Key Points
Question
What is the seroprevalence of SARS-CoV-2 antibodies in a convenience sample of children aged 2 to 17 years in Montreal, Canada, enrolled between October 2020 and March 2021?
Findings
In this cohort study of 1632 participants, the mean baseline seroprevalence of SARS-CoV-2 was 5.8%. Of the 95 participants who were seropositive for SARS-CoV-2 antibodies, 82% were not tested or tested negative, and all experienced either mild or no clinical symptoms.
Meaning
The findings suggest that there was more transmission occurring in children compared with what was being detected, although children experienced few or mild symptoms.
This cohort study estimates the seroprevalence of SARS-CoV-2 antibodies in children and teenagers in 4 neighborhoods of Montreal, Canada.
Abstract
Importance
Quebec prioritized in-person learning after the first wave of the COVID-19 pandemic, with school closures being implemented temporarily in selected schools or in hot-spot areas. Quebec’s decision to keep most schools open was controversial, especially in Montreal, which was the epicenter of Canada’s first and second waves; therefore, understanding the extent to which children were infected with SARS-CoV-2 provides important information for decisions about school closures.
Objective
To estimate the seroprevalence of SARS-CoV-2 antibodies in children and teenagers in 4 neighborhoods of Montreal, Canada.
Design, Setting, and Participants
This cohort study (the Enfants et COVID-19: Étude de séroprévalence [EnCORE] study) enrolled a convenience sample of children aged 2 to 17 years between October 22, 2020, and March 22, 2021, in Montreal, Canada.
Exposures
Potential exposure to SARS-CoV-2.
Main Outcomes and Measures
The main outcome was seroprevalence of SARS-CoV-2 antibodies, collected using dried blood spots (DBSs) and analyzed with a research-based enzyme-linked immunosorbent assay (ELISA). Parents also completed an online questionnaire that included questions on self-reported COVID-19 symptoms and tests, along with sociodemographic questions.
Results
This study included 1632 participants who provided a DBS sample from 30 day cares, 22 primary schools, and 11 secondary schools. The mean (SD) age of the children who provided a DBS sample was 9.0 (4.4) years; 801 (49%) were female individuals, with 354 participants (22%) from day cares, 725 (44%) from primary schools, and 553 (34%) from secondary schools. Most parents had at least a bachelor’s degree (1228 [75%]), and 210 (13%) self-identified as being a racial or ethnic minority. The mean seroprevalence was 5.8% (95% CI, 4.6%-7.0%) but increased over time from 3.2% (95% CI, 0.7%-5.8%) in October to November 2020 to 8.4% (95% CI, 4.4%-12.4%) in March to April 2021. Of the 95 children with positive SARS-CoV-2 antibody results, 78 (82%) were not tested or tested negative with reverse transcription–polymerase chain reaction (RT-PCR) testing, and all experienced mild (49 [52%]) or no clinical symptoms (46 [48%]). The children of parents who self-identified as belonging to a racial and ethnic minority group were more likely to be seropositive compared with children of White parents (adjusted seroprevalence ratio, 1.9; 95% CI, 1.1-2.6).
Conclusions and Relevance
These results provide a benchmark of the seroprevalence status in Canadian children. The findings suggest that there was more transmission occurring in children compared with what was being detected by RT-PCR, although children experienced few or mild symptoms. It will be important to continue monitoring the serological status of children, particularly in the context of new COVID-19 variants of concern and in the absence of mass vaccination campaigns targeting young children.
Introduction
Children and adolescents are susceptible to infection with SARS-CoV-2 but experience much lower rates of severe COVID-19 disease than adults.1 Many children experience no or mild symptoms of infection, and evidence prior to the emergence of variants of concern (VOC) suggests young children probably played a smaller role in transmission than adults,2,3 while older children and adolescents may have transmission rates more similar to adults.3 The role children play in transmission of the virus has been the topic of much debate, especially with respect to the safety of in-person schooling.4
Through the first year of the SARS-CoV-2 pandemic, hundreds of millions of students around the world were affected by the closures of schools and day cares.5 Some countries have kept schools mostly open (eg, Sweden, Taiwan), while others have opted for extended closures (eg, US, India). In Canada, schools were mostly closed during the first few months of the pandemic (March to June 2020), but have opened for in-person learning on a province-by-province basis during the September 2020 to June 2021 school year. Despite having one of the highest provincial incidence and mortality rates, Quebec has prioritized in-person learning, with school closures being implemented temporarily in selected schools or in hot-spot areas such as Quebec City.6 Quebec’s decision to keep most schools open has remained controversial, especially in Montreal, which was the epicenter of Canada’s first and second waves.7,8
Longitudinal seroprevalence studies conducted in schools and day cares are beginning to advance our knowledge of SARS-CoV-2 transmission among children, staff, and families in educational settings. For example, studies conducted in French9 and British primary schools and French day cares10 suggested little evidence of transmission from children to their peers or teachers, and a large Swiss cohort11 found minimal clustering of seropositive cases within classes and schools, despite a clear increase in seroprevalence in children over a 5-month period of very high transmission. Determinants of SARS-CoV-2 seropositivity among children have included contact with a confirmed COVID-19 case,10,12 health care workers in the household,12 belonging to a minority racial or ethnic group, and geographical area.11 One year into the pandemic, little is known regarding the seroprevalence of SARS-CoV-2 infection and its determinants among children and adolescents in Canada.
This article reports baseline seroprevalence results from wave 1 (October 2020 to April 2021) of the Enfants et COVID-19: Étude de séroprévalence (EnCORE13) study, a longitudinal cohort study in primary and secondary schools and day cares in Montreal. In addition, we examined associations between seroprevalence and sociodemographic and household characteristics, reported COVID-19 symptoms and tests, and potential COVID-19 risk factors and prevention efforts.
