Abstract
Estrogen receptor-positive (ER+) breast cancer accounts for approximately 75% of all breast cancers. Endocrine therapies, including selective ER modulators (SERMs), aromatase inhibitors (AIs) and selective ER down-regulators (SERDs) provide substantial clinical benefit by reducing the risk of disease recurrence and mortality. However, resistance to endocrine therapies represents a major challenge, limiting the success of ER+ breast cancer treatment. Mechanisms of endocrine resistance involve alterations in ER signaling via modulation of ER (e.g., ER downregulation, ESR1 mutations or fusions); alterations in ER coactivators/corepressors, transcription factors (TFs), nuclear receptors and epigenetic modulators; regulation of signaling pathways; modulation of cell cycle regulators; stress signaling; alterations in tumor microenvironment, nutrient stress and metabolic regulation. Current therapeutic strategies to improve outcome of endocrine resistant patients in clinics include inhibitors against mechanistic target of rapamycin (mTOR), cyclin-dependent kinase (CDK) 4/6 and the Phosphoinositide 3-kinase (PI3K) subunit, p110α. Preclinical studies reveal novel therapeutic targets, some of which are currently tested in clinical trials as single agents or in combination with endocrine therapies, such as ER partial agonists, ER proteolysis targeting chimeras (PROTACs), next-generation selective estrogen receptor modulators (SERDs), AKT inhibitors, epidermal growth factor receptor 1 &2 (EGFR/HER2) dual inhibitors, HER2 targeting antibody-drug conjugates and histone deacetylase (HDAC) inhibitors. In this review, we summarize the established and emerging mechanisms of endocrine resistance, alterations during metastatic recurrence, and discuss the approved therapies and on-going clinical trials testing the combination of novel targeted therapies with endocrine therapy in endocrine-resistant ER+ breast cancer patients.
Keywords: Endocrine resistance, breast cancer, mechanisms of resistance, therapy
Introduction
Breast cancer is the most common cancer and the leading cause of cancer-related death among women worldwide [1]. ER+ breast cancer has the highest incidence rate and accounts for around 75% of all cases [2]. The growth of ER+ tumors is driven by ER signaling which may function through genomic (canonical) and nongenomic (non-canonical) pathways [3]. Upon estrogen (here: 17β-estradiol or E2) binding, ER dimerizes with another monomer, translocates to the nucleus and either directly binds to specific estrogen response elements (EREs) with the help of pioneer factors, such as Forkhead Box A1 (FOXA1) and GATA Binding Protein 3 (GATA3) [4] or indirectly binds to DNA by interacting with other transcription factors, such as Activator Protein 1 (AP-1), Sp1 Transcription Factor (SP1) and Nuclear Factor Kappa B Subunit 1 (NF-Kβ) (ERE-independent genomic action) [5]. Upon binding to DNA, ER acts as a scaffold for the assembly of a large coactivator complex [6] that results in activation of gene transcription. The first of the ER coactivators discovered was Steroid Receptor Coactivator 3 (SRC-3/AIB1), belonging to the steroid receptor coactivator (SRC)/p160 family. Other classes of ER coactivators include members of the histone acetyltransferase cAMP responsive element binding protein (CREB)-binding protein (CBP)/p300, ATP-dependent chromatin remodeling complexes like SWI/SNF, E3 ubiquitin-protein ligases and steroid RNA activator (SRA) [7]. Among the large number of ER target genes, there are many cell cycle, cell growth, proliferation and differentiation-related genes that trigger malignancy, such as MYC proto-oncogene, BHLH Transcription Factor (MYC) and Cyclin D1 (CCND1), regulating cell cycle progression and Transforming Growth Factor Alpha (TGFA), Epidermal Growth Factor (EGF) and Insulin Like Growth Factor 1 (IGF1), stimulating cellular growth [8]. In the non-genomic action, E2-ER complex may act as a component of cell membrane and cytoplasmic signaling cascades, causing rapid activation of growth factor signaling, including PI3K/AKT and Ras/MAPK pathways [9, 10]. These signal transduction pathways may further converge non-genomic actions of estrogen to genomic regulation of target genes since the functions of many transcription factors, including ER itself, are regulated via phosphorylation by protein kinases belonging to growth factor signaling [9].
Endocrine therapies modulating ER level and/or activity have been the mainstay therapy for decades and improved the quality of life and survival of ER+ breast cancer patients. Tamoxifen, the first Food and Drug Administration (FDA)-approved SERM, has been the backbone of adjuvant hormone therapy, especially for premenopausal women since 1970s. Tamoxifen is recommended to treat early, locally advanced and metastatic ER+ breast cancer (MBC), and it greatly reduces the risk of breast cancer recurrence, regardless of age, stage or menopausal status [11] even when used at low doses to minimize toxicity [12]. Tamoxifen monotherapy significantly improves overall survival, and an extended 10-years use is recommended in early-stage disease to prevent late recurrence [13]. Postmenopausal women with ER+ breast cancer are treated with tamoxifen or AIs, latter one is blocking the production of estrogen by inhibiting the aromatase enzyme (CYP19A1). In first-line settings, AIs, especially the third-generation inhibitor, letrozole have been shown to provide a substantial benefit over tamoxifen in prolonging time to disease progression (9.4 vs. 6.0 months; P=0.0001) [14]. In second-line settings, switching to AIs after 2-3 years of tamoxifen reduces the risk of recurrence although no decrease in mortality has been achieved [11]. Fulvestrant is the only SERD approved by the FDA to be used in postmenopausal patients with advanced disease either alone or in combination with AIs [15]. Fulvestrant showed similar efficacy to tamoxifen in first-line settings for metastatic disease [16], and was shown to be at least as effective as anastrozole in terms of time to progression in postmenopausal patients whose disease was progressed on tamoxifen [17].
Although most ER+ breast cancer patients initially respond well to endocrine therapy, resistance develops over time (acquired resistance), or some patients are unresponsive to endocrine therapy from the beginning (de novo resistance). Switching over different endocrine therapies is an effective strategy to manage metastatic disease, as exemplified by the increased response achieved in tamoxifen resistant patients after treatment with AIs or fulvestrant. However, response rates to second-line hormone therapy are lower than in frontline [18], necessitating the identification and targeting novel mechanisms of endocrine resistance to improve clinical outcome. In this review, we summarize the established and emerging mechanisms of endocrine resistance, the current clinical management of endocrine-resistant ER+ breast cancer, longitudinal analyses of primary and metastatic endocrine-resistant tumors, and the potential future therapeutic strategies originating from pre-clinical studies that are now in clinical trials.
Mechanisms of Endocrine Resistance in Breast Cancer
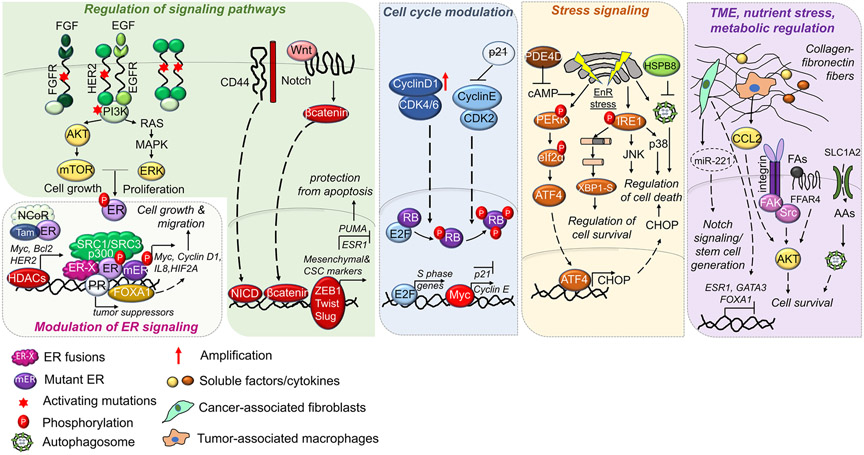
Endocrine resistance is multifactorial and involves modulation of a plethora of different processes and signaling pathways. Mechanisms of endocrine resistance involve alterations in ER signaling via modulation of ER (e.g. ER downregulation, ESR1 mutations or fusions); alterations in ER coactivators/corepressors, transcription factors (TFs), nuclear receptors and epigenetic modulators; regulation of signaling pathways; modulation of cell cycle regulators; stress signaling; alterations in tumor microenvironment, nutrient stress and metabolic regulation. (Fig. 1).
Figure 1.
Mechanisms of endocrine resistance in ER+ breast cancer. ER+ breast cancer cells may acquire resistance to endocrine therapy by various mechanisms: ER modulation: Regulation of ER during endocrine resistance involves loss of ER, ER phosphorylation by growth factor signaling, ER activating mutations or ER fusions which have constitutive transcriptional activity, leading to enhanced cell growth and migration. Coactivators/corepressors, transcription factors (TFs), nuclear receptors and epigenetic modulators: Increased expression of ER co-activators, such as Steroid Receptor Coactivator 1/3 (SRC-1/3), TFs such as Forkhead Box A1 (FOXA1) or histone modifiers, such as histone deacetylase (HDACs), or reduced levels of ER corepressor, such as Nuclear Receptor Corepressor 1 (NCOR) or the Progesterone Receptor (PR) leads to reprograming of ER transcriptional landscape, promoting the transcription of genes related to cell survival. Regulation of Signaling Pathways: Activation or overexpression of growth factor receptors or their ligands trigger downstream PI3K/AKT/mTOR and RAS/MEK/ERK pathways leading to enhanced cell growth and proliferation. Activation of the stemness inducers, Notch and Wnt signaling also triggers stem cell properties and endocrine resistance by activating the transcriptional program controlled by the intracellular domain of the notch protein (NICD) and β-catenin. Activation of EMT transcription factors (TFs), such as ZEB1, Twist and Slug leads to transcription of mesenchymal and cancer stem cell (CSC) markers that further leads to protection from apoptosis. Modulation of cell cycle progression: Amplification or activation of Cyclin D1/cyclin-dependent kinases 4/6 (CDK4/6) or Cyclin E/CDK2 mediates hyperphosphorylation and inactivation of retinoblastoma (RB) in early and late G1, respectively, leading to increased transcription of S phase genes. Furthermore, increased c-Myc modulates the transcription of G1/S transition regulators. Stress signaling: PDE4D upregulation reduces cAMP levels that protects cells from endoplasmic reticulum (EnR) stress-induced cell death, while overexpression of the heat shock protein, Heat Shock Protein Family B (Small) Member 8 (HSPB8) mediates endocrine resistance via suppressing pro-apoptotic autophagy. Tumor microenvironment, nutrient stress, and metabolic regulation: Enhanced collagen/fibronectin fibers activates integrin signaling, leading to PI3K/AKT activation and cell survival in the presence of endocrine therapy. Secreted soluble factors, cytokines or vesicle-embedded miRNA by cancer-associated fibroblasts (CAFs) or tumor-associated macrophages (TAMs) may reduce ER expression, promote cancer cell survival and stemness, leading to endocrine resistance. Endocrine resistant cells may also exhibit enhanced import of acidic amino acids by upregulation of Solute Carrier Family 1 Member 2 (SLC1A2) or activation of fatty acid receptors, such as Free Fatty Acid Receptor 4 (FFAR4), leading to activation of survival signaling.
Modulation of ER
Since ER is the primary target of endocrine therapies, alterations in ER expression and/or activity may cause endocrine resistance. Loss of ER expression is observed in around 20% of advanced ER+ breast cancer and it renders cells resistant to endocrine therapy via loss of estrogen dependence [19]. There are multiple mechanisms that lead to loss of ER expression, such as promoter methylation [20] or histone modifications [21]. Twist Family BHLH Transcription Factor 1 (TWIST), a basic helix-loop-helix transcription factor and a mesenchymal marker, was shown to bind ER promoter and suppress ER transcription, leading to estrogen-independent growth and resistance to tamoxifen [22]. In addition to loss of ER, mutations at the ligand binding domain (LBD) are detected in approximately 20% of metastatic ER+ tumors and are usually acquired following long-term treatment with tamoxifen or Ais [23, 24] (Table 1). D538G mutation was identified to be the most frequent ESR1 mutation followed by the Y537 mutation [24]. Mutated ER is constitutively active and triggers estrogen-independent ER transcriptional activity, leading to endocrine resistance. The three-dimensional (3D) structure analysis of the mutants in comparison with the wild type (WT) ER revealed that the mutations on the LBD induce a structural change that resembles estrogen-bound ER, triggering co-activator binding and transcriptional activity in the absence of the ligand [25]. Overexpression of D538G or Y537S mutants enables ligand-independent binding of the coactivators to ER, promotes transcription of the ER targets, Progesterone Receptor (PGR) and Growth Regulating Estrogen Receptor Binding 1 (GREB1), increases cell viability and migration and confers resistance to tamoxifen and fulvestrant [24, 26]. A covalent Cyclin Dependent Kinase 7 (CDK7) inhibitor, THZ1 was demonstrated to reduce S118 phosphorylation and constitutive activity of the Y527S mutant, leading to enhanced sensitivity to fulvestrant [27, 28]. Importantly, mutant ER exhibits differences in chromatin accessibility and induces the expression of a distinct set of targets [29], regulating cell growth and metastasis [28] in the presence of the ER modulators. Mutations in LBD decreases the affinity of the receptor to tamoxifen and fulvestrant [23], necessitating the use of more potent ER antagonists [24]. In this line, next-generation oral SERMs or SERDs can target both the WT and mutant ER, such as GDC-9545 and elacestrant [30], and they showed clinical activity (NCT03332797 and NCT02338349) [31, 32] with acceptable toxicity profiles in mutant ER-carrying MBC, some of which progressed on prior therapy with SERDs (Table 2).
Table 1.
Genes that are mutated in endocrine resistance, type of the mutation and roles of the mutated genes in mediating endocrine resistance.
| Mutated Gene |
Type of mutation |
Roles of the gene in endocrine resistance | References |
|---|---|---|---|
| ESR1 | Gain of function |
|
23-30 |
| NCOR | Loss of function |
|
52-55 |
| RUNX1 | Loss of function |
|
59-61 |
| CTCF | Loss of function |
|
62, 63 |
| ARID1A | Loss of function |
|
62, 67 |
| PIK3CA | Gain of function |
|
62 |
| FGFR2 | Gain of function |
|
93, 96 |
| ERBB2 | Gain of function |
|
62, 101, 194 |
Table 2.
