Abstract
Conventional π-conjugated luminophores suffer from problems such as emission quenching, biotoxicity, environmental pollution, etc. The emerging nonconjugated and nonaromatic clusteroluminogens (CLgens) are expected to overcome these stubborn drawbacks, so research of CLgens shows great significance not only for practical application but also for the construction of fundamental photophysical theories. This perspective summarizes the unusual features of CLgens in comparison to traditional chromophores, such as nonconjugated molecular structures, unmatched absorption and excitation, excitation-dependent luminescence, multiple emission peaks, and room-temperature phosphorescence. Different from the theory of through-bond conjugation in π-conjugated luminophores, through-space interactions, including through-space n···n interaction and through-space n···π interaction, are regarded as the emitting sources of nonconjugated CLgens. In addition, the formation of network clusters is proposed as an efficient strategy to improve the performance of CLgens, and their potential applications of anticounterfeiting, photoelectronic devices, and bioimaging are prospected.
Keywords: clusteroluminescence, through-space interactions, aggregation-induced emission, aggregate science, network cluster
Introduction
Luminescent materials are widely applied in many high-tech areas such as photoelectronic devices, information storage, sensors, and theranostics. Organic luminogens built on through-bond conjugation (TBC) are well-developed in the past century, and their extended π-electronic structures endow them with high emission efficiency and regulable emission colors.1 However, many problems exist in these artificial chromophores, for example, complicated synthesis with high cost and pollutant generation and inevitable biotoxicity and immunotoxicity; meanwhile, most of the luminogens with planar structures suffer from an aggregation-caused quenching effect, which impede their development for practical applications.2,3 It seems that some natural products with low biotoxicity and immunotoxicity can solve the above-mentioned drawbacks if they possess luminescence in the aggregate state, like the luminous protein and cellulose.4−7
As a matter of fact, such a kind of phenomenon was reported by Francis Bacon in 1605, in which sugar showed triboluminescence, and other natural luminophores were also discovered sporadically.8,9 However, the scientific and systematic investigation on this abnormal performance is very limited and delayed due to the unclear photophysical mechanism and unremovable impurities inside these natural materials.10,11 Moreover, the concept of reductionism has popularized the studies of molecular science in different disciplines, and scientists attempt to decipher all of the phenomena and processes at the molecular level.12 Whereas these natural luminophores only fluoresce in a clustering state and not single-molecule species, their solubility is poor in most solvents.13 Because of these issues, it is difficult to clarify the working mechanism of this abnormal luminescence at the molecular level, and the natural luminophores did not draw researchers’ attention, although it has been discovered for more than 400 years.
In the chemical structures of these materials, some of them only possess n-electrons such as cellulose, or separated n- and π-electrons exist in proteins. Two kinds of structural features could be extracted from these natural luminophores: (i) they are nonconjugated systems without traditional TBC; (ii) heteroatoms are abundant in their structures which have lone pairs. This abnormal luminescence is termed clusteroluminescence (CL),14,15 and clusteroluminogens (CLgens) are used to define these special luminophores.16−19 In nature, almost all biogenetic systems have multifarious heteroatoms, and their biological activity is only activated in the clustering state. For example, the difficulty to realize nitrogen fixation is not the determination of the chemical structure of nitrogenase but the conformation of clusters of nitrogenase,20 indicating that the catalytic activity of enzymes will disappear along with the destruction of its assembly structure. The important role of the cluster, including its conformation and interactions, has been extensively explored in biological areas, but how do these properties of cluster affect its photophysical performance?
Investigating the luminescent behaviors of nonconjugated CLgens is attractive to photophysicists; however, it has been a great challenge to reveal the driving force for the cluster formation and the structure–property relationship of the CLgens. In this perspective, we will focus on the emerging through-space interaction (TSI),21−30 a widespread weak interaction inside the CLgens. Different from the traditional through-space conjugation, TSI is a more universal and accurate concept to elucidate the nonbonding interactions in CLgens as conjugation is conventionally represented as electron delocalization via the bond. After introducing the general features of CL, we summarize the effects of TSI on the cluster formation and CL process and then draw clear mechanistic pictures of through-space n···n and through-space n···π interactions for nonconjugated CLgens. Accordingly, the concept of network clusters is proposed to elucidate the vital role of the cluster structure to CL. In addition, the potential practical applications of CLgens are also demonstrated, especially in the photoelectronic and biological areas. This perspective will not only gain mechanistic insight into the CL but also guide the future development of clusterization-triggered emission.
Unusual Features of CL
Nonconjugated Molecular Structures
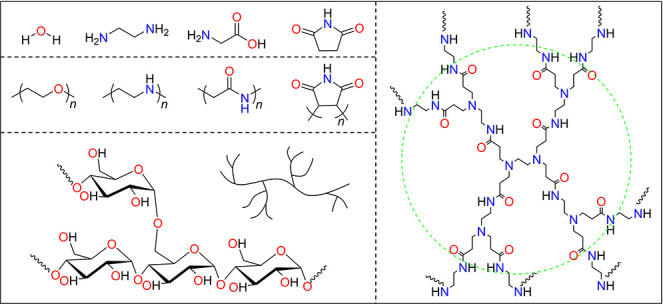
The CL was first discovered in natural systems, such as sugars and proteins. To clarify its photophysical process, many nonconjugated small molecules and polymers were prepared and purified, and the systematic photophysical characterization proved the nature of CL. As shown in Figure 1, water has been reported with blue fluorescence and green phosphorescence at 77 K by Yuan and Tang et al.16,31 Ethanediamine was also characterized with blue fluorescence at room temperature.32 In comparison to these monoheteroatom molecules, their hybrid structures, glycine and succinimide, show stronger CL; for example, the maximum emission wavelength (λem) of succinimide is as long as 470 nm.33,34 From small molecules to polymers, the λem and emission efficiency (Φ) become longer and higher, respectively. Meanwhile, the topological structures of polymers will also affect their CL performance due to the increased structural rigidity from a linear polymer and hyperbranched polymer to the dendrimer.35−44 The typical CLgens summarized in Figure 1 suggest two structural features: (i) they are nonconjugated without aromatic rings and TBC; (ii) heteroatoms such as oxygen and nitrogen are abundant. From the point of an electronic structure, these CLgens could be divided into two categories, a pure n-electron system and n/π-electron hybrid systems.
Figure 1.
Typical structures of nonconjugated clusteroluminogens.
Unmatched Absorption and Excitation
One of the methods to characterize the nonconjugated structure of CLgens is absorption spectra.16,45Figure 2b shows the UV–vis absorption spectra of bovine serum albumin (BSA, Figure 2a) at different concentrations (c).31 The maximum absorption peak (λabs) locates around 280 nm, which corresponds to amide units inside the BSA, suggesting its nonconjugated nature. However, the BSA could be excited by the light whose wavelength is longer than 280 nm. As shown in Figure 2c, the photoluminescence (PL) spectra of BSA aqueous solution were recorded at different excitation wavelengths (λex) from 254 to 420 nm. The emission peak around 360 nm should arise from the n−π* transition of the amide group; meanwhile, CL appeared at longer λex, and its λem is red-shifted along with the increased λex. Then the excitation spectra were measured at λem = 352–500 nm. The excitation peak around 300 nm in Figure 2d could be assigned to the ground-state transition of the amide group, which is almost consistent with the absorption spectra. It is noteworthy that an extra excitation peak longer than 335 nm was detected, which is also red-shifted with the increased λem. Obviously, the absorption and excitation spectra show huge differences in the longer wavelength range, and intense studies demonstrate that it is a general feature for CL.16,46
Figure 2.
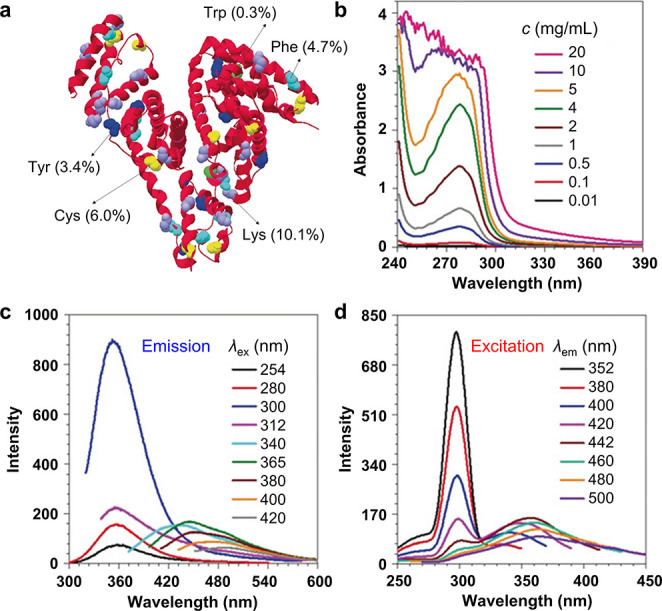
(a) Structure of bovine serum albumin (BSA). (b) UV–vis absorption spectra of aqueous solutions of BSA at different concentrations (c). (c) Photoluminescence (PL) spectra of a BSA solution (20 mg/mL) at different excitation wavelengths (λex). (d) Excitation spectra of a BSA solution (20 mg/mL) at different emission wavelengths (λem). Adapted with permission from ref (31). Copyright 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Currently, there are two hypotheses to explain this extraordinary feature: (i) the longer excitation peak also exists in the absorption spectra, but the corresponding transition is forbidden, so the low-sensitivity absorption characterization could not capture such a signal; (ii) the low-energy transition in excitation spectra only exists in the excited state but not in the ground state. However, the biggest problem for the second hypothesis is how the CLgens could be excited by the light whose energy is lower than its lowest singlet state, and no evidence supports the possibility of nonlinear absorption.
