Abstract
Coronavirus Disease 2019 (COVID-19) is caused by the novel coronavirus, Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) - the culprit of an ongoing pandemic responsible for the loss of over 3 million lives worldwide within a year and a half. While the majority of SARS-CoV-2 infected people develop no or mild symptoms, some become severely ill and may die from COVID-19-related complications. In this review, we compile and comment on a number of biomarkers that have been identified and are expected to enhance the detection, protection and treatment of individuals at high risk of developing severe illnesses, as well as enable the monitoring of COVID-19 prognosis and responsiveness to therapeutic interventions. Consistent with the emerging notion that the majority of COVID-19 deaths occur in older and frail individuals, we researched the scientific literature and report the identification of a subset of COVID-19 biomarkers indicative of increased vulnerability to developing severe COVID-19 in older and frail patients. Mechanistically, increased frailty results from reduced disease tolerance, a phenomenon aggravated by ageing and comorbidities. While biomarkers of ageing and frailty may predict COVID-19 severity, biomarkers of disease tolerance may predict resistance to COVID-19 with socio-economic factors such as access to adequate health care remaining as major non-biomolecular influencers of COVID-19 outcomes.
Keywords: COVID-19, SARS-CoV-2, Biomarker, Coronavirus, Frailty, Disease tolerance
Graphical Abstract
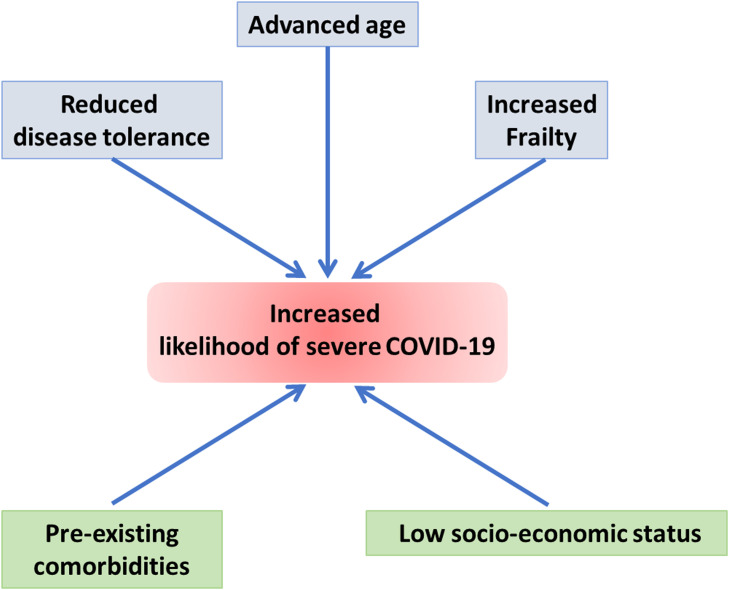
Figure: Biomarkers of ageing and frailty may predict COVID-19 severity as both conditions are associated with reduced disease tolerance - the host’s defense mechanisms to limit tissue damage or reduce immunopathology induced by the infection with a pathogen. While these biomolecular markers inform about the baseline ground for exacerbated viral infection, inflammaging and pre-existing comorbidities, which are common at advanced ages, as well as socio-economic conditions that affect people in underdeveloped nations and underserved communities of developed nations appear to be strong influencers of COVID-19 trajectory - particularly in older and frail individuals.
1. Introduction
In December of 2019, a cluster of patients were admitted to hospital in Wuhan, China showing symptoms including fever, cough, and shortness of breath, which were initially diagnosed as pneumonia caused by an unknown pathogen (Udugama et al., 2020). On Dec 26th, 2019, next generation sequencing (NGS) revealed preliminary sequence data indicating that this was the result of a SARS-related coronavirus (Udugama et al., 2020). This was determined not to be SARS-CoV-1 or MERS-CoV and was instead confirmed to be a novel coronavirus; the genome sequence of which was released to the public on Jan 10th, 2020, added to the GenBank sequence records, and used to design PCR primers allowing for RT-PCR-based testing of SARS-CoV-2 infection (L. F. Wang et al., 2020). The SARS-CoV-2 diagnostic panel from the Centre for Disease Control and Prevention includes three primer-probe mixes: 2019-nCoV_N1and 2019-nCoV_N2 which target the SARS-CoV-2 virus nucleocapsid (N) gene; and RP which targets human RNase P gene for human nucleic acid detection, allowing for specific identification of SARS-CoV-2 RNA in nasopharyngeal swab samples by RT-PCR (Centers for Disease & Prevention; Zhang and Guo, 2020).
In addition to RT-PCR, chest CT scans can be used as an auxiliary method to diagnose SARS-CoV-2 (X. Li et al., 2020). CT scans in conjunction with highly sensitive RT-PCR testing allows for increased sensitivity when diagnosing COVID-19 cases. SARS-CoV-2 can also be detected using emerging clustered regularly interspaced short palindromic repeats (CRISPR) based diagnostic methods, such as the Sherlock Biosciences kit that has received FDA Emergency Use Authorization (Sherlock, 2020, Zhang and Guo, 2020). The Sherlock Biosciences kit involves programming a Cas (CRISPR-associated) protein with the specific high-sensitivity enzymatic reporter unlocking (SHERLOCK) method, to identify SARS-CoV-2 RNA in patient nasal, nasopharyngeal, oropharyngeal, and bronchoalveolar lavage samples (Sherlock).
While the above-described approaches can detect SARS-CoV-2 presence in an individual, predicting disease prognosis remains challenging, which hampers the ability of health care systems to concentrate resources on those individuals at highest risk if developing severe illnesses. Here, we researched the scientific literature for reports on molecular biomarkers capable of informing on COVID-19 susceptibility, severity, or prognosis. The National Institutes of Health Biomarkers Definitions Working Group has described a biomarker as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” ("Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework," 2001). Candidate biomarkers expressed in response to innate immune responses such as inflammation may be useful but non-specific as they tend to be observed in many types of diseases. Many molecular biomarkers in clinical use are serum proteins, in part, because serum specimens are easy to obtain while their analysis by unbiased proteomic approaches can inform about the stage of a disease as well as identify potential targets for therapeutic intervention (Messner et al., 2020). The combined application of serum biomarkers with technologies such as genome sequencing, PCR technology, or CRISPR gene editing can be utilized to characterize disease prognosis in patients with COVID-19 and can ultimately play a role in alleviating the ongoing pandemic.
The pathophysiological mechanisms and manifestations of COVID-19 are strongly influenced by ageing (defined as the diminishing of physiological integrity over time) and frailty (an indicator of biological age rather than chronological age) (Polidori et al., 2021). The relationships between COVID-19 severity, ageing, and frailty are poorly understood and worthy of investigation. The majority of COVID-19 deaths occur in older individuals, with one large scale study finding 79 years of age to be the median age of patients who have died due to COVID-19 (Onder et al., 2020). Ageing can be defined as the diminishing of physiological integrity over time, which leads to defective functioning as well as an increased susceptibility to disease and eventual death in a patient (López-Otín et al., 2013, World Health, 2018). Various biomolecular markers, such as those of inflammation, exhibit increased levels with ageing and may also indicate increased risk of developing severe COVID-19. Frailty has been associated with an accelerated loss of homeostasis and, while typically exhibited at advanced ages, the absence of frailty at an older age may indicate a younger biological age (Polidori et al., 2021). Whether frailty may predict an individuals’ COVID-19 prognosis is not known and merits to be determined (Polidori et al., 2021). The interaction between COVID-19 severity and frailty likely goes far beyond the complexity of the aging process and of the viral infection themselves. Factors like organ reserve, energy imbalance, malnutrition, previous frailty condition, comorbidities, access to healthcare services and psychosocial conditions can influence trajectories massively. Another important factor to consider when discussing how an individual will be impacted by COVID-19 related illnesses could be their innate disease tolerance (Ayres, 2020, Hardy and Fernandez-Patron, 2021, Medzhitov et al., 2012). Disease tolerance can be described as a host’s defense method to limit tissue damage or reduce immunopathology caused by various pathogens; this may include diverting negative effects or perturbations to physiological functions resulting from a host’s immune response, such as inflammatory damage or metabolic changes (Ayres, 2020, Hardy and Fernandez-Patron, 2021, Martins et al., 2019, Medzhitov et al., 2012, Schneider and Ayres, 2008). Furthermore, a patient’s clinical history, including past diagnoses and lifestyle choices, can impact and limit the effectiveness of tolerance mechanisms and thus disease tolerance of the patient as well as the effectiveness of therapies designed to enhance such tolerance (Hardy and Fernandez-Patron, 2021). As we age, disease tolerance declines, which likely reflects a deteriorated immunity (Medzhitov et al., 2012). Tolerance may become impaired with age due to declining of tissue maintenance and capacity to repair damaged cells (Medzhitov et al., 2012). Instances of frailty may reflect a significant decline in disease tolerance (Medzhitov et al., 2012) which could predispose to severe COVID-19 illnesses (Ayres, 2020). Coronaviruses in the past have mediated epigenetic changes, which may accelerate the rate that the immune system ageing, and this dysregulation may be a cause of ageing in itself (Mueller et al., 2020).
In this review, we compile a list of biomarkers of SARS-CoV-1 and SARS-CoV-2 associated illness and discuss how some of these biomarkers have led to therapeutic interventions to treat COVID-19. Although the biomolecular fingerprint discussed is only one of many layers contributing to a patient’s potential risk of developing severe COVID-19, these markers ( Table 1) could be used to create an intelligent biomarkers system for characterization of COVID-19 patients and to enhance the capacity of the health system for proper allocation of resources on those individuals at highest risk of developing severe disease. We identify which of these biomarkers overlap with those of ageing and frailty as well as their impacts on disease tolerance, which may make them predictors of COVID-19 severity and death from COVID-19 complications, both of which have disproportionally occurred in older individuals. Moreover, we explore the relationship between disease tolerance and frailty through presenting recent evidence indicative of disease tolerance as a robust predictor of frailty and COVID-19 trajectory. Finally, we alert that biomolecular markers of aging and frailty alone might not be sufficient to predict COVID-19 trajectory as other factors that cannot be described as biomolecular units including malnutrition, previous frailty condition, comorbidities, access to healthcare services and psychosocial conditions as well as an individual’s innate mechanisms of disease tolerance have an increasingly evident influence on the development and progression of COVID-19.
Table 1.
