Abstract
Cellular senescence is an intricate and multifactorial phenomenon, which is characterized by an irreversible cellular growth arrest, it is caused in response to irretrievably DNA damage, telomere shorting, activation of oncogene, and oxidative stress. Human diploid fibroblasts are a well-established experimental model for premature senescence-related studies, and exposure of fibroblasts to H2O2 is widely used as a SIPS model. Recently, it has been reported many studies of CoQ10 as to anti-aging effects, however the effect of CoQ10 on H2O2-induced SIPS model of human skin fibroblasts has not been understood. So that, we investigated that human skin fibroblasts were used to investigate the prevention effect of CoQ10 against H2O2-induced SIPS model. We created SIPS model fibroblasts with treatment of 100 μM H2O2 for 2 h. In this study, CoQ10 also increased cell viability and mRNA levels of type I, IV collagen and protein level of type I collagen. Moreover, it is shown that CoQ10 suppressed oxidative stress, degradation of collagen by increasing MMP expression, and decreasing senescence-associated phenotypes (e.g. SA-βgal positive staining and SASP) for preventing skin aging via H2O2-induced SIPS model. These results suggested that CoQ10 has possibility to be contributory for extension of healthy life expectancy in Japan.
Keywords: coenzyme Q10 (CoQ10), anti-aging, SIPS, oxidative-stress
Introduction
Senescence of cells is an intricate and multifactorial phenomenon that is characterized by an irreversible cellular growth arrest, and it is brought about by in response to irretrievably DNA damage, telomere shorting, activation of oncogene, protein aggregation, telomere attrition, mitochondrial dysfunction, inflammation, and oxidative stress. In addition, senescent cells exhibit specific morphological alterations, a flat and enlarged cell shape, senescence-associated β-galactosidase (SA-β-gal) positive cells and decrease of synthesis of extracellular matrix (ECM).(1) Oxidative stress is especially play an important role in aging process, and progression of that is postulated in the free radical theory.(2) It is well known that it is a very strong relationship between oxidative stress and aging. Moreover, reactive oxygen species (ROS) induces DNA damage response (DDR) causing for continuous accumulation of cellular damage, so that DDR is trigger for principle pathway that accumulation of senescent cells can contribute to the aging phenotype.(3) Besides, sublethal doses of various compounds such as superoxide anions (O2−), hydroxyl radicals (OH•), and hydrogen peroxide (H2O2) also induce phenomena similar to replicative senescence in normal cells, a phenomenon termed premature senescence. For instance, human diploid fibroblasts are a well-utilized experimental model for premature senescence-related studies, and exposure of normal fibroblasts to H2O2 is widely used as a stress-induced premature senescence (SIPS) model.(4–7) Furthermore, it has reported that exposure to ultraviolet and H2O2 increase intracellular ROS levels and induces premature senescence in fibroblasts.(8,9) Meanwhile, Coppé et al.(10) have reported that in human cells exposed to genotoxic stress, it secretes myriad factors associated with inflammation and malignancy. The phenomenon is called senescence-associated secretory phenotype (SAPS). SASP comprises complex factors including cytokines, growth factors, chemokines, and matrix metalloproteinase (MMP), so that it is reported SASP facilitate the tumorigenesis of normal cells.(11) Additionally, it has also been reported that DDR plays an important role for induction of SASP in vitro.(12) While, SASP observed in both SIPS model and replicative senescence.(13) Therefore, it has been thought that inhibition of SASP is important for prevention to several diseases, anti-aging, suppression of tumorigenesis, and inflammation.
Coenzyme Q10 (CoQ10) is an essential electron carrier for the mitochondrial electron transport system and an important for energy production. In addition, CoQ10 also serves as a free radical scavenger, and prevents oxidative damage in the human body. Furthermore, it is known that CoQ10 participate in inflammation process,(14,15) apoptosis,(16,17) and gene expression.(18,19) In addition, several studies have demonstrated that CoQ10 has broad therapeutic effects including diabetes,(20) cardiovascular disease,(21) and neurological disorder.(22) On the other hand, it also has been known that the intracellular CoQ10 level decreases after the age of 20 years.(23) As an evidence, the antioxidant effect of reduced form CoQ10 has been demonstrated in vivo and in vitro in various biological system. Interestingly, CoQ10 decreased aging speed in SAMP1 mice.(24,25) Moreover, in rats with a polyunsaturated fatty acid (PUFA)-rich diet, CoQ10 treatment protects from age-related DNA double-strand breaks, and prolongs lifespan of the rat.(26) These reports suggest that CoQ10 may involve in aging. Many researchers had been reported in aging skin cells for observation of physiological, biochemical, and molecular characteristics. In particularly, ECM level in aging skin cells is one of the most change, which affect the wrinkle formation, elasticity, and morphology change. MMP plays an important role in regulating the collagen levels, and the elevated level of that induce collagen degradation in aged skin.(27) It has shown that the SIPS model of human diploid fibroblasts disassemble collagen via MMPs regulating.(28) Furthermore, because MMPs are key SAPS factors contribute to degradation of the ECM, it is suggested that MMPs participate the aging process, which are regulated by the intracellular ROS levels.(29) These reports support the notion that CoQ10 with potent antioxidant effect raises the possibility of have the anti-aging effect via inhibition of intracellular MMP and ROS levels. However, the effect of CoQ10 on SIPS model of human skin fibroblasts has not been understood. Therefore, in this study, we investigated for anti-aging effect of CoQ10 on H2O2-indeced SIPS model of human skin fibroblasts.
