Highlights
-
•
Expanded access investigation demonstrating the safety and potential efficacy of amniotic fluid-derived extracellular vesicles in mild-to-moderate COVID-19 patients.
-
•
Application of a new drug for the prevention of COVID-19 progression in a high-risk patient population.
-
•
Association of extracellular vesicle biologic administration with suppression of COVID-19 inflammatory and surrogate biomarkers.
Keywords: COVID-19, Expanded Access, Therapeutic, Extracellular Vesicles, Biologic
Abstract
A pandemic brought on by COVID-19 has created a scalable health crisis. The search to help alleviate COVID-19-related complications through therapeutics has become a necessity. Zofin is an investigational, acellular biologic derived from full-term perinatal amniotic fluid that contains extracellular vesicles. Extracellular nanoparticles as such have been studied for their immunomodulatory benefits via cellular therapeutics and, if applied to COVID-19-related inflammation, could benefit patient outcome. Subjects (n = 8) experiencing mild-to-moderate COVID-19 symptoms were treated with the experimental intervention. Complete blood count, complete metabolic panel, inflammatory biomarkers, and absolute lymphocyte counts were recorded prior to and on days 4, 8, 14, 21, and 30 as markers of disease progression. Additionally, chest x-rays were taken of the patients prior to and on days 8 and 30. Patients experienced no serious adverse events. All COVID-19-associated symptoms resolved or became stable with no indication of disease worsening as found by patient and chest x-ray reports. Inflammatory biomarkers (CRP, IL-6, TNF-) and absolute lymphocyte counts improved throughout the study period. Findings from a proof-of-concept, expanded access trial for COVID-19 patients prove the acellular biologic is safe and potentially effective to prevent disease progression in a high-risk COVID-19 population with mild-to-moderate symptoms.
1. Introduction
Patients with Severe Acute Respiratory Syndrome (SARS) coronavirus 2 (SARS-CoV-2), commonly referred to as the novel coronavirus of 2019, or COVID-19, were first diagnosed in December 2019 in Wuhan, China [1]. The disease rapidly spread across the globe and was declared a pandemic on March 11, 2020, by the World Health Organization (WHO). Despite the availability of vaccines, the disease incidence, hospitalizations, and deaths persist at a high level – largely attributable to the delta variant of mid-2021 that involves younger and healthier populations than originally seen [2].
The most common initial manifestations of COVID-19 – fever, cough, muscle pain, nausea, and vomiting – are due to viral entry into the upper respiratory tract via the ACE-2 receptor [3, 4]. The virus then undergoes local replication and propagation, while infecting ciliated cells in the conducting airways [5]. During this time, an incubation stage persists for approximately 5 days with an early, limited immune response [6]. After virus incubation, a period of mild-to-moderate symptoms can occur to the majority of those infected within 14 days following exposure [6, 7]. After initial infection of the upper respiratory tract, the lungs become a primary target as the disease progresses [8]. Lung complications from COVID-19 can range from pulmonary edema, endothelial and epithelial damage, acute lung injury, and pulmonary fibrosis [8], [9], [10], [11]. The lungs have the capability to regenerate and heal up to a certain threshold of damage; however, significant damage to the lungs often results in irreversible fibrotic scarring and latter functional complications [12].
In some persons – especially those with increased risk factors – an even more severe, multi-organ disease develops, affecting the heart, kidneys, blood coagulation, and central nervous system [7, 13]. The risks factors, or comorbid conditions, that exist and contribute to the host's increased risk for developing severe COVID-19 and retrospective complications are the following: obesity, systemic arterial hypertension, chronic obstructive lung disease, and diabetes mellitus [14]. To note, obesity stands out as having the strongest connection to risk [14, 15].
Despite understanding COVID-19 transmission and clinical manifestations, the precise pathophysiology of COVID-19 human infection is uncertain and the subject of intense, ongoing investigation [16]. The catalyst at the forefront of COVID-19′s initiation and ramification has been postulated as systemic hyper-inflammation, referred to as a “cytokine storm” [17]. An amplified immune response, however, can promote further inflammation to the site, leading to detrimental, self-destructive effects that can overwhelmingly damage the lungs [18], [19], [20], [21]. Inflammation is thus a primary target of therapeutic interventions for COVID-19 in both its early and late stages [22, 23].
Mechanistically, therapeutic agents can be categorized as (i) those that aim to target the viral life cycle, such as remdesivir or lopinavir–ritonavir; (ii) SARS-CoV-2–targeted antibody therapies; and (iii) those that are focused on the host response, such as glucocorticoids and other immunomodulators [24]. Dorward et al. [25] have suggested using an immunomodulator to target COVID-19-associated inflammation rather than targeting the pathogen load itself could more effectively reduce the progression of COVID-19 immunopathology. Additionally, McCullough et al. [26] have proposed that therapeutic approaches based on pathophysiologic principles for early treatment of COVID-19 include the (i) reduction of reinoculation, (ii) combination antiviral therapy, (iii) immunomodulation, and (iv) antiplatelet/antithrombotic therapy. The study described herein is based on immunomodulation.
This investigation is a proof-of-concept trial in which the investigators hypothesize that the study drug, Zofin, possesses immunomodulatory properties that are (i) safe, (ii) ameliorate inflammation in mild-to-moderate COVID-19 patients sufficient to prevent disease progression, and (iii) demonstrate the anti-inflammatory preventative effect by reduction in surrogate inflammatory markers. Zofin is an acellular biologic derived from full-term perinatal amniotic fluid (AF) containing soluble paracrine factors and insoluble extracellular nanoparticles. AF and its components have been hypothesized as being beneficial for regenerative medicine in part due to promoting anti-inflammatory properties, where studies have demonstrated the therapeutic potential of AF-derived stem cells and extracellular vesicles [27], [28], [29], [30]. Because of the correlation between COVID-19 and a pro-inflammatory state, common first-line treatments include anti-inflammatory agents [31, 32]. With previous demonstration of safety, evidence of anti-inflammatory effects, and possible efficacy in treating severely ill COVID-19 patients [33], Zofin has the potential to prevent disease progression.