List of clinical trials that led to approval of current therapies & completed or still ongoing trials testing the potential future therapies to treat endocrine resistance
| Target | Drug | Target population | Phase | ID |
|---|---|---|---|---|
| Key clinical trials leading to approval of current therapies | ||||
| mTOR | Everolimus | in combination with exemestane for postmenopausal women with ER+ locally advanced or metastatic BC refractory to letrozole or anastrozole | Phase III | NCT00863655 198 |
| CDK4/6 | Palbociclib | in combination with letrozole in postmenopausal women with ER+/HER2− advanced BC as a first line therapy | Phase III | NCT01740427 127 |
| CDK4/6 | Palbociclib | in combination with fulvestrant in ER+/HER2− MBC progressed after prior endocrine therapy. | Phase III | NCT01942135 128 |
| CDK4/6 | Palbociclib | in combination with tamoxifen in ER+/HER2− advanced BC as a first line therapy | Phase II | NCT02668666 |
| PI3K | Alpelisib | in combination with fulvestrant in postmenopausal women with ER+/HER2− advanced BC, who received prior treatment with an AI | Phase III | NCT02437318 201 |
| Completed or ongoing clinical trials for potential future therapies | ||||
| mTOR | Everolimus | in combination with letrozole in post-menopausal ER+HER2− patients as a first line therapy | Phase II | NCT01698918 202 |
| ER | TTC-352 | in metastatic ER+ BC patients who received and progressed on at least two lines of endocrine therapy | Phase I | NCT03201913 203 |
| ER | Elacestrant (RAD1901) | heavily pretreated patients with ER+ MBC, including patients with ESR1 mutation as well as those with prior CDK4/6i and SERD treatment | Phase I | NCT02338349 31 |
| ER | Elacestrant (RAD1901) | in combination with an AI or fulvestrant in postmenopausal ER+/HER2− MBC progressed on one or two lines of endocrine therapy and CDK4/6 inhibitor | Phase III | NCT03778931 207 |
| ER | ARV-471 | in combination with palbociclib in patients with ER+/HER2− locally advanced or MBC, progressed on prior hormonal therapy and chemotherapy | Phase I/II | NCT04072952 |
| EGFR/HER2 | Neratinib | in combination with endocrine therapy in pre- and post-menopausal women with locally advanced or metastatic HER2+ or ER+/HER2− BC with recurrence or progression following prior treatment with AIs, tamoxifen or fulvestrant. | Phase II | NCT04460430 |
| AKT | Ipatasertib | in combination with an AI or fulvestrant, with or without Palbociclib in postmenopausal ER+HER− BC patients | Phase I | NCT03959891 208 |
| HER2 | Trastuzumab deruxtecan | In ER+HER2− patients who are resistant to endocrine therapies | Phase II | NCT04132960 |
| HDAC | Entinostat | in combination with exemestane in ER+ BC patients with disease progression after non-steroidal AI | Phase II | NCT00676663 210 |
| HDAC | Entinostat | in combination with exemestane in ER+ BC patients with disease progression after non-steroidal AI | Phase III | NCT02115282 211 |
Besides point mutations, ESR1 fusions are also observed in clinics although to a lesser extent. ESR1 fusions have been identified both in primary breast tumors that are resistant to endocrine therapy [33] as well as in metastatic samples [34]. Most of the ESR1 fusions harbor the N-terminal DNA binding domain of ESR1 fused to the C terminal of different genes, including Coiled-Coil Domain Containing 170 (CCDC170) and Yes1 Associated Transcriptional Regulator (YAP1). The lack of LBD in ESR1 fusions triggers estrogen-independent growth, constitutive expression of ER target genes, and endocrine resistance [35]. One of the ESR1 fusions, ESR1-CCDC170 was found in 6-8% of refractory luminal B breast cancers, and was shown to increase cell motility, and anchorage-independent growth, as well as to reduce the response to endocrine therapy [36]. Recently, it was also demonstrated to bind HER2/HER3/SRC and activate SRC/PI3K/AKT signaling, causing endocrine resistance [37]. Breast cancer cells harboring ESR1-CCDC170 fusion are highly sensitive to the combination of tamoxifen or fulvestrant with HER2 or Src inhibitors [37]. ESR-YAP1 fusion was shown to be fully resistant to fulvestrant, and activate a metastasis-associated transcriptional program, causing increased cell motility and lung metastasis [36]. Importantly, cellular growth induced by ESR-YAP1 and ESR1-(Protocadherin 11 X-Linked) PCDH11X fusions remained sensitive to CDK4/6 inhibition [36, 38], providing a therapeutic strategy to treat ER+ tumors carrying these fusions.
Coactivators/corepressors, transcription factors (TFs), nuclear receptors and epigenetic modulators
Activation of the ER coactivator, SRC-3 (or AIB-1) is implicated in ER+ breast cancer progression, endocrine resistance, and metastasis via regulation of a multitude of mechanisms. During malignant progression of early-stage ER+ breast cancer, SRC-3 represses genes at the 1q21.3 locus, leading to breast cancer progression [39]. SRC-3 overexpression can also revert the inhibitory effect of AR on ER-mediated transcription of cyclin D1 [40]. SRC-3 can lead to tamoxifen resistance via promoting the expression of ER target genes [41], and other oncogenic proteins, such as HER2 [42] in the presence of tamoxifen. Furthermore, it was shown to inhibit the transcription of E-cadherin and promote ER+ breast cancer metastasis [43]. SRC-3 was identified as a novel binding partner of Proline, glutamic acid, leucine-rich protein 1 (PELP1), which is overexpressed in approximately 80% of invasive breast tumors. Inhibiting the cytoplasmic PELP1/AIB1 signaling complex reduces tumorsphere formation in MCF-7 cells and increased survival in vivo [44]. In clinics, high SRC-3 expression is associated with poor survival in early-stage ER+ breast cancer [45]. It also shows prognostic value in ER+/HER2-negative ILC [46]. Another ER coactivator, Steroid Receptor Coactivator 1 (SRC-1) was shown to be overexpressed in AI resistant tumors and trigger metastatic progression via interacting with the transcription factor, ETS Proto-Oncogene 2, Transcription Factor (ETS2) and regulating the transcription of c-Myc and Matrix Metallopeptidase 9 (MMP9) [47]. It can also mediate transcriptional reprogramming by interacting with Signal Transducer and Activator of Transcription 1 (STAT-1), independent of estrogen [48] or by inducing hypermethylation of a set of differentiation genes whose low expression significantly associated with poor clinical outcome [49]. A novel single nucleotide polymorphism (SNP; P1272S; rs1804645) in SRC-1 has recently been identified to be significantly associated with reduced bone metastasis under tamoxifen treatment [50].
In contrast to coactivators which potentiate ER-dependent transcription, ERα corepressors antagonize ER activity by recruiting histone-modifying enzymes, competing with coactivators or interfering with ER dimerization [51]. Loss of ER corepressors is observed in 13% to 55% of ER-positive breast tumors [52], and promotes resistance to endocrine therapy [19]. Nuclear Receptor Corepressor 1 (NCOR) is one of the first ER corepressors identified as frequently mutated and genomically altered in ER+ breast cancer [52]. NCOR depletion causes tamoxifen to behave as a partial agonist, leading to increased transcription of cell cycle inducers, c-Myc, CCND1 and the Stromal Cell-Derived Factor 1 (SDF1), and confers tamoxifen resistance in vitro and in vivo [53] (Table 1). COP9 Signalosome Subunit 5 (COPS5) which is overexpressed/amplified in 9% of the ERα+ primary breast tumors and in 86.7% of tamoxifen-resistant tumors promotes proteasomal degradation of NCOR, thus converting tamoxifen to an ER agonist and conferring resistance [54]. Importantly, low NCOR1 mRNA is associated with shorter relapse-free survival in tamoxifen-treated patients [55].
Alterations in the transcription factors, such as c-Myc, FOXA1, RUNX Family Transcription Factor 1 (RUNX1) and CCCTC-Binding Factor (CTCF) may also confer endocrine resistance via ER-dependent or independent mechanisms. A signature associated with c-Myc activation was found to be enriched upon estrogen deprivation and predicts poor outcome following tamoxifen treatment [56]. Mechanistically, c-Myc can regulate the transcription of numerous targets that are mostly related to cell cycle, such as Cyclin Dependent Kinase Inhibitor 1A (p21), Cyclin A and Cyclin E [57, 58]. RUNX1 is a transcription factor found to be mutated in ER+ breast tumors and associated with poor outcome [59]. Mechanistically RUNX1 can suppress FOXA1 [60] and β-catenin transcription [61] (Table 1). CTCF was found to be altered in endocrine resistant tumors, predominantly in post-treatment samples [62], and it can prevent ER binding to chromatin [63] (Table 1). FOXA1, a key transcription factor promoting ER-mediated transcription has been demonstrated to drive endocrine resistance [64]. FOXA1 can trigger the transcription of classical ER target genes in sensitive models, while the endocrine resistant counterparts exhibit differences in FOXA1 and ER binding events [64], resulting in manifestation of a non-classical ER transcriptional program, involving the transcriptional activation of C-X-C Motif Chemokine Ligand 8 (IL8) or Hypoxia-Inducible Factor 2-Alpha (HIF2A), leading to cellular growth and metastasis [65, 66]. SWItch/Sucrose Non-fermentable (SWI/SNF) nucleosome remodeling complex components, AT-Rich Interaction Domain Proteins, ARID1A and ARID2 have also been shown to be important for endocrine resistance. Loss of function mutations or deletions in ARID1A and ARID2 have been detected in post-endocrine treatment and metastatic specimens [62]. Mechanistically, loss of ARID1A impairs SWI/SNF recruitment to luminal transcription factor foci, leading to acquisition of basal characteristics and endocrine resistance [67] (Table 1).
The nuclear receptors, Androgen Receptor (AR) and PR are also critical in determining response to endocrine therapy. Inhibition of the canonical and non-canonical AR reduced estradiol-dependent growth, synergized with tamoxifen and fulvestrant, and reduced the metastatic burden [68, 69]. Inhibiting AR was also shown to specifically reduce the growth of AI resistant cells whereas it had no effect on AI sensitive models, suggesting a context-dependent contribution of AR to ER+ cancer cell growth [70]. A recent study reported that AR activation, but not inhibition, exerts potent antitumor activity in resistance to standard-of-care ER and CDK4/6 inhibitors, by displacing ER from the chromatin and instead, upregulating AR target genes that include tumor suppressors [71]. This discrepancy in the role of AR in the growth of ER+ breast tumors may be due to crosstalk of ER with other nuclear receptors, such as PR or with cofactors, p300 and SRC-3[71]. PR is also an important ER interacting nuclear receptor that determines clinical outcome. The interaction between ER and PR alters the transcriptional program to favor better clinical outcome in ER+ breast cancer [72]. Treatment of ER+ cell line xenografts and tumor explants with progesterone enhanced the anti-proliferative effects of tamoxifen, by re-directing transcription from proliferation-related genes to genes related to cell death, apoptosis, and differentiation pathways [72].
Epigenetic reprogramming involves post-translational modifications of histones by histone acetyltransferases (HATs), deacetylases (HDACs), methyltransferases (HMTs) or demethylases (HDMs) and can lead to altered chromatin accessibility [73] and result in endocrine resistance. The ER corepressor NCOR suppresses ER-mediated transcription in the presence of tamoxifen via recruiting HDAC3, causing chromatin condensation and loss of RNA polymerase II from ER-bound DNA [52]. Another ER corepressor Nuclear Receptor Subfamily 2 Group F Member 2 (NR2F2) attenuates hormone-dependent signaling by recruiting HDAC1 to ER-bound DNA [52]. Loss of ER corepressors may impede the recruitment of HDACs to ER-bound promoters, disrupting the intricate balance between histone acetylation and deacetylation and predisposing cancer cells to the anti-tumorigenic effects of HDAC inhibitors [52, 74]. For instance, inhibition of HDACs may result in restoration of p21, while suppressing c-Myc, B-cell Lymphoma 2 (Bcl2) and HER2, leading to sensitivity to endocrine therapy [75, 76]. HDAC inhibitors may also restore ER expression via remodeling histone acetylation and methylation without altering the methylation of the ER promoter [77]. In line with these findings, a class II HDAC, HDAC9 negatively regulates ER at mRNA and protein levels and inhibits its transcriptional activity. Furthermore, HDAC9 is overexpressed in tamoxifen resistant cells, and high expression of HDAC9 is associated with worse prognosis in tamoxifen-treated patients [78]. Inhibition of HDAC activity with vorinostat (SAHA) has also been shown to induce autophagic cell death in tamoxifen resistant MCF-7 cells and reduced the growth of MCF-7 tamoxifen resistant (TamR) xenografts [79]. In addition to modifiers of histone acetylation, HMTs and HMDs have also been associated with endocrine resistance [80, 81]. For instance, ectopic expression of zinc-finger E-box binding homeobox 1 (ZEB1) inhibits ER transcription by forming a complex with DNA methyltransferase 3B (DNMT3B) and HDAC1 on ER promoter to induce DNA hypermethylation, resulting in resistance to endocrine therapies in vitro and in vivo [82]. These findings suggest that epigenetic remodeling may represent an attractive strategy to restore sensitivity to endocrine therapy.
Regulation of Signaling pathways
Endocrine resistance may be governed by deregulation of a variety of different signaling pathways, such as growth factor receptor, Notch and Wnt signaling pathways. It is now well-established that there is a bidirectional crosstalk between ER and the growth factor signaling. Estrogen can induce the expression of growth factor receptor ligands, such as TGFα [83] and IGF1 [84] which is followed by activation of the downstream pro-survival signaling cascades [84]. Reciprocally, activation of growth factor signaling results in phosphorylation of ER itself, as well as its coregulators at multiple sites, leading to their activation. For instance, ERK1/2 and PI3K/AKT phosphorylates ER at Serine 118 and 167 residues, leading to enhanced ER/estrogen receptor activation function 1 (AF-1) activity [85, 86]. Phosphorylation of the coactivators upon growth factor stimulation increases their activity even in the absence of E2 [87] or in the presence of endocrine therapy [88]. On the other hand, phosphorylation of corepressors causes their nuclear export, thus relieving the inhibitory effects on ER transcriptional activity [89].
Endocrine resistant tumors often have altered expression or activity of growth factor receptors or their ligands [90, 91]. The increase in growth factor signaling can be mediated by various mechanisms, including loss of ER [92], amplifications of EGFR, HER2 [62] or Fibroblast Growth Factor Receptor (FGFR) [93] or activating mutations in HER2 or PI3K [62] (Table 1). Activation of the EGFR/HER2 signaling, in turn, stimulates cell proliferation and inhibits apoptosis in endocrine resistant tumors. It can also trigger PI3K/AKT-mediated downregulation of ER or ligand-independent ER activation through phosphorylation [85, 86]. Growth factor signaling may also rewire ER-dependent gene transcription via EGF-induced activation of ER that generates a distinct mRNA landscape than E2-induced transcription [94]. FGFR signaling is overactivated in 40% of the post-treatment tumors from ER+ MBCs with endocrine resistance in the form of FGFR1, FGFR2, or FGF3 amplifications or FGFR2 mutations [93] (Table 1), and is associated with shorter time to progression (TTP) [95]. Mechanistically, activation of FGFR signaling stimulates MAPK and AKT signaling pathways, leading to tamoxifen and fulvestrant resistance [93, 96].