Materials and Methods
Materials
CoQ10 powder, PureSorb-QTM40 (P40), which is containing 40 w/v% CoQ10, was kindly donated by Nisshin Pharma Inc. (Tokyo, Japan).(30) Antibody against type I collagen was obtained from COSMO BIO Co., Ltd. (Tokyo, Japan). All other chemicals used were of analytical grade available from commercial suppliers.
Cell culture
Normal human dermal fibroblast (NHDF) was purchased from Lonza Co. Ltd. (Tokyo, Japan). Fibroblasts were grown in Dulbecco’s modified Eagle’s medium (DMEM, Nacalai Tesque, Kyoto, Japan) supplemented with 2% fetal bovine serum (FBS; MP Biomedicals, Illkirch, France), 2 mM glutamine, 100 units/ml penicillin and 100 mg/ml streptomycin (Nacalai Tesque). The cells were incubated under 37°C in a humidified atmosphere of 5% CO2 and 95% air in a CO2 incubator. After 24 h, culture medium was replaced by the DMEM containing 2% FBS and P40, and the cells were subsequently pre-cultured for 1 week in the CO2 incubator until the experiments.
Cell viability
After treatment, cell viability was quantified using Cell Counting Kit-8 (Dojindo Laboratories, Kumamoto, Japan).(31) The assay was carried out in accordance with the instruction manual. On the other hand, cell viability was also visualized by DAPI-staining. The assay was carried out in accordance with the instruction manual. After the fibroblasts on each well of 24-well plate were washed with PBS twice, the cells were incubated with DAPI staining solution (Dojindo Laboratories) for 30 min in CO2 incubator, fluorescence of DAPI-DNA complex meaning cell death was observed by using fluorescence microscopy (IX-71, Olympus). For detection of dead cells, the fibroblasts were imaged by using fluorescence microscopy.
Intracellular ROS production
The fibroblasts were incubated with PBS containing 2.5 μM CM-H2DCFDA (Invitrogen) at 37°C for 30 min in CO2 incubator. Fluorescence of 2',7'-dichlorodihydrofluorescein, which was oxidation product of CH-H2DCFDA by ROS produced in cells, was observed by using fluorescence microscopy. The assay was carried out in accordance with the instruction manual.
Cell senescence
We examined SA-βgal staining, a biomarker of senescence. Senescence of fibroblasts were measured using Senescence Detection Kit (BioVision, Mountain View, CA) based on SA-βgal staining. The assay was carried out in accordance with the instruction manual.
Real-time PCR
Whole RNA of cultured skin fibroblasts was extracted using RNeasy Mini Kit (Qiagen, Chatsworth, CA) in accordance with the instruction manual. The mRNA expression was quantified by methods of TaqMan with real-time reverse transcriptase (RT)-PCR. PCR primers were purchased from SIGMA-ALDRICH custom oligonucleotide synthesis service (Sigma Aldrich, St. Louis, MO). Primer sequences are COL1 (forward, 5'-GGGATTCCCTGGACCTAAAG-3' and reverse, 5'-GGAACACCTCGCTCTCCA-3'), COL4 (forward, 5'-AGGAGAGAAGGGCGCTGT-3' and reverse, 5'-TCCAGGTAAGCCGTCAACA-3'), MMP2 (forward, 5'-CCCCAAAACGGACAAAGAG-3' and reverse, 5'-TGTCCTTCAGCACAAACAGG-3'), MMP8 (forward, 5'-TGACAGAGACCTCATTTTCCTATTTA-3' and reverse, 5'-CTGCGTCAATTGCTTGGA-3'), GLB1 (forward, 5'-GCTCCTCCGACCCAGATT-3' and reverse, 5'-CTGGCCCTCCATTCTGATAG-3'), IL-6 (forward, 5'-CAGGAGCCCAGCTATGAACT-3' and reverse, 5'-GAAGGCAGCAGGCAACAC-3'), IL-8 (forward, 5'-AGACAGCAGAGCACACAAGC-3' and reverse, 5'-AGGAAGGCTGCCAAGAGAG-3'), and p21 (forward, 5'-CCGAGGCACTCAGAGGAG-3' and reverse, 5'-AGCTGCTCGCTGTCCACT-3'). The PCR was carried out using Applied Biosystems Veriti Thermal Cycler and the reaction conditions were followed; 95°C for 10 min, 40 cycles of amplification at 95°C for 15 s, 60°C for 1 min, followed by 95°C for 15 s, 60°C for 30 s and 95°C for 15 s. Target gene expression level was normalized to the housekeeping gene, 18sRNA, expression level.
Immunostaining
After fibroblast was fixed with 4% paraformaldehyde for 20 min, the cells were washed with PBS three times, and then permeabilized and blocked with 0.2% Triton-X and 1% normal goat serum (NGS) containing phosphate-buffered saline (PBS) for 20 min at room temperature. The permeabilized cells were treated with type I collagen antibody (1:300). After overnight incubation at 4°C, the fibroblasts were washed with PBS three times, the fibroblasts were treated with Alexa Fluor 488 probe (1:500, Invitrogen, Carlsbad, CA) for 1 h at room temperature in dark. The fibroblasts were mounted with DAPI and imaged by using fluorescence microscopy.
