Abstract
Purpose
Several observational studies have presented conflicting results on the association between the use of proton pump inhibitors (PPIs) or histamine H2 receptor antagonist (H2RA) and the risk of coronavirus disease 2019 (COVID-19). This systematic review and meta-analysis aimed to examine this association.
Methods
In July 2021, PubMed, Embase, Cochrane Central Register of Controlled Trials, and Web of Science were searched for articles investigating the relationship between the two main acid suppressants and COVID-19. Studies showing the effect estimates as hazard ratio (HR) for severe outcomes or incidence of COVID-19 were evaluated using a random-effects model.
Results
A total of 15 retrospective cohort studies with 18,109 COVID-19 cases were included in the current meta-analysis. PPI use was significantly associated with severe outcomes of COVID-19 (hazard ratio [HR] = 1.53; 95% confidence interval [CI]: 1.20–1.95) but not with the incidence of COVID-19, whereas H2RA use was significantly associated with decreased incidence (HR = 0.86, 95% CI: 0.76–0.97). For subgroup analyses of PPIs, increased severe outcomes of COVID-19 were observed in < 60 years, active use, in-hospital use, and Asians. For subgroup analyses of H2RAs, decreased severe outcomes of COVID-19 were observed in > 60 years, while in-hospital use and use in Asia were associated with higher disease severity.
Conclusions
Close observation can be considered for COVID-19 patients who use PPIs to prevent severe outcomes. However, caution should be taken because of substantial heterogeneity and plausible protopathic bias.
Supplementary information
The online version contains supplementary material available at 10.1007/s00228-021-03255-1.
Keywords: Proton pump inhibitor, H2 receptor antagonists, COVID-19, Observational study, Meta-analysis
Introduction
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused more than 4.2 million deaths from coronavirus disease 2019 (COVID-19) worldwide until early August 2021, causing a global health crisis [1]. Up to 42% of hospitalized patients with COVID-19 developed acute respiratory distress syndrome (ARDS). In addition, the mortality rate of patients admitted to the ICU reached 39–72% [2].
Acidic gastric juice can deactivate the swallowed source of infection and prevent microorganisms from reaching the intestine. Therefore, gastric juice is considered the first line of defense against infection, and the risk of viral infection could be increased by gastric hypoacidity [3]. SARS-CoV-2 causes an infection in the glandular epithelial cells of the gastrointestinal tract via fecal–oral transmission [4], thereby increasing the possibility of more prevalent COVID-19 as the secretion of gastric acid decreases. Proton pump inhibitors (PPIs), the most potent gastric secretion inhibitors, are among the top 10 most widely used drugs in the world; however, PPIs tended to be overused, and only 30% of users follow the drug label instructions [5]. A meta-analysis of 48 observational articles showed that PPI users had a 43% increased risk of pneumonia compared with non-users [6]. In a retrospective cohort study of hospitalized 152 COVID-19 patients [7], the use of PPIs more than doubled the adverse outcomes, such as secondary infection and ARDS. In contrast to PPIs, ranitidine, a histamine H2 receptor antagonist (H2RA), has been shown to prevent the replication of SARS-CoV-2, alleviating virus pneumonia both in vitro and in vivo [8]. Another retrospective hospitalized cohort study reported that famotidine use decreased the risk of intubation or death from COVID-19 by 58% [9].
A few studies examined the relationship between PPIs/H2RAs and adverse outcomes of [10–12], mortality from [11, 13], or incidence of COVID-19 [12, 13] by calculating the meta-estimates. However, all of the aforementioned studies were letters to the editor, not original or review articles. Hence, this study aimed to exhaustively demonstrate the effect of acid suppression treatments on the risk of COVID-19.
Methods
The statement of Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines [14] was used to report the results of the present systematic review and meta-analysis.
Literature search
Four electronic databases (PubMed, Embase, Cochrane Central Register of Controlled Trials, Web of Science) were searched by two authors (H.B.K and J.H.K) using common keywords related to acid-suppressive medication and COVID-19. The last literature search was conducted on July 9, 2021. Supplemental Table 1 describes the comprehensive literature search strategy. A manual review of the bibliographies of appropriate articles was carried out to discover additional studies. No language restriction was indicated to check all plausible articles.
Selection criteria
Two authors (H.B.K and J.H.K) independently evaluated the eligibility of all studies. If there were discrepancies in the selection of studies among reviewers, they were settled by consensus. For the current meta-analysis, the following inclusion criteria were used: (a) observational studies with case–control or cohort studies, (b) studies that reported the association between “current use of PPIs or H2RAs” and “COVID-19,” (c) studies in which PPIs and H2RAs had been taken before patients got COVID-19, and (d) studies of human subjects including adults aged ≥ 18 years. Studies including PPI and H2RA concomitant use or not published in peer-reviewed journals, review articles, case reports, and abstracts only presented at academic conferences were excluded. Severe outcomes of COVID-19 include intubation, ventilator support, development of ARDS, admission to ICU, secondary infection, or death due to COVID-19. The protocol was registered in the PROSPERO registry (registration number CRD42021271760).
Data extraction and assessment of methodological quality
A standardized tool was utilized to obtain the following data from the selected studies: name of the first author; study design; location and study period; demographics (mean age or age range, and sex); sample size; ascertainment of exposure and outcome assessment; confounding variables that were adjusted, duration, and dosage of gastric acid suppressants and antacids used; and adjusted OR and 95% CI. Through contact with the corresponding author taken to acquire sufficient data, additional data from a published article were obtained from co-author (B.J.W).