Here within are the findings of a proof-of-concept, expanded access trial for high-risk patients with mild-to-moderate COVID-19 infection to investigate (i) the primary outcome, the safety of Zofin as an intravenous (IV) therapeutic, (ii) the secondary outcomes, the efficacy of Zofin on surrogate inflammatory biomarkers and patient outcomes, and (iii) whether the results justified further testing in large scale, controlled clinical trials of similar patients who are at high risk for disease progression.
2. Methods
2.1. Ethics
This study involving human participants was reviewed and approved by the Food and Drug Administration (FDA) and the Institute of Regenerative and Cellular Medicine Institutional Review Board. The patient's consent to participate in the study and that the data be used for publication was obtained at the treatment site using an Institutional Review Board (IRB)-approved informed consent form. FDA approval to treat these patients was submitted under the approved parent Investigational New Drug (IND) #19,881 and issued as Expanded Access to Zofin for patients with COVID-19 (IND# 19,881-EA). IRB approval was issued by a letter with IRB approval number IRCM-2020–269 from the Institute of Regenerative and Cellular Medicine Institutional Review Board. The approved clinical trial protocol was listed on ClinicalTrials.org with an identifier of NCT04657406.
2.2. Therapeutic intervention
The therapeutic intervention studied in this proof-of-concept trial is a trademarked allogenic, acellular biologic called Zofin containing AF-derived extracellular vesicles. Zofin is manufactured by Organicell Regenerative Medicine, Inc., in Miami, FL, as previously described [5]. Zofin is derived from human AF donated from consenting adults during routine, planned cesarean sections under IRB approved donor screening (IRB approval agency: IRCM). The final Zofin product was released by Organicell Regenerative Medicine, Inc., after meeting the release criteria requirements. The product specifications, based on specific release criteria, for Zofin administered in these treatments were as follows: sterility (14-day cultures: no growth for aerobic, anaerobic, and fungal contamination), endotoxin (<0.25 EU/mL), nanoparticle composition (concentration=2.63×1011 nanoparticles/mL), mode particle (size=99.7 nm), protein concentration (1.084 mg/mL), and hyaluronic acid concentration (196 ng/mL). Supplement Fig. 1 shows the nanoparticle tracking analysis of Zofin performed by a NanoSight NS300 (Malvern Panalytical, Malvern, UK).
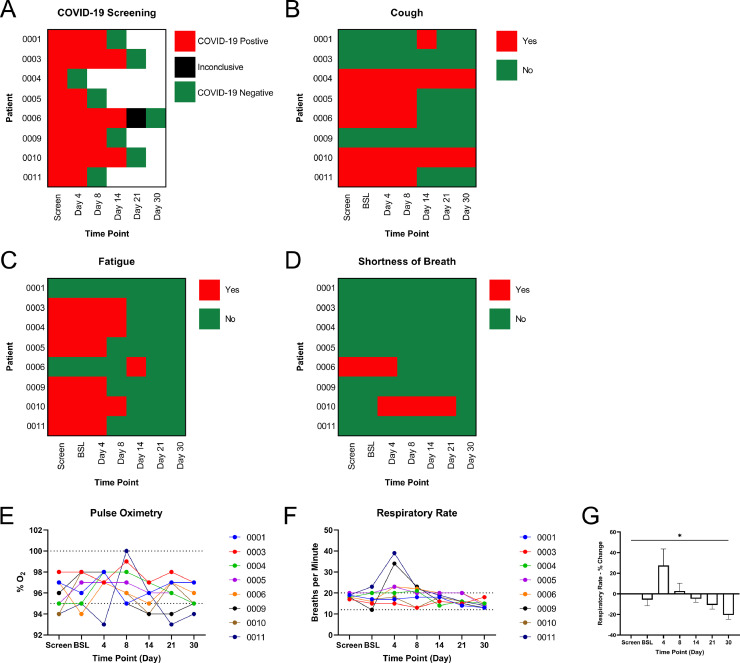
Fig. 1.
Observation of Patient Outcomes. (A) COVID-19 screening results at each testing time point detected by RT-qPCR. Reported cases of (B) cough, (C) fatigue, and (D) shortness of breath in all patients. (E) Pulse oximetry measurements of each patient throughout the study period. Normal range: 95–100%O2. (F) Respiratory rate as recorded per patients in addition to the (G) percentage change in respiratory rate throughout the study. Normal range: 12–20 breaths/minute. * P-value <0.05.
2.3. Patient enrollment and administration of therapeutic intervention
Patient evaluation, testing, and product infusion were performed in a single outpatient site at United Memorial Medical Center (UMMC) in Houston, TX throughout the months of March and June 2021. Study subjects were contacted by phone in chronological order the next day following testing positive for COVID-19 in the UMMC clinic. About 50% declined to participate, primarily for travel reasons. Eleven subjects were enrolled between the ages of 35 and 69 years who had mild-to-moderate COVID-19 symptoms as defined by the inclusion criteria (Table 1). Patient enrollment was completed following successful screening, which included COVID-19 testing and symptom checking. Qualified patients were scheduled for baseline (BSL) treatment up to 72 h after screening. The study duration was 30 days followed by a phone call at 90 days post-infusion. Subjects received an intravenous infusion of 1 mL of Zofin diluted in 100 mL of normal saline on day 0 (baseline), day 4, and day 8, respectively, for a total of three treatments of Zofin. Patients received in-person follow-ups on day 0 (baseline), day 4, day 8, day 14, day 21, and day 30 and a phone call follow-up on day 90. One patient withdrew before receiving any doses of Zofin and 2 subjects were unwilling to come to the study site after receiving the initial 3 infusions. In total, 8 subjects completed the follow-ups and are therefore included in the data analysis (Table 2). The patient population contained patients with a mean BMI of 31.2 ± 4.8. Most of the patients had a BMI considered to be obese with others being overweight and only 1 normal weight. Three of the eight subjects had comorbidities with two of them taking associated medications. Patients 001, 004, and 010 had a medical history of arthritis and osteoporosis; arthritis, gout, type-2 diabetes, and hypertension; and type-2 diabetes, hypertension, and asthma, respectively. Patients 004 and 010 were actively taking medications prescribed by their physicians to address the listed comorbidities. (Note: The 2 subjects who received infusions but did not complete the follow-ups have been contacted by phone 90 days post-infusion and stated that they have fully recovered with no adverse effects.)
