Abstract
We describe a 56-year-old female patient hospitalised with COVID-19 in April 2020 who had persistent respiratory symptoms after radiographic and microbiologic recovery. X-ray of the chest demonstrated an elevated right hemidiaphragm while fluoroscopy confirmed unilateral diaphragmatic paralysis. Symptoms resolved gradually, concurrent with restoration of right hemidiaphragm function. Thus, we describe a rare cause of postacute sequelae of COVID-19 dyspnoea.
Keywords: COVID-19, adult intensive care, lung function
Background
COVID-19, caused by SARS-CoV-2, is a multisystem viral illness predominantly affecting the respiratory system. SARS-CoV-2 also targets the nervous system and patients can present with anosmia, dizziness, seizures, or, rarely, acute cerebrovascular disease.1 Neurologic symptoms are a consequence of direct viral effects on the central nervous system (CNS) or peripheral nervous system (PNS) and collateral damage from the inflammatory response. Case reports have described mononeuritis multiplex, Guillain-Barre syndrome and bilateral diaphragmatic paralysis associated with acute COVID-19; however, it is unclear whether PNS lesions affect patients after recovery from acute illness.2–4 Postacute sequelae of COVID-19 (PASC) affect up to two out of three of patients, up to 60% of which describe persistent dyspnoea more than 60 days after infection.5 Here, we describe a case of COVID-19-related unilateral diaphragmatic paralysis causing PASC dyspnoea.
Case presentation
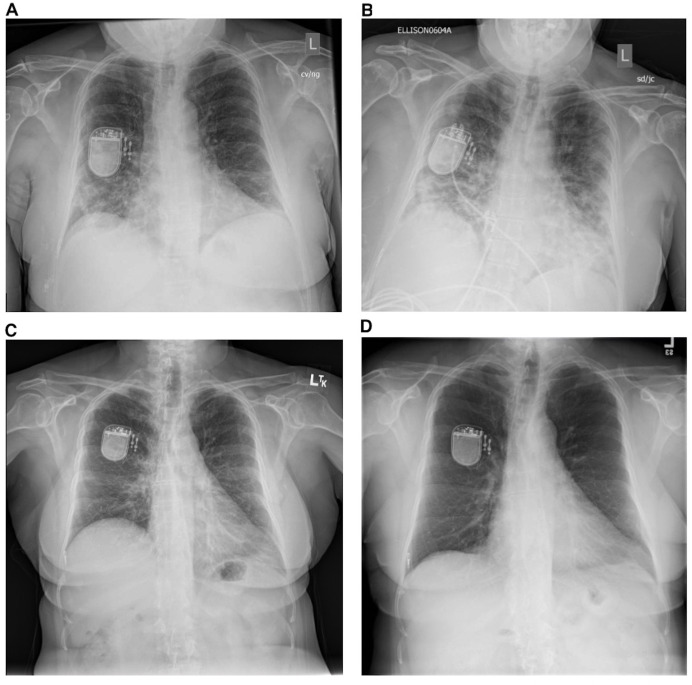
A 56-year-old woman with a body mass index of 32 and medical history of obstructive sleep apnea (OSA), hypertension, hyperlipidaemia and breast cancer presented in April 2020 with progressive dyspnoea, cough, headache, fever and chills after being diagnosed with COVID-19 by nasal swab 6 days earlier. Her admission vital signs were significant for a temperature of 101.1°F, heart rate of 104 beats per minute and oxygen saturation of 95% on room air. X-ray of the chest (XRC) demonstrated bilateral peripheral interstitial infiltrates (figure 1A).
Figure 1.
Evolution of the patient’s X-rays of the chest during acute and postacute COVID-19 course. (A) X-ray of the chest on presentation to the hospital demonstrating bilateral interstitial infiltrates. (B) X-ray of the chest at intensive care unit admission demonstrating progressive bilateral interstitial infiltrates and newly elevated right hemidiaphragm. (C) X-ray of the chest 2 weeks after hospital discharge demonstrating resolution of peripheral infiltrates and persistent right hemidiaphragm elevation. (D) X-ray of the chest showing complete resolution of right hemidiaphragm elevation.
She was admitted to the medical ward and administered supplemental oxygen. On day 5 of admission, she developed progressive hypoxaemia requiring admission to the intensive care unit for close monitoring. She was started on empiric ceftriaxone and azithromycin. On day 7 of admission, an XRC demonstrated worsening multifocal airspace disease consistent with COVID-19 acute respiratory distress syndrome (ARDS) and an elevated right hemidiaphragm (figure 1B). She was administered vancomycin and cefepime, furosemide, and was enrolled in a trial of tocilizumab for COVID-19 ARDS. The patient attempted to prone, but only tolerated lying on her side, without any preference for one side or the other. Her neck was not immobilised. She never received treatment with non-invasive ventilation or intubation and mechanical ventilation. Central venous access was deferred, and she never received steroids, remdesivir or vasopressors. She refused treatment for her OSA throughout her hospitalisation. Her hypoxaemia gradually improved, she was weaned off supplemental oxygen by day 15, and was discharged home on day 20. After discharge, the patient had persistent dyspnoea, dry cough and ambulatory hypoxaemia.