Methods
Study Design and Conduct
This study is a cohort study based on 4 sentinel neighborhoods from the 34 boroughs and independent municipalities that make up the island of Montreal (population, 1.9 million people14). These neighborhoods were selected to reflect diversity in terms of geography, cumulative COVID-19 cases, and neighborhood socioeconomic status.15 The populations living in the West Island and Plateau-Mont-Royal are more affluent and highly educated, while Montreal North is one of the city’s poorest and most racially diverse neighborhoods, and Mercier-Hochelaga-Maisonneuve is a working-class neighborhood with nearly one-third of the population living below the poverty line.16 Schools and day cares within each neighborhood were selected by the research team and school boards to participate in this study. The study team provided a letter of invitation for parents that was sent either by the school board or by the participating school or day care. The letter of invitation was emailed a maximum of 3 times to parents.
Ethics approval was received from the research ethics boards of the Université de Montréal and the Centre Hospitalier Universitaire Sainte-Justine. Details of the full cohort study procedures are available in the study protocol.15 This study followed the reporting requirements of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.17
Eligibility Criteria and Study Procedure
Participants were eligible for enrollment if they attended 1 of the participating schools or day cares, if they were between the ages of 2 and 17 years, and if their parent or legal guardian gave informed consent to participate and the child gave their assent. Multiple children per household were eligible, as long as they all attended a participating school or day care. Parental consent and their child’s assent were provided online, which was followed by an online questionnaire. Once the baseline questionnaire was complete, an at-home dried blood spot (DBS) collection kit was mailed to their residence.
The questionnaire collected the following information from parents: dates and results of prior reverse transcriptase–polymerase chain reaction (RT-PCR) tests and the presence of SARS-CoV-2 symptoms and the time of RT-PCR tests; dates of hospitalizations and COVID-19 vaccinations for the participant and household members; basic sociodemographic and household characteristics; preventive behaviors in the household and at school and/or day care; and health and lifestyle information for the child. Presently, there are 2 waves of data collection planned for spring and fall 2021.
Serological Samples and Laboratory Analysis
Specimen collection kits for finger prick whole blood samples were sent to the participant’s household 1 day after completion of the online questionnaire. Kits contained printed directions for collection, storage, and mailing for each specimen. Participants also had access to tutorial videos explaining each procedure step-by-step, and video conference support from the research team was available on request. Parents used contact-activated retractable lancets (BD Microtainer) and a Whatman 903 protein saver card for their child’s DBS collection. They were asked to dry the samples for at least 2 hours and mail them in a protective envelope with desiccant within 24 hours of collection. On reception in the laboratory, the samples were analyzed for quality control purposes, such as sufficient quantity of blood or layering of blood drops. For the samples that did not pass the quality control, participants and their parents were asked to redo the sample collection. The filter papers with DBS were conserved at −20 °C, and blood was eluted overnight the day before the serological assay. Samples were processed in 96-well plates with 40 samples from participants and 7 control samples (positive and negative) per plate.
The serostatus of participants was determined by an enzyme-linked immunosorbent assay (ELISA) that we developed and validated using the receptor-binding domain (RBD) from the spike protein as an antigen. Prior to beginning the study, we successfully validated the ELISA assay with positive control samples (participants with RT-PCR–confirmed SARS-CoV-2 infection and known to be seropositive for anti–SARS-CoV-2 antibodies) and negative control samples (SARS-CoV-2 seronegative). Based on the results, the assay had a sensitivity of 95% and specificity of 100%. A colorimetric reaction determined by optic density (OD) allowed the detection of IgG in the samples and the evaluation of the signal generated by SARS-CoV-2–specific antibodies against the RBD antigen confirmed whether subjects were seropositive. The OD cutoff for positivity was determined based on the average of OD from negative sera plus 3 SDs. Three samples with an indeterminate result (defined as a good quality sample near the threshold for positive/negative) and 33 with a borderline negative result (defined as a poor quality sample analyzed and found to be negative) were classified as seronegative for the primary analysis. Parents were sent an email that provided their child’s serology result and information about what the result means.
Statistical Analysis
We calculated that a sample size of 457 children per neighborhood (total of 1828 children) would enable estimation of neighborhood-specific seroprevalence with 2% precision.18 This was based on a projected seroprevalence of 5%.
The main outcome was seroprevalence, and we calculated descriptive statistics and univariate associations of seroprevalence with sociodemographic and household characteristics, self-reported COVID-19 symptoms and potential COVID-19 risk factors, and prevention efforts. Previous COVID-19 diagnoses via RT-PCR were self-reported by parents. Higher risk conditions were self-reported and included, eg, diabetes, immunocompromised, serious heart conditions, chronic obstructive pulmonary disease, kidney disease, and cancer. Based on Canadian Census categorization, parents were asked whether they self-identify as Arab, Black, Chinese, Filipino, Japanese, Korean, Latin American, South Asian, Southeast Asian, West Asian, and/or White. Due to small numbers within specific racial and ethnic groups, we generated a binary variable (racial or ethnic minority group vs White). Body mass index (BMI) categories were calculated using Stata’s zbmicat function,19 which uses BMI cutoffs recommended by the Childhood Obesity Working Group of the International Obesity Taskforce. Other essential occupations included day care educator or worker, corrections or prison officer, teacher or other school staff, first responder, public transportation driver, food service industry, grocery store staff, pharmacy staff, hairdresser or barber, aesthetician, flight attendant, and factory worker. Adjusted seroprevalence differences and ratios by setting of attendance (ie, day care, primary school, secondary school), parental education level, and parental race and ethnicity were estimated by calculating average marginal effects from multivariable logistic regression models, which adjusted for age, sex, neighborhood, and timing of the DBS.
Sensitivity analyses were performed to generate seroprevalence estimates under scenarios in which (1) indeterminate samples were categorized as positive and (2) all indeterminate and borderline negative results were excluded. All analyses used robust standard errors to account for clustering by school and were conducted using Stata version 14 (StataCorp). Statistical significance was set at P < .05, and all tests were 2-tailed.