Table 1.
Patient inclusion and exclusion criteria.
| Inclusion Criteria | Exclusion Criteria |
| Age (years) of male or female subjects age >18 years at the time of signing the Informed Consent Form | Have moderate-to-severe respiratory distress syndrome due to COVID-19 |
| Clinical diagnosis of COVID-19 by the qualitative reverse-transcription polymerase chain reaction (RT-PCR) | Females who are pregnant, nursing, or of childbearing potential while not practicing effective contraceptive methods |
| At least 1 of the mild or moderate COVID-19 clinical symptoms according to the NIH categories previously described | Females with a positive blood pregnancy test at screening which will be within 72 h of the intraperitoneal (IP) infusion |
| The main symptoms of mild illness are the following: •low-grade fever (<38ºC, 37.5–37.9) •dry cough •fatigue •feeling slightly breathless •muscle pain •headache •sore throat •diarrhea |
Inability to perform any of the assessments required |
| The main symptoms of moderate illness are the following: •fever (≥38ºC) •a dry and more consistent cough several times an hour •tiredness and need to stay in bed •breathless with moderate exercise •muscle pain •headache •soreness from coughing •diarrhea •dry mouth |
Active listing (or expected future listing) for transplant of any organ |
| Adequate venous access | Be a solid organ transplant recipient. (This does not include prior cell-based therapy (>12 months prior to enrollment) or bone, skin, ligament, tendon, or corneal grafting) |
| For women of child-bearing potential only, agree to use FDA-recommended birth control until 6 months post treatment | Have a history of organ or cell transplant rejection |
| For male subjects, agree to use contraceptives and not donate sperm during the study | History of substance (drug or alcohol) abuse |
| Agree to comply with all protocol requirements and be willing to complete all study visits | Taking prescription medications not being used appropriately for a pre-existing medical condition |
| Untreated HIV infection. (Patients could be enrolled if they have been treated for HIV and test negative for HIV viral load but still test positive for antibodies) |
Table 2.
Participant demographics and baseline characteristics.
| COVID-19 Patient Parameter | |
| Patient Population | N = 8 |
| Age (years) | 51.9 ± 10.5 |
| Gender (Female/Male) | 3/5 |
| Patient BMI | |
| Mean | 31.2 ± 4.8 |
| 25 (normal weight) | N = 1 |
| 2530 (overweight) | N = 2 |
| 30 (obese) | N = 5 |
*Data was presented as mean ± SEM or count.
2.4. COVID-19 testing
Qualitative detection of COVID-19 was performed using BD universal viral transport kit. Specimens were obtained by nasopharyngeal swab and analyzed by real time-polymerase chain reaction (RT-PCR) methodology at a CLIA certified laboratory. Testing was completed from screening and every visit thereafter until a COVID-19 negative result was obtained.
2.5. Laboratory and biomarker testing
Blood collection and testing for complete blood count (CBC), complete metabolic panel (CMP), and inflammatory biomarkers occurred after initiation of Zofin therapy on days 0, 4, 8, 14, 21, and 30. CBC, CMP, C-reactive protein (CRP), and d-Dimer testing were completed at the UMMC Laboratory. Inflammatory biomarkers interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-) measurements were completed by Labcorp. Absolute lymphocyte counts (ALC) were calculated by multiplying the total white blood cell (WBC) count by the lymphocyte percentages determined at each timepoint.
2.6. Patient outcomes and safety monitoring
At each visit from the time of screening and thereafter, patients were asked to report the presence of cough, fatigue, and shortness of breath, in addition to any new symptoms or change in original symptoms. A pulse oximeter was placed on the index finger and the blood oxygen saturation was recorded at each visit. Vital signs, including heart rate, systolic blood pressure, diastolic blood pressure, respiratory rate, and temperature, were recorded at screening and all following visits. The patients were strictly monitored 15 min prior to the initiation of the infusion, during the infusion, and for a minimum of 2 h post-infusion.
A portable chest x-ray (CXR) was used to acquire imaging at baseline (day 0, prior to treatment), day 8, and day 30 to evaluate, identify, and monitor lung abnormalities [34]. After images were acquired, analysis was performed by a staff radiologist at UMMC, and CXR reports were generated to describe the clinical findings.
2.7. Statistical analysis
Statistical differences were calculated using a repeated measures one-way ANOVA test. If statistical significance was found, a Tukey test was used to determine the post hoc analysis. Data analysis was completed using full data sets (n = 8). For example, incomplete data (n<8) for TNF-α and IL-6 at BSL resulted in the removal of that time point for statistical analysis of both biomarkers, and analysis was performed on the complete data sets starting at day 4. For the percent change analysis, patient 003 was excluded for CRP and IL-6 due to an outlier and incomplete data, respectively, resulting in an n-value of 7. Data is presented as the mean ± standard error of the mean (SEM). Figure assembly and data analysis were performed using Prism9 software (GraphPad Software, LLC).
3. Results
3.1. Safety reporting and laboratory testing
During product administration and throughout the study period, no serious adverse events were reported. CBC results were analyzed for each patient and indicated no abnormalities of concern (Supplement Fig. 2). Similarly, CMP results were collected and analyzed per patient and demonstrated no findings of concern (Supplement Fig. 3).
