Abstract
A convoluted poly(4-vinylpyridine) cobalt(II) (P4VP-CoCl2) system was developed as a stable and reusable heterogeneous catalyst. The local structure near the Co atom was determined on the basis of experimental data and theoretical calculations. This immobilized cobalt catalyst showed high selectivity and catalytic activity in the [2 + 2 + 2] cyclotrimerization of terminal aryl alkynes. With 0.033 mol % P4VP-CoCl2, the regioselective formation of 1,3,5-triarylbenzene was realized without 1,2,4-triarylbenzene formation. Further, a multigram-scale (11 g) reaction proceeded efficiently. In addition, the polymer-supported catalyst was successfully recovered and used three times. X-ray photoelectron spectroscopy analysis of the recovered catalyst suggested that cobalt was in the +2 oxidation state. The 1,3,5-triarylbenzene derivatives were applied to the synthesis of a molecular beam electron resist and a polycyclic aromatic hydrocarbon.
Keywords: cobalt catalysis, cyclotrimerization, polymer-supported catalyst, regioselective catalyst, reusable catalyst
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) have long attracted significant research interest because of their potential applicability in nanoparticles, organic semiconductors, and photovoltaics.1−18 Symmetric 1,3,5-triarylbenzenes act as key building blocks for the generation of PAHs.19 Organic light-emitting diodes,20−22 dendrimers,23,24 and fullerene fragments25−28 can also be synthesized from 1,3,5-triarylbenzenes. The common synthesis routes for 1,3,5-triarylbenzenes are multistep Suzuki–Miyaura-type reactions,29−33 acid-catalyzed triple condensation reactions of aryl methyl ketones,34−39 and transition-metal-catalyzed cyclotrimerization reactions of aryl alkynes.40−69 Although several transition metals, including cobalt,40−47 nickel,48−54,54 rhodium,55−57 and ruthenium,58,59 are known to catalyze cyclotrimerization reactions, the selective formation of 1,3,5-triarylbenzene has rarely been achieved.70−74 The transition-metal-catalyzed cyclotrimerization of alkynes usually generates a mixture of 1,2,4-triarylbenzene as a major product and 1,3,5-triarylbenzene as a minor product (Scheme 1a).40−69 Additionally, these reactions typically demand external reducing agents and solvents, resulting in unwanted chemical waste while reducing the atom efficiency of the reaction. These transition-metal-catalyzed reactions also demand high catalyst loadings, and the catalysts are not usually recoverable and reusable.
Scheme 1. Representative Examples of Transition-Metal-Catalyzed Cyclotrimerization.
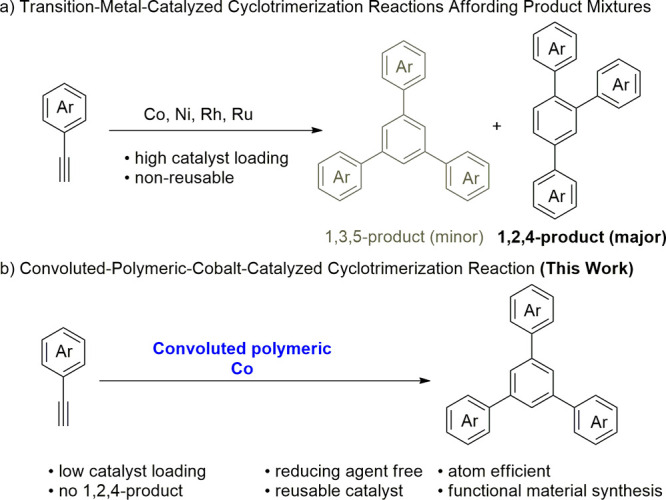
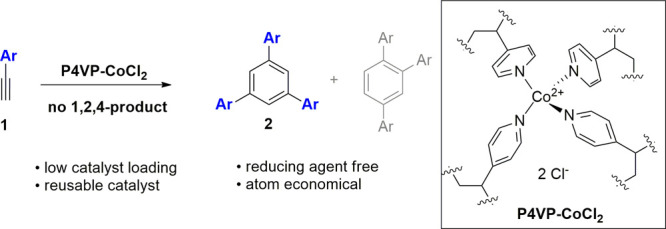
Because steric repulsion between a bulky ligand and an aryl acetylene could suppress the formation of 1,2,4-triarylbenzene,43,44 we assumed that a polymer-supported catalyst might generate the desired 1,3,5-triarylbenzenes with very high selectivity. Polymers like poly(4-vinylpyridine) (P4VP) are advantageous because in addition to being bulky, they enable heterogeneous catalysis75−80 and thus catalyst reuse. Although homogeneous cobalt catalysis for cyclotrimerization has been reported,40−47 to the best of our knowledge, polymer-supported cobalt81−84 has not yet been applied to this reaction. Herein, we report 1,3,5-selective cyclotrimerization using a reusable, stable, heterogeneous, and convoluted polymeric cobalt(II) catalyst under external reducing agent-free conditions (Scheme 1b).
2. Results and Discussion
Preparation and Structural Analysis for Convoluted Cobalt Catalyst
To prepare the convoluted polymeric cobalt catalyst, a methanol solution of commercially available non-cross-linked P4VP (I; MW ∼ 160 000) was treated with an aqueous solution of CoCl2 for 6 h at room temperature. Consequently, molecular convolution produced P4VP-CoCl2 (II) as a blue precipitate (Scheme 2), which was insoluble in water, methanol, 1,4-dioxane, hexane, and ethyl acetate. On the basis of X-ray absorption near edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) data, elemental analysis, and density functional theory (DFT) calculations, the local structure near the Co atom of II was proposed, as shown in Scheme 2 (see Figures S1 and S2 for details). Scanning electron microscopy (SEM) analysis of the synthesized cobalt complex revealed that the catalyst was porous (Figure 1) and it had a regular distribution of cobalt (for details; see Figure S3).
Scheme 2. Synthesis and Calculated Model Structure of P4VP-Supported Cobalt Complex II.
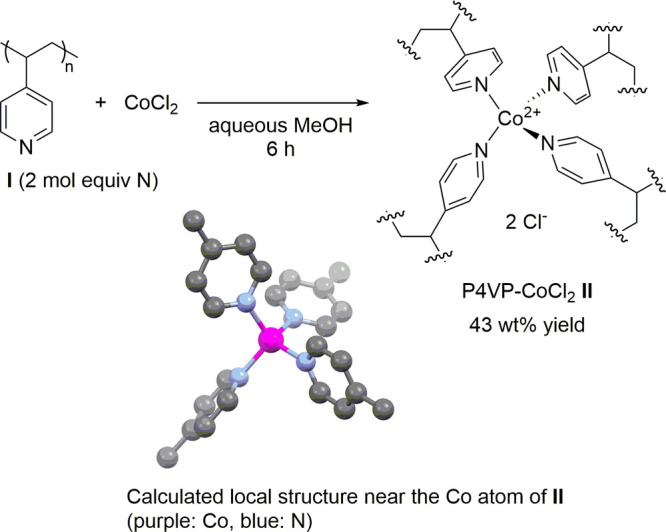
Figure 1.
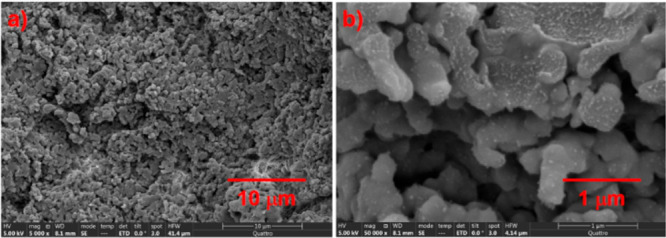
SEM Image of P4VP-CoCl2II.
