disability of 20.50 (14.5–35.00). As to the different evaluated dimensions, medians were as follows: family/home responsibilities – 5.50 (1.25–7.00); recreation – 3.00 (1.00–9.25); social activities – 0.50 (0.00–4.25); occupation – 2.50 (0.00–7.00); sexual behaviour – 0.00 (0.00–2.00); self-care – 2.50 (1.00–5.75); and life-support activities – 2.50 (1.00–7.00), as shown in Table 5.
Table 5.
Pain disability index.
| Variable | Average ± standard deviation | Median (P25–P75) |
|---|---|---|
| Family/home responsibilities | 4.50 ± 3.220 | 5.50 (1.25–7.00) |
| Recreation | 4.35 ± 3.870 | 3.00 (1.00–9.25) |
| Social activity | 2.50 ± 3.332 | 0.50 (0.00–4.75) |
| Occupation | 3.45 ± 3.300 | 2.50 (0.00–7.00) |
| Sexual behaviour | 1.40 ± 2.437 | 0.00 (0.00–2.00) |
| Self-care | 3.15 ± 2.834 | 2.50 (1.00–5.75) |
| Life-support activity | 3.55 ± 3.316 | 2.50 (1.00–7.00) |
| Total PDI | 23.35 ± 12.470 | 20.50 (14.50–35.00) |
PDI: pain disability index.
As to characteristics of neuropathic pain, 30% (n = 6) refers a burning sensation, 15% (n = 3) refer a painful cold sensation, 40% (n = 8) refers an electric shock sensation, 70% (n = 14) reports a tingling sensation, 45% (n = 9) reports a sting sensation, 60% (n = 12) reports numbness and 35% (n = 7) reports itching. Of these patients, 45% (n = 9) reports four or more characteristics of neuropathic pain at the chronic pain location (Table 6).
Table 6.
DN4.
| Variable | N (%) | DN4 result | N (%) | |
|---|---|---|---|---|
| Burning sensation | No | 14 (70.0) | 0 | 3 (15.0) |
| Yes | 6 (30.0) | |||
| Painful cold sensation | No | 15 (75.0) | 1 | 1 (5.0) |
| Yes | 5 (15.0) | |||
| Electric shock sensation | No | 12 (60.0) | 2 | 4 (20.0) |
| Yes | 8 (40.0) | |||
| Tingling | No | 6 (30.0) | 3 | 3 (15.0) |
| Yes | 14 (70.0) | |||
| Pricking | No | 11 (55.0) | 4 | 4 (20.0) |
| Yes | 9 (45.0) | |||
| Numbness | No | 8 (40.0) | 5 | 3 (15.0) |
| Yes | 12 (60.0) | |||
| Itching | No | 13 (65.0) | 6 | 2 (10.0) |
| Yes | 7 (35.0) | |||
Comparison of affected dimensions of quality of life (1 – no problems, 2 – some problems and 3 – extreme problems) between patients with and without pain (Table 7)
Table 7.
EuroQoL EQ-5D-3L.