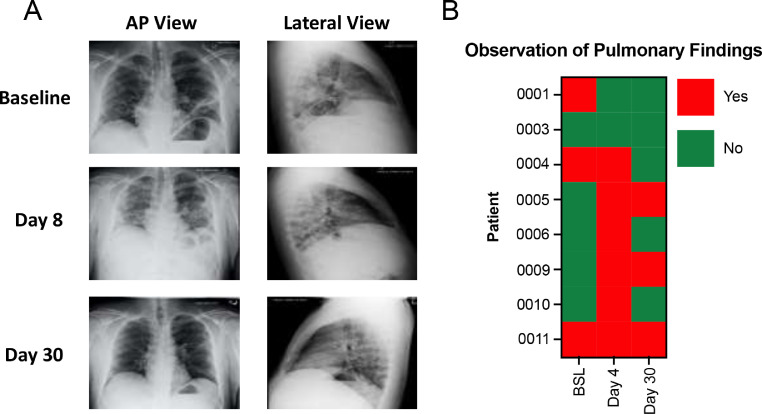
Fig. 2.
Representative chest x-ray (CXR) imaging and summary of pulmonary findings. A) Representative CXR images of patient 011 at baseline, day 8, and day 30. B) The observation of pulmonary findings as indicated from CXR reports at the three time points for all patients.
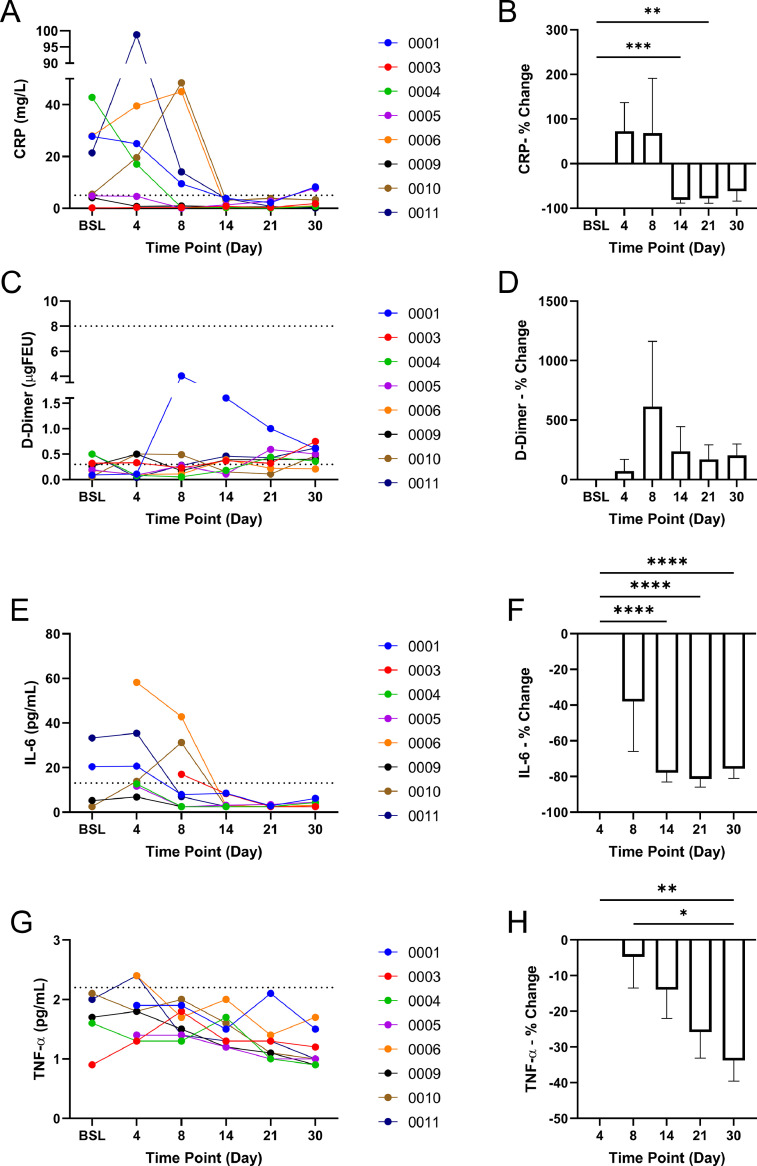
Fig. 3.
Inflammatory Biomarker Testing. A) CRP levels in all patients throughout the study. Normal range: 0–5.0 mg/L. B) Percentage change of CRP concentration compared to baseline. C) d-Dimer level in all patients throughout the study. Normal range: 0.3–8.0 ugFEU. D) Percentage change of d-Dimer concentration compared to baseline. E) IL-6 levels in all patients throughout the study. Normal range: 0–13.0 pg/mL. F) Percentage change of IL-6 concentration compared to day 4. G) TNF-α levels in all patients throughout the study. Normal range: 0–2.2 pg/mL. H) Percentage change of TNF-α concentration compared to day 4. * P-value <0.05, ** P-value <0.01, *** P-value <0.001, **** P-value <0.0001.
3.2. Observation of patient outcomes
At the time of patient screening, all patients were confirmed positive for COVID-19 infection. COVID-19 testing was repeated at day 4 and subsequent visits until a negative test result was obtained (Fig. 1A). All patients resolved from the COVID-19 infection within the 30-day visit period. Five out of 8 patients reported a cough at screening. By the 30-day visit, cough was resolved in 3 out of 5 patients (Fig. 1B). While 6 out of 8 patients originally reported fatigue at screening, the fatigue resolved by day 14 (Fig. 1C). Shortness of breath was reported by 1 patient at screening, which resolved by day 8, and 1 patient at day 4, which resolved by day 30 (Fig. 1D). Pulse oximetry testing of each patient demonstrated 2 patients below normal O2 saturation at screening, 3 patients below normal O2 saturation between day 4 to day 21, and 1 patient with lower than normal O2 saturation at the final 30-day visit (Fig. 1E). The respiratory rate, or breaths per minute, were recorded for all patients (Fig. 1F), and per analysis, a significant decrease was found for the respiratory rate percent change as compared from screening to day 30 (−20.5% ± 4.3%, P-value <0.05)(Fig. 1G).
3.3. Chest X-ray imaging
Chest x-ray (CXR) images were taken at baseline, day 4, and day 30 to observe the presence of pulmonary abnormalities (Fig. 2A). Baseline images demonstrated that 3 out of 8 patients had pulmonary observations or findings prior to Zofin infusion, whereas 6 out of 8 patients had observed pulmonary findings at day 4. By day 30, 3 out of 8 patients had observable pulmonary findings (Fig. 2B). The patients individual CXR and CXR analysis statements (Supplement Fig. 4) were collected at each time point to demonstrate and describe the pulmonary findings indicated in Fig. 2B.
