Abstract
Objective:
To assess the effects of low-level laser irradiation vs ultrasound irradiation on bone healing after distraction osteogenesis.
Materials and Methods:
Distraction osteogenesis was performed with rapid maxillary expansion devices (Hyrax-Morelli, Sorocaba, São Paulo Brazil) in 24 rabbits (Oryctolagus cuniculus). After a 2-day latency period, the distraction devices were activated for 10 days at a rate of 1 mm/d. Four groups of six animals were treated as follows: (1) control, (2) laser irradiation on the right side, (3) ultrasound irradiation on the right side, and (4) laser irradiation on the right side and ultrasound on the left side. Histomorphometric analysis was used to assess the bone healing area. Analysis of variance was used to perform the statistical analyses.
Results:
The influence of low-intensity laser associated with ultrasound irradiation on bone healing was statistically significant. The analyses showed the greatest amount of bone healing in the jaws of animals in group 4, which received treatment with both ultrasound and laser.
Conclusion:
This study concluded that bone healing is accelerated with the application of laser irradiation. The greatest effects were observed with combined ultrasound and laser treatment.
Keywords: Bone healing; Distraction osteogenesis; Histomorphometry, Laser; Rabbits; Ultrasound
INTRODUCTION
Bone healing involves an ongoing process of bone remodeling.1 Low-intensity laser (LIL) and low-intensity ultrasound (LIUS) irradiation can be applied for bone repair in cases involving bone expansion, fractures, and other surgical procedures in which bone healing is needed.2,3 Controversies remain regarding the best type of irradiation to obtain the desired result.2,3 The LIUS technique does not overheat the tissues, and it is indicated for tissue repair, wound healing, increasing bone mass, and bone consolidation2 as a result of the physiological mechanical stress, including stimulation of vascular activity.4,5 Various biostimulatory effects of LIL have been reported with regard to wound healing and collagen synthesis via in vitro and in vivo studies.6 With respect to the bone, LIL has been shown to modulate inflammation, accelerate cell proliferation,7 and enhance healing.7,8
Distraction osteogenesis (DO) elongates bones by creating gaps and filling them with newly formed bone without the need for soft or hard tissue grafting.9–12 Early treatment of patients with oral and/or craniofacial anomalies using orthodontic and maxillary orthopedic therapy and DO is recommended when the patient is a child or an adolescent.13,14
This perspective appears to lead to a question: What would be the best method to use, laser or ultrasound, with regard to the effect the method would have on bone healing after DO? Thus, the aim of this study was to compare the effects of LIL vs those of LIUS with regard to bone healing after performance of DO in rabbits using histomorphometry to analyze the outcome.
MATERIALS AND METHODS
Twenty-four male New Zealand rabbits (Oryctolagus cuniculus), aged approximately 2 years and with body weights between 3.0 and 3.5 kg and fully erupted permanent teeth, were used. The animals, selected by a veterinarian, were quarantined, fed special rations for rabbits, received necessary medication, and were submitted to laboratory and radiographic exams. The study was approved by the Ethics Committee for Animal Experimentation of the Federal University of Piauí (UFPI) under report number 01/09. The procedures performed on the animals met the Proposed International Ethical Guidelines for Biomedical Research involving animals (Council for International Organizations of Medical Sciences/World Health Organization, 1985). The sedation was administered intramuscularly: 4 mg/kg xylazine chloride (50 mg/mL; Anasedan, Vetbrands, Miramar, FL, USA) and 40 mg/kg ketamine hydrochloride (23 mg/mL; Dopalen, Vetbrands, Miramar, FL, USA) were used.
Rapid maxillary expansion (RME) devices (Hyrax-Morelli, 13 mm, Morelli Ortodontia, Sorocaba, SP. Brazil) were used to perform DO. Before surgical insertion of the RME screws in the mandibles, the operative area of the animals was trichotomized, and solutions of 70% alcohol followed by iodated alcohol were used for the aseptic technique. Submandibular incisions were made in three locations: one anterior incision and two posterior incisions (one on the left side and one on the right side). Mandibular osteotomy was performed in the left vestibular region to the corresponding right side (1 mm from the mesial root of the first molar) with an abrasive disk under cooling.
