SUMMARY.
Missouri, United States, is located within the Mississippi Migratory Bird Flyway where wild waterfowl stop to feed and rest during migration and, weather permitting, to overwinter. Historically, Missouri has experienced sporadic influenza A virus (IAV) outbreaks in poultry and commercial swine. The introduction of IAVs from wild, migratory waterfowl is one possible source for the IAV, IAV genomic segments, or both involved in these outbreaks in key agricultural species. During 2005 through 2013, 3984 cloacal swabs were collected from hunter-harvested waterfowl in Missouri as part of an active IAV surveillance effort. Twenty-four avian species were represented in the sample population and 108 (2.7%) of the samples tested positive for IAV recovery. These IAV isolates represented 12 HA and nine NA subtypes and at least 27 distinct HA–NA combinations. An H14 IAV isolate recovered in Missouri during the sample period provided evidence for further establishment of the H14 subtype in North American wild waterfowl and gave proof that the previously rare subtype is more genetically diverse than previously detected. The present surveillance effort also produced IAV isolates that were genomically linked to the highly pathogenic H7N3 IAV strain that emerged in 2012 and caused severe disease in Mexico’s domestic poultry. The presence of antigenically diverse IAV’s circulating in wild waterfowl in the vicinity of commercial poultry and swine, along with the association of several wild-bird–lineage IAV genomic segments in viruses infecting poultry in North America, justifies continued attention to biosecurity efforts in food animal production systems and ongoing active IAV surveillance in wild birds.
Keywords: influenza A virus, wild-waterfowl, Missouri, surveillance
RESUMEN.
Vigilancia para el virus de influenza A en aves acuáticas en Missouri, en los Estados Unidos, 2005–2013.
El estado de Missouri, en los Estados Unidos, se encuentra dentro de la ruta de aves migratorias del Mississippi donde las aves acuáticas silvestres paran para alimentarse y descansar durante la migración y para pasar el invierno bajo mejores condiciones climáticas. Históricamente, Missouri ha experimentado brotes esporádicos del virus de la influenza en las aves y cerdos comerciales. La introducción de virus a partir de este tipo de aves acuáticas silvestres y migratorias es una fuente posible del virus de la influenza aviar, de sus segmentos genómicos, o de ambos que pueden estar implicados en brotes en especies pecuarias importantes. Durante el año 2005 hasta el 2013, se recolectaron 3984 hisopos cloacales de aves acuáticas obtenidas de la caza en Missouri como parte del esfuerzo de vigilancia activa contra la influenza aviar. Veinticuatro especies aviares estuvieron representadas en la población de la muestra y 108 (2.7%) de las muestras resultaron positivas para la recuperación del virus de la influenza aviar. Estos aislamientos representaron 12 subtipos de HA y nueve subtipos de NA y al menos 27 combinaciones de HA-NA distintas. Un aislamiento H14 recuperado en Missouri durante el período de la muestra presentó evidencias de su establecimiento en aves acuáticas silvestres de América del Norte y dio prueba de que este subtipo que raramente se encuentra, es genéticamente más diverso de lo que previamente detectado. El actual esfuerzo de vigilancia también produjo aislamientos que estuvieron genómicamente ligados a la cepa altamente patógena H7N3 que surgió en el 2012 y que causó una enfermedad grave en las aves comerciales de México. La presencia de antigénicamente diversa de los virus de influenza aviar que circulan en las aves acuáticas silvestres en las inmediaciones avicultura comercial y porcina, junto con la asociación de varios segmentos genómicos procedentes de los virus en aves silvestres que infectan a las aves de corral en América del Norte, justifica una atención continua a los esfuerzos de bioseguridad en los sistemas de producción animal y en la continuación de la vigilancia activa de aves silvestres.
Wild, migratory waterfowl are natural reservoirs for influenza A viruses (IAVs). The persistence of IAVs in these far-ranging migratory species makes them potential vehicles for the global dissemination of these viruses (24). When wild waterfowl stopover and wintering grounds coincide with commercial poultry and swine production facilities, the potential for transmission of wild-bird–lineage IAVs to domestic birds and food animals is increased (7,8,26). Most attention is given to waterfowl-origin H5 and H7 IAVs because they are the HA subtypes that have demonstrated potential for high pathogenicity among birds (20). In addition to highly pathogenic (HP) IAVs, low pathogenic (LP) IAVs are also concerning for poultry producers because of the threat they pose to overall flock health and production (3,15). Beyond poultry, avian-lineage IAVs have impacted swine health as well; most notably, North American avian-lineage gene segments (PB2 and PA) are part of the triple-reassortant internal gene cassette that has been circulating in pigs since 1998 (26). Monitoring IAV activity in wild birds is an important component of comprehensive risk assessments for poultry and swine producers in the Midwestern United States.
