Abstract
Background:
Chronic postoperative pain following total joint replacement (TJA) is a substantial clinical problem, and poor sleep may affect predictive factors for postoperative pain, such as pain catastrophizing. However, the magnitude of these associations is currently unknown. This exploratory study investigated (1) the relationship between preoperative sleep quality, clinical pain intensity, pain catastrophizing, anxiety, and depression and (2) their associations with chronic postoperative pain following TJA.
Methods:
This secondary analysis from a larger randomized controlled trial included rest pain intensity (preoperative and 12 months postoperative; visual analogue scale, VAS), preoperative Pittsburgh Sleep Quality Index (PSQI), Pain Catastrophizing Scale (PCS), Hospital Anxiety and Depression Scale (HADS) data from 74 knee and 89 hip osteoarthritis (OA) patients scheduled for TJA. Poor sleepers were identified based on preoperative PSQI scores higher than 5.
Results:
Poor sleepers demonstrated higher preoperative VAS, pain catastrophizing, anxiety, and depression compared with good sleepers (all p < 0.003). Preoperative PSQI (β = 0.23, p = 0.006), PCS (β = 0.44, p < 0.005), and anxiety (β = 0.18, p = 0.036) were independent factors for preoperative VAS. Preoperative VAS (β = 0.32, p < 0.005), but not preoperative sleep quality (β = −0.06, p = 0.5), was an independent factor for postoperative VAS.
Conclusion:
The OA patients reporting poor preoperative sleep quality show higher preoperative pain, pain catastrophizing, anxiety, and depression. High preoperative pain intensity, but not poor sleep quality, was associated with higher chronic postoperative pain intensity. Future studies are encouraged to explore associations between sleep and chronic postoperative pain.
Keywords: Sleep quality, preoperative risk factors, total knee and hip arthroplasty, postoperative pain, pain catastrophizing, depression
Introduction
Knee and hip osteoarthritis (OA) are highly prevalent musculoskeletal disorders that affect the elder population. 1 The treatment for end-stage OA is total joint replacement (total knee or hip replacement: TKA and THA, respectively); however, it is well established that an approximate 20% of patients following TKA and 10% of patients following THA will report chronic postoperative pain. 2
Preoperative pain intensity 3 and cognitive factors such as high preoperative pain catastrophizing predict the presence of chronic postoperative pain 6 and 24 months after TKA.4–8 Furthermore, a recent study demonstrated that preoperative sleep parameters such as Pittsburgh Sleep Quality Index (PSQI) and daily sleepiness were associated with acute postoperative pain after TKA and THA. 9 Sleep may therefore play an important role in maintaining, for example, neural mechanisms such as descending pain inhibitory control 10 and possibly the prevention of, for example, depression 11 known to predict chronic postoperative pain.3,12,13 Furthermore, in a smaller cohort of 18 knee and hip OA patients, Roehrs and Roth 14 reported that simply extending sleep by approximately 1 hour improved early postoperative outcomes. These lines of evidence may suggest an interplay between cognitive factors such as depression, sleep, and postoperative pain. However, no evidence is currently available on how preoperative poor sleep is associated with preoperative risk factors pain catastrophizing, depression, anxiety, or chronic postoperative pain 12 months after TKA and THA.
The aims of the present exploratory study were to (1) assess the association between preoperative sleep quality and preoperative clinical pain intensity, pain catastrophizing, anxiety, and depression and (2) explore the associations between preoperative sleep quality, pain intensity, pain catastrophizing, depression, and anxiety and chronic postoperative pain intensity 12 months after total joint replacement in patients with OA.
Methods
Patients
A total of 185 knee OA patients (82 men and 103 women; mean age ± SD: 68.8 ± 8.92 years) and 189 hip OA patients (120 men and 69 women; mean age ± SD: 66.93 ± 13.75 years) scheduled for TKA or THA were enrolled. The patients took part in a large randomized controlled trial assessing the effect of acute and 7-day postoperative administration of the muscle relaxant chlorzoxazone on postoperative pain (12 months after TKA or THA). Chlorzoxazone is believed to enhance acute postoperative pain recovery, 15 which may improve postoperative pain, 16 but the study demonstrated no effect of chlorzoxazone on acute and chronic postoperative pain compared with placebo. 17 Therefore, these exploratory analyses were carried out. Patients were assessed for eligibility at a prescheduled hospital visit preceding admission for surgery between September 2015 and September 2016, and a follow-up was conducted 12 months post-surgery (2017).
Exclusion criteria involved use of gabapentinoids, glucocorticoids, opioids, anxiolytics, antiepileptics or antidepressants; alcohol abuse; other pain treatments outside of standard care; malignant conditions; pregnancy; BMI > 40 kg/m2; suffering from other peripheral or central acting diseases; allergy towards chlorzoxazone; perioperative complications (e.g. fractures) and liver diseases. All patients signed an informed consent prior to inclusion. The study was approved by the National Medicine Agency, the local ethics committee (VN-20150024) and National Data Protection Agency, preregistered at Clinicaltrials.gov (identifier number: NCT02405104) and conducted in accordance to the Declaration of Helsinki.
Sleep quality
Sleep quality was assessed preoperatively using the PSQI. The PSQI measures sleep quality and disturbances over 1-month intervals using 19 items, including subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, sleep medication usage and daytime dysfunction, and is assessed on a 0- to 21-point scale. 18 A PSQI measure >5 indicates poor sleep, with reported sensitivity of ~90% when distinguishing good from poor sleepers. 18
Pain Catastrophizing Scale
The Pain Catastrophizing Scale (PCS) 19 was administered preoperatively to assess pain catastrophizing. The PCS scores range from 0 to 52 across three subscales (rumination, magnification, and helplessness) based on 13 items (each scored from 0 to 4) reflecting the frequency of catastrophizing cognitions. The PCS is validated in chronic pain patients, pain-free subjects,20,21 and the Danish version in clinical and non-clinical cohorts. 22 The total PCS score was calculated and used for further analysis.