Four perforations, two on each side of the corticotomy site, were made using bone cutters. The distraction screw was placed below the lower edge of the mandible, and its posts were adapted to the four perforations. The surgical wound was sutured, and the distraction screw was left exposed to enable activation of the screw.
A prophylactic antibiotic (0.5 mL of penicillin) and 0.02 mg/kg of buprenorphine were administered subcutaneously before the surgery and 48 and 96 hours after the surgery. The postoperative diet was not altered, as the rabbits were able to eat independently, but an oral vitamin supplement was added. The water and food consumption of the animals were monitored. Their weights were measured daily and compared to the presurgical values.
Activation of Distraction Devices
After a 2-day latency period, DO was initiated in the mandibles of all of the animals (n = 24). The distraction screw was activated for 10 days at a rate of 1 mm/d (4 activations/d of the screw at 0800, 1300, 1800, and 2300 hours) (Figures 1A and 2B). After completing the distraction, the devices were stabilized with acrylic resin.
Figure 1.
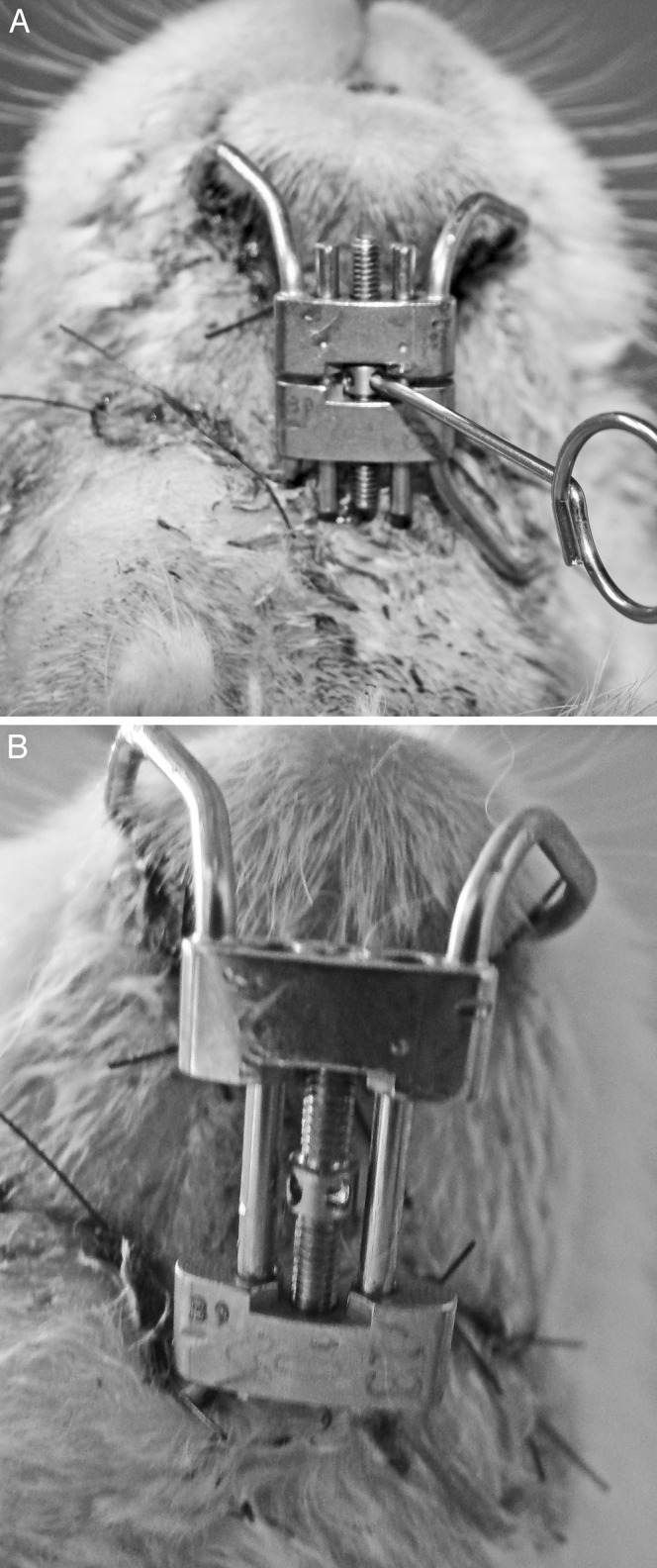
(A) Initial activation of the distraction device; (B) complete opening after the 10th day of activation.