The methodological quality of each selected article was determined using the Newcastle–Ottawa Scale (NOS) to assess the quality of case–control and cohort studies included in the meta-analyses [15]. The NOS uses a star rating system, ranging from 0 to 9, and a maximum of 4, 2, and 3 points are allocated for selection, comparability, and exposure or outcome, respectively. For comparability, if age and sex were adjusted, 1 point was given, and if the medications or comorbidity was described in detail, 2 points were given. Scores 0–3, 4–6, and 7–9 are considered low, moderate, and high methodological qualities.
Data synthesis and statistical analysis
The primary outcome was severe outcomes of COVID-19. We also conducted a series of subgroup analyses according to study design, mean age of participants, research location (hospital or community), administration time of PPIs or H2RAs, active use of PPI (active or inactive), geographical region, number of participants, and confounding factors such as body mass index (BMI), smoking status, and comorbidity. Among the studies in which comorbidity was adjusted for, only those using propensity score matching were selected [16]. The relationship between acid suppressants and the incidence of COVID-19 was also estimated as a secondary outcome.
The HRs (hazard ratios) and 95% CIs of each selected article demonstrating the relationship between PPIs/H2RAs use and risk of COVID-19 were used to calculate the pooled HRs with the corresponding 95% CI. The Higgins I2 test was employed to identify the percentage of total variation to rate the heterogeneity among the articles [17]. The I2 results obtained were between 0% (no observed heterogeneity) and 100% (maximal heterogeneity). An I2 of 0–25% indicated an insignificant heterogeneity, 26–50% low heterogeneity, 51–75% moderate heterogeneity, and 76–100% high heterogeneity [17]. A random-effect model based on the DerSimonian and Laird method was used to present the overall HR and 95% CI values, given that the participant characteristics and research methods of each included paper were dissimilar [18]. When the HR was obtained using multiple adjustment models, the model with the greatest number of confounding variables was selected. Meta-regression analysis was utilized to verify whether covariates at study level might account for heterogeneity.
Lastly, publication bias, which suggests that articles describing statistically significant results are more likely to be published than those describing null results, was estimated using Begg’s funnel plot and Egger’s test. An asymmetric Begg’s funnel plot or a P-value of < 0.05 in the Egger’s test indicated the presence of a publication bias. All statistical analyses were performed using Stata SE version 13.1 (StataCorp, College Station, TX, USA).
Results
Identification of relevant articles
The procedure for selecting suitable articles is shown in Supplemental Fig. 1. In total, our search strategy initially identified 1345 articles after searching the four databases. After eliminating duplicated articles and additional ones that did not meet the predetermined selection criteria, the remaining 26 articles were assessed. And additional 13 articles that did not meet the inclusion criteria were excluded. In addition, a further two studies were identified and included after screening the references, which were extracted after reviewing the full texts. Finally, 15 cohort studies [7, 9, 13, 19–30] were selected in the current meta-analysis.
Characteristics of studies and methodological quality assessment
Characteristics of the included 15 observational studies are described in Supplemental Table 2. The number of participants in the selected articles ranged from 152 to 132,316 and 18,109 people were diagnosed with COVID-19. The mean age of participants ranged from 44.8 to 73.8 years, and the mean proportion of female participants was 48.9%. The included studies were conducted in the following geographical regions: North America (n = 7) [9, 19, 21–24, 26], Europe (n = 5) [7, 13, 28–30], and Asia (n = 3) [20, 25, 27].
Eleven of the studies provided data on adverse outcomes [7, 9, 19, 22–27, 29, 30], two studies reported the incidence of COVID-19 [21, 28], and two studies presented the results of adverse outcomes as well as the COVID-19 incidence [13, 20]. With regard to exposure assessment, seven studies [7, 19, 20, 23, 28–30] investigated PPI use, two [9, 22] used famotidine as H2RAs, and six [13, 21, 24–27] used both PPIs and H2RAs. The variables adjusted in each article are explained in Supplemental Table 3.
The NOS scores of selected studies ranged from 6 to 9 stars (Supplemental Table 4) and the mean scores of the selected cohort studies were 7.7. All included studies except Almario et al. [21] were considered to be of high quality.