Investigations
The patient was referred to a pulmonologist who obtained a repeat XRC demonstrating resolution of pulmonary infiltrates and persistently elevated right hemidiaphragm (figure 1C). At the time of her first clinic visit, pulmonary function tests were obtained that demonstrated a restrictive pattern (forced expiratory volume in 1 second (FEV1) 1.88 L (76% predicted), forced vital capacity (FVC) 2.18 L (69% predicted), FEV1/FVC 87%, total lung capacity 3.64 L (76% predicted), residual volume 1.56 L (85% predicted)). CT of the chest showed normal pulmonary parenchyma and a persistently elevated right hemidiaphragm. Chest fluoroscopy demonstrated paradoxical right hemidiaphragm movement consistent with unilateral diaphragmatic paralysis. Reduced access to ambulatory testing during the pandemic prevented further investigation of diaphragm function (eg, electromyography, diaphragmatic ultrasound, seated/supine spirometry). A repeat XRC 11 months after discharge showed resolution of her right hemidiaphragm elevation (figure 1D).
Outcome and follow-up
The patient’s symptoms resolved over the following year with expectant management. She is now able to perform her own activities of daily living again and walk longer distances without getting short of breath.
Discussion
PASC affects up to two out of three of COVID-19 survivors and commonly manifests with respiratory and neuromuscular symptoms, which can persist for up to 6 months.6 The full spectrum of disease remains uncharacterised and reports of neuromuscular aetiologies of dyspnoea are scarce. Here, we describe a case of a 56-year-old woman with transient right hemidiaphragm paralysis as a cause of PASC-related dyspnoea.
Phrenic neuropathy is the most common cause of diaphragmatic paralysis and can be due to stretching/cooling during cardiothoracic surgery,7 damage to the cervical nerve root,8 9 neuralgic amyotrophy10 or viral infections such as herpes zoster or influenza.11 12 Our case suggests that SARS-CoV-2 could be an additional viral aetiology of phrenic neuropathy. Two possible processes could explain this phenomenon: direct neuroinvasion or an inflammatory process affecting the phrenic nerve. SARS-CoV-2 has been shown to invade the CNS and PNS. Frequently, this begins in the olfactory system after which there is viral dissemination within the CNS and PNS; however, retrograde invasion of the PNS from a non-neuronal reservoir is also possible.12 A study of diaphragm muscle obtained at autopsy demonstrated SARS-CoV-2 infection of diaphragm myofibers, suggesting this could be a potential source for phrenic nerve retrograde invasion.13 A second explanation is that SARS-CoV-2 could have caused mononeuritis multiplex of the phrenic nerve. Two potential mechanisms could induce mononeuritis multiplex. First, a SARS-CoV-2-induced prothrombotic state or systemic vasculitis led to the formation of microthrombi within the vasa nervosum, inducing ischaemia and neuronal dysfunction.14 Second, release of proinflammatory cytokines during SARS-CoV-2 infection directly impaired neuronal function.15
This case report adds important dimensions to our understanding of diaphragmatic paralysis in COVID-19. One prior report describes diaphragmatic paralysis in a patient infected with COVID-19 with a history of OSA who was treated with continuous positive airway pressure (CPAP) and mechanical ventilation.16 A second case report describes respiratory failure in an obese patient with COVID-19 attributable to diaphragmatic paralysis.4 Our patient is obese and has OSA, suggesting these may be risk factors for the development of diaphragmatic paralysis with COVID-19. A preprint describing a cohort of 25 patients with COVID-19 treated with mechanical ventilation identified a high burden of diaphragmatic abnormalities.5 Another study describes 1527 patients with COVID-19, 1.5% of which were diagnosed with diaphragmatic dysfunction on CT scan.17 These reports differ from ours as each patient was treated with CPAP or mechanical ventilation, both known risk factors for the development of diaphragmatic dysfunction, while our patient received no non-invasiveventilation or mechanical ventilation.18 Taken together, our report suggests that OSA and obesity may be risk factors for the development of diaphragmatic paralysis during COVID-19 infection.
Treatment for unilateral diaphragmatic paralysis is reserved for symptomatic patients and involves weight loss and/or nocturnal positive pressure ventilation. Patients with severe or bilateral diaphragmatic paralysis may require diaphragmatic fundoplication. Our patient’s unilateral paralysis resolved with expectant management keeping with its likely viral origin.
Learning points.
We report a case of postacute sequelae of COVID-19 (PACS) dyspnoea due to unilateral diaphragmatic paralysis.