Figure 2.
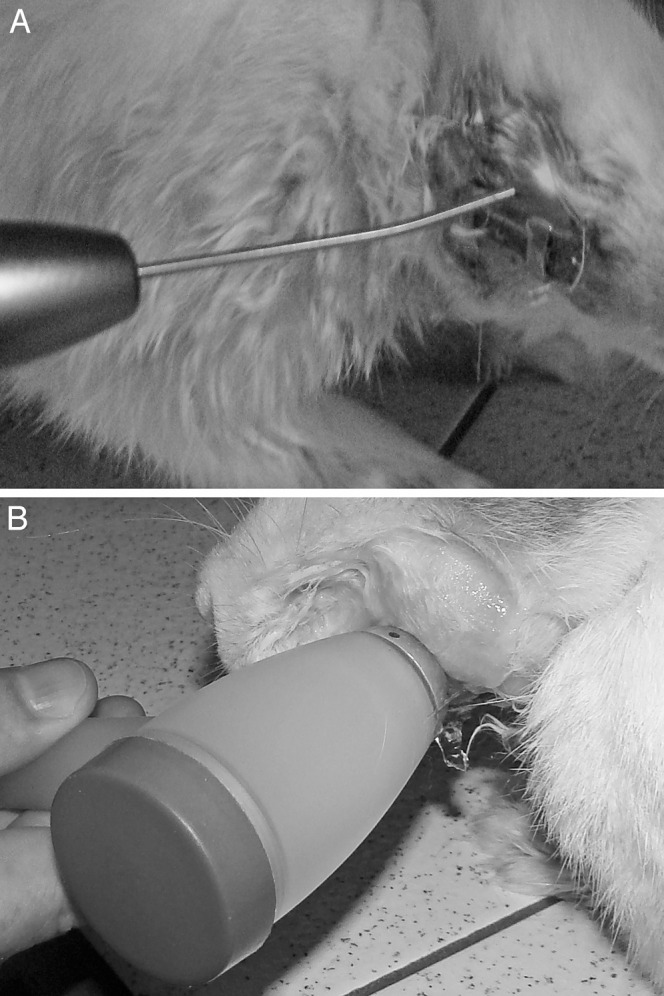
(A) Laser application on the right side and (B) ultrasound on the left side in an animal from group 4.
The application of LIL and LIUS to the mandibles was initiated after the 2-day latency period along with the activation of the distraction screws. Six control animals in group 1 received neither LIL nor LIUS. The other 18 animals, divided into three groups, received the following treatments: (a) LIL on the right side (group 2), (b) LIUS on the right side (group 3), and (c) LIL on the right side and LIUS on the left side (group 4) (Figure 2A,B). The left sides of the animals in groups 2 (subgroup C3) and 3 (subgroup C4) were also used as controls (Table 1).
Table 1.