| Variable | Total N (%) | Pain N (%) | No pain N (%) | p value |
|---|---|---|---|---|
| Mobility | ||||
| I have no problems in walking about | 57 (67.1) | 10 (50.0) | 47 (72.3) | p = 0.124 |
| I have some problems in walking about | 27 (31.8) | 10 (50.0) | 17 (26.2) | |
| I am confined to bed | 1 (1.2) | 0 (0.0) | 1 (1.5) | |
| Self-care | ||||
| I have no problems with self-care | 65 (76.5) | 9 (45.0) | 56 (86.2) | p < 0.001 |
| I have some problems washing or dressing myself | 20 (23.5) | 11 (55.0) | 9 (13.8) | |
| I am unable to wash or dress myself | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Usual activities | ||||
| I have no problems with performing my usual activities | 59 (69.4) | 5 (25.0) | 54 (83.1) | p < 0.001 |
| I have some problems with performing my usual activities | 25 (29.4) | 15 (75.0) | 10 (15.4) | |
| I am unable to perform my usual activities | 1 (1.2) | 0 (0.0) | 1 (1.5) | |
| Pain/discomfort | ||||
| I have no pain or discomfort | 66 (77.6) | 1 (5.0) | 65 (100.0) | p < 0.001 |
| I have moderate pain or discomfort | 17 (20.0) | 17 (85.0) | 0 (0.0) | |
| I have extreme pain or discomfort | 2 (2.4) | 2 (10.0) | 0 (0.0) | |
| Anxiety/depression | ||||
| I am not anxious or depressed | 61 (71.8) | 5 (25.0) | 56 (86.2) | p < 0.001 |
| I am moderately anxious or depressed | 19 (22.4) | 11 (55.0) | 8 (12.3) | |
| I am extremely anxious or depressed | 5 (5.9) | 4 (20.0) | 1 (1.5) | |
| HRQoL | Average ± standard deviation | Median (P25–P75) | ||
| Total | 0.8040 ± 0.2317 | 0.8475 (0.6625–1.0000) | ||
| Pain | 0.5338 ± 0.2077 | 0.5463 (0.4106–0.6735) | ||
| No pain | 0.8872 ± 0.1668 | 1.0000 (0.8062–1.0000) | ||
HRQoL: health-related quality of life.
χ2 test, p < 0.05.
Mobility (level 2 or 3): 50% (n = 10) of patients with pain versus 27.7% (n = 18) patients without pain.
Self-care (level 2 or 3): 55% (n = 11) of patients with pain versus 13.8% (n = 9) patients without pain.
Usual activities (level 2 or 3): 75% (n = 15) of patients with pain versus 16.9% (n = 11) patients without pain.
Pain/discomfort (level 2 or 3): 95% (n = 19) of patients with pain versus 0% (n = 0) patients without pain.
Anxiety/depression (level 2 or 3): 75% (n = 15) of patients with pain versus 13.8% (n = 9) patients without pain.
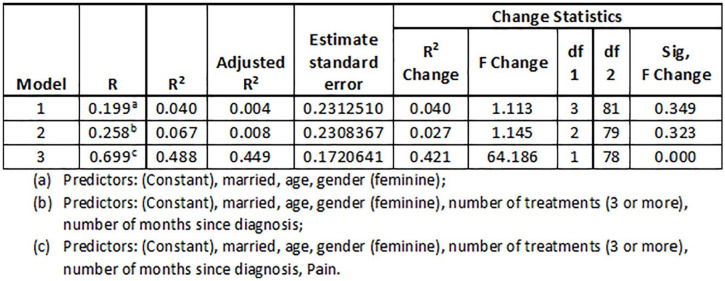
Results (Figure 1) reveal that the different predictors present a high explanatory power for HRQoL (adjusted R² = 0,449). The contribution of variables related to patients and to the disease seems to be residual, with only significant changes when chronic pain is introduced in the model.
Figure 1.
HRQoL predictors.
The different predictors are only significant in the third model, highlighting age and, with greater intensity, pain, both negatively. This means that the older the patients, the lower the HRQoL and, when patients present pain, they present lower HRQoL. It is interesting, in any case, to underline that age only emerges as a significant predictor when pain is introduced into the model.
Discussion
The aim of this study was assessment of chronic pain prevalence and its impact on the quality of life of cancer survivors discharged from the Medical Oncology consultation at Centro Hospitalar Universitário do Porto. We demonstrated that close to one-quarter of patients presented chronic pain (23.5%, n = 20). Of these, 85% reported the presence of at least one neuropathic pain descriptor, with 45% presenting diagnostic criteria for neuropathic pain (DN4 ⩾ 4). Less than half of the patients with chronic pain underwent medical follow-up, and only slightly more than one-third of the patients was taking analgesic medication. There was a median pain-related disability index of 20.50 (14.50–35.00).