Protein quantity
Proteins were quantified using Protein Quantification Kit-Rapid (Dojindo Laboratories) based on the method of Bradford,(32) and standard BSA solution was used to generate a standard curve for this assay.
Statistical analysis
Data are expressed as mean ± SD. The significance of differences between individual data was determined by using Student’s unpaired t test. The p values less than 0.05 were considered significant.
Results
CoQ10 protects H2O2-induced cell death of fibroblasts
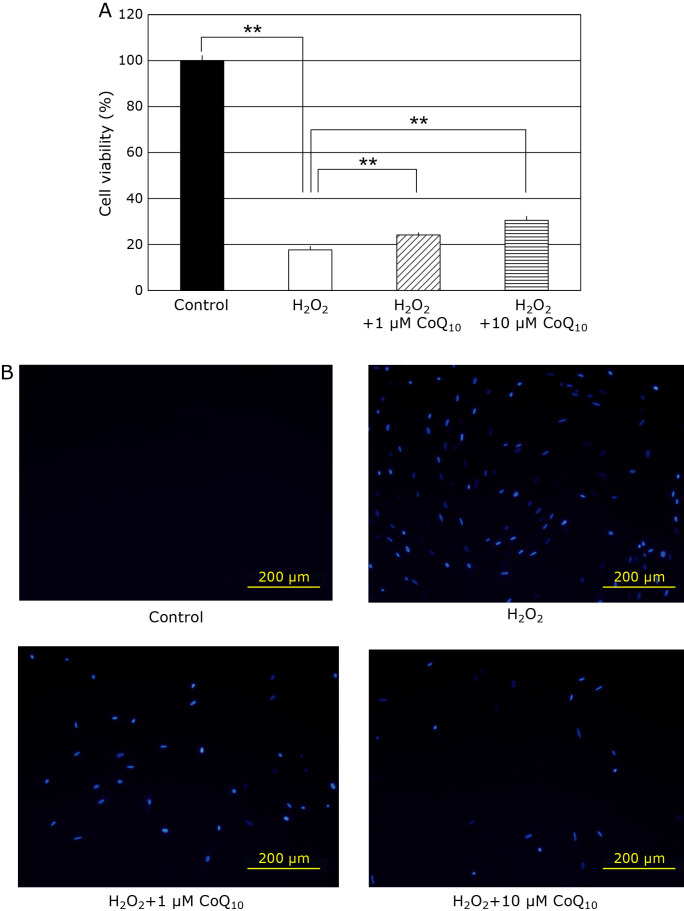
SIPS model of fibroblasts are prepared by H2O2 treatment, high concentration of H2O2 may induce cell death. Therefore, we investigated whether CoQ10 can prevent 100 μM H2O2-induced cell death by MTT and DAPI assay. When SIPS-induced fibroblasts were treated with 100 μM H2O2, the cell viability significantly decreased to 17% (Fig. 1A). 1 μM and 10 μM CoQ10 significantly increased the cell viability to 24.2% and 30.6%, respectively. The same protective effect of CoQ10 was also detected by DAPI staining (Fig. 1B). These results suggest that CoQ10 can prevents H2O2-induce cell death of fibroblast because of its antioxidative activity.
Fig. 1.
Cell viability in fibroblasts were detected with MTT assay and DAPI staining methods. (A) The cell viability was 100% (closed bar), 17.7% (open bar), 24.2% (hatched bar), and 30.6% (horizontal striped bar), respectively. (B) The image of cell death was obtained using DAPI staining in fibroblasts under the fluorescence microscopy. Results are means ± SD. n = 3–5, **p<0.01.