Excitation-Dependent Luminescence
Figure 2c shows that the λem of CL was red-shifted with the increased λex. Actually, it is another general feature for CLgens. Figure 3a,b exhibits the fluorescence microscope images of two CLgens, sodium alginate and P(MA-co-DMAA).47,48 With the increase of excitation wavelength from UV to green, the fluorescence colors change from blue to red. In the meantime, the brightness of the CL was enhanced when the concentration of CLgens increased, and the film was brighter than the solution (Figure 3b). Figure 3c demonstrates the continuous red shift of λem in P(MA-co-DMAA) accompanied by the increased λex, showing an excitation-dependent luminescence effect which is different from the traditional chromophores with TBC.49 According to the molecular photophysical theories, the radiative transition follows Kasha’s rule that the luminescence is only from the lowest excited state. The emission wavelength is permanent for one molecule with a fixed structure, so changing the λex will only affect the PL intensity but not λem.50 Nowadays, the widely accepted explanation for the excitation-dependent luminescence effect is the diverse conformation of CLgens with different extents of electron delocalization in the clustering state, which corresponds to emitting species with different energy gaps.16,51−53
Figure 3.
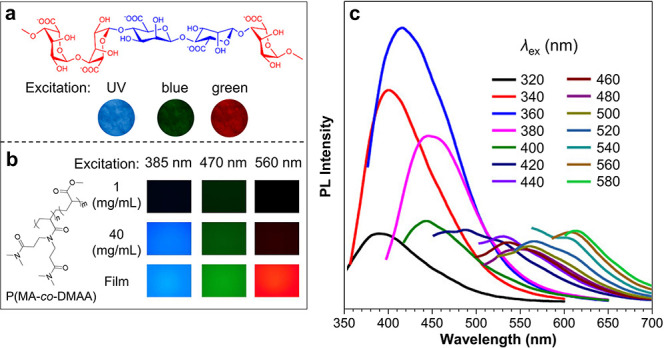
(a) Fluorescence microscope images of sodium alginate powder taken under excitation of UV, blue, and green lights. Adapted from ref (47). Copyright 2018 American Chemical Society. (b) Fluorescence microscope images of DMSO solutions and a solid film of P(MA-co-DMAA) (m = 70, n = 30) excited at 385, 470, and 560 nm. (c) PL spectra of a DMSO solution of P(MA-co-DMAA) (c = 10 mg/mL) excited at 320–580 nm. Adapted from ref (48). Copyright 2020 American Chemical Society.
Multiple Emission Peaks
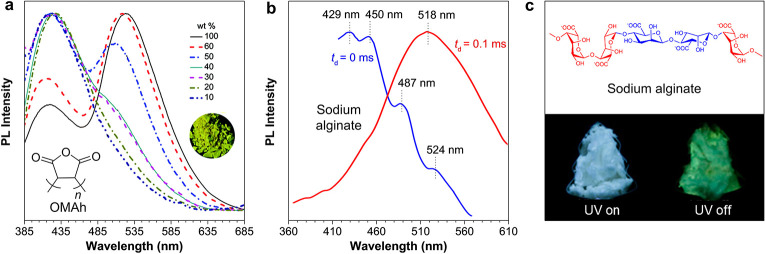
Although multifarious packing structures existed in CLgens and induced the excitation-dependent luminescence property, only one broad CL peak was generated at a specific excitation whose wavelength was longer than the λabs.54 However, using the light around the λabs to excite the CLgens usually generates multiple emission peaks.21,55 As shown in Figure 4a, the PL spectra of oligo(maleic anhydride) (OMAh) doped in poly(methyl methacrylate) (PMMA) have dual emission peaks, a shorter one around 420 nm and the other longer one around 535 nm.56 Meanwhile, the emission intensity (I) ratio of these two peaks (I420/I535) became smaller with the increase of doping ratio from 10 to 100 wt %. Theoretical studies indicate that the shorter peak is derived from the transition of succinic anhydride units, and the longer one stems from the interchain interactions inside the CLgens.
Figure 4.
(a) PL spectra of oligo(maleic anhydride) (OMAh)-doped PMMA films with different doping ratio (wt %; λex = 365 nm). Adapted with permission from ref (56). Copyright 2017 Royal Society of Chemistry. (b) PL spectra of sodium alginate powder recorded with delay time (td) of 0 or 0.1 ms (λex = 330 nm). (c) Photographs of sodium alginate powder taken under excitation of 312 nm light or after turning off the UV irradiation. Adapted from ref (47). Copyright 2018 American Chemical Society.
The above-mentioned dual-emission peaks are from different emitting species in CLgens, but both of them are fluorescence. Another case to generate multiple emission peaks is the coexistence of fluorescence and phosphorescence.57,58Figure 4b demonstrates the PL spectra of sodium alginate powder recorded with delay time (td) of 0 and 0.1 ms, and four emission peaks were observed at td = 0 ms with wavelengths of 429, 450, 487, and 524 nm.47 The delayed spectrum proved the phosphorescent nature of longer-wavelength emission. The photographs of sodium alginate powder taken under UV light further verified the coexistence of singlet and triplet radiative transitions (Figure 4c) and that green phosphorescence was obtained after ceasing the UV irradiation, showing room-temperature phosphorescence (RTP) property. The multiple emission peaks are another intrinsic feature for the CLgens, which endows them with incomparable advantages in producing white-light emission in comparison to the traditional TBC chromophores.
Room-Temperature Phosphorescence
There are many efficient strategies to produce RTP from organic aggregates, three of them are lone-pair electron incorporation, heavy-atom effect, and clusterization, suggesting that the CLgens are the ideal candidate to emit RTP.59 As shown in Figure 5, pentaerythritol is a simple molecule with four hydroxy groups, and obvious RTP is achieved at different excitation from 254 to 365 nm.60 The PL decay curves in Figure 5b suggest that the lifetime of RTP (τp) is as long as 1.4 ms, which is even longer than most of the TBC systems.61,62 Meanwhile, the λem of RTP is longer than 500 nm and red-shifted with the increase of λex (Figure 5c); for example, the λem locates at 500 nm under 255 nm excitation, and a 20 nm red shift of λem is observed when the λex increases to 315 nm, which should have the same mechanism with the effect of excitation-dependent luminescence. From the structural characteristic of CLgens, RTP should be another general feature. However, it is noteworthy that the generation of RTP is highly dependent on the packing structures of CLgens in addition to the chemical structures, so the phosphorescence of some CLgens only emerges at low temperature, which could further rigidify the clustering-state conformation.63,64
Figure 5.
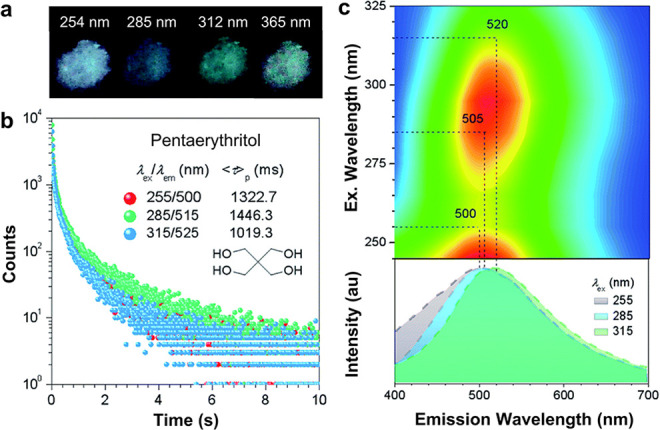
(a) Photographs of room-temperature phosphorescence (RTP) from pentaerythritol crystals after turning off UV irradiation at different wavelengths. (b) Decay of RTP curves of crystals of pentaerythritol. (c) Excitation–RTP mapping and RTP spectra at different excitations (td = 0.1 ms) for pentaerythritol crystals. Adapted with permission from ref (60). Copyright 2020 Royal Society of Chemistry.