Results
Descriptive Characteristics
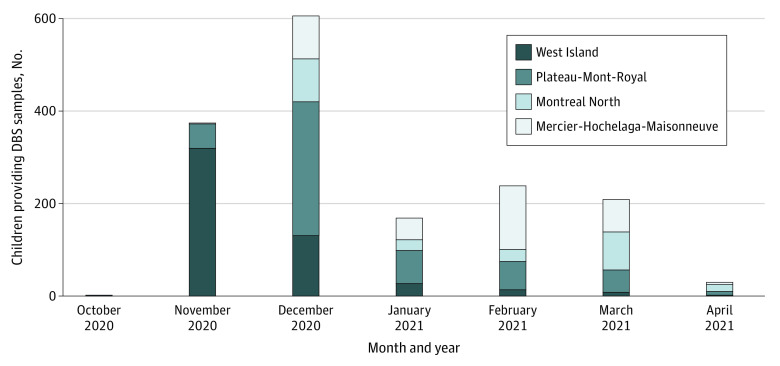
Recruitment followed the approvals of the ethics committees and the school boards; it began in West Island (and surrounding area) on October 16, 2020, followed by Plateau, Mercier-Hochelaga-Maisonneuve, and Montreal North (Figure 1). By the end of the recruitment (March 14, 2021), 30 day cares, 22 primary schools, and 11 secondary schools were participating in the study. The median sizes of the daycares, primary schools, and secondary schools were 90 (range, 46-219), 341 (range, 101-644), and 795 (range, 319-2193) students, respectively. There was a total of 21 564 children who attended the participating day cares or schools, with 20 928 aged between 2 and 17 years who were eligible for inclusion. 1901 children (9%) from 1444 households enrolled in the study and completed the questionnaire. Of these participants, 1632 (86%) provided a DBS sample that was of sufficient quality for the serological analysis. Participation rates varied substantially among the different day care centers and schools (median [IQR], 10% [5%-20%]). Participation rates were lower among parents of secondary school children (7%) compared with day care (14%) and primary school (14%) children. Primary and secondary schools that ranked in the upper half of the school-based socioeconomic distribution also had higher participation rates (24%) vs those in the lower half (5%).
Figure 1. Monthly Participant Recruitment by Neighborhood.
DBS indicates dried blood spot.
The mean (SD) age of the children who provided a DBS sample was 9.0 (4.4) years, and 801 (49%) were female individuals, with 354 participants (22%) from day cares, 724 (44%) from primary schools, and 553 (34%) from secondary schools. The planned sample size of 457 was attained for the 2 more affluent neighborhoods (West Island, 504 participants; Plateau-Mont-Royal, 533 participants), but not for Montreal North (237 participants) or Mercier-Hochelaga-Maisonneuve (357 participants). Most parents had at least a bachelor’s degree (1228 [75%]), and 210 (13%) self-identified as belonging to a racial or ethnic minority groups. On average, there were 2 adults and 2 children in 3 to 4–bedroom households. Among the RT-PCR test results reported by participants, 26 were positive prior to DBS collection, and the only significantly different symptom, compared with RT-PCR negative tests (881 participants), was loss of smell (eFigure 1 in the Supplement). All 95 participants with seropositive results either experienced no symptoms (46 [48%]) or mild symptoms (49 [52%]). The median (IQR) time between an RT-PCR positive test result and the DBS collection date was 109 (66-146) days. Two children were hospitalized with or due to COVID-19.
Seropositivity of SARS-CoV-2
We identified 95 seropositive children out of a total of 1632 children tested, resulting in estimated crude seroprevalence of 5.8% (95% CI, 4.6%-7.0%). This estimate included 36 borderline negative or indeterminate samples that were classified as seronegative. If we excluded these 36 results, the estimated seroprevalence was 6.0% (95% CI, 4.7%-7.2%), and if we assumed the 3 indeterminate samples were instead seropositive, the estimate was 6.1% (95% CI, 4.8%-7.4%).
The seropositive children were from 12 day cares, 15 elementary schools, and 10 secondary schools. Individual and household characteristics associated with seroprevalence are summarized in Table 1. The largest difference in seroprevalence between participants was having had a positive RT-PCR test (prevalence difference, 59.9 percentage points; 95% CI, 39.6-80.2 percentage points) and someone in the household who had a positive RT-PCR test (prevalence difference, 27.4 percentage points; 95% CI, 15.2-39.6 percentage points). Other factors significantly associated with seroprevalence included parental race and ethnicity and neighborhood. The seroprevalence difference for children was 5.8 percentage points (95% CI, 1.3-10.2 percentage points) for children whose parents belonged to racial and ethnic minority groups compared with White parents.
Table 1. Crude Seroprevalence and Seroprevalence Differences and Ratios for Children Aged 2 to 17 Years by Individual and Household Characteristics, October 2020 to March 2021 in Montreal, Canada.