Missouri is an important poultry producing state, with an annual population of over 50 million broilers and laying hens combined and more than 7 million turkeys (22). As of 2013, Missouri ranked ninth in the nation in terms of poultry sales within the year with an estimated value of US$1.4 billion (23). In addition, the state hosts a robust commercial swine industry that produces more than 9.7 million pigs per year, ranking seventh in the nation for the number of swine produced (22). The susceptibility of swine to infection with avian-, human-, and swine-lineage IAV strains garners concern in that IAV reassortment occurring in swine can give rise to novel strains with pandemic potential (14). For these reasons, a collaborative project was established between the Missouri Avian Influenza Task Force and The Ohio State University to monitor IAV activity in Missouri and obtain a contemporary assessment of IAV activity in wild, migratory waterfowl in the state.
Previous IAV surveillance studies conducted in the northern reaches of the Mississippi Migratory Bird Flyway documented that approximately 20% of juvenile birds were shedding IAVs during premigration marshalling periods (1,10,24), whereas studies conducted in the southern wintering grounds report an estimated IAV prevalence closer to 2% (5,21). Few current studies have focused on the IAV dynamics occurring in the Midwestern United States during southern migration periods. Therefore, in 2005 an IAV surveillance effort was initiated in wild waterfowl in Missouri during the fall migration season to gain insight on the prevalence and diversity of IAV strains in hunter-harvested wild waterfowl.
MATERIALS AND METHODS
Sample collection.
Influenza A virus surveillance was conducted during 2005 through 2013 by collecting cloacal swabs as previously described (19). Swabs were collected from hunter-harvested waterfowl during three distinct hunting seasons: teal season during September, open duck season (all duck species) during November and December, and light geese season during February through April. Samples were collected at nine different locations throughout Missouri (Fig. 1). Each bird accessioned into the study was visually examined to determine species, age, and sex. Sampling was contingent upon the success and participation of hunters along with the availability of the research team members. Not all study sites were used each year due to variable bird numbers and prior accessibility.
Fig. 1.
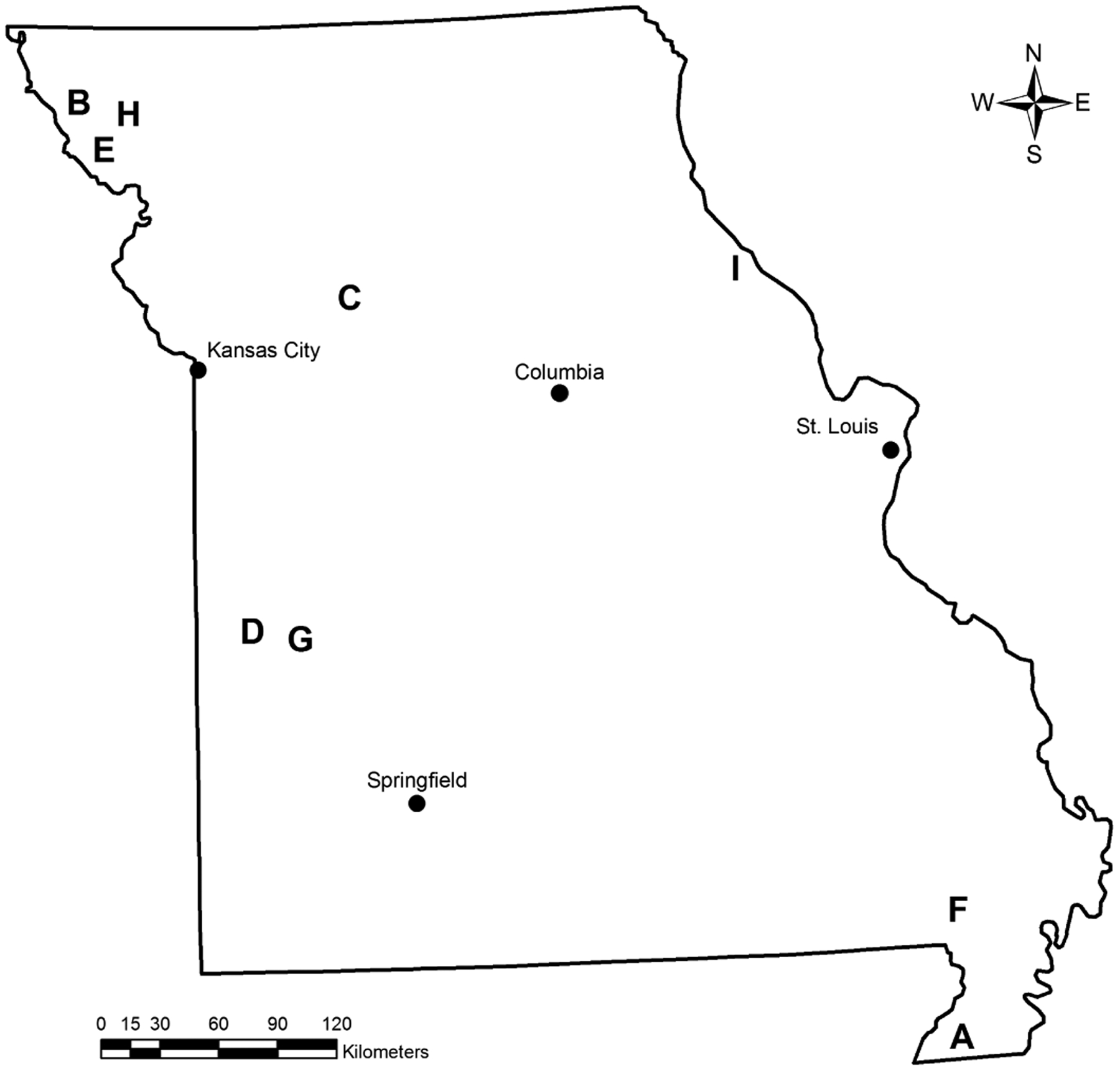
Geographic locations of nine (A–I) study sites for IAV surveillance in wild birds in the state of Missouri, United States, 2005–2013.
