Abstract
Objectives
The CHA2DS2-VASc score is the preferred risk model for anticoagulation decision-making in atrial fibrillation (AF) patients. Recent studies have found this score to have prognostic value in other cardiovascular diseases. We assessed the relationships between CHA2DS2-VASc score and long-term mortality in adults referred for stress testing,
Methods
165 184 consecutive patients from January 1991 to December 2014 from a prospective registry were studied, with CHA2DS2-VASc score calculated for all patients, and AF and anticoagulation status were recorded. The primary endpoint was all-cause mortality.
Results
In this cohort, 12 450 (7.5%) patients had AF and mean CHA2DS2-VASc score was 2.2±1.2. There were 22 152 (18.4%) deaths during mean follow-up of 6.1±4.8 years. In multivariable analysis, CHA2DS2-VASc score, presence of AF and anticoagulation use, along with end-stage renal failure and smoking were all independently associated with mortality with HRs (95% CIs) of 1.23 (1.21 to 1.25), 1.18 (1.10 to 1.27) and 1.50 (1.40 to 1.60), respectively. Higher CHA2DS2-VASc score was incrementally associated with worse survival both in patients with and without AF (log-rank p<0.001). Anticoagulation use was associated with reduced survival in non-AF patients with alternative anticoagulation indications at all CHA2DS2-VASc score categories, and AF patients with lower CHA2DS2-VASc score 0–2, but was protective in AF patients with higher CHA2DS2-VASc score 4–9.
Conclusion
Incrementally higher CHA2DS2-VASc score, a simple clinical tool, is associated with mortality in patients regardless of presence of AF and anticoagulation status. Anticoagulation use was associated with worse survival in non-AF patients and AF patients with low CHA2DS2-VASc scores, but was protective in AF patients with high CHA2DS2-VASc scores.
Keywords: CHA2DS2-VASc score, exercise test, atrial fibrillation, anticoagulation, mortality
Key questions.
What is already known about this subject?
CHA2DS2-VASc score is an establishing thromboembolism risk model in atrial fibrillation (AF) patients, however, its prognostic utility in the other general and low-risk populations are less well established.
What does this study add?
In this large cohort study of 165 184 patients referred for stress tests, the CHA2DS2-VASc score, along with presence of AF, anticoagulation use and reduced metabolic equivalent of task were independently associated with mortality during follow-up. Whereas CHA2DS2-VASc score was associated with worse prognosis regardless of AF status, anticoagulation was associated with reduced survival in all non-AF patients and AF patients with low CHA2DS2-VASc score, but was protective in AF patients with higher CHA2DS2-VASc score.
How might this impact on clinical practice?
CHA2DS2-VASc is a simple score that adequately stratifies mortality risk, and can be embedded as a calculator in electronic medical records for widespread use in adults regardless of AF status.
Introduction
The CHA2DS2-VASc score is the most widely used and guideline-recommended model for estimating thromboembolic risk and deciding on anticoagulation therapy in atrial fibrillation (AF) patients.1–3 A number of contemporary studies have reported the CHA2DS2-VASc score to be associated with adverse clinical outcomes in a variety of cardiovascular diseases beyond AF, such as heart failure, acute coronary syndrome, hypertension, cerebrovascular disease, peripheral arterial disease and even non-cardiovascular disease like chronic obstructive pulmonary disease and COVID-19 infection.4–10 Whether the CHA2DS2-VASc score stratify mortality risk in both patients with AF and without AF (non-AF), regardless of the number of co-morbidities, is not well established, but important to investigate given recent interests in clinical electronic medical records (EMR) embedded calculator based risk prediction in general populations. Patients undergoing stress tests are a relatively low-risk cohort compared with those with established cardiovascular disease, and are often referred for risk stratification. This study aimed to evaluate the prognostic utility of CHA2DS2-VASc score in a large prospective stress testing registry of patients from a tertiary referral centre and its interactions with AF and anticoagulation status.
Methods
Study population
It was not appropriate or possible to involve patients or the public in the design, or conduct, or reporting, or dissemination plans of our research. Data are available on reasonable request. Details regarding the methodology of patient inclusion and study design have been previously published.11 Briefly, consecutive unique patients undergoing exercise stress testing (including exercise ECG, echocardiography or nuclear studies) at our institution between 1 January 1991 and 31 December 2014 were identified from the prospective registry for inclusion. Pharmacologic stress tests were excluded. The main indications for exercise stress tests were ruling out coronary artery disease, symptom evaluation, those with cardiovascular risk factors, and follow-up of known coronary artery disease, with the proportions of each indication reported in online supplemental eTable of the prior publication.11 The time of the stress test, including the first for patients with multiple stress test over the time period, was used as reference point for all clinical characteristics and subsequent follow-up.