Reaction Optimization
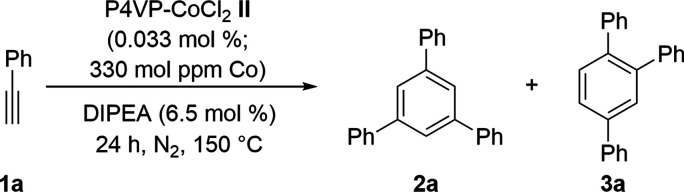
With P4VP-CoCl2II in hand as a heterogeneous Co catalyst, the cyclotrimerization of phenylacetylene (1a) was investigated (Table 1). Pleasingly, when the reaction of 1a was carried out with 0.033 mol % of II (330 mol ppm of Co) in the presence of N,N-diisopropylethylamine (DIPEA, Hünig’s base) at 150 °C for 24 h under neat conditions, the regioselective cyclization proceeded selectively to produce 1,3,5-triphenylbenzene (2a) in 68% yield, and no 1,2,4-triphenylbenzene (3a) was detected (entry 1, Table 1). Although the reaction proceeded well without base (entry 2), the catalyst was not reusable (for details, see the Supporting Information, Section 12). Zn as a reducing agent did not improve the reactivity and slightly reduced the product yield (entry 3). When 0.015 mol % of II (150 mol ppm of Co) was used, the reaction still proceeded to afford 2a in 59% yield (entry 4). The increased catalyst loading (0.066 mol % or 660 mol ppm of Co) was unable to increase the reaction yield (entry 5). The reaction in xylene afforded 2a in 66% yield, whereas those in toluene (40% yield) and DMF (trace) were sluggish (entries 6–8). In the absence of metals, no desired product was obtained (entry 9). A homogeneous cobalt catalyst (CoCl2) without a ligand provided only a trace amount of 2a (entry 10), whereas the yield improved slightly in the presence of pyridine as a ligand (entry 11). The reaction using other homogeneous Co catalysts, including CpCo(CO)2 and (PPh3)3CoCl, produced mixtures of 1,3,5- and 1,2,4-triphenylbenzene (2a and 3a) in yields up to 15% and 21%, respectively (entries 12 and 13). The reaction with more electron-donating 4-t-butyl-pyridine as a ligand gave 16% of 1,3,5-triphenylbenzene (2a) and 20% of 1,2,4-triphenylbenzene (3a), whereas that of 4-i-propyl-pyridine as a monomeric unit of P4VP afforded 19% 1,3,5-triphenylbenzene (2a) and 24% 1,2,4-triphenylbenzene (3a) (entries 14 and 15). These experimental data suggest that molecular convolution of the metal-cross-linked 3D Co species is important for the 1,3,5-selectivity.
Table 1. Cyclotrimerization of 1a Using P4VP-CoCl2a.
| entry | deviation from standard conditions | yield of 2a (%) | yield of 3a (%) |
|---|---|---|---|
| 1 | none | 69 (68b) | 0 |
| 2 | without base | 64 | 0 |
| 3 | zinc (1 mol equiv) | 63 | 0 |
| 4 | 0.015 mol % P4VP-CoCl2 (150 mol ppm Co) | 59 | 0 |
| 5 | 0.066 mol % P4VP-CoCl2 (660 mol ppm Co) | 68 | 0 |
| 6 | in toluene (0.5 mL) | 40 | 0 |
| 7 | in xylene (0.5 mL) | 66 | 0 |
| 8 | in DMF (0.5 mL) | trace | 0 |
| 9 | in the absence of Co | 0 | 0 |
| 10 | CoCl2 (0.3 mol % Co) | trace | 9 |
| 11 | CoCl2 (0.3 mol % Co) and pyridine (1.3 mol %) | 4 | 7 |
| 12 | CpCo(CO)2 (0.3 mol % Co) and pyridine (1.3 mol %) | 15 | 21 |
| 13 | (PPh3)3CoCl (0.3 mol % Co) and pyridine (1.3 mol %) | 1 | 0 |
| 14 | CoCl2 (0.3 mol % of Co) and 4-t-Bu-pyridine (1.3 mol %) | 16 | 20 |
| 15 | CoCl2 (0.3 mol % of Co) and 4-i-Pr-pyridine (1.3 mol %) | 19 | 24 |
Yield determined by 1H NMR using 1,3,5-trimethoxybenzene as an internal standard.
Isolated yield.
Substrate Scope
Using the optimized reaction conditions, the substrate scope for cyclotrimerization was investigated. All the reactions selectively produced 1,3,5-triarylbenzene products (Table 2). For example, p-, m-, and o-methyl-substituted phenyl acetylenes 1b–d were readily converted to 1,3,5-tris(methyl phenyl)benzenes 2b–d in 84, 77, and 67% yield, respectively (entries 2–4). Substrates bearing electron-donating groups, such as p-methoxy (1e) and p-butyl (1f), were converted to the desired C3-symmetric cyclotrimerized products in 86 and 79% yields, respectively (entries 5 and 6). Halogen-containing aryl alkynes 1-bromo-4-ethynylbenzene (1g), 1-chloro-4-ethynylbenzene (1h), and 1-ethynyl-4-fluorobenzene (1i) furnished the desired cycloaddition products in 66, 61, and 53% yield, respectively (entries 7–9). The reaction also afforded the desired product in the presence of electron-withdrawing groups (entries 10–12) with lower yields. The lower yield is due to several byproduct formations (for details, see the Supporting Information, Section S15). Product 2k has been used to synthesize the core of self-assembled dendritic supermolecules.85,86 Interestingly, unprotected 4-ethynyl phenol (1m) was readily converted to desired product 2m in 71% yield (entry 13). The reactions of 4-ethynyl-1,1′-biphenyl (1n) and 2-ethynyl-1,1′-biphenyl (1o) proceeded to afford the corresponding 1,3,5-tris(biphenyl)benzenes in 49 and 42% yield, respectively (entries 14 and 15). Although the reaction did not tolerate 3-ethynylpyridine (1p), 1-ethynylnaphthalene (1q) produced 1,3,5-tri(1-naphthyl)benzene (2q) in 41% yield (entries 16 and 17). The reaction with aliphatic alkynes such as 1-octyne (1r) and (triisopropylsilyl)acetylene (1s) did not proceed under the optimized reaction conditions (entries 18 and 19). The reaction with internal alkyne such as 1,2-diphenylethyne (1t) was also not able to furnish the desired cyclotrimerized product (entry 20).
Table 2. Substrate Scope for Cyclotrimerizationa.
| entry | substrate 1 (Ar) | yield of 2 (%) |
|---|---|---|
| 1 | Ph, 1a | 68 |
| 2 | 4-MeC6H4, 1b | 84 |
| 3 | 3-MeC6H4, 1c | 77 |
| 4 | 2-MeC6H4, 1d | 67 |
| 5 | 4-MeOC6H4, 1e | 86 |
| 6 | 4-BuC6H4, 1f | 79 |
| 7 | 4-BrC6H4, 1g | 66b |
| 8 | 4-ClC6H4, 1h | 61b |
| 9 | 4-FC6H4, 1i | 53 |
| 10 | 4-CF3C6H4, 1j | 42 |
| 11 | 4-EtO2CC6H4, 1k | 46 |
| 12 | 4-MeCOC6H4, 1l | 39b |
| 13 | 4-HOC6H4, 1m | 71b |
| 14 | 4-PhC6H4, 1n | 49b |
| 15 | 2-PhC6H4, 1o | 42b |
| 16 | 3-Py, 1p | 0b |
| 17 | 1-naphthyl, 1q | 41 |
| 18 | 1-octyne (1r) | 0 |
| 19 | (triisopropylsilyl)acetylene (1s) | 0 |
| 20 | 1,2-diphenylethyne (1t) | 0b |
Reaction conditions: 1 (1 mol equiv., 5 mmol), P4VP-CoCl2 (0.033 mol %, 330 mol ppm of Co), DIPEA (6.5 mol %), 150 °C, 24 h, N2 atmosphere. Isolated yield.