Through-Space Interactions in CL
In the section above, five unusual features of CLgens have been introduced, and all of the features are about the properties of CLgen or CL. Generally, the properties of materials are determined by their structures which focus on chemical and clustering structures in molecular and aggregate science studies, respectively.65−68 In the following section, the clustering structures of CLgens and the involved interactions will be discussed from two parts, through-space n···n and n···π interactions.
Through-Space n···n Interaction
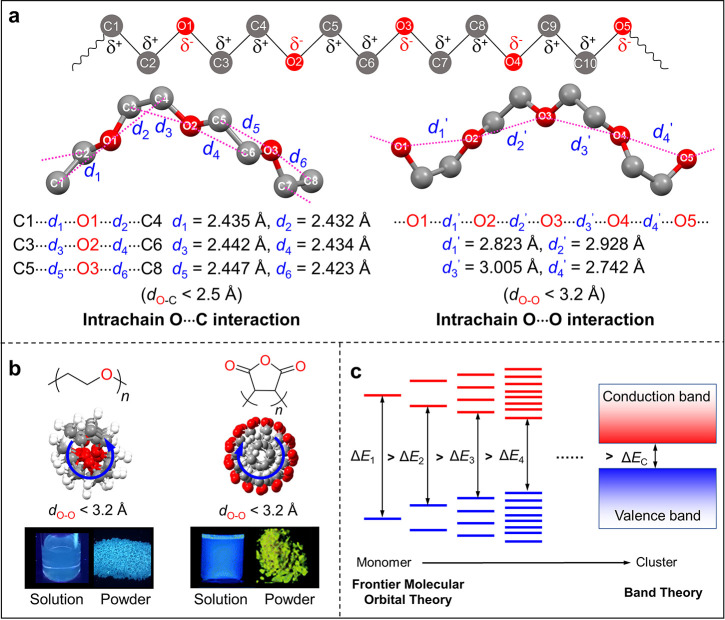
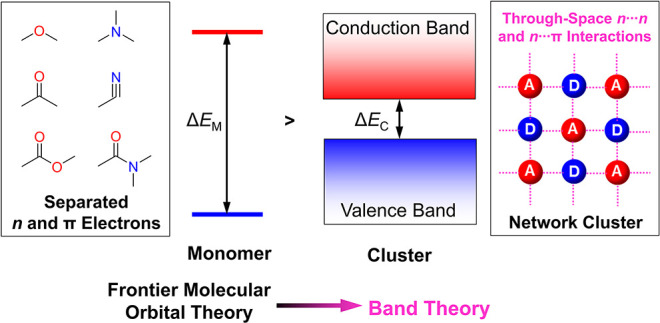
Through-space interaction has been revealed as the working mechanism of CL.16,69,70 Different from TSC, TSI represents the weak interaction that could induce through-space conjugation and narrow the energy gap of the whole system. Figure 6a shows the crystal structure of poly(ethylene glycol) (PEG), the left and right parts demonstrated the intrachain O···C and O···O interactions, respectively.71 It is well-known that the carbon and oxygen atoms in PEG are positively and negatively charged, respectively, so strong electrostatic interactions were generated between oxygen and its adjacent carbons, such as O1 to C1 and O1 to C4 whose distance is 2.435 and 2.432 Å, respectively, shorter than the sum of the van der Waals radius of carbon and oxygen.72 These intrachain O···C interactions are interwoven with each other and rigidify the PEG chain, resulting in the strong and stabilized through-space O···O interaction.73 The average intrachain O···O distance is around 2.9 Å, which is shorter than the sum of the van der Waals radius of two oxygen atoms (3.04 Å). The through-space O···O interactions were connected and formed an n-electron band. Therefore, the crystal structure analysis proved the strong through-space n···n interaction in PEG as no π-electron is involved.
Figure 6.
(a) Intrachain O–C and O–O interactions in poly(ethylene glycol) (PEG) crystals. (b) Examples of helical segmental conformations of PEG (left) and poly(maleic anhydride) (PMAh; right) and fluorescence photographs of their solutions and powders taken under UV excitation. Adapted with permission from ref (56). Copyright 2017 Royal Society of Chemistry. (c) Schematic illustration of band theory for clusteroluminescence.
The through-space n···n interaction in PEG produces blue CL in its high-concentration aqueous solution (Figure 6b). The spatial structure of PEG indicates that the polymer chain is arranged like a helix, and the carbon and oxygen locate at outer and inner layers, respectively, which blocks the interchain through-space n···n interaction. As a result, no obvious red shift of the CL wavelength was observed from solution to PEG powder. The arrangement of the poly(maleic anhydride) (PMAh) chain also shows helical structure whose intrachain O···O distances are smaller than 3.2 Å, and PMAh solution almost fluoresces the same blue color with PEG.15 However, the PMAh powder emits yellow CL, which is red-shifted compared to its solution.56 The optimized PMAh structure suggested that, different from PEG, the carbon and oxygen atoms in PMAh locate at inner and outer layers of the helix, respectively, which facilitates its interchain through-space interaction. So the red-shifted λem of CL in PMAh from solution to powder should be ascribed to the coexistence of intrachain and interchain through-space interactions in the clustering state.
Frontier molecular orbital (FMO) theory has been used to explain the photophysical properties of TBC systems successfully.74 However, it encountered a lot of problems in the area of CL; for example, the FMO cannot explain the CL in PEG which only possesses the n−σ* transition of an isolated oxygen atom. As mentioned above, the through-space n···n interaction in PEG will create an n-electron band along the polymer chain, so it is proposed that the photophysical processes of CLgens follow the band theory, and the energy gap of CLgens (ΔEC) is determined by the energy difference between conduction and valence bands (Figure 6c).16 The band theory has been widely applied to describe the photophysical processes in inorganic emitters,75 and it is anticipated that the CL will be a bridge to integrating the organic and inorganic photophysics.
Through-Space n···π Interaction
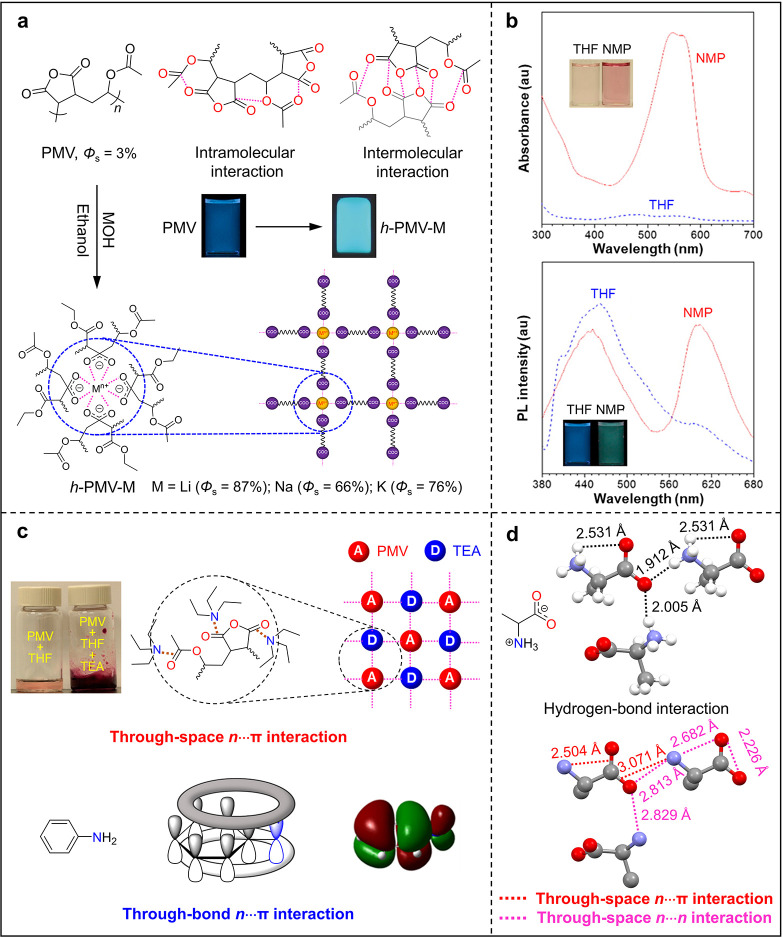
In pure n-electron CLgens, through-space n···n interactions play a vital role in their CL behaviors. There is another major category of CLgens which contain both n- and π-electrons.76 Poly[(maleic anhydride)-alt-(vinyl acetate)] (PMV) shows weak blue CL in solution with a fluorescence quantum yield (Φs) of 3%, which originates from the intrachain and interchain through-space n···π interactions fixed by the intramolecular and intermolecular electrostatic interactions between the negatively charged oxygen and positively charged carbon atoms (Figure 7a). When PMV was hydrolyzed by inorganic bases such as LiOH, NaOH, and KOH, the resultant h-PMV-M (M = Li, Na, and K) complexes show higher Φs, which reaches up to 87%.40 It is proposed that strong complexation interaction between the metal cation and carboxylic anion will form a framework structure in h-PMV-M and increase its structural rigidity, resulting in the increased Φs.