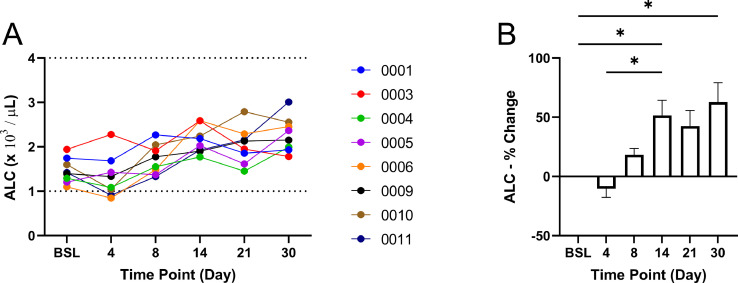
Fig. 4.
Absolute lymphocyte count (ALC) as determined by the white blood cell counts and lymphocyte percentages. Normal range: 1–4 x103/L. The (A) calculated ALC and (B) percentage change of ALC as compared to baseline for all patients. * P-value <0.05.
3.4. Inflammatory biomarker testing
Inflammatory biomarker testing included CRP, d-Dimer, IL-6, and TNF- throughout the study period. At baseline, 5 out of 8 patients had high levels of CRP concentration with 2 patients having increased levels of CRP by the end of the study period (Fig. 3A). The percent change of CRP significantly decreased at the 14-day (−81.1% ± 7.3%, P-value <0.001) and 21-day (−78.0% ± 10.9%, P-value <0.01) time points as compared to BSL (Fig. 3B). d-Dimer was not found to be outside of the normal range for any of the patients at baseline or throughout the study period, and no significant differences between the percent changes were found (Fig. 3C and D). IL-6 measurements were performed at baseline and throughout the study period; however, samples lost for 4 patients at baseline and 1 patient at day 4 reduced the number of complete data sets to be analyzed. Analysis of the complete data sets from day 4 onward found 4 out of 7 patients with elevated IL-6. IL-6 was not elevated in any of the patients by day 14 and thereafter (Fig. 3E). The percent change of IL-6 in the 7 patients with complete data sets from day 4 showed a significant decrease at day 14 (−77.9% ± 5.2%, P-value <0.0001), day 21 (−81.4% ± 4.5%, P-value <0.0001), and day 30 (−75.7% ± 5.3%, P-value <0.0001) (Fig. 3F). Similarly, TNF- measurements were performed; however, samples lost for 3 patients at baseline reduced the number of complete data sets to be analyzed. Higher than normal TNF- was found in 2 out of 8 patients at day 4 (Fig. 3G). Analysis of the percent change from day 4 found TNF- to be significantly reduced from day 4 to day 30 (−33.8% ± 5.8%, P-value <0.01) (Fig. 3H).
3.5. Absolute lymphocyte count
The ALC was calculated using the total WBC count and lymphocyte percentages collected with the CBC analysis. At baseline, all patients were within the normal range of ALC but trended towards the lower limit (Fig. 4A). Throughout the study period, ALC gradually increased with a significant increase by day 14 (51.5% ± 12.8%, P-value <0.05) and day 30 (62.8% ± 16.4%, P-value <0.05)(Fig. 4B).
4. Discussion
The most important goal of treating patients with mild-to-moderate COVID-19 infection is to prevent progression, which, when it occurs, usually requires hospitalization, sometimes accompanied with ventilator assistance, intubation, and death in 40% of those reaching a critical stage [35]. Currently, for ambulatory patients with mild-to-moderate COVID-19 at high risk for progression to severe disease, the Infectious Disease Society of America guideline panel suggests only bamlanivimab/etesevimab, casirivimab/imdevimab, or sotrovimab, which is a conditional recommendation with moderate certainty of evidence [36]. Thus, the development of novel therapeutics is vital.
As a biological agent, Zofin is hypothesized to ameliorate COVID-19 infection via its perinatal-derived immunomodulatory and anti-inflammatory properties. Zofin's composition, which is derived from perinatal tissue paracrine factors, contains two fractions believed to contribute to its therapeutic potential and ultimately its mechanism of action: extracellular vesicles (EVs) and soluble proteins/extracellular matrix components. In nature, AF contains components that play various roles in fetal immunity, development, and overall homeostasis [37]. Some of the components of AF that have been characterized include its proteomics and cell types, including AF stem cells. Proteomics of AF reveal that the majority of its proteins reside extracellularly and share similarity with specific organ tissues with the lung being the third out of the top ten tissues [38]. Mesenchymal stem cells from AF and their paracrine factors have been proven to have a regenerative effect in multiple organs, including the lungs [39, 40], highlighting the potential of perinatal cells to be beneficial in cell therapeutics. Of special importance to COVID-19, mesenchymal cells of different origin have been used to treat disease models of the lung such as pulmonary arterial hypertension and acute lung injury [41, 42]. However, the mechanism of action from cell-derived therapies has been linked to the cells’ secreted factors, such as paracrine factors [39] or exosomes and EVs [42]. As an acellular biologic, Zofin's therapeutic potential likely resides in the EVs, nanoparticles, and paracrine factors found in AF and thus incorporates the perinatal tissue's inherent protective and immunomodulatory ability. Preclinical data investigating the therapeutic potential of Zofin's EV fraction in a neonatal lung injury model demonstrated the product's immunomodulatory and anti-inflammatory properties [30]. When delivered by intratracheal injection, high oxygen-damaged lungs are protected from tissue injury concurrent with a reduction in macrophage invasion and suppression of pro-inflammatory cytokine expression [30]. Preclinical testing of other EV biologics in large animals has established effective EV/nanoparticle single doses ranging from 1 × 1011 to 3 × 1012 particles / dose [43, 44]. The Zofin dose tested within this study contained a nanoparticle concentration of 2.6 × 1011 particles / dose and fell within this referenced range. Together, these findings provide critical developmental insight into Zofin's mechanistic regulation of an inflammatory response during pulmonary injury.