One-half a milliliter of xylene was added.
Catalyst Reusability
To our delight, the cyclotrimerization reaction using recovered cobalt catalyst preceded well (Table 3; for details, see the Supporting Information, Section 9). After the reaction with a fresh catalyst (II), the catalyst was recovered from the reaction mixture with 98% yield (entry 1). The reaction with the recovered catalyst provided 80% yield of desired C3-symmetric triarylbenzene (2b) whereas the catalyst can be recovered again with 95% yield (entry 2). Similarly, a third trial provided 72% yield of 2b with 97% catalyst recovery (entry 3).
Table 3. Application of Recovered Catalyst.

| entry | no. of run | catalyst recovery yield (%) | yield of 2b (%) |
|---|---|---|---|
| 1 | fresh | 98 | 84 |
| 2 | 1st | 95 | 80 |
| 3 | 2nd | 97 | 72 |
Cobalt Contamination Test from Isolated Product
The inductively coupled plasma-mass spectrometry (ICP-MS) analysis of 1,3,5-triphenylbenezene (2a) showed only 0.03 ppm cobalt contamination in the product (for details, see the Supporting Information, Section 11). This low amount of Co contamination in the products meets the criteria of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use.87 The hot filtration test suggested the heterogeneous nature of the catalytic system (for details, see the Supporting Information, Section 9.3).
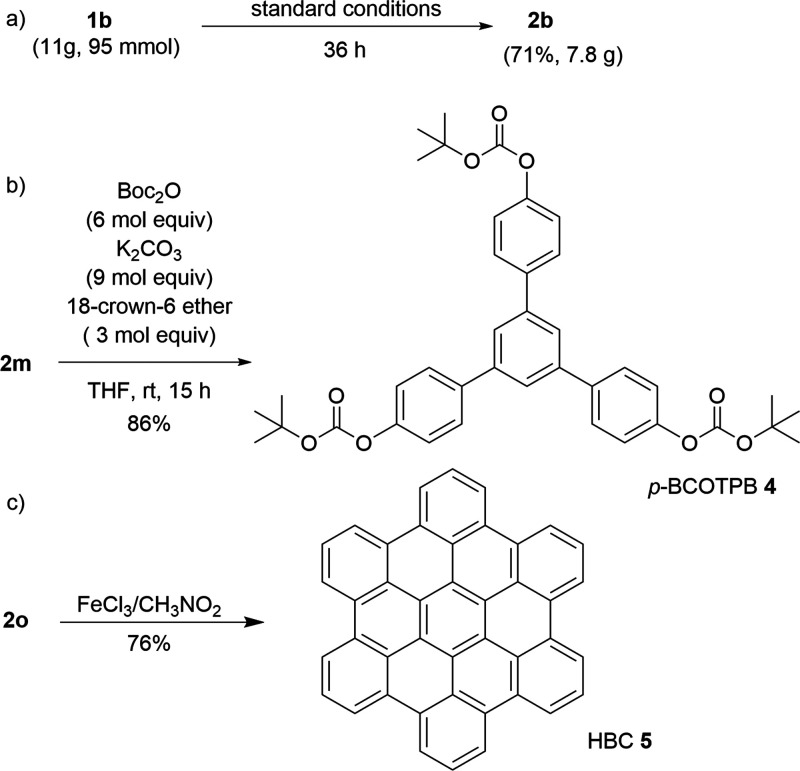
Application of Cyclotrimerization Product
The reaction of 1b also proceeded successfully on a multigram scale (11 g, 95 mmol), yielding 7.8 g (71%) of 2b (Scheme 3a). To demonstrate the synthetic applications of the obtained products, we synthesized functional materials. The reaction of 2m with di-t-butyl dicarbonate afforded 1,3,5-tris(4-t-butoxycarbonyloxyphenyl)benzene (p-BCOTPB, 4) (Scheme 3b). p-BCOTPB is an electron beam molecular resist with high sensitivity and high resolution.88 Furthermore, product 2o was converted to a PAH (hexa-peri-hexabenzocoronene; HBC, 5) after reaction with FeCl3 in nitromethane (Scheme 3c); HBC is an organic semiconductor.19
Scheme 3. Multigram-Scale Reaction and Application of Cyclotrimerized Products.
Speculative Catalytic Pathway
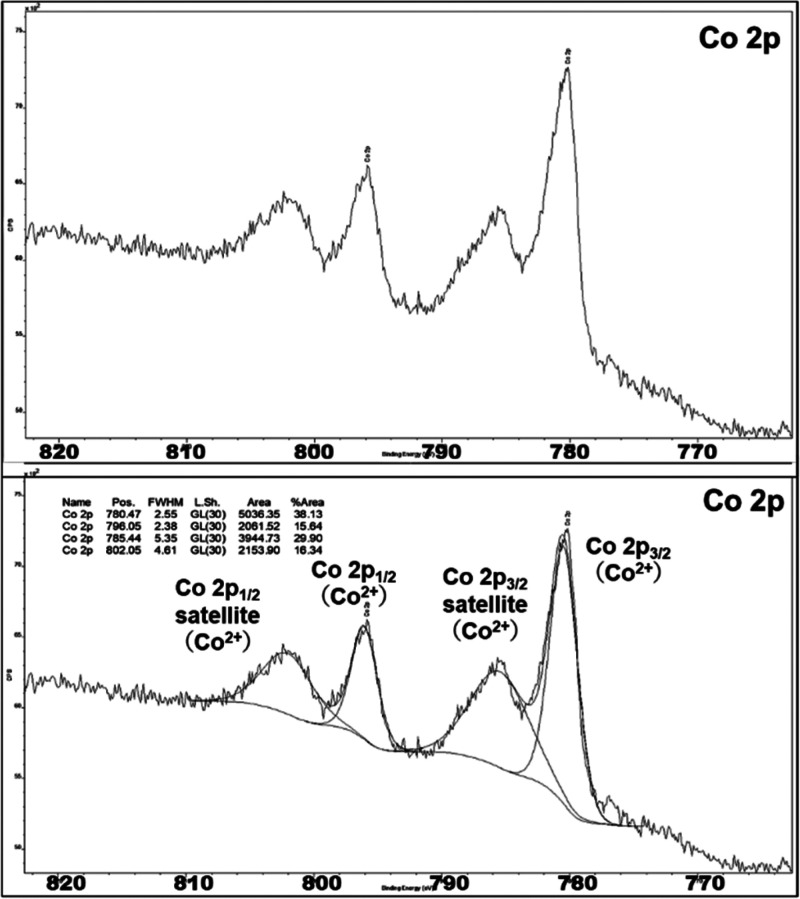
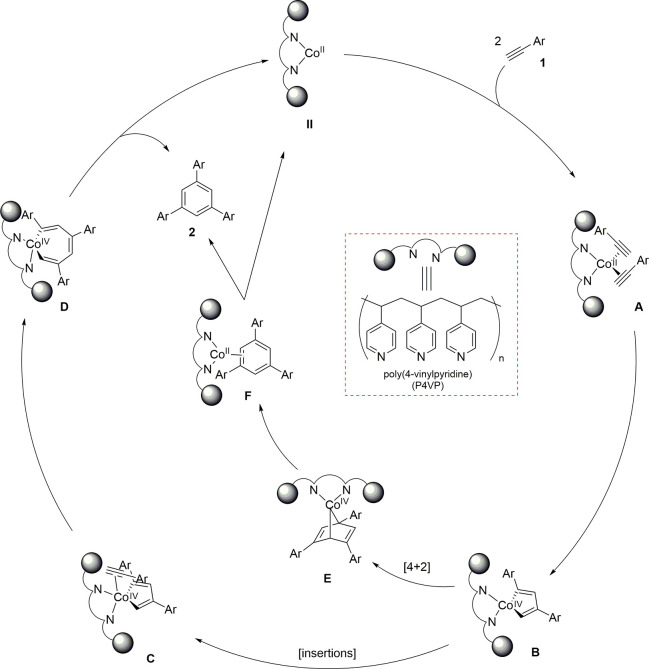
We believe there are two plausible pathways for this transformation. One is the Co(I)/Co(III) catalytic pathway,44,45 where Co(I) is generated via a disproportionation reaction,89 and the other is Co(II)/Co(IV) catalysis.90−92 A disproportionation reaction should generate a mixture of Co(I) and Co(III) species. However, the X-ray photoelectron spectrum of the recovered catalyst was similar to that of the P4VP-CoCl2 catalyst before the reaction, indicating that the recovered catalyst contains only Co(II) (Figure 2; for details, see the Supporting Information, Section S13). Therefore, the cyclotrimerization reaction likely proceed via a Co(II)/Co(IV) pathway, where a high reaction temperature assists in generating the Co(IV) species. As shown in Scheme 4, two equivalents of arylacetylene 1 coordinate to the cobalt(II) species to produce intermediate A. Oxidative cyclization proceeds to produce the corresponding five-membered cobalta(IV)-cycle intermediate (B). Coordination of another equivalent of 1 (C) should proceed via least steric repulsion (Figure S5), which is the critical step for 1,3,5-regioselectivity. Insertion affords seven-membered cobaltacycle intermediate D. Finally, the reductive elimination of D generates desired C3-symmetric cycloaddition product 2 and cobalt(II) catalyst II. Alternatively, a [4 + 2] cycloaddition of aryl acetylene (1) with intermediate B could result the formation of intermediate E. Reductive elimination from E generates the final product 2 via intermediate F. Additionally, the fact that the reaction is described only for terminal alkynes leaves some room for consideration of other mechanistic options, namely, those involving vinylidene complex intermediates.93,94
Figure 2.
XPS spectra of cobalt 2p orbital of P4VP-Co before (top) and after (bottom) the reaction.
Scheme 4. Speculative Catalytic Pathway.
3. Conclusion
In conclusion, we developed P4VP-CoCl2 as a bench-stable convoluted polymeric cobalt catalyst, which showed high catalytic activity for [2 + 2 + 2] cycloaddition and produced 1,3,5-regioselective cyclotrimerized products. The reaction proceeded smoothly using 0.033 mol % of catalyst (330 mol ppm of Co), with little Co leaching. Moreover, the catalyst was recoverable and reusable, and the reaction could be scaled up to the multigram scale. Electron beam molecular resist p-BCOTPB and organic semiconductor HBC were successfully synthesized from the cyclotrimerized products. Detailed mechanistic investigations are currently ongoing in our laboratory.
Acknowledgments