Distribution of the Four Animal Groups (n = 24) that Received Distraction Osteogenesis and Treatment with Low-Intensity Laser and Ultrasound Irradiation After a 2-Day Latency Perioda
The LIL and LIUS applications were adjusted to biostimulation mode according to the functions of each appliance. Laser irradiation (wavelength: 808 nm; output power: 100 mW; 6 J/cm2; continuous guide light) and ultrasound irradiation (1.0 cm2 [era]; 1.0 MHz [ultrasonic frequency]; P100 20% [mode] 2.0 W; 2.0 w/cm2) were applied for 6 minutes each night for 24 days. The protective eyeglasses, which came with the laser therapy appliance (Flash Lase III, DMC, São Carlos, São Paulo, Brazil), were used by the operator during all LIL applications. The ultrasound appliance used was a Sonopulse III for Physiotherapy and Orthopedics (1 MHz–3 MHz; Indústria Brasileira de Equipamentos Médicos-Ibramed-S34, Amparo, São Paulo, Brazil). For the LIUS applications, gel was placed between the skin of the animal and the active tip of the transducer to enable conduction of the impulses. Twenty-four days after the latency period, a veterinarian euthanatized the animals by administering a 50 mg/kg infusion of thiopental and 100 mg/kg potassium chloride via the brachial vein.
Assessment of Bone Healing
For all 24 of the animals, the jaw segments of interest were identified, dissected, placed in 4% paraformaldehyde and phosphate buffer (0.1 M phosphate-buffered saline), and prepared for light microscopy analysis. The tissue segments were prepared using a LEICA RM 2165 microtome and cut with a thickness of 5–7 µm in the vertical direction following the transverse axis of the incisor roots, in the coronal plane. The slices were stained using the Harris hematoxylin-eosin method. The histological analysis was performed using an HM-LUX Nikon E600 microscope (Nikon, Melville, NY, USA) with the following resolutions: 4NF × 0.10 and 10NF × 0.25. A system with computerized image analysis (Qwin Leica D-1000, version 4.1) was used to capture 20 fields, with 10 fields evenly distributed across both the left and right sides of the jaws. The histomorphometric evaluation was performed in a blinded fashion by two researchers who underwent standardized preparatory training. The images were evaluated in a randomized order. The image threshold and the total surface area of this range were automatically calculated by the software. For data collection, a total area of up to 7 mm2 was measured at a magnification of 4×, and the results were an average of the counts. The analyzed parameter was the total area of the trabecular bone silhouettes examined, including the osteoid (B.Ar - mm2), which was used to assess the amount of bone healing in the 20 captured fields.
Statistical Analysis
For the statistical analysis, a completely randomized design was adopted to evaluate the amount of regenerated tissue in square micrometers (mm2) after performing DO. The following parameter was used for the analysis: the means of the bone healing of all the animals (n = 24). The eight treatments considered were as follows: Control 1 and Control 2 (subgroups from group 1), Laser 1 and Control 3 (subgroups from group 2), US 1 and Control 4 (subgroups from group 3), and Laser 2 and US 2 (subgroups from group 4). Thus, it was possible to make comparisons among all four groups of animals and between the right and left sides of the mandibles. The data observed in the experiment were submitted to analysis of variance and Tukey post hoc analysis using the SPSS 13.0 program (SPSS Inc, Chicago, Ill). Descriptive statistics included the means of the evaluated groups (P < .001).