| Demographic characteristics | Children, No./total No. (%) | Seroprevalence, % (95% CI) | Crude prevalence ratio (95% CI) | |
|---|---|---|---|---|
| Seronegative | Seropositive | |||
| Total | 1537 | 95 | 5.8 (4.6 to 7.0) | NA |
| Sex | ||||
| Female | 746/1537 (49) | 55/95 (58) | 6.9 (4.9 to 8.8) | 1 [Reference] |
| Male | 797/1537 (51) | 40/95 (42) | 4.8 (3.2 to 6.4) | 0.7 (0.4 to 1.0) |
| Age, y | ||||
| 2-4 | 316/1529 (21) | 16/95 (17) | 4.8 (2.9 to 6.7) | 1 [Reference] |
| 5-9 | 515/1529 (34) | 27/95 (28) | 5.0 (3.4 to 6.6) | 1.0 (0.5 to 1.5) |
| 10-14 | 493/1529 (32) | 36/95 (38) | 6.8 (4.2 to 9.4) | 1.4 (0.6 to 2.2) |
| 15-17 | 205/1529 (13) | 16/95 (17) | 7.2 (3.4 to 11.0) | 1.5 (0.5 to 2.5) |
| Child attending | ||||
| Day care | 335/1537 (22) | 19/95 (20) | 5.4 (3.3 to 7.4) | 1 [Reference] |
| Primary school | 686/1537 (45) | 39/95 (41) | 5.4 (4.1 to 6.6) | 1.0 (0.6 to 1.5) |
| Secondary school | 516/1537 (34) | 37/95(39) | 6.7 (3.8 to 9.6) | 1.3 (0.5 to 2.0) |
| BMI | ||||
| Underweight or normal weight | 1208/1520 (79) | 80/95 (84) | 6.2 (4.9 to 7.5) | 1 [Reference] |
| Overweight or obesity | 312/1520 (21) | 15/95 (16) | 4.6 (2.3 to 6.9) | 0.7 (0.4 to 1.1) |
| Born premature | ||||
| No | 1447/1520 (95) | 87/93 (94) | 5.7 (4.5 to 6.9) | 1 [Reference] |
| Yes (<37 wk) | 73/1520 (5) | 6/93 (6) | 7.6 (1.9 to 13.3) | 1.3 (0.3 to 2.4) |
| Chronic medical conditions | ||||
| None | 1427/1525 (94) | 90/95 (95) | 5.9 (4.7 to 7.2) | 1 [Reference] |
| Asthma | 48/1525 (3) | 4/95 (4) | 7.4 (0.5 to 14.3) | 1.3 (0.1 to 2.4) |
| Other conditions | 50/1525 (3) | 1/95 (1) | 2.0 (−2.0 to 6.1) | 0.3 (−0.4 to 1.0) |
| Neighborhood | ||||
| West Island | 487/1537 (32) | 17/95 (18) | 3.4 (2.4 to 4.3) | 1 [Reference] |
| Mercier-Hochelaga-Maisonneuve | 329/1537 (21) | 28/95 (29) | 7.8 (5.2 to 10.5) | 2.3 (1.3 to 3.4) |
| Montreal North | 215/1537 (14) | 22/95 (23) | 9.3 (6.4 to 12.2) | 2.8 (1.6 to 3.9) |
| Plateau-Mont-Royal | 506/1537 (33) | 28/95 (29) | 5.3 (3.5 to 7.0) | 1.6 (0.9 to 2.3) |
| Parental respondent’s level of education | ||||
| <Bachelor’s degree | 366/1517 (24) | 18/95 (19) | 4.7 (2.6 to 6.8) | 1 [Reference] |
| Bachelor’s degree | 606/1517 (40) | 41/95 (43) | 6.3 (4.5 to 8.2) | 1.4 (0.8 to 2.0) |
| ≥Master’s degree | 545/1517 (36) | 36/95 (38) | 6.2 (4.2 to 8.2) | 1.3 (0.6 to 2.1) |
| Parental respondent’s race and ethnicity | ||||
| Racial or ethnic minority | 179/1512 (12) | 22/95 (23) | 11.0 (6.9 to 15.1) | 2.1 (1.1 to 3.1) |
| White | 1333/1512 (88) | 73/95 (77) | 5.2 (3.9 to 6.4) | 1 [Reference] |
| Adults in household, No. | ||||
| 1 | 182/1526 (12) | 10/95 (11) | 5.2 (1.8 to 8.6) | 1 [Reference] |
| 2 | 1188/1526 (78) | 70/95 (74) | 5.6 (4.2 to 6.9) | 1.1 (0.3 to 1.8) |
| ≥3 | 156/1526 (10) | 15/95 (16) | 8.8 (4.7 to 12.9) | 1.7 (0.4 to 3.0) |
| Children in household, No. | ||||
| 1 | 326/1526 (21) | 24/95 (25) | 6.9 (3.7 to 10.1) | 1 [Reference] |
| 2 | 834/1526 (55) | 44/95 (46) | 5.0 (3.6 to 6.5) | 0.7 (0.3 to 1.1) |
| ≥3 | 366/1526 (24) | 27/95 (28) | 6.9 (4.3 to 9.4) | 1.0 (0.4 to 1.6) |
| Bedrooms in household, No. | ||||
| 1-2 | 314/1526 (21) | 18/95 (19) | 5.4 (3.2 to 7.7) | 1 [Reference] |
| 3-4 | 1112/1526 (73) | 73/95 (77) | 6.2 (4.8 to 7.5) | 1.1 (0.6 to 1.6) |
| ≥5 | 100/1526 (7) | 4/95 (4) | 3.8 (0 to 7.9) | 0.7 (−0.1 to 1.5) |
| Any household member working in | ||||
| Other occupations | 869/1526 (57) | 44/95 (46) | 4.8 (3.5 to 6.2) | 1 [Reference] |
| Essential occupation other than health care | 132/1526 (9) | 17/95 (18) | 6.8 (3.8 to 9.8) | 1.4 (0.7 to 2.2) |
| Health care | 525/1526 (34) | 34/95 (36) | 7.9 (5.0 to 10.7) | 1.6 (0.8 to 2.4) |
| Household member has underlying health issues that may put them at higher risk if exposed to COVID-19 | ||||
| No | 1275/1526 (84) | 83/95 (87) | 6.2 (4.9 to 7.5) | 1 [Reference] |
| Yes | 251/1526 (16) | 12/95 (13) | 4.6 (1.3 to 7.9) | 0.7 (0.2 to 1.3) |
| Child had positive COVID-19 diagnosis via RT-PCR prior to serology testinga | ||||
| No | ||||
| Child was not tested | 829/1535 (54) | 48/95 (51) | 5.5 (4.2 to 6.7) | 1 [Reference] |
| All tests negative | 697/1535 (45) | 30/95 (32) | 4.1 (2.7 to 5.5) | 0.8 (0.4 to 1.1) |
| Yes, at least 1 positive test | 9/1535 (1) | 17/95 (18) | 65.4 (44.9 to 85.9) | 11.9 (7.7 to 16.2) |
| Household member other than child had confirmed COVID-19 infection via RT-PCR | ||||
| No | 1475/1526 (97) | 71/95 (75) | 4.6 (3.6 to 5.6) | 1 [Reference] |
| Yes | 51/1526 (3) | 24/95 (25) | 32.0 (19.9 to 44.1) | 7.0 (3.9 to 10.0) |
Abbreviations: BMI, body mass index; NA, not applicable; RT-PCR, reverse transcriptase–polymerase chain reaction.