Laboratory testing.
Virus isolation attempts were conducted on each sample using 10-day-old specific-pathogen-free (SPF) embryonating chicken eggs as previously described (19). All allantoic fluid with hemagglutinating activity was tested for the presence of IAV using the Avian Influenza Virus Type A Antigen Test Kit (Synbiotics Corporation, San Diego, CA). The IAV isolates were submitted to the United States Department of Agriculture (USDA) Animal and Plant Health Inspection Service (APHIS), National Veterinary Services Laboratories (NVSL) in Ames, Iowa for antigenic HA and NA subtyping using standard hemagglutination inhibition (HI) and neuraminidase inhibition (NI) tests, respectively.
Statistical analysis.
Data were analyzed using Stata 13.1 (StataCorp LP, College Station, TX). Frequencies and percentages were calculated to assess descriptive information. Cross-tabulations were utilized along with a Pearson chi-square (χ2) test to identify statistically significant associations among nonparametric data. In addition, cross-tabulations were used to identify any potential interactions among the factors.
Binary logistic regression using a forward stepwise model building approach was used to assess differences among potential predictor variables for IAV detection. Variables included in the final model were year, species, and age. Species was recoded into groupings including: mallard (Anas platyrhynchos), gadwall (Anas strepera), American green-winged teal (Anas crecca carolinensis), northern shoveler (Anas clypeata), blue-winged teal (Anas discors), other dabbling ducks, and all other species (i.e., diving ducks, geese, etc.). Age was categorized as hatch year, mature (after hatch year), or unknown. The categories for sex were male, female, or undetermined.
RESULTS
Sampling overview.
A total of 3984 cloacal swab samples were collected representing 24 different species (Table 1). The majority of samples (3571 [89.6%]) were taken from dabbling ducks including mallard, gadwall, American green-winged teal, blue-winged teal, northern shoveler, and other dabblers. The remaining 413 (10.3%) samples consisted of all other species (i.e., diving ducks, geese, etc.). Most of the samples (3335 [83.7%]) were collected during the later open duck season and 405 samples (10.2%) were collected during teal season, with the remaining 244 (6.1%) collected during light goose season. For age, there were 2078 (52.1%) birds identified as mature, 1353 (34.0%) birds classified as hatch year, and 553 (13.9%) coded as unknown. When examining the sex of the sampled birds, they consisted of 2401 (60.3%) males and 1250 (31.4%) females with the sex of 333 (8.4%) birds being undetermined.
Table 1.
Total number of samples collected and IAV virus isolates recovered from individual bird species by study site location in Missouri, United States, 2005–2013.