Association between acid suppressant use and the incidence and severe outcomes of COVID-19
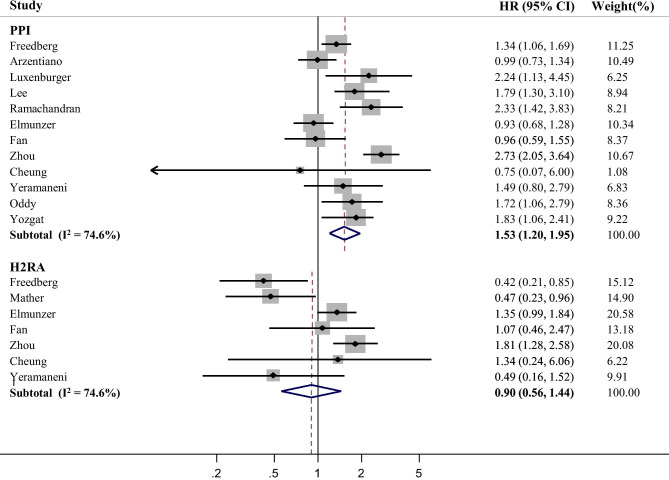
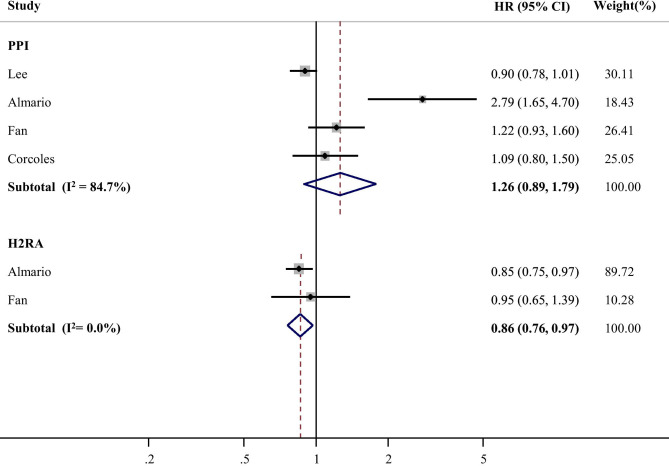
The present meta-analysis revealed a significant relationship between PPI use and severe outcomes of COVID-19, albeit with high heterogeneity (HR = 1.53, 95% CI, 1.20–1.95, I2 = 74.6%; Fig. 1). In contrast, treatment with H2RAs was not associated with increased severe outcomes. Unlike severe outcomes, no significant relationship was observed between PPI use and incidence of COVID-19 (Fig. 2). H2RAs use was associated with decreased incidence of COVID-19 (HR = 0.86, 95% CI: 0.76–0.97, I2 = 0.0%; Fig. 2). With regard to the types of adverse outcomes, the association with PPI remained significant for mortality and ARDS incidence from COVID-19 (Supplemental Fig. 2). Mortality from COVID-19 was not associated with H2RAs (Supplemental Fig. 3).
Fig. 1.
Forest plot estimating association between acid suppressant use and severe outcomes of COVID-19
Fig. 2.
Forest plot estimating association between acid suppressant use and incidence of COVID-19
Subgroup analysis of association between acid suppressants use and adverse outcomes of COVID-19
Table 1 presents the association between PPI use and severe outcomes of COVID-19 in a series of subgroup analyses of diverse factors. The significant association was consistently observed in the subgroup analyses by mean age, research location, administration time of PPIs, number of participants, and studies adjusted for comorbidity using propensity score matching also showed consistent associations. The association was stronger in the subgroup of people aged under 60 years, active use of PPIs, and in-hospital administration of PPIs. In addition, a harmful association was observed in studies conducted in Asia and Europe, but not in those conducted in North America. However, when studies that adjusted for BMI or smoking status were separately analyzed, no significant association was observed.
Table 1.
Association between PPI use and severe outcomes of COVID-19 in the subgroup meta-analysis by various factors
| Factors | No. of studies | Summary HR (95% CI) | Heterogeneity, I2 (%) | Meta-regression |
|---|---|---|---|---|
| All7,9,13,19,20,23–27,29,30 | 10 | 1.48 (1.12–1.97) | 72.7 | |
| Mean age | 0.57 | |||
| < 60 years20,24–26,30 | 5 | 1.66 (1.08–2.57) | 83.9 | |
| > 60 years7,9,13,19,23,29 | 6 | 1.42 (1.08–1.87) | 63.2 | |
| Research location | 0.60 | |||
| Hospital7,9,19,23–27,29,30 | 10 | 1.58 (1.20–2.08) | 77.2 | |
| Community13,20 | 2 | 1.32 (0.72–2.44) | 71.7 | |
| Administration time of PPIs | 0.41 | |||
| At-home7,9,13,19,20,23,24,29,30 | 9 | 1.42 (1.13–1.77) | 64.6 | |
| In-hospital25–27 | 3 | 2.03 (1.17–3.54) | 50.7 | |
| Active use of PPIs | 0.13 | |||
| Active use9,13,20,23–27,29 | 9 | 1.54 (1.15–2.06) | 76.4 | |
| Non-active use7,19,30 | 3 | 1.51 (0.90–2.53) | 75.5 | |
| Geographical region | 0.06 | |||
| North America9,19,23,24,26 | 5 | 1.27 (0.96–1.68) | 67.2 | |
| Europe7,13,29,30 | 4 | 1.57 (1.11–2.23) | 47.3 | |
| Asia20,25,27 | 3 | 2.21 (1.48–3.29) | 43.8 | |
| Number of participants | 0.62 | |||
| Small (≤ 1000)7,19,23,27,29,30 | 6 | 1.64 (1.16–2.32) | 61.9 | |
| Large (> 1000)9,13,20,24,25,26 | 6 | 1.44 (1.01–2.07) | 83.5 | |
| Confounding adjustment | ||||
| BMI7,13,19,26 | 4 | 1.13 (0.98–1.29) | 11.5 | |
| Smoking status13,19,23,30 | 4 | 1.40 (0.91–2.14) | 76.2 | |
| NSAIDs use19,23,29 | 3 | 1.31 (0.96–1.78) | 75.6 | |
| Steroid use20,27,29 | 3 | 1.73 (1.25–2.38) | 0.0 | |
| Comorbidity7,13,20,23,24,26,29,30 | 8 | 1.45 (1.16–1.81) | 57.7 | |
| Gastrointestinal disease13,21,29 | 3 | 1.73 (1.07–2.81) | 74.8 |
ARDS acute respiratory distress syndrome, BMI body mass index, CI confidence interval, COVID-19 coronavirus disease 2019, HR hazard ratio, NA not applicable, NSAID non-steroidal anti-inflammatory drug, PPI proton pump inhibitor
The association between H2RA use and severe outcomes of COVID-19 in a series of subgroup analyses is shown in Table 2. In cases of mean age > 60 years, the use of H2RA was associated with decreased odds of the serious outcome of COVID-19 (HR = 0.57, 95% CI: 0.33–0.98, I2 = 36.7%). Contrary to at-home use of H2RAs, in-hospital use was significantly linked to an increased risk of adverse outcomes (HR = 1.73, 95% CI: 1.29–2.30, I2 = 0.0%). In addition, a harmful association was observed in studies conducted in Asia. According to meta-regression analysis, the overall effect of each subgroup was not statistically significant. A subgroup analysis of active H2RA use was not considered as all studies presented only active use.