Targeting receptor tyrosine kinases or their downstream effectors can increase sensitivity to endocrine therapy [97, 98]. Combined blockade of ER and PI3Kα, TORC1, HER2 and HER3 in ER+ cells harboring HER2 activating mutations restores sensitivity to fulvestrant and to estrogen deprivation [99]. Combination of fulvestrant with the HER2 inhibitor, neratinib has also showed clinical efficacy in HER2-mutant MBC (NCT01953926) [100]. The FGFR-driven endocrine resistance can be reversed by using FGFR inhibitors, MEK inhibitors or mTOR inhibitors in combination with endocrine therapies [93, 95]. Although the frequency of PIK3CA mutants show no difference in primary vs. endocrine resistant metastatic tumors (unlike the ESR1 and ERBB2 mutations), PI3K inhibitors in combination with endocrine therapy were shown to significantly improve survival in metastatic ER+ breast cancer. The P110α inhibitor, alpelisib was recently approved by the FDA in combination with fulvestrant in ER+ MBCs harboring PIK3CA mutations [101] (Table 2). MAPK signaling can also be activated by overexpression of IGF-binding protein 1 (IGFBP-1) in tamoxifen resistant cells, and its inhibition reduces ERK phosphorylation and mediates tamoxifen sensitivity [102].
Notch Receptor 4 (Notch4), a receptor of the Notch signaling, which is an important regulator of cancer stemness, was shown to be overexpressed in breast cancer stem cells (BCSCs) [103]. BCSCs are known to be CD44+CD24−/low and ALDH1+ and are enriched in advanced luminal breast cancer and associated with poor prognosis [104]. In vitro studies of parental (WT) and TamR MCF-7 cells showed an elevation of CD44+CD24−/low cell population [105], as well as enhanced ALDH activity in MCF7-TamR cells as compared to WT counterparts [106]. Consistent with these in vitro findings, short-term treatment of patient-derived xenografts (PDXs) with tamoxifen or fulvestrant decreased cell proliferation, but increased BCSC activity, suggesting that acquisition of stem cell properties is an early event in endocrine resistance [107]. Inhibiting Notch4 reduced the enrichment of BCSCs in acquired tamoxifen resistant PDXs [107] and the number of circulating tumor cells (CTCs) [108]. Treatment with endocrine therapy in ER+ PDXs has also increased STAT-3 phosphorylation along with BCSC enrichment, and targeting STAT-3 using SFX-01 in combination with endocrine therapy prevented BCSC activity, reducing tumor growth and spontaneous lung metastasis [109]. In clinics, letrozole-treated patients exhibit an enrichment of CD44+CD24−/low BCSCs [110]. In addition, global gene expression and methylation analysis in tamoxifen-resistant models identified high expression of SRY-Box Transcription Factor 2 (SOX2) and E2F Transcription Factor 2 (E2F2), indicative of cancer stem-like properties [111].
It is well-known that the generation of stem cells and acquisition of mesenchymal traits can be governed by induction of epithelial-mesenchymal transition (EMT) [110, 112]. EMT is a critical initiator of cancer cell dissemination which is the first step of the metastasis cascade. Intriguingly, activation of the EMT program was reported to drive resistance to anti-cancer therapies [113]. In this line, we and others have shown that endocrine resistant cells acquire EMT-like features [114-116] which are causally important for resistance. For instance, miR-375, a tumor suppressor miRNA, sensitizes TamR cells to tamoxifen along with loss of EMT-like properties, partially via direct targeting of the oncogene metadherin (MTDH) [115]. Snail Family Transcriptional Repressor 2 (Slug), another EMT factor, was also upregulated in endocrine resistant ER+ breast cancer cells. It is a prognostic biomarker of patients with refractory ER+ breast cancer [117], and can promote protection from apoptosis by suppressing the transcription of the pro-apoptotic regulators, such as Puma [118]. Importantly, resistance to anti-cancer therapies has been attributed, in part, to the generation of CSCs as a result of the activation of the EMT program [113], and thus, targeting either EMT or stemness may confer endocrine sensitivity. For instance, Wnt3a and β-catenin signaling which are important for stem cell functioning were shown to be activated upon tamoxifen resistance along with the acquisition of EMT, and their inhibition restored epithelial phenotype and suppressed cell growth [114, 119]. Notch4, the activator of stemness, cross-talks with STAT-3 to regulate EMT in MCF-7 TamR cells, and treatment with Notch inhibitor reduces tumor growth and metastasis in MCF-7 TamR xenografts [120]. In another study, ER in complex with a transcription factor, RUNX Family Transcription Factor 2 (RUNX2) was shown to be important for the transcription of a stem cell transcription factor, SRY-Box Transcription Factor 9 (SOX9) upon tamoxifen treatment that triggers cell proliferation and EMT, thus regulating both drug resistance and metastasis [121]. Along these lines, targeting the common modulators of EMT and CSC phenotypes, such as the Notch and Wnt signaling, may provide a unique opportunity to abrogate not only endocrine resistance, but also metastasis [122].
Modulation of cell cycle progression
Estrogen can induce cell cycle progression from G1 to S phase via transcriptional upregulation of cell cycle regulators, including Cyclin D1 and Cyclin E or activation of Cyclin Dependent Kinases (CDK2 and CDK4) which further phosphorylate downstream substrates, such as retinoblastoma (RB) [123]. Treatment with endocrine therapies blocks cell cycle progression at G1 via altering the ER-dependent transcription of cell cycle modulators [123, 124]. In this line, endocrine resistant tumors often exhibit alterations in cell cycle regulators, such as activation of c-Myc, Cyclin D1 or CDK4/6 kinases [125] to circumvent the inhibitory effects of endocrine therapies on G1 to S transition. The major mechanism of c-Myc-induced endocrine resistance was shown to be the activation of Cyclin E/CDK2 through transcriptional repression of endocrine therapy-induced p21 transcription [58]. Amplification of CCND1, the activator of CDK4/6 kinases, correlates with ER positivity and is associated with worse prognosis and resistance to endocrine therapy in ER+ breast cancer patients [126].
The development of highly potent, small molecule inhibitors of CDK4/6 has revolutionized the treatment of endocrine resistant ER+ breast cancer. There are currently three CDK4/6 inhibitors; palbociclib, ribociclib, and abemaciclib approved by the FDA in combination with endocrine therapies to treat ER+HER2− breast cancer patients at the first line and advanced settings [127, 128] (Table 2). CDK4/6 inhibition showed activity against RB-proficient human tumor xenograft models in preclinical studies [129]. Mechanistically, CDK4/6 inhibitors block the hyperphosphorylation of Rb, thereby halt cell cycle progression at G1 [130]. Given the involvement of G1 to S transition modulation in endocrine resistance, CDK4/6 inhibitors were shown to greatly synergize with endocrine therapy [130-132]. Given the critical involvement of CDK4/6 in driving endocrine resistance, proteins that regulate CDK4 or Rb have also been reported as mediators of resistance, such as Ankyrin Repeat And LEM Domain Containing 2 (LEM4), a nuclear envelope protein, which was demonstrated to bind CDK4 and Rb, inducing their phosphorylation, as well as to induce Aurora A-mediated ER phosphorylation, further enhancing the ER-dependent transcription of Cyclin D1 and c-Myc, causing tamoxifen resistance [133]. The efficacy of CDK4/6 inhibition can further be improved by targeting receptor tyrosine kinases given the extensive crosstalk between CDK4/6 and the mitogenic signaling pathways. For instance, combination of palbociclib with an FGFR inhibitor caused a synergistic growth inhibition in the estrogen-deprived ER+ breast cancer cells harboring FGFR1/CCND1 co-amplification [33]. Along these lines, triple combinations involving endocrine therapy, CDK4/6 inhibitors and PI3K/mTOR of HER2 inhibitors have been tested in clinical trials and showed manageable toxicity and encouraging signs of clinical benefit [134, 135].
Stress Signaling
Unfolded protein response (UPR) is initiated upon detection of misfolded proteins and may cause variable phenotypic responses depending on the severity, duration and type of the stress signal. While acute UPR activation is generally a pro-survival mechanism which reduces the misfolded protein load in the endoplasmic reticulum, and thus maintains cellular homeostasis, chronic UPR activation may initiate the apoptotic cascade by PKR-like ER kinase (PERK)- Eukaryotic Translation Initiation Factor 2A (eIF2α)-Activating Transcription Factor 4 (ATF4)-C/EBP homologous protein (CHOP) and the Inositol-requiring enzyme 1 (IRE1)-C-Jun N-terminal kinase (JNK) pathways [136, 137]. In this line, we demonstrated that combination of tamoxifen with a specific inhibitor of the phosphodiesterase 4D elevates cyclic AMP (cAMP) levels, inducing EnR stress and activation of stress-related kinases p38 and JNK, leading to apoptotic cell death and tamoxifen sensitization [138]. Moreover, a plant toxin, persin was shown to overcome tamoxifen resistance by induction of EnR stress response characterized by increased levels of CHOP, the EnR chaperone Binding Immunoglobulin Protein (Bip), and spliced X-Box Binding Protein 1 (XBP-1), leading to apoptotic cell death [139]. Similar to UPR, induction of autophagy may also be either pro-survival or pro-apoptotic depending on the source and duration of the stressor that ultimately dictates cell fate. Under chronic activation of autophagy, cells undergo apoptosis [136]. It has been demonstrated that cell death upon treatment with endocrine therapy involves extensive formation of autophagic vacuoles [140]. Targeting a nuclear transport protein, Exportin 1 (XPO1) or a heat shock protein, Heat Shock Protein Beta-8 (HSPB8) enhances tamoxifen sensitivity via autophagy induction [141, 142].
UPR activation followed by cell death has also been associated with estrogen-induced apoptosis in estrogen-independent models. For instance, global gene expression analysis upon E2 treatment of the estrogen-independent, endocrine resistant MCF7:5C cells demonstrated that activation of UPR is involved in estrogen-induced apoptosis [143]. Recently, a synthetic selective estrogen mimic, TTC-352 which is currently in clinical trials (NCT03201913), has been shown to trigger a rapid estrogen receptor-induced UPR, characterized by eIF2α phosphorylation, increased expression of ATF4 and CHOP, resulting in apoptosis in MCF7:5C cells [144]. In addition to the activation of PERK-eIF2α arm, estrogen signaling may also activate the IRE1-XBP1 arm of the UPR via direct [145] or c-Myc-driven [146] transcription of XBP1, which is further spliced by IRE1 into XBP1-S, regulating estrogen-induced cell proliferation [147].
Several studies evaluated the role of XBP1-S in ER+ breast cancer and showed that it acts as a pro-survival mechanism that triggers the growth of ER+ cells. XBP1-S was found to be elevated in endocrine resistant breast cancer, confers estrogen independence and endocrine resistance, and is associated with poor clinical outcome [147, 148]. It is also constitutively active in mutant ESR1-carrying breast cancer cells [149]. Several small molecule inhibitors that block IRE1/XBP1-S, e.g., STF-083010 have been developed and shown to overcome endocrine resistance [150]. The stress-related kinases p38 and JNK which are activated upon UPR induction were shown to positively regulate ER signaling in a feed-forward loop via phosphorylation of ER or the cofactors [151, 152]. In this line, increased p38 phosphorylation was detected in tamoxifen resistant tumors and correlated with MAPK signaling activity [19]. Nevertheless, further studies are warranted testing a potential benefit of p38 inhibition in enhancing endocrine sensitivity. Another mechanism through which acute activation of UPR promotes survival is the induction of autophagy which preserves tissue homeostasis. For instance, the EnR chaperone, Bip which is activated upon UPR to participate in protein folding and assembly, was shown to trigger autophagy by reducing mTOR phosphorylation, and block apoptosis in the presence of endocrine therapy [153]. Several additional studies reported upregulation of autophagy markers in endocrine resistant models as reviewed elsewhere [136]. Along these lines, targeting autophagy in combination with tamoxifen restores sensitivity by the induction of apoptotic cell death [154]. Overall, while chronic activation of stress signaling, e.g., UPR or autophagy may trigger cell death, cell recovery from acute stress may involve coordination of the UPR and autophagy regulators and activation of a pro-survival mode of action in endocrine resistance.
Tumor Microenvironment, nutrient stress and metabolic regulation
A growing body of evidence suggest that alterations in tumor microenvironment induced by cancer, stromal or immune cells provide a unique opportunity for tumor growth and invasiveness under endocrine therapy. These include re-modeling of the extracellular matrix (ECM), immune surveillance, re-firing of pro-survival receptor signaling upon interaction with stromal cells, and changes in nutrient availability, leading to metabolic regulation. The soluble factors within the ECM can modulate estrogen responsiveness [155] and thus endocrine resistance that is in part mediated by the activation of PI3K/AKT and MAPK pathways downstream of the ECM-sensing integrin receptor activation [156]. An ECM metalloproteinase inducer (EMMPRIN) was found to be upregulated at protein level in tamoxifen resistant breast tumors, and significantly associated with an earlier tumor progression following first line tamoxifen treatment [157]. In another study, mRNA expression analysis of primary breast cancer samples revealed that high expression of the ECM component, tenascin C is associated with tamoxifen resistance [158]. Importantly, a dense collagen-I matrix was shown to activate the crosstalk of estrogen and prolactin, leading to activation of SRC-family kinases and reduced sensitivity to tamoxifen [159]. A transgenic mouse model of elevated collagen composition (Col1a1tm1Jae/+) revealed a larger primary tumor volume and size of lung metastasis in stiff collagen background under tamoxifen treatment [160].