Anxiety and depression
The Hospital Anxiety and Depression Scale (HADS) is considered part of the (British) National Institute for Health and Care Excellence (NICE) recommendation for diagnosis of depression and anxiety 23 and was employed to determine the level of preoperative anxiety and depression. The scale consists of 14 questions, of which 7 items assess anxiety and 7 items assess depression. 24 Each item is evaluated by a score from 0 to 3 and summated to give a separate score for anxiety and depression. Cut-off values for anxiety and depression scores are 8 or above, which yield good specificity and sensitivity. 25
Pain intensity measures
Clinical pain intensity was defined as pain after 20 minutes of rest and was rated on a visual analogue scale (VAS; 0–10 cm; referred to as pain from hereon). Both pre- and 12-month postoperative clinical pain intensity were obtained.
Statistics
First, the potential effect of chlorzoxazone on the study parameters was tested in a multivariate analysis of co-variance (MANCOVA), with fixed factor randomization (chlorzoxazone or placebo).
Patients (TKA and THA pooled) were grouped based on sleep quality into good or poor sleepers defined as PSQI > 5. Independent-samples t tests were conducted between the two subgroups to explore whether the two groups differed. Chi-square tests on proportions were used to test differences in proportions of gender and surgery type between good and poor sleepers. Linear regression analyses were conducted on postoperative pain intensity 12 months after surgery with independent preoperative factors pain, PSQI, PCS, anxiety, and depression, and on preoperative pain intensity with independent preoperative factors PSQI, PCS, depression, and anxiety. In addition, a linear regression was conducted for preoperative PSQI with independent preoperative factors pain, PCS, anxiety, and depression. All collinearity tolerance and variance inflation factor (VIF) levels were above 0.1 and below 10, 26 respectively, indicating no collinearity or multicollinearity among the independent variables. Since TKA and THA patients may differ in aetiology and experience of pain, associations within the TKA and the THA group were tested separately with Pearson’s product moment correlation tests. A p-value below 0.05 was set to determine statistical significance. Bonferroni correction was applied to correct for multiple comparisons (0.05 / 5 = 0.01). All statistical analyses were performed in Statistical Package for Social Sciences (SPSS; version 26, IBM). Data are reported as mean ± standard error of the mean (SEM) unless otherwise stated.
Results
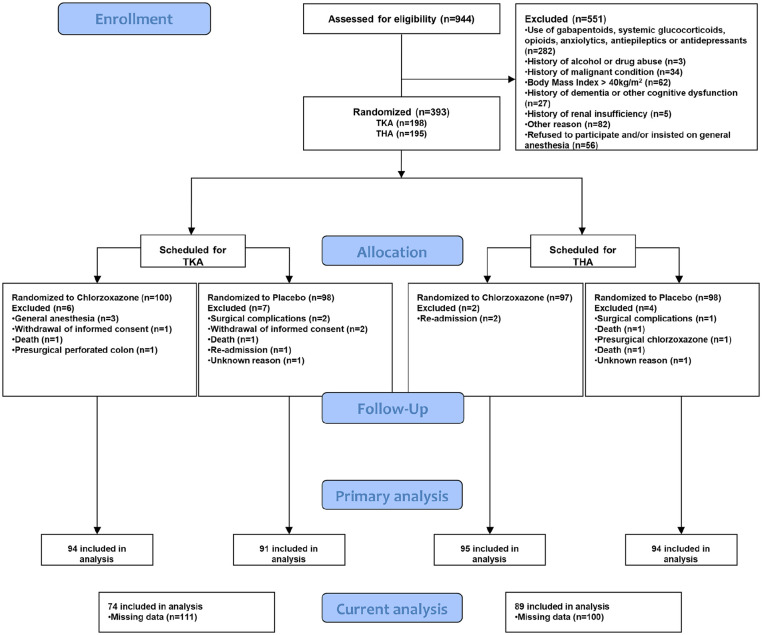
A total of 163 patients all had preoperative and postoperative data available (41.48% of all participants in the primary trial) and were included for further analysis (CONSORT diagram, Figure 1). The excluded patient group displayed a significantly higher proportion of females (62.8%) compared with included patients (37.2%, p = 0.03), but otherwise no significant differences were found for age (t(372) = 1.68, p = 0.09), PCS (t(328) = 0.89, p = 0.4), PSQI (t(230) = −0.54, p = 0.6), anxiety (t(295) = 0.31, p = 0.76), or depression (t(291) = 1.1, p = 0.28). Demographics for the included patients can be seen in Table 1. The MANCOVA demonstrated that randomization (chlorzoxazone or placebo) did not impact preoperative pain (F(1,161) = 1.74, p = 0.19), postoperative pain (F(1,161) = 0.87, p = 0.35), PCS (F(1,161) = 0.14, p = 0.71), PSQI (F(1,161) = 0.39, p = 0.53), anxiety (F(1,161) = 0.41, p = 0.52), or depression (F(1,161) = 2.15, p = 0.14).
Figure 1.
CONSORT diagram.
TKA: total knee arthroplasty, THA: total hip arthroplasty.
Flow of patients throughout the trial and the secondary analysis conducted in the current study.
Table 1.
Demographics and descriptives of the patient cohort (N = 163).
| Age (years) (mean ± SD) | 66.71 ± 13.63 |
| Gender (n (%)) | 99 males (60.74%); 64 females (39.26%) |
| Surgical procedure (TKA/THA), n (%) | 74 (45.4%)/89 (54.6%) |
| Preoperative pain intensity (0–10) (mean ± SD) | 3.55 ± 2.3 |
| PCS (0–52) (mean ± SD) | 17.79 ± 12.25 |
| PSQI (0–21) (mean ± SD) | 7.91 ± 4.61 |
| HADS (Anxiety; 0–21) (mean ± SD) | 3.96 ± 3.36 |
| HADS (Depression; 0–21) (mean ± SD) | 2.31 ± 2.64 |
SD: standard deviation; TKA: total knee arthroplasty, THA: total hip arthroplasty; PCS: Pain Catastrophizing Scale; PSQI: Pittsburgh Sleep Quality Index; HADS: Hospital Anxiety and Depression Scale.