Biomarkers identified for illnesses caused by SARS-CoV-1 and SARS-CoV-2.
| Biomarker | Increase or Decrease | Coronavirus | Marker Indication | Reference |
|---|---|---|---|---|
| Truncated Forms of α1-antitryspin | Increase | SARS-CoV-1 | Lung failure or injury | (Ren et al., 2004) |
| Serum amyloid A | Increase | SARS-CoV-1 | Pneumonia extent | (Yip et al., 2005) |
| Platelet factor 4 and β-thromboglobulin | Decrease and Increase | SARS-CoV-1 | ARDS development, need for supplemental oxygen | (Poon and Lo, 2012) |
| C-reactive protein | Increase | SARS-CoV-2 | Inflammatory status | (Chen et al., 2020, Dugué et al., 2021, Guan Wj, N. Z. Y. H. Y. L. W. H. O. C. Q. H. J. X. L. L. S. H. L. C. L. H. D., 2020, Salminen et al., 2012, Wagner et al., 2016; F. Wang et al., 2020) |
| Interleukin-6 | Increase | SARS-CoV-2 | Inflammatory status, cytokine storm formation | (Chen X, Z. B. Q. Y. C. Y. X. J. F. Y. M. D. H. Q. L. Y. Y. B. D. J. L. F., 2020, Guan Wj, N. Z. Y. H. Y. L. W. H. O. C. Q. H. J. X. L. L. S. H. L. C. L. H. D., 2020, Wagner et al., 2016; F. Wang et al., 2020) |
| Lactate Dehydrogenase | Increase | SARS-CoV-2 | Lung tissue damage, sepsis | (Cardoso et al., 2018, Chen et al., 2020, Galiatsatos, 2020, Guan Wj, N. Z. Y. H. Y. L. W. H. O. C. Q. H. J. X. L. L. S. H. L. C. L. H. D., 2020, Wang et al., 2020) |
| Procalcitonin | Increase | SARS-CoV-2 | Secondary bacterial infection | (Chen et al., 2020, Guan Wj, N. Z. Y. H. Y. L. W. H. O. C. Q. H. J. X. L. L. S. H. L. C. L. H. D., 2020, Henry and Lippi, 2020, Wang et al., 2020, Yang et al., 2018) |
| Serum Amyloid A/Lymphocyte | Increase/ Decrease | SARS-CoV-2 | Inflammatory status, lymphocytopenia | (H. Li et al., 2020; Tang et al., 2020) |
| Acute phase proteins | Increase and decrease | SARS-CoV-2 | Increased cytokine concentration | (Messner et al., 2020, Shen et al., 2020) |
| Complement system proteins | Increase | SARS-CoV-2 | Pathogen presence | (Messner et al., 2020, Shen et al., 2020) |
| Galectin 3-binding protein | Increase | SARS-CoV-2 | IL-6 expression | (Messner et al., 2020) |
| Monocyte differentiating protein CD14 | Increase | SARS-CoV-2 | LPS recognition | (Messner et al., 2020) |
| β and γ-1 actin | Increase | SARS-CoV-2 | Severity | (Messner et al., 2020) |
| α-1B-glycoportein | Increase | SARS-CoV-2 | Severity | (Messner et al., 2020) |
| Gelsolin | Decrease | SARS-CoV-2 | Inflammation status | (Messner et al., 2020) |
| Apolipoproteins | Decrease | SARS-CoV-2 | Macrophage dysregulation | (Goldstein et al., 2020, Messner et al., 2020, Shen et al., 2020) |
| Pro-platelet basic protein and platelet factor 4 | Decrease | SARS-CoV-2 | Thrombocytopenia | (Shen et al., 2020) |
| Cortisol | Increase | SARS-CoV-2 | Potential acute death | (Marcos-Pérez et al., 2019, Tan et al., 2020) |
| Vitamin D | Decrease | SARS-CoV-2 | Acute respiratory tract infection | (Meltzer et al., 2020, Pabst et al., 2015) |
2. The first identified biomarkers of SARS
COVID-19 cases and deaths have vastly outnumbered those reports for previous human coronavirus diseases, notably the SARS and MERS pandemics, which proved to be more deadly but significantly less virulent. SARS is caused by the SARS-CoV-1 coronavirus and provoked the 2002–2003 outbreaks in mainland China, Hong Kong, Singapore, Taiwan, and Toronto (Yip et al., 2005). The SARS pandemic saw at least 8098 infected patients and was responsible for 774 deaths according to the World Health Organization (World Health, 2003). One of the motivations for conducting this literature review was to clarify whether biomarkers of past coronavirus diseases such as SARS can serve as surrogate biomarkers of severe or lethal COVID-19 or provide a complement to emerging COVID-19 specific biomarkers. To this end we first searched PubMed and Medline-deposited papers for biomarkers of SARS using search terms in supplementary Table 1.
2.1. Truncated forms of- α1-antitrypsin (TF-α1-AT)
A 2004 study identified elevated truncated forms of alpha 1-antitrypsin (TF-α1-AT) as useful biomarkers for SARS severity (Ren et al., 2004). In this study, proteins were separated from serum samples using 2-dimensional electrophoresis, selected via MALDI-TOF MS, and identified by searching protein databases. The authors identified serum α1-antitrypsin, complement C4 protein, and serum amyloid A, of which α1-antitrypsin and complement C4 proteins were truncated. Of the identified proteins, TF-α1-AT was found to be the most specific at differentiating between SARS patients and non-SARS patients, reaching a specificity of 92.8% across all controls (Ren et al., 2004).
The three isoforms of N-terminal truncated α1-antitrpysin were found at very low or undetectable levels in serum samples collected from healthy and non-SARS pneumonia patients, whereas TF-α1-AT levels were increased by 4.5-fold in SARS patients (Ren et al., 2004). Following one week of treatment with corticosteroids and ribavirin, TF-α1-AT decreased in SARS patient sera, signifying that TF-α1-AT can serve as a useful biomarker for monitoring the effectiveness of treatment in SARS patients (Ren et al., 2004).
Serum α1-antitrypsin, which is found in bronchoalveolar lavage fluid of the lungs and has been observed to reduce fibrosis and lung injury, was found at low concentrations in SARS patients compared to non-SARS pneumonia patients (Ren et al., 2004). This may indicate lung failure in SARS patients. α1-antitrypsin has been studied as a potential biomarker for other instances, including advanced non-small cell lung cancer with epidermal growth factor receptor tyrosine kinase inhibitors therapies (Zhao et al., 2013), and as a large component of GlycA-associated risk for future morbidity and mortality from various causes (Ritchie et al., 2019) in addition to SARS, so TF-α1-AT cannot be considered a high precision biomarker of SARS.
2.2. Serum Amyloid A (SAA)
Serum Amyloid A (SAA) concentration correlates with the extent of pneumonia in SARS patients (Yip et al., 2005). Increased serum amyloid A production in the liver enhances patient inflammatory responses by inducing chemotaxis of monocytes and polymorphonuclear neutrophils (Badolato et al., 1995; H. Li et al., 2020). SAA is an acute phase protein formed primarily in liver cells by cytokines including interleukins 6 (IL-6) and 1β (IL-1β) as well as tumor necrosis factor-α (H. Li et al., 2020). To determine peaks that were differentially expressed substantially in SARS patients vs. non-SARS patients (including those with infections caused by influenza A virus, influenza B virus, respiratory adenovirus, respiratory syncytial virus, hepatitis B virus, tuberculosis, various other bacteria, as well as healthy individuals functioning as a negative control) protein chip array profiling analysis was used (Yip et al., 2005). The authors found that SAA increased considerably in sera of SARS patients and also gradually decreased over the course of treatment with ribavirin and systemic steroids and could, therefore, be utilized to assess patients’ responses to treatment. To mark the extent of pneumonia, patients were evaluated using serial chest x-ray opacity (Yip et al., 2005). SAA concentrations were found to peak earlier than radiographical scores in this study and thus may be used as an earlier marker for disease activity (Yip et al., 2005). However, using SAA as a SARS-CoV-1 biomarker has been the subject of controversies (Pang and Lo, 2006). A team of researchers compared sera samples of SARS patients to non-SARS patients who presented similar symptoms as the SARS patients and were suspected cases during the outbreak (before later testing negative for the SARS-CoV-1 antibody) (Pang and Lo, 2006). The authors argued that previous studies analyzing SAA as a SARS biomarker, such as the one mentioned above, used insufficient non-SARS patients to draw comparisons from. They found that the SAA peaks from SELDI analysis of sera were not identified as SARS-specific features; ELISA analysis confirmed that SAA concentrations were elevated in both SARS and non-SARS patients (Pang and Lo, 2006). SAA concentration may reflect the severity of an illness, rather than the cause of the illness itself. Thus, SAA itself may be insufficient at differentiating SARS from non-SARS patients, but in conjunction with other markers, SAA could substantiate a specific SARS diagnosis classification model (Pang and Lo, 2006).
2.3. Platelet factor 4 and β-thromboglobulin
A 2012 study detailed the correlation of decreased serum platelet factor 4 and increased serum β-thromboglobulin levels with poor SARS prognosis after comparing 39 SARS patients to 39 non-SARS patients showing symptoms similar to SARS who later tested negative for the SARS-CoV-1 antibody (Poon and Lo, 2012). SELDI ProteinChip technology was used to gather serum proteomic profiling data, and initially identified 20 candidate biomarkers for SARS detection; these were then narrowed down to 4 proteomic features considered to be statistically relevant prognostic biomarkers for intensive care unit admission or supplemental oxygen administration later on (Poon and Lo, 2012). The researchers determined two of these features to be platelet factor 4 and β-thromboglobulin (β-TG). Serum platelet factor 4 levels were found to be distinctively lower in SARS patients compared to non-SARS patients whereas β-thromboglobulin was found to be distinctively higher; likewise, decreased platelet factor 4 and increased β-thromboglobulin were also observed in SARS patients who required supplemental oxygen administration when compared to those who did not (Poon and Lo, 2012).
Platelet factor 4 and β-thromboglobulin are both CXC chemokines and play roles in immune response regulation (Poon and Lo, 2012). The authors have identified elevated β-thromboglobulin and decreased platelet factor 4 in SARS patients in a ratio that has not been previously observed for any other disease (Poon and Lo, 2012). Platelet factor 4 and β-thromboglobulin in serum may serve as high precision predictors of poor SARS prognosis and are correlated with an increased need for supplemental oxygen in later stages of treatment (Poon and Lo, 2012).
3. Biomarkers of ageing and frailty may predict COVID-19 severity
Bats may be a reservoir host of SARS-CoV-2, which was eventually transmitted to an unknown intermediate host, before reaching humans (Andersen Kg, 2020). COVID-19 illnesses caused by SARS-CoV-2 have been seen to be more harmful to humans than seasonal flues caused by other coronaviruses, although the mortality rate of COVID-19 is lower than that of SARS or MERS (Terpos et al., 2020). A meta-analysis published in April of 2020 identified the mortality rate of COVID-19 to be much lower than that of SARS and MERS (35% and 13%, respectively) as the mortality rate of COVID-19, at the time, was 5.6% - although the authors acknowledge that the true rate is almost certainly lower than this (Pormohammad et al., 2020). Perhaps the most shocking thing about the current COVID-19 pandemic is how quickly and vastly it has spread. Since its emergence, COVID-19 has reached 235 countries and territories, infected over 234,000,000 people, and resulted in over 4800,000 deaths (World Health).
The lungs are the most affected organ, but the heart, liver, and brain can also be compromised (Tang et al., 2020). This is another reason why older individuals may be more severely affected by COVID-19 infection, as age-associated changes to the lungs occur; however, there is variability across individuals and the rate of these changes (Polidori, 2020).