This presentation is likely due to direct effects of SARS-CoV-2 on phrenic nerve function.
Peripheral neuropathies should be considered as a cause of PASC-related dyspnoea.
Obesity and obstructive sleep apnea could be risk factors for development of diaphragm paralysis in the setting of COVID-19 infection.
Footnotes
Twitter: @nd1920, @danokin
Contributors: ND wrote the first draft of the manuscript and edited all subsequent versions. CH and PG provided intellectual guidance and edited and approved the final manuscript. DO supervised the project, wrote the first draft of the manuscript and edited all subsequent versions.
Funding: National Heart, Lung, and Blood Institute (T32 HL116275).
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020;77:683–90. 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caress JB, Castoro RJ, Simmons Z, et al. COVID-19-associated Guillain-Barré syndrome: the early pandemic experience. Muscle Nerve 2020;62:485–91. 10.1002/mus.27024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Estévez-Rivera E, Benavides-Hinestroza J, Rubiano H. Mononeuritis multiplex associated with Sars-Cov2-Covid-19 infection: case report. Int J Neurol Neurother 2020;7:102. [Google Scholar]
- 4.Maurier F, Godbert B, Perrin J. Respiratory distress in SARS-CoV-2 without lung damage: phrenic paralysis should be considered in COVID-19 infection. Eur J Case Rep Intern Med 2020;7:001728. 10.12890/2020_001728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farr E, Wolfe AR, Deshmukh S. Short of breath for the long Haul: diaphragm muscle dysfunction in survivors of severe COVID-19 as determined by neuromuscular ultrasound. medRxiv 2020. [Google Scholar]
- 6.Nalbandian A, Sehgal K, Gupta A, et al. Post-Acute COVID-19 syndrome. Nat Med 2021;27:601–15. 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canbaz S, Turgut N, Halici U, et al. Electrophysiological evaluation of phrenic nerve injury during cardiac surgery--a prospective, controlled, clinical study. BMC Surg 2004;4:2. 10.1186/1471-2482-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman SA, Holmes MD, Taylor DJ. Unilateral diaphragmatic paralysis following bronchial artery embolization for hemoptysis. Chest 2000;118:269–70. 10.1378/chest.118.1.269 [DOI] [PubMed] [Google Scholar]
- 9.McCaul JA, Hislop WS. Transient hemi-diaphragmatic paralysis following neck surgery: report of a case and review of the literature. J R Coll Surg Edinb 2001;46:186–8. [PubMed] [Google Scholar]
- 10.Tsao BE, Ostrovskiy DA, Wilbourn AJ, et al. Phrenic neuropathy due to neuralgic amyotrophy. Neurology 2006;66:1582–4. 10.1212/01.wnl.0000216140.25497.40 [DOI] [PubMed] [Google Scholar]
- 11.Parker GW, Ramos ED. Paralysis of the phrenic nerve following herpes zoster. JAMA 1962;180:408–10. 10.1001/jama.1962.03050180054016 [DOI] [Google Scholar]
- 12.Matsuda K, Park CH, Sunden Y, et al. The vagus nerve is one route of transneural invasion for intranasally inoculated influenza A virus in mice. Vet Pathol 2004;41:101–7. 10.1354/vp.41-2-101 [DOI] [PubMed] [Google Scholar]
- 13.McFarland AJ, Yousuf MS, Shiers S, et al. Neurobiology of SARS-CoV-2 interactions with the peripheral nervous system: implications for COVID-19 and pain. Pain Rep 2021;6:e885. 10.1097/PR9.0000000000000885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi Z, de Vries HJ, Vlaar APJ, et al. Diaphragm pathology in critically ill patients with COVID-19 and postmortem findings from 3 medical centers. JAMA Intern Med 2021;181:122–4. 10.1001/jamainternmed.2020.6278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Needham E, Newcombe V, Michell A, et al. Mononeuritis multiplex: an unexpectedly frequent feature of severe COVID-19. J Neurol 2021;268:1–5. 10.1007/s00415-020-10321-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.FitzMaurice TS, McCann C, Walshaw M, et al. Unilateral diaphragm paralysis with COVID-19 infection. BMJ Case Rep 2021;14:e243115. 10.1136/bcr-2021-243115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdeldayem EH, Abdelrahman AS, Mansour MG. Recognition of phrenic paralysis as atypical presentation during CT chest examination of COVID-19 infection and its correlation with CT severity scoring: a local experience during pandemic era. Egypt J Radiol Nucl Med 2021;52. 10.1186/s43055-021-00527-9 [DOI] [Google Scholar]
- 18.Dres M, Goligher EC, Heunks LMA, et al. Critical illness-associated diaphragm weakness. Intensive Care Med 2017;43:1441–52. 10.1007/s00134-017-4928-4 [DOI] [PubMed] [Google Scholar]