We are grateful to the Materials Characterization Support Team (RIKEN Center for Emergent Matter Science) for elemental analysis. We thank Dr. Tetsuo Honma (JASRI, SPring-8) for assistance with XAFS studies (SPring-8, BL14B2), Dr. Takehiro Suzuki (RIKEN Center for Sustainable Resource Science) for MALDI-TOFMS measurements, and Mr. Makoto Tokunaga (Comprehensive Analysis Center for Science, Saitama University) for XPS studies. We gratefully acknowledge financial support from AMED (19ak0101115h), the JSPS (21H01979), the Fugaku Trust for Medical Research, and RIKEN.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacsau.1c00360.
Experimental details for catalyst preparation, cyclotrimerization reaction procedure, and analytical data (1H NMR, 13C NMR, and 19F-NMR spectra, XAFS data, XPS spectra, SEM images, elemental analysis results, and melting points) (PDF)
Author Contributions
The manuscript was written through the contributions of all the authors. All authors have approved the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Li C.; Liu M.; Pschirer N. G.; Baumgarten M.; Müllen K. Polyphenylene-Based Materials for Organic Photovoltaics. Chem. Rev. 2010, 110, 6817–6855. 10.1021/cr100052z. [DOI] [PubMed] [Google Scholar]
- Coropceanu V.; Cornil J.; da Silva Filho D. A.; Olivier Y.; Silbey R.; Brédas J.-L. Charge Transport in Organic Semiconductors. Chem. Rev. 2007, 107, 926–952. 10.1021/cr050140x. [DOI] [PubMed] [Google Scholar]
- Uribe-Romo F. J.; Doonan C. J.; Furukawa H.; Oisaki K.; Yaghi O. M. Crystalline Covalent Organic Frameworks with Hydrazone Linkages. J. Am. Chem. Soc. 2011, 133, 11478–11481. 10.1021/ja204728y. [DOI] [PubMed] [Google Scholar]
- Jin W.; Yamamoto Y.; Fukushima T.; Ishii N.; Kim J.; Kato K.; Takata M.; Aida T. Systematic Studies on Structural Parameters for Nanotubular Assembly of Hexa-peri-hexabenzocoronenes. J. Am. Chem. Soc. 2008, 130, 9434–9440. 10.1021/ja801179e. [DOI] [PubMed] [Google Scholar]
- Bao C.; Lu R.; Jin M.; Xue P.; Tan C.; Xu T.; Liu G.; Zhao Y. Helical Stacking Tuned by Alkoxy Side Chains in p-Conjugated Triphenylbenzene Discotic Derivatives. Chem. - Eur. J. 2006, 12, 3287–3294. 10.1002/chem.200501058. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Cao X.-Y.; Zi H.; Pei J. Single-Molecule Nanosized Polycyclic Aromatics with Alternant Five- and Six-Membered Rings: Synthesis and Optical Properties. Org. Lett. 2005, 7, 959–962. 10.1021/ol047625c. [DOI] [PubMed] [Google Scholar]
- van de Craats A. M.; Stutzmann N.; Bunk O.; Nielsen M. M.; Watson M.; Müllen K.; Chanzy H. D.; Sirringhaus H.; Friend R. H. Meso-Epitaxial Solution-Growth of Self-Organizing Discotic Liquid-Crystalline Semiconductors. Adv. Mater. 2003, 15, 495–499. 10.1002/adma.200390114. [DOI] [Google Scholar]
- Transition-Metal-Mediated Aromatic Ring Construction; Tanaka K., Ed.; John Wiley & Sons: Hoboken, NJ, 2013. [Google Scholar]
- Agenet N.; Buisine O.; Slowinski F.; Gandon V.; Aubert C.; Malacria M. Cotrimerizations of Acetylenic Comounds. Org. React. 2007, 68, 1–302. 10.1002/0471264180.or068.01. [DOI] [Google Scholar]
- Shibata Y.; Tanaka K. Rhodium-Catalyzed [2 + 2 + 2] Cycloaddition of Alkynes for the Synthesis of Substituted Benzenes: Catalysts, Reaction Scope, and Synthetic Applications. Synthesis 2012, 44, 323–350. 10.1055/s-0031-1289665. [DOI] [Google Scholar]
- Domínguez G.; Pérez-Castells J. Recent Advances in [2 + 2 + 2] Cycloaddition Reactions. Chem. Soc. Rev. 2011, 40, 3430–3444. 10.1039/c1cs15029d. [DOI] [PubMed] [Google Scholar]
- Nagata T.; Obora Y. Transition-Metal-Mediated/Catalyzed Synthesis of Pyridines, Pyrimidines, and Triazines by [2 + 2 + 2] Cycloaddition Reactions. Asian J. Org. Chem. 2020, 9, 1532–1547. 10.1002/ajoc.202000240. [DOI] [Google Scholar]
- Kotha S.; Lahiri K.; Sreevani G. Design and Synthesis of Aromatics through [2 + 2 + 2] Cyclotrimerization. Synlett 2018, 29, 2342–2361. 10.1055/s-0037-1609584. [DOI] [Google Scholar]
- Röse P.; Hilt G. Cobalt-Catalysed Bond Formation Reactions; Part 2. Synthesis 2016, 48, 463–492. 10.1055/s-0035-1560378. [DOI] [Google Scholar]
- Thakur A.; Louie J. Advances in Nickel-Catalyzed Cycloaddition Reactions to Construct Carbocycles and Heterocycles. Acc. Chem. Res. 2015, 48, 2354–2365. 10.1021/acs.accounts.5b00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S.; Sugiyama Y.-K. From the Development of Catalysts for Alkyne and Alkyne–Nitrile [2 + 2 + 2] Cycloaddition Reactions to Their Use in Polymerization Reactions. Synlett 2013, 24, 1044–1060. 10.1055/s-0032-1316913. [DOI] [Google Scholar]
- Broere D. L. J.; Ruijter F. Recent Advances in Transition-Metal-Catalyzed [2 + 2 + 2] Cyclo(co)trimerization Reactions. Synthesis 2012, 44, 2639–2672. 10.1055/s-0032-1316757. [DOI] [Google Scholar]
- Hapke M. Transition metal-free formal [2 + 2 + 2] cycloaddition reactions of alkynes. Tetrahedron Lett. 2016, 57, 5719–5729. 10.1016/j.tetlet.2016.10.083. [DOI] [Google Scholar]
- Zhang S.-L.; Xue Z.-F.; Gao Y.-R.; Mao S.; Wang Y.-Q. Triple Condensation of Aryl Methyl Ketones Catalyzed by Amine and Trifluoroacetic Acid: Straightforward Access to 1,3,5-Triarylbenzenes under Mild Conditions. Tetrahedron Lett. 2012, 53, 2436–2439. 10.1016/j.tetlet.2012.03.008. [DOI] [Google Scholar]