CoQ10 suppresses senescent phenotypes in the fibroblasts
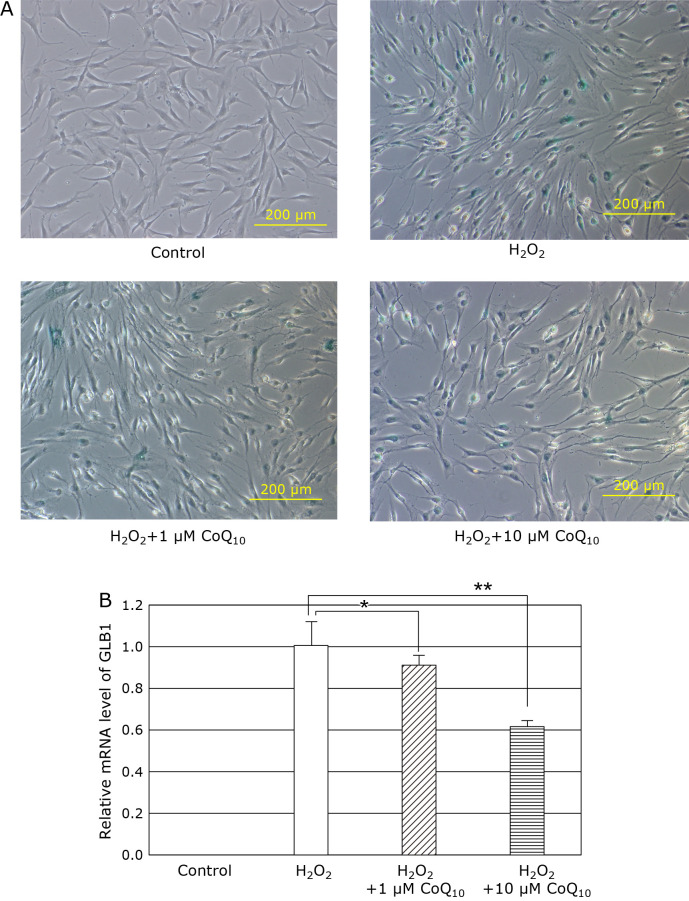
GLB1 genes which encodes lysosomal enzyme, SA-βgal is an important markers of replicative senescence and induced senescence.(33) SA-βgal is using a biomarker of cell aging. When SIPS-induced fibroblasts were treated with H2O2 for 2 h, intracellular SA-βgal and GLB1 mRNA level were increased (Fig. 2A and B), whereas SA-βgal positive cell number and GLB1 mRNA level were diminished by addition of 1 or 10 µM CoQ10. These results indicated that CoQ10 suppresses the senescent phenotype induced by H2O2-treatment in fibroblasts.
Fig. 2.
We experimented the role of CoQ10 in the fibroblasts of SIPS model induced byH2O2. GLB1 genes expression encoding the SA-βgal activity is marker of cell senescence. (A) The mRNA level of GLB1 was increased by treatment of H2O2 (open bar), and CoQ10 treatment also dose-dependently decreased in fibroblasts of SIPS model. Results are means ± SD. n = 3–7, *p<0.05, **p<0.01. (B) We performed an immunohistochemical analysis of SA-βgal positive, and CoQ10 decreased the number of SA-βgal positive cells of fibroblasts of SIPS model.
Effect of CoQ10 on the H2O2-induced changes of Collagen and MMP levels of fibroblasts
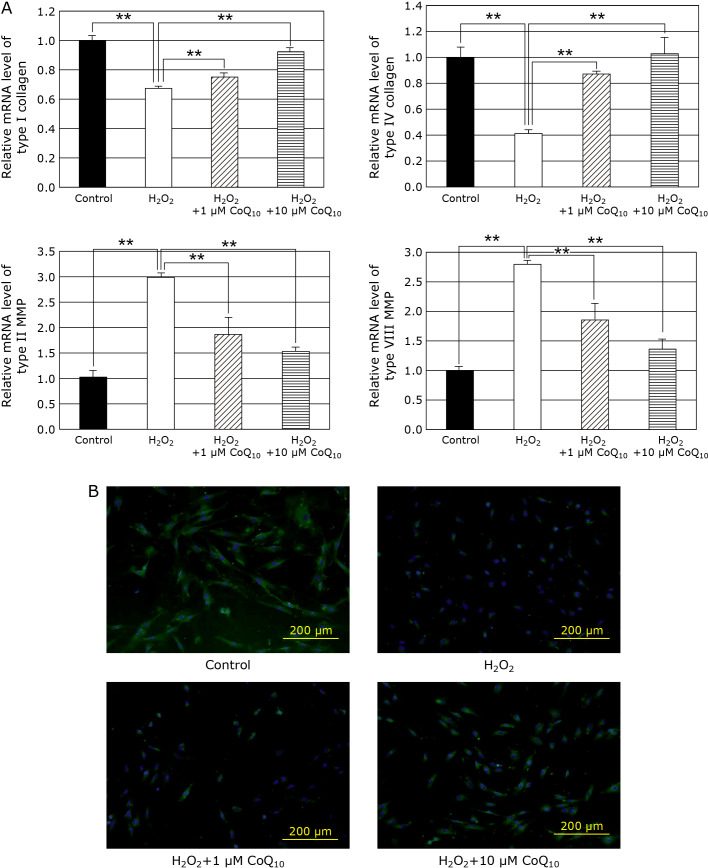
Although, Collagen is the most abundant protein in human body, its expression decreased in aging. While, MMP have been known as catabolic enzyme of ECM, such as collagen, elastin, and laminin, and plays notably an important role in degradation of collagen. Therefore, we examined the effect of H2O2 on collagen and MMP expression levels of human skin fibroblasts. As the results, H2O2 decreased the mRNA levels of type I and IV collagens, but increased the levels of type II and VIII MMPs. On the other hands, CoQ10 returned the H2O2-induced changes of collagen mRNA and MMP levels to control levels dose-dependently (Fig. 3A). In addition, H2O2 also decreased the protein level of type I collagen of the fibroblasts (Fig. 3B). These results suggest that CoQ10 suppresses cell senescence via maintenance of intracellular collagen level induced by H2O2-treatment in fibroblasts.
Fig. 3.