The impact of sleep quality on psychological factors
The good sleepers were older than the poor sleepers (t(151) = −0.25, p = 0.02). In addition, a higher proportion of females were found in the poor sleepers’ group (poor sleepers = 50 males/44 females; good sleepers = 41 males/18 females) (χ2 = 3.9, p = 0.046). No difference was observed for proportion of surgery type in the subgroups (χ2 = 3.7, p = 0.055) (see Table 2).
Table 2.
Differences in main comparisons.
| Good sleepers | Poor sleepers | t test | |
|---|---|---|---|
| Age (years) (mean ± SD) | 70.47 ± 15.8 | 64.27 ± 11.47 | t(161) = −2.9, p = 0.004 |
| Gender (M/F) (n) | 45/19 | 54/45 | χ 2 = 4.05, p = 0.044 |
| Surgical procedure (TKA/THA) (n) | 35/29 | 39/60 | χ2 = 3.7, p = 0.055 |
| Preoperative pain intensity (0–10) (mean ± SD) | 2.76 ± 2.11 | 4.06 ± 2.29 | t(161) = 3.62, p < 0.001 |
| PCS (0–52) (mean ± SD) | 12.39 ± 8.85 | 21.29 ± 12.89 | t(160.38) = 5.22, p < 0.001 |
| HADS (Anxiety; 0–21) (mean ± SD) | 2.39 ± 2.26 | 4.97 ± 3.57 | t(160.96) = 5.64, p < 0.001 |
| HADS (Depression; 0–21) (mean ± SD) | 1.28 ± 1.67 | 2.98 ± 2.92 | t(158.79) = 4.71, p < 0.001 |
TKA: total knee arthroplasty, THA: total hip arthroplasty; PCS: Pain Catastrophizing Scale, PSQI: Pittsburgh Sleep Quality Index, HADS: Hospital Anxiety and Depression Scale.
Good sleepers were significantly older, were represented by less women, and had lower preoperative pain, lower PCS, lower depression, and lower anxiety than the poor sleepers.Boldfaced values represent significant findings (P < 0.05).
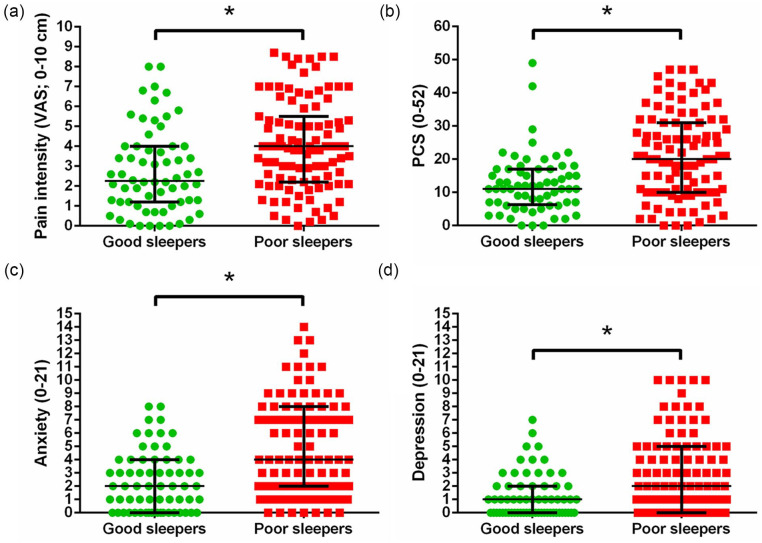
The poor sleepers reported significantly increased preoperative pain intensity (t(151) = 3.06, p = 0.003; Figure 2(a)), higher pain catastrophizing thoughts (t(149.16) = 4.96, p < 0.001; Figure 2(b)), higher anxiety scores (t(150.88) = 5.32, p< 0.001; Figure 2(c)), and higher depression scores (t(150.51) = 4.5, p < 0.001; Figure 2(d)) compared with the good sleepers.
Figure 2.
Differences between OA patients suffering from poor sleep versus good sleep (median ± interquartile ranges). Poor sleepers reported (a) significantly higher pain, (b) pain catastrophizing thoughts, (c) anxiety, and (d) depression.
PCS: Pain Catastrophizing Scale; Anxiety/Depression: Hospital Anxiety and Depression Scale.
Associations between preoperative sleep, preoperative pain intensity and preoperative cognitive factors
For preoperative pain, the linear regression model was significant (F(4,158) = 13.71, p < 0.005) with independent preoperative factors PSQI (β = 0.23, p = 0.006), PCS (β = 0.44, p < 0.005), and anxiety (β = 0.18, p = 0.036) explaining 23.9% of the variance (R2 = 0.239) in preoperative pain. Pre-operative depression was not a significantly independent predictor for preoperative pain (β < 0.005, p = 0.99). For preoperative PSQI, the linear regression model was significant (F(4,158) = 21.26, p < 0.005) with independent preoperative factors pain (β = 0.201, p = 0.006), PCS (β = 0.177, p = 0.044), anxiety (β = 0.18, p = 0.036), and depression (β = 0.23, p = 0.005) explaining 33.3% of the variance (R2 = 0.333) in preoperative PSQI.
Associations between preoperative sleep quality, pain intensity, cognitive factors and postoperative pain intensity 12 months after surgery
For postoperative pain intensity 12 months after surgery, the linear regression model was significant (F(5,157) = 4.25, p = 0.001) and explained 9.1% of the variance (R2 = 0.091) with independent preoperative factor pain (β = 0.32, p < 0.005). Preoperative PCS (β = 0.091, p = 0.38), anxiety (β = −0.137, p = 0.18), depression (β = 0.036, p = 0.72), and PSQI (β = −0.06, p = 0.5) were not independent factors for postoperative pain intensity 12 months after surgery.