Clinical characteristics
Patient demographics, relevant history and medication use at the time of presentation for stress testing and metabolic equivalent of task (METs) on exercise test were collected. Patients were classified as having AF if this was previously documented on at least one prior ECG, at the time of the test, or a home monitoring device (Holter, Zio and others). The CHA2DS2-VASc score (congestive heart failure, hypertension, age, diabetes mellitus, stroke or transient ischemic attack, vascular disease, sex) was calculated retrospectively for every patient at the time of presentation for stress testing, with the constituents of this score and other clinical characteristics obtained from the stress test registry and clinical records for the documented history of these factors.1 Patients were classified as taking anticoagulation at time of stress test if they were prescribed warfarin, rivaroxaban, apixaban, dabigatran, heparin or edoxaban.
Outcome
The primary outcome was all cause mortality during follow-up from the time of stress test. For the time period prior to 1 November 2011, mortality data was obtained from the Social Security Death Index. Due to access restrictions implemented after this date, mortality data after 1 November 2011 was obtained from the Institutional Death Index. The final censoring date was through 31 December 2017.
Statistical analyses
Continuous variables are expressed as mean±SD and categorical variables as frequency (percentage). Student’s t-test and Pearson χ2 tests were used to compare continuous and categorical variables respectively between AF and non-AF patients. CHA2DS2-VASc score was divided into 0–1, 2, 3, 4 and 5–9 for analysis. Trend analyses such as anticoagulation use by AF status and CHA2DS2-VASc score were determined using the Cochran-Armitage test. Kaplan-Meier survival curves were constructed to compare survival by CHA2DS2-VASc score in all patients, and separately for patients without AF and patients with AF. They were also constructed to compare survival by patients without and with AF and on and not on anticoagulation medications, separately for different CHA2DS2-VASc score categories. Survival between groups was compared using log-rank test. To identify factors associated with mortality during follow-up in all patients, AF patients and non-AF patients, univariable Cox proportional hazards regression analysis was performed for the CHA2DS2-VASc score and other clinical variables collected that are not components of the CHA2DS2-VASc score. Multivariable Cox proportional hazards regression analysis for the outcome of death was then performed using all variables having a p value less than 0.05 on univariable analysis. Statistical analyses were performed using R V.3.1.3 (Vienna, Austria), and all tests were two tailed, with p values below 0.05 deemed statistically significant.
Results
Baseline characteristics
The study population included 165 184 consecutive patients referred for stress testing. AF diagnosis was present in 12 450 (7.5%) patients. Baseline characteristics are shown in table 1, with mean age 55.8±12.9, and 69 412 (42.0%) females. AF patients were older, with greater proportion being male, and had a higher prevalence of all comorbidities in the past history and medications recorded, including anticoagulation use in 6460 (51.9%) of AF patients and 7397 (4.8%) of non-AF patients. The mean CHA2DS2-VASc score overall was 2.2±1.2, higher in AF patients 2.8±1.4 than non-AF patients 2.1±1.2. The mean METs overall was 9.0±2.8, lower in AF patients 7.4±2.8 than non-AF patients 9.1±2.7. Trends in baseline age, clinical factors and medication use have been previously reported.11 Number of patients per year increased sharply from 104 in 1991 to 6064 in 1995, and subsequent steadily to averaging 8857 in 2011–2014; while proportion of patients on anticoagulation apart from being lower in 1991 and 1992 with stable afterwards at 7.1%–10.3%.
Table 1.
Cohort characteristics by atrial fibrillation (AF) status
| Characteristic | All | Non-AF | AF |
| No of patients | 165 184 | 152 734 | 12 450 |
| Demographics | |||
| Age (years) | 55.8±12.9 | 55.2±12.8 | 63.5±11.5 |
| Female | 69 412 (42.0) | 65 459 (42.9) | 3953 (31.8) |
| (Delete this row) | |||
| Past history | |||
| Congestive heart failure | 12 540 (9.1) | 9424 (7.5) | 3116 (25.0) |
| Hypertension | 102 079 (61.8) | 91 108 (59.7) | 10 971 (88.1) |
| Diabetes | 28 178 (17.1) | 25 461 (16.7) | 2717 (21.8) |
| Cerebrovascular event | 8563 (5.2) | 7076 (4.6) | 1487 (11.9) |
| Coronary artery disease | 36 622 (22.2) | 32 120 (21) | 4502 (36.2) |
| Vascular disease | 8690 (5.3) | 7660 (5.0) | 1030 (8.3) |
| Smoking history | 80 329 (48.6) | 73 823 (48.3) | 6506 (52.3) |
| End-stage renal disease | 4346 (3.1) | 3653 (2.9) | 693 (5.6) |
| CHA2DS2-VASc score | 2.2±1.2 | 2.1±1.2 | 2.8±1.4 |
| 0–1 | 80 048 (48.4) | 77 167 (50.5) | 2881 (23.1) |
| 2 | 40 730 (24.7) | 37 552 (24.6) | 3178 (25.5) |
| 3 | 24 833 (15) | 21 895 (14.3) | 2938 (23.6) |
| 4 | 12 222 (7.4) | 10 263 (6.7) | 1959 (15.7) |
| 5–9 | 7351 (4.5) | 5857 (3.8) | 1494 (12.0) |
| Medications | |||
| Anticoagulation | 13 857 (8.4) | 7397 (4.8) | 6460 (51.9) |
| Aspirin | 60 096 (36.4) | 54 707 (35.8) | 5389 (43.3) |
| Statin use | 48 812 (29.6) | 44 296 (29.0) | 4516 (36.3) |
| Beta blocker use | 49 251 (29.8) | 42 292 (27.7) | 6959 (55.9) |
| ACEi/ARB | 47 551 (28.8) | 42 128 (27.6) | 5423 (43.6) |
| Insulin | 9343 (5.7) | 8411 (5.5) | 932 (7.5) |
| Metabolic equivalent of tasks | 9.0±2.8 | 9.1±2.7 | 7.4±2.8 |
All numbers are frequency (percentage) or mean±SD.