ER+ tumors have been considered as immunologically cold with low levels of tumor infiltrating lymphocytes and to be resistant to immune checkpoint blockers that may, in part, be explained by the negative association between ER and Programmed Cell Death 1 Ligand 1 (PD-L1) expression [161] and the secretion of immunosuppressive cytokines upon endocrine therapy [162]. However, although immune cell infiltration has no prognostic value in low-risk ER+ patients, in highly proliferative tumors, immune cells can indeed predict better prognosis [163]. A potential benefit of immunotherapy might be especially considered for AI resistant luminal B tumors as it has recently been demonstrated that these tumors express higher levels of the immune checkpoint components Indoleamine 2,3-Dioxygenase 1 (IDO1), Lymphocyte Activating 3 (LAG3), and Programmed Cell Death Protein 1 (PD-1) [164]. A phase I trial testing the combination of exemestane with tremelimumab (anti–CTLA-4 antibody) in hormone responsive breast cancer demonstrated stable disease for 12 weeks in 11 patients (42%) as the best overall response [165]. Currently, a Phase II trial is testing pembrolizumab (anti–PD-1 antibody) in patients with localized ER+ inflammatory breast cancer, who are receiving endocrine therapy and did not achieve a pathological complete response to chemotherapy (NCT02971748). Furthermore, analysis of 61 primary breast cancer tissues, 85% of which were ER+/HER2−, revealed that the CD8+ tumor infiltrating lymphocytes retained robust capacity for production of effector cytokines [166]. Further studies analyzing the effects of tumor infiltrating lymphocytes (TILs) in endocrine resistant ER+ breast cancer may result in novel therapeutic options that involve immune modulators to treat endocrine resistant ER+ breast cancer.
The importance of cancer-associated fibroblasts (CAFs) and tumor-associated macrophages (TAMs) within the tumor microenvironment in endocrine resistance is also recognized. It has been reported that pretreatment with a cocktail of CAF-derived factors reduced the expression of ESR1, FOXA1 and GATA3 and conferred tamoxifen resistance, while blocking the secretion of these factors by inhibiting the CAF-activating Platelet Derived Growth Factor C (PDGF-CC) induced the expression of ER and sensitizes tumors to endocrine therapy [167]. A miRNA, miR-221 that was carried to ER+ tumor cells within the microvesicles secreted by CAFs activates Notch signaling leading to generation of CD133hi CSCs, conferring endocrine resistance [168]. The M2 phenotype (CD163+) of TAMs which have poor antigen-presenting ability, may secrete anti-inflammatory cytokines to promote tumor progression [169]. The Kaplan-Meier survival analyses of 145 ER+ breast cancer patients treated with endocrine therapies, mainly tamoxifen, revealed a strong association of macrophage markers (CD68 and CD163) with disease recurrence [170]. Mechanistically, when macrophages were co-cultured with MCF-7 TamR cells, they turned into TAMs and secreted CC-Chemokine Ligand 2 (CCL2) to increase tamoxifen resistance via activating PI3K/AKT/mTOR pathway [171]. Collectively, markers of CAFs and TAMs in endocrine-treated ER+ tumors may predict patient outcome and targeting the signaling pathways driving CAF/TAM activation represent an opportunity to inhibit endocrine resistant tumors.
Nutrient availability within the tumor microenvironment may also determine the metabolic dependencies of cancer cells by inducing reprogramming of amino acid, glucose or fatty acid metabolism, driving endocrine resistance and metastasis. Treatment with endocrine therapy may lead to nutrient deprivation by reducing glucose and glutamine uptake and total cellular ATP production which was followed by cell cycle arrest and cell death [172]. Endocrine resistant cells exhibit several metabolic alterations that counteract the effects of endocrine therapy on cancer cell metabolism. mTOR is the major kinase sensing the changes in cellular amino acid levels, such as glutamine [173] and it plays an important role in endocrine resistance. A higher glutamine dependency was reported in estrogen-independent and endocrine resistant ER+ tumors and inhibiting Glutaminase (GLS), an enzyme that converts glutamine to glutamate, in combination with the mTOR inhibitor, everolimus reduced cell growth [174]. Long-term estrogen-deprived (LTED) ER+ breast cancer cells were characterized by altered amino acid metabolism via activation of autophagy and enhanced import of acidic amino acids, mediated by Solute Carrier Family 1 Member 2 (SLC1A2), leading to endocrine resistance [175]. In another study, hypoxia was shown to revert the fulvestrant-dependent repression of the glutamine transporter, Solute Carrier Family 38 Member 2 (SNAT2) in a HIF1A-dependent manner, leading to fulvestrant resistance in vitro and in vivo [176].
Endocrine resistant cells were shown to exhibit higher glucose and glutamine uptake that is, in part, mediated by c-Myc overexpression whose inhibition reduced the uptake of glucose and glutamine leading to decreased cell growth [177]. Furthermore, glucose consumption rate was found to be higher in MCF-7 TamR cells which correlates with upregulation of PI3K/AKT/PTEN axis [178]. The ESR1 Y537S mutant carrying cells were shown to exhibit elevated tricarboxylic acid (TCA) cycle activity, glucose independence and reliance on glutamine as a carbon source [179]. The glucose catabolizing pentose phosphate pathway (PPP) has also been associated with endocrine resistance via upregulation of Nuclear Receptor Binding SET Domain Protein 2 (NSD2), a histone H3K36 methyltransferase which increased PPP activity, elevated NADPH production, and reduced reactive oxygen species (ROS) levels, without significantly altering glycolysis [81].
AI resistant invasive lobular breast carcinoma (ILC) cells were shown to activate key regulators of fatty acid and cholesterol metabolism, driving estrogen-independent growth and AI resistance [180]. High expression of Free Fatty Acid Receptor 4 (FFAR4), a receptor for long-chain free fatty acids was associated with worse survival in tamoxifen-treated ER+ breast cancer patients, and a synthetic FFAR4 agonist drives tamoxifen resistance via activating ERK and AKT pathways [181]. Furthermore, the sterol regulatory element-binding protein 1 (SREBP1), a regulator of fatty acid and cholesterol synthesis was shown to predict lack of endocrine response and found to be overexpressed in a LTED ER+ cell variant which was hypersensitive to genetic or pharmacological inhibition of SREBPs [180]. More recently, the fatty acid synthase (FASN) was shown to be critical for HER2-driven tamoxifen resistance, and a FASN inhibitor was demonstrated to fully restore the anti-estrogenic activity of tamoxifen in ER+/HER2+ breast cancer xenografts [182].
Metabolic reprogramming is also essential for metastasis in ER+ breast cancer. PFKFB4 which is a metabolic enzyme synthesizing fructose 2,6-bisphosphate, was found to be elevated in ER+ breast tumors, and to mediate SRC-3 phosphorylation, leading to enhanced target gene expression. Inhibiting PFKFB4 significantly reduced breast tumor growth and metastases [183]. Furthermore, inhibiting a Krebs cycle gene, Isocitrate dehydrogenase 1 (IDH1), significantly increased the migration rate in MCF-7 cells through HIF1A-dependent expression of SNAIL and TWIST [184]. A point mutation in IDH1 has been identified in a recurrent ER+ breast cancer patient at the primary tumor as well as at all metastatic sites, suggesting an involvement of metabolic adaptation also in metastatic tumors [185]. Likewise, several alterations that are known to drive endocrine resistance and have roles in metabolic regulation, such as c-Myc which facilitates the uptake of glutamine by inducing the expression of glutamine transporters and glutamine-metabolizing enzymes [186], was also identified in metastatic tumors of ER+ breast cancer patients [187]. However, depending on the metastatic site, cancer cells may also undergo differential metabolic reprogramming and acquire changes that are distinct from primary tumors. For instance, breast cancer cells metastasizing to lungs rewire their metabolism towards oxidative phosphorylation (OXPHOS) from glycolysis due to the oxygen-rich microenvironment in the lungs. This is in contrast with primary cells which exhibit high glycolysis rate [188]. On the other hand, breast cancer cells metastasizing to liver have reduced OXPHOS [189], whereas brain metastatic cells exhibit increased rates of glycolysis coupled to increased activity of Krebs cycle [190]. Overall, therapies targeting different elements of cancer cell metabolism have the potential to reduce the growth of metastatic colonies in MBC; although the therapeutic potential of metabolic therapy in combination with endocrine therapy still needs testing at the preclinical and clinical settings [191].
Alterations during metastatic recurrence in endocrine resistance
It is known that the recurrence risk in ER+ breast cancer patients is prolonged with approximately half of all distant recurrences occurring after 5 years and a persistence of relapse until 20 years [192]. The recent efforts on the genomic characterization of matched primary vs. metastases provides a unique opportunity to identify novel biomarkers and functional drivers of distant recurrence in MBC. Analyses of the alterations in the clonal composition of primary tumor as compared to their matched metastases samples from 242 MBC patients revealed an increase in clonality in genes with potential clinical impact, including ESR1 in ER+/HER2− MBC (endocrine resistance) and RB1 in ER+/HER2− and HER2+ MBC (resistance to CDK4/6 inhibitors) [187]. ESR1 mutations were also identified in metastatic samples of luminal breast cancer patients treated with AIs, consistent with the findings on the role of ESR1 mutations in AI resistance [193]. Activating HER2 mutations were identified in metastatic biopsies of ER+ breast cancer patients who had developed resistance to aromatase inhibitors, tamoxifen or fulvestrant whereas treatment-naïve primary tumors showed no evidence of these mutations [194]. HER2 mutations were found to be mutually exclusive with ESR1 mutations, suggesting distinct mechanisms of acquired endocrine resistance [194]. An FGFR4-induced signature was found to be significantly higher in ER+ tumor metastases compared with their primaries, and to predict clinical outcome [195]. Interestingly, FGFR4 signature also predicts a subtype switch from luminal A primary tumor to HER2-enriched metastatic tumor, suggesting that FGFR4 might be a functional important target for metastatic ER+ breast cancer. Furthermore, recurrent gains in RET expression and HER2 signaling were identified in brain metastases as compared to primary tumors of MBC patients and using a RET inhibitor, cabozantinib or a pan-HER pathway inhibitor, afatinib significantly reduced the proliferation of ex vivo cultures of a brain metastatic tumor obtained from an endocrine resistant patient [196]. Alterations in actionable genes in CDK/Rb/E2F and FGFR signaling pathways were also identified in bone metastases of ER+ breast cancer patients as compared to matched primary samples [197].
Clinical Management of Endocrine Resistance and Ongoing Trials
Current therapies
Current therapeutic strategies used in endocrine resistant disease include inhibitors against mTOR, CDK4/6 and PI3K subunit, p110α (Fig. 2). Everolimus, an mTOR inhibitor, was approved by the FDA in 2012 in combination with exemestane for treating postmenopausal women with advanced ER+HER2− breast cancer who progressed on prior treatment with letrozole or anastrozole. The approval was based on the results of a Phase III BOLERO-2 trial (NCT00863655) which demonstrated a 4.1-month improvement in median progression-free survival [198] (Table 2). Palbociclib, a highly selective serine/threonine kinase inhibitor of CDK4/6, had been tested in preclinical studies and early clinical trials and demonstrated anti-tumor efficacy as a monotherapy, as well as in combination with endocrine therapy in ER+ breast cancer models. Two pivotal clinical studies; PALOMA-2 and PALOMA-3 tested Palbociclib as a first-line agent in combination with letrozole (NCT01740427), and as a combination therapy after progression on prior endocrine therapy (NCT01942135), respectively [127, 128]. Based on the significant improvement in progression-free survival in these studies, palbociclib was granted the FDA approval in 2015 in post-menopausal women with ER+HER2− advanced breast cancer as a first-line therapy in combination with letrozole. Later in 2016, the approval was extended as a combination with any aromatase inhibitor, and also to be used in advanced or metastatic breast cancer with disease progression following endocrine therapy. Currently, Palbociclib is also being tested in combination with tamoxifen as a first line therapy (NCT02668666). In addition to palbociclib, there are two other CDK4/6 inhibitors; ribociclib and abemaciclib used in clinics. Both are approved in combination with an AIs as first-line therapy for ER+/HER2− advanced breast cancer, and abemaciclib is also approved in combination with fulvestrant in ER+/HER2− advanced breast cancer with progressive disease [199]. Their efficacy are likely to be equivalent [199] although there might be differences in terms of treatment-related adverse effects [200]. The p110α inhibitor, Alpelisib is the first and only PI3K inhibitor that was approved by the FDA in 2019 in combination with fulvestrant for postmenopausal women with ER+HER2−, PIK3CA-mutated, advanced, or metastatic breast cancer (NCT02437318) [201].
Figure 2.
Current and potential future therapeutic options to treat endocrine resistant ER+ breast cancer patients. Current treatment options to treat endocrine resistant ER+ patients include targeting of the cell cycle modulator kinases, cyclin-dependent kinases 4/6 (CDK4/6) by palbociclib, ribociclib and abemaciclib; targeting of mechanistic target of rapamycin (mTOR) kinase by Everolimus; and targeting of P110α subunit of Phosphoinositide 3-kinase (PI3K) by alpelisib. In addition to these strategies, there are several inhibitors that are currently under clinical investigation for the treatment of endocrine resistant ER+ patients such as the AKT Serine/Threonine Kinase (AKT) inhibitor, ipatasertib; the ER partial agonist, TTC-352; the ER proteolysis targeting chimeras (PROTACs), ARV-471; the next-generation selective ER down-regulator (SERD), elacestrant; the epidermal growth factor receptor 1 &2 (EGFR/HER2) dual inhibitor, neratinib; the HER2 targeting antibody-drug conjugate, trastuzumab deruxtecan; and the histone deacetylase (HDAC) inhibitor, entinostat.
Ongoing clinical testing for future therapies
Currently, there are several clinical trials completed or ongoing, testing the safety and efficacy of novel therapy options in endocrine resistant disease (Table 2, Fig. 2). The mTOR inhibitor, everolimus which had initially been approved in combination with exemestane in postmenopausal women progressed on prior endocrine therapy, has recently been tested in a Phase II trial as a first line therapy in combination with letrozole in post-menopausal ER+HER2− patients and demonstrated enhanced PFS as compared to letrozole alone (NCT01698918) [202], suggesting that combination of everolimus and endocrine therapy might be a good first line treatment option in postmenopausal women with ER+HER2− breast cancer.
A Phase I trial with the selective ER partial agonist, TTC-352 in metastatic ER+ breast cancer patients who received and progressed on at least two lines of endocrine therapy has been completed (NCT03201913). It demonstrated safety and early clinical evidence of antitumor activity, encouraging the follow up Phase II trials [203]. Second-generation SERDs have proven to be effective against endocrine resistant disease in preclinical studies in in vitro and in vivo settings. Elacestrant (RAD1901) is a novel, nonsteroidal, orally bioavailable SERD which induces dose-dependent ER degradation and inhibition of the estrogen-dependent gene transcription, leading to reduced cell proliferation in multiple ER+ breast cancer cell lines and xenografts, including those that were derived from heavily pretreated patients [204, 205]. It is also effective against models resistant to CDK4/6 inhibitors and fulvestrant [204, 206]. In a Phase I trial, RAD1901 showed an acceptable safety profile and also exhibited single-agent activity with confirmed partial responses in heavily pretreated patients with ER+ MBC, including patients with ESR1 mutation as well as those with prior CDK4/6i and SERD treatment (NCT02338349) [31]. A Phase III trial (EMERALD, NCT03778931 [207]) is still ongoing evaluating the safety and efficacy of RAD1901 for treatment of postmenopausal women with ER+/HER2 advanced or MBC who have progressed on one or two prior lines of endocrine therapy and CDK4/6 inhibitor therapy in combination with an AI or fulvestrant. The ER degrader, ARV-471 is the first ER PROTAC that is now being tested in a Phase I/II trial alone or in combination with palbociclib in patients with ER+/HER2− locally advanced or metastatic breast cancer, progressed on prior hormonal therapy and chemotherapy (NCT04072952).