- Kotha S.; Kashinath D.; Kumar S. Synthesis of Liquid Crystalline Materials Based on 1,3,5-Triphenylbenzene and 2,4,6-Triphenyl-1,3,5-s-triazine. Tetrahedron Lett. 2008, 49, 5419–5423. 10.1016/j.tetlet.2008.07.021. [DOI] [Google Scholar]
- Luo J.; Zhou Y.; Niu Z.-Q.; Zhou Q.-F.; Ma Y.; Pei J. Three-Dimensional Architectures for Highly Stable Pure Blue Emission. J. Am. Chem. Soc. 2007, 129, 11314–11315. 10.1021/ja073466r. [DOI] [PubMed] [Google Scholar]
- Pang J.; Marcotte E. J.-P.; Seward C.; Brown R. S.; Wang S. A Blue Luminescent Star-Shaped ZnII Complex that Can Detect Benzene. Angew. Chem., Int. Ed. 2001, 40, 4042–4045. . [DOI] [PubMed] [Google Scholar]
- He Q.; Huang H.; Yang J.; Lin H.; Bai F. Synthesis and Spectroscopic Properties of a Series of Hyperbranched Conjugated Molecules with 1,3,5-Triphenylbenzene as Cores. J. Mater. Chem. 2003, 13, 1085–1089. 10.1039/b210297h. [DOI] [Google Scholar]
- Brunel J.; Ledoux I.; Zyss J.; Blanchard-Desce M. Propeller-Shaped Molecules with Giant Off-Resonance Optical Nonlinearities. Chem. Commun. 2001, 923–924. 10.1039/b101425k. [DOI] [Google Scholar]
- Vivekanand P. A.; Balakrishnan T. Synthesis and Characterization of a Novel Multi-Site Phase Transfer Catalyst and a Kinetic Study of the Intramolecular Cyclopentanation of Indene. Appl. Catal., A 2009, 364, 27–34. 10.1016/j.apcata.2009.05.014. [DOI] [Google Scholar]
- Gómez-Lor B.; Echavarren A. M. Synthesis of a Triaza Analogue of Crushed-Fullerene by Intramolecular Palladium-Catalyzed Arylation. Org. Lett. 2004, 6, 2993–2996. 10.1021/ol048760s. [DOI] [PubMed] [Google Scholar]
- Gómez-Lor B.; González-Cantalapiedra E.; Ruiz M.; de Frutos Ó.; Cárdenas D. J.; Santos A.; Echavarren A. M. Synthesis of C3 Benzo[1,2-e:3,4-e′:5,6-e″]tribenzo[l]acephenanthrylenes (“Crushed Fullerene” Derivatives) by Intramolecular Palladium-Catalyzed Arylation. Chem. - Eur. J. 2004, 10, 2601–2608. 10.1002/chem.200306023. [DOI] [PubMed] [Google Scholar]
- Mongin O.; Brunel J.; Porrès L.; Blanchard-Desce M. Synthesis and Two-Photon Absorption of Triphenylbenzene-Cored Dendritic Chromophores. Tetrahedron Lett. 2003, 44, 2813–2816. 10.1016/S0040-4039(03)00455-6. [DOI] [Google Scholar]
- Schnobrich J. K.; Lebel O.; Cychosz K. A.; Dailly A.; Wong-Foy A. G.; Matzger A. J. Linker-Directed Vertex Desymmetrization for the Production of Coordination Polymers with High Porosity. J. Am. Chem. Soc. 2010, 132, 13941–13948. 10.1021/ja107423k. [DOI] [PubMed] [Google Scholar]
- Noguchi H.; Shioda T.; Chou C.-M.; Suginome M. Differentially Protected Benzenediboronic Acids: Divalent Cross-Coupling Modules for the Efficient Synthesis of Boron-Substituted Oligoarenes. Org. Lett. 2008, 10, 377–380. 10.1021/ol702420x. [DOI] [PubMed] [Google Scholar]
- Bonvallet P. A.; Breitkreuz C. J.; Kim Y. S.; Todd E. M.; Traynor K.; Fry C. G.; Ediger M. D.; McMahon R. J. Organic Glass-Forming Materials: 1,3,5-Tris(naphthyl)benzene Derivatives. J. Org. Chem. 2007, 72, 10051–10057. 10.1021/jo701921m. [DOI] [PubMed] [Google Scholar]
- Ramakrishna V.; Dastagiri Reddy N. Synthesis of Zwitterionic Palladium Complexes and Their Application as Catalysts in Cross-Coupling Reactions of Aryl, Heteroaryl and Benzyl Bromides with Organoboron Reagents in Neat Water. Dalton Trans. 2017, 46, 8598–8610. 10.1039/C7DT01433C. [DOI] [PubMed] [Google Scholar]
- Yoshikai N.; Matsuda H.; Nakamura E. Hydroxyphosphine Ligand for Nickel-Catalyzed Cross-Coupling through Nickel/Magnesium Bimetallic Cooperation. J. Am. Chem. Soc. 2009, 131, 9590–9599. 10.1021/ja903091g. [DOI] [PubMed] [Google Scholar]
- Yang J.-X.; Tao X.-T.; Yuan C. X.; Yan Y. X.; Wang L.; Liu Z.; Ren Y.; Jiang M. H. A Facile Synthesis and Properties of Multicarbazole Molecules Containing Multiple Vinylene Bridges. J. Am. Chem. Soc. 2005, 127, 3278–3279. 10.1021/ja043510s. [DOI] [PubMed] [Google Scholar]
- Cao X.-Y.; Liu X.-H.; Zhou X.-H.; Zhang Y.; Jiang Y.; Cao Y.; Cui Y.-X.; Pei J. Giant Extended π-Conjugated Dendrimers Containing the 10,15-Dihydro-5H-diindeno[1,2-a;1’,2’-c]fluorene Chromophore: Synthesis, NMR Behaviors, Optical Properties, and Electroluminescence. J. Org. Chem. 2004, 69, 6050–6058. 10.1021/jo049268p. [DOI] [PubMed] [Google Scholar]
- Kumar A.; Dixit M.; Singh S. P.; Raghunandan R.; Maulik P. R.; Goel A. Reusable Resin Amberlyst 15 Catalyzed New Convenient Protocol for Accessing Arylated Benzene Scaffolds. Tetrahedron Lett. 2009, 50, 4335–4339. 10.1016/j.tetlet.2009.05.029. [DOI] [Google Scholar]
- Jing X.; Xu F.; Zhu Q.; Ren X.; Yan C.; Wang L.; Wang J. Novel Method for the Synthesis of 1,3,5-Triarylbenzenes from Ketones. Synth. Commun. 2005, 35, 3167–3171. 10.1080/00397910500283370. [DOI] [Google Scholar]
- Wagh G. D.; Akamanchi K. G. Sulfated Tungstate Catalyzed Synthesis of C3-Symmetric 1,3,5-Triaryl Benzenes under Solvent-Free Condition. Tetrahedron Lett. 2017, 58, 3032–3036. 10.1016/j.tetlet.2017.06.055. [DOI] [Google Scholar]