ACEi/ARB, ACE inhibitor/angiotensin receptor blocker.
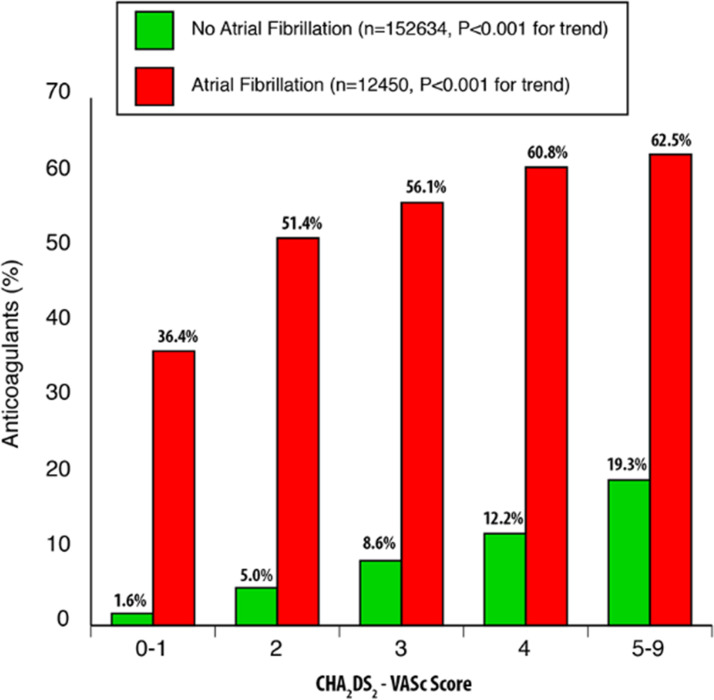
Figure 1 illustrates anticoagulation prescription in the cohort by AF status and CHA2DS2-VASc score. Anticoagulation use increased with the CHA2DS2-VASc score category in AF and non-AF patients (p<0.001 for trend in both). In AF patients however, utilisation of anticoagulation in CHA2DS2-VASc score 3 or higher was only 56%–63%, while in CHA2DS2-VASc score 0–1 category it was 36%.
Figure 1.
Anticoagulation use by atrial fibrillation status and CHA2DS2-VASc score (congestive heart failure, hypertension, age, diabetes mellitus, stroke or transient ischemic attack, vascular disease, sex).
Survival by CHA2DS2-VASc score, AF and anticoagulation status
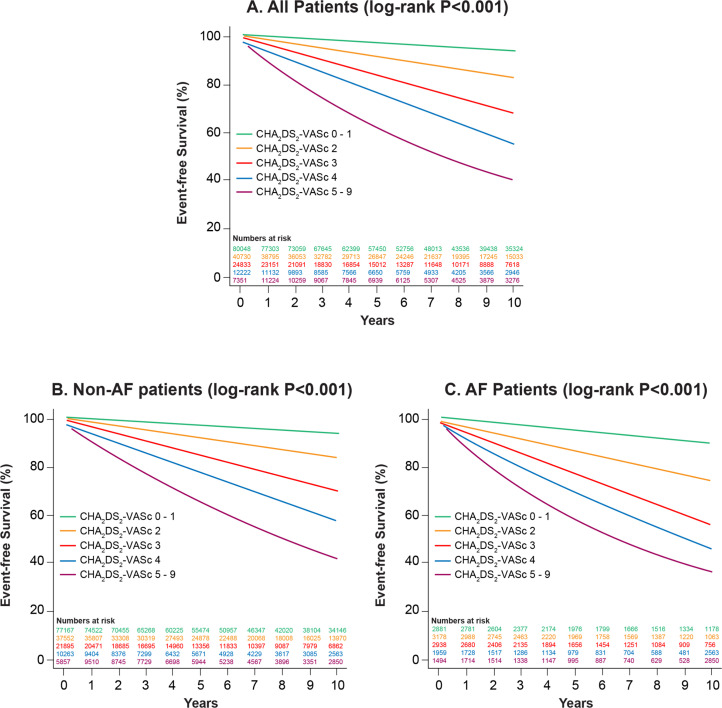
During mean follow-up of 6.1±4.8 years for the cohort, there were 22 152 (13.4%) deaths. Figure 2 (and Central illustration) displays survival by CHA2DS2-VASc score for the total cohort, non-AF and AF subgroups. Increasing CHA2DS2-VASc score was associated with reduced survival in all three cohorts (log-rank p<0.001). In addition, for all CHA2DS2-VASc score categories, AF patients had higher mortality during follow-up than non-AF patients.