Previous studies have established that chronic pain could be present in up to 40% of cancer survivors, depending on several factors such as location of the primary tumour, type of treatment or time since diagnosis. In most of the studies carried out, prevalence of pain was assessed in patients still being followed-up in Oncology. Thanks to innovation in medicine, more patients have been surviving and for a longer period after diagnosis, making chronic pain a reality increasingly present in their lives. In this study, we estimated the prevalence of chronic pain in almost one-quarter (23.5%) of cancer survivors after curative treatment and discharge from oncology services.
The development of chronic pain after curative treatment raises questions about the therapeutic approach to be taken with these patients, and untreated or incorrectly treated pain can have quite deleterious effects on patients’ quality of life and functionality, as well as it may persist up to 20 years after treatment conclusion.8,34,35 Thus, we found that less than half the patients had medical follow-up on pain, all of which was done by their assistant physician, and only 35% of these patients underwent analgesic medication to control chronic pain. Considering the high prevalence and impact of chronic pain in cancer survivors, it would be crucial for their quality of life management to have a higher percentage of referrals to specialized pain management units. These results may reflect the historical perspective on the oncological disease that focuses, essentially, on eradicating the tumour and prolonging average life expectancy after diagnosis, neglecting the medium- and long-term impact of oncological disease and its treatment.1,6,14,15,36
In order to proceed with the correct therapeutic approach, it will also be necessary to understand pain characteristics, namely, to understand the presence of pain mechanisms: nociceptive, neuropathic or mixed. It is also fundamental to emphasize the importance of acute pain effective treatment, which often follows treatments applied to these patients, in order to prevent the peripheral and central sensitization mechanisms that underlie chronic pain. However, the correct and timely identification of a neuropathic pain component often presents a relevant impact on the outcome and therapeutic adjustment of these patients. Accordingly, within chronic pain patients in this study, the vast majority (85%) reported the presence of at least one neuropathic pain descriptor, with 45% of these patients having diagnostic criteria for neuropathic pain based on DN4 (DN4 ⩾ 4), even though the physical examination components of this questionnaire were not performed.8,9,28
Several studies have been demonstrating the negative impact of chronic pain presence on functionality and quality of life of cancer survivors, an impact that may even be superior to the impact caused by the diagnosis of cancer disease.1,9,19,31–33 Several studies have shown that the most affected dimensions were work activity,13,42–45 as well as domestic and leisure activities and sexual behaviour.43,44,46 Azevedo et al. 47 described that, for the Portuguese population, the main dimensions affected by chronic pain were family and home responsibilities, leisure time, work and sleep. In order to assess the impact of pain on this population study, we assessed the PDI, the result of which shows that the main dimensions affected by pain were family and home responsibilities (5.50; 1.25–7.00) and recreational activities (3.00; 1.00–9.25). It should be noted that the advanced average age and the employment situation of most members of this population (unemployed or retired – 77.6%) have played a significant role in reducing the level of disability related to some of the tasks surveyed, namely, at the occupational level (2.50; 0.00–7.00) and sexual behaviour (0.00; 0.00–2.00). Finally, social activities’ dimension tends to be one mostly affected by pain, accounting for the frequent association between chronic pain and depression, which often leads to these patients’ greater social isolation. In this study, this association was not verified, possibly because we are facing a population of cancer survivors who may show greater resilience from the onset. 48
We also assessed HRQoL based on a five-dimensional questionnaire, with a lower absolute HRQoL value in chronic pain patients compared to patients without pain, as well as a higher score in each of the evaluated dimensions. We also found that chronic pain is the main negative predictor of HRQoL, therefore demonstrating its negative impact on the quality of life of cancer survivors.