- Tayebee R.; Jarrahi M. H6P2W18O62/Nanoclinoptilolite as an Efficient Nanohybrid Catalyst in the Cyclotrimerization of Aryl Methyl Ketones under Solvent-Free Conditions. RSC Adv. 2015, 5, 21206–21214. 10.1039/C5RA01344E. [DOI] [Google Scholar]
- Wang W.; Inoue S.; Enthaler S.; Driess M. Bis(silylenyl)- and Bis(germylenyl)-Substituted Ferrocenes: Synthesis, Structure, and Catalytic Applications of Bidentate Silicon(II)–Cobalt Complexes. Angew. Chem., Int. Ed. 2012, 51, 6167–6171. 10.1002/anie.201202175. [DOI] [PubMed] [Google Scholar]
- Geny A.; Agenet N.; Iannazzo L.; Malacria M.; Aubert C.; Gandon V. Air-Stable {(C5H5)Co} Catalysts for [2 + 2 + 2] Cycloadditions. Angew. Chem., Int. Ed. 2009, 48, 1810–1813. 10.1002/anie.200806001. [DOI] [PubMed] [Google Scholar]
- Hilt G.; Vogler T.; Hess W.; Galbiati F. A Simple Cobalt Catalyst System for the Efficient and Regioselective Cyclotrimerisation of Alkynes. Chem. Commun. 2005, 1474–1475. 10.1039/b417832g. [DOI] [PubMed] [Google Scholar]
- Yong L.; Butenschön H. The First Cobalt Catalyzed [2 + 2 + 2] Alkyne Cyclotrimerization in Aqueous Medium at Room Temperature. Chem. Commun. 2002, 2852–2853. 10.1039/B207978J. [DOI] [PubMed] [Google Scholar]
- Hilt G.; Hengst C.; Hess W. Solvent-Dependent Regiochemical Cyclotrimerisation of Phenylacetylene with Cobalt Catalysts Containing Disulfide Ligands: A Case Study. Eur. J. Org. Chem. 2008, 2008, 2293–2297. 10.1002/ejoc.200800106. [DOI] [Google Scholar]
- Xu L.; Yu R.; Wang Y.; Chen J.; Yang Z. Highly Regioselective Syntheses of Substituted Triphenylenes from 1,2,4-Trisubstituted Arenes via a Co-Catalyzed Intermolecular Alkyne Cyclotrimerization. J. Org. Chem. 2013, 78, 5744–5750. 10.1021/jo400570b. [DOI] [PubMed] [Google Scholar]
- Goswami A.; Ito T.; Okamoto S. Efficient Activation of 2-Iminomethylpyridine/Cobalt-Based Alkyne [2 + 2 + 2] Cycloaddition Catalyst by Addition of a Silver Salt. Adv. Synth. Catal. 2007, 349, 2368–2374. 10.1002/adsc.200700188. [DOI] [Google Scholar]
- Sierra M. A.; Torres M. R.; de la Torre M. C.; Álvaro E. Synthesis of Terpene and Steroid Dimers and Trimers Having Cyclobutadienyl–Co and Aromatic Tethers. J. Org. Chem. 2007, 72, 4213–4219. 10.1021/jo0703698. [DOI] [PubMed] [Google Scholar]
- Orsino A. F.; Gutiérrez del Campo M.; Lutz M.; Moret M.-E. Enhanced Catalytic Activity of Nickel Complexes of an Adaptive Diphosphine–Benzophenone Ligand in Alkyne Cyclotrimerization. ACS Catal. 2019, 9, 2458–2481. 10.1021/acscatal.8b05025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi C.; Sun Z.; Liu Y. Cyclotrimerization of Terminal Alkynes Catalyzed by the System of NiCl2/Zn and (Benzimidazolyl)-6-(1-(arylimino)ethyl)pyridines. Dalton Trans. 2013, 42, 13327–13330. 10.1039/c3dt51674a. [DOI] [PubMed] [Google Scholar]
- Shen L.; Zhao Y.; Luo Q.; Li Q.-S.; Liu B.; Redshaw C.; Wu B.; Yang X.-J. Cyclotrimerization of Alkynes Catalyzed by a Self-Supported Cyclic Tri-Nuclear Nickel(0) Complex with α-Diimine Ligands. Dalton Trans. 2019, 48, 4643–4649. 10.1039/C9DT00819E. [DOI] [PubMed] [Google Scholar]
- Pal S.; Uyeda C. Evaluating the Effect of Catalyst Nuclearity in Ni-Catalyzed Alkyne Cyclotrimerizations. J. Am. Chem. Soc. 2015, 137, 8042–8045. 10.1021/jacs.5b04990. [DOI] [PubMed] [Google Scholar]
- Tamizmani M.; Sivasankar C. Synthesis, Characterization and Catalytic Application of Some Novel PNP-Ni(II) Complexes: Regio-Selective [2 + 2 + 2] Cycloaddition Reaction of Alkyne. J. Organomet. Chem. 2017, 845, 82–89. 10.1016/j.jorganchem.2017.02.039. [DOI] [Google Scholar]
- Rodrigo S. K.; Powell I. V.; Coleman M. G.; Krause J. A.; Guan H. Efficient and Regioselective Nickel-Catalyzed [2 + 2 + 2] Cyclotrimerization of Ynoates and Related Alkynes. Org. Biomol. Chem. 2013, 11, 7653–7657. 10.1039/c3ob41872c. [DOI] [PubMed] [Google Scholar]
- Yang K.; Wang P.; Sun Z.; Guo M.; Zhao W.; Tang X.; Wang G. Hydrogen-Bonding Controlled Nickel-Catalyzed Regioselective Cyclotrimerization of Terminal Alkynes. Org. Lett. 2021, 23, 3933–3938. 10.1021/acs.orglett.1c01095. [DOI] [PubMed] [Google Scholar]
- Thiel I.; Horstmann M.; Jungk P.; Keller S.; Fischer F.; Drexler H.-J.; Heller D.; Hapke M. Insight into the Activation of in Situ Generated Chiral RhI Catalysts and Their Application in Cyclotrimerizations. Chem. - Eur. J. 2017, 23, 17048–17057. 10.1002/chem.201703198. [DOI] [PubMed] [Google Scholar]
- Yoshida K.; Morimoto I.; Mitsudo K.; Tanaka H. RhCl3/Amine-Catalyzed [2 + 2 + 2] Cyclization of Alkynes. Tetrahedron 2008, 64, 5800–5807. 10.1016/j.tet.2008.03.079. [DOI] [Google Scholar]
- Torres Ò.; Fernàndez M.; Díaz-Jiménez À.; Pla-Quintana A.; Roglans A.; Solà M. Examining the Factors That Govern the Regioselectivity in Rhodium-Catalyzed Alkyne Cyclotrimerization. Organometallics 2019, 38, 2853–2862. 10.1021/acs.organomet.9b00347. [DOI] [Google Scholar]