Figure 2.
Kaplan-Meier survival curves stratified by CHA2DS2-VASc score (congestive heart failure, hypertension, age, diabetes mellitus, stroke or transient ischemic attack, vascular disease, sex) for (A) the entire cohort, (B) patients without atrial fibrillation (non-AF) and (C) patients with AF.
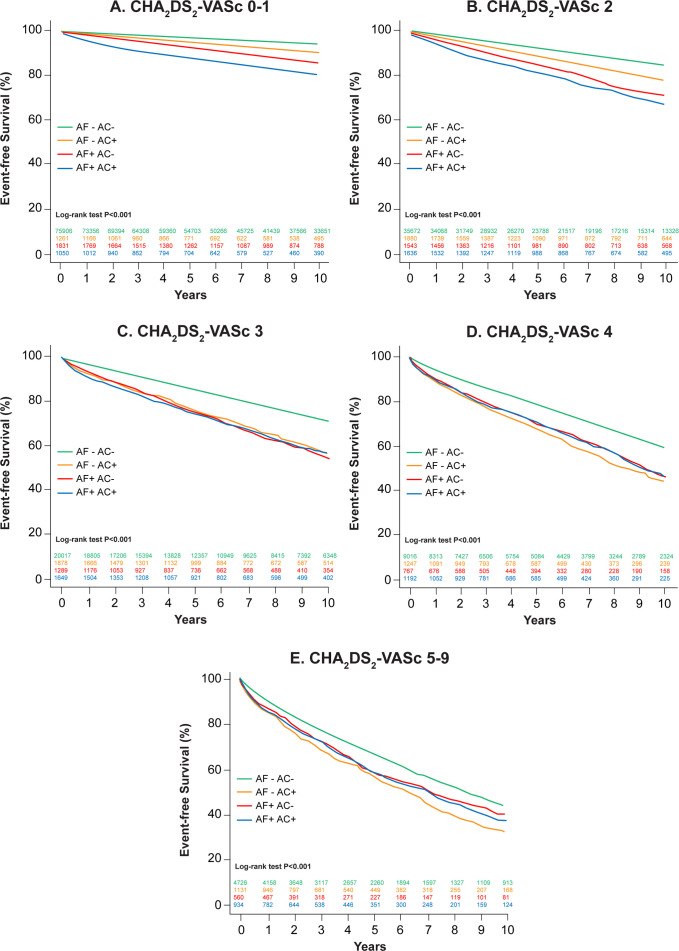
Figure 3 shows the survival curves of AF and anticoagulation status for each CHA2DS2-VASc score category. Patients without AF and no anticoagulation use had consistently the best prognosis at all CHA2DS2-VASc score categories. Patients without AF but with anticoagulation use had the worst prognosis in CHA2DS2-VASc score 0–1 and 2, but were similar to AF patients at higher CHA2DS2-VASc scores. In patients with AF, anticoagulation use and survival differed depending on the in CHA2DS2-VASc score category. Anticoagulation use was associated with increased mortality during follow-up in AF patients at CHA2DS2-VASc score 0–1 and 2, no difference in CHA2DS2-VASc score 3, but lower mortality especially later on during follow-up at CHA2DS2-VASc score of 4 and 5–9.
Figure 3.
Kaplan-Meier survival curves by atrial fibrillation (AF) and anticoagulation (AC) status for (A) CHA2DS2-VASc score 0 or 1, (B) CHA2DS2-VASc score 2, (C) CHA2DS2-VASc score 3, (D) CHA2DS2-VASc score (congestive heart failure, hypertension, age, diabetes mellitus, stroke or transient ischemic attack, vascular disease, sex) four and (E) CHA2DS2-VASc score 5–9.