Figure 7.
(a) Enhancing clusteroluminescence efficiency in solid state (Φs) through physical cross-linking between metallic and carboxylic ions in hydrolyzed poly[(maleic anhydride)-alt-(vinyl acetate)] (h-PMV-M). Adapted with permission from ref (40). Copyright 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (b) UV–vis absorption and PL spectra of poly[(maleic anhydride)-alt-(vinyl acetate)] (PMV) in THF and N-methyl-2-pyrrolidone (NMP; c = 5 mM, λex = 330 nm). Adapted from ref (15). Copyright 2014 American Chemical Society. (c) Top panel: photographs of PMV in THF before (PMV + THF) and after adding triethylamine or TEA (PMV + THF + TEA); illustration of through-space n···π interaction in the charge-transfer complex between PMV and TEA. Bottom panel: through-bond n···π interaction in aniline. (d) Hydrogen bonds and through-space n···π and n···n interactions in alanine crystal.
It has been reported that anhydride is easy to form complex with nitrogen-containing molecules.14 As shown in Figure 7b, when the PMV was dissolved in THF and N-methyl-2-pyrrolidone (NMP), an extra absorption peak around 550 nm emerged in the NMP solution, but no such peak was observed in THF, which verified the formation of the PMV–NMP complex.15 Their PL spectra further proved that a new emitting species formed in NMP solution, whose CL was located around 600 nm. However, the present data could not conclude that the longer CL peak was definitely from the PMV–NMP complex because an inconspicuous peak at the same range was observed in THF. To confirm the complexation interaction, triethylamine (TEA) was added to the THF solution of PMV. Figure 7c shows the obvious color change from light red to dark red after TEA was added. In this case, TEA and PMA could be regarded as donor and acceptor, respectively. The electrostatic D–A interaction in the PMV–TEA complex further increases the structural rigidity and improves the number and strength of through-space n···π interactions, including the (n)O···C = O(π) and (n)N···C = O(π) interactions. The n···π interaction widely exists in different systems, such as the through-bond n···π interactions in aniline.
Although through-space n···n and n···π interactions are discussed individually in this section, they always work synergistically, especially for the CLgens with π-electrons. Glycine has been reported with blue CL in the solid state, and its crystal structure shows abundant intramolecular and intermolecular hydrogen-bond interactions which improve and stabilize the through-space n···n (O···O or N···O) and n···π (N···C=O or O···C=O) interactions inside glycine, which is the reason why such a simple structure could emit blue CL.13
Perspectives of CL
Clusteroluminescence as an independent scientific concept has a relatively short history, and it still needs a lot of experimental and theoretical works to develop efficient CLgens for advanced applications and build systematic aggregate photophysics based on TSI. In this part, two perspectives of CL will be introduced: (i) network clusters that can improve the CL emission efficiency and (ii) promising practical applications of CLgens.
Network Clusters
Research of carbon dots starts from the fluorescent single-walled carbon nanotube with an extremely low Φ of 1.6%.77 At present, the concept of carbon dots contains different materials such as graphene quantum dots, carbon quantum dots, carbonized polymer dots, and cross-linking polymer dots.78−80 Although all of these materials are called carbon dots, they possess completely different mechanisms and structures. For example, graphene and carbon quantum dots are mainly composed of carbon core, which is prepared by the “top-down” method. Both of them always show low Φ, and quantum confinement effect is regarded as the emitting source. The carbonized polymer dots are synthesized via hydro-/solvothermal methods at high temperature and pressure, and the resultant dots always show core–shell structure, which is made up of carbon core and the shell of a cross-linking polymer network.81,82 The Φ of carbonized polymer dots is generally higher than that of the first two materials but with an unambiguous photophysical mechanism.83 The cross-linking polymer dots could be obtained from the same monomers to the synthesis of carbonized polymer dots but with a mild reaction condition such as low temperature and atmospheric pressure.84,85 It is believed that the cross-linking polymer dots are pure network clusters without a carbon core, but its Φ is sometimes even higher than the carbonized polymer dots, suggesting the emitting species in carbonized polymer dots and cross-linking polymer dots are both the network clusters.
As shown in Figure 8a, two CLgens were prepared from the cross-linking reaction between p-benzoquinone and N1,N1′-(ethane-1,2-diyl)bis(ethane-1,2-diamine) at room temperature, one with green emission (λem = 518 nm, Φ = 35.3%) and the other one with yellow emission (λem = 543 nm, Φ = 17.5%).85 The proposed chemical structures of these two CLgens are drawn in Figure 8a, which should be the cross-linking polymer networks. The red-shifted CL is ascribed to larger network clusters and the corresponding extended n···n/n···π interactions, which are demonstrated in Figure 8b. The n···π interactions consist of a through-bond n···π interaction between nitrogen and its neighbor double bond and a through-space n···π interaction between nitrogen and the carbonyl group. The n···n interaction is from the through-space interaction between nitrogen and oxygen. The formation of a network cluster provides an efficient strategy to boost the emission efficiency of CLgens.
Figure 8.
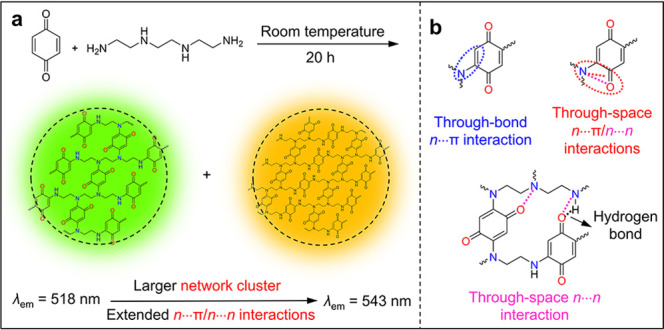
(a) Preparation of network clusters by cross-linking reaction between p-benzoquinone and N1,N1′-(ethane-1,2-diyl)bis(ethane-1,2-diamine) at room temperature; change in emission color with a variation in cluster size and extent of n···π and n···n interactions.85 (b) Examples of through-bond n···π interaction and through-space n···π and n···n interactions in the network clusters.
Potential Applications
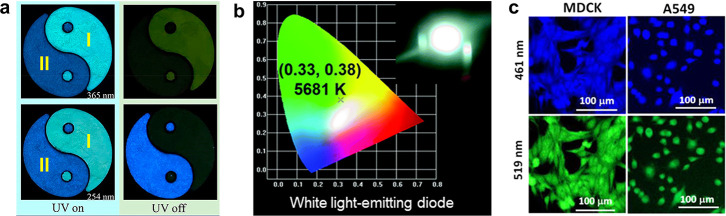
In comparison to traditional TBC-based chromophores, CLgens have many advantages, such as low cost, less pollution, excellent photophysical properties, and good biocompatibility, which shows huge potential in practical applications in the areas of anticounterfeiting technology, photoelectronic devices, bioimaging, etc.86−90Figure 9a demonstrates the anticounterfeiting application of two CLgens I and II, which are the dehydration products of boric acid with ethylenediamine and oxalic acid, respectively. These two CLgens emit cyan and blue colors, respectively, under both 365 and 254 nm irradiation.91 However, CLgen I exhibits yellow phosphorescence only at 365 nm excitation but not at 254 nm; in contrast, a blue phosphoresce is observed in CLgen II at 254 nm excitation but at 365 nm does not work. Therefore, the two excitation channels give the same fluorescence signal but different phosphoresce information, which provides a simple but reliable anticounterfeiting technique. Moreover, the feature of multiple emission peaks in CLgens is the best candidate to fabricate white-light emission. As shown in Figure 9b, poly(hydroxyurethane) has been characterized with broad CL peak, and its film fluoresces bright white light with CIE chromaticity coordinates of (0.33, 0.38).92 The inset in Figure 9b is the fabricated white-light-emitting diode device. The nonconjugated structure of CLgens endows this material with excellent biocompatibility. Figure 9c shows the superb bioimaging quality of CLgens for both cancer (A549) and normal (MDCK) cells.93 By virtue of the excitation-dependent luminescence effect in CLgens, the dual-channel bioimaging is realized in one bioprobe in which blue and green imaging is recorded upon 461 and 519 nm excitations, respectively, which could improve the accuracy of bioimaging.
Figure 9.