Table 2.
Association between H2RA use and adverse outcomes of COVID-19 in the subgroup meta-analysis by various factors
| Factors | No. of studies | Summary HR (95% CI) | Heterogeneity, I2 (%) | Meta-regression |
|---|---|---|---|---|
| All9,13,22,24–27 | 7 | 0.90 (0.56–1.44) | 74.6 | |
| Mean age | 0.10 | |||
| < 60 years24–26 | 3 | 1.36 (0.88–1.10) | 62.3 | |
| > 60 years9,13,22 | 3 | 0.57 (0.33–0.98) | 36.7 | |
| Administration time of H2RAs | 0.43 | |||
| At-home9,13,22,24 | 4 | 0.76 (0.39–1.45) | 78.3 | |
| In-hospital25–27 | 3 | 1.73 (1.29–2.30) | 0.0 | |
| Specific type of H2RAs | 0.42 | |||
| Famotidine9,22,25–27 | 5 | 0.74 (0.33–1.66) | 82.2 | |
| All13,24 | 2 | 1.31 (0.98–1.76) | 0.0 | |
| Geographical region | 0.17 | |||
| North America9,22,24,26 | 4 | 0.64 (0.31–1.33) | 80.3 | |
| Europe13 | 1 | 1.07 (0.46–2.47) | NA | |
| Asia25,27 | 2 | 1.79 (1.27–2.52) | 53.9 | |
| Number of participants | 0.46 | |||
| Small (≤ 1000)22,27 | 2 | 0.61 (0.25–1.49) | 26.1 | |
| Large (> 1000)9,13,24–26 | 5 | 1.00 (0.60–1.65) | 75.9 | |
| Confounding adjustment | ||||
| BMI7,13,22,24 | 4 | 0.76 (0.39–1.45) | 78.3 | |
| Smoking status13,22 | 2 | 0.69 (0.31–1.54) | 53.2 | |
| Steroid use22,27 | 2 | 0.61 (0.25–1.49) | 26.1 | |
| Comorbidity7,13,22,24,26 | 5 | 0.71 (0.40–1.28) | 73.8 | |
| Gastrointestinal disease13,24 | 2 | 1.31 (0.98–1.76) | 0.0 |
BMI body mass index, CI confidence interval, COVID-19 coronavirus disease 2019, HR hazard ratio, NA not applicable, H2RA histamine H2 receptor antagonist
Publication bias
No publication bias was found in this meta-analysis of the effect of PPI use on adverse outcomes; the Begg’s funnel plots were all symmetrical, and the Egger’s test yield a P-value of 0.57 (Supplemental Fig. 4).
Discussion
Based on the results from the current meta-analysis, PPI use was weakly associated with increased odds of severe outcomes of COVID-19, especially in patients aged under 60 years, in-hospital use, and in those living in Asia. Unlike PPIs, the use of H2RAs was weakly associated with the decreased incidence of COVID-19, although in-hospital use was moderately linked to increased severe outcomes.
Recently, four meta-analyses [31–34] were published regarding the association between the use of acid suppressants and COVID-19. Three meta-analyses [31–33] identified the relationship for PPIs and one by Kow et al. [34] for famotidine. Supplemental Table 5 describes the differences in study characteristics and the main results among the past meta-analysis and the present study. Most meta-analyses showed similar findings to our results, except for Zippi et al. [32], which found that PPIs use was not significantly associated with an increased risk of severe COVID-19 outcomes. The differences between our study and previous meta-analyses are that the most recent articles were searched, the search formulas were detailed, and subgroup analyses for a variety of factors were performed. Moreover, the exclusion of studies that only looked at the past use of acid suppressant is thought to be attributable to different selection criteria. Above all, the inclusion of only published cohort studies has strength compared to previous meta-analyses. All of the aforementioned meta-analyses were partly based on unpublished data. Thus, their accuracy cannot be guaranteed because unpublished data did not go through peer-review process. In addition, unpublished studies are susceptible to bias and may have lower methodological quality than published studies [35].