Associations within subgroups of OA patients undergoing TKA or THA
Both the TKA and THA subgroup showed significant correlations between preoperative PSQI and PCS with preoperative pain (all r > 0.325, all p < 0.005; similar to the full cohort). Only the TKA group demonstrated associations between preoperative pain and preoperative PCS and postoperative pain 12 months after surgery (r = 0.507 and 0.286, both p < 0.013), whereas the THA group did not (r = 0.085 and −0.019, respectively, p = 0.428). Only the THA group showed an association between preoperative pain and preoperative depression (r = 0.285, p = 0.007). All subgroup correlations are shown in Table 3.
Table 3.
Correlation analyses within each subgroup of OA patients.
| TKA only | THA only | Full cohort | |
|---|---|---|---|
| Preoperative PSQI & preoperative pain | r = 0.325, p = 0.005 | r = 0.393, p < 0.001 | r = 0.36, p < 0.001 |
| Preoperative pain and preoperative PCS | r = 0.419, p < 0.001 | r = 0.489, p < 0.001 | r = 0.46, p < 0.001 |
| Preoperative pain and preoperative depression | r = 0.132, p = 0.263 | r = 0.285, p = 0.007 | r = 0.23, p = 0.004 |
| Preoperative pain and preoperative anxiety | r = 0.09, p = 0.43 | r = 0.202, p = 0.057 | r = 0.16, p = 0.12 |
| Preoperative pain and postoperative pain | r = 0.507, p < 0.001 | r = 0.085, p = 0.428 | r = 0.32, p < 0.001 |
| Postoperative pain and preoperative PCS | r = 0.286, p = 0.013 | r = −0.019, p = 0.86 | r = 0.15, p = 0.057 |
| Postoperative pain and preoperative anxiety | r = −0.123, p = 0.298 | r = 0.106, p = 0.323 | r = −0.045, p = 0.57 |
| Postoperative pain and preoperative depression | r = 0.094, p = 0.427 | r = 0.07, p = 0.513 | r = 0.042, p = 0.59 |
| Postoperative pain and preoperative PSQI | r = 0.116, p = 0.325 | r = 0.048, p = 0.654 | r = 0.05, p = 0.52 |
TKA: total knee arthroplasty, THA: total hip arthroplasty; PCS: Pain Catastrophizing Scale, PSQI: Pittsburgh Sleep Quality Index.
Preoperative pain was significantly correlated with preoperative PSQI and PCS in each group. For the TKA group, both preoperative pain and preoperative PCS were associated with postoperative pain intensity 12 months after surgery, whereas preoperative depression was associated with preoperative pain within the THA group.Boldfaced values represent significant findings (P < 0.05).
Discussion
The current study aimed to assess the association between poor sleep quality, preoperative factors known to influence postoperative pain and pain intensity 12 months after surgery. The results show that poor sleepers demonstrate higher preoperative pain intensity, higher levels of pain catastrophizing thoughts and higher levels of anxiety and depression. Finally, higher preoperative pain intensity, but not preoperative sleep quality, was an independent factor for higher pain intensity 12 months after surgery.
Sleep quality, its impact on preoperative pain and relation to chronic postoperative pain
Up to 50% of chronic pain patients report poor sleep, 27 and 1-month postoperative sleep disruption may mediate the association between pain and functional limitations reported 1 and 3 months after surgery, respectively. 28 A recent systematic review and meta-analysis identified sleep difficulties as a strong preoperative predictor of poor postoperative pain control; however, this was only based on two studies. 29 In addition, Mamie et al. 30 showed preoperative chronic sleep difficulties increased the risk of severe acute postoperative pain following intraperitoneal or orthopaedic surgery. A recent study reported that preoperative PSQI and daily sleepiness were associated with acute postoperative pain, up to 3 months and 3 days after TKA and THA, respectively. 9 The current study extends these findings by demonstrating that patients who are considered poor sleepers, based on the PSQI cut-off value, also report higher preoperative pain, pain catastrophizing, anxiety, and depression. Conversely, no significant association between preoperative sleep quality and pain intensity 12 months after surgery was found. It is worth nothing that the primary trial did not aim to assess the association(s) between sleep quality and pain after surgery, and therefore also present with large attrition which will be further discussed in the limitations. These considerations should be factored in when comparing the current findings with those of Luo et al. 9 Nonetheless, there may be an association between poor sleep quality preoperatively and pain after surgery; however, the timeline of which this remains relevant and the underlying mechanisms should be elucidated in future studies. In healthy participants, partial and total sleep deprivation have shown to affect both peripheral10,31 and central facilitatory and inhibitory pain mechanisms.10,32 These pain mechanisms are also altered in chronic pain patients suffering from, for example, fibromyalgia or knee OA12,33–35 and may predict the analgesic response to standard pain treatment in OA36,37 and reports of chronic postoperative pain following TKA12,13,38–41 and THA.42,43 Together with the current findings, these studies indicate that OA patients reporting poor sleep also suffer from higher preoperative pain which is predictive of chronic postoperative pain. 3 Interestingly, the subgroup correlations provided in the current report may suggest that preoperative pain intensity is associated with pain intensity 12 months after surgery only in TKA patients. Future studies may investigate whether such difference exists in a design specifically aimed towards this. In a randomized controlled feasibility trial, Roehrs and Roth 14 demonstrated, in a cohort of 18 knee and hip OA patients scheduled for TKA or THA, that extending sleep by approximately 1 hour yielded an improvement in acute postoperative pain and opiate use, indicating that improving sleep might yield better postoperative outcomes for patients. Of note, earlier large-scale studies,44,45 albeit not related to postoperative pain, have demonstrated associations between sleep quality and sleep duration, however, often of low-to-moderate strength. Therefore, sleep quality and sleep duration should not be considered synonymous parameters and should be considered in the present discussion and future studies on the relationship between sleep parameters and pre- and postoperative measures of pain. Since the current study found a difference in age between those patients who sleep poorly compared with those who sleep well, the possible effect of age on postoperative pain should be considered, despite the availability of contrasting evidence on the association between age and postoperative pain after various surgical procedures. 46 The risk of postoperative pain may decline as a linear function of age per decade increase, across a large variety of different surgical procedures, including TKA and THA. 47 In the current study, this may partially explain why the preoperative pain intensity was higher in the younger poor sleep group and may have indirectly influenced postoperative pain intensity due to the well-established association between pre- and postoperative pain (see, for example, Lewis et al. 3 ). However, this remains a speculation as of now and requires further investigation. Furthermore, a larger proportion of women was found in the poor sleep group, and women, in general, report higher preoperative pain intensity 48 and more frequently develop chronic postoperative pain.49,50 It is worth noting that our findings are in line with earlier epidemiological studies investigating the differences in, for example, sleep quality, where a larger proportion of women report poor sleep quality among other sleep parameters compared with men. 51 This study cannot rule out a possible influence of the difference in proportion of females in the poor sleepers group when comparing, for example, preoperative pain intensity, but it is acknowledged and should be controlled in future studies.