(a) Photographs of the Taichi patterns fabricated by filling parts I and II with dehydration products of boric acid with ethylenediamine and oxalic acid, respectively, upon turning on and off UV excitations at 365 nm (top panel) and 254 nm (bottom panel). Adapted from ref (91). Copyright 2021 American Chemical Society. (b) CIE chromaticity coordinates of the white light-emitting diode fabricated from poly(hydroxyurethane). Adapted with permission from ref (92). Copyright 2017 Royal Society of Chemistry. (c) Fluorescence imaging of MDCK and A549 cells stained by nonconjugated polymer clusteroluminogens upon excitations at 461 nm (top panel) and 519 nm (bottom panel). Adapted from ref (93). Copyright 2020 American Chemical Society.
Although CLgens show some advantages over traditional TBC-based luminogens, such as low cost, environmental friendliness, and good biocompatibility, which gives CLgens many potential applications, the relatively low emission efficiency and blue emission color have impeded the development of the above-mentioned applications. For example, the fabricated light-emitting devices always show low efficiency, and the concentration of CLgens used for cell imaging is 1000 times higher than traditional luminogens. It becomes an urgent task to overcome these drawbacks in CLgens, and the formation of network clusters is one of the promising strategies.
In summary, as an emerging luminescent material, clusteroluminogens are revealed with many unusual features in comparison to traditional chromophores, such as nonconjugated molecular structures, unmatched absorption and excitation, excitation-dependent luminescence, multiple emission peaks, and room-temperature phosphorescence, which bring a lot of extraordinary properties and applications to CLgens. The important role of through-space interactions to CL is emphasized and elaborated in this perspective, which is summarized into two categories: through-space n···n interaction for pure n-electron-based CLgens and through-space n···π interaction for the n/π-electron hybrid system. In addition, the theory of network cluster is proposed to disclose the emitting species in polymer dot systems, which could be a feasible strategy to improve the emission efficiency of CLgens. It is noteworthy that the impurities in the CLgens are still the biggest problem for CL research, especially for a mechanistic study. Removing the impurities (e.g., initiator and catalyst) from the polymer CLgens and demonstrating the CL property in the small molecules are two promising directions to investigate the working mechanisms of CL. It is expected that a systematic aggregate photophysics based on TSI will be built for CL and different kinds of efficient CLgens, and their advanced applications could be developed soon.
Acknowledgments
This work was supported by the Fundamental Research Funds for the Central Universities (2021QNA4032) and the Open Fund of Guangdong Provincial Key Laboratory of Luminescence from Molecular Aggregates and South China University of Technology (2019B030301003), National Natural Science Foundation of China grants (51773076, 21871060, 81271476, and 31870991), the Innovation and Technology Commission (ITC-CNERC14SC01), the Research Grants Council of Hong Kong (16305518, 16307020, C6014-20W, C6009-17G, and N-HKUST609/19), and the Material Science Foundation of Guangdong Province (2019B121205012).
The authors declare no competing financial interest.
References
- Xu S. D.; Duan Y. K.; Liu B. Precise Molecular Design for High-Performance Luminogens with Aggregation-Induced Emission. Adv. Mater. 2020, 32 (1), 1903530. 10.1002/adma.201903530. [DOI] [PubMed] [Google Scholar]
- Deng X.; Yu X.; Xiao J.; Zhang Q. Our research progress in heteroaggregation and homoaggregation of organic π-conjugated systems. Aggregate 2021, 2 (3), e35. 10.1002/agt2.35. [DOI] [Google Scholar]
- Mei J.; Leung N. L. C.; Kwok R. T. K.; Lam J. W. Y.; Tang B. Z. Aggregation-Induced Emission: Together We Shine, United We Soar!. Chem. Rev. 2015, 115 (21), 11718–11940. 10.1021/acs.chemrev.5b00263. [DOI] [PubMed] [Google Scholar]
- Cai X. M.; Lin Y. T.; Li Y.; Chen X. F.; Wang Z. Y.; Zhao X. Q.; Huang S. L.; Zhao Z.; Tang B. Z. BioAIEgens derived from rosin: how does molecular motion affect their photophysical processes in solid state?. Nat. Commun. 2021, 12 (1), 1773. 10.1038/s41467-021-22061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y. A.; Zhao Z.; Su H. F.; Zhang P. F.; Liu J. K.; Niu G. L.; Li S. W.; Wang Z. Y.; Kwok R. T. K.; Ni X. L.; Sun J. Z.; Qin A. J.; Lam J. W. Y.; Tang B. Z. Exploration of biocompatible AIEgens from natural resources. Chem. Sci. 2018, 9 (31), 6497–6502. 10.1039/C8SC01635F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y.; Tan Y.; Mei J.; Zhang Y.; Yuan W.; Zhang Y.; Sun J.; Tang B. Z. Room temperature phosphorescence from natural products: Crystallization matters. Sci. China: Chem. 2013, 56 (9), 1178–1182. 10.1007/s11426-013-4923-8. [DOI] [Google Scholar]
- Liu Y.; Wolstenholme C. H.; Carter G. C.; Liu H.; Hu H.; Grainger L. S.; Miao K.; Fares M.; Hoelzel C. A.; Yennawar H. P.; Ning G.; Du M.; Bai L.; Li X.; Zhang X. Modulation of Fluorescent Protein Chromophores To Detect Protein Aggregation with Turn-On Fluorescence. J. Am. Chem. Soc. 2018, 140 (24), 7381–7384. 10.1021/jacs.8b02176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon F.The Advancement of Learning; Macmillan and Co.: London, 1605. [Google Scholar]
- Shukla A.; Mukherjee S.; Sharma S.; Agrawal V.; Radha Kishan K. V.; Guptasarma P. A novel UV laser-induced visible blue radiation from protein crystals and aggregates: scattering artifacts or fluorescence transitions of peptide electrons delocalized through hydrogen bonding?. Arch. Biochem. Biophys. 2004, 428 (2), 144–53. 10.1016/j.abb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Tomalia D. A.; Klajnert-Maculewicz B.; Johnson K. A. M.; Brinkman H. F.; Janaszewska A.; Hedstrand D. M. Non-traditional intrinsic luminescence: inexplicable blue fluorescence observed for dendrimers, macromolecules and small molecular structures lacking traditional/conventional luminophores. Prog. Polym. Sci. 2019, 90, 35–117. 10.1016/j.progpolymsci.2018.09.004. [DOI] [Google Scholar]
- Zhao Z.; Wang Z.; Tavakoli J.; Shan G.; Zhang J.; Peng C.; Xiong Y.; Zhang X.; Cheung T. S.; Tang Y.; Huang B.; Yu Z.; Lam J. W. Y.; Tang B. Z. Revisiting an ancient inorganic aggregation-induced emission system: An enlightenment to clusteroluminescence. Aggregate 2021, 2 (2), e36. 10.1002/agt2.53. [DOI] [Google Scholar]
- Tang B. Z. Aggregology: Exploration and innovation at aggregate level. Aggregate 2020, 1 (1), 4–5. 10.1002/agt2.9. [DOI] [Google Scholar]
- Chen X. H.; Luo W. J.; Ma H. L.; Peng Q.; Yuan W. Z.; Zhang Y. M. Prevalent intrinsic emission from nonaromatic amino acids and poly(amino acids). Sci. China: Chem. 2018, 61 (3), 351–359. 10.1007/s11426-017-9114-4. [DOI] [Google Scholar]