Median time between RT-PCR positive test and serology test was 109 days (IQR, 66-146 days).
Table 2 shows adjusted seroprevalence differences and ratios for type of establishment, household occupations, parental race and ethnicity, and neighborhood. With adjustment for sex, age, and timing of DBS, there were no longer significant differences in seroprevalence rates by neighborhood. The differences in seroprevalence between day care, primary school, and secondary school aged children remained insignificant, with secondary school aged children having the highest seroprevalence at 6.7% (95% CI, 4.6%-8.8%). Having a household member working in health care was consistent with increased seroprevalence (adjusted seroprevalence ratio, 1.5%; 95% CI, 0.8%-2.3%). The children of parents from racial and ethnic minority groups were more likely to be seropositive compared with children of White parents (adjusted prevalence ratio, 1.9%; 95% CI, 1.1%-2.8%).
Table 2. Adjusted Seroprevalence Differences and Ratios by Type of Establishment, Parental Education, and Parental Race and Ethnicity, October 2020 to March 2021 in Montreal, Canada.
| Category | Adjusted seroprevalence (95% CI) | Adjusted seroprevalence | |
|---|---|---|---|
| Difference (95% CI) | Ratio (95% CI) | ||
| Neighborhooda | |||
| West Island | 4.8 (2.8 to 6.8) | 0 [Reference] | 1 [Reference] |
| Mercier-Hochelaga-Maisonneuve | 6.2 (3.8 to 8.6) | 1.3 (−2.3 to 5.2) | 1.3 (0.4 to 2.2) |
| Montreal North | 7.3 (4.5 to 10.1) | 2.5 (−1.5 to 6.5) | 1.5 (0.5 to 2.5) |
| Plateau-Mont-Royal | 5.4 (3.2 to 7.6) | 0.6 (−2.3 to 3.6) | 1.1 (0.5 to 1.8) |
| Child attendingb | |||
| Day care | 5.0 (3.0 to 7.0) | 0 [Reference] | 1 [Reference] |
| Primary school | 5.6 (4.3 to 6.9) | 0.6 (−1.9 to 3.1) | 1.1 (0.6 to 1.7) |
| Secondary school | 6.7 (4.6 to 8.8) | 1.7 (−1.4 to 4.7) | 1.3 (0.6 to 2.1) |
| Any household member working inc | |||
| Other occupations | 4.9 (3.5 to 6.4) | 0 [Reference] | 1 [Reference] |
| Essential occupation other than health care | 6.7 (4.3 to 9.1) | 1.7 (−1.3 to 4.8) | 1.4 (0.7 to 2.0) |
| Health care | 7.5 (4.8 to 10.4) | 2.6 (−0.5 to 5.8) | 1.5 (0.8 to 2.3) |
| Parental respondent’s race and ethnicityc | |||
| Racial or ethnic minority group | 10.2 (6.5 to 13.9) | 4.9 (1.0 to 8.8) | 1.9 (1.1 to 2.8) |
| White | 5.3 (4.2 to 6.3) | 0 [Reference] | 1 [Reference] |
Adjusted for age, sex, and date of dried blood spot.
Adjusted for sex, neighborhood, and date of dried blood spot.
Adjusted for age, sex, neighborhood, and date of dried blood spot.
Our study population was more educated, with fewer participants from racial and ethnic minority groups compared with neighborhood characteristics of adults from the 2016 census. When standardizing our seroprevalence estimate of 5.8% (95% CI, 4.6%-7.0%) to the race and ethnicity distribution of the 2016 census, it increased slightly to 6.2% (95% CI, 5.3%-7.1%).
Seroprevalence estimates by month of DBS collection (presented alongside confirmed daily COVID-19 case numbers for the province of Quebec) suggest an increasing trend over time, from 3.2% (95% CI, 0.7%-5.8%) in October to November 2020 to 8.4% (95% CI, 4.4%-12.4%) in March to April 2021 (Figure 2). These estimates are adjusted for age, sex, and neighborhood (to account for differential timing of recruitment across the neighborhoods). Finally, crude seroprevalence rates were calculated for different prevention practices that parents reported for behaviors at the household level. No practices were significantly associated with the serological status of children (eFigure 2 in the Supplement).
Figure 2. Estimated Seroprevalence for Participants Compared With Overall Daily Confirmed COVID-19 Cases in Quebec.