Several plausible biological mechanisms can account for the relationship between PPI use and increased severe outcomes of COVID-19. First, PPIs can strongly inhibit the secretion of gastric acid, thereby increasing the likelihood of SARS-CoV-2 infiltrating the digestive tract [36]. This state can increase the spread of the virus, consequently raising the likelihood of a cytokine storm, further exacerbating the severe consequences of the COVID-19 [37]. Second, the substantial hypochlorhydria can lead to dysbiosis of the gut microbiota, which can increase enteric infections or sepsis. Hypochlorhydria can also increase the overgrowth of bacteria in the small intestine, which amplifies the severity of COVID-19 [38]. Finally, by inhibiting the anti-inflammatory activity of neutrophils, PPIs can reduce defense against infectious agents, thereby increasing the severity of COVID-19 [39].
Apart from plausible biological mechanisms, the relationship between PPI use and adverse outcomes of COVID-19 can be caused by protopathic bias. Protopathic bias, or reverse causality, is a source of systematic bias that takes place when exposure conditions change in response to the demonstration of potential consequences. For example, people using PPIs tend to have more severe gastroesophageal reflux disease (GERD) than those not taking these medications [40]. Tobacco use, non-steroidal anti-inflammatory drugs (NSAIDs) use, and obesity are associated with the risk of gastroesophageal reflux symptoms [41]. Since GERD is associated with the risk of developing pneumonia [42], an increase of severe outcomes from COVID-19 might have occurred due to obesity and tobacco or NSAIDs use rather than PPIs use. As can be seen in Table 2, when only the studies that adjusted for obesity, smoking status, and use of NSAIDs analyzed separately, the significant association disappeared.
It remains uncertain why H2RA intake, especially pre-admission administration, can reduce the incidence of COVID-19. Mast cells in the nasal cavity and respiratory tract secrete histamine [43] and can increase the inflammatory response by secreting inflammatory cytokines [44]. Moreover, it has an angiotensin-converting enzyme 2 receptor, which SARS-CoV-2 utilizes to enter the body and proliferate [45]. Therefore, H2RAs may play a role in reducing the secretion of histamine and inflammatory reactions, which are at least partially involved in the development of COVID-19 [46].
Contrary to at-home use, increased severe outcomes were observed with in-hospital use of H2RAs. This result might be due to the occurrence of gastrointestinal symptoms of COVID-19 or other medical conditions (e.g., for the purpose of preventing digestive side effects that may occur during COVID-19 treatment) rather than the medication per se. SARS-CoV-2 can also spread to the digestive system aside from the respiratory system and can cause gastrointestinal symptoms [47]. Another reason might be a decreased gastric acid inhibition as a result of tolerance to H2RAs [48]. Among population who used acid suppressants such as H2RAs, those more vulnerable to COVID-19 get prone to this disease early after beginning to take acid-suppressive medications, while those who can tolerate such medications due to a longer dosing period can lessen the adverse outcomes [49].
The association between PPIs use and severe outcomes from COVID-19 was most prominent in Asia. The first possible mechanism to explain this is that the use of PPI may suppress gastric acid secretion to a greater extent in Asians due to a lower mass of parietal cell [50]. Second, the incidence of genetic polymorphism for cytochrome P450 2C19 is higher in Asians compared to other regions, which makes it easier to slow down the metabolism of PPIs, and thus the degree of gastric acid inhibition may be stronger [51, 52]. Finally, the prevalence of Helicobacter pylori infection in Asia is higher than in Europe or North America [53] and PPIs may inhibit gastric acid secretion greater in the presence of Helicobacter pylori infection.
The current study has several important limitations. First, all of the selected articles had an observational design, and many studies were conducted in a single hospital; hence, the results of these studies can be difficult to generalize. Second, several essential factors associated with antacid use and COVID-19 may not have been adjusted for in the selected articles. For example, the use of concomitant medications such as angiotensin-converting enzyme inhibitors, angiotensin receptor blockers (ARBs), or statins has been linked to decreased disease severity [54, 55]. When three studies [19, 23, 27] that were adjusted for these factors were collected, a significant relationship between PPI use and severity was not observed (HR = 1.24, 95% CI: 0.76–2.00, I2 = 68.7%). Third, most included studies did not specify the dosage, duration, and particular type of acid suppressant used.
Conclusion
Our meta-analysis presents evidence that the use of PPIs but not H2RAs was associated with increased adverse outcomes from COVID-19. Despite the fact that our study showed a lower quality of evidence due to a lack of prospective cohort studies and RCTs, close observation is recommended for COVID-19 patients who use PPIs to prevent serious consequences. More evidence is required to verify our results, and prospective cohort studies and RCTs must be utilized.
Supplementary information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to express our appreciation to Prof. B. Joseph Elmunzer for help with data collection.