| Species | Location | Total (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | ||
| American black duck × mallard hybrid (Anas rubripes × platy) | — | — | — | — | 0/1 | — | — | — | 0/1 (0) | |
| American black duck (Anas rubripes) | — | — | — | — | — | 0/2 | 0/1 | — | — | 0/3 (0) |
| American green-winged teal (Anas crecca carolinensis) | 0/10 | — | — | — | — | 6/305 | 2/133 | — | — | 8/448 (1.8) |
| American coot (Fulica americana) | 0/1 | — | — | — | — | 0/7 | — | — | — | 0/8 (0) |
| American widgeon (Anas americana) | 0/2 | — | — | — | — | 1/33 | 1/9 | — | — | 2/44 (4.5) |
| Blue-winged teal (Anas discors) | — | — | — | 0/1 | 34/374 | 0/5 | — | 1/8 | 35/388 (9.0) | |
| Bufflehead (Bucephala albeola) | — | — | — | — | — | 0/3 | 1/1 | — | — | 1/4 (25) |
| Canada goose (Branta canadensis) | — | — | — | — | — | 0/1 | 0/9 | — | — | 0/10 (0) |
| Canvasback (Aythya valisineria) | — | — | — | — | — | 0/9 | — | — | — | 0/9 (0) |
| Common loon (Gavia immer) | — | — | — | — | — | — | 0/1 | — | — | 0/1 (0) |
| Gadwall (Anas strepera) | 0/47 | — | — | — | — | 3/411 | 0/86 | — | — | 3/544 (0.6) |
| Greater white-fronted goose (Anser albifrons) | 0/2 | — | — | — | — | 0/2 | — | — | — | 0/4 (0) |
| Hooded merganser (Lophodytes cucullatus) | 0/1 | — | — | — | — | 0/5 | 0/1 | — | — | 0/7 (0) |
| Lesser scaup (Aythya affinis) | — | — | — | — | — | 0/14 | 0/4 | — | — | 0/18 (0) |
| Lesser snow goose (Chen caerulescens caerulescens) | — | — | — | — | — | 0/2 | — | — | — | 0/2 (0) |
| Mallard (Anas platyrhynchos) | 3/104 | 0/31 | 21/885 | 9/576 | — | — | 33/1596 (2.1) | |||
| Northern pintail (Anas acuta) | — | 0/2 | 0/48 | 1/23 | — | — | 1/73 (1.4) | |||
| Northern shoveler (Anas clypeata) | 0/10 | — | — | — | — | 17/313 | 3/48 | — | — | 20/371 (5.4) |
| Redhead (Aythya americana) | — | — | — | — | — | 0/10 | 0/2 | — | — | 0/12 (0) |
| Ring-necked duck (Aythya collaris) | 0/7 | — | — | — | — | 0/55 | 1/12 | — | — | 1/74 (1.4) |
| Ross’s goose (Chen rossii) | — | — | — | — | 0/28 | — | — | 0/3 | — | 0/31 (0) |
| Ruddy duck (Oxyura jamaicensis) | — | — | — | — | — | 0/1 | — | — | — | 0/1 (0) |
| Snow goose (Chen caerulescens) | — | 0/32 | 1/80 | — | 0/98 | 0/4 | 0/7 | 0/3 | — | 1/224 (0.4) |
| Unknown | — | — | — | — | — | 2/8 | — | — | — | 2/8 (25) |
| Wood duck (Aix sponsa) | 1/39 | — | — | — | — | 0/40 | 0/24 | — | — | 1/103 (1) |
| Total | 4/223 | 0/32 | 1/80 | 0/34 | 0/126 | 84/2533 | 18/942 | 0/6 | 1/8 | 108/3984 |
| Percent recovery | 1.8 | 0 | 1.3 | 0 | 0 | 3.3 | 1.9 | 0 | 12.5 | 2.7 |
IAV recovery.
Of the 3984 samples collected, 108 (2.7%) were positive for IAV isolation. The proportion of IAV-positive samples varied during the study years from a low of 1.30% in 2006 to a high of 5.76% in 2013 (Table 2). Overall, the evaluation of the frequency of IAV recovery showed a significant relationship between year and the proportion of IAV positive samples (χ2 = 33.04, P < 0.0001). The Marascuilo post hoc procedure was used to compare multiple proportions to investigate the differences between years, but no statistically significant differences could be identified (data not shown). Differences between years could be attributed to the addition of teal sampling during 2010 and subsequent years.
Table 2.
HA and NA subtype combinations for IAV isolates recovered by year of surveillance activity in Missouri, United States, 2005–2013. Numbers in parentheses equals total number of isolates with respective antigenic subtype.
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | |
|---|---|---|---|---|---|---|---|---|---|
| Samples collected | 300 | 383 | 400 | 339 | 329 | 607 | 652 | 523 | 451 |
| Recovered isolates (% positive) | 4 (1.33) | 5 (1.30) | 9 (2.25) | 9 (2.65) | 8 (2.43) | 27 (4.45) | 10 (1.53) | 10 (1.91) | 26 (5.76) |
| Identified subtypes | H2N3 (1) | H6N2 (1) | H?N? (1) | H6N1,2,4 (2) | H?N2 (1) | H?N2 (1) | H3,4N2 (1) | H3N6 (1) | H?N? (7) |
| H4N6 (1) | H8N3 (1) | H?N1,4 (1) | H6N2 (1) | H?N9 (1) | H?N3 (1) | H3N6 (2) | H4N6 (2) | H1N2 (1) | |
| H11N3 (1) | H9N3 (1) | H4N2 (1) | H10N7 (1) | H6N2 (1) | H?N6 (1) | H4N8 (3) | H4N8 (2) | H2N3 (1) | |
| H11N9 (1) | H11N9 (2) | H5N2 (1) | H11N9 (2) | H7N3 (1) | H?N7 (1) | H5N2 (1) | H5N3 (1) | H3N6,8 (1) | |