An EGFR/HER2 dual inhibitor, neratinib, is currently being tested in an open-label, single arm, multicenter phase II study in combination with endocrine therapy in pre- and post-menopausal women with locally advanced or metastatic HER2+ or ER+/HER2-negative breast cancer who had recurrence or progression following prior treatment with AIs, tamoxifen or fulvestrant (NCT04460430). Ipatasertib, an AKT inhibitor, is currently being tested in a Phase I trial (TAKTIC, NCT03959891) in ER+HER2− breast patients and in postmenopausal women in combination with an AI or fulvestrant, with or without Palbociclib. The initial results demonstrated that the triple combination was well tolerated, and a subset of the patients showed signs of clinical benefit, encouraging the follow-up studies [208]. Recently, HER2-targeting antibody drug conjugates (ADC) were shown to have clinical efficacy in ER+HER2− endocrine resistant patients. The anti-tumorigenic activity of these HER2-targeting agents even in HER2-low tumors was attributed to the release of ADC payload before internalization or high membrane permeability causing the entry of the ADC into the cells without HER2 binding [209]. The DAISY trial is currently recruiting a wide group of metastatic breast cancer patients including HER2-low, ER+ patients who are resistant to endocrine therapies to test the efficacy of Trastuzumab deruxtecan, a highly promising second-generation ADC (NCT04132960).
Motivated by the large numbers of preclinical studies showing sensitization to endocrine therapy upon HDAC inhibition, an HDAC inhibitor, entinostat has been tested in combination with exemestane in AI resistant patients. The first of these clinical trials was a phase II randomized, placebo-controlled study (ENCORE 301, NCT00676663) which demonstrated a significant improvement in progression-free survival (PFS) and overall survival (OS), with the addition of entinostat to exemestane in patients with ER+ advanced breast cancer with disease progression after prior non-steroidal AI therapy [210]. Based on these promising results, a phase III trial (E2112, NCT02115282) was performed to investigate the efficacy of entinostat or placebo in combination with exemestane in ER+HER2− patients with locally advanced or metastatic breast cancer progressed on AI treatment. The initial findings seem to fail to confirm the results of the ENCORE 301 trial, as the combination of exemestane and entinostat did not improve survival in AI resistant patients [211]. Further clinical testing is warranted to assess the potential benefit of HDAC inhibitors in overcoming endocrine resistance in clinics.
Conclusions and Future Perspectives
Endocrine resistance is a major challenge in clinics that hinders the short- or long-term efficacy of endocrine therapy in ER+ breast cancer patients, leading to poor clinical outcome. Among the established mechanisms of resistance, alterations in ER in terms of expression level, mutations or fusions have been one of the major mechanisms given that ER is the main target of endocrine therapy. Second generation SERDs which were proven to be effective against ESR1 mutant carrying tumors, and the latest application of the PROTAC technology that enables stronger ER degradation represents an opportunity for more effective targeting of the estrogen signaling that will ultimately result in better clinical responses.
The extensive crosstalk between ER and the cellular signaling pathways involves not only the receptor tyrosine kinases, and the downstream PI3K/AKT/mTOR and ERK/MAPK pathways, but also involves an interplay with cellular stress signaling (e.g., EnR stress and autophagy) that is fueled by the genomic as well as and non-genomic activity of ER. This intricate network of signaling pathways, on one hand, provides growth advantage to the estrogen-independent resistant cells, and on the other hand, fuels ER signaling in those that still rely on estrogen. Further studies are needed to uncover the key players that coordinate the crosstalk between ER and cellular stress signaling which will also result in identification of potential targets to eradicate resistant tumors.
The compelling preclinical evidence supported by analyses of clinical samples, on the acquisition and maintenance of a mesenchymal, stem-like state during endocrine resistance suggests that targeting the key players that simultaneously regulate both endocrine resistance and metastasis will result in inhibition the growth of resistant tumors as well as metastatic dissemination and distant recurrence. Considering that late recurrence upon endocrine therapy is common in ER+ breast cancer due to tumor cell dormancy, extensive characterization of the EMT, CTCs and invasiveness in endocrine resistance will likely identify useful biomarkers, as well as targetable molecules to combat endocrine resistance and metastasis. Despite the traditional view of the lack of immunogenicity in ER+ breast tumors, a growing body of evidence pointed out a potential benefit of combining immunotherapy with endocrine therapy, especially in certain disease settings. More work on the causality of immune checkpoint components in endocrine resistance is needed to determine the clinical benefit of combining endocrine therapy with immunotherapy. Furthermore, the longitudinal sample analyses will help us better understand the disease progression and molecular alterations in metastatic setting/recurrence, which will further enable identification of biomarkers and druggable targets.
Overall, endocrine resistance can be manifested in a variety of different phenotypes accompanied by different molecular alterations. As our knowledge on the role of these alterations and the potential therapeutic strategies to eradicate endocrine resistant tumors harboring these alterations expands, careful design of the clinical trials based on these alterations would allow efficient testing of the preclinical findings. This will ultimately result in rapid approval of new treatment options that will improve the outcome of resistant and metastatic disease.
Funding:
This work was supported by the American Cancer Society Research Scholar Grant RSG-19-194-01-CSM (OS), National Institutes of Health Grant 2P20GM109091-06 (OS), and Susan G. Komen Interdisciplinary Graduate Training to Eliminate Cancer Disparities (IGniTE-CD) GTDR17500160 (OzgeS).
Footnotes
Conflict of interest: O. Sahin is co-founder of OncoCube Therapeutics LLC; founder and president of LoxiGen, Inc.; and is also a recipient of a research grant from Halozyme Therapeutics, Inc. The other authors declare that they have no conflict of interest.
Consent for publication
Consent to publish has been obtained from all authors.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries (2018) CA: a cancer journal for clinicians 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A et al. Breast cancer statistics, 2019 (2019) CA: a cancer journal for clinicians 69: 438–451. [DOI] [PubMed] [Google Scholar]
- 3.Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer (2009) Nat Rev Cancer 9: 631–643. [DOI] [PubMed] [Google Scholar]
- 4.Theodorou V, Stark R, Menon S, Carroll JS. GATA3 acts upstream of FOXA1 in mediating ESR1 binding by shaping enhancer accessibility (2013) Genome Res 23: 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Safe S, Kim K. Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathways (2008) J Mol Endocrinol 41: 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glass CK, Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors (2000) Genes Dev 14: 121–141. [PubMed] [Google Scholar]
- 7.Lonard DM, O'Malley BW. The expanding cosmos of nuclear receptor coactivators (2006) Cell 125: 411–414. [DOI] [PubMed] [Google Scholar]
- 8.Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and actions of estrogens (2002) The New England journal of medicine 346: 340–352. [DOI] [PubMed] [Google Scholar]
- 9.Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes (2005) Mol Endocrinol 19: 833–842. [DOI] [PubMed] [Google Scholar]
- 10.McDonnell DP, Norris JD. Connections and regulation of the human estrogen receptor (2002) Science 296: 1642–1644. [DOI] [PubMed] [Google Scholar]
- 11.Early Breast Cancer Trialists' Collaborative G, Davies C, Godwin J, Gray R, Clarke M, Cutter D et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials (2011) Lancet 378: 771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeCensi A, Puntoni M, Guerrieri-Gonzaga A, Caviglia S, Avino F, Cortesi L et al. Randomized Placebo Controlled Trial of Low-Dose Tamoxifen to Prevent Local and Contralateral Recurrence in Breast Intraepithelial Neoplasia (2019) Journal of clinical oncology : official journal of the American Society of Clinical Oncology: JCO1801779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial (2013) Lancet 381: 805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group (2001) Journal of clinical oncology : official journal of the American Society of Clinical Oncology 19: 2596–2606. [DOI] [PubMed] [Google Scholar]
- 15.Lumachi F, Santeufemia DA, Basso SM. Current medical treatment of estrogen receptor-positive breast cancer (2015) World journal of biological chemistry 6: 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howell A, Robertson JF, Abram P, Lichinitser MR, Elledge R, Bajetta E et al. Comparison of fulvestrant versus tamoxifen for the treatment of advanced breast cancer in postmenopausal women previously untreated with endocrine therapy: a multinational, double-blind, randomized trial (2004) Journal of clinical oncology : official journal of the American Society of Clinical Oncology 22: 1605–1613. [DOI] [PubMed] [Google Scholar]
- 17.Vergote I, Robertson JF. Fulvestrant is an effective and well-tolerated endocrine therapy for postmenopausal women with advanced breast cancer: results from clinical trials (2004) Br J Cancer 90 Suppl 1: S11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Augereau P, Patsouris A, Bourbouloux E, Gourmelon C, Abadie Lacourtoisie S, Berton Rigaud D et al. Hormonoresistance in advanced breast cancer: a new revolution in endocrine therapy (2017) Therapeutic advances in medical oncology 9: 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutierrez MC, Detre S, Johnston S, Mohsin SK, Shou J, Allred DC et al. Molecular changes in tamoxifen-resistant breast cancer: relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase (2005) Journal of clinical oncology : official journal of the American Society of Clinical Oncology 23: 2469–2476. [DOI] [PubMed] [Google Scholar]
- 20.Ottaviano YL, Issa JP, Parl FF, Smith HS, Baylin SB, Davidson NE. Methylation of the estrogen receptor gene CpG island marks loss of estrogen receptor expression in human breast cancer cells (1994) Cancer Res 54: 2552–2555. [PubMed] [Google Scholar]
- 21.Yang X, Ferguson AT, Nass SJ, Phillips DL, Butash KA, Wang SM et al. Transcriptional activation of estrogen receptor alpha in human breast cancer cells by histone deacetylase inhibition (2000) Cancer Res 60: 6890–6894. [PubMed] [Google Scholar]
- 22.Vesuna F, Lisok A, Kimble B, Domek J, Kato Y, van der Groep P et al. Twist contributes to hormone resistance in breast cancer by downregulating estrogen receptor-alpha (2012) Oncogene 31: 3223–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toy W, Shen Y, Won H, Green B, Sakr RA, Will M et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer (2013) Nat Genet 45: 1439–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toy W, Weir H, Razavi P, Lawson M, Goeppert AU, Mazzola AM et al. Activating ESR1 Mutations Differentially Affect the Efficacy of ER Antagonists (2017) Cancer Discov 7: 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katzenellenbogen JA, Mayne CG, Katzenellenbogen BS, Greene GL, Chandarlapaty S. Structural underpinnings of oestrogen receptor mutations in endocrine therapy resistance (2018) Nat Rev Cancer 18: 377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merenbakh-Lamin K, Ben-Baruch N, Yeheskel A, Dvir A, Soussan-Gutman L, Jeselsohn R et al. D538G mutation in estrogen receptor-alpha: A novel mechanism for acquired endocrine resistance in breast cancer (2013) Cancer Res 73: 6856–6864. [DOI] [PubMed] [Google Scholar]
- 27.Harrod A, Fulton J, Nguyen VTM, Periyasamy M, Ramos-Garcia L, Lai CF et al. Genomic modelling of the ESR1 Y537S mutation for evaluating function and new therapeutic approaches for metastatic breast cancer (2017) Oncogene 36: 2286–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeselsohn R, Bergholz JS, Pun M, Cornwell M, Liu W, Nardone A et al. Allele-Specific Chromatin Recruitment and Therapeutic Vulnerabilities of ESR1 Activating Mutations (2018) Cancer Cell 33: 173–186 e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnesen S, Blanchard Z, Williams MM, Berrett KC, Li Z, Oesterreich S et al. Estrogen Receptor Alpha Mutations in Breast Cancer Cells Cause Gene Expression Changes through Constant Activity and Secondary Effects (2021) Cancer Res 81: 539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fanning SW, Greene GL. Next-Generation ERalpha Inhibitors for Endocrine-Resistant ER+ Breast Cancer (2019) Endocrinology 160: 759–769. [DOI] [PubMed] [Google Scholar]
- 31.Bardia A, Kaklamani V, Wilks S, Weise A, Richards D, Harb W et al. Phase I Study of Elacestrant (RAD1901), a Novel Selective Estrogen Receptor Degrader, in ER-Positive, HER2-Negative Advanced Breast Cancer (2021) Journal of clinical oncology : official journal of the American Society of Clinical Oncology 39: 1360–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim Elgene KLJ, Perez-Fidalgo Jose Alejandro, Bellet Meritxell, Boni Valentina, Garcia Jose Manuel Perez, Estevez Laura, Bardia Aditya, Turner Nicholas C., Villanueva Rafael, Cobo Sara Lopez-Tarruella, Im Seock-Ah, Kim Sung-Bae, Gates Mary R., Monemi Sharareh, Chen Ya-Chi, Moore Heather, Loi Sherene, Sohn Joohyuk. A phase Ib study to evaluate the oral selective estrogen receptor degrader GDC-9545 alone or combined with palbociclib in metastatic ER-positive HER2-negative breast cancer (2020) Journal of Clinical Oncology 38, no. 15_suppl: 1023–1023. [Google Scholar]