RESULTS
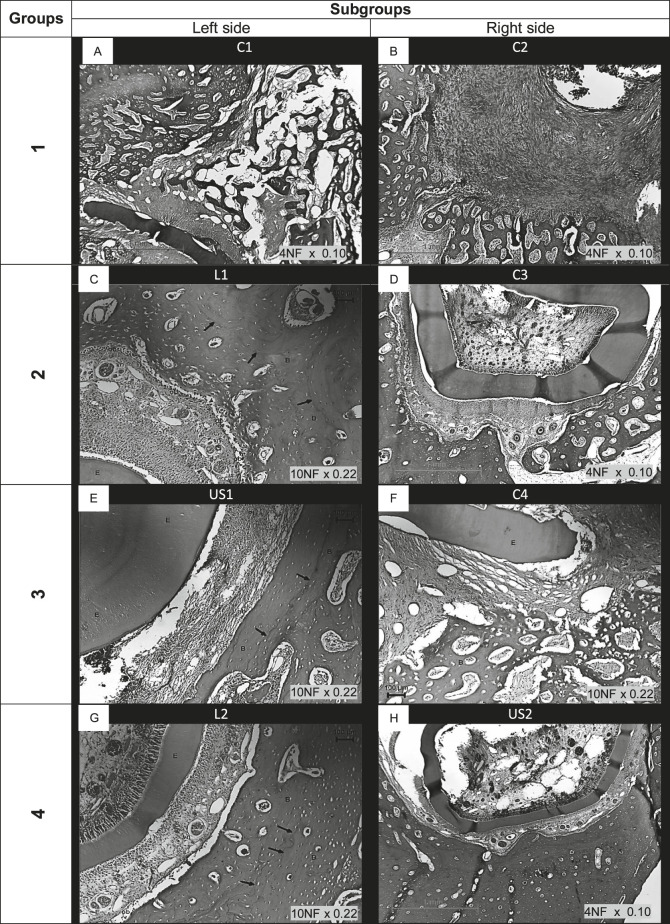
Figure 3A–H shows photomicrographs of the histological features of bone healing after DO in the four studied groups. In all of the sections, bone formation was examined on the outer surface of the mandible because the deeper layers contained the growing parts of the lower incisors. Immature woven bone with some trabecular bone is visible in the photomicrographs, although in the LIL and LIUS irradiation groups, less marrow space and densely packed trabeculae can be observed, and the bony structures appear to be more compact. Increased osteoblastic activity is indicated by an increased number of osteoblasts lining the newly formed bony trabeculae, where incremental growth lines (Figure 3C,E,G,H) can be observed in the animals that received LIL and LIUS irradiation. Numerous osteocytes can be observed in the lacunae in the newly formed bone matrix, and bony lamellae have begun to arrange around blood vessels to form Haversian canals. In Figure 3D and F, osteoclastic activity indicative of ongoing bone remodeling can be observed.
Figure 3.
Photomicrographs of subgroups: (A) C1 = control 1; (B) C2 = control 2; (C) L1 = laser 1; (D) C3 = control 3; (E) U1 = ultrasound 1; (F) C4 = control 4; (G) L2 = laser 2; (H) US2 = ultrasound 2. Legends: C = control; L = laser application; US = ultrasound application; arrows = incremental growth lines; E = enamel; and B = bone. Hematoxylin and eosin–stained sections.
Assessment of Bone Healing
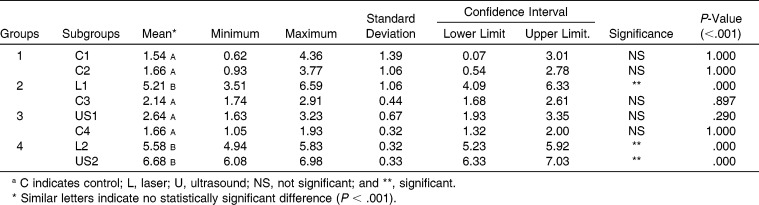
Table 2 shows that when the means of all of the controls and those of the LIL and LIUS treatments were evaluated without considering the mandibular side, controls 1, 2 (subgroups of group 1), 3 (subgroup of group 2), and 4 (subgroup of group 3) showed the least new bone regeneration (means from 1.54 to 2.14 mm2), and US2 (subgroup of group 4) showed the greatest amount of new bone generation (6.68 mm2), followed by the subgroups L2 (5.58 mm2), L1 (5.21 mm2), and US1 (2.64 mm2). When all of the controls were assessed, no significant difference was observed; however, in the sides showing the jaws that received LIL and LIUS irradiation, significant bone healing was observed. The greatest bone healing was observed in group 2 (L1 = 5.21 mm2; C3 = 2.14 mm2) and group 4 (L2 = 5.58 mm2; US2 = 6.68 mm2). Group 4 showed the greatest effect (6.68 mm2) in the subgroup US2, which received LIL application on the opposite side, but the group that received LIUS irradiation alone (group 3, subgroup US1) did not show a significant difference (US1 = 2.64 mm2).