Multivariable analysis for long-term mortality
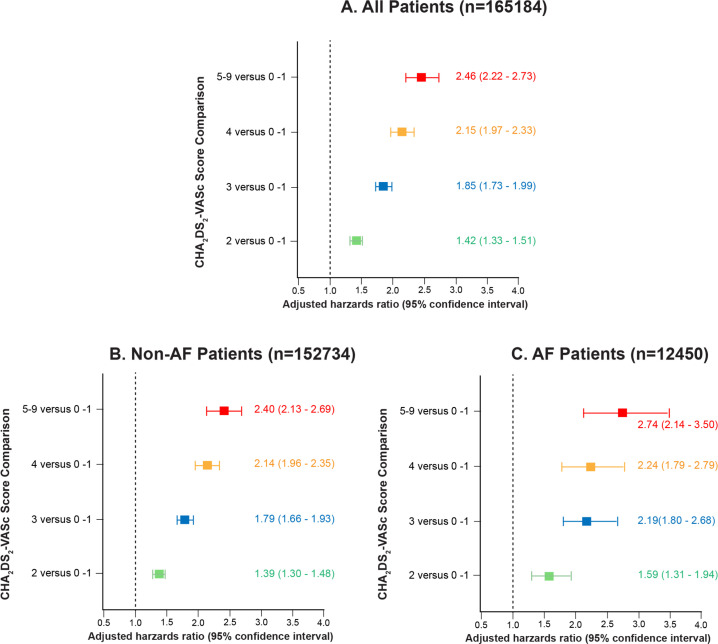
Results of multivariable analysis for all-cause mortality during follow-up are listed in table 2 in all patients, AF patients only and non-AF patients only. Increasing CHA2DS2-VASc score was independently associated with long term mortality in all three groups of patients, with adjusted HR (95% CI) of 1.23 (1.21 to 1.25) for the all patients model. Separate multivariable models in patients from 1991 to 1995, 1996–2000, 2001–2005, 2006–2010 and 2011–2014 showed similar results, including CHA2DS2-VASc score being consistently associated with long-term mortality with HRs 1.17–1.29, p<0.001 for all. AF was also independently associated with long-term mortality, adjusted HR (95% CI) of 1.18 (1.10 to 1.27), as was smoking history and end-stage renal disease. Both anticoagulation and aspirin use were also independently associated with increased long-term mortality for the entire cohort, adjusted HRs (95% CIs) of 1.50 (1.40 to 1.60) and 1.08 (1.03 to 1.13) respectively, while increasing METs was protective, hazards ratio (95% CI) of 0.73 (0.72 to 0.74). Statin use was associated with lower mortality during follow-up, adjusted HRs (95% CIs) 0.73 (0.72 to 0.74). Figure 4 shows the adjusted hazards ratios for mortality during follow-up by CHA2DS2-VASc score category in all patients, non-AF and AF patients. There is a markedly increase in risk of death as CHA2DS2-VASc score increased in adjusted analyses whether AF was present or not.
Table 2.
Multivariable Cox proportional hazards regression analyses for predictors of death in all patients, and patients with and without atrial fibrillation (AF)
| Analysis | All patients (n=165 184) | AF patients (n=12 450) | Non-AF patients (n=152 734) | |||
| Parameters | HR | P value | HR | P value | HR | P value |
| (95% CI) | (95% CI) | (95% CI) | ||||
| CHA2DS2-VASc score | 1.23 (1.21 to 1.25) | <0.001 | 1.21 (1.16 to 1.26) | <0.001 | 1.23 (1.21 to 1.26) | <0.001 |
| Atrial fibrillation | 1.18 (1.10 to 1.27) | <0.001 | N/A | N/A | N/A | N/A |
| Smoking history | 1.65 (1.58 to 1.73) | <0.001 | 1.45 (1.30 to 1.62) | <0.001 | 1.69 (1.61 to 1.77) | <0.001 |
| End-stage renal disease | 2.33 (2.10 to 2.59) | <0.001 | 2.12 (1.71 to 2.64) | <0.001 | 2.43 (2.15 to 2.73) | <0.001 |
| Anticoagulation | 1.50 (1.40 to 1.60) | <0.001 | 1.21 (1.08 to 1.36) | <0.001 | 1.63 (1.51 to 1.77) | <0.001 |
| Aspirin | 1.08 (1.03 to 1.13) | 0.001 | 0.97 (0.86 to 1.09) | 0.569 | 1.08 (1.03 to 1.14) | 0.001 |
| Statin | 0.85 (0.81 to 0.9) | <0.001 | 0.83 (0.74 to 0.94) | 0.003 | 0.86 (0.92 to 0.91) | <0.001 |
| Metabolic equivalents of task | 0.73 (0.72 to 0.74) | <0.001 | 0.74 (0.72 to 0.76) | <0.001 | 0.73 (0.72 to 0.74) | <0.001 |
CHA2DS2-VASc, congestive heart failure, hypertension, age, diabetes mellitus, stroke or transient ischemic attack, vascular disease, sex; N/A, not applicable.
Figure 4.