- Cadierno V.; García-Garrido S. E.; Gimeno J. Efficient Intermolecular [2 + 2 + 2] Alkyne Cyclotrimerization in Aqueous Medium Using a Ruthenium(IV) Precatalyst. J. Am. Chem. Soc. 2006, 128, 15094–15095. 10.1021/ja066552k. [DOI] [PubMed] [Google Scholar]
- Nogami J.; Tanaka Y.; Sugiyama H.; Uekusa H.; Muranaka A.; Uchiyama M.; Tanaka K. Enantioselective Synthesis of Planar Chiral Zigzag-Type Cyclophenylene Belts by Rhodium-Catalyzed Alkyne Cyclotrimerization. J. Am. Chem. Soc. 2020, 142, 9834–9842. 10.1021/jacs.0c03684. [DOI] [PubMed] [Google Scholar]
- Sugahara T.; Guo J.-D.; Sasamori T.; Nagase S.; Tokitoh N. Regioselective Cyclotrimerization of Terminal Alkynes Using a Digermyne. Angew. Chem., Int. Ed. 2018, 57, 3499–3503. 10.1002/anie.201801222. [DOI] [PubMed] [Google Scholar]
- Brenna D.; Villa M.; Gieshoff T. N.; Fischer F.; Hapke M.; Jacobi von Wangelin A. Iron-Catalyzed Cyclotrimerization of Terminal Alkynes by Dual Catalyst Activation in the Absence of Reductants. Angew. Chem., Int. Ed. 2017, 56, 8451–8454. 10.1002/anie.201705087. [DOI] [PubMed] [Google Scholar]
- Okamoto S.; Yamada T.; Tanabe Y.; Sakai M. Alkyne [2 + 2 + 2] Cyclotrimerization Catalyzed by a Low-Valent Titanium Reagent Derived from CpTiX3 (X = Cl, O-i-Pr), Me3SiCl, and Mg or Zn. Organometallics 2018, 37, 4431–4438. 10.1021/acs.organomet.8b00678. [DOI] [Google Scholar]
- Batrice R. J.; McKinven J.; Arnold P. L.; Eisen M. S. Selective Oligomerization and [2 + 2 + 2] Cycloaddition of Terminal Alkynes from Simple Actinide Precatalysts. Organometallics 2015, 34, 4039–4050. 10.1021/acs.organomet.5b00455. [DOI] [Google Scholar]
- Joosten A.; Soueidan M.; Denhez C.; Harakat D.; Hélion F.; Namy J.-L.; Vasse J.-L.; Szymoniak J. Multimetallic Zirconocene-Based Catalysis: Alkyne Dimerization and Cyclotrimerization Reactions. Organometallics 2008, 27, 4152–4157. 10.1021/om8002703. [DOI] [Google Scholar]
- Morohashi N.; Yokomakura K.; Hattori T.; Miyano S. Highly Regioselective [2 + 2 + 2] Cycloaddition of Terminal Alkynes Catalyzed by Titanium Complexes of p-tert-Butylthiacalix[4]arene. Tetrahedron Lett. 2006, 47, 1157–1161. 10.1016/j.tetlet.2005.12.034. [DOI] [Google Scholar]
- Fu Y.-S.; Yu S. J. Immobilization of Homogeneous Palladium(II) Complex Catalysts on Novel Polysiloxanes with Controllable Solubility: Important Implications for the Study of Heterogeneous Catalysis on Silica Surfaces. Angew. Chem., Int. Ed. 2001, 40, 437–440. . [DOI] [PubMed] [Google Scholar]
- Shibata T.; Fujimoto T.; Yokota K.; Takagi K. Iridium Complex-Catalyzed Highly Enantio- and Diastereoselective [2 + 2 + 2] Cycloaddition for the Synthesis of Axially Chiral Teraryl Compounds. J. Am. Chem. Soc. 2004, 126, 8382–8383. 10.1021/ja048131d. [DOI] [PubMed] [Google Scholar]
- Sato Y.; Tamura T.; Mori M. Arylnaphthalene Lignans through Pd-Catalyzed [2 + 2 + 2] Cocyclization of Arynes and Diynes: Total Synthesis of Taiwanins C and E. Angew. Chem., Int. Ed. 2004, 43, 2436–2440. 10.1002/anie.200453809. [DOI] [PubMed] [Google Scholar]
- Gervasio G.; Sappa E.; Marko L. Synthesis and crystal structure of [Co2(CO)4{PhC = CC(O) CH3}3]. Its role in the cyclotrimerization of 1-phenylbut-1-yn-3-one to 1,3,5 triphenyltris(carboxymethyl) benzene. J. Organomet. Chem. 1993, 444, 203–209. 10.1016/0022-328X(93)83075-7. [DOI] [Google Scholar]
- Kakeya M.; Fujihara T.; Kasaya T.; Nagasawa A. Dinuclear Niobium(III) Complexes [{NbCl2(L)}2(μ-Cl)2(μ-L)] (L = tetrahydrothiophene, dimethyl sulfide): Preparation, Molecular Structures, and the Catalytic Activity for the Regioselective Cyclotrimerization of Alkynes. Organometallics 2006, 25, 4131–4137. 10.1021/om0601100. [DOI] [Google Scholar]
- Xu Y.; Pan Y.; Wu Q.; Wang H.; Liu P. Regioselective Synthesis of 1,3,5-Substituted Benzenes via the InCl3/2-Iodophenol-Catalyzed Cyclotrimerization of Alkynes. J. Org. Chem. 2011, 76, 8472–8476. 10.1021/jo201010d. [DOI] [PubMed] [Google Scholar]
- Huang Y.-S.; Huang G.-T.; Liu Y.-L.; Yu J.-S. K.; Tsai Y.-C. Reversible Cleavage/Formation of the Chromium–Chromium Quintuple Bond in the Highly Regioselective Alkyne Cyclotrimerization. Angew. Chem., Int. Ed. 2017, 56, 15427–15431. 10.1002/anie.201709583. [DOI] [PubMed] [Google Scholar]
- Li J.-H.; Xie Y.-X. Carbon Dioxide Promoted Palladium-Catalyzed Cyclotrimerization of Alkynes in Water. Synth. Commun. 2004, 34, 1737–1743. 10.1081/SCC-120030761. [DOI] [Google Scholar]
- Doll J. S.; Eichelmann R.; Hertwig L. E.; Bender T.; Kohler V. J.; Bill E.; Wadepohl H.; Roşca D.-A. Iron-Catalyzed Trimerization of Terminal Alkynes Enabled by Pyrimidinediimine Ligands: A Regioselective Method for the Synthesis of 1,3,5-Substituted Arenes. ACS Catal. 2021, 11, 5593–5600. 10.1021/acscatal.1c00978. [DOI] [Google Scholar]
- Sen A.; Dhital R. N.; Sato T.; Ohno A.; Yamada Y. M. A. Switching from Biaryl Formation to Amidation with Convoluted Polymeric Nickel Catalysis. ACS Catal. 2020, 10, 14410–14418. 10.1021/acscatal.0c03888. [DOI] [Google Scholar]