Table 2.
Means (mm2) of the Histomorphometric Measurements (B.Ar = Total Area of Trabecular Bone Silhouettes, Including Osteoid) on the Left and Right Sides of the Jaw After Performing Distraction Osteogenesisa
DISCUSSION
Several methods15–17 can be used to evaluate bone structure. The variable we chose to measure (B.Ar = total area of trabecular bone silhouettes examined including osteoid, in mm2) is considered necessary and sufficient to show the complete picture of the histomorphometric findings in any human or animal experiment.18
In our study, we investigated the effects of laser and ultrasound irradiation on bone healing in 24 rabbits subjected to DO. The ideal animals in which to obtain clear observations of bony and sutural changes under stress are the rabbit and the rat.18,19
Laser and ultrasound treatment stimulated cellular multiplication, verifying that the consolidation was accelerated independently of the period or duration of the treatment.20–22 The period evaluated in our research was 24 days, with a consolidation period of 14 days, and resulted in considerable bone healing; however, ultrasound treatment elicited a greater response in the US2 mandibles (subgroup from group 4 that received LIL on the opposite side).
Despite the lack of a statistically significant difference in the B.Ar (mm2), for the animals in group 3, the value for the subgroup US1 (2.64 mm2) was greater than that for the control subgroup C3 (2.14 mm2). Our results are consistent with the previous study of Moinnes et al.,23 which showed that ultrasound accelerated bone healing compared with a control.
The clinical success of laser treatment depends on the wavelength, potency, duration of the pulses, exposure time, and nature of the tissue being irradiated.24–26 In our study, the laser settings were 808 nm, 100 mW, 6 J, and continuous with guide light; the treatment resulted in significantly greater B.Ar with greater bone healing on the sides to which it was applied, which could be observed in the subgroups L2 (group 4) and L1 (group 2). This finding is in agreement with those from the literature,27 in which shorter times for bone healing have been reported as a result of its stimulation of osteoblast proliferation, absorption of exudates, the growth of new blood vessels, and increased collagen deposition by fibroblasts.
In our work, jaws treated with LIL and LIUS (Figure 3C,E,G,H) showed numerous incremental growth lines; these findings are supported by the work of Yoshida et al.,28 which indicated that when LIL is applied, the number of osteoblasts can increase by a 1.7-fold measure, and the area with newly formed bone islands can increase by a 3.4-fold measure. Table 2 shows the accelerated bone healing effects in the jaws treated with LIL (groups 2 and 4) and LIUS (group 4). The C3 subgroup (2.14 mm2) in group 2 showed a greater bone healing response compared with the C1 (1.54 mm2) and C2 (1.66 mm2) subgroups in group 1 and the C4 subgroup (1.66 mm2) in group 3.
Significant bone healing was observed for subgroup US2 (6.68 mm2) in group 4 but was not observed for subgroup US1 (2.64 mm2) in group 3. Group 4, which received LIUS on one side of the jaw and LIL on the opposite side, showed the greatest amount of healing. Our outcome is in accord with those of other studies2,18,25 that state that laser and ultrasound accelerate the process of forming new bone. From the results of our study, it was observed that the dosage of the laser used was sufficient to accelerate bone healing because the LIL also appeared to accelerate the healing on the side opposite to its application; this finding can be observed in the C3 subgroup in group 2 and the US2 subgroup in group 4.
The results of our study using LIL and LIUS treatment after DO suggest that patients with certain syndromes11,14 and microsomias8,13 may benefit from such therapies; this suggestion is in accord with the literature,23,25 which states that laser irradiation accelerates the healing process of bone fractures and, together with LIUS, can enhance the healing process of a fractured bone more than can LIL therapy alone.
CONCLUSIONS
Application of LIL accelerated bone healing.
The greatest effects were observed for LIUS combined with LIL.