Forest plot of adjusted HR for mortality during follow-up by CHA2DS2-VASc score (congestive heart failure, hypertension, age, diabetes mellitus, stroke or transient ischemic attack, vascular disease, sex) two or above compared with 0–1 in (A) all patients, (B) patients without atrial fibrillation (AF) and (C) patients with AF.
Discussion
In this prospective cohort of 165 184 adults with a small proportion having AF (7.5%) and on anticoagulation (8.4%) overall, there were important findings: (1) Increasing CHA2DS2-VASc score was associated with higher mortality during follow-up in both univariable and multivariable analysis, and in both AF and non-AF patients, (2) AF itself was also independently associated with worse survival, (3) Furthermore, anticoagulation use in the overall cohort was also associated with mortality during follow-up, and this was true in CHA2DS2-VASc score categories of non-AF patients but only the lower CHA2DS2-VASc score categories 0–3 in AF patients and (4) Other factors adjusting for CHA2DS2-VASc score associated with reduced survival were identified, including smoking history, end-stage renal disease, aspirin use and reduced METs achieved during stress test.
Current guidelines continue to advocate using the CHA2DS2-VASc score to decide on anticoagulation in AF patients for stroke prevention.1–3 Other recent studies have also demonstrated the prognostic value of CHA2DS2-VASc score in various cardiovascular and non-cardiovascular diseases, for clinical outcomes beyond stroke such as death, major adverse cardiovascular events, heart failure hospitalisations and cardiac hospitalisations.4–10 Most of the patients in these studies have established cardiovascular diseases, are hospitalised and/or undergoing cardiovascular procedures, whereas our study differs with having a large proportion of patients who are stable outpatients. Even so, the CHA2DS2-VASc score was an important risk prognosticator in our lower risk population, and this comes as no surprise given that most of the CHA2DS2-VASc components are known cardiovascular risk factors. Importantly, the CHA2DS2-VASc score which was traditionally used for stroke (and to lesser extent mortality) risk stratification and anticoagulation guidance in AF patients was shown in our large ‘all comers’ cohort of stress testing patients to be an elegant tool for long term mortality risk stratification. Furthermore, the prognostic value of CHA2DS2-VASc score was present in both AF and non-AF patients.
Given that the CHA2DS2-VASc score is simple and based solely on clinical history and no laboratory or imaging parameters compared with existing Framingham-based cardiovascular risk calculators, ACC/AHA ASCVD risk estimator or SCORE (all of which require either a blood test, an imaging test, or a geographical European location), CHA2DS2-VASc score may provide a simpler clinical alternative for mortality risk stratification.12–14 One parameter of the CHA2DS2-VASc score that is not a known predictor of mortality is female sex. Indeed, there was a recent change in AF guidelines to using a gender-based threshold, where anticoagulation is not recommended for scores of 0 in men and 0 or 1 in women, can be considered for scores of 1 in men and 2 in women, and recommended with scores of 2 or more in men and 3 or more in women. Removing the female sex parameter may improve the prognostic utility of this score for death as well. CHA2DS2-VASc can be simply imbedded in any EMR to generate a risk for stroke based on published data,1 or total mortality risk in patients with or without AF based on the data from this cohort. We believe that this simple yet informative tool can be used by healthcare providers, administrators and most importantly patients to assess treatment strategies, health economics and healthcare/treatment decision making, respectively.
Another important observation is the association of AF with mortality during follow-up in our population, which reiterates results from prior studies.15–17 AF is well known to be associated with multiple adverse cardiovascular outcomes especially stroke and heart failure, which partly explains the impact on prognosis.2 3 18 19 Other reasons include adverse events of the therapies used to mitigate the consequences of AF such as bleeding risk associated with anticoagulation and antiarrhythmics. The association between AF and mortality in our cohort persisted after adjusting for the clinical CHA2DS2-VASc score and anticoagulation, so other mechanisms are at play. AF ablation has been shown to improve cardiovascular outcomes in selected patients meeting inclusion criteria of recent randomised trials, including as initial therapy or with concurrent heart failure.20–22 Our findings again highlight the importance of optimal management of AF including risk factor modification, medical therapy and interventions where indicated, and ongoing research necessary to further reduce the mortality risk gap between AF and the general population.2 3 Our findings also show that AF is a risk marker associated with other unadjusted co-morbidities (such as obesity, sleep apnoea, sedentary life style, untreated hypertension and valvular disease), and potentially contributes to a higher risk of death rather than just an innocent bystander.