- 33.Giltnane JM, Hutchinson KE, Stricker TP, Formisano L, Young CD, Estrada MV et al. Genomic profiling of ER(+) breast cancers after short-term estrogen suppression reveals alterations associated with endocrine resistance (2017) Sci Transl Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartmaier RJ, Trabucco SE, Priedigkeit N, Chung JH, Parachoniak CA, Vanden Borre P et al. Recurrent hyperactive ESR1 fusion proteins in endocrine therapy-resistant breast cancer (2018) Ann Oncol 29: 872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lei JT, Shao J, Zhang J, Iglesia M, Chan DW, Cao J et al. Functional Annotation of ESR1 Gene Fusions in Estrogen Receptor-Positive Breast Cancer (2018) Cell Rep 24: 1434–1444 e1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veeraraghavan J, Tan Y, Cao XX, Kim JA, Wang X, Chamness GC et al. Recurrent ESR1-CCDC170 rearrangements in an aggressive subset of oestrogen receptor-positive breast cancers (2014) Nat Commun 5: 4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L, Lin L, Veeraraghavan J, Hu Y, Wang X, Lee S et al. Therapeutic role of recurrent ESR1-CCDC170 gene fusions in breast cancer endocrine resistance (2020) Breast Cancer Res 22: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeong JH, Yun JW, Kim HY, Heo CY, Lee S. Elucidation of Novel Therapeutic Targets for Breast Cancer with ESR1-CCDC170 Fusion (2021) J Clin Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kushner MH, Ory V, Graham GT, Sharif GM, Kietzman WB, Thevissen S et al. Loss of ANCO1 repression at AIB1/YAP targets drives breast cancer progression (2020) EMBO Rep 21: e48741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Amicis F, Chiodo C, Morelli C, Casaburi I, Marsico S, Bruno R et al. AIB1 sequestration by androgen receptor inhibits estrogen-dependent cyclin D1 expression in breast cancer cells (2019) BMC Cancer 19: 1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA et al. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer (2003) Journal of the National Cancer Institute 95: 353–361. [DOI] [PubMed] [Google Scholar]
- 42.Hurtado A, Holmes KA, Geistlinger TR, Hutcheson IR, Nicholson RI, Brown M et al. Regulation of ERBB2 by oestrogen receptor-PAX2 determines response to tamoxifen (2008) Nature 456: 663–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vareslija D, Ward E, Purcell SP, Cosgrove NS, Cocchiglia S, O'Halloran PJ et al. Comparative analysis of the AIB1 interactome in breast cancer reveals MTA2 as a repressive partner which silences E-Cadherin to promote EMT and associates with a pro-metastatic phenotype (2021) Oncogene 40: 1318–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Truong TH, Hu H, Temiz NA, Hagen KM, Girard BJ, Brady NJ et al. Cancer Stem Cell Phenotypes in ER(+) Breast Cancer Models Are Promoted by PELP1/AIB1 Complexes (2018) Mol Cancer Res 16: 707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alkner S, Jensen MB, Rasmussen BB, Bendahl PO, Ferno M, Ryden L et al. Prognostic and predictive importance of the estrogen receptor coactivator AIB1 in a randomized trial comparing adjuvant letrozole and tamoxifen therapy in postmenopausal breast cancer: the Danish cohort of BIG 1-98 (2017) Breast Cancer Res Treat 166: 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Narbe U, Sjostrom M, Forsare C, Bendahl PO, Alkner S, Leeb-Lundberg LMF et al. The estrogen receptor coactivator AIB1 is a new putative prognostic biomarker in ER-positive/HER2-negative invasive lobular carcinoma of the breast (2019) Breast Cancer Res Treat 175: 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McBryan J, Theissen SM, Byrne C, Hughes E, Cocchiglia S, Sande S et al. Metastatic progression with resistance to aromatase inhibitors is driven by the steroid receptor coactivator SRC-1 (2012) Cancer Res 72: 548–559. [DOI] [PubMed] [Google Scholar]
- 48.Browne AL, Charmsaz S, Vareslija D, Fagan A, Cosgrove N, Cocchiglia S et al. Network analysis of SRC-1 reveals a novel transcription factor hub which regulates endocrine resistant breast cancer (2018) Oncogene 37: 2008–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ward E, Vareslija D, Charmsaz S, Fagan A, Browne AL, Cosgrove N et al. Epigenome-wide SRC-1-Mediated Gene Silencing Represses Cellular Differentiation in Advanced Breast Cancer (2018) Clin Cancer Res 24: 3692–3703. [DOI] [PubMed] [Google Scholar]
- 50.Watters RJ, Verdelis K, Lucas PC, Jiang S, Chen Y, Lu F et al. A Novel Mouse Model for SNP in Steroid Receptor Co-Activator-1 Reveals Role in Bone Density and Breast Cancer Metastasis (2021) Endocrinology 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dobrzycka KM, Townson SM, Jiang S, Oesterreich S. Estrogen receptor corepressors -- a role in human breast cancer? (2003) Endocr Relat Cancer 10: 517–536. [DOI] [PubMed] [Google Scholar]
- 52.Legare S, Basik M. Minireview: The Link Between ERalpha Corepressors and Histone Deacetylases in Tamoxifen Resistance in Breast Cancer (2016) Mol Endocrinol 30: 965–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keeton EK, Brown M. Cell cycle progression stimulated by tamoxifen-bound estrogen receptor-alpha and promoter-specific effects in breast cancer cells deficient in N-CoR and SMRT (2005) Mol Endocrinol 19: 1543–1554. [DOI] [PubMed] [Google Scholar]
- 54.Lu R, Hu X, Zhou J, Sun J, Zhu AZ, Xu X et al. COPS5 amplification and overexpression confers tamoxifen-resistance in ERalpha-positive breast cancer by degradation of NCoR (2016) Nat Commun 7: 12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Girault I, Lerebours F, Amarir S, Tozlu S, Tubiana-Hulin M, Lidereau R et al. Expression analysis of estrogen receptor alpha coregulators in breast carcinoma: evidence that NCOR1 expression is predictive of the response to tamoxifen (2003) Clin Cancer Res 9: 1259–1266. [PubMed] [Google Scholar]
- 56.Miller TW, Balko JM, Ghazoui Z, Dunbier A, Anderson H, Dowsett M et al. A gene expression signature from human breast cancer cells with acquired hormone independence identifies MYC as a mediator of antiestrogen resistance (2011) Clin Cancer Res 17: 2024–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jansen-Durr P, Meichle A, Steiner P, Pagano M, Finke K, Botz J et al. Differential modulation of cyclin gene expression by MYC (1993) Proc Natl Acad Sci U S A 90: 3685–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mukherjee S, Conrad SE. c-Myc suppresses p21WAF1/CIP1 expression during estrogen signaling and antiestrogen resistance in human breast cancer cells (2005) J Biol Chem 280: 17617–17625. [DOI] [PubMed] [Google Scholar]
- 59.Ellis MJ, Ding L, Shen D, Luo J, Suman VJ, Wallis JW et al. Whole-genome analysis informs breast cancer response to aromatase inhibition (2012) Nature 486: 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Bragt MP, Hu X, Xie Y, Li Z. RUNX1, a transcription factor mutated in breast cancer, controls the fate of ER-positive mammary luminal cells (2014) Elife 3: e03881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chimge NO, Little GH, Baniwal SK, Adisetiyo H, Xie Y, Zhang T et al. RUNX1 prevents oestrogen-mediated AXIN1 suppression and beta-catenin activation in ER-positive breast cancer (2016) Nat Commun 7: 10751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Razavi P, Chang MT, Xu G, Bandlamudi C, Ross DS, Vasan N et al. The Genomic Landscape of Endocrine-Resistant Advanced Breast Cancers (2018) Cancer Cell 34: 427–438 e426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fiorito E, Sharma Y, Gilfillan S, Wang S, Singh SK, Satheesh SV et al. CTCF modulates Estrogen Receptor function through specific chromatin and nuclear matrix interactions (2016) Nucleic Acids Res 44: 10588–10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hurtado A, Holmes KA, Ross-Innes CS, Schmidt D, Carroll JS. FOXA1 is a key determinant of estrogen receptor function and endocrine response (2011) Nat Genet 43: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fu X, Jeselsohn R, Pereira R, Hollingsworth EF, Creighton CJ, Li F et al. FOXA1 overexpression mediates endocrine resistance by altering the ER transcriptome and IL-8 expression in ER-positive breast cancer (2016) Proc Natl Acad Sci U S A 113: E6600–E6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fu X, Pereira R, De Angelis C, Veeraraghavan J, Nanda S, Qin L et al. FOXA1 upregulation promotes enhancer and transcriptional reprogramming in endocrine-resistant breast cancer (2019) Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu G, Chhangawala S, Cocco E, Razavi P, Cai Y, Otto JE et al. ARID1A determines luminal identity and therapeutic response in estrogen-receptor-positive breast cancer (2020) Nat Genet 52: 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chia K, Milioli H, Portman N, Laven-Law G, Coulson R, Yong A et al. Non-canonical AR activity facilitates endocrine resistance in breast cancer (2019) Endocr Relat Cancer 26: 251–264. [DOI] [PubMed] [Google Scholar]
- 69.D'Amato NC, Gordon MA, Babbs B, Spoelstra NS, Carson Butterfield KT, Torkko KC et al. Cooperative Dynamics of AR and ER Activity in Breast Cancer (2016) Mol Cancer Res 14: 1054–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Creevey L, Bleach R, Madden SF, Toomey S, Bane FT, Vareslija D et al. Altered Steroid Milieu in AI-Resistant Breast Cancer Facilitates AR Mediated Gene-Expression Associated with Poor Response to Therapy (2019) Mol Cancer Ther 18: 1731–1743. [DOI] [PubMed] [Google Scholar]
- 71.Hickey TE, Selth LA, Chia KM, Laven-Law G, Milioli HH, Roden D et al. The androgen receptor is a tumor suppressor in estrogen receptor-positive breast cancer (2021) Nat Med 27: 310–320. [DOI] [PubMed] [Google Scholar]
- 72.Mohammed H, Russell IA, Stark R, Rueda OM, Hickey TE, Tarulli GA et al. Progesterone receptor modulates ERalpha action in breast cancer (2015) Nature 523: 313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications (2011) Cell Res 21: 381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zou Z, Luo X, Nie P, Wu B, Zhang T, Wei Y et al. Inhibition of SRC-3 enhances sensitivity of human cancer cells to histone deacetylase inhibitors (2016) Biochem Biophys Res Commun 478: 227–233. [DOI] [PubMed] [Google Scholar]
- 75.Raha P, Thomas S, Thurn KT, Park J, Munster PN. Combined histone deacetylase inhibition and tamoxifen induces apoptosis in tamoxifen-resistant breast cancer models, by reversing Bcl-2 overexpression (2015) Breast Cancer Res 17: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sabnis GJ, Goloubeva OG, Kazi AA, Shah P, Brodie AH. HDAC inhibitor entinostat restores responsiveness of letrozole-resistant MCF-7Ca xenografts to aromatase inhibitors through modulation of Her-2 (2013) Mol Cancer Ther 12: 2804–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou Q, Atadja P, Davidson NE. Histone deacetylase inhibitor LBH589 reactivates silenced estrogen receptor alpha (ER) gene expression without loss of DNA hypermethylation (2007) Cancer Biol Ther 6: 64–69. [DOI] [PubMed] [Google Scholar]
- 78.Linares A, Assou S, Lapierre M, Thouennon E, Duraffourd C, Fromaget C et al. Increased expression of the HDAC9 gene is associated with antiestrogen resistance of breast cancers (2019) Mol Oncol 13: 1534–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu S, Gong X, Ma Z, Zhang M, Huang L, Zhang J et al. Endocrine resistant breast cancer cells with loss of ERalpha expression retain proliferative ability by reducing caspase7-mediated HDAC3 cleavage (2020) Cell Oncol (Dordr) 43: 65–80. [DOI] [PubMed] [Google Scholar]
- 80.Hinohara K, Wu HJ, Vigneau S, McDonald TO, Igarashi KJ, Yamamoto KN et al. KDM5 Histone Demethylase Activity Links Cellular Transcriptomic Heterogeneity to Therapeutic Resistance (2018) Cancer Cell 34: 939–953 e939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang J, Duan Z, Nugent Z, Zou JX, Borowsky AD, Zhang Y et al. Reprogramming metabolism by histone methyltransferase NSD2 drives endocrine resistance via coordinated activation of pentose phosphate pathway enzymes (2016) Cancer letters 378: 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang J, Zhou C, Jiang H, Liang L, Shi W, Zhang Q et al. ZEB1 induces ER-alpha promoter hypermethylation and confers antiestrogen resistance in breast cancer (2017) Cell Death Dis 8: e2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ciana P, Ghisletti S, Mussi P, Eberini I, Vegeto E, Maggi A. Estrogen receptor alpha, a molecular switch converting transforming growth factor-alpha-mediated proliferation into differentiation in neuroblastoma cells (2003) J Biol Chem 278: 31737–31744. [DOI] [PubMed] [Google Scholar]
- 84.Lee AV, Cui X, Oesterreich S. Cross-talk among estrogen receptor, epidermal growth factor, and insulin-like growth factor signaling in breast cancer (2001) Clin Cancer Res 7: 4429s–4435s; discussion 4411s-4412s. [PubMed] [Google Scholar]
- 85.Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance (2001) J Biol Chem 276: 9817–9824. [DOI] [PubMed] [Google Scholar]
- 86.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase (1995) Science 270: 1491–1494. [DOI] [PubMed] [Google Scholar]
- 87.Font de Mora J, Brown M. AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor (2000) Mol Cell Biol 20: 5041–5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer (2004) Journal of the National Cancer Institute 96: 926–935. [DOI] [PubMed] [Google Scholar]
- 89.Hong SH, Privalsky ML. The SMRT corepressor is regulated by a MEK-1 kinase pathway: inhibition of corepressor function is associated with SMRT phosphorylation and nuclear export (2000) Mol Cell Biol 20: 6612–6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ellis MJ, Tao Y, Young O, White S, Proia AD, Murray J et al. Estrogen-independent proliferation is present in estrogen-receptor HER2-positive primary breast cancer after neoadjuvant letrozole (2006) Journal of clinical oncology : official journal of the American Society of Clinical Oncology 24: 3019–3025. [DOI] [PubMed] [Google Scholar]
- 91.Fox EM, Miller TW, Balko JM, Kuba MG, Sanchez V, Smith RA et al. A kinome-wide screen identifies the insulin/IGF-I receptor pathway as a mechanism of escape from hormone dependence in breast cancer (2011) Cancer Res 71: 6773–6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Arpino G, Wiechmann L, Osborne CK, Schiff R. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance (2008) Endocr Rev 29: 217–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mao P, Cohen O, Kowalski KJ, Kusiel JG, Buendia-Buendia JE, Cuoco MS et al. Acquired FGFR and FGF Alterations Confer Resistance to Estrogen Receptor (ER) Targeted Therapy in ER(+) Metastatic Breast Cancer (2020) Clin Cancer Res 26: 5974–5989. [DOI] [PubMed] [Google Scholar]