Relative mRNA expression of type I, IV collagens and type II, VIII MMP were determined by RT-PCR method and the target gene levels were normalized to the housekeeping gene 18sRNA. (A) The mRNA level of type I, IV collagen was increased by treatment of CoQ10, and it also dose-dependently enhanced in fibroblasts. Conversely, CoQ10 treatment decreased the mRNA levels of type type II, VIII MMP. Results are means ± SD. n = 3–7, **p<0.01. (B) Type I collagen protein expression in fibroblasts of SIPS model was determined by immunofluorescence, cell nuclei are stained blue with DAPI, and type I collagen protein is stained green with Alexa Fluor 488 probe.
Effect of CoQ10 on the H2O2-indeced change of SASP levels of skin fibroblasts
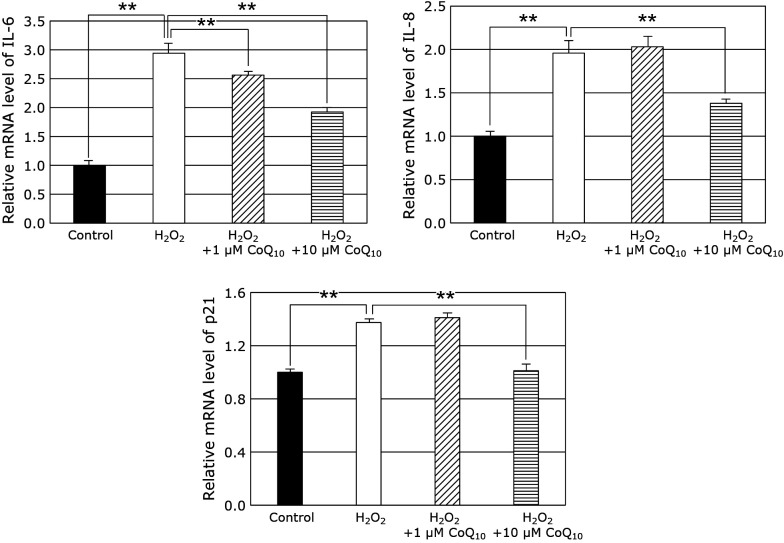
SASP, which is released from senescent cells, stimulates tumorigenesis promotion, evokes of chronic inflammation, and promotes development of age-related diseases.(34) Therefore, we examined the effect of H2O2 on mRNA levels of p21, IL-6, and IL-8 of fibroblasts. As the results, the mRNA levels of p21, IL-6, and IL-8 increased of fibroblasts. On the other hands, 10 μM CoQ10 suppressed the H2O2-induced up-regulations of p21, IL-6, and IL-8 mRNA levels (Fig. 4). These results suggest that CoQ10 suppresses cell senescence via suppression of induction of SASP induced by H2O2-treatment in fibroblasts.
Fig. 4.
We confirmed effects of CoQ10 on IL-6, IL-8, and p21 gene expression in fibroblasts of SIPS model. The SASP (such as IL-6, IL-8, and p21) related to aging was increased by the addition of H2O2, while it decreased the mRNA levels of IL-6, IL-8, and p21 with CoQ10 treatment. Results are means ± SD. n = 3–6, **p<0.01.
CoQ10 depress ROS of H2O2-induced SIPS model
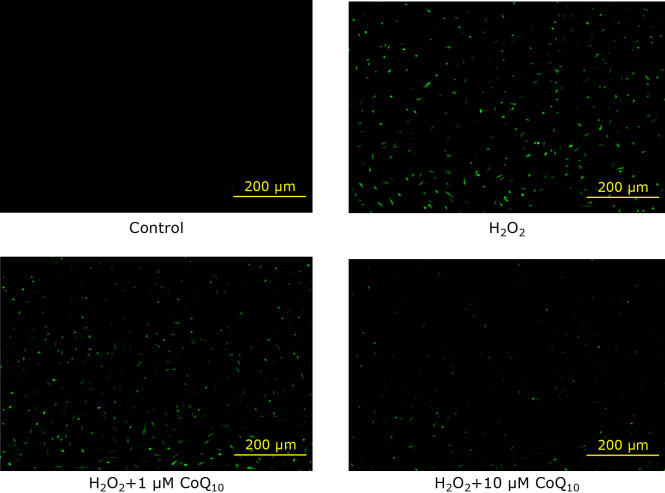
It is known that DNA damage caused by ROS induces the DDR, which is process for cellular senescence.(35) Therefore, we examined H2O2-induced ROS production and its suppression by CoQ10-treatment. As the results, H2O2 increased the green fluorescence meaning intracellular ROS production. On the other hands, CoQ10-treatment suppressed the intracellular ROS production concentration-dependently (Fig. 5). This result suggests that CoQ10 decreased the H2O2-induced intracellular ROS production of fibroblasts, which caused SIPS.
Fig. 5.
We observed intracellular ROS levels with CM-H2DCFDA in fibroblast of SIPS model, and the green fluorescence intensity was monitored by fluorescence microscopy.
Discussion
Cellular senescence is complexly and represent multiple dysfunction such as growth arrest and secretory of SASP factors, so that it has been recognized as an important feature during tumorigenesis suppression and contribution of aging.(36) Among them, oxidative stress is particularly important role for cellular senescence process.(2) Therefore, SIPS model cells induced by oxidative stress (especially H2O2) are widely used in senescence-related studies in recently.(4,5) However, the fundamental effectiveness or mechanism of CoQ10 on H2O2-induced SIPS model of human skin fibroblasts have not been reported. Consequently, this study evaluated mainly the effects of CoQ10 as to collagen synthesis and degradation, it is purpose to elucidate their underlying mechanism in preventing skin aging of SIPS model.