| — | — | H6N2 (1) | H?N? (2) | H9N2 (1) | H?N8 (1) | H6N2 (1) | H10N3 (1) | H3N8 (6) | |
| — | — | H7N1 (1) | H?N5 (1) | H10N3 (1) | H3N1 (1) | H11N9 (1) | H10N7 (1) | H4N6 (1) | |
| — | — | H7N7 (1) | — | H10N7 (1) | H3N6 (3) | H12N1,4 (1) | H11N9 (1) | H4N8 (6) | |
| — | — | H11N? (1) | — | H12N5 (1) | H4N6 (1) | — | H?N? (1) | H6N8 (1) | |
| — | — | H11N2 (1) | — | — | H5N2 (1) | — | — | H10N3 (1) | |
| — | — | — | — | — | H5N9 (1) | — | — | H10N7 (1) | |
| — | — | — | — | — | H6N1 (1) | — | — | — | |
| — | — | — | — | — | H6N1,2,4 (1) | — | — | — | |
| — | — | — | — | — | H7N1,4 (1) | — | — | — | |
| — | — | — | — | — | H7N3 (3) | — | — | — | |
| — | — | — | — | — | H7N7 (2) | — | — | — | |
| — | — | — | — | — | H9,12N2 (1) | — | — | — | |
| — | — | — | — | — | H10N7 (1) | — | — | — | |
| — | — | — | — | — | H11N3,9 (1) | — | — | — | |
| — | — | — | — | — | H11N9 (3) | — | — | — | |
| — | — | — | — | — | H12N4 (1) | — | — | — |
The frequency of IAV recovery did differ (χ2 = 7.21, P = 0.027) between sexes of sampled bird; males 59/2401 (2.5%), females 45/1250 (3.6%), and undetermined 4/333 (1.2%). The relationship between age and IAV recovery was also significant (χ2 = 22.0900, P < 0.001); mature birds 42/2078 (2.0%), hatch year 59/1353 (4.4%), and unknown 7/553 (1.3%). Table 1 shows the varied distribution of IAV isolations across the represented species. Notably, 35/388 (9.0%) blue-winged teal and 20/371 (5.4%) northern shovelers tested positive for IAV.
The multivariable model shown in Table 3 displays the adjusted odds ratios of the included variables. Year was forced into the model due to the high variability in IAV recovery during the sampled years. The final model shows that the adjusted odds of IAV recovery for blue-winged teal and northern shovelers, when compared to all other species, were 5.04 and 2.76, respectively. The final model also shows the odds of IAV recovery were 1.58 times higher for hatch-year birds when compared to mature birds.
Table 3.
Odds ratios from multivariable logistic regression model for predictors of IAV infection of wild birds sampled in Missouri, United States, 2005–2013.
| Variable | Odds ratio | 95% Confidence interval | P value |
|---|---|---|---|
| Age | |||
| Mature | 1.0A | ||
| Hatch year | 1.58 | 0.99–2.52 | 0.056 |
| Unknown | 0.84 | 0.36–2.01 | 0.706 |
| Species | |||
| All other species | 1.0A | ||
| Mallard | 1.36 | 0.51–3.67 | 0.539 |
| Gadwall | 0.26 | 0.06–1.15 | 0.075 |
| American green-winged teal | 0.94 | 0.29–3.02 | 0.913 |
| Northern shoveler | 2.76 | 0.98–7.79 | 0.054 |
| Blue-winged teal | 5.04 | 1.86–13.67 | 0.001 |
| Other dabbling ducks | 0.89 | 0.23–3.49 | 0.866 |
| Year | |||
| 2005 | 1.0A | ||
| 2006 | 0.77 | 0.20–2.93 | 0.702 |
| 2007 | 1.20 | 0.36–4.00 | 0.770 |
| 2008 | 1.66 | 0.50–5.52 | 0.410 |
| 2009 | 1.60 | 0.47–5.49 | 0.454 |
| 2010 | 2.10 | 0.71–6.26 | 0.183 |
| 2011 | 0.59 | 0.17–2.01 | 0.401 |
| 2012 | 0.66 | 0.20–2.25 | 0.513 |
| 2013 | 1.94 | 0.64–5.94 | 0.244 |
Referent group.
Subtype distribution.
Antigenic subtyping identified at least 12 HA and nine NA subtypes. The 27 HA–NA combinations detected were represented in 78 isolates where a single HA and NA antigenic subtype was detected. There were nine isolates where antigenic subtyping indicated the presence of two or more HA, NA, or both subtypes, which may represent mixed infections. In addition, antigenic subtyping results did not identify an HA or NA (or both together) subtype for 21 isolates (Table 2).
DISCUSSION
The findings of the current study are comparable to the results of other investigators where the recorded IAV prevalence varies over seasons and is influenced by the age and species of the sampled birds (3,5,18,25). The present surveillance study demonstrated an IAV prevalence of 2.7% in the sampled wild bird population. This is similar to studies conducted in Texas during the same time period (5) but is much lower than the IAV prevalence of 13.5% documented concurrently in Minnesota (25). While migration stops can serve as comingling points which allow for IAV transmission, our data indicate that IAV activity among the southern migrating birds is relatively low by the time they reach Missouri.