Our findings pertaining to anticoagulation status is also interesting. In patients without AF, those taking anticoagulants have worse survival than those without, and this is related to the other underlying medical conditions warranting anticoagulation such as prosthetic heart valves, venous thromboembolism and thrombophilia. In AF patients, the association between anticoagulation use and death is more complex, whereby anticoagulation use had higher mortality during follow-up in lower CHA2DS2-VASc scores (0–2), and lower mortality CHA2DS2-VASc at higher scores (4 or more). Our findings are despite the well-established role of thromboembolic stroke prevention of anticoagulants in AF compared with placebo or aspirin, and suggest that the absolute risk reduction of stroke is only greater enough to lead to mortality reduction at higher CHA2DS2-VASc score.23–25 Also, other comorbidities and events that are not adjusted for in our multivariable analysis may increase the risk of death in those on anticoagulation whether AF is present or not, such as malignancy and bleeding. Indeed, most anticoagulation trials did not show significant reduction in mortality, except the very original warfarin versus placebo in AF study, along with apixaban versus warfarin.25 26 Aspirin use increasing the risk of death is likely related to both the presence of cardiovascular disease and additional bleeding risk, including when taken together with anticoagulants.27 28 On the other hand, statin use was associated with improved prognosis, likely related to a substantial proportion of patients having elevated risk of or established cardiovascular diseases.12 Other factors identified including end-stage renal failure, smoking history and lower METS at time of test are also known to signal poor survival.11
Study limitations
This study has some expected limitations. It is a single-centre observational registry but reflects real-world experience, data were prospectively collected, and is the largest stress testing registry in the literature. The stress testing cohort is not equivalent to the general healthy population to allow direct generalisability, but is of lower risk than established cardiovascular disease cohorts in the literature using the CHA2DS2-VASc score, and the next step would be testing the score in a general population. The time spanning the study is another limitation given the changes in cardiovascular risk factor burden, definitions of cardiovascular risk factors and diseases, and therapies during the study period. AF diagnosis relied on clinical records without routine monitoring, and AF variables during follow-up such as antiarrhythmic therapy, ablations, and incident AF were not available to us for evaluation. The type of anticoagulation was not available for analysis which have changed substantially over the study period, and their use were based on at the time of stress test, so subsequent use and adherence during follow-up were also not studied. The cause of death data were not available, and other outcomes beyond all-cause mortality such as stroke, myocardial infarction and bleeding were not assessed.
Conclusion
In conclusion, the CHA2DS2-VASc score was independently associated all-cause mortality during follow-up in this large registry of adult patients undergoing stress tests. This was true in both patients with and without AF, and also whether patients were taking anticoagulation medications or not. AF patients also had lower survival than non-AF patients at the same CHA2DS2-VASc score category. Anticoagulation use was associated with lower survival in non-AF patients and AF patients with low CHA2DS2-VASc scores 0–2, but protective in AF patients with high CHA2DS2-VASc scores 4–9. Overall, the CHA2DS2-VASc score can be embedded in EMR as a simple clinical tool to assess long term mortality risk in adults regardless of AF status.
Acknowledgments
Dr Tom Kai Ming Wang is supported by the National Heart Foundation of New Zealand Overseas Clinical and Research Fellowship (grant number 1775).
Footnotes
Twitter: @SergeHarbMD, @TomKMWang
SCH and TKMW contributed equally.
Contributors: SCH, TKMW, DN, PCC and WJ were involved in the planning and conduct of the study, and YW assisted with statistical analyses. SCH, TKMW and WJ were involved in the writing of the manuscript, and all authors were involved in the critical revision of the manuscript and approved the submission. WJ is responsible for the overall study content as guarantor.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Institutional review board approval was obtained prior to the commencement of the study (IRB 15–596), and patient consent was waived.
References
- 1.Lip GYH, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro heart survey on atrial fibrillation. Chest 2010;137:263–72. 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American heart association Task force on clinical practice guidelines and the heart rhythm Society. J Am Coll Cardiol 2019;74:104–32. 10.1016/j.jacc.2019.01.011 [DOI] [PubMed] [Google Scholar]