- Ohno A.; Sato T.; Mase T.; Uozumi Y.; Yamada Y. M. A. A Convoluted Polyvinylpyridine-Palladium Catalyst for Suzuki-Miyaura Coupling and C–H Arylation. Adv. Synth. Catal. 2020, 362, 4687–4698. 10.1002/adsc.202000742. [DOI] [Google Scholar]
- Hu H.; Ohno A.; Sato T.; Mase T.; Uozumi Y.; Yamada Y. M. A. Self-Assembled Polymeric Pyridine Copper Catalysts for the Huisgen Cycloaddition with Alkynes and Acetylene Gas: Application in Synthesis of Tazobactam. Org. Process Res. Dev. 2019, 23, 493–498. 10.1021/acs.oprd.8b00429. [DOI] [Google Scholar]
- Yamada Y. M. A.; Sarkar S. M.; Uozumi Y. An Amphiphilic Self-Assembled Polymeric Copper Catalyst to Parts per Million Levels: Click Chemistry. J. Am. Chem. Soc. 2012, 134, 9285–9290. 10.1021/ja3036543. [DOI] [PubMed] [Google Scholar]
- Yamada Y. M. A.; Sarkar S. M.; Uozumi Y. Self-Assembled Poly(imidazole-palladium): Highly Active, Reusable Catalyst at Parts per Million to Parts per Billion Levels. J. Am. Chem. Soc. 2012, 134, 3190–3198. 10.1021/ja210772v. [DOI] [PubMed] [Google Scholar]
- Jungk P.; Fischer F.; Thiel I.; Hapke H. CoCl(PPh3)3 as Cyclotrimerization Catalyst for Functionalized Triynes under Mild Conditions. J. Org. Chem. 2015, 80, 9781–9793. 10.1021/acs.joc.5b01623. [DOI] [PubMed] [Google Scholar]
- Baydoun H.; Burdick J.; Thapa B.; Wickramasinghe L.; Li D.; Niklas J.; Poluektov O. G.; Schlegel H. B.; Verani C. N. Immobilization of an Amphiphilic Molecular Cobalt Catalyst on Carbon Black for Ligand-Assisted Water Oxidation. Inorg. Chem. 2018, 57, 9748–9756. 10.1021/acs.inorgchem.7b03252. [DOI] [PubMed] [Google Scholar]
- Jiang W.; Yang X.; Li F.; Zhang Q.; Li S.; Tong H.; Jiang Y.; Xia L. Immobilization of a Molecular Cobalt Cubane Catalyst on Porous BiVO4 via Electrochemical Polymerization for Efficient and Stable Photoelectrochemical Water Oxidation. Chem. Commun. 2019, 55, 1414–1417. 10.1039/C8CC08802K. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Chen H.; Tian H. Immobilization of a Cobalt Catalyst on Fullerene in Molecular Devices for Water Reduction. Chem. Commun. 2015, 51, 11508–11511. 10.1039/C5CC03856A. [DOI] [PubMed] [Google Scholar]
- Pirouzmand M.; Amini M. M.; Safari N.; Hamoule T. Immobilization of Cobalt Phthalocyanine and Tetrasulfophthalocyanine onto MCM-41 and MCM-48: Effect of Immobilization Method on Catalytic Activity. J. Braz. Chem. Soc. 2013, 24, 1864–1870. 10.5935/0103-5053.20130233. [DOI] [Google Scholar]
- Bednaříková T.; Tošner Z.; Horský J.; Jindřich J. Synthesis of C3-Symmetric Tri(alkylamino) Guests and Their Interaction with Cyclodextrins. J. Inclusion Phenom. Macrocyclic Chem. 2015, 81, 141–152. 10.1007/s10847-014-0443-1. [DOI] [Google Scholar]
- Gibson H. W.; Hamilton L.; Yamaguchi N. Molecular Self-Assembly of Dendrimers, Non-covalent Polymers and Polypseudorotaxanes. Polym. Adv. Technol. 2000, 11, 791–797. . [DOI] [Google Scholar]
- ICH Harmonised Guideline, Guideline for Elemental Impurities, Q3D(R1). International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, Final Version, March 22, 2019. https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-32.pdf.
- Kadota T.; Kageyama H.; Wakaya F.; Gamo K.; Shirota Y. Novel Electron-Beam Molecular Resists with High Resolution and High Sensitivity for Nanometer Lithography. Chem. Lett. 2004, 33, 706–707. 10.1246/cl.2004.706. [DOI] [Google Scholar]
- Socol S. M.; Verkade J. G. Steric and Electronic Effects in Cobalt(II) Disproportionation with Phosphorus Ligands. Inorg. Chem. 1986, 25, 2658. 10.1021/ic00235a034. [DOI] [Google Scholar]
- Nguyen A. I.; Hadt R. G.; Solomon E. I.; Tilley T. D. Efficient C–H Bond Activations via O2 Cleavage by a Dianionic Cobalt(II) Complex. Chem. Sci. 2014, 5, 2874–2878. 10.1039/C4SC00108G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopmann K. H.; Ghosh A. Mechanism of Cobalt-Porphyrin–Catalyzed Aziridination. ACS Catal. 2011, 1, 597–600. 10.1021/cs1001114. [DOI] [Google Scholar]
- Lyaskovskyy V.; Suarez A. I. O.; Lu H.; Jiang H.; Zhang X. P.; de Bruin B. Mechanism of Cobalt(II) Porphyrin-Catalyzed C–H Amination with Organic Azides: Radical Nature and H-Atom Abstraction Ability of the Key Cobalt(III)–Nitrene Intermediates. J. Am. Chem. Soc. 2011, 133, 12264–12273. 10.1021/ja204800a. [DOI] [PubMed] [Google Scholar]
- Bianchini C.; Peruzzini M.; Vacca A.; Zanobini F. Metal-Hydride Alkyl-Metal-Vinylidene Rearrangements Occurring in both Solid State and Solution. Role of the 1-alkyne Substituent in Determining the Relative Stability of pi-Alkyne, Hydride Alkynyl, and Vinylidene Forms at Cobalt. Organometallics 1991, 10, 3697–3737. 10.1021/om00056a048. [DOI] [Google Scholar]
- Foerstner J.; Kakoschke A.; Goddard R.; Rust J.; Wartchow R.; Butenschön H. New Carbene and Vinylidene Chelate Complexes of Cyclopentadienylcobalt Bearing a Phosphorus Tether. J. Organomet. Chem. 2001, 617–618, 412–422. 10.1016/S0022-328X(00)00676-8. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.