Sleep quality and its relation to anxiety, depression and pain catastrophizing
Sleep abnormalities have been associated with high risk of depression,52,53 anxiety, 11 and pain catastrophizing. 54 For instance, depression was shown to be independently associated with sleep problems in rheumatoid arthritis patients, 55 however, the direction in which insomnia, depression, and even chronic pain interact remains elusive. 56 In temporomandibular disorder patients, sleep disturbance was shown to exert a mediating role on pain catastrophizing. 57 In support, indirect evidence for the role of sleep on pain catastrophizing was shown in another study, where cognitive behavioural therapy for insomnia in knee OA patients reduced pain catastrophizing. 58
These studies support early interventions aimed at managing depression, anxiety, and pain catastrophizing, since they, especially the latter, are known to predict chronic postoperative pain,4,5 and this exploratory study extends these findings by showing higher levels of pain catastrophizing in knee OA patients who report poor sleep prior to TKA and THA. A recent multi-site randomized clinical trial was performed to lower catastrophizing thoughts in TKA patients but showed no effect on the incidence of chronic postoperative pain. 59 Therefore, future studies may explore the possibility of managing this triad of cognitive impairment through, for instance, sleep therapy or cognitive behavioural therapy, to evaluate its effect on chronic postoperative pain.
Cognitive factors and their relation to chronic postoperative pain
A systematic review and meta-analysis showed that preoperative anxiety and pain catastrophizing hold predictive value for chronic postoperative pain 3–12 months after various surgeries. 60 Another study reported that patients enrolled for abdominal surgery who underwent a preoperative pain management intervention for understanding and managing chronic postoperative pain exhibited lower preoperative anxiety and pain attitude and, importantly, reported lower acute pain intensity up to 24 hours after surgery. 61 In knee OA, preoperative anxiety and depression was shown to be associated with chronic postoperative pain 6 62 and 12 months after TKA. 63 Furthermore, in TKA patients, pain intensity and pain-related distress may be associated with anxiety, depression, and pain catastrophizing. 64 In addition, preoperative pain catastrophizing has been demonstrated to predict chronic postoperative pain 6 4 and 24 months 5 after TKA, albeit contradictory evidence exist. 65 In this respect, the association between pain and functional limitation 1 and 3 months after surgery, respectively, may be mediated by reported sleep disruption 1 month after TKA. 28 Furthermore, depression has been shown to predict higher postoperative pain, whereas catastrophizing was shown to be a unique predictor of higher postoperative night-time pain. 66 In populations suffering from sleep-related disorders such as sleep apnea, the prevalence of depression and anxiety is more pronounced when compared with a non-apnea group. 67 The current exploratory study adds to this growing body of evidence by showing that hip and knee OA patients reporting poor sleep exhibit higher preoperative anxiety, depression, and pain catastrophizing levels. In a 12-month longitudinal study, Scott et al. 68 demonstrated that following a telecare intervention optimizing pain monitoring and analgesic management, improvements in depression, pain catastrophizing, and anxiety predicted better pain outcomes. Therefore, these factors may play a direct or an indirect role in chronic postoperative pain, and further research similar to the study by Riddle et al. 59 is needed to further our understanding of preoperative interventions on cognitive factors associated with postoperative pain.
Limitations
Several limitations should be considered for this study. Large attrition from the primary trial (i.e. only 41.48% of the participants of the original trial 17 were included in this report, due to a lack of pre- or post-measures of the included variables) may introduce issues with both external and internal validity; hence, we opted to demonstrate that baseline values for the included cohort did not differ significantly compared with the excluded patients. However, this does not conclusively demonstrate that the included cohort is representative for the full population of TKA and THA patients from which they originated from, and the results should be interpreted with care. Since a larger proportion of females were found in the excluded and poor sleepers’ groups, and that females are known to report higher anxiety, 69 higher preoperative pain intensity, 48 and more frequently develop chronic postoperative pain,49,50 this should be considered when interpreting the current findings. Furthermore, since the current study investigated preoperative depression and anxiety, it is important to highlight that the primary trial excluded patients based on the use of antidepressants and anxiolytics within the past 4 weeks. 17 As such, the prevalence (~20% for both types of OA)70,71 or severity of depression or anxiety symptoms in the patient cohort may be underrepresented compared with the general TKA or THA population undergoing surgery. No exclusion criteria on common sleep disturbances such as obstructive sleep apnea were implemented in the primary trial, and since no predictive value of the PSQI has been shown for sleep disturbances such as obstructive sleep apnea 72 or other sleep behaviour disorders, 73 this study cannot rule out that other underlying sleep disorders were present in the cohort.