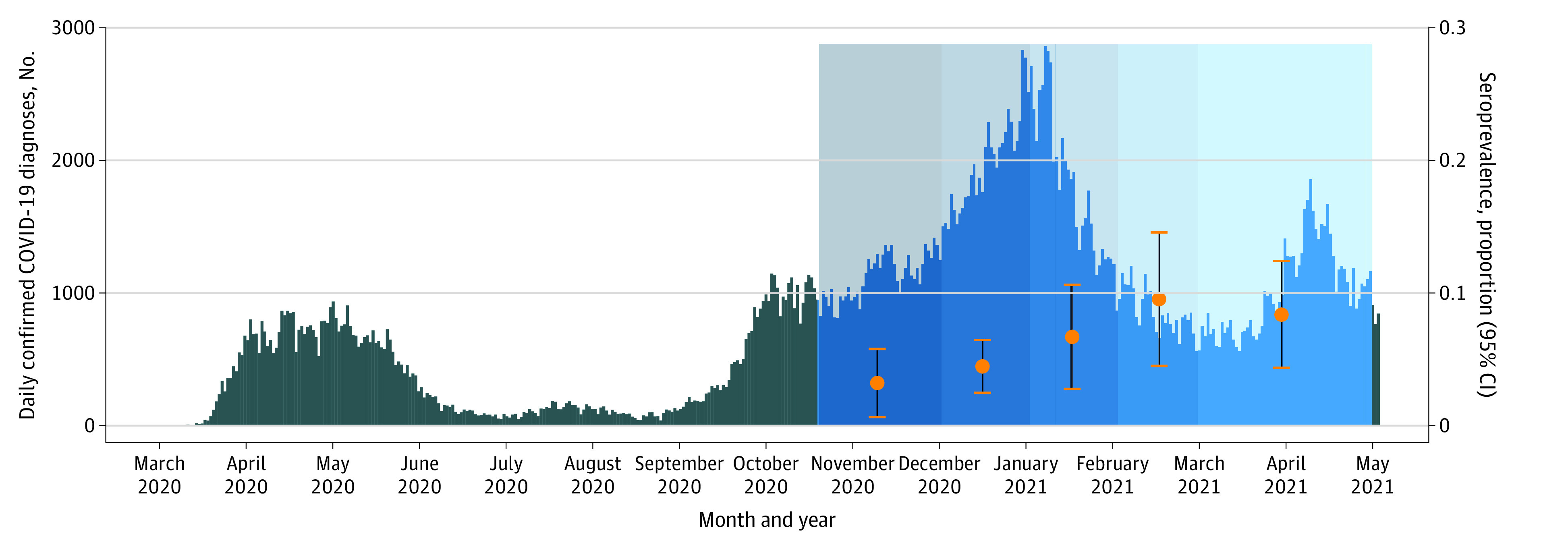
The dots and whiskers indicate seroprevalence estimates and 95% CIs for the study population for different months during the first wave of data collection. The shaded area marks the study period, with the gradient indicating different months.
Discussion
The baseline results of our seroprevalence study provide important insight into the proportion of children who have detectable levels of SARS-CoV-2 antibodies from one of Canada’s most affected cities during the first (February to August 2020) and second (September 2020 to March 2021) waves of the COVID-19 pandemic.6 A particular strength of our study is the selection of schools and day cares that represent socioeconomically and ethnically diverse populations that will be observed over time.
The increase in seroprevalence over time was consistent with the confirmed case trend in Quebec, with school-aged children younger than 18 years contributing 19% to the total incidence in the second wave, compared with only 6% in the first wave.20,21 Our estimated seroprevalence among children was comparable with seroprevalence among Montreal’s adult population for the first wave (3.2% vs 3.1%) but was lower for the second wave (8.4% vs 13.8%).22 In other parts of Canada, seroprevalence estimates for children from the first and second waves have been considerably lower, ranging from 0.3% to 1.6%.23,24 Internationally, seroprevalence estimates for children and youth from the first and second waves are quite varied, ranging from 0.6% in Germany,2 4.3% in France,10 2.4% to 22.3% in Switzerland,3,11 and 2.4% to 11.2% in the UK,25,26 although the trend of increasing seroprevalence over time was consistently found in the cohort studies.11,26,27
In our study, seropositivity was associated with racial and ethnic minority status, which is consistent with the results of other seroprevalence studies26,28,29 and the disproportionate impact COVID-19 has had on racial and ethnic minority communities.30,31 We did not find any significant differences between the age groups nor did we find any significant associations with the risk of seropositivity and sociodemographic characteristics, such as sex and parent’s educational level, which is similar to other studies.2,25,32 We found that only 18% of our seropositive participants had previously received positive results from RT-PCR testing. This finding further confirms the value of serology as a tool for SARS-CoV-2 surveillance and, although RT-PCR and antigen tests remain essential for acute infection detection, having a more accurate understanding of previous infections allows us to better understand and manage community transmission.33
Limitations
Our study had some important limitations, including the potential for selection bias in our results. The seroprevalence estimates could be an overestimation if the participating children were more likely to have COVID-19 compared with nonparticipating children. For example, households that believed that their child may have been exposed to COVID-19 may have been more motivated to participate. Participation rates varied substantially among the different day care centers and schools (median [IQR], 10% [5%-20%]) and were associated with an index of school-level socioeconomic status (SES). Only 5% of eligible children participated from schools in the lower half of the SES distribution vs 24% of eligible children from schools in the upper half. We were not able to capture the characteristics of nonparticipating children or why parents did not wish to participate, although we received anecdotal reports that the DBS procedure was a barrier to participation and that the letter of invitation sometimes went unnoticed. Reluctance to participate due to the DBS procedure may have lowered participation rates, thus affecting the precision of our estimates; however, we do not expect this introduced bias, as we have no reason to believe it differed according to the participants’ eventual serology result. Given the challenges of conducting this study during the pandemic, we were also not able to select our sample in such a way as to ensure it was representative of our target population.
Comparing our study population with neighborhood characteristics of adults from the 2016 census, our study population was more educated and fewer were from racial and ethnic minority groups across all neighborhoods (eTable 1 and eTable 2 in the Supplement).16 However, when we standardized our seroprevalence estimates to the race and ethnicity distribution of the 2016 census, our seroprevalence estimate increased slightly to 6.2% (95% CI, 5.3%-7.1%). We also used research-based ELISA with a 95% sensitivity, which is in line with sensitivity estimates of currently available commercial assays,34 but which nonetheless may have led to a small number of false negatives. Furthermore, there were 9 children with positive RT-PCR tests prior to the DBS collection, as reported by their parents, who had a negative serology result. These children were largely asymptomatic compared with children who were seropositive and had positive RT-PCR test results, likely causing a weaker immune response to SARS-CoV-2.35,36 Among children who reported at least 1 RT-PCR test prior to providing the DBS sample, the length of time between the most recent RT-PCR test date and DBS date did not significantly differ between seropositive and seronegative children. We may also have not detected children who were previously infected with the virus as antibody levels can wane over time.37,38
Conclusions
Our results provide evidence of the seroprevalence status of Canadian children and highlight the benefits of serological testing in children for SARS-CoV-2. They also support the observation that children experience fewer symptoms from infection with the virus than adults,4,39 although they represent a potential risk to their households and communities.40,41 Contributing to further evidence of COVID-19 inequities, our results were consistent with higher rates of seropositivity among racial and ethnic minority groups. It will be important to continue monitoring the serological status of children, particularly in the context of VOC and in the absence of high vaccine coverage.