- 94.Lupien M, Meyer CA, Bailey ST, Eeckhoute J, Cook J, Westerling T et al. Growth factor stimulation induces a distinct ER(alpha) cistrome underlying breast cancer endocrine resistance (2010) Genes Dev 24: 2219–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Drago JZ, Formisano L, Juric D, Niemierko A, Servetto A, Wander SA et al. FGFR1 Amplification Mediates Endocrine Resistance but Retains TORC Sensitivity in Metastatic Hormone Receptor-Positive (HR(+)) Breast Cancer (2019) Clin Cancer Res 25: 6443–6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tomlinson DC, Knowles MA, Speirs V. Mechanisms of FGFR3 actions in endocrine resistant breast cancer (2012) Int J Cancer 130: 2857–2866. [DOI] [PubMed] [Google Scholar]
- 97.Bosch A, Li Z, Bergamaschi A, Ellis H, Toska E, Prat A et al. PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer (2015) Sci Transl Med 7: 283ra251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ribas R, Pancholi S, Guest SK, Marangoni E, Gao Q, Thuleau A et al. AKT Antagonist AZD5363 Influences Estrogen Receptor Function in Endocrine-Resistant Breast Cancer and Synergizes with Fulvestrant (ICI182780) In Vivo (2015) Mol Cancer Ther 14: 2035–2048. [DOI] [PubMed] [Google Scholar]
- 99.Croessmann S, Formisano L, Kinch LN, Gonzalez-Ericsson PI, Sudhan DR, Nagy RJ et al. Combined Blockade of Activating ERBB2 Mutations and ER Results in Synthetic Lethality of ER+/HER2 Mutant Breast Cancer (2019) Clin Cancer Res 25: 277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smyth LM, Piha-Paul SA, Won HH, Schram AM, Saura C, Loi S et al. Efficacy and Determinants of Response to HER Kinase Inhibition in HER2-Mutant Metastatic Breast Cancer (2020) Cancer Discov 10: 198–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Andre F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer (2019) The New England journal of medicine 380: 1929–1940. [DOI] [PubMed] [Google Scholar]
- 102.Zheng Y, Sowers JY, Houston KD. IGFBP-1 Expression Promotes Tamoxifen Resistance in Breast Cancer Cells via Erk Pathway Activation (2020) Front Endocrinol (Lausanne) 11: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Harrison H, Farnie G, Howell SJ, Rock RE, Stylianou S, Brennan KR et al. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor (2010) Cancer Res 70: 709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome (2007) Cell Stem Cell 1: 555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Piva M, Domenici G, Iriondo O, Rabano M, Simoes BM, Comaills V et al. Sox2 promotes tamoxifen resistance in breast cancer cells (2014) EMBO Mol Med 6: 66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dubrovska A, Hartung A, Bouchez LC, Walker JR, Reddy VA, Cho CY et al. CXCR4 activation maintains a stem cell population in tamoxifen-resistant breast cancer cells through AhR signalling (2012) Br J Cancer 107: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Simoes BM, O'Brien CS, Eyre R, Silva A, Yu L, Sarmiento-Castro A et al. Anti-estrogen Resistance in Human Breast Tumors Is Driven by JAG1-NOTCH4-Dependent Cancer Stem Cell Activity (2015) Cell Rep 12: 1968–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McClements L, Annett S, Yakkundi A, O'Rourke M, Valentine A, Moustafa N et al. FKBPL and its peptide derivatives inhibit endocrine therapy resistant cancer stem cells and breast cancer metastasis by downregulating DLL4 and Notch4 (2019) BMC Cancer 19: 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Simoes BM, Santiago-Gomez A, Chiodo C, Moreira T, Conole D, Lovell S et al. Targeting STAT3 signaling using stabilised sulforaphane (SFX-01) inhibits endocrine resistant stem-like cells in ER-positive breast cancer (2020) Oncogene 39: 4896–4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features (2009) Proc Natl Acad Sci U S A 106: 13820–13825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lin X, Li J, Yin G, Zhao Q, Elias D, Lykkesfeldt AE et al. Integrative analyses of gene expression and DNA methylation profiles in breast cancer cell line models of tamoxifen-resistance indicate a potential role of cells with stem-like properties (2013) Breast Cancer Res 15: R119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY et al. The epithelial-mesenchymal transition generates cells with properties of stem cells (2008) Cell 133: 704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications (2017) Nature reviews Clinical oncology 14: 611–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hiscox S, Jiang WG, Obermeier K, Taylor K, Morgan L, Burmi R et al. Tamoxifen resistance in MCF7 cells promotes EMT-like behaviour and involves modulation of beta-catenin phosphorylation (2006) Int J Cancer 118: 290–301. [DOI] [PubMed] [Google Scholar]
- 115.Ward A, Balwierz A, Zhang JD, Kublbeck M, Pawitan Y, Hielscher T et al. Re-expression of microRNA-375 reverses both tamoxifen resistance and accompanying EMT-like properties in breast cancer (2013) Oncogene 32: 1173–1182. [DOI] [PubMed] [Google Scholar]
- 116.Yuan J, Liu M, Yang L, Tu G, Zhu Q, Chen M et al. Acquisition of epithelial-mesenchymal transition phenotype in the tamoxifen-resistant breast cancer cell: a new role for G protein-coupled estrogen receptor in mediating tamoxifen resistance through cancer-associated fibroblast-derived fibronectin and beta1-integrin signaling pathway in tumor cells (2015) Breast Cancer Res 17: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Alves CL, Elias D, Lyng MB, Bak M, Ditzel HJ. SNAI2 upregulation is associated with an aggressive phenotype in fulvestrant-resistant breast cancer cells and is an indicator of poor response to endocrine therapy in estrogen receptor-positive metastatic breast cancer (2018) Breast Cancer Research 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim S, Yao J, Suyama K, Qian X, Qian BZ, Bandyopadhyay S et al. Slug promotes survival during metastasis through suppression of Puma-mediated apoptosis (2014) Cancer Res 74: 3695–3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Loh YN, Hedditch EL, Baker LA, Jary E, Ward RL, Ford CE. The Wnt signalling pathway is upregulated in an in vitro model of acquired tamoxifen resistant breast cancer (2013) BMC Cancer 13: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bui QT, Im JH, Jeong SB, Kim YM, Lim SC, Kim B et al. Essential role of Notch4/STAT3 signaling in epithelial-mesenchymal transition of tamoxifen-resistant human breast cancer (2017) Cancer letters 390: 115–125. [DOI] [PubMed] [Google Scholar]
- 121.Jeselsohn R, Cornwell M, Pun M, Buchwalter G, Nguyen M, Bango C et al. Embryonic transcription factor SOX9 drives breast cancer endocrine resistance (2017) Proc Natl Acad Sci U S A 114: E4482–E4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gooding AJ, Schiemann WP. Epithelial-Mesenchymal Transition Programs and Cancer Stem Cell Phenotypes: Mediators of Breast Cancer Therapy Resistance (2020) Mol Cancer Res 18: 1257–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nair BC, Vadlamudi RK. Regulation of hormonal therapy resistance by cell cycle machinery (2008) Gene Ther Mol Biol 12: 395. [PMC free article] [PubMed] [Google Scholar]
- 124.Osborne CK, Boldt DH, Clark GM, Trent JM. Effects of tamoxifen on human breast cancer cell cycle kinetics: accumulation of cells in early G1 phase (1983) Cancer Res 43: 3583–3585. [PubMed] [Google Scholar]
- 125.Butt AJ, McNeil CM, Musgrove EA, Sutherland RL. Downstream targets of growth factor and oestrogen signalling and endocrine resistance: the potential roles of c-Myc, cyclin D1 and cyclin E (2005) Endocr Relat Cancer 12 Suppl 1: S47–59. [DOI] [PubMed] [Google Scholar]
- 126.Stendahl M, Kronblad A, Ryden L, Emdin S, Bengtsson NO, Landberg G. Cyclin D1 overexpression is a negative predictive factor for tamoxifen response in postmenopausal breast cancer patients (2004) Br J Cancer 90: 1942–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K et al. Palbociclib and Letrozole in Advanced Breast Cancer (2016) The New England journal of medicine 375: 1925–1936. [DOI] [PubMed] [Google Scholar]
- 128.Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer (2018) The New England journal of medicine 379: 1926–1936. [DOI] [PubMed] [Google Scholar]
- 129.Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy (2015) Nat Rev Drug Discov 14: 130–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro (2009) Breast Cancer Res 11: R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Thangavel C, Dean JL, Ertel A, Knudsen KE, Aldaz CM, Witkiewicz AK et al. Therapeutically activating RB: reestablishing cell cycle control in endocrine therapy-resistant breast cancer (2011) Endocr Relat Cancer 18: 333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Miller TW, Balko JM, Fox EM, Ghazoui Z, Dunbier A, Anderson H et al. ERalpha-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancer (2011) Cancer Discov 1: 338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gao A, Sun T, Ma G, Cao J, Hu Q, Chen L et al. LEM4 confers tamoxifen resistance to breast cancer cells by activating cyclin D-CDK4/6-Rb and ERalpha pathway (2018) Nat Commun 9: 4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bardia A, Hurvitz SA, DeMichele A, Clark AS, Zelnak A, Yardley D et al. Phase I/II trial of exemestane, ribociclib, and everolimus in women with HR+/HER2− advanced breast cancer after progression on CDK4/6 inhibitors (TRINITI-1) (2021) Clin Cancer Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mayer IA, Abramson VG, Formisano L, Balko JM, Estrada MV, Sanders ME et al. A Phase Ib Study of Alpelisib (BYL719), a PI3Kalpha-Specific Inhibitor, with Letrozole in ER+/HER2− Metastatic Breast Cancer (2017) Clin Cancer Res 23: 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Clarke R, Cook KL, Hu R, Facey CO, Tavassoly I, Schwartz JL et al. Endoplasmic reticulum stress, the unfolded protein response, autophagy, and the integrated regulation of breast cancer cell fate (2012) Cancer Res 72: 1321–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hetz C, Papa FR. The Unfolded Protein Response and Cell Fate Control (2018) Mol Cell 69: 169–181. [DOI] [PubMed] [Google Scholar]
- 138.Mishra RR, Belder N, Ansari SA, Kayhan M, Bal H, Raza U et al. Reactivation of cAMP Pathway by PDE4D Inhibition Represents a Novel Druggable Axis for Overcoming Tamoxifen Resistance in ER-positive Breast Cancer (2018) Clin Cancer Res 24: 1987–2001. [DOI] [PubMed] [Google Scholar]
- 139.McCloy RA, Shelley EJ, Roberts CG, Boslem E, Biden TJ, Nicholson RI et al. Role of endoplasmic reticulum stress induction by the plant toxin, persin, in overcoming resistance to the apoptotic effects of tamoxifen in human breast cancer cells (2013) Br J Cancer 109: 3034–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bursch W, Ellinger A, Kienzl H, Torok L, Pandey S, Sikorska M et al. Active cell death induced by the anti-estrogens tamoxifen and ICI 164 384 in human mammary carcinoma cells (MCF-7) in culture: the role of autophagy (1996) Carcinogenesis 17: 1595–1607. [DOI] [PubMed] [Google Scholar]
- 141.Gonzalez-Malerva L, Park J, Zou L, Hu Y, Moradpour Z, Pearlberg J et al. High-throughput ectopic expression screen for tamoxifen resistance identifies an atypical kinase that blocks autophagy (2011) Proc Natl Acad Sci U S A 108: 2058–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kulkoyluoglu-Cotul E, Smith BP, Wrobel K, Zhao YC, Chen KLA, Hieronymi K et al. Combined Targeting of Estrogen Receptor Alpha and XPO1 Prevent Akt Activation, Remodel Metabolic Pathways and Induce Autophagy to Overcome Tamoxifen Resistance (2019) Cancers (Basel) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ariazi EA, Cunliffe HE, Lewis-Wambi JS, Slifker MJ, Willis AL, Ramos P et al. Estrogen induces apoptosis in estrogen deprivation-resistant breast cancer through stress responses as identified by global gene expression across time (2011) Proc Natl Acad Sci U S A 108: 18879–18886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Abderrahman B, Maximov PY, Curpan RF, Fanning SW, Hanspal JS, Fan P et al. Rapid Induction of the Unfolded Protein Response and Apoptosis by Estrogen Mimic TTC-352 for the Treatment of Endocrine-Resistant Breast Cancer (2021) Mol Cancer Ther 20: 11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1 (2005) Cell 122: 33–43. [DOI] [PubMed] [Google Scholar]
- 146.Zhao N, Cao J, Xu L, Tang Q, Dobrolecki LE, Lv X et al. Pharmacological targeting of MYC-regulated IRE1/XBP1 pathway suppresses MYC-driven breast cancer (2018) The Journal of clinical investigation 128: 1283–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Barua D, Gupta A, Gupta S. Targeting the IRE1-XBP1 axis to overcome endocrine resistance in breast cancer: Opportunities and challenges (2020) Cancer letters 486: 29–37. [DOI] [PubMed] [Google Scholar]
- 148.Davies MP, Barraclough DL, Stewart C, Joyce KA, Eccles RM, Barraclough R et al. Expression and splicing of the unfolded protein response gene XBP-1 are significantly associated with clinical outcome of endocrine-treated breast cancer (2008) Int J Cancer 123: 85–88. [DOI] [PubMed] [Google Scholar]
- 149.Mao C, Livezey M, Kim JE, Shapiro DJ. Antiestrogen Resistant Cell Lines Expressing Estrogen Receptor alpha Mutations Upregulate the Unfolded Protein Response and are Killed by BHPI (2016) Sci Rep 6: 34753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ming J, Ruan S, Wang M, Ye D, Fan N, Meng Q et al. A novel chemical, STF-083010, reverses tamoxifen-related drug resistance in breast cancer by inhibiting IRE1/XBP1 (2015) Oncotarget 6: 40692–40703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Feng W, Webb P, Nguyen P, Liu X, Li J, Karin M et al. Potentiation of estrogen receptor activation function 1 (AF-1) by Src/JNK through a serine 118-independent pathway (2001) Mol Endocrinol 15: 32–45. [DOI] [PubMed] [Google Scholar]
- 152.Lee H, Bai W. Regulation of estrogen receptor nuclear export by ligand-induced and p38-mediated receptor phosphorylation (2002) Mol Cell Biol 22: 5835–5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cook KL, Shajahan AN, Warri A, Jin L, Hilakivi-Clarke LA, Clarke R. Glucose-regulated protein 78 controls cross-talk between apoptosis and autophagy to determine antiestrogen responsiveness (2012) Cancer Res 72: 3337–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Qadir MA, Kwok B, Dragowska WH, To KH, Le D, Bally MB et al. Macroautophagy inhibition sensitizes tamoxifen-resistant breast cancer cells and enhances mitochondrial depolarization (2008) Breast Cancer Res Treat 112: 389–403. [DOI] [PubMed] [Google Scholar]