Conclusion
This study investigated associations between preoperative quality of sleep, preoperative risk factors for chronic postoperative pain and pain intensity 12 months after TKA and THA. The results showed that OA patients with poor preoperative sleep have higher preoperative pain intensities and higher levels of pain catastrophizing, anxiety, and depression compared with patients who sleep well. Preoperative pain intensity, but not sleep quality, was an independent factor for pain intensity 12 months after total knee and hip arthroplasty. As such, the present findings do not support an association between preoperative sleep quality and postoperative pain intensity 12 months after surgery, but that differences may exist in preoperative measures important to resolve pain after surgery based on sleep quality.
Footnotes
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributorship: K.K.P., M.L., O.S., and L.A.-N. contributed to the conceptual development of the study. Data were collected by M.L. and O.S. and analysed by D.B.L. All authors interpreted and discussed the data. D.B.L. wrote the first draft, which was critically revised by K.K.P., L.A.-N., O.S., and M.L. All authors approved the final version.
Data availability: Data are available upon request from the author.
Ethical approval: Ethical approval for this study was obtained from the Northern Jutland Scientific Ethics Committee (VN-20150024).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the University Talent Management Programme (J. No. 771126), the Shionogi Science Program, and the TaNeDS Europe grant. None of the funders had any role in the study other than to provide funding. K.K.P. received a grant from the Ministry of Higher Education and Science in collaboration with Cortex Technology Aps to develop the cuff algometer. Center for Neuroplasticity and Pain (CNAP) is supported by the National Research Foundation (DNRF121).
Guarantor: K.K.P. is the guarantor of this article.
Informed consent: Written informed consent was obtained from all subjects before the study.
Trial registration: Clinicaltrials.gov (identifier number: NCT02405104)
ORCID iD: Dennis Boye Larsen  https://orcid.org/0000-0002-2844-7408
https://orcid.org/0000-0002-2844-7408
References
- 1.Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390: 1211–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beswick AD, Wylde V, Gooberman-Hill R, et al. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of Prospective studies in unselected patients. BMJ Open 2012; 2(1): e000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis GN, Rice DA, McNair PJ, et al. Predictors of persistent pain after total knee arthroplasty: a systematic review and meta-analysis. Br J Anaesth 2015; 114(4): 551–561. [DOI] [PubMed] [Google Scholar]
- 4.Riddle DL, Wade JB, Jiranek WA, et al. Preoperative pain catastrophizing predicts pain outcome after knee arthroplasty. Clin Orthop Relat Res 2010; 468(3): 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forsythe ME, Dunbar MJ, Hennigar AW, et al. Prospective relation between catastrophizing and residual pain following knee arthroplasty: two-year follow-up. Pain Res Manag 2008; 13(4): 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Somers TJ, Keefe FJ, Pells JJ, et al. Pain catastrophizing and pain-related fear in osteoarthritis patients: relationships to pain and disability. J Pain Symptom Manage 2009; 37(5): 863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birch S, Stilling M, Mechlenburg I, et al. Association between pain catastrophizing, physical function and pain at first visit in the outpatient knee clinic. Knee 2019; 26(6): 1286–1291. [DOI] [PubMed] [Google Scholar]
- 8.Odole A, Ekediegwu E, Ekechukwu END, et al. Correlates and predictors of pain intensity and physical function among individuals with chronic knee osteoarthritis in Nigeria. Musculoskelet Sci Pract 2019; 39: 150–156. [DOI] [PubMed] [Google Scholar]
- 9.Luo ZY, Li LL, Wang D, et al. Preoperative sleep quality affects postoperative pain and function after total joint arthroplasty: a prospective cohort study. J Orthop Surg Res 2019; 14: 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staffe AT, Bech MW, Clemmensen SLK, et al. Total sleep deprivation increases pain sensitivity, impairs conditioned pain modulation and facilitates temporal summation of pain in healthy participants. PLoS ONE 2019; 14(12): e0225849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alvaro PK, Roberts RM, Harris JK. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep 2013; 36: 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersen KK, Graven-Nielsen T, Simonsen O, et al. Preoperative pain mechanisms assessed by cuff algometry are associated with chronic postoperative pain relief after total knee replacement. Pain 2016; 157(7): 1400–1406. [DOI] [PubMed] [Google Scholar]
- 13.Petersen KK, Arendt-Nielsen L, Simonsen O, et al. Presurgical assessment of temporal summation of pain predicts the development of chronic postoperative pain 12 months after total knee replacement. Pain 2015; 156(1): 55–61. [DOI] [PubMed] [Google Scholar]
- 14.Roehrs TA, Roth T. Increasing presurgery sleep reduces postsurgery pain and analgesic use following joint replacement: a feasibility study. Sleep Med 2017; 33: 109–113. [DOI] [PubMed] [Google Scholar]
- 15.van Tulder MW, Touray T, Furlan AD, et al. Muscle relaxants for non-specific low-back pain. Cochrane Database Syst Rev 2003; 2017: CD004252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kehlet H, Jensen TS, Woolf CJ, et al. Persistent postsurgical pain: risk factors and prevention. Lancet 2006; 367: 1618–1625. [DOI] [PubMed] [Google Scholar]