Zofin has previously been approved for testing in a phase I/II clinical trial for patients with moderate-to-severe acute respiratory distress syndrome related to COVID-19 infection (Clinicaltrials.gov identifier: NCT04384445); this study population represents those patients with moderate-to-severe COVID-19 who are hospitalized but not yet intubated. Similarly, single patient emergency use authorizations were also approved to treat severe cases of acute respiratory distress syndrome induced by COVID-19 [33]. In continuation of these studies, expanded access was granted by the FDA to test Zofin in this discussed population of patients with milder disease. Mild-to-moderate, addressed within this study, and moderate-to-severe illness represent two distinct populations of patients at various stages of COVID-19 infection.
This proof-of-concept expanded access trial sought to primarily determine the safety of Zofin when administered intravenously to mild-to-moderate COVID-19-infected patients in addition to observing its efficacy via patient outcome, inflammatory biomarker status, and absolute lymphocyte count (ALC). All subjects were closely observed for adverse events and serious adverse event: no serious adverse events were observed throughout the study. Vital signs including heart rate, body temperature, and blood pressure (data not shown) as well as CBC and CMP tests were conducted to monitor the treatment's influence on overall patient health. No areas of concern were derived from these parameters, and the effect of Zofin via IV therapy for mild-to-moderate COVID-19 patients was concluded to be safe.
The presence and severity of COVID-19 infection within the lungs and their capacity to function is central to its pathophysiology [8, 9]; thus, pulmonary symptoms such as cough and shortness of breath as well as fatigue were carefully monitored as measures of outcome. The length of time necessary for patients to test negative for COVID-19 and to exhibit symptom onset and/or recovery varied. COVID-19 so far has demonstrated itself to have a varying disease phenotype in addition to symptom heterogeneity in patients [45], which could contribute to the variable outcomes as seen in Fig. 1. Nonetheless, by the end of the study, all patients tested negative for COVID-19, and a majority resolved from all associated symptoms, not including 2 out of the 8 patients who experienced a lingering cough. Image qualification of the patients’ lungs further supported an improvement in patient outcome for the majority. Seven patients exhibited pulmonary findings. Four of the 7 patients resolved pulmonary findings by day 30, while 3 of the 7 patients completed the study with unresolved pulmonary findings. None of the patients displayed pulmonary worsening by day 30; therefore, no COVID-19-induced lung findings progressed throughout this study. Additionally, the respiratory rate significantly decreased throughout the study, suggestive of an improvement to lung health.
The induction of prolonged inflammation is associated with COVID-19 infection severity and worsening [18, 19]. Regardless of the viral RNA and protein presence of COVID-19 within multiple organs, inflammation has been reported to aggregate predominantly in the lungs [25]. Additionally, a reduction in ALC as an indication of leukopenia has been observed in COVID-19 patients and found to correlate with disease severity [46]. Therefore, measurement of pro-inflammatory cytokines and ALC as COVID-19 disease surrogate markers throughout injury progression in addition to lung pathology are critical components in COVID-19 patient outcome. Although the study population had elevated concentrations of CRP, IL-6, and TNF- at either baseline or the earlier time point of day 4, analysis found significant decreases in all three inflammatory cytokines. Additionally, ALC levels originally trended towards the lower limit but elevated with a maximum percent change at the endpoint of the study. These results indicate inflammation subsided and ALC levels were stabilized, which lessened inflammatory-related disease severity for COVID-19 patients.
A special note must be made about the role of obesity in this study. Although body weight was not a factor in the inclusion or exclusion criteria, all but 1 of the 8 patients reported were overweight with most being overtly obese. Over 73% of Americans aged ≥20 years are overweight, including 42.5% who are obese [47]; therefore, a majority of the study population having excess weight was unsurprising. The finding that almost all study patients were overweight was not realized until conducting data analysis after the study had been completed (90 days after initial infusion for all study patients). The importance of this demographic is that obesity has emerged as a strong risk factor – perhaps second only to advanced age – for hospitalization among persons with acute COVID-19 [48, 49]. Obesity, which is a component of the metabolic syndrome, is associated with the secretion of inflammatory adipokines [50, 51]. The role of inflammation in COVID-19 disease progression is thus magnified in obese individuals, and this study, regardless of the population's high risk, established evidence to attenuate COVID-19-associated inflammation. Aside from obesity, additional comorbidities existed within three of the patients with two patients actively being treated for their medical conditions. The prescribed medications were unrelated to COVID-19 treatments and unlikely to be interactive with COVID-19-related therapeutics. Because only a minority of the patients were treated with their comorbidity-prescribed medications, we do not believe the medications had a strong influence on patient outcome.
5. Conclusion
Results of this proof-of-concept study, particularly the significant trending effect of Zofin on inflammatory biomarkers and ALC, suggests that Zofin provided a safe and efficacious therapeutic to prevent COVID-19 progression to more serious stages. Although COVID-19 was in the mild-to-moderate stage at the time of initial treatment, some patients in this cohort could have worsened throughout the treatment course by chance, which was not observed. Analysis of patient outcomes, including COVID-19-associated symptoms, chest x-ray images, and inflammatory biomarkers demonstrated the beneficial outcomes that may indicate Zofin's efficacy and therapeutic value for preventing disease progression. Because the patient size was relatively low and lacked a placebo control, these results warrant further investigation in a larger, randomized and placebo-controlled study; however, evidence supports Zofin's ability to prevent disease progression for this generalized cohort of mild-to-moderate COVID-19 patients—especially those with a high-risk factor of obesity. The completed study supports Zofin as a feasible, safe, and potentially efficacious therapy for patients with mild-to-moderate COVID-19 who are at increased risk for progression, including the need for hospitalization, ventilation, and death.