Author contribution
JHK and HBK conceived and designed the study. JHK and HBK searched the articles and retrieved the data. HBK and BJW performed the statistical analysis. HBK drafted the manuscript. JHK and BJW supervised the systematic review and meta-analysis and significantly amended the manuscript. All authors took part in the interpretation of the results and approved the version of the manuscript. JHK had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Availability of data and material
Data supporting the results of this study are available upon reasonable request of the corresponding author.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/17/2022
A Correction to this paper has been published: 10.1007/s00228-022-03335-w
References
- 1.World Health Organization (2021) WHO coronavirus (COVID-19) dashboard. https://www.covid19.who.int. Accessed 5 August 2021
- 2.CDC (2020) Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID–19). 2020
- 3.Martinsen TC, Bergh K, Waldum HL. Gastric juice: a barrier against infectious diseases. Basic Clin Pharmacol Toxicol. 2005;96:94–102. doi: 10.1111/j.1742-7843.2005.pto960202.x. [DOI] [PubMed] [Google Scholar]
- 4.Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marks D (2016) Time to halt the overprescribing of proton pump inhibitors. Clin Pharm. [https://www.pharmaceutical-journal.com/20201548.article]. Accessed 20 July 2021
- 6.Wang CH, Li CH, Hsieh R, Fan CY, Hsu TC, Chang WC, Hsu WT, Lin YY, Lee CC (2019) Proton pump inhibitors therapy and the risk of pneumonia: a systematic review and meta–analysis of randomized controlled trials and observational studies. Expert Opin Drug Saf 18:163–172. 10.1080/14740338.2019.1577820 [DOI] [PubMed]
- 7.Luxenburger H, Sturm L, Biever P, Rieg S, Duerschmied D, Schultheiss M, Neumann-Haefelin C, Thimme R, Bettinger D. Treatment with proton pump inhibitors increases the risk of secondary infections and ARDS in hospitalized patients with COVID-19: coincidence or underestimated risk factor? J Intern Med. 2021;289:121–124. doi: 10.1111/joim.13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan S, Wang R, Chan JF, Zhang AJ, Cheng T, Chik KK, Ye Z, Wang S, Lee AC, Jin L, Li H, Jin D, Yuen K, Sun H. Metallodrug ranitidine bismuth citrate suppresses SARS-CoV-2 replication and relieves virus-associated pneumonia in Syrian hamsters. Nat Microbiol. 2020;5:1439–1448. doi: 10.1038/s41564-020-00802-x. [DOI] [PubMed] [Google Scholar]
- 9.Freedberg DE, Conigliaro J, Wang TC, Tracey KJ, Callahan MV, Abrams JA; Famotidine Research Group Famotidine use is associated with improved clinical outcomes in hospitalized COVID–19 patients: a retrospective cohort study. Gastroenterology. 2020;159:1129–1131. doi: 10.1053/j.gastro.2020.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kow CS, Hasan SS. Use of proton pump inhibitors and risk of adverse clinical outcomes from COVID-19: a meta-analysis. J Intern Med. 2021;289:125–128. doi: 10.1111/joim.13183. [DOI] [PubMed] [Google Scholar]
- 11.Hariyanto TI, Prasetya IB, Kurniawan A. Proton pump inhibitor use is associated with increased risk of severity and mortality from coronavirus disease 2019 (COVID-19) infection. Dig Liver Dis. 2020;52:1410–1412. doi: 10.1016/j.dld.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li GF, An XX, Yu Y, Jiao LR, Canarutto D, Yu G, Wang G, Wu DN, Xiao Y (2020) Do proton pump inhibitors influence SARS-CoV-2 related outcomes? A meta-analysis. Gut gutjnl-2020–323366. Online ahead of print. 10.1136/gutjnl-2020-323366 [DOI] [PMC free article] [PubMed]
- 13.Fan X, Liu Z, Miyata T, Dasarathy S, Rotroff DM, Wu X, Poulsen KL, Nagy LE. Effect of acid suppressants on the risk of COVID-19: a propensity score-matched study using UK Biobank. Gastroenterology. 2020;160:455–458.e5. doi: 10.1053/j.gastro.2020.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells GA, Shea B, O’Connell D (2014) The Newcastle-Ottawa Scale (NOS) for assessing the quality of non randomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 24 July 2021
- 16.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79:516–524. doi: 10.2307/2288398. [DOI] [Google Scholar]
- 17.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 18.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 19.Argenziano MG, Bruce SL, Slater CL, Tiao JR, Baldwin MR, Barr RG, Chang BP, Chau KH, Choi JJ, Gavin N, Goyal P, Mills AM, Patel AA, Romney MS, Safford MM, Schluger NW, Sengupta S, Sobieszczyk ME, Zucker JE, Asadourian PA, Bell FM, Boyd R, Cohen MF, Colquhoun MI, Colville LA, de Jonge JH, Dershowitz LB, Dey SA, Eiseman KA, Girvin ZP, Goni DT, Harb AA, Herzik N, Householder S, Karaaslan LE, Lee H, Lieberman E, Ling A, Lu R, Shou AY, Sisti AC, Snow ZE, Sperring CP, Xiong Y, Zhou HW, Natarajan K, Hripcsak G, Chen R (2020) Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ 369:m1996. 10.1136/bmj.m1996 [DOI] [PMC free article] [PubMed]