Oxidative stress especially plays a major role in the aging process, which is postulated in the free radical theory of aging.(2) In addition, DNA damage induced by oxidative stress have been known to cause cell death, so that aging process evoked by DDR.(3) Therefore, it has been thought that inhibition of oxidative stress with antioxidant reagent leads to the suppression of DDR, which eventually prevent cellular senescence. In this study, CoQ10 protected cell death induced by H2O2, then it is likely involved in the potent antioxidant effects of CoQ10 (Fig. 1). Moreover, it is reported that CoQ10 has broad therapeutic effects, including diabetes,(20) cardiovascular disease,(21) and neurological disorder,(22) besides these are likely to be involved in potent antioxidative effects of CoQ10. Furthermore, CoQ10 significantly decreased the number of SA-βgal senescence positive cells on SIPS model in this study (Fig. 2). These results suggested that CoQ10 attenuated the cellular senescence, thereby CoQ10 have the possibility of adaptation to age-related diseases.
Senescent cells also secreted SASP factors, such as IL-6, IL-8, and MMP, which promote even more the cellular senescence and tumor progression, further it also induced the cycle inhibitors p16 and p21.(11,13) In contrast, chronic inflammation is associated with aging and play an important role in several age-related diseases, and this phenomenon has been termed as inflammaging,(37) which mainly activate NF-κB as a master regulator of the SASP.(38) Besides, it is reported senescent cells often downregulate ECM protein and upregulate ECM degrading enzymes in replicative senescence.(39) Among them, MMP has attracted a lot of attention for skin aging. MMP belonging to the family of zinc-binding, have been known as chemokine which degrade components of the ECM such as collagen. It has been reported that collagen expression and protein levels decrease in aged skin, because the event caused by downregulation of collagen expression and upregulation of MMP expression. In addition, dermal fibroblasts express increased levels of collagen-degrading MMP-1 in aged compared with young human skin in vivo.(40) Moreover, Inui et al.(41) have reported that collagen is degradated via increasing MMP expression by UVB. Oxidative stress, inflammation, and aging are intimately related to each other, it is possible that skin aging can be improved by suppressing these factors. Therefore, assessing the loss of collagen, either decreased synthesis or increased degradation, is important in analyzing the factors that may contribute to skin aging.(42) In this study, we examined as to collagen and MMP expression levels in H2O2-induced SIPS model of human skin fibroblasts. It is showed that CoQ10 effectively enhanced collagen synthesis and inhibited collagen degradation indicated by upregulation of collagen genes, type I, IV collagen synthesis and downregulation of type II, VIII MMP genes on SIPS model of fibroblasts (Fig. 3A). Furthermore, CoQ10 increased protein levels of type I collagen in this result (Fig. 3B). Meanwhile, the mRNA of p21, IL-6, and IL-8, it is better elevated in SIPS model, whereas CoQ10 treatment suppressed upregulation of p21, IL-6, and IL-8 mRNA levels (Fig. 4). These results suggested that CoQ10 treatment ameliorate the skin aging, and inhibited synthesis of SASP factors in SIPS model.
Intracellular excessive oxidative stress has harmful effects on biomolecules such as lipids, proteins, and DNA and eventually leads to cellular senescence. In addition, Sublethal doses of various compounds such as O2•−, OH•, as well as H2O2 also induce phenomena similar to replicative senescence in normal cells, a phenomenon termed premature senescence. For instance, the exposure of human skin fibroblasts to a sublethal concentration of H2O2 induces phenotypic changes that mimic those seen in replicative senescence.(8,9) Therefore, a senescence model of H2O2-induced SIPS was used to investigate the protective effects of CoQ10 against the oxidative stress induced by H2O2. In this study, H2O2 increased green fluorescence indicating intracellular ROS levels, whereas suppression of ROS was observed by CoQ10 treatment in SIPS model (Fig. 5). This effect is thought to be a potent antioxidant effect possessed by CoQ10, Mellors and Tapple(43) reported that the antioxidant effects of CoQ10 is comparable to α-tocopherol in antioxidant potential. In recent study, it is known that senescent cells increase chromosomal DNA fragments on cytoplasm, which is one cause, and it provoke the SASP factors secretion induced by ROS-dependent DDR.(44) In general, CoQ10 is lipid-soluble, which have a low absorption rate on the human body, although P40 exhibited higher uptake compared with CoQ10.(32) In this study, it is shown that CoQ10 suppress oxidative stress, degradation of collagen by increasing MMP expression, and decreasing senescence-associated phenotypes (e.g., SA-βgal positive staining and SASP) for preventing skin aging via H2O2-induced SIPS model (Fig. 6). Therefore, it is considered CoQ10 had an anti-aging action in SIPS model of human skin fibroblasts. These results suggested that CoQ10 has possibility to be contributory for extension of healthy life expectancy in Japan. We hope that this study will help further clarify the mechanism of aging and development of aging control method.
Fig. 6.