Data availability
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
Declaration of Competing Interest
Michael Bellio, Cassie Bennett, Alissa Arango, and Maria Ines Mitrani represent Organicell Regenerative Medicine employees. Maria Ines Mitrani is the chief science officer, serves on the Organicell Regenerative Medicine Board of Directors, has a patent pending, and holds equity in the company. Michael Bellio is the laboratory director of the company and holds equity in the company. Aisha Khan and Xiumin Xu disclose a relationship with AssureImmune Cord Blood Bank that includes equity. Aisha Khan and Xuimin Xu provides regulatory consulting for Organicell and hold equity in the company. Vincent Friedewald and Cesar Barrera have no commercial, proprietary, or financial interests.
Acknowledgments
This clinical trial and its research were sponsored by Organicell Regenerative Medicine.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.bbiosy.2021.100031.
Appendix. Supplementary materials
References
- 1.Wu F., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. 03/01 2020, doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Callaway E. Delta coronavirus variant: scientists brace for impact. Nature. Jul 2021;595(7865):17–18. doi: 10.1038/d41586-021-01696-3. [DOI] [PubMed] [Google Scholar]
- 3.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol. Mar 17 2020;94(7) doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.M. Hoffmann et al., "SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor," Cell, vol. 181, no. 2, pp. 271–280 e8, Apr 16 2020, doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed]
- 5.Sims A.C., Baric R.S., Yount B., Burkett S.E., Collins P.L., Pickles R.J. Severe acute respiratory syndrome coronavirus infection of human ciliated airway epithelia: role of ciliated cells in viral spread in the conducting airways of the lungs. J Virol. Dec 2005;79(24):15511–15524. doi: 10.1128/JVI.79.24.15511-15524.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lauer S.A., et al. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann Intern Med. 2020;172(9):577–582. doi: 10.7326/M20-0504. 05/05 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y., Wang Y., Chen Y., Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. (in eng), J Med Virol. 2020;92(6):568–576. doi: 10.1002/jmv.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bösmüller H., Matter M., Fend F., Tzankov A. The pulmonary pathology of COVID-19. (in eng), Virchows Arch. 2021;478(1):137–150. doi: 10.1007/s00428-021-03053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calabrese F., et al. Pulmonary pathology and COVID-19: lessons from autopsy. The experience of European Pulmonary Pathologists. (in eng), Virchows Arch. Sep 2020;477(3):359–372. doi: 10.1007/s00428-020-02886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye Z., Zhang Y., Wang Y., Huang Z., Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. (in eng), Eur Radiol. Aug 2020;30(8):4381–4389. doi: 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.X. Cui et al., "Pulmonary Edema in COVID-19 Patients: Mechanisms and Treatment Potential," (in English), Front Pharmacol Rev vol. 12, no. 1444, 2021-June-07 2021, doi: 10.3389/fphar.2021.664349. [DOI] [PMC free article] [PubMed]
- 12.Lucas A., Yasa J., Lucas M. Regeneration and repair in the healing lung. Clin Transl Immunol. 2020;9(7):e1152. doi: 10.1002/cti2.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y., et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. 2020/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallo Marin B., et al. Predictors of COVID-19 severity: A literature review. Rev Med Virol. Jan 2021;31(1):1–10. doi: 10.1002/rmv.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernandez-Garduno E. Obesity is the comorbidity more strongly associated for Covid-19 in Mexico. A case-control study. Obes Res Clin Pract. 2020;14(4):375–379. doi: 10.1016/j.orcp.2020.06.001. Jul - Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parasher A. COVID-19: Current understanding of its Pathophysiology, Clinical presentation and Treatment. Postgrad Med J. May 2021;97(1147):312–320. doi: 10.1136/postgradmedj-2020-138577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calabretta E., et al. COVID-19-induced endotheliitis: emerging evidence and possible therapeutic strategies. Br J Haematol. Apr 2021;193(1):43–51. doi: 10.1111/bjh.17240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang L., et al. COVID-19: immunopathogenesis and Immunotherapeutics. Signal Transduction and Targeted Therapy. 2020;5(1):128. doi: 10.1038/s41392-020-00243-2. 2020/07/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishra K.P., Singh A.K., Singh S.B. Hyperinflammation and Immune Response Generation in COVID-19. NeuroImmunoModulation. 2020;27(2):80–86. doi: 10.1159/000513198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGonagle D., Sharif K., O'Regan A., Bridgewood C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. (in eng), Autoimmun Rev. Jun 2020;19(6) doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.C. Nedeva, J. Menassa, and H. Puthalakath, "Sepsis: Inflammation Is a Necessary Evil," (in English), Front Cell Dev Biol Mini Rev vol. 7, no. 108, 2019-June-20 2019, doi: 10.3389/fcell.2019.00108. [DOI] [PMC free article] [PubMed]
- 22.Reyes A.Z., et al. Anti-inflammatory therapy for COVID-19 infection: the case for colchicine. Ann Rheum Dis. Dec 8 2020 doi: 10.1136/annrheumdis-2020-219174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie C., et al. Association of Early Inflammation with Age and Asymptomatic Disease in COVID-19. J Inflamm Res. 2021;14:1207–1216. doi: 10.2147/JIR.S304190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stebbing J., Lauschke V.M. JAK Inhibitors - More Than Just Glucocorticoids. N Engl J Med. Jul 29 2021;385(5):463–465. doi: 10.1056/NEJMe2108667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorward D.A., et al. Tissue-Specific Immunopathology in Fatal COVID-19. Am J Respir Crit Care Med. 2021;203(2):192–201. doi: 10.1164/rccm.202008-3265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCullough P.A., et al. Pathophysiological Basis and Rationale for Early Outpatient Treatment of SARS-CoV-2 (COVID-19) Infection. Am J Med. Jan 2021;134(1):16–22. doi: 10.1016/j.amjmed.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loukogeorgakis S.P., De Coppi P. Concise Review: Amniotic Fluid Stem Cells: The Known, the Unknown, and Potential Regenerative Medicine Applications. Stem Cells. 2017;35(7):1663–1673. doi: 10.1002/stem.2553. [DOI] [PubMed] [Google Scholar]