A challenge that has been seen in the past, by testing for the SARS-CoV-1 antibody, is that antibody concentration increases in late stages of the disease, and thus is an inefficient marker for measuring the severity of the disease (Yip et al., 2005). This challenge is being seen, yet again, with COVID-19 as antibody testing can only be used to confirm a past infection. For these reasons, molecular biomarkers that indicate the severity of COVID-19 may be essential to effectively allocate resources necessary to treat patients who are most susceptible to COVID-19 illnesses (I. Huang et al., 2020).
Most COVID-19 cases are asymptomatic or show only mild influenza-like symptoms; however, some cases display severe pneumonia, organ failure, acute respiratory distress syndrome, or death (I. Huang et al., 2020). Moreover, it is unknown, at this point, what the likelihood is of a recovered COVID-19 patient being faced with a reinfection is (Zhang and Guo, 2020). Various potential biomarkers of COVID-19 infections have been proposed as outlined below and contribute in part to the many complex factors determining the likelihood of an individual to develop severe COVID-19 illness.
3.1. C-reactive protein
C-reactive protein is an acute phase inflammatory protein that is typically detected only at very low levels; however, it can dramatically escalate as a part of acute inflammatory responses, which may be indicative of bacterial or viral infections (Liu et al., 2020). As C-reactive protein can increase in response to inflammation, it can also increase with age (Dugué et al., 2021) “Inflammaging” is the phenomenon of chronic activation of the innate immune system, resulting in inflammation, that occurs alongside ageing (Franceschi et al., 2018). More specifically, this can refer to the increase in inflammatory markers such as C-reactive protein, interleukin-6, interleukin-1β, and tumor necrosis factor-α with age (Wagner et al., 2016, Zuo et al., 2019) and may be caused by (i) a build-up of pro-inflammatory tissue damage, (ii) ageing cells tendency to secrete pro-inflammatory cytokines, and (iii) the failure to clear pathogens effectively (Salminen et al., 2012, Wagner et al., 2016). Elevated serum C-reactive protein levels are affiliated with increasing age and, in the case of COVID-19 patients may indicate poor prognosis and increased risk of mortality among older individuals (Dugué et al., 2021), based on thirteen studies (I. Huang et al., 2020).
One study published in February of 2020 described clinical characteristics from 1099 confirmed COVID-19 patients throughout mainland China; 926 of these patients were deemed nonsevere, while the remaining 173 were categorized as severe (Guan Wj, 2020). Most patients were reported to have elevated C-reactive protein levels (60.7%) and especially so in severe cases (81.5%), where elevated C-reactive protein is defined as equal to or greater than 10 mg/L. Elevated C-reactive protein was also seen in 91.1% of cases that resulted in a composite primary end point – which the authors defined as a need for intensive care unit admission, mechanical ventilation, or death (Guan Wj, 2020). A much smaller scale study of 60 COVID-19 patients admitted to the Zhongnan Hospital of Wuhan University, using the same parameters, found that C-reactive protein was abnormal in 72% of patients and ranged from 9 to 67 mg/L (F. Wang et al., 2020). Another study in Wuhan, China recording clinical characteristics of 99 COVID-19 patients demonstrated similarly elevated C-reactive protein levels as the previously mentioned studies, showing an increase in 63/73 (or 86%) patients; however, this study used a different operational definition for elevated C-reactive protein, defined as equal to or greater than 5.0 mg/L (Chen N and et al., 2020).
Serum C-reactive protein was used to track COVID-19 patient improvement following treatment with tocilizumab; this study saw C-reactive protein levels decreased dramatically over the course of treatment, indicating that C-reactive protein levels could be utilized to monitor patients’ response to treatment (Conrozier T, 2020). Serum C-reactive protein levels may enable monitoring of COVID-19 progression, being representative of patients’ inflammatory status. However, C-reactive protein is released in many inflammatory responses; on its own, it cannot be a high precision biomarker of COVID-19.
3.2. Interleukin-6
Interleukin-6 is a, pleiotropic, proinflammatory cytokine found in stromal and immune cells and is the primary trigger for cytokine storms formation (Montesarchio et al., 2020). Cytokine storms are caused by sustained cytokine and chemokine responses as a part of a hyperactive innate immune response (Sinha P, 2020), which undermines organ function and augments systemic vascular permeability (Chen X, Z. B. Q. Y. C. Y. X. J. F. Y. M. D. H. Q. L. Y. Y. B. D. J. L. F., 2020, Liu et al., 2020). One study has found that 82% of fatal COVID-19 cases that experience a cytokine storm occur in individuals over age 60 (Paranjpe et al., 2020). Elevated IL-6 may result in acute lung injury as well as acute respiratory distress syndrome (ARDS) (Henry and Lippi, 2020). Much like C-reactive protein, IL-6 levels increase with age being an integral part of inflammaging (Wagner et al., 2016). Establishing whether or not baseline inflammaging increases the predisposition to the COVID-19 induced cytokine storm merits further investigation.
IL-6 levels may serve as a biomarker of poor COVID-19 prognosis, as indicated by a study of sera collected from 48 confirmed COVID-19 patients who were further classified as 21 moderate cases, 10 severe cases, and 17 critically ill cases (Chen X, 2020). It was observed that IL-6 levels were dramatically escalated in all deaths within the study, as well as sharply raised in critically ill patients - nearly 10 times higher than that of patients with severe cases (Chen X, 2020). The examination of 60 COVID-19 patients hospitalized in Wuhan, China found IL-6 ranged from 6 to 29 pg/mL in patients compared to a defined normal range of 0–7 pg/mL, with 70% of patients exceeding this range (F. Wang et al., 2020). Another study in Wuhan, using the same normal range, displayed an average of 7.9 pg/mL from 99 patients of varying severities, 52% of which were considered to have elevated IL-6 levelS (Chen X, 2020).
IL-6 is a marker of inflammation in many circumstances; hence, it is not COVID-19-specific and would need to be considered along with other marker proteins, and perhaps RT-PCR, to be considered a biomarker of COVID-19 illnesses in particular.
3.3. Lactate dehydrogenase
The study of 1099 patients previously described detected elevated lactate dehydrogenase in 37.2% of patients, 58.1% of severe cases, as well as 70.5% of cases with a primary composite end point (ICU admission, mechanical ventilation, or death); here, elevated lactate dehydrogenase was described as equal to or greater than 250 U/L (Guan Wj, 2020). A study of 99 COVID-19 patients similarly saw increased lactate dehydrogenase in 76% of patients, with an average of 336 U/L, based on the same operational definition (Chen N and et al., 2020). Another study described clinical characteristics of 138 patients with COVID-19 hospitalized in Wuhan, China (Wang D and et al., 2020). This study found median lactate dehydrogenase levels to be 261 U/L in all patients and 435 U/L in patients requiring intensive care – those levels are higher than the normal range of serum lactate dehydrogenase (125–243 U/L) (Wang D and et al., 2020). Knowing this, elevated lactate dehydrogenase may be indicative of a poor COVID-19 prognosis. Lactate dehydrogenase is released in response to tissue damage; hence, a high serum concentration of lactate dehydrogenase could indicate lung damage in COVID-19 (Henry, 2020) or sepsis which is one of the most severe complications of COVID-19 (Galiatsatos, 2020). However, lactate dehydrogenase on its own is not a specific biomarker of COVID-19 and again needs to be used in combination with other markers in order to specifically track COVID-19 severity.
Similar to the markers mentioned above, elevated lactate dehydrogenase may indicate ageing, frailty, and could be utilized to identify those elderly individuals most prone to developing severe disease and death (Cardoso et al., 2018).
3.4. Procalcitonin
Procalcitonin is a glycoprotein precursor of calcitonin and is typically present at very low or undetectable amounts in serum (Liu et al., 2020). Elevated procalcitonin can be used to differentiate bacterial infections from viral infections, as procalcitonin is raised in response to bacterial infections yet usually remains low in viral infections (Liu et al., 2020). Therefore, elevated procalcitonin in severe cases of COVID-19 may indicate a concomitant bacterial infection. Furthermore, procalcitonin has also been studied as a possible marker of ageing and frailty. One retrospective study of 435 patients analyzing the ability of procalcitonin to predict frailty compared to IL-6 and C-reactive protein found that procalcitonin (but not IL-6 or C-reactive protein) was associated with frailty in elderly patients without infection (Yang et al., 2018). This may make procalcitonin even more indicative of patients who will suffer from severe COVID-19 cases. A meta-analysis of 16 studies identified elevated procalcitonin in severe and lethal COVID-19 cases (I. Huang et al., 2020).
Procalcitonin levels in 1099 patients were equal to or greater than 0.5 ng/mL (Guan Wj, 2020). Interestingly, procalcitonin was present in only 5.5% of all cases, but 13.7% of severe cases, and 24% of cases with a composite primary end point. Similar to this study, procalcitonin levels were not significantly raised in another similar, but smaller, study - showing only 6% of cases had elevated procalcitonin - where the normal range was classified as 0.0–5.0 ng/mL (Chen N and et al., 2020).
While it is apparent that procalcitonin is not overwhelmingly present in all COVID-19 cases, it is increasingly present in severe COVID-19. One study found 35.5% of hospitalized patients, and 75% of those in intensive care units, had elevated procalcitonin in their study of hospitalized patients, with median values of 49 ng/mL and 27 ng/mL respectively (Wang D and et al., 2020). Additionally, a meta-analysis found that procalcitonin levels only differed by 0.2 ng/mL between non-severe and severe cases; however, there was an increased risk of severe COVID-19 infection – by nearly 5-fold – in patients with increased procalcitonin (Henry and Lippi, 2020). Because of this, it can be concluded that procalcitonin should be monitored as a marker of secondary bacterial infection, as this is frequently found in non-survivors, and is an important marker that presents a clear way to distinguish patients likely to develop very severe or critical disease from those likely to develop mild conditions.
3.5. Serum Amyloid A / Lymphocyte count
As in SARS patients, serum amyloid A levels have been investigated in COVID-19 patients. One study has proposed that serum amyloid A concentration and lymphocyte counts are sensitive indicators of COVID-19 severity and may be used to monitor inflammation conditions in COVID-19 infected patients (H. Li et al., 2020). 132 COVID-19 patients were monitored for compelling changes of blood serum amyloid A and lymphocyte counts, the authors found that serum amyloid A increased, while lymphocyte decreased, as the disease advanced from mild to severe and were of greater significance to disease classification than other factors studied. Similarly, serum amyloid A levels fell, and lymphocyte counts raised as patients’ clinical conditions improved, indicating that they may be used to monitor improvement as well (H. Li et al., 2020).
Similar to SARS infections, cytokines such as interleukin-1β, IL-6, and tumor necrosis factor-α, are more prominent in critically ill COVID-19 patients compared to milder patients, and promote elevated serum amyloid A production, which may be indicative of COVID-19 severity (H. Li et al., 2020). The ratio of serum amyloid A to lymphocyte counts was established to be more sensitive than of serum amyloid A or lymphocyte counts used on their own at indicating inflammation in COVID-19 patients. However, another study identified that a low lymphocyte count in itself may be an indicator of COVID-19 diagnosis; as decreased lymphocyte count, including B cells, T cells, and natural killer cells, may allow uncontrolled and increasingly harmful infections to occur (Tang et al., 2020). Low T cell counts, in particular, have been seen in COVID-19 infected patients, and may be a direct result of the cytokine storm (Tavakolpour et al., 2020). These decreased lymphocyte counts have the potential to accelerate to lymphopenia and are clear indicators of the weakening in a patients’ ability to combat a viral infection (Tang et al., 2020). Because of this, lymphocyte count on its own is representative of the severity of the illness in patients and may correlate with COVID-19 severity.