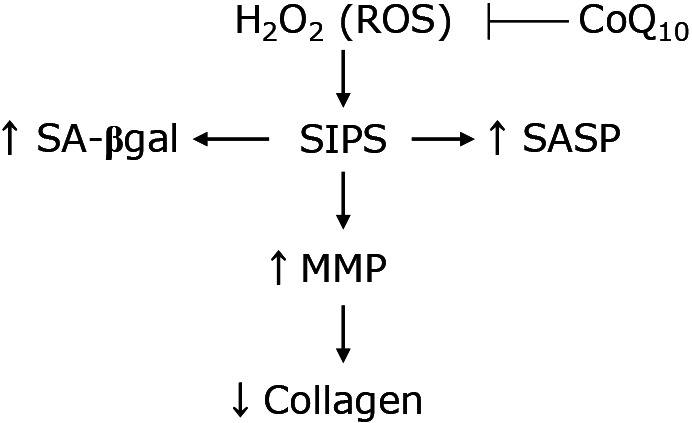
Proposed scheme for anti-aging effects of CoQ10 in SIPS model. H2O2 has been used in various studies as the senescence induction agent, that modeled of SIPS in this study. In this study, SA-βgal activity and SASP synthesis increased in SIPS model of fibroblasts. In addition, increasing of MMP expression levels induced the collagen degradation and decrease of intracellular collagen levels. Furthermore, intracellular ROS levels increased in SIPS model. This change is similar to a well-known phenomenon of replicative senescence in human skin fibroblasts. So, the scheme suggested that CoQ10 could show that anti-aging effects to inhibit ROS that induce SIPS model of fibroblasts.
Acknowledgments
This research was supported Nisshin Pharma Inc., for the donation of P40.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sic U S A 1995; 92: 9363–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol 1956; 11: 298–300. [DOI] [PubMed] [Google Scholar]
- 3.Rodier F, Campisi J, Bhaumik D. Two faces of p53: aging and tumor suppression. Nucleic Acids Res 2007; 35: 7475–7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.So MJ, Cho EJ. Phloroglucinol attenuates free radical-induced oxidative stress. Prev Nutr Food Sci 2014; 19: 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makpol S, Jam FA, Khor SC, Ismail Z, Yusof YAM, Ngah WZW. Comparative effects of biodynes, tocotrienol-rich fraction, and tocopherol in enhancing collagen synthesis and inhibiting collagen degradation in stress-induced premature senescence model of human diploid fibroblasts. Oxid Med Cell Longev 2013; 2013: 298574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen QM, Prowse KR, Tu VC, Purdom S, Linskens MH. Uncoupling the senescent phenotype from telomere shortening in hydrogen peroxide-treated fibroblasts. Exp Cell Res 2001; 265: 294–303. [DOI] [PubMed] [Google Scholar]
- 7.Chen Q, Ames BN. Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells. Proc Natl Acad Sic U S A 1994; 91: 4130–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin D, Ren R, Jia C, et al. Rapamycin protects skin fibroblasts from ultraviolet B-induced photoaging by suppressing the production of reactive oxygen species. Cell Physiol Biochem 2018; 46: 1849–1860. [DOI] [PubMed] [Google Scholar]
- 9.Chen QM. Replicative senescence and oxidant-induced premature senescence. Byond the control of cell cycle checkpoints. Ann N Y Acad Sci 2000; 908: 111–125. [DOI] [PubMed] [Google Scholar]
- 10.Coppé JP, Patil CK, Rodier F, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 2008; 6: 2853–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura M, Ohsawa S, Igaki T. Mitochondrial defects trigger proliferation of neighbouring cells via a senescence-associated secretory phenotype in Drosophila. Nat Commun 2014; 5: 5264. [DOI] [PubMed] [Google Scholar]
- 12.Rodier F, Coppé JP, Patil CK, et al. Persistent DNA damage signaling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol 2009; 11: 973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maciel-Barón LA, Morales-Rosales SL, Aquino-Cruz AA, et al. Senescence associated secretory phenotype profile from primary lung mice fibroblasts depends on the senescence induction stimuli. Age (Dordr) 2016; 38: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmelzer C, Lorenz G, Rimbach G, Döring F. In vitro effects of the reduced form of Coenzyme Q10 on secretion levels of TNF-α and chemokines in response to LPS in the human monocytic cell line THP-1. J Clin Biochem Nutr 2009; 44: 62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmelzer C, Lorenz G, Lindner I, et al. Effects of Coenzyme Q10 on TNF-α secretion in human and murine monocytic cell lines. Biofactors 2007; 31: 35–41. [DOI] [PubMed] [Google Scholar]
- 16.Sumi K, Okura T, Fujioka Y, et al. Coenzyme Q10 suppresses apoptosis of mouse pancreatic β-cell line MIN6. Diabetol Metab Syndr 2018; 10: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen CC, Liou SW, Chen CC, et al. Coenzyme Q10 rescues ethanol-induced corneal fibroblast apoptosis through the inhibition of caspase-2 activation. J Biol Chem 2013; 288: 11689–11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groneberg DA, Kindermann B, Althammer M, et al. Coenzyme Q10 affects expression of genes involved in cell signaling, metabolism and transport in human CaCo-2 cells. Int J Biochem Cell Biol 2005; 37: 1208–1218. [DOI] [PubMed] [Google Scholar]
- 19.Schmelzer C, Döring F. Identification of LPS-inducible genes downregulated by ubiquinone in human THP-1 monocytes. Biofactors 2010; 36: 222–228. [DOI] [PubMed] [Google Scholar]