eFigure 1. Prevalence of Symptoms at Time of RT-PCR Test by Serostatus of Participant
eFigure 2. Household Protective Behaviors by Serostatus of Child
eTable 1. Comparison of EnCORE Sample and 2016 Census Distribution for Self-reported Race and Ethnicity and Education Level (Adult Population Aged 0-65 Years) and Neighborhood of Residence
eTable 2. Comparison of EnCORE Adjusted Seroprevalence Estimates Standardized to the Covariate Distributions of 2016 Census Data on Race and Ethnicity and Neighborhood of Residence
References
- 1.Buonsenso D, Valentini P, De Rose C, et al. ; Gemelli Against COVID-19 Post-Acute Care Study Group . Seroprevalence of anti-SARS-CoV-2 IgG antibodies in children with household exposure to adults with COVID-19: Preliminary findings. Pediatr Pulmonol. 2021;56(6):1374-1377. doi: 10.1002/ppul.25280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tönshoff B, Müller B, Elling R, et al. Prevalence of SARS-CoV-2 infection in children and their parents in southwest Germany. JAMA Pediatr. 2021;175(6):586-593. doi: 10.1001/jamapediatrics.2021.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stringhini S, Wisniak A, Piumatti G, et al. Seroprevalence of anti–SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396(10247):313-319. doi: 10.1016/S0140-6736(20)31304-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lessler J, Grabowski MK, Grantz KH, et al. Household COVID-19 risk and in-person schooling. Science. 2021;372(6546):1092-1097. doi: 10.1126/science.abh2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UNESCO . UNESCO figures show two thirds of an academic year lost on average worldwide due to COVID-19 school closures. February 2, 2021. Accessed October 25, 2021. https://en.unesco.org/news/unesco-figures-show-two-thirds-academic-year-lost-average-worldwide-due-covid-19-school
- 6.Government of Canada. COVID-19 daily epidemiology update. February 7, 2021. Accessed February 8, 2021. https://health-infobase.canada.ca/covid-19/epidemiological-summary-covid-19-cases.html?stat=rate&measure=deaths&map=pt#a2
- 7.Bruemmer R. Should Quebec be closing schools in its COVID-19 hot zones? Montreal Gazette. January 16, 2021. Accessed February 8, 2021. https://montrealgazette.com/news/local-news/should-quebec-be-closing-schools-in-its-covid-19-hot-zones
- 8.Coletta A. The coronavirus is surging, but Canada is keeping schools open. The Washington Post. November 3, 2020. Accessed February 8, 2021. https://www.washingtonpost.com/world/the_americas/coronavirus-canada-schools-second-wave/2020/11/02/fb8c6c54-0e52-11eb-bfcf-b1893e2c51b4_story.html
- 9.Fontanet A, Grant R, Tondeur ML, et al. SARS-CoV-2 infection in primary schools in northern France: a retrospective cohort study in an area of high transmission. medRxiv. Preprint posted June 29, 2020. doi: 10.1101/2020.06.25.20140178 [DOI]
- 10.Lachassinne E, de Pontual L, Caseris M, et al. ; COVIDOCRECHE collaborators . SARS-CoV-2 transmission among children and staff in daycare centres during a nationwide lockdown in France: a cross-sectional, multicentre, seroprevalence study. Lancet Child Adolesc Health. 2021;5(4):256-264. doi: 10.1016/s2352-4642(21)00024-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ulyte A, Radtke T, Abela IA, et al. Clustering and longitudinal change in SARS-CoV-2 seroprevalence in school children in the canton of Zurich, Switzerland: prospective cohort study of 55 schools. BMJ. 2021;372(n616):n616. doi: 10.1136/bmj.n616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boey L, Roelants M, Merckx J, et al. Age-dependent seroprevalence of SARS-CoV-2 antibodies in school-aged children from areas with low and high community transmission. Eur J Pediatr. 2021;1-8. doi: 10.1007/s00431-021-04222-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.EnCORE Study. EnCORE: a COVID-19 seroprevalence study. Accessed October 26, 2021. https://www.encorestudy.ca/
- 14.Wikipedia . Montreal. Accessed Feb 8, 2021. https://en.wikipedia.org/wiki/Montreal#cite_note-cp2016-CD-10
- 15.Zinszer K, McKinnon B, Bourque N, et al. Seroprevalence of anti-SARS-CoV-2 antibodies among school and daycare children and personnel: protocol for a cohort study in Montreal, Canada. medRxiv. Preprint published April 16, 2021. doi: 10.1101/2021.04.14.21255499 [DOI] [PMC free article] [PubMed]
- 16.Statistics Canada . Census profile, 2016 Census: Montréal [Census metropolitan area], Quebec and Canada [Country]. Accessed May 5, 2021. https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/details/page.cfm?Lang=E&Geo1=CMACA&Code1=462&Geo2=PR&Code2=01&Data=Count&SearchText=Montreal&SearchType=Begins&SearchPR=01&TABID=1&B1=All
- 17.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573-577. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 18.Niang L, Winn T, Rusli B. Practical issues in calculating the sample size for prevalence studies. Arch Orofacial Sci. 2006;1: 9-14. Accessed November 4, 2021. https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.504.2129&rep=rep1&type=pdf [Google Scholar]
- 19.Vidmar S, Carlin J, Hesketh K, Cole T. Standardizing anthropometric measures in children and adolescents with new functions for egen. Stata J. 2004;4(1): 50-55. doi: 10.1177/1536867X0100400104 [DOI] [Google Scholar]
- 20.Québec. Les variants de la COVID-19 se propagent rapidement au Québec. March 26, 2021. Accessed May 6, 2021. https://www.quebec.ca/nouvelles/actualites/details/les-variants-de-la-covid-19-se-propagent-rapidement-au-quebec/
- 21.Québec. Données sur les variants du SRAS-CoV-2 au Québec. May 5, 2021. Accessed May 6, 2021. https://www.inspq.qc.ca/covid-19/donnees/variants#metho