REFERENCES
- 1.Jabbour Z, El-Hakim M, Henderson JE, de Albuquerque RF., Jr Bisphosphonates inhibit bone remodeling in the jaw bones of rats and delay healing following tooth extractions. Oral Oncol. 2014;50:485–490. doi: 10.1016/j.oraloncology.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Kravitz ND, Kusnoto B. Soft-tissue lasers in orthodontics: an overview. Am J Orthod Dentofacial Orthop. 2008;133:S110–S114. doi: 10.1016/j.ajodo.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 3.Oyonarte R, Zarate M, Rodriguez F. Low-intensity pulsed ultrasound stimulation of condylar growth in rats. Angle Orthod. 2009;79:964–970. doi: 10.2319/080708-414.1. [DOI] [PubMed] [Google Scholar]
- 4.Colvard M, Kuo P. Managing aphthous ulcers: laser treatment applied. J Am Dent Assoc. 1991;122:51–53. doi: 10.1016/s0002-8177(91)26017-1. [DOI] [PubMed] [Google Scholar]
- 5.Cook-Mills JM, Johnson JD, Deem TL, Ochi A, Wang L, Zheng Y. Calcium mobilization and Rac1 activation are required for VCAM-1 (vascular cell adhesion molecule-1) stimulation of NADPH oxidase activity. Biochem J. 2004;378:539–547. doi: 10.1042/BJ20030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mester E, Mester AF, Mester A. The biomedical effects of laser application. Lasers Surg Med. 1985;5:31–39. doi: 10.1002/lsm.1900050105. [DOI] [PubMed] [Google Scholar]
- 7.Ueda Y, Shimizu N. Pulse irradiation of low-power laser stimulates bone nodule formation. J Oral Sci. 2001;43:55–60. doi: 10.2334/josnusd.43.55. [DOI] [PubMed] [Google Scholar]
- 8.Khadra M, Ronold HJ, Lyngstadaas SP, Ellingsen JE, Haanaes HR. Low-level laser therapy stimulates bone-implant interaction: an experimental study in rabbits. Clin Oral Implants Res. 2004;15:325–332. doi: 10.1111/j.1600-0501.2004.00994.x. [DOI] [PubMed] [Google Scholar]
- 9.Azuma Y, Ito M, Harada Y, Takagi H, Ohta T, Jingushi S. Low-intensity pulsed ultrasound accelerates rat femoral fracture healing by acting on the various cellular reactions in the fracture callus. J Bone Miner Res. 2001;16:671–680. doi: 10.1359/jbmr.2001.16.4.671. [DOI] [PubMed] [Google Scholar]
- 10.do Nascimento LEAG, Pithon MM, Sant'Anna EF. Treatment of bilateral Brodie bite in a periodontally compromised patient using distraction osteogenesis: case report. J World Fed Orthod. 2013;2:e137–e146. [Google Scholar]
- 11.Choi SH, Kang DY, Hwang CJ. Adult patient with hemifacial microsomia treated with combined orthodontics and distraction osteogenesis. Am J Orthod Dentofacial Orthop. 2014;145:72–84. doi: 10.1016/j.ajodo.2013.02.033. [DOI] [PubMed] [Google Scholar]
- 12.Olmez S, Dogan S, Pekedis M, Yildiz H. Biomechanical evaluation of sagittal maxillary internal distraction osteogenesis in unilateral cleft lip and palate patient and noncleft patients: a three-dimensional finite element analysis. Angle Orthod. 2014;84:815–824. doi: 10.2319/080613-586.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monasterio FO, Molina F, Berlanga F, et al. Swallowing disorders in Pierre Robin sequence: its correction by distraction. J Craniofac Surg. 2004;15:934–941. doi: 10.1097/00001665-200411000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Pithon MM, de Andrade AC, de Andrade AP, dos Santos RL. Sturge-Weber syndrome in an orthodontic patient. Am J Orthod Dentofacial Orthop. 2011;140:418–422. doi: 10.1016/j.ajodo.2009.10.049. [DOI] [PubMed] [Google Scholar]