- 20.Zahedi H, Eghtesadi S, Seifirad S, et al. Effects of CoQ10 supplementation on lipid profiles and glycemic control in patients with type 2 diabetes: a randomized, double blind, placebo-controlled trial. J Diabetes Metab Disord 2014; 13: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar A, Kaur H, Devi P, Mohan V. Role of coenzyme Q10 (CoQ10) in cardiac disease, hypertension and Meniere-like syndrome. Pharmacol Ther 2009; 124: 259–268. [DOI] [PubMed] [Google Scholar]
- 22.Muthukumaran K, Leahy S, Harrison K, et al. Orally delivered water soluble Coenzyme Q10 (Ubisol-Q10) blocks on-going neurodegeneration in rats exposed to paraquat: potential for therapeutic application in Parkinson’s disease. BMN Nuerosci 2014; 15: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalén A, Appelkvist EL, Dallner G. Age-related changes in the lipid compositions of rat and human tissue. Lipids 1989; 24: 579–584. [DOI] [PubMed] [Google Scholar]
- 24.Stocker R, Bowry VW, Frei B. Ubiquinol-10 protects human low density lipoprotein more efficiently against lipid peroxidation than does α-tocopherol. Proc Natl Acad Sci U S A 1991; 88: 1646–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan J, Fujii K, Yao J, et al. Reduced coenzyme Q10 supplementation decelerates senescence in SAMP1 mice. Exp Gerontol 2006; 41: 130–140. [DOI] [PubMed] [Google Scholar]
- 26.Quiles JL, Ochoa JJ, Huertas JR, Mataix J. Coenzyme Q supplementation protects from age-related DNA double-strand breaks and increases lifespan in rats fed on a PUFA-rich diet. Exp Gerontol 2004; 39: 189–194. [DOI] [PubMed] [Google Scholar]
- 27.Varani J, Warner RL, Gharaee-Kermani M, et al. Vitamin A antagonizes decreased cell growth and elevated collagen-degradation matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol 2000; 114: 480–486. [DOI] [PubMed] [Google Scholar]
- 28.Chen BH, Wen Y, Li H, Polan ML. Collagen metabolism and turnover in women with stress urinary incontinence and pelvic prolapse. Int Urogynecol J Pelvic Floor Dysfunct 2002; 13: 80–87. [DOI] [PubMed] [Google Scholar]
- 29.Dasgupta J, Kar S, Liu R, et al. Reactive oxygen species control senescence-associated matrix metalloproteinase-1 through c-Jun-N-terminal Kinase. J Cell Physiol 2010; 225: 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nukui K, Yamagishi T, Miyawaki H, Kettawan A, Okamoto T, Sato K. Comparison of uptake between PureSorb-QTM40 and regular hydrophobic coenzyme Q10 in rats and humans after single oral intake. J Nutr Sci Vitaminol 2007; 53: 187–190. [DOI] [PubMed] [Google Scholar]
- 31.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 16: 55–63. [DOI] [PubMed] [Google Scholar]
- 32.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 33.Lee BY, Han JA, Im JS, et al. Senescence-associated β-galactosidase is lysosomal β-galactosidase. Aging Cell 2006; 5: 187–195. [DOI] [PubMed] [Google Scholar]
- 34.Yoshimoto S, Loo TM, Atarashi K, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013; 499: 97–101. [DOI] [PubMed] [Google Scholar]
- 35.Hara E, Smith R, Parry D, Tahara H, Stone S, Peters G. Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol Cell Biol 1996; 16: 859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol 2011; 192: 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 2014; 69 Suppl 1: S4–S9. [DOI] [PubMed] [Google Scholar]
- 38.Chien Y, Scuoppo C, Wang X, et al. Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes Dev 2011; 25: 2125–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shelton DN, Chang E, Whittier PS, Choi D, Funk WD. Microarray analysis of replicative senescence. Curr Biol 1999; 9: 939–945. [DOI] [PubMed] [Google Scholar]
- 40.Fisher GJ, Quan T, Purohit T, et al. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am J Pathol 2009; 174: 101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inui M, Ooe M, Fujii K, Matsunaka H, Yoshida M, Ichihashi M. Mechanisms of inhibitory effects of CoQ10 on UVB-induced wrinkle formation in vitro and in vivo. Biofactors 2008; 32: 237–243. [DOI] [PubMed] [Google Scholar]
- 42.Péterszegi G, Andrès E, Molinari J, Ravelojaona V, Robert L. Effect of cellular aging on collagen biosynthesis: I. Methodological consideration and pharmacological applications. Arch Gerontol Geriatr 2008; 47: 356–367. [DOI] [PubMed] [Google Scholar]
- 43.Mellors A, Tappel AL. The inhibition of mitochondrial peroxidation by ubiquinone and ubiquinol. J Biol Chem 1966; 241: 4353–4356. [PubMed] [Google Scholar]
- 44.Takahashi A, Okada R, Nagao K, et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat Commun 2017; 8: 15287. [DOI] [PMC free article] [PubMed] [Google Scholar]