3.6. Complement system and acute phase proteins
Mass spectrometry-based protein identification and quantitation has revealed sets of serum proteins that may serve as biomarkers of COVID-19. One such study profiled sera from 28 severe COVID-19 cases, 25 non-severe cases, 25 non-COVID-19 patients presenting similar symptoms as COVID-19 patients, and 28 healthy individuals (Shen et al., 2020). These researchers built a random forest machine learning model, to identify numerous serum proteins that were differentially expressed in COVID-19 patients, compared to non-COVID-19 patients (Shen et al., 2020). Another study following the SARS-CoV-2 outbreak in Germany, studied 31 SARS-CoV-2 infected patients for candidate biomarkers, and validated their claims using 17 additional COVID-19 patients and 15 healthy individuals (Messner et al., 2020). Both of these studies found many of the same markers, including complement factors, inflammation modulators, and pro-inflammatory factors upstream and downstream of IL-6 (Messner et al., 2020, Shen et al., 2020).
The complement system is essential to eliminate pathogens in the early phase of an acute infection, such as COVID-19, and involves many acute phase proteins being released by the liver in response to increased cytokine concentration – particularly IL-6 (Gabay and Kushner, 1999). Among the acute phase proteins found to be upregulated and suggested to be useful markers of poor COVID-19 prognosis in the study by Shen and Coauthors were serum amyloid A1, serum amyloid A2, C-reactive protein, alpha-1-antichymotrypsin, and serum amyloid P-component (Shen et al., 2020). Messner and coauthors also observed increased levels of serum amyloid A, serum amyloid A2, and C-reactive protein in their study, along with other increased acute phase proteins including Z-dependent protease inhibitor, inter-α-trypsin inhibitor heavy chains 3 and 4, haptoglobin, leucine-rich α-2-glycoprotein 1, lipopolysaccharide binding protein, as well as decreased albumin and transferrin (Messner et al., 2020).
Paradoxically, transferrin levels were found to be increased in COVID-19 patients, in a different study aiming to identify gene products contributing to coagulopathy related to COVID-19, which investigated the Genotype-Tissue Expression database to study the expression of genes associated with “blood coagulation” (McLaughlin et al., 2020). In this latter study, transferrin was upregulated in SARS-CoV-2 infected individuals, increased with age, and was higher in males than females (McLaughlin et al., 2020). Transferrin levels were also found to rise with disease progression in this study (McLaughlin et al., 2020). Transferrin itself is a procoagulant and an iron-carrier molecule, and its elevation may be indicative of iron-deficient anemia development in COVID-19 patients (McLaughlin et al., 2020). Moreover, a study of 65 older individuals showed that transferrin increases with frailty as found using a non-targeted glycoproteomic method (Darvin et al., 2013). Elevated transferrin may identify COVID-19 patients who are more frail and likely to experience severe illness.
Among the complement system proteins elevated in COVID-19 patients are complement 6, complement factor B, properdin, complement complement 1r, complement complement 1 s, complement C8 α chain, complement factor H, and complement factor I - all of which play roles in regulating the complement pathway and may be markers of increased COVID-19 severity (Messner et al., 2020, Shen et al., 2020).
Among the serum proteins upregulated by COVID-19 are mediators of the IL-6 signaling including galectin 3-binding protein (which promotes IL-6 expression) and monocyte differentiated antigen CD14 (which is downstream of IL-6 and is involved in LPS recognition) (Messner et al., 2020). Also elevated were β and γ-1 actin, which normally play roles in cell shape and movement, and α-1B-glycoprotein, which is of an unknown function, all of which are associated with increased severity (Messner et al., 2020). Lastly, decreased gelsolin, an actin modulating protein, may also be associated with inflammation and poor prognosis in COVID-19 patients (Messner et al., 2020).
3.7. Apolipoproteins
Apolipoproteins are known to play roles in disrupting the commencement of the innate immune response (Cho and Seong, 2009). Shen and coauthors found apolipoprotein A1, apolipoprotein A2, apolipoprotein H, apolipoprotein D, apolipoprotein L1 and apolipoprotein M were also found to be downregulated in COVID-19 patients (Shen et al., 2020). The decreased levels of apolipoprotein A1 as well as apolipoprotein C1 were confirmed by a separate study (Messner et al., 2020).
Serum amyloid A, which as discussed previously, is increased under inflammation circumstances, is responsible for lowering apolipoprotein A1 levels in high density lipoprotein particles (Sorokin et al., 2020). Apolipoprotein A1, and apolipoprotein M, are associated with high-density lipoprotein particles and interact with lipid rafts on cell membranes that have immune cell receptors, which aid in regulating immune responses (Sorokin et al., 2020). Declining apolipoprotein A1 seems to be associated with the transition from mild to severe COVID-19 illness and may signify dysregulation of the immune response.
Apolipoprotein E levels may influence COVID-19 infection susceptibility (Goldstein et al., 2020). Apolipoprotein E has three possible isoforms, and the authors propose that presence of an apoE4 allele may predispose an individual to an increased risk of developing severe COVID-19 illnesses, finding it to be associated with an augmented innate immune response resulting in cytokine storm formation and acute respiratory distress syndrome development (Goldstein et al., 2020). Thus, downregulation of apolipoproteins may indicate progressing COVID-19 disease.
3.8. Pro-platelet
Suppressed platelet degranulation was observed in COVID-19 patients, in association with an inflammatory response with potential to damage blood vessel walls (Shen et al., 2020). This degranulation may be provoked by SARS-CoV-2 entry via the ACE-2 receptor releasing angiotensin II (Biancardi et al., 2017, Kuchi Bhotla et al., 2020) resulting in downregulation of pro-platelet basic protein and platelet factor 4, which were also associated with poor COVID-19 prognosis (Shen et al., 2020) (and SARS, when found in combination increased β-thromboglobulin, as discussed earlier).
Pro-platelet basic protein and platelet factor 4 can also be linked to thrombocytopenia (low platelet count) in cases with severe, or even lethal, COVID-19 and usually indicates organ malfunction and intravascular coagulation (Lippi 2020; Shen et al., 2020). One meta-analysis found that patients exhibiting thrombocytopenia had a three-fold increased risk of developing severe COVID-19 (Lippi 2020). Due to these potentially fatal side effects, pro-platelet basic protein and platelet factor 4 should be used as biomarkers of poor COVID-19 prognosis and potential thrombocytopenia in patients.
3.9. Cortisol
Another study found elevated cortisol, which plays a key role in the body’s stress response, to be associated with a higher chance of death by COVID-19 (Tan et al., 2020). The researchers conducted a cohort study on acute cortisol concentrations in 535 patients admitted to various hospitals in London, UK; 403 of which were diagnosed with or had strong clinical and radiological suspected cases of COVID-19, while the remaining 132 were suspected to have COVID-19 but tested negative and did not have strong clinical or radiological suspicion (Tan et al., 2020). Furthermore, these patients did not have pre-existing conditions that could account for raised cortisol levels (Tan et al., 2020). The researchers found that elevated cortisol, as well as C-reactive protein, creatinine, and neutrophil-to-leukocyte ratio were predictors of potential acute death - with cortisol out-performing the other markers in this regard (Tan et al., 2020). This can indicate that cortisol may be useful as a predictor of COVID-19 illnesses severity. In their study, COVID-19 patients were shown to have significantly higher acute cortisol stress responses than patients who were not suspected or confirmed cases of COVID-19 (Tan et al., 2020). The researchers found that COVID-19 patients with baseline cortisol concentrations of 744 nmol/L or less displayed a median survival rate of 36 days, compared to those with cortisol concentrations greater than this value had a survival rate median of only 15 days, with cortisol concentrations observed in this study peaking at 3241nmol/L (Tan et al., 2020). Furthermore, it was identified that cortisol concentration doubling correlates to a 42% increase in the risk of mortality within this study (Tan et al., 2020). Moreover, increased cortisol may also be indicative of increased frailty. One study determined the amounts of cortisol in 252 older adults (who were at least 65 years of age) of varying frailty statuses and found that there was significantly increasing cortisol concentrations in more frail patients (Marcos-Pérez et al., 2019). Hence, elevated cortisol (itself a marker of stress and possibly frailty) may indicate potentially fatal COVID-19.
3.10. Vitamin D
Decreased vitamin D levels correlate with acute respiratory tract infections in COVID-19 patients (Mercola et al., 2020). Sufficient levels of Vitamin D correlate with reduced risk; presumably by restricting viral replication as well as raising the concentration of ACE2 and reducing pro-inflammatory cytokines (Mercola et al., 2020). Vitamin D generally has anti-inflammatory effects on immune cells and promotes them to produce antimicrobial peptides, including cathelicidins (Beard et al., 2011, Dimitrov and White, 2016). Cathelicidin can carry out antiviral effects by disrupting SARS-CoV-2 envelope proteins (Barlow et al., 2014, Kara et al., 2020). One of the causes for vitamin deficiency is darker skin pigmentation and may be in part why COVID-19 is disproportionately affecting people of color (Mercola et al., 2020). If vitamin D deficiency raises the risk of COVID-19, then those with vitamin D deficiencies may be more likely to test positive for COVID-19 than those without (Meltzer et al., 2020), this claim derives from a retroactive study looking at patients who were tested for COVID-19 and had vitamin D levels recorded within a year prior to testing (Meltzer et al., 2020). In this study, Vitamin D deficiency was determined based on most recent 25-hydroxycholecalciferol < 20 ng/mL or 1,25-dihydroxycholecalciferol < 18 pg/mL measurements before testing for COVID-19 (Meltzer et al., 2020). Vitamin D levels were combined with any treatment received to determine patient vitamin D statuses when tested for COVID-19 (Meltzer et al., 2020). The authors found that 18% of vitamin D deficient patients had positive COVID-19 testing results compared to 11% of patients who were not Vitamin D deficient (Meltzer et al., 2020). Thus, insufficient vitamin D levels have been proposed to increase the risk of COVID-19 (Meltzer et al., 2020).
Low levels of vitamin-D have also been proposed as a biomarker of frailty. One large study 940 adults (who were at least 65 years of age) found that participants with serum amounts of vitamin D below 15 ng/mL were less often frail than those who had vitamin D exceeding this amount (Pabst et al., 2015). Hence, COVID-19 patients with low levels of vitamin D are more likely to be frail and at an increased risk of a poor COVID-19 prognosis.