- 17.Skrejborg P, Petersen KK, Beck J, et al. Investigating the effect of perioperative chlorzoxazone on acute postoperative pain after total hip and knee replacement surgery. Clin J Pain 2020; 36(5): 352–358. [DOI] [PubMed] [Google Scholar]
- 18.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989; 28(2): 193–213. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess 1995; 7: 524–532. [Google Scholar]
- 20.Osman A, Barrios FX, Gutierrez PM, et al. The pain catastrophizing scale: further psychometric evaluation with adult samples. J Behav Med 2000; 23(4): 351–365. [DOI] [PubMed] [Google Scholar]
- 21.Edwards RR, Calahan C, Mensing G, et al. Pain, catastrophizing, and depression in the rheumatic diseases. Nat Rev Rheumatol 2011; 7: 216–224. [DOI] [PubMed] [Google Scholar]
- 22.Kjøgx H, Zachariae R, Pfeiffer-Jensen M, et al. Pain frequency moderates the relationship between pain catastrophizing and pain. Front Psychol 2014; 5: 1421–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kendrick T, Pilling S. Common mental health disorders-Identification and pathways to care: NICE clinical guideline. Br J Gen Pract 2012; 62: 47–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snaith RP. The hospital anxiety and depression scale. Health Qual Life Outcomes 2003; 10: 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjelland I, Dahl AA, Haug TT, et al. The validity of the Hospital Anxiety and Depression Scale. J Psychosom Res 2002; 52: 69–77. [DOI] [PubMed] [Google Scholar]
- 26.Hair J., Black WC, Babin BJ, et al. Multivariate data analysis: international version. Upper saddle River, NJ: Pearson, 2010. [Google Scholar]
- 27.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev 2004; 8(2): 119–132. [DOI] [PubMed] [Google Scholar]
- 28.Cremeans-Smith JK, Millington K, Sledjeski E, et al. Sleep disruptions mediate the relationship between early postoperative pain and later functioning following total knee replacement surgery. J Behav Med 2006; 29(2): 215–222. [DOI] [PubMed] [Google Scholar]
- 29.Yang MMH, Hartley RL, Leung AA, et al. Preoperative predictors of poor acute postoperative pain control: a systematic review and meta-analysis. BMJ Open 2019; 9: e025091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mamie C, Bernstein M, Morabia A, et al. Are there reliable predictors of postoperative pain. Acta Anaesthesiol Scand 2004; 48(2): 234–242. [DOI] [PubMed] [Google Scholar]
- 31.Schuh-Hofer S, Wodarski R, Pfau DB, et al. One night of total sleep deprivation promotes a state of generalized hyperalgesia: a surrogate pain model to study the relationship of insomnia and pain. Pain 2013; 154(9): 1613–1621. [DOI] [PubMed] [Google Scholar]
- 32.Eichhorn N, Treede RD, Schuh-Hofer S. The role of sex in sleep deprivation related changes of nociception and conditioned pain modulation. Neuroscience 2018; 387: 191–200. [DOI] [PubMed] [Google Scholar]
- 33.Sivertsen B, Lallukka T, Petrie KJ, et al. Sleep and pain sensitivity in adults. Pain 2015; 156: 1433–1439. [DOI] [PubMed] [Google Scholar]
- 34.Arendt-Nielsen L, Nie H, Laursen MB, et al. Sensitization in patients with painful knee osteoarthritis. Pain 2010; 149: 573–581. [DOI] [PubMed] [Google Scholar]
- 35.Staud R, Cannon RC, Mauderli AP, et al. Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain 2003; 102(1–2): 87–95. [DOI] [PubMed] [Google Scholar]
- 36.Petersen KK, Olesen AE, Simonsen O, et al. Mechanistic pain profiling as a tool to predict the efficacy of 3-week nonsteroidal anti-inflammatory drugs plus paracetamol in patients with painful knee osteoarthritis. Pain 2019; 160(2): 486–492. [DOI] [PubMed] [Google Scholar]
- 37.Petersen KK, Simonsen O, Olesen AE, et al. Pain inhibitory mechanisms and response to weak analgesics in patients with knee osteoarthritis. Eur J Pain 2019; 23(10): 1904–1912. [DOI] [PubMed] [Google Scholar]
- 38.Petersen KK, Simonsen O, Laursen MB, et al. The role of preoperative radiologic severity, sensory testing, and temporal summation on chronic postoperative pain following total knee arthroplasty. Clin J Pain 2018; 34(3): 193–197. [DOI] [PubMed] [Google Scholar]
- 39.Kurien T, Arendt-Nielsen L, Petersen KK, et al. Preoperative neuropathic pain-like symptoms and central pain mechanisms in knee osteoarthritis predicts poor outcome 6 months after total knee replacement surgery. J Pain 2018; 19(11): 1329–1341. [DOI] [PubMed] [Google Scholar]
- 40.Vaegter HB, Handberg G, Emmeluth C, et al. Preoperative hypoalgesia after cold pressor test and aerobic exercise is associated with pain relief 6 months after total knee replacement. Clin J Pain 2017; 33(6): 475–484. [DOI] [PubMed] [Google Scholar]
- 41.Wylde V, Palmer S, Learmonth ID, et al. The association between pre-operative pain sensitisation and chronic pain after knee replacement: an exploratory study. Osteoarthritis Cartilage 2013; 21(9): 1253–1256. [DOI] [PubMed] [Google Scholar]
- 42.Izumi M, Petersen KK, Laursen MB, et al. Facilitated temporal summation of pain correlates with clinical pain intensity after hip arthroplasty. Pain 2017; 158(2): 323–332. [DOI] [PubMed] [Google Scholar]
- 43.Wylde V, Sayers A, Lenguerrand E, et al. Preoperative widespread pain sensitization and chronic pain after hip and knee replacement: a cohort analysis. Pain 2015; 156(1): 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kripke DF, Garfinkel L, Wingard DL. Mortality associated with sleep duration and insomnia. Prim Care Companion J Clin Psychiatry 2002; 4: 131–136. [DOI] [PubMed] [Google Scholar]
- 45.Takeuchi H, Taki Y, Nouchi R, et al. Shorter sleep duration and better sleep quality are associated with greater tissue density in the brain. Sci Rep 2018; 8: 5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gagliese L, Weizblit N, Ellis W, et al. The measurement of postoperative pain: a comparison of intensity scales in younger and older surgical patients. Pain 2005; 117(3): 412–420. [DOI] [PubMed] [Google Scholar]