- 15.Parfitt AM. A new approach to iliac bone histomorphometry: implications for biomechanics and cell biology. J Clin Invest. 2014;124:70–71. doi: 10.1172/JCI73843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibanez R. Bone mineral density measurement techniques. An Sist Sanit Navar. 2003;26(suppl 3):19–27. [PubMed] [Google Scholar]
- 17.Vandeweghe S, Coelho PG, Vanhove C, Wennerberg A, Jimbo R. Utilizing micro-computed tomography to evaluate bone structure surrounding dental implants: a comparison with histomorphometry. J Biomed Mater Res B Appl Biomater. 2013;101:1259–1266. doi: 10.1002/jbm.b.32938. [DOI] [PubMed] [Google Scholar]
- 18.Recker RR, Kimmel DB, Dempster D, Weinstein RS, Wronski TJ, Burr DB. Issues in modern bone histomorphometry. Bone. 2011;49:955–964. doi: 10.1016/j.bone.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Storey E. Tissue response to the movement of bones. Am J Orthod. 1973;64:229–247. doi: 10.1016/0002-9416(73)90017-1. [DOI] [PubMed] [Google Scholar]
- 20.Babuccu C, Keklikoglu N, Baydogan M, Kaynar A. Cumulative effect of low-level laser therapy and low-intensity pulsed ultrasound on bone repair in rats. Int J Oral Maxillofac Surg. 2014;43:769–776. doi: 10.1016/j.ijom.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Andrade Gomes do Nascimento LE, Sant'Anna EF, Carlos de Oliveira Ruellas A, Issamu Nojima L, Goncalves Filho AC, Antonio Pereira Freitas S. Laser versus ultrasound on bone density recuperation after distraction osteogenesis—a cone-beam computer tomographic analysis. J Oral Maxillofac Surg. 2013;71:921–928. doi: 10.1016/j.joms.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Kocyigit ID, Coskunses FM, Pala E, Tugcu F, Onder E, Mocan A. A comparison of the low-level laser versus low intensity pulsed ultrasound on new bone formed through distraction osteogenesis. Photomed Laser Surg. 2012;30:438–443. doi: 10.1089/pho.2012.3263. [DOI] [PubMed] [Google Scholar]
- 23.Moinnes JJ, Vidula N, Halim N, Othman SF. Ultrasound accelerated bone tissue engineering monitored with magnetic resonance microscopy. Conf Proc IEEE Eng Med Biol Soc. 2006;1:484–488. doi: 10.1109/IEMBS.2006.260306. [DOI] [PubMed] [Google Scholar]
- 24.Romanos GE, Yukna RA, Serio FG. Debate lingers over role of lasers in periodontal therapy. Compend Contin Educ Dent. 2014;35:154–156. [PubMed] [Google Scholar]
- 25.Obradovic R, Kesic L, Mihailovic D, Jovanovic G, Antic S, Brkic Z. Low-level lasers as an adjunct in periodontal therapy in patients with diabetes mellitus. Diabetes Technol Ther. 2012;14:799–803. doi: 10.1089/dia.2012.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malali E, Kadir T, Noyan U. Er:YAG lasers versus ultrasonic and hand instruments in periodontal therapy: clinical parameters, intracrevicular micro-organism and leukocyte counts. Photomed Laser Surg. 2012;30:543–550. doi: 10.1089/pho.2011.3202. [DOI] [PubMed] [Google Scholar]
- 27.Coleton S. The use of lasers in periodontal therapy. Gen Dent. 2008;56:612–616. [PubMed] [Google Scholar]
- 28.Yoshida T, Yamaguchi M, Utsunomiya T, et al. Low-energy laser irradiation accelerates the velocity of tooth movement via stimulation of the alveolar bone remodeling. Orthod Craniofac Res. 2009;12:289–298. doi: 10.1111/j.1601-6343.2009.01464.x. [DOI] [PubMed] [Google Scholar]