3.11. Inflammaging as a likely contributor to frailty and COVID-19 susceptibility
Ageing is accompanied by low-grade unresolved sterile inflammation, a process coined inflammaging. Inflammaging involves the persistent physiological activation of innate immune responses as well as metabolic remodeling of immune cells, which can be damaging as well as being a catalyst of ageing thus creating a vicious circle that is likely to increase frailty. While many markers of COVID-19 are discussed above, the ones that are most key to identifying patients risk of developing severe COVID-19, particularly older patients, include the inflammatory markers, lactate dehydrogenase, procalcitonin, cortisol, and vitamin D. These could be used in a clinical setting to identify patients with the highest risk of developing severe and possibly fatal COVID-19. Inflammatory markers, such as C-reactive protein and IL-6, are involved in inflammaging and should be closely monitored in old COVID-19 patients. Could elevated levels of C-reactive protein and IL-6 indicate an increased probability of cytokine storms, poor prognosis and multisystem inflammation? This would not be surprising as the immune system response to COVID-19 is very dependent on production of IL-6; furthermore, in the elderly, elevated IL-6 may indicate presence of significant inflammaging (Dugué et al., 2021; I. Huang et al., 2020). Elevated lactate dehydrogenase can indicate ageing and frailty (Cardoso et al., 2018) as well as being indicative of COVID-19 potential lung tissue damage. Procalcitonin predicts frailty even more than C-reactive protein or IL-6 in elderly patients with no infection present (Yang et al., 2018), and has been associated with secondary bacterial infection in COVID-19 patients. Most importantly, procalcitonin has been particularly present in patients who face very severe COVID-19 illness and those who have not survived, making procalcitonin even more indicative of patients who will suffer from severe cases of COVID-19. Similarly, elevated cortisol has been associated with frailty (Marcos-Pérez et al., 2019) and a higher chance of death by COVID-19 (Tan et al., 2020), making it a key biomarker to monitor in COVID-19 patients. Lastly, low levels of vitamin-D, another proposed biomarker of frailty, is correlated with yet another symptom of severe COVID-19 (acute respiratory tract infections) (Mercola et al., 2020), making it another key indicator of severe COVID-19 risk. Overall, it appears that the levels of inflammaging markers along with those of lactate dehydrogenase, procalcitonin, cortisol, and vitamin D may be useful in predicting who will develop severe and potentially fatal COVID-19, based on their indication of general inflammation status as well as the most severe symptoms of COVID-19. Moreover, these markers overlap with those which indicate ageing and frailty and should be closely monitored in patients, particularly those of advanced ages.
4. Clinical translation of this research: integrating the mechanisms of viral infectivity with biomolecular markers of ageing, frailty, disease tolerance and non-biomolecular factors to assess the susceptibility to severe COVID-19
4.1. Mechanisms of viral infectivity
Hundreds of viruses are known to infect humans through attaching to target cell receptors (Dimitrov, 2004) and the novel SARS-CoV-2 virus is no exception. Following exposure to the SARS-CoV-2 virus, various biomolecular mechanisms of viral infectivity are elicited which impact the magnitude of antiviral immune response as well as viral replication, thus influencing disease severity. SARS-CoV-2 can enter target cells by direct fusion of the viral envelope with the host cell membrane as well as by endocytosis with subsequent membrane fusion (Zhang et al., 2021). SARS-CoV-2 cell membrane receptors include angiotensin-converting enzyme 2 (ACE2), CD147 (cluster of differentiation 147 –a transmembrane glycoprotein of the immunoglobulin superfamily also known as basigin or EMMPRIN) (K. Wang et al., 2020) CD26 (cluster of differentiation 26 –membrane-anchored glycoprotein with dipeptidyl peptidase activity on its ectodmain) via trimeric spike protein) (Tay et al., 2020). High ACE2 expression may not only promote infection of cells by SARS-CoV-2 but may also be associated with cell-intrinsic characteristics predisposing to the development of a more severe disease phenotype upon infection (Lavorgna et al., 2021). Spike is a class I viral fusion protein, which requires proteolytic cleavage for activation of the fusion potential. The protein is cleaved by TMPRSS-2, TMPRSS-4, furin, trypsin, cathepsins, or human airway trypsin-like protease, depending on the cell type. Availability of these proteases on target cells determines whether viral cell entry occurs through the host cell membrane or via endocytosis (Zhang et al., 2021). The spike protein itself is comprised of two subunits (S1 and S2) which share an extracellular domain, while the S1 subunit comprises a receptor binding domain, through which SARS-CoV-2 attaches to ACE2 during infection (Tay et al., 2020). This mechanism is the basis of many vaccine preparations designed to induce the production of host neutralizing antibodies against the receptor binding domain of spike (Tai et al., 2020, Yang et al., 2020). However, an ever-increasing number of SARS-CoV-2 variants carry mutations in the receptor binding domain with the potential to diminish the neutralization of SARS-CoV-2 by antibodies-based immune responses (Baum et al., 2020; Q. Li et al., 2020). In addition to the spike protein, ACE2 expression patterns and therefore viral entry potential, are susceptible to epigenetic pressure - a phenomenon involving DNA methylation and histone modifications, whose cumulative effects over the course of a lifetime can profoundly alter ACE2 expression as we age (Rath et al., 2021). This genetic variability of SARS-CoV-2 is likely to disproportionately increase the risk of developing severe disease in older and frail infected, vaccinated, or re-infected individuals.
4.2. Why would older age and frailty disproportionately predispose to severe COVID-19 illness?
The answer to this question may be that in ageing and frailty there may be a reduction in ‘disease tolerance’ - the host’s defense mechanisms to limit tissue damage or reduce immunopathology induced by the infection with a pathogen (Ayres, 2020, Hardy and Fernandez-Patron, 2021, Medzhitov et al., 2012). Disease tolerance declines with increased age, and as a result of deteriorated immunity (Medzhitov et al., 2012) and may differentially affect older or frail individuals infected by SARS-CoV-2. For example, a recent study found that significantly different molecular and cellular responses are mounted in response to SARS-CoV-2 infection by symptomatic patients, compared to asymptomatic patients (Chan et al., 2021) The study, which involved 178 symptomatic patients (age range: 39.4 ± 12.5 years) and 48 asymptomatic patients (48.2 ± 15.9), was not designed to test aging or frailty; however, it identified potentially new biomarkers predictive of disease trajectory which may be differentially expressed in older and frail individuals (Chan et al., 2021). The authors found that symptomatic patients had higher counts of mature neutrophils and lower proportion of CD169 + expressing monocytes in the peripheral blood tolerance (Chan et al., 2021). Their systemic levels of pro-inflammatory cytokines were increased while their virus-specific Th17 cells levels were lower –despite their neutralizing antibody profile against SARS-CoV-2. Most interestingly, asymptomatic COVID-19 patients had higher systemic levels of growth factors that are associated with cellular repair (Chan et al., 2021). In contrast to the patients who developed symptoms, the asymptomatic patients were found to mount less pro-inflammatory and more protective immune responses against SARS-CoV-2 indicative of a superior disease tolerance (Chan et al., 2021). Moreover, ACE2 expression in the lung has been found to positively correlate with age among middle-aged and older adults but does not seem to be associated with sex or race (e.g., Asian individuals do not differ from other populations for ACE2 expression and do not harbor unique genetic polymorphisms in the ACE2 locus) (Asselta et al., 2020, Chen et al., 2020; Q. Li et al., 2020). This latter observation has been proposed to explain why the elderly are more susceptible to SARS-CoV-2 (Chen N and et al., 2020; C. Huang et al., 2020; L. F. Wang et al., 2020).
4.3. Non-biomolecular factors may increase the susceptibility to severe COVID-19 in parallel with ageing
The evidence show that clinical history, lifestyle, and socio-economic factors can have a strong influence on disease trajectory, not just in COVID-19 patients (Hassan et al., 2021, Takahashi et al., 2018).
Together with the pandemic proportions of COVID-19, there are many non-communicable conditions have also reached worldwide pandemic proportions, such as hypertension, obesity, diabetes, neurodegeneration as well as highly inflammatory conditions such as cancer and immunosuppression, which predominantly affect older individuals and may interact to increase susceptibility to severe COVID-19 (Bambra, 2016, Bambra et al., 2020, England, 2018, Guo et al., 2019) likely through impairing disease tolerance mechanisms, with a resultant increase in frailty.
For example, hypertension (sustained high blood pressure) is a major risk factor for vascular, cardiac, cerebral and renal events as well as increases the risk of developing a severe COVID-19, with one meta-analysis finding hypertension may cause an up to 2.5-fold increased risk of severe COVID-19, particularly in patients who are older (G. Lippi et al., 2020b). As the burden of hypertension increases with ageing, so does the risk of death in older COVID-19 patients (G. Lippi et al., 2020b) due to vascular, cardiac, cerebral, renal complications (Tehrani et al., 2021). Hypertension is a risk factor for neurodegeneration and a common comorbidity of Alzheimer’s disease (Kim et al., 2017). In the elderly, cognitive impairment (e.g., Alzheimer’s disease) appears to be one of the most common comorbidities that negatively impacts COVID-19 prognosis (Martins et al., 2019, Wu et al., 2020, Xia et al., 2021). Alzheimer’s patients often have increased levels of inflammatory cytokines such as IL-6, which as discussed above is elevated in COVID-19 patients with hyperinflammation (Kim et al., 2017) and may serve as a clinical marker of reduced disease tolerance (Chan et al., 2021).
Beyond the above-mentioned comorbidities, as with many disease conditions COVID-19 trajectory is negatively influenced by socio-economic and psychosocial factors. Lack of access to adequate healthcare service predominantly affects people in underdeveloped countries and in underserved communities of developed nations and is certain to have a disproportionate impact in older and frail individuals. Malnutrition primarily affects the underserved communities of the world and has been previously linked to increased predisposition to severe viral pneumonia and, more recently, to the SARS-CoV-2 pneumonia with a prevalence of 42% in COVID-19 patients hospitalized in non-intensive medical units and 67% in those placed in intensive care (Bedock et al., 2020, Short et al., 2018).
Psychosocial issues (work-related stress, family problems, sexual abuse, violence, depression, anxiety) predispose to severe COVID-19, even if there are no pre-existing medical comorbidities (Bambra et al., 2020, Segerstrom and Miller, 2004). For example, social isolation, which has become a part of many people’s daily life throughout the pandemic, is a risk factor for psychosocial stress, morbidity, and mortality (Mattos Dos Santos, 2020). Social isolation can translate into suppression of the immune system and an increased general susceptibility to infectious diseases, including COVID-19 (Dhabhar et al., 2020, Takahashi et al., 2018) (Segerstrom and Miller, 2004). Persistent stress due to social isolation can exacerbate aggression traits and depression traits which have been mechanistically linked to reduced disease tolerance indicators such as increased levels of inflammatory cytokines, immune suppression, slow healing with delayed clearing of infection (Takahashi et al., 2018).