- 47.Gerbershagen HJ, Pogatzki-Zahn E, Aduckathil S, et al. Development of severe postoperative pain. Anesthesiology 2014; 120: 1237–1245. [DOI] [PubMed] [Google Scholar]
- 48.Keefe FJ, Lefebvre JC, Egert JR, et al. The relationship of gender to pain, pain behavior, and disability in osteoarthritis patients: the role of catastrophizing. Pain 2000; 87(3): 325–334. [DOI] [PubMed] [Google Scholar]
- 49.Rosseland LA, Stubhaug A. Gender is a confounding factor in pain trials: women report more pain than men after arthroscopic surgery. Pain 2004; 112(3): 248–253. [DOI] [PubMed] [Google Scholar]
- 50.Pereira MP, Pogatzki-Zahn E. Gender aspects in postoperative pain. Curr Opin Anaesthesiol 2015; 28(5): 546–558. [DOI] [PubMed] [Google Scholar]
- 51.Middelkoop HAM, Smilde-van den Doel DA, Neven AK, et al. Subjective sleep characteristics of 1,485 males and females aged 50-93: effects of sex and age, and factors related to self-evaluated quality of sleep. J Gerontol A Biol Sci Med Sci 1996; 51: M108–M115. [DOI] [PubMed] [Google Scholar]
- 52.Rao U, Hammen CL, Poland RE. Risk markers for depression in adolescents: sleep and HPA measures. Neuropsychopharmacology 2009; 34(8): 1936–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goesling J, Clauw DJ, Hassett AL. Pain and depression: an integrative review of neurobiological and psychological factors. Curr Psychiatry Rep 2013; 15(12): 421. [DOI] [PubMed] [Google Scholar]
- 54.Goodin BR, Fillingim RB, Machala S, et al. Subjective sleep quality and ethnicity are interactively related to standard and situation-specific measures of pain catastrophizing. Pain Med 2011; 12(6): 913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nicassio PM, Wallston KA. Longitudinal relationships among pain, sleep problems, and depression in rheumatoid arthritis. J Abnorm Psychol 1992; 101(3): 514–520. [DOI] [PubMed] [Google Scholar]
- 56.Finan PH, Smith MT. The comorbidity of insomnia, chronic pain, and depression: dopamine as a putative mechanism. Sleep Med Rev 2013; 17(3): 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buenaver LF, Quartana PJ, Grace EG, et al. Evidence for indirect effects of pain catastrophizing on clinical pain among myofascial temporomandibular disorder participants: the mediating role of sleep disturbance. Pain 2012; 153(6): 1159–1166. [DOI] [PubMed] [Google Scholar]
- 58.Lerman SF, Finan PH, Smith MT, et al. Psychological interventions that target sleep reduce pain catastrophizing in knee osteoarthritis. Pain 2017; 158(11): 2189–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riddle DL, Keefe FJ, Ang DC, et al. Pain Coping Skills Training for Patients Who Catastrophize About Pain Prior to Knee Arthroplasty. J Bone Jt Surg 2019; 101: 218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Theunissen M, Peters ML, Bruce J, et al. Preoperative anxiety and catastrophizing: a systematic review and meta-analysis of the association with chronic postsurgical pain. Clin J Pain 2012; 28(9): 819–841. [DOI] [PubMed] [Google Scholar]
- 61.Lin LY, Wang RH. Abdominal surgery, pain and anxiety: preoperative nursing intervention. J Adv Nurs 2005; 51(3): 252–260. [DOI] [PubMed] [Google Scholar]
- 62.Noiseux NO, Callaghan JJ, Clark CR, et al. Preoperative predictors of pain following total knee arthroplasty. J Arthroplasty 2014; 29(7): 1383–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brander VA, Stulberg SD, Adams AD, et al. Predicting total knee replacement pain: a Prospective, Observational Study. Clin Orthop Relat Res 2003(416): 27–36. [DOI] [PubMed] [Google Scholar]
- 64.Hadlandsmyth K, Sabic E, Zimmerman MB, et al. Relationships among pain intensity, pain-related distress, and psychological distress in pre-surgical total knee arthroplasty patients: a secondary analysis. Psychol Health Med 2017; 22(5): 552–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Høvik LH, Winther SB, Foss OA, et al. Preoperative pain catastrophizing and postoperative pain after total knee arthroplasty: a prospective cohort study with one year follow-up. BMC Musculoskelet Disord 2016; 17: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Edwards RR, Haythornthwaite JA, Smith MT, et al. Catastrophizing and depressive symptoms as prospective predictors of outcomes following total knee replacement. Pain Res Manag 2009; 14(4): 307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharafkhaneh A, Giray N, Richardson P, et al. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep 2005; 28(11): 1405–1411. [DOI] [PubMed] [Google Scholar]
- 68.Scott EL, Kroenke K, Wu J, et al. Beneficial effects of improvement in depression, pain catastrophizing, and anxiety on pain outcomes: a 12-month longitudinal analysis. J Pain 2016; 17(2): 215–222. [DOI] [PubMed] [Google Scholar]
- 69.Wood TJ, Thornley P, Petruccelli D, et al. Preoperative predictors of pain catastrophizing, anxiety, and depression in patients undergoing total joint arthroplasty. J Arthroplasty 2016; 31(12): 2750–2756. [DOI] [PubMed] [Google Scholar]
- 70.Marks R. Comorbid depression and anxiety impact hip osteoarthritis disability. Disabil Health J 2009; 2(1): 27–35. [DOI] [PubMed] [Google Scholar]
- 71.Sharma A, Kudesia P, Shi Q, et al. Anxiety and depression in patients with osteoarthritis: impact and management challenges. Open Access Rheumatol 2016; 8: 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buysse DJ, Hall ML, Strollo PJ, et al. Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. J Clin Sleep Med 2008; 4: 563–571. [PMC free article] [PubMed] [Google Scholar]
- 73.Nishiyama T, Mizuno T, Kojima M, et al. Criterion validity of the Pittsburgh Sleep Quality Index and Epworth sleepiness scale for the diagnosis of sleep disorders. Sleep Med 2014; 15(4): 422–429. [DOI] [PubMed] [Google Scholar]