- Xing C.; Lam J. W. Y.; Qin A.; Dong Y.; Haeussler M.; Yang W.; Tang B. Z. Unique photoluminescence from nonconjugated alternating copolymer poly[(maleic anhydride)-alt-(vinyl acetate)]. Polym. Mater. Sci. Eng. 2007, 96, 418–419. [Google Scholar]
- Zhao E.; Lam J. W. Y.; Meng L.; Hong Y.; Deng H.; Bai G.; Huang X.; Hao J.; Tang B. Z. Poly[(maleic anhydride)-alt-(vinyl acetate)]: A Pure Oxygenic Nonconjugated Macromolecule with Strong Light Emission and Solvatochromic Effect. Macromolecules 2015, 48 (1), 64–71. 10.1021/ma502160w. [DOI] [Google Scholar]
- Zhang H.; Zhao Z.; McGonigal P. R.; Ye R.; Liu S.; Lam J. W. Y.; Kwok R. T. K.; Yuan W. Z.; Xie J.; Rogach A. L.; Tang B. Z. Clusterization-triggered emission: Uncommon luminescence from common materials. Mater. Today 2020, 32, 275–292. 10.1016/j.mattod.2019.08.010. [DOI] [Google Scholar]
- Wang Z.; Zhang H.; Li S.; Lei D.; Tang B. Z.; Ye R. Recent Advances in Clusteroluminescence. Top. Curr. Chem. 2021, 379 (2), 14. 10.1007/s41061-021-00326-w. [DOI] [PubMed] [Google Scholar]
- Huang T.; Wang Z.; Qin A.; Sun J.; Tang B. Luminescent Polymers Containing Unconventional Chromophores. Acta Chim. Sin. 2013, 71 (07), 979–990. 10.6023/A13010071. [DOI] [Google Scholar]
- Wang H.; Zhao E.; Lam J. W. Y.; Tang B. Z. AIE luminogens: emission brightened by aggregation. Mater. Today 2015, 18 (7), 365–377. 10.1016/j.mattod.2015.03.004. [DOI] [Google Scholar]
- Lee S. C.; Holm R. H. The Clusters of Nitrogenase: Synthetic Methodology in the Construction of Weak-Field Clusters. Chem. Rev. 2004, 104 (2), 1135–1158. 10.1021/cr0206216. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Zheng X.; Xie N.; He Z.; Liu J.; Leung N. L. C.; Niu Y.; Huang X.; Wong K. S.; Kwok R. T. K.; Sung H. H. Y.; Williams I. D.; Qin A.; Lam J. W. Y.; Tang B. Z. Why Do Simple Molecules with “Isolated” Phenyl Rings Emit Visible Light?. J. Am. Chem. Soc. 2017, 139 (45), 16264–16272. 10.1021/jacs.7b08592. [DOI] [PubMed] [Google Scholar]
- Li J.; Shen P.; Zhao Z.; Tang B. Z. Through-Space Conjugation: A Thriving Alternative for Optoelectronic Materials. CCS Chem. 2019, 1 (2), 181–196. 10.31635/ccschem.019.20180020. [DOI] [Google Scholar]
- Hoffmann R. Interaction of orbitals through space and through bonds. Acc. Chem. Res. 1971, 4 (1), 1–9. 10.1021/ar50037a001. [DOI] [Google Scholar]
- Shao S.; Wang L. Through-space charge transfer polymers for solution-processed organic light-emitting diodes. Aggregate 2020, 1 (1), 45–56. 10.1002/agt2.4. [DOI] [Google Scholar]
- Zhang J.; Hu L.; Zhang K.; Liu J.; Li X.; Wang H.; Wang Z.; Sung H. H. Y.; Williams I. D.; Zeng Z.; Lam J. W. Y.; Zhang H.; Tang B. Z. How to Manipulate Through-Space Conjugation and Clusteroluminescence of Simple AIEgens with Isolated Phenyl Rings. J. Am. Chem. Soc. 2021, 143 (25), 9565–9574. 10.1021/jacs.1c03882. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Du L.; Wang L.; Liu J.; Wan Q.; Kwok R. T. K.; Lam J. W. Y.; Phillips D. L.; Tang B. Z. Visualization and Manipulation of Molecular Motion in the Solid State through Photoinduced Clusteroluminescence. J. Phys. Chem. Lett. 2019, 10 (22), 7077–7085. 10.1021/acs.jpclett.9b02752. [DOI] [PubMed] [Google Scholar]
- Liu B.; Zhang H.; Liu S.; Sun J.; Zhang X.; Tang B. Z. Polymerization-induced emission. Mater. Horiz. 2020, 7 (4), 987–998. 10.1039/C9MH01909J. [DOI] [Google Scholar]
- Sturala J.; Etherington M. K.; Bismillah A. N.; Higginbotham H. F.; Trewby W.; Aguilar J. A.; Bromley E. H. C.; Avestro A.-J.; Monkman A. P.; McGonigal P. R. Excited-State Aromatic Interactions in the Aggregation-Induced Emission of Molecular Rotors. J. Am. Chem. Soc. 2017, 139 (49), 17882–17889. 10.1021/jacs.7b08570. [DOI] [PubMed] [Google Scholar]
- Morisaki Y.; Gon M.; Chujo Y. Conjugated microporous polymers consisting of tetrasubstituted [2.2]Paracyclophane junctions. J. Polym. Sci., Part A: Polym. Chem. 2013, 51 (10), 2311–2316. 10.1002/pola.26600. [DOI] [Google Scholar]
- Zhu Q.; Zhang Y.; Nie H.; Zhao Z.; Liu S.; Wong K. S.; Tang B. Z. Insight into the strong aggregation-induced emission of low-conjugated racemic C6-unsubstituted tetrahydropyrimidines through crystal-structure–property relationship of polymorphs. Chem. Sci. 2015, 6 (8), 4690–4697. 10.1039/C5SC01226K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.; Dou X.; Chen X.; Zhao Z.; Wang S.; Wang Y.; Sui K.; Tan Y.; Gong Y.; Zhang Y.; Yuan W. Z. Reevaluating Protein Photoluminescence: Remarkable Visible Luminescence upon Concentration and Insight into the Emission Mechanism. Angew. Chem., Int. Ed. 2019, 58 (36), 12667–12673. 10.1002/anie.201906226. [DOI] [PubMed] [Google Scholar]
- Zhang H.Aggregation-Induced Emission: Mechanistic Study, Clusteroluminescence and Spontaneous Resolution. Ph.D. thesis, Hong Kong University of Science and Technology, 2019. [Google Scholar]
- He B.; Zhang J.; Zhang J.; Zhang H.; Wu X.; Chen X.; Kei K. H. S.; Qin A.; Sung H. H. Y.; Lam J. W. Y.; Tang B. Z. Clusteroluminescence from Cluster Excitons in Small Heterocyclics Free of Aromatic Rings. Adv. Sci. 2021, 8 (7), 2004299. 10.1002/advs.202004299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravanfar R.; Bayles C. J.; Abbaspourrad A. Structural Chemistry Enables Fluorescence of Amino Acids in the Crystalline Solid State. Cryst. Growth Des. 2020, 20 (3), 1673–1680. 10.1021/acs.cgd.9b01430. [DOI] [Google Scholar]
- Larson C. L.; Tucker S. A. Intrinsic Fluorescence of Carboxylate-Terminated Polyamido Amine Dendrimers. Appl. Spectrosc. 2001, 55 (6), 679–683. 10.1366/0003702011952596. [DOI] [Google Scholar]
- Wang D.; Imae T. Fluorescence Emission from Dendrimers and Its pH Dependence. J. Am. Chem. Soc. 2004, 126 (41), 13204–13205. 10.1021/ja0454992. [DOI] [PubMed] [Google Scholar]
- Song G.; Lin Y.; Zhu Z.; Zheng H.; Qiao J.; He C.; Wang H. Strong fluorescence of poly(N-vinylpyrrolidone) and its oxidized hydrolyzate. Macromol. Rapid Commun. 2015, 36 (3), 278–85. 10.1002/marc.201400516. [DOI] [PubMed] [Google Scholar]
- Pastor-Pérez L.; Chen Y.; Shen Z.; Lahoz A.; Stiriba S.-E. Unprecedented Blue Intrinsic Photoluminescence from Hyperbranched and Linear Polyethylenimines: Polymer Architectures and pH-Effects. Macromol. Rapid Commun. 2007, 28 (13), 1404–1409. 10.1002/marc.200700190. [DOI] [Google Scholar]
- Liu S. G.; Luo D.; Li N.; Zhang W.; Lei J. L.; Li N. B.; Luo H. Q. Water-Soluble Nonconjugated Polymer Nanoparticles with Strong Fluorescence Emission for Selective and Sensitive Detection of Nitro-Explosive Picric Acid in Aqueous Medium. ACS Appl. Mater. Interfaces 2016, 8 (33), 21700–9. 10.1021/acsami.6b07407. [DOI] [PubMed] [Google Scholar]
- Guo Z.; Ru Y.; Song W.; Liu Z.; Zhang X.; Qiao J. Water-Soluble Polymers with Strong Photoluminescence through an Eco-Friendly and Low-Cost Route. Macromol. Rapid Commun. 2017, 38 (14), 1700099. 10.1002/marc.201700099. [DOI] [PubMed] [Google Scholar]