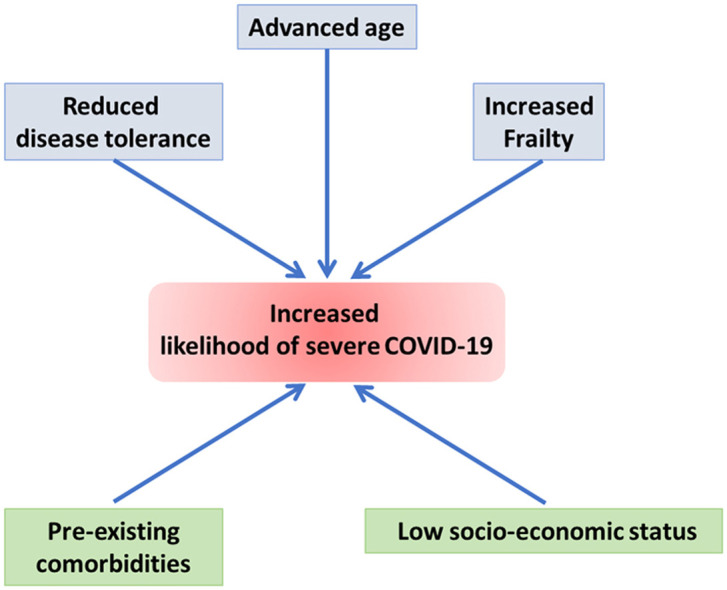
Interestingly, a recent study has identified a simple virus-free model, based on publicly available transcriptional data from human cell lines, which can recapitulate features of the clinically relevant infections leading the authors to suggest that "in patients with severe COVID-19, a baseline ground could be already present and, as a consequence, the viral infection might simply exacerbate a variety of latent (or inherent) pre-existing conditions, representing therefore a tipping point at which they become clinically significant" (Lavorgna et al., 2021). In the light of our previous analysis, we suggest that advanced age, increased frailty, and reduced disease tolerance are likely factors to determine COVID-19 severity through providing a suitable 'baseline ground' for exacerbated viral infection, with socio-economic and psycho-social conditions being strong influencers of COVID-19 trajectory, particularly in older individuals ( Fig. 1).
Fig. 1.
Biomarkers of ageing and frailty may predict COVID-19 severity as both conditions are associated with reduced disease tolerance - the host’s defense mechanisms to limit tissue damage or reduce immunopathology induced by the infection with a pathogen. While these biomolecular markers inform about the baseline ground for exacerbated viral infection, inflammaging and pre-existing comorbidities, which are common at advanced ages, as well as socio-economic conditions that affect people in underdeveloped nations and underserved communities of developed nations appear to be strong influencers of COVID-19 trajectory - particularly in older and frail individuals.
5. Conclusion
We have compiled and discussed a spectrum of candidate biomarkers of the illnesses associated with COVID-19, ageing and frailty. Most of them are induced under general circumstances, such as inflammation or tissue damage. However, some have also been studied as markers of ageing and frailty. The expression of biomarkers common to COVID-19, ageing and frailty, such as elevated C-reactive protein, interleukin-6, lactate dehydrogenase, procalcitonin, transferrin, cortisol, as well as low vitamin D levels, might identify those individuals who will have poor COVID-19 prognoses, as COVID-19 deaths have occurred disproportionately in old and frail patients. These biomarkers, each individually and the fingerprint as whole, alone are not a sufficient risk evaluation of COVID-19 in patients, however they do provide one aspect within an individual that can be studied to in part predict and characterize how severely a patient will be impacted by a COVID-19 diagnosis, which could enhance the capacity of the health system for proper allocation of resources for those patients with the highest risk of developing severe disease or possible death. While our literature research indicates that biomarkers of aging and frailty may predict severe COVID-19 illnesses, emerging research suggests that biomarkers of disease tolerance may predict resistance to COVID-19. Having measurements of both types of biomarkers (ageing/frailty and tolerance) could be a great tool in the hands of clinicians as they try to predict the trajectories of their COVID-19 patients.
CRediT authorship contribution statement
Kailyn J. Wanhella, Carlos Fernandez-Patron: Conceived the study and wrote the manuscript. Carlos Fernandez-Patron: Secured funding acquisition and supervised the project. All authors participated performed critical revisions of the manuscript and approved the final version of the manuscript.
Declaration of Interest
None.
Acknowledgments
Funding: This work was supported by the Natural Sciences and Engineering Council of Canada [RES0034250]; and the University of Alberta Hospital Foundation [RES0048483].
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.arr.2021.101513.
Appendix A. Supplementary material
Supplementary material
.
References
- Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26(4):450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselta R., Paraboschi E.M., Mantovani A., Duga S. ACE2 and TMPRSS2 variants and expression as candidates to sex and country differences in COVID-19 severity in Italy. Aging (Albany NY) 2020;12(11):10087–10098. doi: 10.18632/aging.103415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres J.S. Surviving COVID-19: A disease tolerance perspective. Sci. Adv. 2020;6(18) doi: 10.1126/sciadv.abc1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badolato R., Johnston J.A., Wang J.M., McVicar D., Xu L.L., Oppenheim J.J., Kelvin D.J. Serum amyloid A induces calcium mobilization and chemotaxis of human monocytes by activating a pertussis toxin-sensitive signaling pathway. J. Immunol. 1995;155(8):4004–4010. 〈http://www.jimmunol.org/cgi/content/abstract/155/8/4004〉 (1950) [PubMed] [Google Scholar]
- Bambra C. Health Divides Where you Live can Kill You. 1 ed.., Bristol University Press; 2016. [DOI] [Google Scholar]
- Bambra C., Riordan R., Ford J., Matthews F. The COVID-19 pandemic and health inequalities. J. Epidemiol. Community Health. 2020;74(11):964–968. doi: 10.1136/jech-2020-214401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow P.G., Findlay E.G., Currie S.M., Davidson D.J. Antiviral potential of cathelicidins. Future Microbiol. 2014;9(1):55–73. doi: 10.2217/fmb.13.135;2210.2217/fmb.13.135. [DOI] [PubMed] [Google Scholar]
- Baum A., Fulton B.O., Wloga E., Copin R., Pascal K.E., Russo V., Giordano S., Lanza K., Negron N., Ni M., Wei Y., Atwal G.S., Murphy A.J., Stahl N., Yancopoulos G.D., Kyratsous C.A. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science. 2020;369(6506) doi: 10.1126/science.abd0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard J.A., Bearden A., Striker R. Vitamin D and the anti-viral state. J. Clin. Virol. 2011;50(3):194–200. doi: 10.1016/j.jcv.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedock D., Bel Lassen P., Mathian A., Moreau P., Couffignal J., Ciangura C., Poitou-Bernert C., Jeannin A.C., Mosbah H., Fadlallah J., Amoura Z., Oppert J.M., Faucher P. Prevalence and severity of malnutrition in hospitalized COVID-19 patients. Clin. Nutr. ESPEN. 2020;40:214–219. doi: 10.1016/j.clnesp.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biancardi V.C., Bomfim G.F., Reis W.L., Al-Gassimi S., Nunes K.P. The interplay between Angiotensin II, TLR4 and hypertension. Pharmacol. Res. 2017;120:88–96. doi: 10.1016/j.phrs.2017.03.017. [DOI] [PubMed] [Google Scholar]
- Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clinical Pharmacology & Therapeutics. 2001;69(3):89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- Cardoso A.L., Fernandes A., Aguilar-Pimentel J.A., de Angelis M.H., Guedes J.R., Brito M.A., Ortolano S., Pani G., Athanasopoulou S., Gonos E.S., Schosserer M., Grillari J., Peterson P., Tuna B.G., Dogan S., Meyer A., van Os R., Trendelenburg A.U. Towards frailty biomarkers: Candidates from genes and pathways regulated in aging and age-related diseases. Ageing Res. Rev. 2018;47:214–277. doi: 10.1016/j.arr.2018.07.004. https://doi.org/S1568-1637(18)30093-X[pii] [DOI] [PubMed] [Google Scholar]
- Centers for Disease & Prevention. CDC’s Diagnostic Test for COVID-19 Only and Supplies. In (Vol. 2020).
- Chan Y.-H., Fong S.-W., Poh C.-M., Carissimo G., Yeo N.K.-W., Amrun S.N., Goh Y.S., Lim J., Xu W., Chee R.S.-L., Torres-Ruesta A., Lee C.Y.-P., Tay M.Z., Chang Z.W., Lee W.-H., Wang B., Tan S.-Y., Kalimuddin S., Young B.E., Leo Y.-S., Wang C.-I., Lee B., Rötzschke O., Lye D.C., Renia L., Ng L.F.P. Asymptomatic COVID-19: disease tolerance with efficient anti-viral immunity against SARS-CoV-2. EMBO Mol. Med. 2021;13(6) doi: 10.15252/emmm.202114045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiology and clinical characteristics of 99 Cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Z. B. Q. Y. C. Y. X. J. F. Y. M. D. H. Q. L. Y. Y. B. D. J. L. F Detectable Serum Severe Acute Respiratory Syndrome Coronavirus 2 Viral Load (RNAemia) Is Closely Correlated With Drastically Elevated Interleukin 6 Level in Critically Ill Patients With Coronavirus Disease 2019. Clin. Infect. Dis.: Off. Publ. Infect. Dis. Soc. Am. 2020;7(8) doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y., Shan, K., & Qian, W., 2020, Asians Do Not Exhibit Elevated Expression or Unique Genetic Polymorphisms for ACE2, the Cell-Entry Receptor of SARS-CoV-2. 10.20944/preprints202002.0258.v2. [DOI]
- Cho N.-H., Seong S.-Y. Apolipoproteins inhibit the innate immunity activated by necrotic cells or bacterial endotoxin. Immunology. 2009;128(1):e479–e486. doi: 10.1111/j.1365-2567.2008.03002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrozier T, L. A. B. J. C. D. P. R. P. Y. B. M. B. A. M. G. V. C. A. T. L. B. J Biomarker variation in patients successfully treated with tocilizumab for severe coronavirus disease 2019 (COVID-19): results of a multidisciplinary collaboration. Clin. Exp. Rheumatol. 2020;38(4):742–747. 〈https://www.clinexprheumatol.org/article.asp?a=15887〉 [PubMed] [Google Scholar]
- Darvin K., Randolph A., Ovalles S., Halade D., Breeding L., Richardson A., Espinoza S.E. Plasma Protein Biomarkers of the Geriatric Syndrome of Frailty. J. Gerontol.: Ser. A. 2013;69 A(2):182–186. doi: 10.1093/gerona/glt183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar F.S., Meaney M.J., Sapolsky R.M., Spencer R.L. Reflections on Bruce S. McEwen’s contributions to stress neurobiology and so much more. Stress. 2020;23(5):499–508. doi: 10.1080/10253890.2020.1806228. [DOI] [PubMed] [Google Scholar]
- Dimitrov D.S. Virus entry: molecular mechanisms and biomedical applications. Nat. Rev. Microbiol. 2004;2(2):109–122. doi: 10.1038/nrmicro817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov V., White J.H. Species-specific regulation of innate immunity by vitamin D signaling. J. Steroid Biochem. Mol. Biol. 2016;164:246–253. doi: 10.1016/j.jsbmb.2015.09.016. [DOI] [PubMed] [Google Scholar]
- Dugué P.A., Hodge A.M., Ulvik A., Ueland P.M., Midttun Ø., Rinaldi S., Macinnis R.J., Li S.X., Meyer K., Navionis A.S., Flicker L., Severi G., English D.R., Vineis P., Tell G.S., Southey M.C., Milne R.L., Giles G.G. Association of markers of inflammation, the kynurenine pathway and B vitamins with age and mortality, and a signature of inflammaging. J. Gerontol. Ser. A, Biol. Sci. Med. Sci. 2021 doi: 10.1093/gerona/glab163.glab163[pii]. [DOI] [PubMed] [Google Scholar]
- England, P.H., 2018, Local action on health inequalities understanding and reducing ethnic inequalities in health. London: Public Health England. 〈https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/730917/local_action_on_health_inequalities.pdf〉.