- 22.Lewin A, Therrien R, De Serres G, et al. SARS-CoV-2 seroprevalence among blood donors in Québec, and analysis of symptoms associated with seropositivity: a nested case-control study. Can J Public Health. 2021;112(4):576-586. doi: 10.17269/s41997-021-00531-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kellner J. Alberta Childhood COVID-19 Cohort (AB3C) Aim 3: longitudinal sero-epidemiology study: first interim report, January 31, 2021. Accessed October 25, 2021. https://prism.ucalgary.ca/bitstream/handle/1880/113084/AB3C%20Aim%203%201st%20Interim%20Report-final.pdf?sequence=5&isAllowed=y
- 24.Manny E, Carroll A, Charlton C, et al. Increased mask use and fewer gatherings associated with lower SARS-CoV-2 seropositivity among young school-age children. Preprint Lancet. Preprint published December 3, 2020. doi: 10.2139/ssrn.3728570 [DOI]
- 25.Waterfield T, Watson C, Moore R, et al. Seroprevalence of SARS-CoV-2 antibodies in children: a prospective multicentre cohort study. Arch Dis Child. 2021;106(7):680-686. doi: 10.1136/archdischild-2020-320558 [DOI] [PubMed] [Google Scholar]
- 26.Ladhani SN, Baawuah F, Beckmann J, et al. SARS-CoV-2 infection and transmission in primary schools in England in June-December, 2020 (sKIDs): an active, prospective surveillance study. Lancet Child Adolesc Health. 2021;5(6):417-427. doi: 10.1016/S2352-4642(21)00061-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stringhini S, Zaballa M-E, Perez-Saez J, et al. ; Specchio-COVID19 Study Group . Seroprevalence of anti-SARS-CoV-2 antibodies after the second pandemic peak. Lancet Infect Dis. 2021;21(5):600-601. doi: 10.1016/S1473-3099(21)00054-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward H, Atchison C, Whitaker M, et al. SARS-CoV-2 antibody prevalence in England following the first peak of the pandemic. Nat Commun. 2021;12(1):905. doi: 10.1038/s41467-021-21237-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biggs HM, Harris JB, Breakwell L, et al. ; CDC Field Surveyor Team . Estimated community seroprevalence of SARS-CoV-2 antibodies—two Georgia counties, April 28-May 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(29):965-970. doi: 10.15585/mmwr.mm6929e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Statistics Canada . COVID-19 mortality rates in Canada’s ethno-cultural neighbourhoods. October 28, 2020. Accessed October 25, 2021. https://www150.statcan.gc.ca/n1/en/pub/45-28-0001/2020001/article/00079-eng.pdf?st=ngPjcBbD
- 31.Pan D, Sze S, Minhas JS, et al. The impact of ethnicity on clinical outcomes in COVID-19: a systematic review. EClinicalMedicine. 2020;23:100404. doi: 10.1016/j.eclinm.2020.100404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richard A, Wisniak A, Perez-Saez J, et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies, risk factors for infection and associated symptoms in Geneva, Switzerland: a population-based study. medRxiv. Preprint posted December 18, 2020. doi: 10.1101/2020.12.16.20248180 [DOI] [PMC free article] [PubMed]
- 33.Peeling RW, Wedderburn CJ, Garcia PJ, et al. Serology testing in the COVID-19 pandemic response. Lancet Infect Dis. 2020;20(9):e245-e249. doi: 10.1016/S1473-3099(20)30517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng MP, Yansouni CP, Basta NE, et al. Serodiagnostics for severe acute respiratory syndrome-related coronavirus 2: a narrative review. Ann Intern Med. 2020;173(6):450-460. doi: 10.7326/M20-2854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chia WN, Zhu F, Ong SWX, et al. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: a longitudinal study. Lancet Microbe. 2021;2(6):e240-e249. doi: 10.1016/S2666-5247(21)00025-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Post N, Eddy D, Huntley C, et al. Antibody response to SARS-CoV-2 infection in humans: a systematic review. PLoS One. 2020;15(12):e0244126. doi: 10.1371/journal.pone.0244126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020;5(12):1598-1607. doi: 10.1038/s41564-020-00813-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bloise S, Marcellino A, Testa A, et al. Serum IgG levels in children 6 months after SARS-CoV-2 infection and comparison with adults. Eur J Pediatr. 2021;1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forbes H, Morton CE, Bacon S, et al. Association between living with children and outcomes from COVID-19: OpenSAFELY cohort study of 12 million adults in England. BMJ. 2021;372(n628):n628. doi: 10.1136/bmj.n628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gurdasani D, Alwan NA, Greenhalgh T, et al. School reopening without robust COVID-19 mitigation risks accelerating the pandemic. Lancet. 2021;397(10280):1177-1178. doi: 10.1016/S0140-6736(21)00622-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chu VT, Yousaf AR, Chang K, et al. ; Georgia Camp Investigation Team . Household transmission of SARS-CoV-2 from children and adolescents. N Engl J Med. 2021;385(10):954-956. doi: 10.1056/NEJMc2031915 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Prevalence of Symptoms at Time of RT-PCR Test by Serostatus of Participant
eFigure 2. Household Protective Behaviors by Serostatus of Child
eTable 1. Comparison of EnCORE Sample and 2016 Census Distribution for Self-reported Race and Ethnicity and Education Level (Adult Population Aged 0-65 Years) and Neighborhood of Residence
eTable 2. Comparison of EnCORE Adjusted Seroprevalence Estimates Standardized to the Covariate Distributions of 2016 Census Data on Race and Ethnicity and Neighborhood of Residence