Surprisingly, Mallards sampled in the present study were shedding IAVs at a relatively lower frequency, contradicting surveillance data from other geographic locations within the Mississippi Flyway (25). However, just as reported by Ferro et al. (5) from IAV surveillance in Texas, both blue-winged teal and northern shovelers sampled within the context of the present surveillance effort in Missouri had the highest prevalence of IAV infections. Blue-winged teal and northern shovelers are dabbling ducks, like mallards and green-winged teal, but have life cycle differences that may contribute to their susceptibility to IAVs in the more-southern regions of the United States. Blue-winged teal leave northern latitudes before other dabbling duck species, making the migratory trek earlier in the autumn and are among the last species to return north in the spring (4). As postulated by Ferro et al. (5) the high energy costs of long-distance migrations of the blue-winged teal may make the species more susceptible to IAV infections, thus constituting the high prevalence of IAVs being shed in this sampled population. In addition, during the present study green-winged teal were sampled during both the early hunting seasons, which allow hunters to only harvest teal species, and during the later duck hunting seasons when hunters can harvest all species of dabbling ducks. Because the blue-winged teal migrate before the green-winged teal, samples are only collected from the blue-winged teal during the earlier hunting season, which could contribute to the appearance of an intraseason trend and thus bias the overall prevalence data (18). No matter the reason for IAV recovery differences, blue-winged teal are known to be an important species to the ecology of IAV and IAV subtype diversity (17,21).
The higher recovery from hatch-year birds is similar to several previous surveillance studies (9,11,16,21), and the observed inverse relationship between age and viral shedding has been demonstrated in an experimental setting (2). The significant difference in the frequency of IAV recovery between sexes is an interesting finding. Even when the 333 birds for which the sex could not be identified are removed from the dataset, the difference in IAV recovery between males and females remains significant (P = 0.048). Although statistically significant on its own, sex was not included in the final model because the included variables of age, species, and year provided maximum explanation of the variation.
The present surveillance effort produced several unique IAV isolates, some of which were genomically linked to severe disease in domestic poultry. In summer of 2012, poultry farms in Jalisco, Mexico experienced outbreaks of highly pathogenic avian influenza (HPAI) virus H7N3, which spread into three additional Mexican states. The H7N3 HPAI affected broilers, breeders, layers, and backyard poultry from 2012 through the latest outbreak in spring 2014 and was transmitted to at least two poultry workers in Jalisco (12,13). A recent investigation into the ancestry of this H7N3 HPAI virus revealed that the most-closely related strain of the H7 HA was that of A/northern shoveler/Missouri/10OS4750/2010(H7N3), an isolate recovered through the present surveillance effort. While this analysis does not infer evolutionary history, Lu et al. (13) did show, through Bayesian phylogenetic methods, that the most-common ancestor of the HPAI HA gene was from a wild bird LP H7 clade which gained basic amino acid residues at the cleavage site, becoming HP in March 2012. Additionally, further investigation, through the National Center for Biotechnology Information (NCBI) BLAST tool (http://www.ncbi.nlm.nih.gov/) of selectively sequenced Missouri IAV isolates, shows that A/blue-winged teal/Missouri/11OS2563/2011(H12N4) and A/mallard/Missouri/12OS5756/2012(H10N7) have PB1 genes that are also closely matched (≥98.9% nucleotide identities) to the H7N3 HPAI isolates (data not shown).
Although outbreaks of H7N3 HPAI were not reported in Missouri, several cases of LP IAV in commercial poultry operations were reported in Missouri shortly before and during our surveillance period. In 2004, a year before our study commenced, there were H3N2 infections in commercial turkey breeding operations, and several serotypes were present in two large backyard chicken flocks, both of which were depopulated (Massengill, unpubl. data). In 2011, there was also a case of LP H7N3 detected in commercially raised turkeys, although viruses were never isolated (Massengill, unpubl. data). Genomically, an H1N1 IAV isolate recovered from turkeys in Missouri in 1987 matches with ≥98% nucleotide identity to seven of eight wild-bird–origin IAV isolate segments recovered in the United States in 1986–87. The NA segment was of swine lineage (data not shown). These examples provide evidence that even LP IAVs circulating in wild birds have had detrimental impacts on food animal production agriculture in Missouri, and must be considered a potential threat into the future, therefore justifying ongoing biosecurity programs.
Another interesting finding of our IAV surveillance in Missouri waterfowl was the molecular detection of another lineage of H14 IAV associated with the apparent intraflyway spread of the H14 subtype. As reported by Fries et al. (6), A/northern-shoveler/Missouri/10OS4637/2010(H14N6) was antigenically characterized as an H4, but genomic sequencing revealed this to be an H14 lineage HA. This H14 isolate is genetically diverse from the five H14 isolates recovered from wild waterfowl in Wisconsin during the same year (97.5% amino acid identity between Wisconsin and Missouri), indicating that at least two lineages of these uncommonly recovered H14 viral isolates could be circulating and adapting in an unsampled host in the United States (6). Although there is presently no indication that the H14 subtype is a significant threat to poultry or swine health, it is concerning that Eurasian gene segments can suddenly appear in, and rapidly spread throughout, the Mississippi Flyway where major poultry and swine production units exist.