- 20.Lee SW, Ha EK, Yeniova AÖ, Moon SY, Kim SY, Koh HY, Yang JM, Jeong SJ, Moon SJ, Cho JY, Yoo IK, Yon DK. Severe clinical outcomes of COVID-19 associated with proton pump inhibitors: a nationwide cohort study with propensity score matching. Gut. 2021;70:76–84. doi: 10.1136/gutjnl-2020-322248. [DOI] [PubMed] [Google Scholar]
- 21.Almario CV, Chey WD, Spiegel BMR (2020) Increased risk of COVID-19 among users of proton pump inhibitors. Am J Gastroenterol 115:1707–1715. 10.14309/ajg.0000000000000798 [DOI] [PMC free article] [PubMed]
- 22.Mather JF, Seip RL, McKay R (2020) Impact of famotidine use on clinical outcomes of hospitalized patients with COVID-19. Am J Gastroenterol 115:1617–1623. 10.14309/ajg.0000000000000832 [DOI] [PMC free article] [PubMed]
- 23.Ramachandran P, Perisetti A, Gajendran M, Jean-Louis F, Bansal P, Dwivedi AK, Goyal H (2020) Pre-hospitalization proton pump inhibitor use and clinical outcomes in COVID-19. Eur J Gastroenterol Hepatol Online ahead of print. 10.1097/MEG.0000000000002013 [DOI] [PubMed]
- 24.Elmunzer BJ, Wolf BJ, Scheiman JM, Tierney WM, Taylor JR, North American Alliance for the Study of Digestive Manifestations of COVID-19 Association between pre-admission acid suppressive medication exposure and severity of illness in patients hospitalized with COVID-19. Gastroenterology. 2021;160:1417–1422. doi: 10.1053/j.gastro.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou J, Wang X, Lee S, Wu WKK, Cheung BMY, Zhang Q, Tse G (2020) Proton pump inhibitor or famotidine use and severe COVID-19 disease: a propensity score-matched territory-wide study. Gut gutjnl-2020–323668. 10.1136/gutjnl-2020-323668 [DOI] [PubMed]
- 26.Yeramaneni S, Doshi P, Sands K, Cooper M, Kurbegov D, Fromell G (2021) Famotidine use is not associated with 30-day mortality: a coarsened exact match study in 7158 hospitalized patients with coronavirus disease 2019 from a large healthcare system. Gastroenterology 160:919–921. 10.1053/j.gastro.2020.10.011 [DOI] [PMC free article] [PubMed]
- 27.Cheung KS, Hung IF, Leung WK (2020) Association between famotidine use and COVID-19 severity in Hong Kong: a territory-wide study. Gastroenterology S0016–5085(20)34940–4. 10.1053/j.gastro.2020.05.098. [DOI] [PMC free article] [PubMed]
- 28.Vila-Corcoles A, Satue-Gracia E, Ochoa-Gondar O, Torrente-Fraga C, Gomez-Bertomeu F, Vila-Rovira A, Hospital-Guardiola I, de Diego-Cabanes C, Bejarano-Romero F, Rovira-Veciana D, Basora-Gallisa J (2020) Use of distinct anti-hypertensive drugs and risk for COVID-19 among hypertensive people: a population-based cohort study in Southern Catalonia, Spain. J Clin Hypertens (Greenwich) 22:1379–1388. 10.1111/jch.13948 [DOI] [PMC free article] [PubMed]
- 29.Oddy C, McCaul J, Keeling P, Allington J, Senn D, Soni N, Morrison H, Mawella R, Samuel T, Dixon J. Pharmacological predictors of morbidity and mortality in COVID-19. J Clin Pharmacol. 2021 doi: 10.1002/jcph.1878.10.1002/jcph.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yozgat A, Kasapoğlu B, Can G, Tanoğlu A, Sakin YS, Yalçin KS, Gürler M, Kaplan M, Kaban MG, Kirsoy M, Kara U, Kekilli M (2021) Long-term proton pump inhibitor use is a risk factor for mortality in patients hospitalized for COVID-19. Turk J Med Sci 2021. Online ahead of print. 10.3906/sag-2103-80 [DOI] [PMC free article] [PubMed]
- 31.Toubasi AA, AbuAnzeh RB, Khraisat BR, Al-Sayegh TN, AlRyalat SA. A meta-analysis: proton pump inhibitors current use and the risk of coronavirus infectious disease 2019 development and its related mortality. Arch Med Res. 2021;S0188–4409(21):00075–78. doi: 10.1016/j.arcmed.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zippi M, Fiorino S, Budriesi R, Micucci M, Corazza I, Pica R, de Biase D, Gallo CG, Hong W (2021) Paradoxical relationship between proton pump inhibitors and COVID-19: a systematic review and meta-analysis. World J Clin Cases 9:2763–2777. 10.12998/wjcc.v9.i12.2763 [DOI] [PMC free article] [PubMed]
- 33.Yan C, Chen Y, Sun C, Ahmed MA, Bhan C, Guo Z, Yang H, Zuo Y, Yan Y, Hu L, Sun Y, Li Y, Zhou Q (2021) Will proton pump inhibitors lead to a higher risk of COVID-19 infection and progression to severe disease? A meta-analysis. Jpn J Infect Dis. 10.7883/yoken.JJID.2021.074 [DOI] [PubMed]
- 34.Kow CS, Abdul Sattar Burud I, Hasan SS (2021) Use of famotidine and risk of severe course of illness in patients with COVID-19: a meta-analysis. Mayo Clin Proc 96:1365–1367. 10.1016/j.mayocp.2021.03.001 [DOI] [PMC free article] [PubMed]
- 35.Higgins JPT, Green S (editors) (2011) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.handbook.cochrane.org. Accessed October 14, 2021