Conclusion
This study has some limitations, such as the small number of sampled patients and the sole inclusion of patients who were followed-up by oncology service, leaving out patients not followed by this service, being translated into a distribution by type of tumour different from the cancer patients’ population. Exclusion criteria also included the absence of informed consent or still being under some type of treatment, namely, continued care, palliative care, or end-of-life comfort care. Considering these limitations, these results cannot be extrapolated to the general population of cancer patients.
However, the demonstration of a significant prevalence of chronic pain in these cancer survivors as well as its negative impact on functionality and quality of life of these patients represent a strong indication for the need to raise awareness among health professionals who treat these patients to the importance of timely diagnosis and treatment of pain, the necessity to carry out additional and more comprehensive studies on pain, functionality and quality of life in cancer surviving patients, as well as the need to change clinical practice in order to provide better long-term healthcare for this group of patients.
Acknowledgments
The authors would like to thank Professor Isabel Menezes for all the guidance and assistance in this research.
Footnotes
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Contributorship: P.G.J. conducted the data collection and the telephone interviews with the supervision of V.D. P.G.J. created the database and wrote the first draft of the article. All authors reviewed, edited and approved the final version of the article.
Ethical approval: Ethical approval for this study was obtained from Comissão de Ética CHUP/ICBAS (Research Project No. 2019.225)
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Guarantor: P.G.J. is the guarantor of this article.
Informed consent: Verbal informed consent was obtained from all subjects before the study. Written informed consent was not obtained because the main part of information was obtained directly from the patients through a telephone interview whose purpose was thoroughly explained and only those who consented were interviewed. On top of that, in order to obtain written informed consent, the patients would have to come to the hospital which was not possible or recommendable, giving the age and medical condition of the subjects and the fact that we are amid of a global pandemic.
ORCID iD: João Poço Gonçalves  https://orcid.org/0000-0002-0702-4342
https://orcid.org/0000-0002-0702-4342
References
- 1.Paice JA, Portenoy R, Lacchetti C, et al. Management of chronic pain in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. JCO 2016; 34(27): 3325–3345. [DOI] [PubMed] [Google Scholar]
- 2.Van den Beuken-van Everdingen M. Chronic pain in cancer survivors: a growing issue. J Pain Palliat Care Pharmacother 2012; 26(4): 385–387. [DOI] [PubMed] [Google Scholar]
- 3.Van Leeuwen M, Husson O, Alberti P, et al. Understanding the quality of life (QOL) issues in survivors of cancer: towards the development of an EORTC QOL cancer survivorship questionnaire. Health Qual Life Outcomes 2018; 16(1): 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiltink LM, White K, King MT, et al. Systematic review of clinical practice guidelines for colorectal and anal cancer: the extent of recommendations for managing long-term symptoms and functional impairments. Support Care Cancer 2020; 28(6): 2523–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox-Martin E, Anderson-Mellies A, Borges V, et al. Chronic pain, health-related quality of life, and employment in working-age cancer survivors. J Cancer Surviv 2020; 14(2): 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurita GP, Sjøgren P. Pain management in cancer survivorship. Acta Oncologica 2015; 54(5): 629–634. [DOI] [PubMed] [Google Scholar]