- Franceschi C., Garagnani P., Parini P., Giuliani C., Santoro A. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018;14(10):576–590. doi: 10.1038/s41574-018-0059-4. [DOI] [PubMed] [Google Scholar]
- Gabay C., Kushner I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999;340(6):448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- Galiatsatos, P., 2020, What Coronavirus Does to the Lungs. In (Vol. 2020): John Hopkins Medicine.
- Goldstein M.R., Poland G.A., Graeber C.W. Does apolipoprotein E genotype predict COVID-19 severity? QJM: Int. J. Med. 2020;113(8):529–530. doi: 10.1093/qjmed/hcaa142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Wj, N. Z. Y. H. Y. L. W. H. O. C. Q. H. J. X. L. L. S. H. L. C. L. H. D Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Wei D., Zhang X., Wu Y., Li Q., Zhou M., Qu J. Clinical Features Predicting Mortality Risk in Patients With Viral Pneumonia: The MuLBSTA Score. Front Microbiol. 2019;10:2752. doi: 10.3389/fmicb.2019.02752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy E., Fernandez-Patron C. Targeting MMP-Regulation of Inflammation to Increase Metabolic Tolerance to COVID-19 Pathologies: A Hypothesis. Biomolecules. 2021;11(3) doi: 10.3390/biom11030390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan I., Mukaigawara M., King L., Fernandes G., Sridhar D. Hindsight is 2020? Lessons in global health governance one year into the pandemic. Nat. Med. 2021;27(3):396–400. doi: 10.1038/s41591-021-01272-2. [DOI] [PubMed] [Google Scholar]
- Henry B.M. d O.M.H.S.B.S.P.M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin. Chem. Lab. Med. 2020;58(7):1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang I., Pranata R., Lim M.A., Oehadian A., Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther. Adv. Respir. Dis. 2020;14 doi: 10.1177/1753466620937175. 22 10.1177/1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara M., Ekiz T., Ricci V., Kara Ö., Chang K.-V., Özçakar L. ‘Scientific Strabismus’ or two related pandemics: coronavirus disease and vitamin D deficiency. Br. J. Nutr. 2020;124(7):736–741. doi: 10.1017/S0007114520001749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.S., Lee K.J., Kim H. Serum tumour necrosis factor-α and interleukin-6 levels in Alzheimer’s disease and mild cognitive impairment. Psychogeriatrics. 2017;17(4):224–230. doi: 10.1111/psyg.12218. [DOI] [PubMed] [Google Scholar]
- Kuchi Bhotla H., Kaul T., Balasubramanian B., Easwaran M., Arumugam V.A., Pappusamy M., Muthupandian S., Meyyazhagan A. Platelets to surrogate lung inflammation in COVID-19 patients. Med. Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.110098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavorgna G., Cavalli G., Dagna L., Gregori S., Larcher A., Landoni G., Ciceri F., Montorsi F., Salonia A. A virus-free cellular model recapitulates several features of severe COVID-19. Sci. Rep. 2021;11(1):17473. doi: 10.1038/s41598-021-96875-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Xiang X., Ren H., Xu L., Zhao L., Chen X., Long H., Wang Q., Wu Q. Serum Amyloid A is a biomarker of severe Coronavirus Disease and poor prognosis. J. Infect. 2020;80(6):646–655. doi: 10.1016/j.jinf.2020.03.035. https://doi.org/https://doi-org.login.ezproxy.library.ualberta.ca/10.1016/j.jinf.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Cao Z., Rahman P. Genetic variability of human angiotensin-converting enzyme 2 (hACE2) among various ethnic populations. Mol. Genet. Genom. Med. 2020;8(8) doi: 10.1002/mgg3.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020;10(2):102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G., Wong J., Henry B.M. Hypertension in patients with coronavirus disease 2019 (COVID-19): a pooled analysis. Pol. Arch. Intern Med. 2020;130(4):304–309. doi: 10.20452/pamw.15272. [DOI] [PubMed] [Google Scholar]
- Liu F., Li L., Xu M., Wu J., Luo D., Zhu Y., Li B., Song X., Zhou X. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 2020;127 doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos-Pérez D., Sánchez-Flores M., Maseda A., Lorenzo-López L., Millán-Calenti J.C., Pásaro E., Laffon B., Valdiglesias V. Serum cortisol but not oxidative stress biomarkers are related to frailty: results of a cross-sectional study in Spanish older adults. null. 2019;82(14):815–825. doi: 10.1080/15287394.2019.1654639. [DOI] [PubMed] [Google Scholar]
- Martins R., Carlos A.R., Braza F., Thompson J.A., Bastos-Amador P., Ramos S., Soares M.P. Disease Tolerance as an Inherent Component of Immunity. Annu Rev. Immunol. 2019;37:405–437. doi: 10.1146/annurev-immunol-042718-041739. [DOI] [PubMed] [Google Scholar]
- Mattos Dos Santos R. Isolation, social stress, low socioeconomic status and its relationship to immune response in Covid-19 pandemic context. Brain, Behav., Immun. - Health. 2020;7 doi: 10.1016/j.bbih.2020.100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.M., Bechtel M., Bojkova D., Münch C., Ciesek S., Wass M.N., Michaelis M., Cinatl J., Jr. COVID-19-Related Coagulopathy—Is Transferrin a Missing Link? Diagnostics. 2020;10(8):539. doi: 10.3390/diagnostics10080539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R., Schneider D.S., Soares M.P. Disease tolerance as a defense strategy. Science. 2012;335(6071):936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer D.O., Best T.J., Zhang H., Vokes T., Arora V., Solway J. Association of Vitamin D Deficiency and Treatment with COVID-19 Incidence. medRxiv. 2020 doi: 10.1101/2020.05.08.20095893. [DOI] [Google Scholar]
- Mercola J., Grant W.B., Wagner C.L. Evidence Regarding Vitamin D and Risk of COVID-19 and Its Severity. Nutrients. 2020;12(11):3361. doi: 10.3390/nu12113361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner C.B., Demichev V., Wendisch D., Michalick L., White M., Freiwald A., Textoris-Taube K., Vernardis S.I., Egger A.-S., Kreidl M., Ludwig D., Kilian C., Agostini F., Zelezniak A., Thibeault C., Pfeiffer M., Hippenstiel S., Hocke A., von Kalle C., Campbell A., Hayward C., Porteous D.J., Marioni R.E., Langenberg C., Lilley K.S., Kuebler W.M., Mülleder M., Drosten C., Suttorp N., Witzenrath M., Kurth F., Sander L.E., Ralser M. Ultra-High-Throughput Clinical Proteomics Reveals Classifiers of COVID-19 Infection. Cell Syst. 2020;11(1):11–24. doi: 10.1016/j.cels.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesarchio V., Parrella R., Iommelli C., Bianco A., Manzillo E., Fraganza F., Palumbo C., Rea G., Murino P., De Rosa R., Atripaldi L., D’Abbraccio M., Curvietto M., Mallardo D., Celentano E., Grimaldi A.M., Palla M., Trojaniello C., Vitale M.G., Million-Weaver S.L., Ascierto P.A. Outcomes and biomarker analyses among patients with COVID-19 treated with interleukin 6 (IL-6) receptor antagonist sarilumab at a single institution in Italy. J. Immunother. Cancer. 2020;8(2) doi: 10.1136/jitc-2020-001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller A.L., McNamara M.S., Sinclair D.A. Why does COVID-19 disproportionately affect older people? Aging (Albany NY) 2020;12(10):9959–9981. doi: 10.18632/aging.103344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder G., Rezza G., Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- Pabst G., Zimmermann A.K., Huth C., Koenig W., Ludwig T., Zierer A., Peters A., Thorand B. Association of low 25-hydroxyvitamin D levels with the frailty syndrome in an aged population: Results from the KORA-Age Augsburg study. J. Nutr., Health Aging. 2015;19(3):258–264. doi: 10.1007/s12603-014-0546-9. [DOI] [PubMed] [Google Scholar]
- Pang, R. T. P. T. C. C. K. C. L. N. L. C. R. W. T. Y. K. C. S. S. S. J. J, Lo Y.M. Serum amyloid A is not useful in the diagnosis of severe acute respiratory syndrome. Clin. Chem. 2006;52(6):1202–1204. doi: 10.1373/clinchem.2006.068395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranjpe, I., Russak, A.J., De Freitas, J.K., Lala, A., Miotto, R., Vaid, A., Johnson, K.W., Danieletto, M., Golden, E., Meyer, D., Singh, M., Somani, S., Kapoor, A., O'Hagan, R., Manna, S., Nangia, U., Jaladanki, S.K., O'Reilly, P., Huckins, L.M., Glowe, P., Kia, A., Timsina, P., Freeman, R.M., Levin, M.A., Jhang, J., Firpo, A., Kovatch, P., Finkelstein, J., Aberg, J.A., Bagiella, E., Horowitz, C.R., Murphy, B., Fayad, Z.A., Narula, J., Nestler, E.J., Fuster, V., Cordon-Cardo, C., Charney, D., Reich, D.L., Just, A., Bottinger, E.P., Charney, A.W., Glicksberg, B.S., Nadkarni, G.N., & Mount Sinai, C.I. C. (2020). Retrospective cohort study of clinical characteristics of 2199 hospitalised patients with COVID-19 in New York City. BMJ open, 10(11), e040736. Retrieved 2020/11//, from http://europepmc.org/abstract/MED/33247020. https://doi.org/10.1136/bmjopen-2020–040736. https://europepmc.org/articles/PMC7702220. https://europepmc.org/articles/PMC7702220?pdf=render. [DOI] [PMC free article] [PubMed]
- Polidori M.C. In: Encyclopedia of Gerontology and Population Aging. Gu D., Dupre M.E., editors. Springer International Publishing; 2020. Physiology of aging as a basis of complexity in aging medicine and geriatrics; pp. 1–6. [DOI] [Google Scholar]
- Polidori M.C., Sies H., Ferrucci L., Benzing T. COVID-19 mortality as a fingerprint of biological age. Ageing Res. Rev. 2021;67 doi: 10.1016/j.arr.2021.101308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon, T. C. P. R. T. C. K. C. L. N. L. C. R. W. T. Y. K. C. S. S. N. S. M, Lo Y.M. Proteomic analysis reveals platelet factor 4 and beta‐thromboglobulin as prognostic markers in severe acute respiratory syndrome. Electrophoresis. 2012;33(12):1894–1900. doi: 10.1002/elps.201200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pormohammad A., Ghorbani S., Khatami A., Farzi R., Baradaran B., Turner D.L., Turner R.J., Bahr N.C., Idrovo J.-P. Comparison of confirmed COVID-19 with SARS and MERS cases - Clinical characteristics, laboratory findings, radiographic signs and outcomes: A systematic review and meta-analysis. Rev. Med. Virol. 2020;30(4) doi: 10.1002/rmv.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath S., Perikala V., Jena A.B., Dandapat J. Factors regulating dynamics of angiotensin-converting enzyme-2 (ACE2), the gateway of SARS-CoV-2: Epigenetic modifications and therapeutic interventions by epidrugs. Biomed. Pharmacother. 2021;143 doi: 10.1016/j.biopha.2021.112095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y., He Q.-Y., Fan J., Jones B., Zhou Y., Xie Y., Cheung C.-Y., Wu A., Chiu J.-F., Peiris J.S.M., Tam P.K.H. The use of proteomics in the discovery of serum biomarkers from patients with severe acute respiratory syndrome. Proteomics. 2004;4(11):3477–3484. doi: 10.1002/pmic.200400897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie S.C., Kettunen J., Brozynska M., Nath A.P., Havulinna A.S., Männistö S., Perola M., Salomaa V., Ala-Korpela M., Abraham G., Würtz P., Inouye M. Elevated serum alpha-1 antitrypsin is a major component of GlycA-associated risk for future morbidity and mortality. PLOS ONE. 2019;14(10) doi: 10.1371/journal.pone.0223692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A., Kaarniranta K., Kauppinen A. Inflammaging: disturbed interplay between autophagy and inflammasomes. Aging. 2012;4(3):166–175. doi: 10.18632/aging.100444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider D.S., Ayres J.S. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat. Rev. Immunol. 2008;8(11):889–895. doi: 10.1038/nri2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom S.C., Miller G.E. Psychological Stress and the Human Immune System: A Meta-Analytic Study of 30 Years of Inquiry. Psychol. Bull. 2004;130(4):601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B., Yi X., Sun Y., Bi X., Du J., Zhang C., Quan S., Zhang F., Sun R., Qian L., Ge W., Liu W., Liang S., Chen H., Zhang Y., Li J., Xu J., He Z., Chen B., Wang J., Yan H., Zheng Y., Wang D., Zhu J., Kong Z., Kang Z., Liang X., Ding X., Ruan G., Xiang N., Cai X., Gao H., Li L., Li S., Xiao Q., Lu T., Zhu Y., Liu H., Chen H., Guo T. Proteomic and Metabolomic Characterization of COVID-19 Patient Sera. Cell. 2020;182(1):59–72. doi: 10.1016/j.cell.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherlock, B. Sherlock™ CRISPR SARS-CoV-2. In (Vol. 2020).