- 28.Joo S., Ko I.K., Atala A., Yoo J.J., Lee S.J. Amniotic fluid-derived stem cells in regenerative medicine research. Arch Pharm Res. 2012;35(2):271–280. doi: 10.1007/s12272-012-0207-7. 2012/02/01. [DOI] [PubMed] [Google Scholar]
- 29.Parolini O., Soncini M., Evangelista M., Schmidt D. Amniotic membrane and amniotic fluid-derived cells: potential tools for regenerative medicine? (in eng), Regen Med. Mar 2009;4(2):275–291. doi: 10.2217/17460751.4.2.275. [DOI] [PubMed] [Google Scholar]
- 30.Bellio M.A., et al. Amniotic fluid-derived extracellular vesicles: characterization and therapeutic efficacy in an experimental model of bronchopulmonary dysplasia. Cytotherapy. 2021;23(12):1097–1107. doi: 10.1016/j.jcyt.2021.07.011. 2021/09/17. [DOI] [PubMed] [Google Scholar]
- 31.Soy M., Keser G., Atagündüz P., Tabak F., Atagündüz I., Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. (in eng), Clin Rheumatol. Jul 2020;39(7):2085–2094. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang W., et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. (in eng), Clin Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. 108393–108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.M.I. Mitrani et al., "Case Report: Administration of Amniotic Fluid-Derived Nanoparticles in Three Severely Ill COVID-19 Patients," (in English), Front Med Case Rep vol. 8, no. 242, 2021-March-17 2021, doi: 10.3389/fmed.2021.583842. [DOI] [PMC free article] [PubMed]
- 34.Jacobi A., Chung M., Bernheim A., Eber C. Portable chest X-ray in coronavirus disease-19 (COVID-19): A pictorial review. Clin Imaging. Aug 2020;64:35–42. doi: 10.1016/j.clinimag.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macedo A., Goncalves N., Febra C. COVID-19 fatality rates in hospitalized patients: systematic review and meta-analysis. Ann Epidemiol. May 2021;57:14–21. doi: 10.1016/j.annepidem.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.M. Bhimjaf A, R.L., Shumaker AH, Lavergne V., Baden L., Cheng V.C., Edwards K.M., Gandhi R., Gallagher J., Muller W.J., O'Horo J.C., Shoham S., Murad M.H., Mustafa R.A., Sultan S., Falck-Ytter Y., "Infectiour Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19.," April 11, 2020. [Online]. Available: idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/
- 37.Underwood M.A., Gilbert W.M., Sherman M.P. Amniotic Fluid: Not Just Fetal Urine Anymore. J Perinatol. 2005;25(5):341–348. doi: 10.1038/sj.jp.7211290. 2005/05/01. [DOI] [PubMed] [Google Scholar]
- 38.Cho C.K., Shan S.J., Winsor E.J., Diamandis E.P. Proteomics analysis of human amniotic fluid. (in eng), Mol Cell Proteomics. Aug 2007;6(8):1406–1415. doi: 10.1074/mcp.M700090-MCP200. [DOI] [PubMed] [Google Scholar]
- 39.Pederiva F., Ghionzoli M., Pierro A., De Coppi P., Tovar J.A. Amniotic Fluid Stem Cells Rescue Both in Vitro and in Vivo Growth, Innervation, and Motility in Nitrofen-Exposed Hypoplastic Rat Lungs through Paracrine Effects. Cell Transplant. 2013;22(9):1683–1694. doi: 10.3727/096368912x657756. 2013/09/01. [DOI] [PubMed] [Google Scholar]
- 40.Carraro G., et al. Human Amniotic Fluid Stem Cells Can Integrate and Differentiate into Epithelial Lung Lineages. Stem Cells. 2008;26(11):2902–2911. doi: 10.1634/stemcells.2008-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen J.-y., et al. Therapeutic effects of mesenchymal stem cell-derived microvesicles on pulmonary arterial hypertension in rats. Acta Pharmacol Sin. 2014;35(9):1121–1128. doi: 10.1038/aps.2014.61. 2014/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kordelas L., et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28(4):970–973. doi: 10.1038/leu.2014.41. 2014/04/01. [DOI] [PubMed] [Google Scholar]
- 43.Gallet R., et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J. 2016;38(3):201–211. doi: 10.1093/eurheartj/ehw240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang L., et al. Selective intrarenal delivery of mesenchymal stem cell-derived extracellular vesicles attenuates myocardial injury in experimental metabolic renovascular disease. (in eng), Basic Res Cardiol. Jan 14 2020;115(2):16. doi: 10.1007/s00395-019-0772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samadizadeh S., Masoudi M., Rastegar M., Salimi V., Shahbaz M.B., Tahamtan A. COVID-19: Why does disease severity vary among individuals? Respir Med. 2021;180 doi: 10.1016/j.rmed.2021.106356. 2021/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Illg Z., Muller G., Mueller M., Nippert J., Allen B. Analysis of absolute lymphocyte count in patients with COVID-19. Am J Emerg Med. Mar 2 2021;46:16–19. doi: 10.1016/j.ajem.2021.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.CDC. Obesity and Overweight [Online] Available: cdc.gov/nchs/fastats/obesity-overweight.htm
- 48.Bellini B., et al. Obesity as a risk factor for hospitalization in COronaVirus Disease-19 (COVID-19) patients: Analysis of the Tuscany regional database. Nutr Metab Cardiovasc Dis. Mar 10 2021;31(3):769–773. doi: 10.1016/j.numecd.2020.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rottoli M., et al. How important is obesity as a risk factor for respiratory failure, intensive care admission and death in hospitalised COVID-19 patients? Results from a single Italian centre. Eur J Endocrinol. Oct 2020;183(4):389–397. doi: 10.1530/EJE-20-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ellulu M.S., Patimah I., Khaza'ai H., Rahmat A., Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. Jun 2017;13(4):851–863. doi: 10.5114/aoms.2016.58928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alarcon P.C., et al. Adipocyte inflammation and pathogenesis of viral pneumonias: an overlooked contribution. Mucosal Immunol. May 6 2021 doi: 10.1038/s41385-021-00404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.