- 155.Haslam SZ, Woodward TL. Host microenvironment in breast cancer development: epithelial-cell-stromal-cell interactions and steroid hormone action in normal and cancerous mammary gland (2003) Breast Cancer Res 5: 208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pontiggia O, Sampayo R, Raffo D, Motter A, Xu R, Bissell MJ et al. The tumor microenvironment modulates tamoxifen resistance in breast cancer: a role for soluble stromal factors and fibronectin through beta1 integrin (2012) Breast Cancer Res Treat 133: 459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Umar A, Kang H, Timmermans AM, Look MP, Meijer-van Gelder ME, den Bakker MA et al. Identification of a putative protein profile associated with tamoxifen therapy resistance in breast cancer (2009) Mol Cell Proteomics 8: 1278–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Helleman J, Jansen MP, Ruigrok-Ritstier K, van Staveren IL, Look MP, Meijer-van Gelder ME et al. Association of an extracellular matrix gene cluster with breast cancer prognosis and endocrine therapy response (2008) Clin Cancer Res 14: 5555–5564. [DOI] [PubMed] [Google Scholar]
- 159.Barcus CE, Holt EC, Keely PJ, Eliceiri KW, Schuler LA. Dense collagen-I matrices enhance pro-tumorigenic estrogen-prolactin crosstalk in MCF-7 and T47D breast cancer cells (2015) PLoS One 10: e0116891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jallow F, O'Leary KA, Rugowski DE, Guerrero JF, Ponik SM, Schuler LA. Dynamic interactions between the extracellular matrix and estrogen activity in progression of ER+ breast cancer (2019) Oncogene 38: 6913–6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ali HR, Glont SE, Blows FM, Provenzano E, Dawson SJ, Liu B et al. PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes (2015) Ann Oncol 26: 1488–1493. [DOI] [PubMed] [Google Scholar]
- 162.Joffroy CM, Buck MB, Stope MB, Popp SL, Pfizenmaier K, Knabbe C. Antiestrogens induce transforming growth factor beta-mediated immunosuppression in breast cancer (2010) Cancer Res 70: 1314–1322. [DOI] [PubMed] [Google Scholar]
- 163.Bianchini G, Qi Y, Alvarez RH, Iwamoto T, Coutant C, Ibrahim NK et al. Molecular anatomy of breast cancer stroma and its prognostic value in estrogen receptor-positive and -negative cancers (2010) Journal of clinical oncology : official journal of the American Society of Clinical Oncology 28: 4316–4323. [DOI] [PubMed] [Google Scholar]
- 164.Anurag M, Zhu M, Huang C, Vasaikar S, Wang J, Hoog J et al. Immune Checkpoint Profiles in Luminal B Breast Cancer (Alliance) (2020) Journal of the National Cancer Institute 112: 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vonderheide RH, LoRusso PM, Khalil M, Gartner EM, Khaira D, Soulieres D et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells (2010) Clin Cancer Res 16: 3485–3494. [DOI] [PubMed] [Google Scholar]
- 166.Egelston CA, Avalos C, Tu TY, Simons DL, Jimenez G, Jung JY et al. Human breast tumor-infiltrating CD8(+) T cells retain polyfunctionality despite PD-1 expression (2018) Nat Commun 9: 4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Roswall P, Bocci M, Bartoschek M, Li H, Kristiansen G, Jansson S et al. Microenvironmental control of breast cancer subtype elicited through paracrine platelet-derived growth factor-CC signaling (2018) Nat Med 24: 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sansone P, Berishaj M, Rajasekhar VK, Ceccarelli C, Chang Q, Strillacci A et al. Evolution of Cancer Stem-like Cells in Endocrine-Resistant Metastatic Breast Cancers Is Mediated by Stromal Microvesicles (2017) Cancer Res 77: 1927–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy (2006) Eur J Cancer 42: 717–727. [DOI] [PubMed] [Google Scholar]
- 170.Sotgia F, Fiorillo M, Lisanti MP. Mitochondrial markers predict recurrence, metastasis and tamoxifen-resistance in breast cancer patients: Early detection of treatment failure with companion diagnostics (2017) Oncotarget 8: 68730–68745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Li D, Ji H, Niu X, Yin L, Wang Y, Gu Y et al. Tumor-associated macrophages secrete CC-chemokine ligand 2 and induce tamoxifen resistance by activating PI3K/Akt/mTOR in breast cancer (2020) Cancer Sci 111: 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Clarke R, Tyson JJ, Dixon JM. Endocrine resistance in breast cancer--An overview and update (2015) Mol Cell Endocrinol 418 Pt 3: 220–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kim J, Guan KL. mTOR as a central hub of nutrient signalling and cell growth (2019) Nat Cell Biol 21: 63–71. [DOI] [PubMed] [Google Scholar]
- 174.Demas DM, Demo S, Fallah Y, Clarke R, Nephew KP, Althouse S et al. Glutamine Metabolism Drives Growth in Advanced Hormone Receptor Positive Breast Cancer (2019) Front Oncol 9: 686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bacci M, Lorito N, Ippolito L, Ramazzotti M, Luti S, Romagnoli S et al. Reprogramming of Amino Acid Transporters to Support Aspartate and Glutamate Dependency Sustains Endocrine Resistance in Breast Cancer (2019) Cell Rep 28: 104–118 e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Morotti M, Bridges E, Valli A, Choudhry H, Sheldon H, Wigfield S et al. Hypoxia-induced switch in SNAT2/SLC38A2 regulation generates endocrine resistance in breast cancer (2019) Proc Natl Acad Sci U S A 116: 12452–12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shajahan-Haq AN, Cook KL, Schwartz-Roberts JL, Eltayeb AE, Demas DM, Warri AM et al. MYC regulates the unfolded protein response and glucose and glutamine uptake in endocrine resistant breast cancer (2014) Mol Cancer 13: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hamadneh L, Abuarqoub R, Alhusban A, Bahader M. Upregulation of PI3K/AKT/PTEN pathway is correlated with glucose and glutamine metabolic dysfunction during tamoxifen resistance development in MCF-7 cells (2020) Sci Rep 10: 21933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zinger L, Merenbakh-Lamin K, Klein A, Elazar A, Journo S, Boldes T et al. Ligand-binding Domain-activating Mutations of ESR1 Rewire Cellular Metabolism of Breast Cancer Cells (2019) Clin Cancer Res 25: 2900–2914. [DOI] [PubMed] [Google Scholar]
- 180.Du T, Sikora MJ, Levine KM, Tasdemir N, Riggins RB, Wendell SG et al. Key regulators of lipid metabolism drive endocrine resistance in invasive lobular breast cancer (2018) Breast Cancer Res 20: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chu X, Zhou Q, Xu Y, Jiang J, Li Q, Zhou Q et al. Aberrant fatty acid profile and FFAR4 signaling confer endocrine resistance in breast cancer (2019) J Exp Clin Cancer Res 38: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Menendez JA, Papadimitropoulou A, Vander Steen T, Cuyas E, Oza-Gajera BP, Verdura S et al. Fatty Acid Synthase Confers Tamoxifen Resistance to ER+/HER2+ Breast Cancer (2021) Cancers (Basel) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dasgupta S, Rajapakshe K, Zhu B, Nikolai BC, Yi P, Putluri N et al. Metabolic enzyme PFKFB4 activates transcriptional coactivator SRC-3 to drive breast cancer (2018) Nature 556: 249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liu WS, Chan SH, Chang HT, Li GC, Tu YT, Tseng HH et al. Isocitrate dehydrogenase 1-snail axis dysfunction significantly correlates with breast cancer prognosis and regulates cell invasion ability (2018) Breast Cancer Res 20: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fathi AT, Sadrzadeh H, Comander AH, Higgins MJ, Bardia A, Perry A et al. Isocitrate dehydrogenase 1 (IDH1) mutation in breast adenocarcinoma is associated with elevated levels of serum and urine 2-hydroxyglutarate (2014) Oncologist 19: 602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yue M, Jiang J, Gao P, Liu H, Qing G. Oncogenic MYC Activates a Feedforward Regulatory Loop Promoting Essential Amino Acid Metabolism and Tumorigenesis (2017) Cell Rep 21: 3819–3832. [DOI] [PubMed] [Google Scholar]
- 187.Aftimos P, Oliveira M, Irrthum A, Fumagalli D, Sotiriou C, Nili Gal-Yam E et al. Genomic and transcriptomic analyses of breast cancer primaries and matched metastases in AURORA, the Breast International Group (BIG) molecular screening initiative (2021) Cancer Discov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Davis RT, Blake K, Ma D, Gabra MBI, Hernandez GA, Phung AT et al. Transcriptional diversity and bioenergetic shift in human breast cancer metastasis revealed by single-cell RNA sequencing (2020) Nat Cell Biol 22: 310–320. [DOI] [PubMed] [Google Scholar]
- 189.Dupuy F, Tabaries S, Andrzejewski S, Dong Z, Blagih J, Annis MG et al. PDK1-Dependent Metabolic Reprogramming Dictates Metastatic Potential in Breast Cancer (2015) Cell Metab 22: 577–589. [DOI] [PubMed] [Google Scholar]
- 190.Chen EI, Hewel J, Krueger JS, Tiraby C, Weber MR, Kralli A et al. Adaptation of energy metabolism in breast cancer brain metastases (2007) Cancer Res 67: 1472–1486. [DOI] [PubMed] [Google Scholar]
- 191.Wang L, Zhang S, Wang X. The Metabolic Mechanisms of Breast Cancer Metastasis (2020) Front Oncol 10: 602416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P et al. 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years (2017) The New England journal of medicine 377: 1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Siegel MB, He X, Hoadley KA, Hoyle A, Pearce JB, Garrett AL et al. Integrated RNA and DNA sequencing reveals early drivers of metastatic breast cancer (2018) The Journal of clinical investigation 128: 1371–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nayar U, Cohen O, Kapstad C, Cuoco MS, Waks AG, Wander SA et al. Acquired HER2 mutations in ER(+) metastatic breast cancer confer resistance to estrogen receptor-directed therapies (2019) Nat Genet 51: 207–216. [DOI] [PubMed] [Google Scholar]
- 195.Garcia-Recio S, Thennavan A, East MP, Parker JS, Cejalvo JM, Garay JP et al. FGFR4 regulates tumor subtype differentiation in luminal breast cancer and metastatic disease (2020) The Journal of clinical investigation 130: 4871–4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Vareslija D, Priedigkeit N, Fagan A, Purcell S, Cosgrove N, O'Halloran PJ et al. Transcriptome Characterization of Matched Primary Breast and Brain Metastatic Tumors to Detect Novel Actionable Targets (2019) Journal of the National Cancer Institute 111: 388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Priedigkeit N, Watters RJ, Lucas PC, Basudan A, Bhargava R, Horne W et al. Exome-capture RNA sequencing of decade-old breast cancers and matched decalcified bone metastases (2017) JCI Insight 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer (2012) The New England journal of medicine 366: 520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Shah M, Nunes MR, Stearns V. CDK4/6 Inhibitors: Game Changers in the Management of Hormone Receptor-Positive Advanced Breast Cancer? (2018) Oncology (Williston Park) 32: 216–222. [PMC free article] [PubMed] [Google Scholar]
- 200.Desnoyers A, Nadler MB, Kumar V, Saleh R, Amir E. Comparison of treatment-related adverse events of different Cyclin-dependent kinase 4/6 inhibitors in metastatic breast cancer: A network meta-analysis (2020) Cancer treatment reviews 90: 102086. [DOI] [PubMed] [Google Scholar]
- 201.Narayan P, Prowell TM, Gao JJ, Fernandes LL, Li E, Jiang X et al. FDA Approval Summary: Alpelisib Plus Fulvestrant for Patients with HR-positive, HER2-negative, PIK3CA-mutated, Advanced or Metastatic Breast Cancer (2021) Clin Cancer Res 27: 1842–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Royce M, Bachelot T, Villanueva C, Ozguroglu M, Azevedo SJ, Cruz FM et al. Everolimus Plus Endocrine Therapy for Postmenopausal Women With Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: A Clinical Trial (2018) JAMA Oncol 4: 977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Dudek AZ, Liu LC, Fischer JH, Wiley EL, Sachdev JC, Bleeker J et al. Phase 1 study of TTC-352 in patients with metastatic breast cancer progressing on endocrine and CDK4/6 inhibitor therapy (2020) Breast Cancer Res Treat 183: 617–627. [DOI] [PubMed] [Google Scholar]
- 204.Bihani T, Patel HK, Arlt H, Tao N, Jiang H, Brown JL et al. Elacestrant (RAD1901), a Selective Estrogen Receptor Degrader (SERD), Has Antitumor Activity in Multiple ER(+) Breast Cancer Patient-derived Xenograft Models (2017) Clin Cancer Res 23: 4793–4804. [DOI] [PubMed] [Google Scholar]
- 205.Wardell SE, Nelson ER, Chao CA, Alley HM, McDonnell DP. Evaluation of the pharmacological activities of RAD1901, a selective estrogen receptor degrader (2015) Endocr Relat Cancer 22: 713–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Patel HK, Tao N, Lee KM, Huerta M, Arlt H, Mullarkey T et al. Elacestrant (RAD1901) exhibits anti-tumor activity in multiple ER+ breast cancer models resistant to CDK4/6 inhibitors (2019) Breast Cancer Res 21: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bardia A, Aftimos P, Bihani T, Anderson-Villaluz AT, Jung J, Conlan MG et al. EMERALD: Phase III trial of elacestrant (RAD1901) vs endocrine therapy for previously treated ER+ advanced breast cancer (2019) Future Oncol 15: 3209–3218. [DOI] [PubMed] [Google Scholar]
- 208.Wander SA, Juric D, Supko JG, Micalizzi DS, Spring L, Vidula N et al. Phase Ib trial to evaluate safety and anti-tumor activity of the AKT inhibitor, ipatasertib, in combination with endocrine therapy and a CDK4/6 inhibitor for patients with hormone receptor positive (HR+)/HER2 negative metastatic breast cancer (MBC) (TAKTIC). (2020) Journal of Clinical Oncology 38. [Google Scholar]
- 209.Rinnerthaler G, Gampenrieder SP, Greil R. HER2 Directed Antibody-Drug-Conjugates beyond T-DM1 in Breast Cancer (2019) Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yeruva SLH, Zhao F, Miller KD, Tevaarwerk AJ, Wagner LI, Gray RJ et al. E2112: randomized phase iii trial of endocrine therapy plus entinostat/placebo in patients with hormone receptor-positive advanced breast cancer (2018) NPJ Breast Cancer 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Roisin M Connolly FZ, Miller Kathy D, Lee Min-Jung, Piekarz Richard L, Smith Karen L, Brown-Glaberman Ursa, Winn Jennifer S, Faller Bryan A, Onitilo Adedayo A, Burkard Mark E, Budd George T, Levine Ellis G, Royce Melanie E, Kaufman Peter A, Thomas Alexandra, Trepel Jane B, Wolff Antonio C and Sparano Joseph A. Abstract GS4-02: E2112: Randomized phase 3 trial of endocrine therapy plus entinostat/placebo in patients with hormone receptor-positive advanced breast cancer. A trial of the ECOG-ACRIN cancer research group (2021) Cancer Research 81. [DOI] [PMC free article] [PubMed] [Google Scholar]