- Sherlock, B. (2020). Sherlock Biosciences Receives FDA Emergency Use Authorization for CRISPR SARS-CoV-2 Rapid Diagnostic. In.
- Short K.R., Kedzierska K., van de Sandt C.E. Back to the Future: Lessons Learned From the 1918 Influenza Pandemic. Front Cell Infect. Microbiol. 2018;8:343. doi: 10.3389/fcimb.2018.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha P, M.M. A.C. C.S. (2020). Is a “Cytokine Storm” Relevant to COVID-19? Is a “Cytokine Storm” Relevant to COVID-19?, 180(9), 1152–1154. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed]
- Sorokin A.V., Karathanasis S.K., Yang Z.-H., Freeman L., Kotani K., Remaley A.T. COVID-19—Associated dyslipidemia: Implications for mechanism of impaired resolution and novel therapeutic approaches. FASEB J. 2020;34(8):9843–9853. doi: 10.1096/fj.202001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai W., Zhang X., Drelich A., Shi J., Hsu J.C., Luchsinger L., Hillyer C.D., Tseng C.-T.K., Jiang S., Du L. A novel receptor-binding domain (RBD)-based mRNA vaccine against SARS-CoV-2. Cell Res. 2020;30(10):932–935. doi: 10.1038/s41422-020-0387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A., Flanigan M.E., McEwen B.S., Russo S.J. Aggression, Social Stress, and the Immune System in Humans and Animal Models [Review] Front. Behav. Neurosci. 2018;12(56):56. doi: 10.3389/fnbeh.2018.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan T., Khoo B., Mills E.G., Phylactou M., Patel B., Eng P.C., Thurston L., Muzi B., Meeran K., Prevost A.T., Comninos A.N., Abbara A., Dhillo W.S. Association between high serum total cortisol concentrations and mortality from COVID-19. Lancet Diabetes Endocrinol. 2020;8(8):659–660. doi: 10.1016/S2213-8587(20)30216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D., Comish P., Rui K. The hallmarks of COVID-19 disease. PLOS Pathog. 2020;16(5) doi: 10.1371/journal.ppat.1008536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakolpour S., Rakhshandehroo T., Wei E.X., Rashidian M. Lymphopenia during the COVID-19 infection: What it shows and what can be learned. Immunol. Lett. 2020;225:31–32. doi: 10.1016/j.imlet.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehrani S., Killander A., Åstrand P., Jakobsson J., Gille-Johnson P. Risk factors for death in adult COVID-19 patients: Frailty predicts fatal outcome in older patients. Int J. Infect. Dis. 2021;102:415–421. doi: 10.1016/j.ijid.2020.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpos E., Ntanasis-Stathopoulos I., Elalamy I., Kastritis E., Sergentanis T.N., Politou M., Psaltopoulou T., Gerotziafas G., Dimopoulos M.A. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020;95(7):834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udugama B., Kadhiresan P., Kozlowski H.N., Malekjahani A., Osborne M., Li V.Y.C., Chen H., Mubareka S., Gubbay J., Chan W.C.W. Diagnosing COVID-19: The Disease and Tools for Detection. ACS Nano. 2020;14(4):3822–3835. doi: 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]
- Wagner K.-H., Cameron-Smith D., Wessner B., Franzke B. Biomarkers of aging: from function to molecular biology. Nutrients. 2016;8(6):338. doi: 10.3390/nu8060338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Nie J., Wang H., Zhao Q., Xiong Y., Deng L., Song S., Ma Z., Mo P., Zhang Y. Characteristics of Peripheral Lymphocyte Subset Alteration in COVID-19 Pneumonia. J. Infect. Dis. 2020;22(11):1762–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Chen W., Zhang Z., Deng Y., Lian J.-Q., Du P., Wei D., Zhang Y., Sun X.-X., Gong L., Yang X., He L., Zhang L., Yang Z., Geng J.-J., Chen R., Zhang H., Wang B., Zhu Y.-M., Nan G., Jiang J.-L., Li L., Wu J., Lin P., Huang W., Xie L., Zheng Z.-H., Zhang K., Miao J.-L., Cui H.-Y., Huang M., Zhang J., Fu L., Yang X.-M., Zhao Z., Sun S., Gu H., Wang Z., Wang C.-F., Lu Y., Liu Y.-Y., Wang Q.-Y., Bian H., Zhu P., Chen Z.-N. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct. Target. Ther. 2020;5(1):283. doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.F., Anderson D.E., Mackenzie J.S., Merson M.H. From Hendra to Wuhan: what has been learned in responding to emerging zoonotic viruses. Lancet. 2020;395(10224):e33–e34. doi: 10.1016/S0140-6736(20)30350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health, O. Coronavirus disease (COVID-19) pandemic. In (Vol. 2020).
- World Health, O., 2003, Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. In.
- World Health, O., 2018, Ageing and health. In (Vol. 2021).
- Wu J.T., Leung K., Bushman M., Kishore N., Niehus R., de Salazar P.M., Cowling B.J., Lipsitch M., Leung G.M. Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat. Med. 2020;26(4):506–510. doi: 10.1038/s41591-020-0822-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X., Wang Y., Zheng J. COVID-19 and Alzheimer’s disease: how one crisis worsens the other. Transl. Neurodegener. 2021;10(1):15. doi: 10.1186/s40035-021-00237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Wang W., Chen Z., Lu S., Yang F., Bi Z., Bao L., Mo F., Li X., Huang Y., Hong W., Yang Y., Zhao Y., Ye F., Lin S., Deng W., Chen H., Lei H., Zhang Z., Luo M., Gao H., Zheng Y., Gong Y., Jiang X., Xu Y., Lv Q., Li D., Wang M., Li F., Wang S., Wang G., Yu P., Qu Y., Yang L., Deng H., Tong A., Li J., Wang Z., Yang J., Shen G., Zhao Z., Li Y., Luo J., Liu H., Yu W., Yang M., Xu J., Wang J., Li H., Wang H., Kuang D., Lin P., Hu Z., Guo W., Cheng W., He Y., Song X., Chen C., Xue Z., Yao S., Chen L., Ma X., Chen S., Gou M., Huang W., Wang Y., Fan C., Tian Z., Shi M., Wang F.-S., Dai L., Wu M., Li G., Wang G., Peng Y., Qian Z., Huang C., Lau J.Y.-N., Yang Z., Wei Y., Cen X., Peng X., Qin C., Zhang K., Lu G., Wei X. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature. 2020;586(7830):572–577. doi: 10.1038/s41586-020-2599-8. [DOI] [PubMed] [Google Scholar]
- Yang Y., Hao Q., Flaherty J.H., Cao L., Zhou J., Su L., Shen Y., Dong B. Comparison of procalcitonin, a potentially new inflammatory biomarker of frailty, to interleukin-6 and C-reactive protein among older Chinese hospitalized patients. Aging Clin. Exp. Res. 2018;30(12):1459–1464. doi: 10.1007/s40520-018-0964-3. [DOI] [PubMed] [Google Scholar]
- Yip T.T.C., Chan J.W.M., Cho W.C.S., Yip T.-T., Wang Z., Kwan T.-L., Law S.C.K., Tsang D.N.C., Chan J.K.C., Lee K.-C., Cheng W.-W., Ma V.W.S., Yip C., Lim C.K.P., Ngan R.K.C., Au J.S.K., Chan A., Lim W.W.L. Protein Chip Array Profiling Analysis in Patients with Severe Acute Respiratory Syndrome Identified Serum Amyloid A Protein as a Biomarker Potentially Useful in Monitoring the Extent of Pneumonia. Clin. Chem. 2005;51(1):47–55. doi: 10.1373/clinchem.2004.031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Guo H. Biomarkers of COVID-19 and technologies to combat SARS-CoV-2. Adv. Biomark. Sci. Technol. 2020;2:1–23. doi: 10.1016/j.abst.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Xiang R., Huo S., Zhou Y., Jiang S., Wang Q., Yu F. Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy. Signal Transduct. Target. Ther. 2021;6(1):233. doi: 10.1038/s41392-021-00653-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Yang Z., Liu X., Tian Q., Lv Y., Liang Y., Li C., Gao X., Chen L. Identification of α1-antitrypsin as a potential prognostic biomarker for advanced nonsmall cell lung cancer treated with epidermal growth factor receptor tyrosine kinase inhibitors by proteomic analysis. J. Int Med Res. 2013;41(3):573–583. doi: 10.1177/0300060513476582. [DOI] [PubMed] [Google Scholar]
- Zuo L., Prather E.R., Stetskiv M., Garrison D.E., Meade J.R., Peace T.I., Zhou T. Inflammaging and oxidative stress in human diseases: from molecular mechanisms to novel treatments. Int. J. Mol. Sci. 2019;20(18):4472. doi: 10.3390/ijms20184472. 〈https://www.mdpi.com/1422-0067/20/18/4472〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material