- Yan S.; Gao Z.; Yan H.; Niu F.; Zhang Z. Combination of aggregation-induced emission and clusterization-triggered emission in mesoporous silica nanoparticles for the construction of an efficient artificial light-harvesting system. J. Mater. Chem. C 2020, 8 (41), 14587–14594. 10.1039/D0TC03619F. [DOI] [Google Scholar]
- Wang R.; Yuan W.; Zhu X. Aggregation-induced emission of non-conjugated poly(amido amine)s: Discovering, luminescent mechanism understanding and bioapplication. Chinese J. Polym. Sci. 2015, 33 (5), 680–687. 10.1007/s10118-015-1635-x. [DOI] [Google Scholar]
- Wang Y.; Bin X.; Chen X.; Zheng S.; Zhang Y.; Yuan W. Z. Emission and Emissive Mechanism of Nonaromatic Oxygen Clusters. Macromol. Rapid Commun. 2018, 39 (21), 1800528. 10.1002/marc.201800528. [DOI] [PubMed] [Google Scholar]
- Du L.; Jiang B.; Chen X.; Wang Y.; Zou L.; Liu Y.; Gong Y.; Wei C.; Yuan W. Clustering-triggered Emission of Cellulose and Its Derivatives. Chinese J. Polym. Sci. 2019, 37 (4), 409–415. 10.1007/s10118-019-2215-2. [DOI] [Google Scholar]
- Han T.; Deng H.; Qiu Z.; Zhao Z.; Zhang H.; Zou H.; Leung N. L. C.; Shan G.; Elsegood M. R. J.; Lam J. W. Y.; Tang B. Z. Facile Multicomponent Polymerizations toward Unconventional Luminescent Polymers with Readily Openable Small Heterocycles. J. Am. Chem. Soc. 2018, 140 (16), 5588–5598. 10.1021/jacs.8b01991. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Zhao Z.; Yuan W. Z. Intrinsic Luminescence from Nonaromatic Biomolecules. ChemPlusChem 2020, 85 (5), 1065–1080. 10.1002/cplu.202000021. [DOI] [PubMed] [Google Scholar]
- Dou X.; Zhou Q.; Chen X.; Tan Y.; He X.; Lu P.; Sui K.; Tang B. Z.; Zhang Y.; Yuan W. Z. Clustering-Triggered Emission and Persistent Room Temperature Phosphorescence of Sodium Alginate. Biomacromolecules 2018, 19 (6), 2014–2022. 10.1021/acs.biomac.8b00123. [DOI] [PubMed] [Google Scholar]
- Yang H.; Ren Z.; Zuo Y.; Song Y.; Jiang L.; Jiang Q.; Xue X.; Huang W.; Wang K.; Jiang B. Highly Efficient Amide Michael Addition and Its Use in the Preparation of Tunable Multicolor Photoluminescent Polymers. ACS Appl. Mater. Interfaces 2020, 12 (45), 50870–50878. 10.1021/acsami.0c15260. [DOI] [PubMed] [Google Scholar]
- Sheta S. M.; Akl M. A.; Saad H. E.; El-Gharkawy E.-S. R. H. A novel cerium(iii)–isatin Schiff base complex: spectrofluorometric and DFT studies and application as a kidney biomarker for ultrasensitive detection of human creatinine. RSC Adv. 2020, 10 (10), 5853–5863. 10.1039/C9RA10133K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasha M. Characterization of electronic transitions in complex molecules. Discuss. Faraday Soc. 1950, 9 (0), 14–19. 10.1039/df9500900014. [DOI] [Google Scholar]
- Zhang Yuan W.; Zhang Y. Nonconventional macromolecular luminogens with aggregation-induced emission characteristics. J. Polym. Sci., Part A: Polym. Chem. 2017, 55 (4), 560–574. 10.1002/pola.28420. [DOI] [Google Scholar]
- Bai L.; Yan H.; Bai T.; Guo L.; Lu T.; Zhao Y.; Li C. Energy-Transfer-Induced Multiexcitation and Enhanced Emission of Hyperbranched Polysiloxane. Biomacromolecules 2020, 21 (9), 3724–3735. 10.1021/acs.biomac.0c00823. [DOI] [PubMed] [Google Scholar]
- Wang P.; Liu C.; Tang W.; Ren S.; Chen Z.; Guo Y.; Rostamian R.; Zhao S.; Li J.; Liu S.; Li S. Molecular Glue Strategy: Large-Scale Conversion of Clustering-Induced Emission Luminogen to Carbon Dots. ACS Appl. Mater. Interfaces 2019, 11 (21), 19301–19307. 10.1021/acsami.8b22605. [DOI] [PubMed] [Google Scholar]
- Fang L.; Huang C.; Shabir G.; Liang J.; Liu Z.; Zhang H. Hyperbranching-Enhanced-Emission Effect Discovered in Hyperbranched Poly(4-(cyanomethyl)phenyl methacrylate). ACS Macro Lett. 2019, 8 (12), 1605–1610. 10.1021/acsmacrolett.9b00864. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Y.; He B. R.; Luo W. W.; Peng H. R.; Chen S. M.; Hu R. R.; Qin A. J.; Zhao Z. J.; Tang B. Z. Aggregation-enhanced emission and through-space conjugation of tetraarylethanes and folded tetraarylethenes. J. Mater. Chem. C 2016, 4 (39), 9316–9324. 10.1039/C6TC03767D. [DOI] [Google Scholar]
- Zhou X.; Luo W.; Nie H.; Xu L.; Hu R.; Zhao Z.; Qin A.; Tang B. Z. Oligo(maleic anhydride)s: a platform for unveiling the mechanism of clusteroluminescence of non-aromatic polymers. J. Mater. Chem. C 2017, 5 (19), 4775–4779. 10.1039/C7TC00868F. [DOI] [Google Scholar]
- Yang T.; Dai S.; Yang S.; Chen L.; Liu P.; Dong K.; Zhou J.; Chen Y.; Pan H.; Zhang S.; Chen J.; Zhang K.; Wu P.; Xu J. Interfacial Clustering-Triggered Fluorescence-Phosphorescence Dual Solvoluminescence of Metal Nanoclusters. J. Phys. Chem. Lett. 2017, 8 (17), 3980–3985. 10.1021/acs.jpclett.7b01736. [DOI] [PubMed] [Google Scholar]
- Zhang T.; Zhao Z.; Ma H.; Zhang Y.; Yuan W. Z. Polymorphic Pure Organic Luminogens with Through-Space Conjugation and Persistent Room-Temperature Phosphorescence. Chem. - Asian J. 2019, 14 (6), 884–889. 10.1002/asia.201801727. [DOI] [PubMed] [Google Scholar]
- Zhao W. J.; He Z. K.; Tang B. Z. Room-temperature phosphorescence from organic aggregates. Nat. Rev. Mater. 2020, 5 (12), 869–885. 10.1038/s41578-020-0223-z. [DOI] [Google Scholar]
- Zhou Q.; Yang T.; Zhong Z.; Kausar F.; Wang Z.; Zhang Y.; Yuan W. Z. A clustering-triggered emission strategy for tunable multicolor persistent phosphorescence. Chem. Sci. 2020, 11 (11), 2926–2933. 10.1039/C9SC06518K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H. T.; Zeng J. J.; Yin P. A.; Wang X. D.; Peng Q.; Zhao Z. J.; Lam J. W. Y.; Tang B. Z. Tuning molecular emission of organic emitters from fluorescence to phosphorescence through push-pull electronic effects. Nat. Commun. 2020, 11 (1), 2617. 10.1038/s41467-020-16412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. J.; Chi Z. G.; Chong K. C.; Batsanov A. S.; Yang Z.; Mao Z.; Yang Z. Y.; Liu B. Carbazole isomers induce ultralong organic phosphorescence. Nat. Mater. 2021, 20 (2), 175–180. 10.1038/s41563-020-0797-2. [DOI] [PubMed] [Google Scholar]
- Yang J. S.; Zhang M. M.; Han Z.; Li H. Y.; Li L. K.; Dong X. Y.; Zang S. Q.; Mak T. C. W. A new silver cluster that emits bright-blue phosphorescence. Chem. Commun. 2020, 56 (16), 2451–2454. 10.1039/C9CC09439C. [DOI] [PubMed] [Google Scholar]
- Yang J.; Fang M.; Li Z. Organic luminescent materials: The concentration on aggregates from aggregation-induced emission. Aggregate 2020, 1 (1), 6–18. 10.1002/agt2.2. [DOI] [Google Scholar]
- Zhang H. K.; Zhao Z.; Turley A. T.; Wang L.; McGonigal P. R.; Tu Y. J.; Li Y. Y.; Wang Z. Y.; Kwok R. T. K.; Lam J. W. Y.; Tang B. Z. Aggregate Science: From Structures to Properties. Adv. Mater. 2020, 32 (36), 2001457. 10.1002/adma.202001457. [DOI] [PubMed] [Google Scholar]
- Tu Y. J.; Zhao Z.; Lam J. W. Y.; Tang B. Z. Perspective Aggregate Science: Much to Explore in the Meso World. Matter 2021, 4 (2), 338–349. 10.1016/j.matt.2020.12.005. [DOI] [Google Scholar]
- Zhao Z.; Zhang H.; Lam J. W. Y.; Tang B. Z. Aggregation-Induced Emission: New Vistas at the Aggregate Level. Angew. Chem., Int. Ed. 2020, 59 (25), 9888–9907. 10.1002/anie.201916729. [DOI] [PubMed] [Google Scholar]