- 7.Jemal A, Torre L, Soerjomataram I, et al. (eds). The cancer atlas, 3rd edn. Atlanta: American Cancer Society, 2019, 69 p, https://canceratlas.cancer.org/ [Google Scholar]
- 8.Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clin Oncol 2014; 32(16): 1739–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown MR, Ramirez JD, Farquhar-Smith P. Pain in cancer survivors. Br J Pain 2014; 8(4): 139–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kline-Quiroz C, Nori P, Stubblefield MD. Cancer rehabilitation. Med Clin N Am 2020; 104(2): 239–250. [DOI] [PubMed] [Google Scholar]
- 11.Leysen L, Adriaenssens N, Nijs J, et al. Chronic pain in breast cancer survivors: nociceptive, neuropathic, or central sensitization pain? Pain Pract 2019; 9(2): 183–195. [DOI] [PubMed] [Google Scholar]
- 12.Schreiber JA. Understanding the cancer pain experience. Curr Pain Headache Rep 2014; 18(8): 440. [DOI] [PubMed] [Google Scholar]
- 13.Breivik H, Eisenberg E, O’Brien T. The individual and societal burden of chronic pain in Europe: the case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health 2013; 13(1): 1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cramer JD, Johnson JT, Nilsen ML. Pain in head and neck cancer survivors: prevalence, predictors, and quality-of-life impact. Otolaryngol Head Neck Surg 2018; 159(5): 853–858. [DOI] [PubMed] [Google Scholar]
- 15.Van den Beuken-van Everdingen MH, Hochstenbach LMJ, Joosten EAJ, et al. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage 2016; 51(6): 1070–1090.e9. [DOI] [PubMed] [Google Scholar]
- 16.Boland EG, Ahmedzai SH. Persistent pain in cancer survivors. Curr Opin Support Palliat Care 2017; 11(3): 181–190. [DOI] [PubMed] [Google Scholar]
- 17.Nori P, Kline-Quiroz C, Stubblefield MD. Cancer rehabilitation. Med Clin N Am 2020; 104(2): 251–262. [DOI] [PubMed] [Google Scholar]
- 18.Bouhassira D, Luporsi E, Krakowski I. Prevalence and incidence of chronic pain with or without neuropathic characteristics in patients with cancer. Pain 2017; 158(6): 1118–1125. [DOI] [PubMed] [Google Scholar]
- 19.Lowery AE, Starr T, Dhingra LK, et al. Frequency, characteristics, and correlates of pain in a pilot study of colorectal cancer survivors 1-10 years post-treatment. Pain Med 2013; 14(11): 1673–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. International Classification of Diseases for mortality and morbidity statistics (11th Revision), 2018, https://icd.who.int/browse11/l-m/en
- 21.Bennett MI, Kaasa S, Barke A, et al. The IASP classification of chronic pain for ICD-11: chronic cancer-related pain. Pain 2019; 160(1): 38–44. [DOI] [PubMed] [Google Scholar]
- 22.Mifflin KA, Kerr BJ. The transition from acute to chronic pain: understanding how different biological systems interact. Can J Anesth/J Can Anesth 2014; 61(2): 112–122. [DOI] [PubMed] [Google Scholar]
- 23.Feizerfan A, Sheh G. Transition from acute to chronic pain. Contin Educ Anaesth Crit Care Pain 2015; 15(2): 98–102. [Google Scholar]
- 24.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009; 10(9): 895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nijs J, Leysen L, Adriaenssens N, et al. Pain following cancer treatment: guidelines for the clinical classification of predominant neuropathic, nociceptive and central sensitization pain. Acta Oncol 2016; 55(6): 659–663. [DOI] [PubMed] [Google Scholar]
- 26.Peuckmann V, Ekholm O, Rasmussen NK, et al. Chronic pain and other sequelae in long-term breast cancer survivors: nationwide survey in Denmark. Eur J Pain 2009; 13(5): 478–485. [DOI] [PubMed] [Google Scholar]
- 27.Feddern M-L, Jensen TS, Laurberg S. Chronic pain in the pelvic area or lower extremities after rectal cancer treatment and its impact on quality of life: a population-based cross-sectional study. Pain 2015; 156(9): 1765–1771. [DOI] [PubMed] [Google Scholar]