- 3.Hindricks G, Potpara T, Dagres N. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for Cardio-Thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J 2020;42:373–498. 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 4.Shuvy M, Zwas DR, Keren A, et al. Value of the CHA2 DS2 -VASc score for predicting outcome in patients with heart failure. ESC Heart Fail 2020;7:2553–60. 10.1002/ehf2.12831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borovac JA, Kwok CS, Mohamed MO, et al. The Predictive Value of CHA2DS2-VASc Score on In-Hospital Death and Adverse Periprocedural Events Among Patients With the Acute Coronary Syndrome and Atrial Fibrillation Who Undergo Percutaneous Coronary Intervention: A 10-Year National Inpatient Sample (NIS) Analysis. Cardiovasc Revasc Med 2021;29:61–8. 10.1016/j.carrev.2020.08.003 [DOI] [PubMed] [Google Scholar]
- 6.Mazzone C, Cioffi G, Carriere C, et al. Predictive role of CHA2DS2-VASc score for cardiovascular events and death in patients with arterial hypertension and stable sinus rhythm. Eur J Prev Cardiol 2017;24:1584–93. 10.1177/2047487317726068 [DOI] [PubMed] [Google Scholar]
- 7.Cerşit S, Öcal L, Keskin M, et al. Usefulness of CHA2DS2-VASc Score to predict clinical outcomes of patients undergoing carotid artery stenting. Int J Cardiovasc Imaging 2021;37:783–9. 10.1007/s10554-020-02078-y [DOI] [PubMed] [Google Scholar]
- 8.Yalim Z, Aldemir M, Yalim SA. Assessment of the relationship between death and CHA2DS2-VASc score in peripheral artery disease. Int Angiol 2020;39:509–16. 10.23736/S0392-9590.20.04498-3 [DOI] [PubMed] [Google Scholar]
- 9.Ooi H, Chen L-H, Ni Y-L, et al. CHA2DS2-VASc scores predict major adverse cardiovascular events in patients with chronic obstructive pulmonary disease. Clin Respir J 2018;12:1038–45. 10.1111/crj.12624 [DOI] [PubMed] [Google Scholar]
- 10.Cetinkal G, Kocas BB, Ser OS, et al. Assessment of the modified CHA2DS2VASc risk score in predicting mortality in patients hospitalized with COVID-19. Am J Cardiol 2020;135:143–9. 10.1016/j.amjcard.2020.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mandsager K, Harb S, Cremer P, et al. Association of cardiorespiratory fitness with long-term mortality among adults undergoing exercise treadmill testing. JAMA Netw Open 2018;1:e183605. 10.1001/jamanetworkopen.2018.3605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnett DK, Blumenthal RS, Albert MA. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 20192019;74:e177–232. 10.1016/j.jacc.2019.03.010 [DOI] [Google Scholar]
- 13.D'Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham heart study. Circulation 2008;117:743–53. 10.1161/CIRCULATIONAHA.107.699579 [DOI] [PubMed] [Google Scholar]
- 14.Piepoli MF, Hoes AW, et al. , Authors/Task Force Members . 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur J Prev Cardiol 2016;23:Np1–96. 10.1177/2047487316653709 [DOI] [PubMed] [Google Scholar]
- 15.Andersson T, Magnuson A, Bryngelsson I-L, et al. All-Cause mortality in 272,186 patients hospitalized with incident atrial fibrillation 1995-2008: a Swedish nationwide long-term case-control study. Eur Heart J 2013;34:1061–7. 10.1093/eurheartj/ehs469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benjamin EJ, Wolf PA, D'Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham heart study. Circulation 1998;98:946–52. 10.1161/01.cir.98.10.946 [DOI] [PubMed] [Google Scholar]
- 17.Wang TJ, Massaro JM, Levy D, et al. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham heart study. JAMA 2003;290:1049–56. 10.1001/jama.290.8.1049 [DOI] [PubMed] [Google Scholar]
- 18.Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke Statistics-2019 update: a report from the American heart association. Circulation 2019;139:e56–28. 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 19.Kotecha D, Lam CSP, Van Veldhuisen DJ, et al. Heart Failure With Preserved Ejection Fraction and Atrial Fibrillation: Vicious Twins. J Am Coll Cardiol 2016;68:2217–28. 10.1016/j.jacc.2016.08.048 [DOI] [PubMed] [Google Scholar]
- 20.Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–16. 10.1056/NEJMoa2019422 [DOI] [PubMed] [Google Scholar]
- 21.Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med 2021;384:316–24. 10.1056/NEJMoa2029554 [DOI] [PubMed] [Google Scholar]
- 22.Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–27. 10.1056/NEJMoa1707855 [DOI] [PubMed] [Google Scholar]
- 23.Ezekowitz MD, Bridgers SL, James KE, et al. Warfarin in the prevention of stroke associated with nonrheumatic atrial fibrillation. Veterans Affairs stroke prevention in nonrheumatic atrial fibrillation Investigators. N Engl J Med 1992;327:1406–12. 10.1056/NEJM199211123272002 [DOI] [PubMed] [Google Scholar]
- 24.Rockson SG, Albers GW. Comparing the guidelines: anticoagulation therapy to optimize stroke prevention in patients with atrial fibrillation. J Am Coll Cardiol 2004;43:929–35. 10.1016/j.jacc.2003.11.028 [DOI] [PubMed] [Google Scholar]
- 25., Singer DE, Hughes RA, et al. , Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators . The effect of low-dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation. N Engl J Med 1990;323:1505–11. 10.1056/NEJM199011293232201 [DOI] [PubMed] [Google Scholar]
- 26.Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–92. 10.1056/NEJMoa1107039 [DOI] [PubMed] [Google Scholar]
- 27.Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med 2017;377:1513–24. 10.1056/NEJMoa1708454 [DOI] [PubMed] [Google Scholar]
- 28.Dewilde WJM, Oirbans T, Verheugt FWA, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013;381:1107–15. 10.1016/S0140-6736(12)62177-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.