- Liu B.; Tang B. Z. Aggregation-Induced Emission: More Is Different. Angew. Chem., Int. Ed. 2020, 59 (25), 9788–9789. 10.1002/anie.202005345. [DOI] [PubMed] [Google Scholar]
- Zheng S.; Zhu T.; Wang Y.; Yang T.; Yuan W. Z. Accessing Tunable Afterglows from Highly Twisted Nonaromatic Organic AIEgens via Effective Through-Space Conjugation. Angew. Chem., Int. Ed. 2020, 59 (25), 10018–10022. 10.1002/anie.202000655. [DOI] [PubMed] [Google Scholar]
- Viglianti L.; Leung N. L. C.; Xie N.; Gu X. G.; Sung H. H. Y.; Miao Q.; Williams I. D.; Licandro E.; Tang B. Z. Aggregation-induced emission: mechanistic study of the clusteroluminescence of tetrathienylethene. Chem. Sci. 2017, 8 (4), 2629–2639. 10.1039/C6SC05192H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y.; Tadokoro H. Structural Studies of Polyethers, (-(CH2)m-O-)n. X. Crystal Structure of Poly(ethylene oxide). Macromolecules 1973, 6 (5), 672–675. 10.1021/ma60035a005. [DOI] [Google Scholar]
- Ensing B.; Tiwari A.; Tros M.; Hunger J.; Domingos S. R.; Pérez C.; Smits G.; Bonn M.; Bonn D.; Woutersen S. On the origin of the extremely different solubilities of polyethers in water. Nat. Commun. 2019, 10 (1), 2893. 10.1038/s41467-019-10783-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viglianti L.; Xie N.; Sung H. H. Y.; Voityuk A. A.; Leung N. L. C.; Tu Y. J.; Baldoli C.; Williams I. D.; Kwok R. T. K.; Lam J. W. Y.; Licandro E.; Blancafort L.; Tang B. Z. Unusual Through-Space Interactions between Oxygen Atoms that Mediate Inverse Morphochromism of an AIE Luminogen. Angew. Chem., Int. Ed. 2020, 59 (22), 8552–8559. 10.1002/anie.201908573. [DOI] [PubMed] [Google Scholar]
- Zhang H. K.; Liu J. K.; Du L. L.; Ma C.; Leung N. L. C.; Niu Y. L.; Qin A. J.; Sun J. Z.; Peng Q.; Sung H. H. Y.; Williams I. D.; Kwok R. T. K.; Lam J. W. Y.; Wong K. S.; Phillips D. L.; Tang B. Z. Drawing a clear mechanistic picture for the aggregation-induced emission process. Mater. Chem. Front. 2019, 3 (6), 1143–1150. 10.1039/C9QM00156E. [DOI] [Google Scholar]
- Shu Y. F.; Lin X.; Qin H. Y.; Hu Z.; Jin Y. Z.; Peng X. G. Quantum Dots for Display Applications. Angew. Chem., Int. Ed. 2020, 59 (50), 22312–22323. 10.1002/anie.202004857. [DOI] [PubMed] [Google Scholar]
- Zhang Q.; Mao Q. Y.; Shang C.; Chen Y. N.; Peng X.; Tan H. W.; Wang H. L. Simple aliphatic oximes as nonconventional luminogens with aggregation-induced emission characteristics. J. Mater. Chem. C 2017, 5 (15), 3699–3705. 10.1039/C7TC00783C. [DOI] [Google Scholar]
- Xu X. Y.; Ray R.; Gu Y. L.; Ploehn H. J.; Gearheart L.; Raker K.; Scrivens W. A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126 (40), 12736–12737. 10.1021/ja040082h. [DOI] [PubMed] [Google Scholar]
- Liu J.; Li R.; Yang B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6 (12), 2179–2195. 10.1021/acscentsci.0c01306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.; Gui B.; Zhi S.; Wang H.; Han S.; Wang D.; Wang X.; Yang J. A Mini Review on Polymer Dots: Synthesis, Properties and Optical Applications. Chin. J. Lumin. 2021, 42 (6), 774–792. 10.37188/CJL.20210044. [DOI] [Google Scholar]
- Du X. Y.; Wang C. F.; Wu G.; Chen S. The Rapid and Large-Scale Production of Carbon Quantum Dots and their Integration with Polymers. Angew. Chem., Int. Ed. 2021, 60 (16), 8585–8595. 10.1002/anie.202004109. [DOI] [PubMed] [Google Scholar]
- Liu B.; Chu B.; Wang Y. L.; Hu L. F.; Hu S. L.; Zhang X. H. Carbon dioxide derived carbonized polymer dots for multicolor light-emitting diodes. Green Chem. 2021, 23 (1), 422–429. 10.1039/D0GC03333B. [DOI] [Google Scholar]
- Ding H.; Yu S. B.; Wei J. S.; Xiong H. M. Full-Color Light-Emitting Carbon Dots with a Surface-State-Controlled Luminescence Mechanism. ACS Nano 2016, 10 (1), 484–91. 10.1021/acsnano.5b05406. [DOI] [PubMed] [Google Scholar]
- Xu A.; Wang G.; Li Y.; Dong H.; Yang S.; He P.; Ding G. Carbon-Based Quantum Dots with Solid-State Photoluminescent: Mechanism, Implementation, and Application. Small 2020, 16 (48), 2004621 10.1002/smll.202004621. [DOI] [PubMed] [Google Scholar]
- Han L.; Liu S. G.; Dong J. X.; Liang J. Y.; Li L. J.; Li N. B.; Luo H. Q. Facile synthesis of multicolor photoluminescent polymer carbon dots with surface-state energy gap-controlled emission. J. Mater. Chem. C 2017, 5 (41), 10785–10793. 10.1039/C7TC03314A. [DOI] [Google Scholar]
- Liu M. L.; Yang L.; Li R. S.; Chen B. B.; Liu H.; Huang C. Z. Large-scale simultaneous synthesis of highly photoluminescent green amorphous carbon nanodots and yellow crystalline graphene quantum dots at room temperature. Green Chem. 2017, 19 (15), 3611–3617. 10.1039/C7GC01236E. [DOI] [Google Scholar]
- Yu X.; Zhang H.; Yu J. Luminescence anti-counterfeiting: From elementary to advanced. Aggregate 2021, 2 (1), 20–34. 10.1002/agt2.15. [DOI] [Google Scholar]
- Wang S.; Wu D.; Yang S.; Lin Z.; Ling Q. Regulation of clusterization-triggered phosphorescence from a non-conjugated amorphous polymer: a platform for colorful afterglow. Mater. Chem. Front. 2020, 4 (4), 1198–1205. 10.1039/D0QM00018C. [DOI] [Google Scholar]
- Feng Y.; Yan H.; Ding F.; Bai T.; Nie Y.; Zhao Y.; Feng W.; Tang B. Z. Multiring-induced multicolour emission: hyperbranched polysiloxane with silicon bridge for data encryption. Mater. Chem. Front. 2020, 4 (5), 1375–1382. 10.1039/D0QM00075B. [DOI] [Google Scholar]
- Ye R.; Liu Y.; Zhang H.; Su H.; Zhang Y.; Xu L.; Hu R.; Kwok R. T. K.; Wong K. S.; Lam J. W. Y.; Goddard W. A.; Tang B. Z. Non-conventional fluorescent biogenic and synthetic polymers without aromatic rings. Polym. Chem. 2017, 8 (10), 1722–1727. 10.1039/C7PY00154A. [DOI] [Google Scholar]
- Wang L.; Zhang H. K.; Qin A. J.; Jin Q.; Tang B. Z.; Ji J. Theranostic hyaluronic acid prodrug micelles with aggregation-induced emission characteristics for targeted drug delivery. Sci. China: Chem. 2016, 59 (12), 1609–1615. 10.1007/s11426-016-0246-9. [DOI] [Google Scholar]
- Zhang C.; Wang H.; Lan X.; Shi Y. E.; Wang Z. Modulating Emission of Nonconventional Luminophores from Nonemissive to Fluorescence and Room-Temperature Phosphorescence via Dehydration-Induced Through-Space Conjugation. J. Phys. Chem. Lett. 2021, 12 (5), 1413–1420. 10.1021/acs.jpclett.0c03614. [DOI] [PubMed] [Google Scholar]
- Liu B.; Wang Y. L.; Bai W.; Xu J. T.; Xu Z. K.; Yang K.; Yang Y. Z.; Zhang X. H.; Du B. Y. Fluorescent linear CO2-derived poly(hydroxyurethane) for cool white LED. J. Mater. Chem. C 2017, 5 (20), 4892–4898. 10.1039/C7TC01236E. [DOI] [Google Scholar]
- Mahapatra M.; Dutta A.; Roy J. S. D.; Deb M.; Das U.; Banerjee S.; Dey S.; Chattopadhyay P. K.; Maiti D. K.; Singha N. R. Synthesis of Biocompatible Aliphatic Terpolymers via In Situ Fluorescent Monomers for Three-in-One Applications: Polymerization of Hydrophobic Monomers in Water. Langmuir 2020, 36 (22), 6178–6187. 10.1021/acs.langmuir.0c00636. [DOI] [PubMed] [Google Scholar]