- 28.Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain 2014; 155(12): 2461–2470. [DOI] [PubMed] [Google Scholar]
- 29.Lee E, Takita C, Wright JL, et al. Characterization of risk factors for adjuvant radiotherapy-associated pain in a tri-racial/ethnic breast cancer population. Pain 2016; 157(5): 1122–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Logan HL, Bartoshuk L, Fillingim R, et al. Metallic taste phantom predicts oral pain among 5-year survivors of head and neck cancer. Pain 2008; 140(2): 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curtis SA, Pirie KI. Chronic pain, loss and the future – development and evaluation of an innovative, interactive pain education tool. Br J Pain 2018; 12(3): 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manfuku M, Nishigami T, Mibu A, et al. Comparison of central sensitization-related symptoms and health-related quality of life between breast cancer survivors with and without chronic pain and healthy controls. Breast Cancer 2019; 26(6): 758–765. [DOI] [PubMed] [Google Scholar]
- 33.The British Pain Society, Healthcare Quality Improvement Partnership. National pain audit final report. London: The British Pain Society, Healthcare Quality Improvement Partnership, 2013. [Google Scholar]
- 34.Burton AW, Fanciullo GJ, Beasley RD, et al. Chronic pain in the cancer survivor: a new frontier. Pain Med 2007; 8(2): 189–198. [DOI] [PubMed] [Google Scholar]
- 35.Fathers E, Thrush D, Huson SM, et al. Radiation-induced brachial plexopathy in women treated for carcinoma of the breast. Clin Rehabil 2002; 16(2): 160–165. [DOI] [PubMed] [Google Scholar]
- 36.Breivik H, Cherny N, Collett B, et al. Cancer-related pain: a Pan-European Survey of prevalence, treatment, and patient attitudes. Ann Oncol 2009; 20(8): 1420–1433. [DOI] [PubMed] [Google Scholar]
- 37.Bruera E, Kim HN. Cancer pain. JAMA 2003; 290(18): 2476–2479. [DOI] [PubMed] [Google Scholar]
- 38.Agualusa L, Castro Lopes J, Vaz Patto T, et al. Volume monotemático sobre Questionários sobre Dor Crónica. Revista da Dor 2007; 15(4): 5–56. [Google Scholar]
- 39.Greiner W, Weijnen T, Nieuwenhuizen M, et al. A single European currency for EQ-5D health states. Eur J Health Econ 2003; 4(3): 222–231. [DOI] [PubMed] [Google Scholar]
- 40.Szende A, Oppe M, Devlin NJ. (eds). EQ-5D value sets: inventory, comparative review, and user guide (EuroQol Group monographs). Dordrecht: Springer, 2007, 102 p. [Google Scholar]
- 41.EuroQol Research Foundation. EQ-5D-3L user guide, 2018, https://euroqol.org/publications/user-guides/
- 42.Blyth FM, March LM, Nicholas MK, et al. Chronic pain, work performance and litigation. Pain 2003; 103(1): 41–47. [DOI] [PubMed] [Google Scholar]
- 43.Breivik H, Collett B, Ventafridda V, et al. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006; 10(4): 287–333. [DOI] [PubMed] [Google Scholar]
- 44.Duenas M, Ojeda B, Salazar A, et al. A review of chronic pain impact on patients, their social environment and the health care system. J Pain Res 2016; 9: 457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel AS, Farquharson R, Carroll D, et al. The impact and burden of chronic pain in the workplace: a qualitative systematic review. Pain Practice 2012; 12(7): 578–589. [DOI] [PubMed] [Google Scholar]
- 46.Moulin DE, Clark AJ, Speechley M, et al. Chronic pain in Canada – prevalence, treatment, impact and the role of opioid analgesia. Pain Res Manag 2002; 7(4): 179–184. [DOI] [PubMed] [Google Scholar]
- 47.Azevedo LF, Costa-Pereira A, Mendonça L, et al. Epidemiology of chronic pain: a population-based nationwide study on its prevalence, characteristics and associated disability in Portugal. J Pain 2012; 13(8): 773–783. [DOI] [PubMed] [Google Scholar]
- 48.Rowland JH, Baker F. Introduction: resilience of cancer survivors across the lifespan. Cancer 2005; 104(S11): 2543–2548. [DOI] [PubMed] [Google Scholar]