Further understanding of the natural history, genomic diversity, routes of transmission, and ecology of waterfowl-origin IAVs will assist both animal and public health officials in their control of IAV in the respective populations. This can be seen in recently reported events across Eurasia of apparently related HP H5N1, HP H5N8, and LP H7N9. Ultimately, the ongoing need for up-to-date information about IAV threats to animal populations advocates the need for additional, ongoing surveillance.
ACKNOWLEDGMENTS
We greatly appreciate the cooperation and patience of the waterfowl hunters in Missouri who allowed us to collect samples from their harvested birds over several years. We also thank Aaron Yetter, Tommy Marshall, Pam Ward, Keith Cordelle, Kevin Brunke, Ryan Kelly, Jennifer Weber, Andy West, Clayton Light, Dennis Epperly, Justin Dickey, Anthony Fries, and Omary Nkullo for their support and efforts in sample collection. Mary Lea Killian at the USDA National Veterinary Service Laboratories was also instrumental in the antigenic subtyping of all isolates. We also thank Sujan Rana for his assistance with data analysis.
This work has been funded in part by the Ohio Department of Health; USDA Cooperative State Research, Education, and Extension Service (CSREES) Coordinated Agriculture Project (CAP), Avian Influenza Prevention and Control under award no. 20085520418863 and with federal funds from the Centers of Excellence for Influenza Research and Surveillance (CEIRS), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract Nos. HHSN266200700007C and HHSN272201400006C.
Abbreviations:
- HI
hemagglutination inhibition
- HP
highly pathogenic
- HPAI
highly pathogenic avian influenza
- IAV
influenza A virus
- LP
low pathogenic
- NI
neuraminidase inhibition
REFERENCES
- 1.Bahl AK, Pomeroy BS, Easterday BC, and Mangundimedjo S. Isolation of type A influenza viruses from the migratory waterfowl along the Mississippi Flyway. J. Wildl. Dis 11:360–363. 1975. [DOI] [PubMed] [Google Scholar]
- 2.Costa TP, Brown JD, Howerth EW, and Stallknecht DE. The effect of age on avian influenza viral shedding in mallards (Anas platyrhynchos). Avian Dis. 54:581–585. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dusek RJ, Bortner JB, DeLiberto TJ, Hoskins J, Franson JC, Bales BD, Yparraguirre D, Swafford SR, and Ip HS. Surveillance for high pathogenicity avian influenza virus in wild birds in the Pacific Flyway of the United States, 2006–2007. Avian Dis. 53:222–230. 2009. [DOI] [PubMed] [Google Scholar]
- 4.Bellrose FC Ducks, geese, and swans of North America, 2nd ed. Stackpole Books, Harrisburg, PA. 1978. [Google Scholar]
- 5.Ferro PJ, Budke CM, Peterson MJ, Cox D, Roltsch E, Merendino T, Nelson M, and Lupiani B. Multiyear surveillance for avian influenza virus in waterfowl from wintering grounds, Texas coast, USA. Emerg. Infect. Dis 16:1224–1230. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fries AC, Nolting JM, Bowman AS, Killian ML, Wentworth DE, and Slemons RD. Genomic analyses detect Eurasian-lineage H10 and additional H14 influenza A viruses recovered from waterfowl in the central United States. Influenza Other Respir. Virus 8:493–498. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halvorson D, Karunakaran D, Senne D, Kelleher C, Bailey C, Abraham A, Hinshaw V, and Newman J. Epizootiology of avian influenza— simultaneous monitoring of sentinel ducks and turkeys in Minnesota. Avian Dis. 27:77–85. 1983. [PubMed] [Google Scholar]
- 8.Halvorson DA, Kelleher CJ, and Senne DA. Epizootiology of avian influenza: effect of season on incidence in sentinel ducks and domestic turkeys in Minnesota. Appl. Environ. Microbiol 49:914–919. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinshaw VS, Webster RG, and Turner B. The perpetuation of orthomyxoviruses and paramyxoviruses in Canadian waterfowl. Can. J. Microbiol 26:622–629. 1980. [DOI] [PubMed] [Google Scholar]
- 10.Hinshaw VS, Wood JM, Webster RG, Deibel R, and Turner B. Circulation of influenza viruses and paramyxoviruses in waterfowl originating from two different areas of North America. Bull. WHO 63:711–719. 1985. [PMC free article] [PubMed] [Google Scholar]