- 36.Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10:528–534. doi: 10.1007/s11894-008-0098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dibner J. Fecal-oral transmission of COVID-19: could hypochlorhydria play a role? J Med Virol. 2021;93:166–167. doi: 10.1002/jmv.26265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corsonello A, Lattanzio F, Bustacchini S, Garasto S, Cozza A, Schepisi R, Lenci F, Luciani F, Maggio MG, Ticinesi A, Butto V, Tagliaferri S, Corica F. Adverse events of proton pump inhibitors: potential mechanisms. Curr Drug Metab. 2018;19:142–154. doi: 10.2174/1389200219666171207125351. [DOI] [PubMed] [Google Scholar]
- 39.Namazi MR, Jowkar F. A succinct review of the general and immunological pharmacologic effects of proton pump inhibitors. J Clin Pharm Ther. 2008;33:215–217. doi: 10.1111/j.1365-2710.2008.00907.x. [DOI] [PubMed] [Google Scholar]
- 40.Sarkar M, Hennessy S, Yang YX. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med. 2008;149:391–398. doi: 10.7326/0003-4819-149-6-200809160-00005. [DOI] [PubMed] [Google Scholar]
- 41.Eusebi LH, Ratnakumaran R, Yuan Y, Solaymani-Dodaran M, Bazzoli F, Ford AC (2018) Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut 67:430–440. 10.1136/gutjnl-2016-313589 [DOI] [PubMed]
- 42.Morehead RS. Gastro-oesophageal reflux disease and non-asthma lung disease. Eur Respir Rev. 2009;18:233–243. doi: 10.1183/09059180.00002509. [DOI] [PubMed] [Google Scholar]
- 43.Mukai K, Tsai M, Saito H, Galli SJ. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev. 2018;282:121–150. doi: 10.1111/imr.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Theoharides TC, Alysandratos KD, Angelidou A, Delivanis DA, Sismanopoulos N, Zhang B, Asadi S, Vasiadi M, Weng Z, Miniati A, Kalogeromitros D. Mast cells and inflammation. Biochim Biophys Acta. 2012;1822:21–33. doi: 10.1016/j.bbadis.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caughey GH, Raymond WW, Wolters PJ. Angiotensin II generation by mast cell alpha- and beta-chymases. Biochim Biophys Acta. 2000;1480:245–257. doi: 10.1016/s0167-4838(00)00076-5. [DOI] [PubMed] [Google Scholar]
- 46.Kritas SK, Ronconi G, Caraffa A, Gallenga CE, Ross R, Conti P (2020) Mast cells contribute to coronavirus-induced inflammation: new anti-inflammatory strategy. J Biol Regul Homeost Agents 34:9–14.10.23812/20-Editorial-Kritas [DOI] [PubMed]
- 47.Gu J, Han B, Wang J. COVID-19: gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020;158:1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilder-Smith CH, Merki HS. Tolerance during dosing with H2-receptor antagonists. An overview Scand J Gastroenterol Suppl. 1992;193:14–19. doi: 10.3109/00365529209096000. [DOI] [PubMed] [Google Scholar]
- 49.Yola M, Lucien A. Evidence of the depletion of susceptible effect in non-experimental pharmacoepidemiologic research. J Clin Epidemiol. 1994;47:731–737. doi: 10.1016/0895-4356(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 50.Lam SK, Hasan M, Sircus W, Wong J, Ong GB, Prescott RJ. Comparison of maximal acid output and gastrin response to meals in Chinese and Scottish normal and duodenal ulcer subjects. Gut. 1980;21:324–328. doi: 10.1136/gut.21.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caraco Y, Lagerstrom PO, Wood AJJ. Ethnic and genetic determinants of omeprazole disposition and effect. Clin Pharmacol Ther. 1996;60:157–167. doi: 10.1016/S0009-9236(96)90131-9. [DOI] [PubMed] [Google Scholar]
- 52.Caraco Y, Wilkinson GR, Wood AJJ. Differences between white subjects and Chinese subjects in the in vivo inhibition of cytochrome P450s 2C19, 2D6, and 3A by omeprazole. Clin Pharmacol Ther. 1996;60:396–404. doi: 10.1016/S0009-9236(96)90196-4. [DOI] [PubMed] [Google Scholar]
- 53.van Herwaarden MA, Samson M, van Nispen CHM, Mulder PGH, Smout AJPM. The effect of Helicobacter pylori eradication on intragastric pH during dosing with lansoprazole or ranitidine. Aliment Pharmacol Ther. 1999;13:731–740. doi: 10.1046/j.1365-2036.1999.00531.x. [DOI] [PubMed] [Google Scholar]
- 54.Zhang X, Yu J, Pan LY, Jiang H (2020) ACEI/ARB use and risk of infection or severity or mortality of COVID-19: a systematic review and meta-analysis. Pharmacol Res 158:104927. 10.1016/j.phrs.2020.104927 [DOI] [PMC free article] [PubMed]
- 55.Zhang XJ, Qin JJ, Cheng X, Shen L, Zhao YC, Yuan Y, Lei F, Chen MM, Yang H, Bai L, Song X, Lin L, Xia M, Zhou F, Zhou J, She ZG, Zhu L, Ma X, Xu Q, Ye P, Chen G, Liu L, Mao W, Yan Y, Xiao B, Lu Z, Peng G, Liu M, Yang J, Yang L, Zhang C, Lu H, Xia X, Wang D, Liao X, Wei X, Zhang BH, Zhang X, Yang J, Zhao GN, Zhang P, Liu PP, Loomba R, Ji YX, Xia J, Wang Y, Cai J, Guo J, Li H. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab. 2020;32:176–187. doi: 10.1016/j.cmet.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the results of this study are available upon reasonable request of the corresponding author.