- 11.Ip HS, Flint PL, Franson JC, Dusek RJ, Derksen DV, Gill RE Jr., Ely CR, Pearce JM, Lanctot RB, Matsuoka SM, Irons DB, Fischer JB, Oates RM, Petersen MR, Fondell TF, Rocque DA, Pedersen JC, and Rothe TC. Prevalence of influenza A viruses in wild migratory birds in Alaska: patterns of variation in detection at a crossroads of intercontinental flyways. Virology J. 5:71. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez-Martinez I, Balish A, Barrera-Badillo G, Jones J, Nunez-Garcia TE, Jang Y, Aparicio-Antonio R, Azziz-Baumgartner E, Belser JA, Ramirez-Gonzalez JE, Pedersen JC, Ortiz-Alcantara J, Gonzalez-Duran E, Shu B, Emery SL, Poh MK, Reyes-Teran G, Vazquez-Perez JA, Avila-Rios S, Uyeki T, Lindstrom S, Villanueva J, Tokars J, Ruiz-Matus C, Gonzalez-Roldan JF, Schmitt B, Klimov A, Cox N, Kuri-Morales P, Davis CT, and Diaz-Quinonez JA. Highly pathogenic avian influenza A (H7N3) virus in poultry workers, Mexico, 2012. Emerg. Infect. Dis 19:1531–1534. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu L, Lycett SJ, and Leigh Brown AJ. Determining the phylogenetic and phylogeographic origin of highly pathogenic avian influenza (H7N3) in Mexico. PloS ONE 9:e107330. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma W, Kahn RE, and Richt JA. The pig as a mixing vessel for influenza viruses: human and veterinary implications. J. Mol. Genet. Med 3:158–166. 2008. [PMC free article] [PubMed] [Google Scholar]
- 15.Munster VJ, Wallensten A, Baas C, Rimmelzwaan GF, Schutten M, Olsen B, Osterhaus AD, and Fouchier RA. Mallards and highly pathogenic avian influenza ancestral viruses, northern Europe. Emerg. Infect. Dis 11:1545–1551. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parmley EJ, Bastien N, Booth TF, Bowes V, Buck PA, Breault A, Caswell D, Daoust PY, Davies JC, Elahi SM, Fortin M, Kibenge F, King R, Li Y, North N, Ojkic D, Pasick J, Pryor SP, Robinson J, Rodrigue J, Whitney H, Zimmer P, and Leighton FA. Wild bird influenza survey, Canada, 2005. Emerg. Infect. Dis 14:84–87. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramey AM, Poulson RL, Gonzalez-Reiche AS, Wilcox BR, Walther P, Link P, Carter DL, Newsome GM, Muller ML, Berghaus RD, Perez DR, Hall JS, and Stallknecht DE. Evidence for seasonal patterns in the relative abundance of avian influenza virus subtypes in blue-winged teal (Anas discors). J. Wildl. Dis 50:916–922. 2014. [DOI] [PubMed] [Google Scholar]
- 18.Sharp GB, Kawaoka Y, Wright SM, Turner B, Hinshaw V, and Webster RG. Wild ducks are the reservoir for only a limited number of influenza A subtypes. Epidemiol. Infect 110:161–176. 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slemons RD, Hansen WR, Converse KA, and Senne DA. Type A influenza virus surveillance in free-flying, nonmigratory ducks residing on the eastern shore of Maryland. Avian Dis. 47:1107–1110. 2003. [DOI] [PubMed] [Google Scholar]
- 20.Stallknecht DE, Nagy E, Hunter DB, and Slemons RD. Avian influenza. In: Infectious Diseases of Wild Birds. Thomas NJ, Hunter DB, and Atkinson CT, eds. Blackwell Publishing, Ames, IA. pp. 108–130. 2007. [Google Scholar]
- 21.Stallknecht DE, Shane SM, Zwank PJ, Senne DA, and Kearney MT. Avian influenza viruses from migratory and resident ducks of coastal Louisiana. Avian Dis. 34:398–405. 1990. [PubMed] [Google Scholar]
- 22.[USDA] United States Department of Agriculture. 2012. Census of Agriculture, USDA. [cited 2015 Feb 23]. Available from: http://www.agcensus.usda.gov/Publications/2012/Full_Report/Census_by_State/Missouri/
- 23.USDA. 2013. Missouri State Agriculture Overview. [cited 2015 Feb 23]. Available from: http://www.nass.usda.gov/Quick_Stats/Ag_Overview/stateOverview.php?state=MISSOURI
- 24.Webster RG, Bean WJ, Gorman OT, Chambers TM, and Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol. Rev 56:152–179. 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilcox BR, Knutsen GA, Berdeen J, Goekjian V, Poulson R, Goyal S, Sreevatsan S, Cardona C, Berghaus RD, Swayne DE, Yabsley MJ, and Stallknecht DE. Influenza-A viruses in ducks in northwestern Minnesota: fine scale spatial and temporal variation in prevalence and subtype diversity. PloS ONE 6:e24010. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou NN, Senne DA, Landgraf JS, Swenson SL, Erickson G, Rossow K, Liu L, Yoon K, Krauss S, and Webster RG. Genetic reassortment of avian, swine, and human influenza A viruses in American pigs. J. Virol 73:8851–8856. 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


